Abstract
Current suboptimal treatment options of invasive fungal infections and emerging resistance of the corresponding pathogens urge the need for alternative therapy strategies and require the identification of novel antifungal targets. Aspergillus fumigatus is the most common airborne opportunistic mold pathogen causing invasive and often fatal disease. Establishing a novel in vivo conditional gene expression system, we demonstrate that downregulation of the class 1 lysine deacetylase (KDAC) RpdA leads to avirulence of A. fumigatus in a murine model for pulmonary aspergillosis. The xylP promoter used has previously been shown to allow xylose-induced gene expression in different molds. Here, we demonstrate for the first time that this promoter also allows in vivo tuning of A. fumigatus gene activity by supplying xylose in the drinking water of mice. In the absence of xylose, an A. fumigatus strain expressing rpdA under control of the xylP promoter, rpdAxylP, was avirulent and lung histology showed significantly less fungal growth. With xylose, however, rpdAxylP displayed full virulence demonstrating that xylose was taken up by the mouse, transported to the site of fungal infection and caused rpdA induction in vivo. These results demonstrate that (i) RpdA is a promising target for novel antifungal therapies and (ii) the xylP expression system is a powerful new tool for in vivo gene silencing in A. fumigatus.
Keywords: KDAC, RpdA, murine virulence model, Aspergillus fumigatus, xylose, xylP, conditional in vivo expression, HDAC
Introduction
Aspergillus fumigatus is the most common airborne mold pathogen, capable of causing systemic disease, termed invasive pulmonary aspergillosis, mostly in immunocompromised patients (van de Veerdonk et al., 2017). Difficulties in diagnosis and the emergence of azole-resistant clinical isolates result in high mortality rates associated with invasive pulmonary aspergillosis (Meis et al., 2016; Fisher et al., 2018). Additionally, antifungal drugs used in the clinic suffer from poor specificity and side effects in patients (Campoy and Adrio, 2017). Consequently, there is an urgent need for improvement of antifungal prophylaxis, diagnosis, and therapy (Denning and Bromley, 2015).
Chromatin modulators represent potential antifungal targets. Chromatin is composed of DNA, histones and other proteins ensuring compact organization of the genetic material. The highly conserved N-terminal tails of histones are subject to a variety of post-translational modifications, which significantly impact the expression of genes. One of these modifications is the reversible acetylation of distinct lysine residues, catalyzed by lysine acetyltransferases and their counterparts, lysine deacetylases (KDACs), originally termed histone deacetylases (HDACs). The term KDAC appears more appropriate as it became evident that non-histone proteins are also subject to acetylation by the very same enzymes (reviewed by Narita et al., 2019). One example for an A. fumigatus non-histone protein, whose acetylation status has been proposed to have significant implications on virulence, is the heat shock protein 90 (Lamoth et al., 2014).
Most fungi have four classical KDACs (Brosch et al., 2008) belonging to two different classes. In Aspergillus nidulans, two class 1 enzymes (orthologs of yeast RPD3 and HOS2) and two class 2 enzymes (orthologs of yeast HDA1 and HOS3) were identified (Graessle et al., 2000). Several reports have linked KDACs to fungal virulence pathways, e.g., of the plant pathogens Cochliobolus carbonum (Baidyaroy et al., 2001), Ustilago maydis (Elías-Villalobos et al., 2015) or the human pathogens Candida albicans (Hnisz et al., 2009), and Cryptococcus neoformans (Brandão et al., 2018). Furthermore, application of KDAC inhibitors has been proposed to have an additive effect on antifungal treatment with triazoles, however, with contrasting results depending on the fungi examined (e.g., Pfaller et al., 2009; Pidroni et al., 2018).
In A. fumigatus, the KDAC RpdA was recently shown to be essential and was therefore proposed as a potential new target for antifungal therapies (Bauer et al., 2016). RpdA-type KDACs share a catalytic domain that is highly conserved throughout eukaryotes and that is druggable; several such inhibitors are FDA-approved for cancer treatment (West and Johnstone, 2014). Furthermore, RpdA proteins contain a conserved fungal-specific C-terminal motif required for nuclear localization, catalytic activity, and viability in axenic growth (Tribus et al., 2010; Bauer et al., 2016), which might allow development of fungal-specific KDAC inhibitors.
In order clarify the significance of RpdA during infection, an appropriate conditional gene expression system in a murine aspergillosis model was required. Different conditional gene expression systems have been established in A. fumigatus based on different promoters, e.g., the nitrate-inducible A. fumigatus niiA promoter, the alcohol-inducible A. nidulans alcA promoter, the xylose-inducible Penicillium chrysogenum xylP promoter (Zadra et al., 2000; Romero et al., 2003; Hu et al., 2007; Hartmann et al., 2010), and tetracycline-inducible/repressible Tet-On/Off systems exploiting the Escherichia coli tetracycline-resistance operon (Vogt et al., 2005). Of these, the Tet-On/Off systems have been applied for both up- and downregulation, while the niiA promoter was employed for downregulation of gene expression during infection (Hu et al., 2007; Lin et al., 2015; Sasse et al., 2016; Peng et al., 2018; Souza et al., 2019). The promoter of the P. chrysogenum β-1,4-endoxylanase-encoding xylP gene has been shown previously to allow xylose-mediated activation of gene expression (Figure 1A), even in the presence of glucose, during axenic growth in several molds including A. nidulans, A. fumigatus, P. chrysogenum, and Penicillium marneffei (Zadra et al., 2000; Pongsunk et al., 2005; Hartmann et al., 2010; Sigl et al., 2010; Tribus et al., 2010; Yasmin et al., 2012; Bauer et al., 2016; Vaknin et al., 2016; Gsaller et al., 2018; Misslinger et al., 2018). Moreover, xylose has been shown to be well absorbed, poorly metabolized, and quickly excreted by monogastric mammals (Huntley and Patience, 2018). These properties are already exploited in medicine to study absorption and, consequently, to assay the integrity of the gastrointestinal mucosa (Weiner et al., 1984).
FIGURE 1.
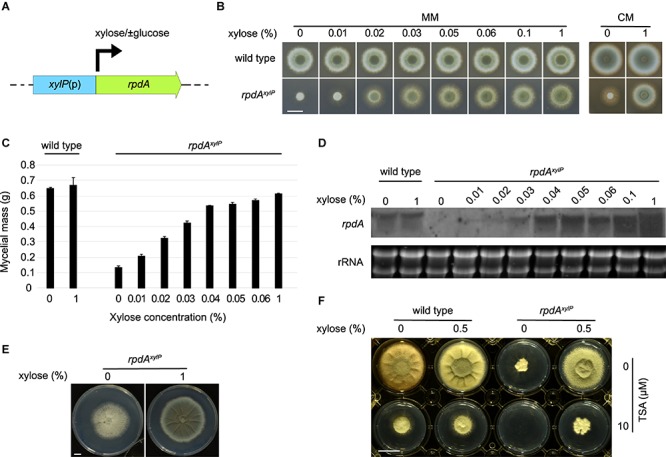
Expression of rpdA is tunable by xylose in strain rpdAxylP during axenic growth. (A) Scheme of rpdA expression under control of the xylP promoter, xylP(p), in strain rpdAxylP. The arrow indicates strong induction by xylose even in the presence of glucose. The transcription start sites (34 and 38 bp upstream of the translation start) have been determined previously (Haas et al., 1993). Promoter motifs including putative binding sites for the xylose-sensing transcription factor XlnR have been predicted previously (Zadra et al., 2000). (B) Growth of rpdAxylP and wild type on solid minimal (MM) and complex (CM) media with different xylose concentrations. Fungal strains were point inoculated (1 × 104 conidia), pictures were taken after incubation for 48 h at 37°C. (C) Biomass measurements of rpdAxylP mycelia grown in liquid minimal medium. Media were inoculated with 106 conidia per ml and biomass production was determined after growth for 24 h at 37°C. Error bars represent the standard deviation of three replicates. (D) Northern analysis of rpdA expression in wild type and rpdAxylP grown under different xylose concentrations. RNA was isolated after 20 h of growth in liquid minimal medium supplemented with xylose as indicated. (E) Growth of rpdAxylP on solid MM with and without xylose. Fungal strains were point inoculated (1 × 104 conidia), pictures were taken after incubation for 5 days at 37°C. (F) Effect of trichostatin A (TSA) on growth of wild type and rpdAxylP with and without xylose induction. Fungal strains were point inoculated (1 × 104 conidia) on solid MM containing 10 μM TSA solubilized in DMSO or the same DMSO concentration (0.2%) without TSA. Picture was taken after 3 days of incubation at 37°C. Scale bars represent 1 cm.
Here, we demonstrate for the first time, that (i) the xylP promoter allows the control of A. fumigatus gene expression during infection in a non-neutropenic murine pulmonary aspergillosis model and (ii) downregulation of rpdA renders A. fumigatus avirulent. These data confirm RpdA as promising target for KDAC inhibitors with potential as novel agents against invasive fungal infections.
Results
Depletion of RpdA Leads to a Drastic but Reversible Growth Reduction Under Axenic Growth Conditions
To clarify the role of RpdA (Afu2g03390) during infection, we used for the first time the xylP promoter for conditional gene expression of rpdA in a murine aspergillosis model. Therefore, the endogenous rpdA promoter was replaced by the xylP promoter with concomitant integration of a pyrithiamine resistance cassette via homologous recombination in A. fumigatus AfS35, termed wild type here, resulting in rpdAxylP transformants, which were verified by Southern blot analysis (Supplementary Figure S1).
Although RpdA has been shown to be essential in A. fumigatus by heterokaryon rescue analysis (Bauer et al., 2016), rpdAxylP displayed weak growth without sporulation on solid minimal medium (Figure 1B), solid complex medium (Figure 1B) and in liquid medium without xylose-induction (Figure 1C). Xylose rescued growth of rpdAxylP in a concentration-dependent manner confirming the functionality of this expression system. Interestingly, radial growth of rpdAxylP did not fully reach the wild-type degree (Figure 1B), which might reflect differences in the 5′-UTR of rpdA in the rpdAxylP strain, as the rpdA mRNA in rpdAxylP contains the 5′-UTR of the xylP mRNA. Alternatively, fine-tuned regulation of rpdA expression during growth via the endogenous promoter might be of importance. Northern analysis confirmed downregulation of rpdA in the absence of xylose and a xylose-concentration-dependent induction in strain rpdAxylP (Figure 1D), while xylose did not affect rpdA expression in the wild type.
The weak growth of rpdAxylP without xylose induction might be caused by slight leakiness of the xylP promoter and/or by parental carry-over of xylose or RpdA via the conidia used for inoculation (see below). After 5 days of growth the colony area of rpdAxylP without xylose induction reached 75% of that of rpdAxylP with xylose induction (Figure 1E). An impact of parental RpdA carry-over during germination cannot be completely excluded. Nevertheless, the presented data indicate that growth of rpdAxylP without xylose induction is caused by leakiness of the xylP promoter as dilution of parental RpdA via growth would be expected to decrease and finally block the growth rpdAxylP without xylose induction within this time frame. Notably, radial growth of rpdAxylP without xylose displayed significant differences to rpdAxylP with xylose induction: The mycelial layer was significantly thinner (data not shown) and conidiation was largely blocked in rpdAxylP without induction (Figure 1E). Similarly, RpdA was found to play a crucial role in conidiation in A. nidulans (Tribus et al., 2010).
The KDAC inhibitor trichostatin A (TSA, 10 μM) decreased the growth of wild type and xylose-induced rpdAxylP but completely blocked growth of rpdAxylP without xylose induction (Figure 1F). These data strongly indicate that RpdA is indeed the target for the growth-inhibitory activity of TSA as suggested previously in A. nidulans (Bauer et al., 2016).
Xylose Feeding of Mice Leads to Rising Xylose Concentrations in Plasma
To test if xylose is absorbed by mice to levels sufficient for in vivo induction of the xylP promoter, 0.2 ml of a 10% (w/v) xylose solution was administered to mice by oral gavage, followed by determination of the plasma xylose concentration 15–240 min after xylose ingestion (Figure 2). No xylose was detected in plasma samples taken directly after the gavage, indicating the absence of xylose under conventional nutritional conditions. Already after 30 min, a plasma xylose concentration of 0.055% was reached. The xylose concentration decreased after 60 min and was hardly detectable after 120 min indicating efficient clearing. As reported previously, mice drink 4–8 ml water within 24 h (Bachmanov et al., 2002), resulting in approx. 0.25 ml/h calculated water uptake (Li et al., 2016). These data indicated that drinking of xylose results in sufficient concentrations for in vivo induction of the xylP promoter.
FIGURE 2.
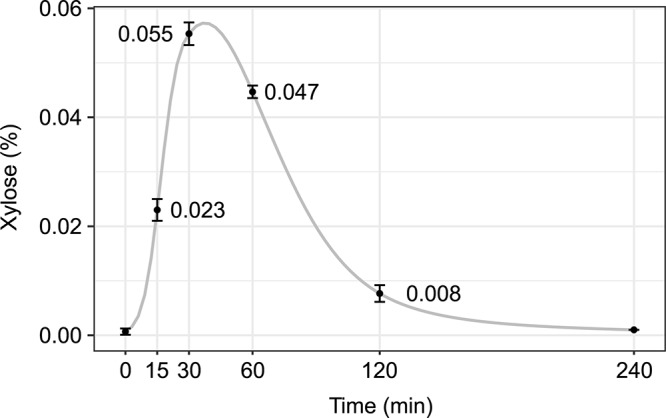
Xylose plasma levels reach concentrations that are sufficient to promote growth of rpdAxylP in vitro. 0.2 ml of a 10% xylose solution were administered to a mouse by oral gavage. Blood samples were taken after the time points indicated and plasma xylose concentrations were measured in triplicates (mean ± standard deviation is indicated). A cubic spline interpolation line illustrating the course of xylose plasma levels is shown in gray.
Downregulation of rpdA Causes Avirulence
Next, the rpdAxylP strain was tested for virulence in a non-neutropenic pulmonary infection model, in which mice are immunosuppressed with cortisone acetate. Three days after the first immunosuppression, cohorts of ten mice were infected intranasally with wild-type or rpdAxylP conidia and were supplied with drinking water with or without xylose (10% m/v). For mice infected with the wild-type strain, the xylose diet did not affect mortality (Supplementary Figure S2), weight loss, or drinking behavior (data not shown). The latter is interesting as xylose supplementation results in a sweet taste of the drinking water, which might affect drinking behavior.
The rpdAxylP strain with xylose supplementation showed mortality rates similar to that of the wild-type strain without xylose in the drinking water (Figure 3). In contrast, rpdAxylP without xylose was avirulent (P < 0.0001, Figure 3). Histological analysis 2 days post infection revealed a similar progress in germination of wild-type and rpdAxylP strains with and without xylose (Figure 4C), which is in agreement with the growth of rpdAxylP under both inducing and non-inducing axenic conditions (Figures 1B,C), although the growth was very limited under non-inducing conditions. The rpdAxylP mutant without xylose caused a lower lung fungal burden compared to rpdAxylP with xylose (P = 0.0292, Figure 4A) or wild type without xylose (P = 0.0094, Figure 4A). Moreover, examination of histological lung sections revealed less and smaller fungal lesions in mice infected with rpdAxylP maintained without xylose, as determined by measuring the total length of fungal hyphae within 10 infection foci each of rpdAxylP with and without xylose and wild-type (P < 0.0001, Figures 4B,C). These results match the partial growth of rpdAxylP in the absence of xylose during axenic growth conditions (Figures 1B,C).
FIGURE 3.
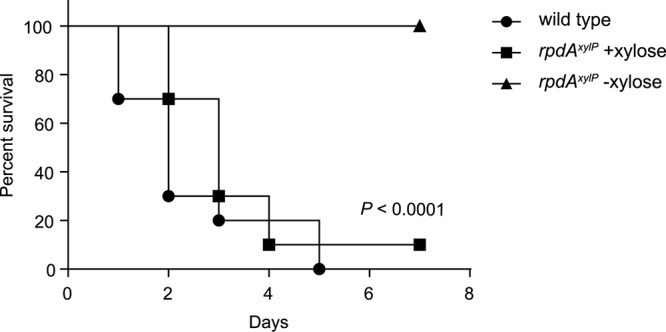
Downregulation of rpdA causes avirulence. Mouse survival curves (Kaplan–Meier plot) following intranasal infection of cortisone-acetate immunocompromised mice (n = 10 animals/group) with (+) and without (–) 10% xylose in the drinking water. Comparison of survival curves for rpdAxylP−xylose vs. wild type or rpdAxylP +xylose using the log-rank test (Mantel-Cox) revealed significance at P < 0.0001.
FIGURE 4.
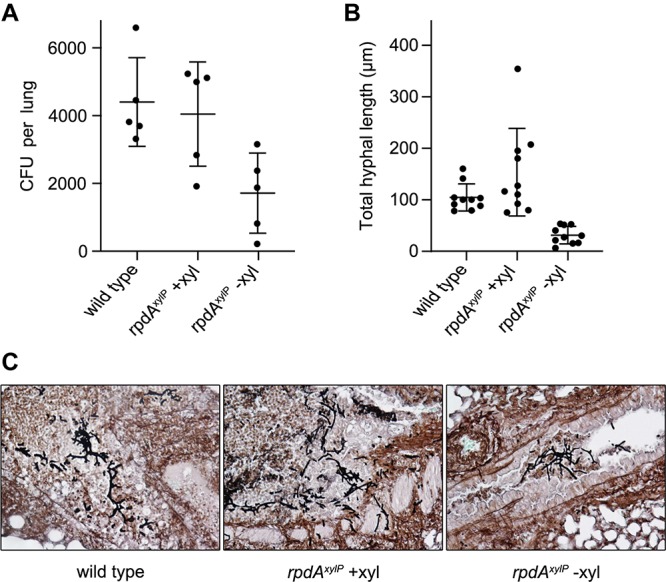
Histology reveals reduced fungal colonization by avirulent rpdAxylP in the absence of xylose. Lungs were extracted 2 days post intranasal infection of cortisone-acetate immunocompromised mice. (A) Mouse lung fungal burden is reduced in mice infected with rpdAxylP−xylose (xyl) compared to wild type (P = 0.0094) and rpdAxylP +xylose (P = 0.0292). (B) Hyphal lesions from rpdAxylP –xylose contain significantly less fungal hyphae (P < 0.0001) than those from rpdAxylP +xylose and wild type. The extend of fungal growth was measured by manually tracing the combined length of hyphae in each lesion (n = 10) using ImageJ software. (C) Representative lung sections of infected mice. Staining, performed with gomori methenamine silver (GMS), stains fungi black.
Taken together, these data demonstrate that downregulation of rpdA (rpdAxylP under non-inducing conditions), which allows limited axenic growth (Figures 1B,C), permits germination during infection (Figures 4B,C) but is insufficient to support virulence (Figure 3). Moreover, the avirulence of rpdAxylP without xylose supplementation compared to the virulence of rpdAxylP with xylose supplementation (Figure 3) demonstrates that the xylP promoter allows control of expression during infection by xylose feeding.
Discussion
It has been shown that the class 1 KDAC RpdA is essential for growth of both A. nidulans and A. fumigatus under axenic conditions (Tribus et al., 2010; Bauer et al., 2016). Concordantly, pharmacological KDAC inhibition resulted in strong growth inhibition (Bauer et al., 2016). Here, we employed for the first time the xylP promoter, which has previously been shown to allow xylose-mediated induction of gene expression in several mold species during axenic conditions, for modulation of A. fumigatus gene expression during murine infection.
The observed wild-type-like virulence of rpdAxylP under xylose supplementation demonstrates that the xylP inducer xylose indeed reaches the infected tissue in amounts sufficient to induce rpdA to a level that can rescue virulence. The employed xylP system therefore appears to be a suitable new tool to conduct virulence studies as an interesting alternative to gene deletion mutants or expression systems that are induced or repressed by tetracycline/doxycycline (Tet-On/Tet-Off promoter systems). The latter systems have been introduced for A. fumigatus in several axenic approaches (Vogt et al., 2005; Helmschrott et al., 2013; Kalb et al., 2015; Macheleidt et al., 2015; Wanka et al., 2016) and there are four studies employing these promoters for control of A. fumigatus gene expression during infection: three for Tet-On (Lin et al., 2015; Sasse et al., 2016; Souza et al., 2019) and one for the Tet-Off system (Peng et al., 2018). A disadvantage of administration of tetracycline-type antibiotics might be their potential side effects in host mitochondria and on the animal microbiome (Ahler et al., 2013; Chatzispyrou et al., 2015; Moullan et al., 2015). Moreover, the Tet-On/Tet-Off systems require not only exchange of the promoter, as in the xylP system, but additionally incorporation of the Tet transcription factor (Lin et al., 2015; Sasse et al., 2016). The half-life of doxycycline in blood was determined to be approximately 170 min in healthy mice (Böcker et al., 1981, 1984). Due to the shorter in vivo half-life of xylose in blood of approximately 35 min, the xylP promoter system might be an attractive alternative, providing different kinetics.
As RpdA has been shown previously to be essential for growth (Bauer et al., 2016), the limited growth of the rpdAxylP strain in the absence of xylose indicates slight leakiness of the xylP promoter. Expression of various genes under control of the xylP promoter in several mold fungi caused a loss-of-function phenotype under non-inducing conditions (Pongsunk et al., 2005; Hartmann et al., 2010; Sigl et al., 2010; Fazius et al., 2012; Gsaller et al., 2012; Bugeja et al., 2013; Baldin et al., 2015; Vaknin et al., 2016), e.g., growth of mutant strains expressing essential genes of the iron sulfur cluster biosynthetic machinery under control of the xylP promoter was completely blocked in non-inducing conditions in A. fumigatus (Misslinger et al., 2018). In contrast, use of the xylP promoter for expression of the essential monothiol glutaredoxin encoding grxD gene in A. fumigatus indicated leakiness of the xylP promoter dependent on the grxD allele (Misslinger et al., 2019). Most likely, the consequence of promoter leakiness, as monitored by growth pattern, depends on the gene expressed; i.e., it is affected by the required amount of the gene product, whereby the efficiency of expression is influenced by mRNA and protein stability, which are gene-specific features. Moreover, the genomic integration site might affect promoter leakiness. Similar to A. fumigatus, xylP promoter-driven rpdA without induction allowed minimal growth also in A. nidulans (Tribus et al., 2010).
At first glance, promoter leakiness might appear unfavorable. Under certain circumstances, however, it actually might be advantageous. The avirulence of rpdAxylP without xylose induction underlines that even a decrease and not only absence of rpdA expression is sufficient to impair pathogenicity. This result actually emphasizes the importance of RpdA as a virulence factor. Moreover, residual RpdA activity in rpdAxylP strains under repression mimics a realistic drug treatment scenario much more than an entire blocking of gene expression, as drug-mediated therapies hardly ever cause total inhibition of the respective targets. Consequently, only few therapy regimens achieve the effect of a total inactivation of the corresponding targets or physiological pathways. Furthermore, in studies evaluating potential drug targets with molds, generally conidia are used for pathogen inoculation. Very often it is observed that the avirulence of mutants is based on the lack of germination of the conidia in the lung. However, this does not reflect the real scenario for therapeutic intervention, as treatment of patients has to combat hyphae but not conidia. In this respect, xylP promoter-mediated downregulation of gene expression represents an interesting alternative to gene deletion, not only for the evaluation of the role in virulence of essential, but also for non-essential genes. On the other hand, regarding a potential broader application, promoter leakiness could indeed impede significant knock-down, particularly of weakly expressed genes.
Growth of rpdAxylP without xylose induction was completely blocked by the KDAC inhibitor TSA in a concentration that caused only partial growth inhibition of wild type or xylose-induced rpdAxylP. These data indicate that the antifungal effect of TSA is mainly due to inhibition of catalytic RpdA activity.
The avirulence of the rpdAxylP strain under non-inducing conditions might be attributed to its reduced growth. Remarkably, deletion of the mitogen-activated protein kinase MpkA-encoding gene caused a dramatic growth defect without diminishing virulence (Valiante et al., 2008). The latter indicates that growth rate and virulence are not always strictly linked. If avirulence of rpdAxylP under non-inducing conditions is caused by the growth defect or a deregulation of virulence-relevant pathways remains to be elucidated.
Irrespective of the mode of action of RpdA, several KDAC inhibitors have already been clinically approved by the FDA for therapeutical use against certain types of cancer: e.g., vorinostat, panobinostat, belinostat, and romidepsin (West and Johnstone, 2014). It is, however, not clear yet, to which extent these compounds inhibit RpdA activity or affect growth of A. fumigatus in vitro or in vivo. Importantly, RpdA itself seems to be a promising candidate for the development of fungal-specific KDAC inhibitors due to specific structure variations within its catalytic domain and the presence of a conserved fungal-specific C-terminal motif (Bauer et al., 2016).
Taken together, in this study we demonstrate the crucial role of RpdA in virulence of A. fumigatus employing a novel expression system, which enables tuning of expression during the infection process.
Materials and Methods
Strains and Media
For this study, the rpdA promoter was exchanged by the xylP promoter in the strain AfS35, which lacks non-homologous end joining due to deletion of the akuA gene (Krappmann et al., 2006). Information on these strains is given in Table 1. Fungal strains were propagated in Aspergillus minimal medium (AMM: 1% (m/v) glucose, 20 mM glutamine, salt solution and trace elements according to Pontecorvo et al., 1953) and in Aspergillus complete medium (CM: 2% (m/v) glucose, 0.2% (m/v) peptone, 0.1% (m/v) yeast extract, 0.1% (m/v) casamino acids, salt solution and trace elements). Addition of xylose is indicated separately.
TABLE 1.
Strains used in this study.
| Name | Genotype | Background/FGSC# | References |
| AfS35 (wild type) | akuAΔ:loxP | D141/A1159 | Krappmann et al., 2006 |
| TIB104 (rpdAxylP) | rpdA(p):ptrA, xylP(p):rpdA; akuAΔ:loxP | AfS35 | This study |
Gene Cassette and Strain Generation
For generation of the rpdAxylP allele, protoplast fusion (Tilburn et al., 1983) combined with the split-marker technique (Nielsen et al., 2006) was used. A. fumigatus AfS35 was co-transformed with 2 DNA fragments, each containing 0.5-kb overlapping but incomplete fragments of the pyrithiamine resistance-conferring ptrA allele from Aspergillus oryzae (Kubodera et al., 2000) ligated to 1.2- and 1-kb of A. fumigatus rpdA 5′-UTR and rpdA coding regions, respectively. These fragments were amplified from plasmid pIB24, which was constructed as follows: First, the rpdA 5′-UTR was amplified using primers AfrpdA5UTRfwdKpnI (AAATGGTACCATCCAACGACTGTTTACCATCCAACGACT GTT) and AfrpdA5UTRrevNdeI (TTTATAATACATATGTGAG AAAGGTCGTGTATGTGAGAAAGGTCGTG). The resulting product was digested with KpnI and NdeI and cloned into pSK275 (Szewczyk and Krappmann, 2010) harboring the pyrithiamine resistance cassette digested with the same enzymes, yielding pSK275-rpdA5′UTR. Next, a fragment containing the P. chrysogenum xylP promoter (Zadra et al., 2000) fused to the rpdA coding sequence was inserted at the SmaI site of pSK275-rpdA5′UTR and screened for proper orientation by restriction analysis. To this end, the xylP promoter fragment was amplified using primers xylPf2 (CCCGGGCACTGATGCGAGC AACAG) and AfrpdAxylPr (CGGAGGAGCAGCCATGTT GGTTCTTCGAGTCG) with pxylPrpdATRU0 (Tribus et al., 2010) as template and then spliced by overlap extension (Shevchuk et al., 2004) to a part of the coding sequence of rpdA that was amplified from A. fumigatus genomic DNA with primers xylPAfrpdAf (CGACTCGAAGAACCAACATGGCTGCTCCTC CGATTG) and AfrpdA7r (ACTGTCACCACCACACTG). All PCR reactions were performed using the Pfu-X7 polymerase (Nørholm, 2010).
RNA Isolation and Northern Blot Analysis
RNA was isolated with TRI Reagent (Sigma) according to the manufacturer’s manual. 10 μg of RNA was used for electrophoresis on a 0.6 M formaldehyde agarose gel and blotted on Hybond-N-membranes (Amersham). A digoxigenin (DIG)-labeled rpdA probe was amplified by PCR using primers AfrpdA5f (CAAGTACGGAGAATACTTCC) and AfrpdA6r (CTTCGTCAAGGTCATCCAG) and detected with an anti-DIG-AP conjugate and the chemiluminescent substrate CSPD (Sigma).
Southern Blot Analysis
Genomic DNA was extracted by PCI and precipitated with isopropanol. For verification of correct xylP promoter integration (xylP(p):rpdA), DNA was digested with PstI and signals were detected with DIG-labeled probes against the 5′ part of the rpdA coding sequence. Correct integration at the rpdA locus yielded a signal at 3422 bp. The DIG-labeled rpdA probe was amplified from pIB24 by PCR using oligonucleotides xylPf3 (TTCATCGACTCGAAGAAC) and AfrpdAr2 (GTCGCATTTGTTGCGGTTAAG).
Plasma Xylose Measurements
Mouse plasma xylose concentrations were determined spectrophotometrically using the commercially available D-Xylose-Assay-Kit (Megazyme, Ireland) according to the manufacturer’s instructions.
Virulence Studies
Six-week-old female ICR mice were immunocompromised by subcutaneous injection with cortisone acetate (300 mg/kg) 3 days prior to infection, on the day of infection, and 3 and 7 days after infection. Conidia were collected in PBS with 0.2% Tween 20. Mice were infected intranasally with 5 × 105 dormant conidia, suspended in 20 μl of PBS with 0.2% Tween 20 (10 μl in each nostril). Mortality was monitored for 21 days. The statistical differences for mouse survival were calculated using the log-rank (Mantel-Cox) test. Differences were considered significant at P-values ≤0.05. For histology and lung fungal burden estimation, 5 mice/group were treated and infected as described above and sacrificed after 48 h for lung extraction. For histology, lungs were paraffin-embedded, sectioned (3–4 μm) and stained with Gomori methenamine silver. Histology section preparations were observed using a BX51 microscope (Olympus, Milan, Italy), and images were captured using a high-resolution DP71 camera (Olympus). Lung fungal burden was assessed by homogenizing the lungs and plating dilutions on Sabouraud dextrose agar (Difco) +0.5% xylose plates for CFU (colony forming unit) enumeration. This study was carried out in accordance with the recommendations of the Ministry of Health (MOH) Animal Welfare Committee, Israel. The protocol was approved by the MOH Animal Welfare Committee, Israel.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The animal study was reviewed and approved by Ministry of Health (MOH) Animal Welfare Committee, Israel.
Author Contributions
IB, MM, YS, A-MD, VP, TO, and BA generated the data. IB, MM, SG, NO, and HH conceived and designed the experiments, and wrote the manuscript. IB, MM, YS, SG, NO, and HH analyzed the data.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. We acknowledge funding by the Austrian Science Fund (FWF, P24803 to SG and Infect-ERA I1616 to HH), by the Israeli Ministry of Health (Infect-ERA 3-0000-11080 to NO), and by the Medical University of Innsbruck (MUI Start ST201405031 to IB). A-MD and TO participated in the HOROS program (W1253) funded by the FWF.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.02773/full#supplementary-material
References
- Ahler E., Sullivan W. J., Cass A., Braas D., York A. G., Bensinger S. J., et al. (2013). Doxycycline alters metabolism and proliferation of human cell lines. PLoS One 8:e64561. 10.1371/journal.pone.0064561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov A. A., Reed D. R., Beauchamp G. K., Tordoff M. G. (2002). Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav. Genet. 32 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baidyaroy D., Brosch G., Ahn J. H., Graessle S., Wegener S., Tonukari N. J., et al. (2001). A gene related to yeast HOS2 histone deacetylase affects extracellular depolymerase expression and virulence in a plant pathogenic fungus. Plant Cell 13 1609–1624. 10.1105/tpc.13.7.1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldin C., Valiante V., Krüger T., Schafferer L., Haas H., Kniemeyer O., et al. (2015). Comparative proteomics of a tor inducible Aspergillus fumigatus mutant reveals involvement of the Tor kinase in iron regulation. Proteomics 15 2230–2243. 10.1002/pmic.201400584 [DOI] [PubMed] [Google Scholar]
- Bauer I., Varadarajan D., Pidroni A., Gross S., Vergeiner S., Faber B., et al. (2016). A class 1 histone deacetylase with potential as an antifungal target. mBio 7:e00831-16. 10.1128/mBio.00831-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böcker R., Estler C. J., Maywald M., Weber D. (1981). Comparison of distribution of doxycycline in mice after oral and intravenous application measured by a high-performance liquid chromatographic method. Arzneimittelforschung 31 2116–2117. [PubMed] [Google Scholar]
- Böcker R., Warnke L., Estler C. J. (1984). Blood and organ concentrations of tetracycline and doxycycline in female mice. Comparison to males. Arzneimittelforschung 34 446–448. [PubMed] [Google Scholar]
- Brandão F., Esher S. K., Ost K. S., Pianalto K., Nichols C. B., Fernandes L., et al. (2018). HDAC genes play distinct and redundant roles in Cryptococcus neoformans virulence. Sci. Rep. 8:5209. 10.1038/s41598-018-21965-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch G., Loidl P., Graessle S. (2008). Histone modifications and chromatin dynamics: a focus on filamentous fungi. FEMS Microbiol. Rev. 32 409–439. 10.1111/j.1574-6976.2007.00100.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugeja H. E., Hynes M. J., Andrianopoulos A. (2013). HgrA is necessary and sufficient to drive hyphal growth in the dimorphic pathogen Penicillium marneffei. Mol. Microbiol. 88 998–1014. 10.1111/mmi.12239 [DOI] [PubMed] [Google Scholar]
- Campoy S., Adrio J. L. (2017). Antifungals. Biochem. Pharmacol. 133 86–96. 10.1016/j.bcp.2016.11.019 [DOI] [PubMed] [Google Scholar]
- Chatzispyrou I. A., Held N. M., Mouchiroud L., Auwerx J., Houtkooper R. H. (2015). Tetracycline antibiotics impair mitochondrial function and its experimental use confounds research. Cancer Res. 75 4446–4449. 10.1158/0008-5472.CAN-15-1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning D. W., Bromley M. J. (2015). Infectious disease. How to bolster the antifungal pipeline. Science 347 1414–1416. 10.1126/science.aaa6097 [DOI] [PubMed] [Google Scholar]
- Elías-Villalobos A., Fernández-Álvarez A., Moreno-Sánchez I., Helmlinger D., Ibeas J. I. (2015). The Hos2 histone deacetylase controls Ustilago maydis virulence through direct regulation of mating-type genes. PLoS Pathog. 11:e1005134. 10.1371/journal.ppat.1005134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazius F., Shelest E., Gebhardt P., Brock M. (2012). The fungal α-aminoadipate pathway for lysine biosynthesis requires two enzymes of the aconitase family for the isomerization of homocitrate to homoisocitrate. Mol. Microbiol. 86 1508–1530. 10.1111/mmi.12076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. C., Hawkins N. J., Sanglard D., Gurr S. J. (2018). Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 360 739–742. 10.1126/science.aap7999 [DOI] [PubMed] [Google Scholar]
- Graessle S., Dangl M., Haas H., Mair K., Trojer P., Brandtner E. M., et al. (2000). Characterization of two putative histone deacetylase genes from Aspergillus nidulans. Biochim. Biophys. Acta 1492 120–126. 10.1016/s0167-4781(00)00093-2 [DOI] [PubMed] [Google Scholar]
- Gsaller F., Eisendle M., Lechner B. E., Schrettl M., Lindner H., Müller D., et al. (2012). The interplay between vacuolar and siderophore-mediated iron storage in Aspergillus fumigatus. Metallomics 4 1262–1270. 10.1039/c2mt20179h [DOI] [PubMed] [Google Scholar]
- Gsaller F., Furukawa T., Carr P. D., Rash B., Jöchl C., Bertuzzi M., et al. (2018). Mechanistic basis of ph-dependent 5-flucytosine resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 62:e02593-17. 10.1128/AAC.02593-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas H., Friedlin E., Stöffler G., Redl B. (1993). Cloning and structural organization of a xylanase-encoding gene from Penicillium chrysogenum. Gene 126 237–242. 10.1016/0378-1119(93)90372-a [DOI] [PubMed] [Google Scholar]
- Hartmann T., Dümig M., Jaber B. M., Szewczyk E., Olbermann P., Morschhäuser J., et al. (2010). Validation of a self-excising marker in the human pathogen Aspergillus fumigatus by employing the beta-rec/six site-specific recombination system. Appl. Environ. Microbiol. 76 6313–6317. 10.1128/AEM.00882-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmschrott C., Sasse A., Samantaray S., Krappmann S., Wagener J. (2013). Upgrading fungal gene expression on demand: improved systems for doxycycline-dependent silencing in Aspergillus fumigatus. Appl. Environ. Microbiol. 79 1751–1754. 10.1128/AEM.03626-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnisz D., Schwarzmüller T., Kuchler K. (2009). Transcriptional loops meet chromatin: a dual-layer network controls white-opaque switching in Candida albicans. Mol. Microbiol. 74 1–15. 10.1111/j.1365-2958.2009.06772.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Sillaots S., Lemieux S., Davison J., Kauffman S., Breton A., et al. (2007). Essential gene identification and drug target prioritization in Aspergillus fumigatus. PLoS Pathog. 3:e24. 10.1371/journal.ppat.0030024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley N. F., Patience J. F. (2018). Xylose: absorption, fermentation, and post-absorptive metabolism in the pig. J. Anim. Sci. Biotechnol. 9:4. 10.1186/s40104-017-0226-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb D., Heinekamp T., Lackner G., Scharf D. H., Dahse H.-M., Brakhage A. A., et al. (2015). Genetic engineering activates biosynthesis of aromatic fumaric acid amides in the human pathogen Aspergillus fumigatus. Appl. Environ. Microbiol. 81 1594–1600. 10.1128/AEM.03268-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krappmann S., Sasse C., Braus G. H. (2006). Gene targeting in Aspergillus fumigatus by homologous recombination is facilitated in a nonhomologous end- joining-deficient genetic background. Eukaryot. Cell 5 212–215. 10.1128/EC.5.1.212-215.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubodera T., Yamashita N., Nishimura A. (2000). Pyrithiamine resistance gene (ptrA) of Aspergillus oryzae: cloning, characterization and application as a dominant selectable marker for transformation. Biosci. Biotechnol. Biochem. 64 1416–1421. 10.1271/bbb.64.1416 [DOI] [PubMed] [Google Scholar]
- Lamoth F., Juvvadi P. R., Soderblom E. J., Moseley M. A., Asfaw Y. G., Steinbach W. J. (2014). Identification of a key lysine residue in heat shock protein 90 required for azole and echinocandin resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 58 1889–1896. 10.1128/AAC.02286-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Su T., He Y., Lu J., Mo W., Wei Y., et al. (2016). Brain formaldehyde is related to water intake behavior. Aging Dis. 7 561–584. 10.14336/AD.2016.0323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.-J., Sasse C., Gerke J., Valerius O., Irmer H., Frauendorf H., et al. (2015). Transcription factor SomA is required for adhesion, development and virulence of the human pathogen Aspergillus fumigatus. PLoS Pathog. 11:e1005205. 10.1371/journal.ppat.1005205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macheleidt J., Scherlach K., Neuwirth T., Schmidt-Heck W., Straßburger M., Spraker J., et al. (2015). Transcriptome analysis of cyclic AMP-dependent protein kinase A-regulated genes reveals the production of the novel natural compound fumipyrrole by Aspergillus fumigatus. Mol. Microbiol. 96 148–162. 10.1111/mmi.12926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meis J. F., Chowdhary A., Rhodes J. L., Fisher M. C., Verweij P. E. (2016). Clinical implications of globally emerging azole resistance in Aspergillus fumigatus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371:20150460. 10.1098/rstb.2015.0460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misslinger M., Lechner B. E., Bacher K., Haas H. (2018). Iron-sensing is governed by mitochondrial, not by cytosolic iron-sulfur cluster biogenesis in Aspergillus fumigatus. Metallomics 10 1687–1700. 10.1039/c8mt00263k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misslinger M., Scheven M. T., Hortschansky P., López-Berges M. S., Heiss K., Beckmann N., et al. (2019). The monothiol glutaredoxin GrxD is essential for sensing iron starvation in Aspergillus fumigatus. PLoS Genet. 15:e1008379. 10.1371/journal.pgen.1008379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moullan N., Mouchiroud L., Wang X., Ryu D., Williams E. G., Mottis A., et al. (2015). Tetracyclines disturb mitochondrial function across eukaryotic models: a call for caution in biomedical research. Cell Rep. 10 1681–1691. 10.1016/j.celrep.2015.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita T., Weinert B. T., Choudhary C. (2019). Functions and mechanisms of non-histone protein acetylation. Nat. Rev. Mol. Cell Biol. 20 156–174. 10.1038/s41580-018-0081-3 [DOI] [PubMed] [Google Scholar]
- Nielsen M. L., Albertsen L., Lettier G., Nielsen J. B., Mortensen U. H. (2006). Efficient PCR-based gene targeting with a recyclable marker for Aspergillus nidulans. Fungal Genet. Biol. 43 54–64. 10.1016/j.fgb.2005.09.005 [DOI] [PubMed] [Google Scholar]
- Nørholm M. H. H. (2010). A mutant Pfu DNA polymerase designed for advanced uracil-excision DNA engineering. BMC Biotechnol. 10:21. 10.1186/1472-6750-10-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., Zhang H., Xu M., Tan M.-W. (2018). A Tet-Off gene expression system for validation of antifungal drug targets in a murine invasive pulmonary aspergillosis model. Sci. Rep. 8:443. 10.1038/s41598-017-18868-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller M. A., Messer S. A., Georgopapadakou N., Martell L. A., Besterman J. M., Diekema D. J. (2009). Activity of MGCD290, a Hos2 histone deacetylase inhibitor, in combination with azole antifungals against opportunistic fungal pathogens. J. Clin. Microbiol. 47 3797–3804. 10.1128/JCM.00618-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidroni A., Faber B., Brosch G., Bauer I., Graessle S. (2018). A class 1 histone deacetylase as major regulator of secondary metabolite production in Aspergillus nidulans. Front. Microbiol. 9:2212. 10.3389/fmicb.2018.02212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongsunk S., Andrianopoulos A., Chaiyaroj S. C. (2005). Conditional lethal disruption of TATA-binding protein gene in Penicillium marneffei. Fungal Genet. Biol. 42 893–903. 10.1016/j.fgb.2005.07.002 [DOI] [PubMed] [Google Scholar]
- Pontecorvo G., Roper J. A., Hemmons L. M., MacDonald K. D., Bufton A. W. J. (1953). The genetics of Aspergillus nidulans. Adv. Genet. 5 141–238. [DOI] [PubMed] [Google Scholar]
- Romero B., Turner G., Olivas I., Laborda F., De Lucas J. R. (2003). The Aspergillus nidulans alcA promoter drives tightly regulated conditional gene expression in Aspergillus fumigatus permitting validation of essential genes in this human pathogen. Fungal Genet. Biol. 40 103–114. 10.1016/s1087-1845(03)00090-2 [DOI] [PubMed] [Google Scholar]
- Sasse A., Hamer S. N., Amich J., Binder J., Krappmann S. (2016). Mutant characterization and in vivo conditional repression identify aromatic amino acid biosynthesis to be essential for Aspergillus fumigatus virulence. Virulence 7 56–62. 10.1080/21505594.2015.1109766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchuk N. A., Bryksin A. V., Nusinovich Y. A., Cabello F. C., Sutherland M., Ladisch S. (2004). Construction of long DNA molecules using long PCR-based fusion of several fragments simultaneously. Nucleic Acids Res. 32:e19. 10.1093/nar/gnh014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigl C., Handler M., Sprenger G., Kürnsteiner H., Zadra I. (2010). A novel homologous dominant selection marker for genetic transformation of Penicillium chrysogenum: overexpression of squalene epoxidase-encoding ergA. J. Biotechnol. 150 307–311. 10.1016/j.jbiotec.2010.09.941 [DOI] [PubMed] [Google Scholar]
- Souza A. C. O., Al Abdallah Q., DeJarnette K., Martin-Vicente A., Nywening A. V., DeJarnette C., et al. (2019). Differential requirements of protein geranylgeranylation for the virulence of human pathogenic fungi. Virulence 10 511–526. 10.1080/21505594.2019.1620063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk E., Krappmann S. (2010). Conserved regulators of mating are essential for Aspergillus fumigatus cleistothecium formation. Eukaryot. Cell 9 774–783. 10.1128/EC.00375-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilburn J., Scazzocchio C., Taylor G. G., Zabicky-Zissman J. H., Lockington R. A., Davies R. W. (1983). Transformation by integration in Aspergillus nidulans. Gene 26 205–221. 10.1016/0378-1119(83)90191-9 [DOI] [PubMed] [Google Scholar]
- Tribus M., Bauer I., Galehr J., Rieser G., Trojer P., Brosch G., et al. (2010). A novel motif in fungal class 1 histone deacetylases is essential for growth and development of Aspergillus. Mol. Biol. Cell 21 345–353. 10.1091/mbc.E09-08-0750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaknin Y., Hillmann F., Iannitti R., Ben Baruch N., Sandovsky-Losica H., Shadkchan Y., et al. (2016). Identification and characterization of a novel Aspergillus fumigatus rhomboid family putative protease, RbdA, Involved in hypoxia sensing and virulence. Infect. Immun. 84 1866–1878. 10.1128/IAI.00011-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiante V., Heinekamp T., Jain R., Härtl A., Brakhage A. A. (2008). The mitogen-activated protein kinase MpkA of Aspergillus fumigatus regulates cell wall signaling and oxidative stress response. Fungal Genet. Biol. 45 618–627. 10.1016/j.fgb.2007.09.006 [DOI] [PubMed] [Google Scholar]
- van de Veerdonk F. L., Gresnigt M. S., Romani L., Netea M. G., Latgé J.-P. (2017). Aspergillus fumigatus morphology and dynamic host interactions. Nat. Rev. Microbiol. 15 661–674. 10.1038/nrmicro.2017.90 [DOI] [PubMed] [Google Scholar]
- Vogt K., Bhabhra R., Rhodes J. C., Askew D. S. (2005). Doxycycline-regulated gene expression in the opportunistic fungal pathogen Aspergillus fumigatus. BMC Microbiol. 5:1. 10.1186/1471-2180-5-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanka F., Cairns T., Boecker S., Berens C., Happel A., Zheng X., et al. (2016). Tet-on, or Tet-off, that is the question: advanced conditional gene expression in Aspergillus. Fungal Genet. Biol. 89 72–83. 10.1016/j.fgb.2015.11.003 [DOI] [PubMed] [Google Scholar]
- Weiner R., Laue R., Dietze F., Hartig W. (1984). A modified D-xylose absorption test. Infusionsther Klin Ernahr 11 333–337. 10.1159/000221689 [DOI] [PubMed] [Google Scholar]
- West A. C., Johnstone R. W. (2014). New and emerging HDAC inhibitors for cancer treatment. J. Clin. Invest. 124 30–39. 10.1172/JCI69738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasmin S., Alcazar-Fuoli L., Gründlinger M., Puempel T., Cairns T., Blatzer M., et al. (2012). Mevalonate governs interdependency of ergosterol and siderophore biosyntheses in the fungal pathogen Aspergillus fumigatus. Proc. Natl. Acad. Sci. U.S.A. 109 E497–E504. 10.1073/pnas.1106399108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadra I., Abt B., Parson W., Haas H. (2000). XylP promoter-based expression system and its use for antisense downregulation of the Penicillium chrysogenum nitrogen regulator NRE. Appl. Environ. Microbiol. 66 4810–4816. 10.1128/aem.66.11.4810-4816.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.


