Abstract
Background.
Rapid industrialization and anthropogenic activities such as the unmanaged use of agro-chemicals, fossil fuel burning and dumping of sewage sludge have caused soils and waterways to be severely contaminated with heavy metals. Heavy metals are non-biodegradable and persist in the environment. Hence, remediation is required to avoid heavy metal leaching or mobilization into environmental segments and to facilitate their extraction.
Objectives.
The present work briefly outlines the environmental occurrence of heavy metals and strategies for using microorganisms for bioremediation processes as reported in the scientific literature.
Methods.
Databases were searched from different libraries, including Google Scholar, Medline and Scopus. Observations across studies were then compared with the standards for discharge of environmental pollutants.
Discussion.
Bioremediation employs microorganisms for removing heavy metals. Microorganisms have adopted different mechanisms for bioremediation. These mechanisms are unique in their specific requirements, advantages, and disadvantages, the success of which depends chiefly upon the kind of organisms and the contaminants involved in the process.
Conclusions.
Heavy metal pollution creates environmental stress for human beings, plants, animals and other organisms. A complete understanding of the process and various alternatives for remediation at different steps is needed to ensure effective and economic processes.
Competing interests.
The authors declare no competing financial interests.
Keywords: bioremediation, microorganisms, heavy metals, contaminants, environment, organic matter, biosorption
Introduction
Heavy metal pollution is a serious concern due to hazardous impacts at even very small concentrations. Heavy metals are non-biodegradable, bioaccumulate in tissues and are biomagnified along with the trophic levels.1Weathering of geological bedrock and volcanic eruptions can discharge heavy metals into the surrounding environment.2 The type of heavy metals released from the rock substratum depends on its composition and other factors such as the inherent chemistry of the bedrock/soil, climate, nature, and composition of the soil and other anthropogenic activities in the region.2,3 Subsequent releases and the entry of heavy metals into the food chain depends on their concentration and uptake by the local flora and fauna. Atmospheric deposition has also been reported to be one of the major causes of deposits in urban and sub-urban areas. Heavy metal sources can be categorized as shown in Figure 1.
Figure 1.
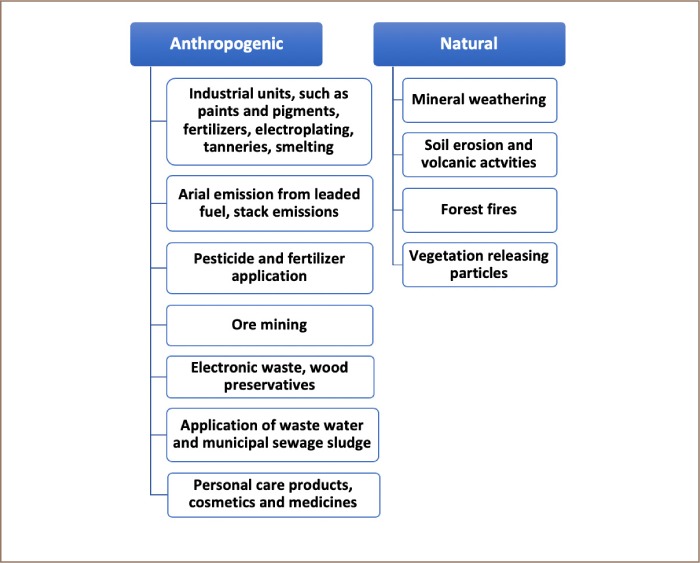
Sources of heavy metals
Heavy metal pollution occurs directly from industries (tannery, electroplating, dyeing, mining), agricultural fields, sewage sludge, and waste treatment plants. Recent studies have established that the long-term use of untreated wastewater from industrial sources can adversely affect water quality, making it unfit for human consumption.2 Untreated industrial wastewater is often colored, frothy, and contains hazardous chemicals including heavy metals, toxic dyes, acids, alkalis, and other toxic chemicals.4 The resulting pollution leads to hazardous impacts on the health of occupants/residents and occupational health hazards for workers.5 The electroplating industry releases hazardous wastewater laden with heavy metals.6 Heavy metals such as chromium (Cr) and nickel (Ni) discharged in untreated effluents by electroplating plants have been reported to surpass permissible limits.6–10 Heavy metals like copper (Cu), Cr, iron (Fe), manganese (Mn), and zinc (Zn) are present in tannery wastewater.11 Various studies have analyzed the quality of industrial wastewater and groundwater supplies in terms of heavy metals compared with standards for discharge of environmental pollutants (Table 1).
Table 1.
Sources of Heavy Metal Contamination in Water
| Element | References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | |
| As | • | x | x | ||||||||
| Cd | • | • | x | x | x | x | x | x | |||
| Cr | • | x | x | x | x | x | |||||
| Cu | x | x | • | • | • | • | • | x | x | x | |
| Fe | x | • | x | x | |||||||
| Hg | • | ||||||||||
| Mn | • | ||||||||||
| Ni | • | x | • | x | • | • | x | ||||
| Pb | • | x | • | • | x | x | x | ||||
| Zn | • | • | • | • | • | x | x | x | |||
| Source | Textile mill effluent | Electroplating industry wastewater sample | Ganga river water quality | Ground water quality | Ground water quality | Water supply | Irrigation water | Irrigation water | Irrigation water | Lake water | Irrigation water |
x indicates the concentration of heavy metals found above permissible limits;
• is the concentration of heavy metals found below permissible limits.
Bureau of Indian Standards for drinking water (BIS-10500-2012): Accepted value in mg/L (permissible limits in the absence of alternate source in mg/L) – As, 0.01 (0.05); Cd, 0.003 (no relaxation); Cu: 0.05 (1.5); Pb,: 0.01 (no relaxation); Hg, 0.001 (no relaxation); Fe: 0.3 (no relaxation); Ni: 0.02 (no relaxation); Cr: 0.05 (no relaxation); Zn: 5 (15); Mn: 0.1 (0.3).
World Health Organization standards for surface waters (μg/L): As, 10; Cr: 50; Cd, 3; Cu: 2000; Ni: 70; Pb,10; Hg, 01.
Abbreviations: As, arsenic; Cd, cadmium; Cr, chromium; Cu, copper; Fe, iron; Hg, mercury; Mn, manganese; Ni, nickel; Pb, lead; Zn, zinc.
Various reports have demonstrated that industrial wastewater contains heavy metals beyond permissible limits for drinking water or surface/irrigation water. Wastewater containing heavy metals (above permissible levels) is used to irrigate fields in various parts of India. Application of heavy metal-contaminated water in agricultural fields has led to their bioaccumulation in crops and associated food chains. Indirect heavy metal pollution results from contaminated surface or groundwater and rainwater. Rivers are one of the most important resources for fresh water and are severely affected by pollution sources.23 According to the report ‘Status of trace and toxic metals in Indian rivers’, out of 414 river water quality stations across various rivers in India, 57 stations have been found to contain two or more heavy metals beyond permissible limits.24 The situation demands immediate action and remediation of contaminated rivers. The chances of exposure to heavy metals have increased due to increased use in the technology, domestic, industrial, and agricultural sectors.2 Heavy metals' effects depend largely upon their chemical nature. Inorganic arsenic (As) compounds are readily absorbed and interfere to a greater extent with cellular reactions compared to the organic forms due to their poor cellular absorption.25 Heavy metals have been reported to attach themselves to protein binding sites and remove the original metals, causing toxicity and cellular malfunctioning.26
Different exposure routes, including dermal (through the skin), ingestion (food or drink), and inhalation (as dust or fume) have been studied, along with the effects of heavy metals, mechanism of toxic action, impacts, and conventional and bioremediation techniques. A number of studies have examined various bioremediation techniques including algae, bacteria, and fungi as biosorbents. The current study aims to provide insight into the environmental occurrence and sources of heavy metals as published in the scientific literature.
Methods
The present study was conducted by searching databases from different libraries: Google Scholar, Medline and Scopus. Studies, irrespective of the place/area of research, were restricted to publications available in English and those published in the last twenty years. The search outcomes were collected, studied and thoroughly mined as applicable to the study objectives. Observations across studies were compared with standards for the discharge of heavy metals.
A total of 301 articles or records were searched in various databases. Most of the articles were identified from electronic bibliographic sources. After the initial adjustment with duplicates (n = 29), 271 articles were further screened on the basis of title (n = 15), relevance (n = 15), and availability of full text (n = 07). Articles related to biosorption with biomass and phytoremediation were discarded (n = 19). The remaining articles (n = 215) were assessed for eligibility and included in the study (Figure 2).
Figure 2.
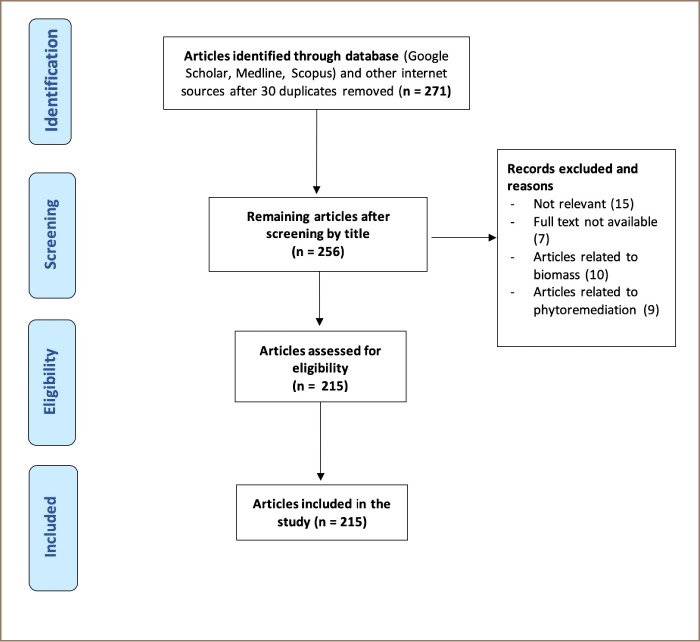
Flow diagram of article selection process
Results
Table 2 demonstrates some of the toxic heavy metals, their occurrence/applications, exposure routes and toxicity mechanisms.
Table 2.
Environmental Occurrence, Routes of Exposure and Toxicity Profile of Toxic Heavy Metals
| Toxic heavy metals | Environmental occurrences | Anthropogenic sources/commercial applications | Exposure routes | Effects | Toxicity and mechanisms | References |
|---|---|---|---|---|---|---|
| As | Low concentrations in all environmental segments; released through soil erosion and volcanic eruptions | Coal combustion; pesticide formulations; smelting; wood preservative; metal refining; drugs/medicines to treat amoebic dysentery and syphilis; veterinary drugs to treat against parasitic diseases | Dermal; ingestion of contaminated food and water; inhalation of contaminated dust; accidental and occupational exposure; wood preservation; pesticide application and manufacturing; glass manufacturing | Carcinogenic; cardiovascular and neurobehavioral disorders; diabetes | Enzymatic biomethylation of inorganic As to its intermediate monomethylarsonic acid, a potential carcinogen; As(III) has the potential to inactivate approximately 200 enzymes; As(V) can substitute phosphate; inhibit repair mechanism of DNA; cellular respiration inhibition | 2,27–29 |
| Cadmium | Earth's crust, especially sedimentary rocks and water; small amounts in crustaceans, potatoes, leafy vegetables, mushrooms, etc. | Production of batteries, alloys, pigments, fertilizers, pesticides; welding; mining; combustion of fossil fuels and municipal wastes; recycling of electronic and cadmium-plated waste | Ingestion of contaminated food; inhalation (smoking); occupational exposure | Acute and chronic exposures lead to lung and stomach cancer; ingestion leads to vomiting, pain, nausea, renal injury, consciousness loss, coma; acute inhalation damages lung tissues and causes chest pain, fever, tachycardia; in vitro/in vivo studies have reported genotoxic effects in animals; cadmium exposure leads to chromosomal aberrations; chromosomal damage; multi-organ dysfunction including liver kidney, lungs and heart; kidney toxicity leads to irreversible proteins loss in urine; osteo-toxicity due to bone resorption or inhibiting bone formation; osteoporosis anemia | DNA damage; interruption of protein and nucleic acid synthesis; blocking repair mechanisms; initiation of cellular multiplication; complex formation with metallothionein causing nephrotoxicity | 2,30–35 |
| Cr | Low concentrations in all environmental segments (Cr (II) to Cr(VI) forms) | Metal processing; dyeing; leather tanning; pigments; chrome plating; wood preservation; metallurgy; welding; boilers and cooking systems as anti-corrosives | Ingestion of Cr contaminated food items and water; inhalation; dermal | Cr(VI) is the main toxic form; dermatitis; kidney damage; asthma, allergies; respiratory tract cancer; inhalation causes ulcers of nose; ingestion causes severe gastrointestinal; cardiovascular, renal, respiratory and neurological disorders; carcinogenic | Cr(VI) causes chromosomal aberrations and DNA strand breaks | 2,36,37 |
| Lead | Occurs in earth's crust (soil) | Lead batteries, soldered metal products; X-ray shields; glass manufacturing, paints and pigments; ammunition; coal combustion | Ingestion of contaminated water and food; inhalation of leaded dust | Anemia, appetite loss; systemic toxicity has multi-organ effects: kidney, liver and central nervous system reproductive and gastrointestinal systems | Mimics calcium and inhibits body calcium metabolism and cycling; interrupts synthesis and repair of DNA and tumor suppressor proteins | 2,38 |
| Mercury | Environmental occurrence in water, soil and air | Electrical industries (switches, batteries); paint industry; dentistry; mining | Ingestion, inhalation and dermal | Absorbed by the gastrointestinal tract and crosses blood-brain and placental barriers; accumulates in the kidneys, liver and the nervous tissue; displays neurotoxic, gastrointestinal toxicity and nephrotoxic effects | Bonds covalently with proteins and exhausts antioxidants | 2 |
Abbreviation: DNA, deoxyribonucleic acid.
Discussion
Rapid population growth has created excessive pressure on terrestrial and aquatic ecosystems, leading to increasing exploitation/extraction of water, food, and water resources. Apart from anthropogenic discharge of heavy metals, natural sources contribute significantly to heavy metal pollution. Their occurrence in soil and release due to soil weathering is an important source of heavy metal pollution. Arsenic is present in various igneous and sedimentary rocks in high concentrations. Approximately 45,000 tons of As is reported to be released by coal sources.28,39 Many countries, including India, Pakistan, Australia, Canada, Nepal, and Japan are severely impacted by As occurrence.40 Arsenic contamination from geogenic sources has been found throughout West Bengal, India.39The bioavailability of heavy metals and their effects further depend on the metal, its physico-chemical properties and lipid solubility which imparts a characteristic toxicological property. Features like age, nutritional status, trophic interactions and physiological adaptations of organisms play an important role in their toxicity. With absorption, a metal is distributed in body tissues and tends to persist in the body in organs such as bones, liver and kidneys for a prolonged time. Heavy metals have been reported to affect cellular fractions and organelles.2 With increasing awareness regarding the persistence, nature and deleterious effects of heavy metals, there has been growing interest in the development of technologies to remediate this contamination.
Heavy metals removal through conventional techniques
Conventional techniques like adsorption, electro-dialysis, precipitation and ion exchange used to remove heavy metals have limitations. The process of chemical precipitation involves adding anions for precipitating metals as suspended particles, which are then removed. The process is not specific and cannot remove heavy metals at low concentrations.41 Through the ion exchange process, heavy metals can be removed to the level of parts per billion.42 However, it's a non-specific, pH-sensitive and expensive method.43 The method of reverse osmosis makes use of membranes. These conventional techniques have drawbacks such as slow and inefficient removal, generation of contaminated sludge requiring careful disposal, high cost and energy involved in the processes, and blockage of membranes.44–47 There is a need for a cheap and effective technology to remove heavy metals with an eco-friendly approach. There has been increasing interest in the use of biological agents for heavy metal removal as an alternative to these methods.
Bioremediation
Microorganisms are ubiquitously present in nature and play a crucial role in elemental biogeochemical cycles of metal transformations between soluble and insoluble species. Metal-microbe interactions can have beneficial or harmful consequences. Apart from the nature of the microbes and chemistry of metals involved, these transformations are dependent on other environmental factors like pH, moisture, temperature, presence of other ions, humic colloidal substances and other living organisms and their competitors which play an important part in microbial colonization and biofilm formation.36
Bioremediation is a technique for removing/converting harmful contaminants like heavy metals into less harmful substances; and/or removing toxic elements from the contaminated environment; or degrading organic substances and ultimate mineralization of organic substances into carbon dioxide, water, nitrogen gas, etc., employing dead or alive biomass. The process of bioremediation can be applied to soil and water media through in- and ex-situ techniques. A brief description of the different techniques of bioremediation for various contaminants is given in Figure 3.
Figure 3.
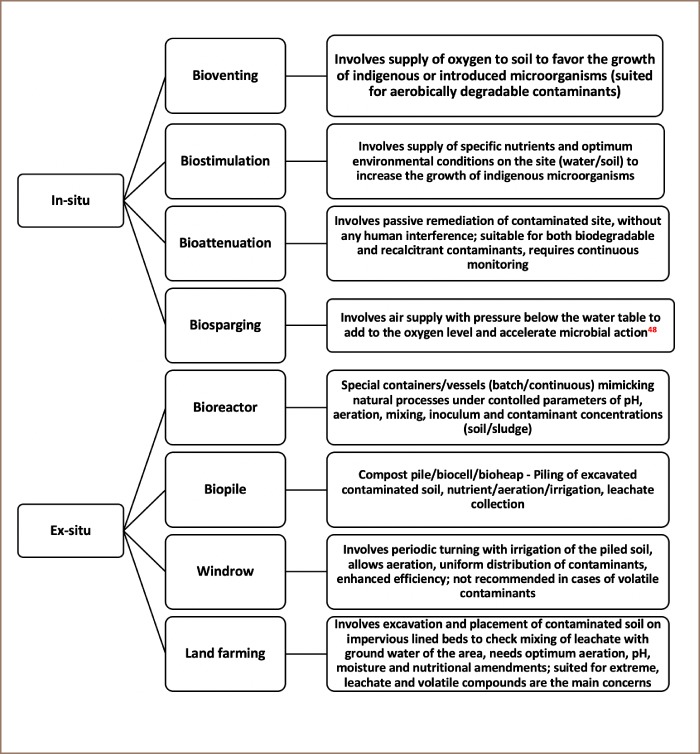
Types of bioremediation techniques for various contaminants49
The in-situ process does not involve excavation or removal and does not disturb the soil structure. It can make use of stimulation of indigenous microbial flora (intrinsic) or introduction of microorganisms (engineered) bioremediation.50 The type and the nature of contaminants, degree of contamination, soil type or site geochemistry and geographical location are some factors to be considered for this technique.51 Contaminants may get adsorbed on soil particles and become unavailable for bioremediation. During bioremediation there are other challenges, like microbial competition and death after inoculation, temperature, and moisture condition of the media.52–54 High temperature increases the solubility of contaminants and hence their mobilization.55 In-situ treatment generally involves pumping oxygen/nutrients (bioventing/biostimulation) into the soil. The texture also plays an important part during bioventing and biostimulation. In coarse-textured soils, it is easier to pump and disperse oxygen and nutrients compared to fine-textured soils. Fine soils like clay retain moisture in their numerous smaller pores with high surface area and prevent oxygen from dispersing uniformly throughout the contaminated soil. However, this process may not be suitable for all types of soils when natural conditions (like temperature) become limiting. Higher sorption capacity by microbial cells compared to clay particles has been indicated by various reports.56 Indigenous species isolated from contaminated sites has been reported to demonstrate exceptional resistance and biosorption efficiency towards heavy metals. Two indigenous strains, AK1 and AK9, belonging to the genus of Pseudomonas, have been isolated from As-contaminated water of the Ganga basin. The strains have been reported to be resistant towards As and other heavy metals, like silver (Ag), cadmium (Cd), cobalt (Co), Cr, Cu, mercury (Hg), Ni, and lead (Pb).57 Ex-situ technique involves the transport of contaminated soil and water from the contaminated area to another site for further treatment. It may be classified as a solid-phase technique (for land treatment), slurry-phase and pile techniques (for a mixed medium containing solid and liquid phases in bioreactors).58 It uses techniques like bioreactors, biopiles, and land farming. For the slurry-phase technique, contaminated soil is mixed with water along with other additives in a bioreactor. However, the efficiency of the bioreactor depends on biosorbent (live/dead), optimal conditions required for microbial growth and adaptability of biomass to the configuration of the bioreactor.59
Mercury removal from synthetic wastewater using a bioreactor has been reported.60 The wastewater bioremediation is dependent on various factors like pH.42 The pH affects their bioavailability by influencing the solution chemistry through processes like complexation, hydrolysis, redox and precipitation.61 Microbial biomass surface area and pretreatment processes (modifying the surface area) tend to influence the bioremediation process.62 Microbial biomass may be required to be immobilized in matrices like alginate and silica gel to develop a suitable commercial biosorbent with appropriate strength and porosity.63 Encapsulation imparts physicochemical stability and heat resistance. Encapsulated Agrobacterium sp. in alginate with nano-particles of Fe has shown an excellent adsorption capacity for continuously five cycles.64 Poor selectivity and difficulties in reusing biomass are some of the limitations of the process. Bioremediation has also been mediated through microbial biofilms having high resistance and tolerance for metal ions. Rhodotorula sp. have a removal efficiency of up to 95.39%.65
Biosorbent materials
Selecting an efficient, highly selective and economical biosorbent is a major concern.63 The biosorbent should be easily available or should demonstrate quick growth. The efficiency of the biosorbent depends on the experimental requirements and pretreatment of the bioagent. There have been many reports on wastewater treatment using various biosorbents.66–72 Various types of biological agents have been employed for remediation in ex- and in-situ conditions. These include agro-wastes like wheat/rice straw, tea/coffee/yeast waste, cotton waste, etc. Microorganisms (bacteria, fungi, yeast or algae) sourced from their natural habitats can be excellent biosorbents.73 These biosorbents can absorb heavy metals at very low concentrations. The functional groups like amide, amine, carbonyl, carboxyl, etc. facilitate the removal of heavy metals. Microorganisms possess characteristic enzymatic profiles required specifically for heavy metal resistance.74,75 However, steric and conformational factors along with the number and availability of reactive sites affect the biosorption process. Microorganisms like fungi convert heavy metals into less toxic compounds and utilize them for their growth; e.g., Pleurotus sp., Klebsiella oxytoca, etc. display metal binding capacity.76,77 Cephalosporium aphidicola has been found to be effective in lead-contaminated soil.78 Some of the most commonly used biosorbents are shown below in Table 3.
Table 3.
Different Types of Organisms as Biosorbents (Adapted)79
| Biosorbent | Examples/species |
|---|---|
| Agricultural residues | Rice/wheat straw |
| Algae | Fucus, Chlamydomonas, Cladophora, Spirogyra sp. |
| Bacteria | Bacillus sp., Vibrio sp., Geobactor sp., Pseudomonas sp., Desulfovibrio sp. |
| Fungi | Pleurotus sp., Aspergillus sp., Penicillium sp., Rhizopus sp., Saccharomyces sp. |
| Industrial wastes | Fermentation/food/beverage wastes |
| Others | Chitosan- and cellulose-based substances |
Bioremediation mechanisms
Microorganisms adapt to and resist heavy metals in highly contaminated areas. Extra-cellular polymeric substances present on the biomass cell wall can attach to heavy metals by mechanisms like proton exchange or micro-precipitation of metals.80 Biomass surfaces have a negative charge because of the presence of carboxyl, amino, phosphoryl, and sulfo groups as potential ion exchange sites and metal sinks. The process of bioremediation takes place through various mechanisms like redox process, adsorption, complexation, ion-exchange, precipitation, and electrostatic attraction.
Microorganisms may initiate metal mobilization/immobilization by redox reactions; and hence, impact bioremediation processes. Heavy metals like Fe, As, Cr, and Hg undergo oxidation and reduction cycles. Bioremediation is facilitated by converting an element from its insoluble and stationary form in sediments into its mobile and soluble phase. Mobilization can also have deleterious impacts when toxic metal ions are redistributed and released from their solid phase from sediments into the solution phase.59 This increases their bioavailability and heavy metals can reach microbial metabolic systems. The bacteria reduces Hg(II) to the elemental and more volatile form of Hg(0).81 Microbial reduction can also enhance the solubility of ions like Fe(III) and As(V) by reducing them to Fe(II) and As(III), respectively, and can facilitate leaching from soil.82,83 Studies have reported bacteria from different natural aquifers which can transform As.84–86 Pokhrel and Viraraghavan employed Aspergillus niger to remove As(V) and As(III).87 Heavy metal biomethylation is an important process in soil and water and may modify toxicity, volatility, and mobility of heavy metals. It also serves as an important means of detoxification as volatile methylated species can be removed from cells.88 Dimethylmercury and alkyl arsines, the methylated products of Hg and As, respectively, are volatile and evaporate and are lost from soil. The organic matter fraction of soil serves as the methyl donor. Yet another indirect mechanism of metal mobilization involves the microbial decomposition of organic matter, which accelerates the release of these ions. Schizophyllum commune has been found to release heavy metals along with dissolved organic matter.89 Excretion of metabolites like carboxylic acids and amino acids by microbes is an important mechanism of chelating metal ions.
Microbes perform metal immobilization and act as sinks for metals by adopting different mechanisms (ex- or in-situ) like biosorption, bioaccumulation, bioconversion and/or inter/intracellular precipitation (as oxalates of Zn, Cu, Co, Cd, Ni) operating in different ways.36,90,91 By immobilization, an element can be easily removed from its aqueous phase in groundwater or wastewater.36,92 Bacterial oxidation of As(III) to As(V) makes them immobilized and retained by the sediments.82,83Methanothermobacter thermautotrophicus has been employed to reduce Cr(VI) to Cr(III) and immobilize it in hydroxide-/oxide-forms. 93 Bacterial reduction and immobilization for Cr(VI) has also been reported for Bacillus cereus and Shewanella sp.94,95 Cellular structures like the cell wall and plasma membrane act as barriers and check the entry of metal ions into cells.96
Biosorption and bioaccumulation
Biosorption and bioaccumulation are attractive options to substitute conventional methods for heavy metal remediation. Bioaccumulation involves heavy metal uptake by living biomass (metabolism dependent/active uptake) and is characterized by the uptake of contaminants by living biomass/cells. Employing living biomass for remediation may not be a viable option owing to highly toxic metals which can accumulate in cells and interrupt metabolic activities resulting in cell death. However, dead biomass (biosorption) remains unaffected by toxicity, does not require any growth/nutritional medium and is flexible to environmental conditions. Heavy metals are adsorbed on the surface in a passive mode without involving energy expenditure (independent of metabolism) until equilibrium is achieved.97 Therefore, biosorption is advantageous, compared to active uptake/bioaccumulation, as it is metabolism independent, however is it largely dependent on the biomass/biosorbent type and contaminants involved.98 For these advantages, microbial biomasses of fungi, algae or yeast have been utilized for bioremediation for in-situ processes. Heavy metal bioremediation in the form of metallic nanoparticles with the help of bacteria and the use of genetically modified microorganisms as a part of the bioremediation process have also been reported. 99–101
Intracellular sequestration is the concentration of metal ions within the microbial cells. It involves complexation of heavy metal ions due to surface interactions and their subsequent transport into the cell.96 Extra-cellular sequestration comprises a concentration of metal ions in the periplasm or their complexation as insoluble precipitates. Cadmium precipitation has been reported in Pseudomonas aeruginosa and Klebsiella planticola.102,103
Bacterial bioremediation
Bacteria are ubiquitously present in the environment. Bacteria are found in different shapes, including rods (Bacillus), cocci (Streptococcus), filamentous (Actinomyces) and spiral (Vibrio cholera). Biosorption by bacteria is an inexpensive and efficient technique to remove pollutants, including non-biodegradable elements, like heavy metals, from wastewater. Bacterial biomass can be living or non-living cells. Bacterial species have adapted and developed mechanisms for metals ions resistance and remediation for their survival.104 Heavy metal ions bioremediation by bacterial agents has been widely researched.105–109 Bacterial biomass accomplishes the rapid removal of metals such as Cu, Zn, Pb, Cd, and Cr.110 biosorption efficiency depends on heavy metal ions and bacterial species (owing to their different cellular structures in terms of peptidoglycans like N-acetylmuramic acid and poly-N-acetylglucosamine). 42 The bacterial cell wall is the primary physical contact linking metal ions and the bacterial biomass. The overall negative charge due to anionic functional groups (like amine, hydroxyl, carboxyl, sulphate, phosphate) present in Gram-positive bacteria (in peptidoglycan, teichoic acids, and teichuronic acids) and in Gram-negative bacteria (in peptidoglycan, lipopolysaccharides, and phospholipids) imparts metal-binding capacity on or within the cell wall.111 The heavy metal removal by dead biomass cells is extracellular. Functional groups, including carboxyl, phosphonate, amine and hydroxyl groups on the cell wall are responsible for these interactions.112,113
The carboxyl groups can bind Cd on the surface by complexation.114 The amino groups have displayed efficient removal of Cr by chelation and electrostatic interactions.115 Bacterial species need to be exposed to the contaminants for enzymatic induction before using them for bioremediation. There is a minimum requirement of contaminant concentration to initiate enzymatic expression necessary for the process.116 Species like Pseudomonas, Desulfovibrio, Bacillus, and Geobacter have been used for bioremediation (Table 4).
Table 4.
Biosorption by Bacterial Species
| Bacterial species | As | Cd | Cr | Co | Cu | Hg | Ni | Pb | Zn | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Arthrobacter sp. | x | 117 | ||||||||
| Bacillus sp. | x | x | 118 | |||||||
| Bacillus sp., | x | 119 | ||||||||
| Aneurinibacillus sp. | ||||||||||
| Bacillus laterosporus | x | x | 120 | |||||||
| Corynebacterium glutamicum | x | 121 | ||||||||
| Desulfovibrio desulfuricans | x | x | x | 122 | ||||||
| Bacillus sp., Pseudomonas sp., Micrococcus sp. | x | x | x | 123 | ||||||
| Bacillus licheniformis | x | x | 124 | |||||||
| Geobacter metallireducens | x | 125 | ||||||||
| Geobacter sulfurreducens | x | 126 | ||||||||
| B. licheniformis | x | x | 127 | |||||||
| Klebsiella planticola | x | 103 | ||||||||
| Micrococcus luteus | x | x | 128 | |||||||
| Pseudomonas aeruginosa | x | 102,129 | ||||||||
| Pseudomonas fluorescens | x | x | x | x | x | x | x | x | 130 | |
| Pseudomonas putida | x | x | x | x | 131,132 | |||||
| P. aeruginosa | x | 115 | ||||||||
| P. aeruginosa | x | 133 | ||||||||
| Rhizopus arrhizus | x | 134 | ||||||||
| Rhodopseudomonas palustris | x | 135 | ||||||||
| Streptococcus equisimilis | x | 136 | ||||||||
| Staphylococcus xylosus | x | 137 | ||||||||
| Vibrio harveyi | x | 138 |
Abbreviations: As, arsenic; Cd, cadmium; Cr, chromium; Co, cobalt; Cu, copper; Fe, iron; Hg, mercury; Mn, manganese; Ni, nickel; Pb, lead; Zn, zinc.
Algal bioremediation (phycoremediation)
Different species of algae are present in large amounts in marine ecosystems. Algae are autotrophic organisms, have low nutritional requirements and generate vast biomass.79 Among the three algal groups; i.e., Phaeophyta, Rhodophyta and Chlorophyta (i.e. brown, green and red, respectively), brown algae have been reported to possess better biosorption capacity (phycoremediation). Metal ion biosorption varies with the kind and structure of the algal biomass, charge and chemical constitution of the heavy metal ion.139,140 Different algae, in live or dead forms, have been used, as single or in combination, in batch or column, for in-situ remediation. The presence of amine, hydroxyl, carboxyl, sulphate, and phosphate are potential metal sites in algal proteins, which operate by complex formation methods during heavy metal remediation.79,141 Calcium, magnesium, and sodium ions present in the cell wall get replaced by heavy metal ions via ion exchange. Table 5 depicts various types of algae as biosorbents.
Table 5—
Biosorption by Algal Species
| Algal species | As | Cd | Cr | Co | Cu | Fe | Hg | Mn | Ni | Pb | Zn | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asparagopsis sp., Codium sp., Spirogyra sp., Chondrus sp.,Fucus sp., Ascophyllum sp. | x | x | x | x | x | 141 | ||||||
| Ceramium virgatum | x | 142 | ||||||||||
| Chlamydomonas reinhardtii | x | x | x | 143 | ||||||||
| Chlorella vulgaris | x | x | x | x | 144 | |||||||
| C. vulgaris | x | x | x | 145 | ||||||||
| C. vulgaris, Scenedesmus armatus | x | x | x | 146 | ||||||||
| Chlorella kessleri | x | x | 147 | |||||||||
| Corallina mediterranea, Galaxaura oblongata, | x | x | x | x | 148 | |||||||
| Jania rubens, Pterocladia capillaceaCladophora fascicularis | x | 149 | ||||||||||
| Caulerpa fastigiata | x | 150 | ||||||||||
| Caulerpa lentillifera | x | x | x | 151 | ||||||||
| Spirogyra hyalina | x | x | x | x | x | x | 152 | |||||
| Fucus vesiculosus | x | x | x | 153 | ||||||||
| F. vesiculosus | x | 154 | ||||||||||
| Isochrysis galbana | x | 155 | ||||||||||
| Pithophora oedogonia | x | x | 156 | |||||||||
| Sargassum polycystum | x | 157 | ||||||||||
| Sargassum sp., Padina sp., Ulva sp., Gracillaria sp. | x | x | x | x | x | 158 | ||||||
| Scenedesmus quadricauda | x | x | x | 159 | ||||||||
| Schizosaccharomyces pombe | x | 160 | ||||||||||
| S. hyalina | x | x | x | x | x | 161 | ||||||
| Spirogyra sp. | x | x | x | x | 162 | |||||||
| Spirulina platensis | x | 163 | ||||||||||
| Ulva fascia | x | x | 164 | |||||||||
| Ulva lactuca, Jania rubens, Sargassum asperifolium | x | 165 | ||||||||||
Abbreviations: As, arsenic; Cd, cadmium; Cr, chromium; Co, cobalt; Cu, copper; Fe, iron; Hg, mercury; Mn, manganese; Ni, nickel; Pb, lead; Zn, zinc.
Fungal bioremediation (mycoremediation)
Fungi are known for their pervasive presence in the natural environment and are exploited extensively in industrial applications.166 Fungi are adapted (in terms of their morphology, ecology and metabolism) according to environmental conditions and are responsible for processes like decomposition and nutrient cycling under natural conditions.167 They have been reported to withstand and survive under stress conditions of moisture, nutrients, pH, etc. Mycoremediation involves use of fungus (live or dead) for the removal of contaminants from different environmental segments.168,169 Mycoremediation is a cost-effective process and does not leave harmful waste products. Hence, it poses a complete solution because of the full mineralization of the pollutants in nature.170 The success of mycoremediation depends on the identification and usage of a suitable fungal species for the target heavy metal or other contaminants. Fungi have the ability to accumulate heavy metals in their fruit bodies in an efficient manner, making them unavailable or decreasing their concentration in the media.171 The future availability of heavy metals and other contaminants in the media depend upon the life of the fungi, chemical behavior of the elements and presence or absence of the fungi after sequestration. Saccharomyces cerevisiae has been reported to sequester up to 65–79% of Pb and Cd from polluted soil.172 The process of biosorption involves fungal cell walls (having chitin, proteins, glucans, lipids, pigments, polysaccharides) and functional groups like hydroxyl, carboxyl, amino, sulphate, or phosphate and is mediated through interactions like adsorption, ion-exchange and complexation.173–175 Aspergillus sp. have been reported to remove Cr from tannery wastewater; it removed 65% of the Cr from the wastewater as compared to 85% from the synthetic medium.176
The phylum basidiomycetes includes wood-decaying species (white- and brown-rot fungi), mushrooms and other fungi.177 Mushrooms have played an important role in the human diet throughout history due to their nutritional and medicinal properties. Besides their use as food, they are used for mycoremediation due to their potential for heavy metal uptake. Metal uptake in mushrooms is affected by contact time, age of mycelia and fructification.178 Some edible wild varieties of mushrooms can accumulate heavy metals over their background concentrations.179 Heavy metals scatter disproportionately in the mushroom fruiting body.180,181 Different species of white-rot fungi, including Pleurotus ostreatus and Termitomyces clypeatus have been reported to degrade persistent pollutants (Table 6).181,182
Table 6.
Bioremediation by Fungal Species
| Fungal species | As | Cd | Cr | Co | Cu | Fe | Hg | Mn | Ni | Pb | Zn | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agaricus bisporus, Pleurotus ostreatus | x | x | x | 183 | ||||||||
| A. bisporus | x | x | x | x | 184 | |||||||
| Alternaria alternata, Penicillium aurantiogriseum | x | x | 185 | |||||||||
| Aspergillus sp. | x | 186 | ||||||||||
| Aspergillus flavus, Aspergillus fumigatus, Paecilomyces sp., Cladosporium sp., Mucor sp. | x | 187 | ||||||||||
| A. flavus, A. gracilis, A. penicillioides, A. restrictus, Sterigmatomyces halophilus | x | x | x | x | x | x | 188 | |||||
| Aspergillus sp., Rhizopus sp. | x | x | 189 | |||||||||
| Aspergillus sp., Penicillium sp. | x | 190 | ||||||||||
| Schizophyllum commune | x | x | x | x | 191 | |||||||
| P. ostreatus | x | x | x | x | 192 | |||||||
| Pleurotus ferulae | x | 193 | ||||||||||
| P. ostreatus | x | 194 | ||||||||||
| Pleurotus floridaTrichoderma viride | x | 195 | ||||||||||
| Rhizopus nigricans | x | 196 | ||||||||||
| Rhizopus arrhizus | x | 197 | ||||||||||
| Penicillium coffeae | x | 198 | ||||||||||
| A. flavus, A. fumigatus, Helminthosporium sp., Cladosporium sp., Mucor sp. | x | 199 | ||||||||||
| Penicillium janthinellum | x | 200 | ||||||||||
| Termitomyces clypeatus | x | 201 | ||||||||||
| Talaromyces helicus | x | 202 | ||||||||||
| Trichoderma viride | x | 203 | ||||||||||
Abbreviations: As, arsenic; Cd, cadmium; Cr, chromium; Co, cobalt; Cu, copper; Fe, iron; Hg, mercury; Mn, manganese; Ni, nickel; Pb, lead; Zn, zinc.
Recommendations
The unregulated discharge of industrial effluents in agricultural fields or water bodies increases their chances of entering the food chain through crops and aquatic animals and subsequent bioaccumulation. Various in-situ and ex-situ methods of bioremediation suitable to different environmental conditions have been investigated and recommended.204–207 The design, development, and application of these techniques require careful selection of biological agents. Extensive research is being carried out using specific strains of microorganisms for bioremediation. Microorganisms carry out redox reactions; and hence, impact the bioremediation processes by metal mobilization/immobilization. Manganese(II)-oxidizing Bacillus sp. strain indirectly oxidizes Cr(III) into the mobile and bioavailable form of Cr(VI) by producing oxidized Mn.208 The process of heavy metal bioremediation is more efficient using different microbial strains concurrently instead of only a single species.63 Advances in genetic engineering and optimization techniques suggest that the future of these technologies is promising.209,210 Genetically modified microorganisms may have a better bioremediation potential for various contaminants. In addition, the potential of agricultural and industrial waste biomass as bioremediators on a lab/commercial scale is currently being tested; e.g., sugarcane bagasse, coconut shell waste, rice husk, and beer waste yeast.211–214 The biosorption capacity of various biosorbents is improved after various physical and chemical modifications and further research is needed in order to use these biosorbents on a commercial scale across industries. The bioremediation approach requires a holistic and inclusive method for systematic, feasible and sustainable strategies which can be easily customized for each scenario. Moreover, there is an urgent need for coordination at all levels, including research organizations, the general public, governmental institutions, and industries.215
Conclusions
Heavy metal pollution occurs from various anthropogenic and natural sources. Heavy metals, due to their non-biodegradable and hazardous nature, should be removed from the environment. Industrial wastewater discharged into environmental segments, such as soil and rivers, requires immediate intervention by governmental agencies, regular monitoring and remediation using appropriate methods. Conventional methods of treatment have limitations and should be replaced by efficient, cost-effective and eco-friendly techniques such as bioremediation employing biological agents. Adopting an appropriate biosorbent in terms of efficiency and economy is a major concern. Microorganisms carry out redox reactions; and hence, impact the bioremediation processes by metal mobilization/immobilization. Metal-microbe interaction influences microbial processes, such as their growth, colonization and microbial biofilm formation for remediation. Further research is needed translating these lab scale options into industrial applications (e.g. microbial films) considering factors such as appropriate and adapted microorganisms/appropriate biomass waste and/or mixtures of different kinds of microbial biomass with conventional technologies and suitable conditions in ex- or in-situ environments.
Acknowledgements
This study was funded as part of employment.
References
- 1.Gray JS. Biomagnification in marine systems: the perspective of an ecologist. Mar Pollut Bull [Internet] 2002;45(1–12):46–52. doi: 10.1016/S0025-326X(01)00323-X. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 2.Wuana RA, Okieimen FE. Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. Int Sch Res Notices. 2011;2011:20. doi: 10.5402/2011/402647. [cited 2019 Aug 21] Article 402647 [ p.]. Available from: [DOI] [Google Scholar]
- 3.Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. Exp Suppl. 2012;101:133–64. doi: 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaushik P, Garg VK, Singh B. Effect of textile effluents on growth performance of wheat cultivars. Bioresour Technol [Internet] 2005 Jul;96(10):1189–93. doi: 10.1016/j.biortech.2004.09.020. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 5.Jarup L. Hazards of heavy metal contamination. Br Med Bull [Internet] 2003 Dec;68(1):167–82. doi: 10.1093/bmb/ldg032. [cited 2019 Aug 21] Available from: [DOI] [PubMed] [Google Scholar]
- 6.Srisuwan G, Thongchai P. Removal of heavy metals from electroplating wastewater by membrane. Songklanakarin J Sci Technol. 2002;24(Suppl.):965–76. [Google Scholar]
- 7.Sivasangari S, Suseendhar S, Kumar KS, Vijayaprasath N, Thirumurugan M. Characteristic study of electroplating and dye industrial effluents. Int J Innov Res Sci En Technol. 2016 Dec;5(12):20810–16. [Google Scholar]
- 8.Venkateswaran P, Vellaichamy S, Palanivelu K. Speciation of heavy metals in electroplating industry sludge and wastewater residue using inductively coupled plasma. Int J Environ Sci Technol [Internet] 2007 Sep;4(4):497–504. doi: 10.1007/BF03325986. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [Google Scholar]
- 9.Nriagu JO. A global assessment of natural sources of atmospheric trace metals. Nat [Internet] 1989 Mar;338:47–9. doi: 10.1038/338047a0. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [Google Scholar]
- 10.Orescanin V, Kollar R, Mikelic IL, Nad K. Electroplating wastewater treatment by the combined electrochemical and ozonation methods. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2013;48(11):1450–5. doi: 10.1080/10934529.2013.781904. [DOI] [PubMed] [Google Scholar]
- 11.Devi R. Bioremediation of tannery effluent by Aspergillus flavus. Poll Res. 2011;6(4):141–8. [Google Scholar]
- 12.Chhikara S, Rana L, Poonam Physico-chemical characterization of textile mill effluent: a case study of Haryana, India. Environ We Int J Sci Tech [Internet] 2013 Mar;8(1):19–23. [cited 2019 Aug 21] Available from: http://www.ewijst.org/issues/vol_8/ewijst080123080.pdf. [Google Scholar]
- 13.Husain A, Javed I, Khan NA. Characterization and treatment of electroplating industry wastewater using Fenton's reagent. J Chem Pharm Res. 2014;6(1):622–7. [Google Scholar]
- 14.Haritash AK, Gaur S, Garg S. Assessment of water quality and suitability analysis of River Ganga in Rishikesh, India. Appl Water Sci [Internet] 2016 Nov;6(4):383–92. doi: 10.1007/s13201-014-0235-1. [cited 2019 Aug 21] Available from: [DOI] [Google Scholar]
- 15.Tyagi S, Sarma K. Assessment of groundwater quality in different land uses in Ghaziabad District of Uttar Pradesh, India. Environ We Int J Sci Tech [Internet] 2018 Jul-Dec;13(2):99–117. [cited 2019 Aug 21] Available from: http://www.ewijst.org/issues/vol13/vol13_files/vol132.htm. [Google Scholar]
- 16.Siddiqui WA, Sharma RR. Assessment of the impact of industrial effluents on groundwater quality in Okhla industrial area, New Delhi, India. E-J Chem [Internet] 2009;6(S1):S41–6. doi: 10.1155/2009/525707. [cited 2019 Aug 21] Available from: [DOI] [Google Scholar]
- 17.Srivastava S, Sharma YK. Arsenic occurrence and accumulation in soil and water of eastern districts of Uttar Pradesh, India. Environ Monit Assess [Internet] 2013 Jun;185(6):4995–5002. doi: 10.1007/s10661-012-2920-6. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 18.Sharma RK, Agrawal M, Marshall F. Heavy metal contamination in vegetables grown in wastewater irrigated areas of Varanasi, India. Bull Environ Contam Toxicol [Internet] 2006 Aug;77(2):312–8. doi: 10.1007/s00128-006-1065-0. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 19.Rai PK, Tripathi BD. Heavy metals in industrial wastewater, soil and vegetables in Lohta village, India. Toxicol Environ Chem [Internet] 2008;90(2):247–57. doi: 10.1080/02772240701458584. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [Google Scholar]
- 20.Singh R, Verma RS, Yadav Y. Use of industrial waste water for agricultural purpose: Pb and Cd in vegetables in Bikaner city, India. Curr World Environ [Internet] 2012;7(2):287. doi: 10.12944/CWE.7.2.14. [cited 2019 Aug 21] Available from: [DOI] [Google Scholar]
- 21.Lokeshwari H, Chandrappa GT. Impact of heavy metal contamination of Bellandur lake on soil and cultivated vegetation. Curr Sci [Internet] 2006 Sep 10;91(5):622–7. [cited 2019 Aug 21] Available from: https://www.jstor.org/stable/24094365 Subscription required to view. [Google Scholar]
- 22.Puttaiah ET, Kiran BR. Heavy metal transport in a sewage-fed lake of Karnataka, India. Proceedings of Taal: The 12th World Lake Conference; 2007 Oct 28–Nov 2; Jaipur, Rajasthan, India. Japan: International Lake Environment Committee; 2008. pp. 347–54. p. [Google Scholar]
- 23.Adesiyan IM, Bisi-Johnson M, Aladesanmi OT, Okoh AI, Ogunfowokan AO. Concentrations and human health risk of heavy metals in rivers in Southwest Nigeria. J Health Poll [Internet] 2018 Sep;8(19):14. doi: 10.5696/2156-9614-8.19.180907. [cited 2019 Aug 21] Article 180907 [ p.]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Status of trace and toxic metals in Indian rivers [Internet] New Delhi, India: Central Water Commission; 2018. Apr, p. 251. [cited 2019 Feb 6] p. Available from: http://www.indiaenvironmentportal.org.in/files/file/status_trace_toxic_materials_indian_rivers.pdf. [Google Scholar]
- 25.Akter KF, Owens G, Davey DE, Naidu R. Arsenic speciation and toxicity in biological systems. Rev Environ Contam Toxicol. 2005;184:97–149. doi: 10.1007/0-387-27565-7_3. [DOI] [PubMed] [Google Scholar]
- 26.J Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014 Jun;7(2):60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung JY, Yu SD, Hong YS. Environmental source of arsenic exposure. J Prev Med Public Health [Internet] 2014 Sep;47(5):253–7. doi: 10.3961/jpmph.14.036. [cited 2019 Aug 21] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferguson JE, Gavis J. A review of the arsenic cycle in natural waters. Water Res [Internet] 1972 Nov;6(11):1259–74. doi: 10.1016/0043-1354(72)90052-8. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [Google Scholar]
- 29.Singh N, Kumar D, Sahu AP. Arsenic in the environment: effects on human health and possible prevention. J Environ Biol. 2007 Apr;28(2 Suppl):359–65. [PubMed] [Google Scholar]
- 30.Singh KK, Singh AK, Hasan SH. Low cost biosorbent ‘wheat bran’ for the removal of cadmium from wastewater: kinetic and equilibrium studies. Bioresour Technol [Internet] 2006 May;97(8):994–1001. doi: 10.1016/j.biortech.2005.04.043. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 31.Health risks of heavy metals from long-range transboundary air pollution [Internet] Copenhagen, Denmark: World Health Organization; 2007. p. 144. [cited 2019 Feb 6] p. Available from: http://www.euro.who.int/document/E91044.pdf. [Google Scholar]
- 32.Draft final review of scientific information on cadmium. Nairobi, Kenya: United Nations Environment Programme, Chemicals Branch; 2008. [Google Scholar]
- 33.Cadmium: environmental health criteria 134 [Internet] Geneva, Switzerland: World Health Organization; 1992. p. 130. [cited 2019 Feb 6] p. Available from: http://www.inchem.org/documents/ehc/ehc/ehc134.htm. [Google Scholar]
- 34.Rani A, Kumar A, Lal A, Pant M. Cellular mechanisms of cadmium-induced toxicity: a review. Int J Environ Health Res [Internet] 2014 Aug;24(4):378–99. doi: 10.1080/09603123.2013.835032. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 35.Coonse KG, Coonts AJ, Morrison EV, Heggland SJ. Cadmium induces apoptosis in the human osteoblast-like cell line Saos-2. J Toxicol Environ Health A [Internet] 2007;70(7):575–81. doi: 10.1080/15287390600882663. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 36.Gadd GM. Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiol [Internet] 2010 Mar;156(3):609–43. doi: 10.1099/mic.0.037143-0. [cited 2019 Aug 21] Available from: [DOI] [PubMed] [Google Scholar]
- 37.Chen JM, Hao OJ. Microbial chromium (VI) reduction. Critic Rev Environ Sci Technol [Internet] 1998;28(3):219–51. doi: 10.1080/10643389891254214. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [Google Scholar]
- 38.Toxicological profile for lead. Atlanta, GA: Agency for Toxic Substances and Disease Registry; 1999. [Google Scholar]
- 39.Mahimairaja S, Bolan NS, Adriano DC, Robinson B. Arsenic contamination and its risk management in complex environmental settings. Adv Agron [Internet] 2005;86:1–82. doi: 10.1016/S0065-2113(05)86001-8. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [Google Scholar]
- 40.Basu A, Saha D, Saha R, Ghosh T, Saha B. A review on sources, toxicity and remediation technologies for removing arsenic from drinking water. Res Chem Intermed [Internet] 2014 Feb;40(2):447–85. doi: 10.1007/s11164-012-1000-4. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [Google Scholar]
- 41.Gray NF. Water technology: an introduction for environmental scientists and engineers. London, UK: Hodder Headline Group; 1999. pp. 473–4. p. [Google Scholar]
- 42.Hassan SH, Awad YM, Kabir MH, Oh SE, Joo JH. Biotechnology cracking new pastures. New Delhi, India: MD Publications Pvt. Ltd.; 2010. Jan, Bacterial biosorption of heavy metals; pp. 79–110. p. [Google Scholar]
- 43.Aziz HA, Adlan MN, Ariffin KS. Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) removal from water in Malaysia: post treatment by high quality limestone. Bioresour Technol [Internet] 2008 Apr;99(6):1578–83. doi: 10.1016/j.biortech.2007.04.007. [cited 2019 Aug 21] Available from: [DOI] [PubMed] [Google Scholar]
- 44.Gunatilake SK. Methods of removing heavy metals from industrial wastewater. J Multidiscip Eng Sci Stud. 2015 Nov;1(1):12–8. [Google Scholar]
- 45.Ayangbenro AS, Babalola OO. A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Res Public Health [Internet] 2017 Jan;14(1):16. doi: 10.3390/ijerph14010094. [cited 2019 Aug 21] Article 94 [ p.]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Firdousi SA. Bioaccumulation and bio-absorptions of heavy metals by the mushroom from the soil. J Med Chem Drug Discov. 2017;2(3):25–33. [Google Scholar]
- 47.Ahalya N, Ramachandra TV, Kanamadi RD. Biosorption of heavy metals. Res J Chem Environ. 2003 Dec;7(4):71–9. [Google Scholar]
- 48.Adams JA, Reddy KR. Extent of benzene biodegradation in saturated soil column during air sparging. Groundw Monit Remediat [Internet] 2003 Aug;23(3):85–94. doi: 10.1111/j.1745-6592.2003.tb00686.x. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [Google Scholar]
- 49.Azubuike CC, Chikere CB, Okpokwasili GC. Bioremediation techniques-classification based on site of application: principles, advantages, limitations and prospects. World J Microbiol Biotechnol [Internet] 2016 Nov;32(11):18. doi: 10.1007/s11274-016-2137-x. [cited 2019 Aug 21] Article 180 [ p.]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hazen TC. In situ: groundwater bioremediation. In: Timmis KN, editor. Handbook of hydrocarbon and lipid microbiology. Berlin, Germany: Springer; 2010. pp. 2583–94. editor. p. [Google Scholar]
- 51.Philp JC, Atlas RM. Bioremediation of contaminated soils and aquifers. In: Atlas RM, Philp JC, editors. Bioremediation: applied microbial solutions for real-world environmental cleanup. Washington, DC: American Society for Microbiology Press; 2005. pp. 139–236. p. [Google Scholar]
- 52.Bouchez T, Patureau D, Dabert P, Juretschko S, Dore J, Delgenes P, Moletta R, Wagner M. Ecological study of a bioaugmentation failure. Environ Microbiol [Internet] 2000 Apr;2(2):179–90. doi: 10.1046/j.1462-2920.2000.00091.x. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 53.Liu W, Luo Y, Teng Y, Li Z, Christie P. Prepared bed bioremediation of oily sludge in an oilfield in northern China. J Hazard Mater [Internet] 2009 Jan 15;161(1):479–84. doi: 10.1016/j.jhazmat.2008.03.123. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 54.Thompson IP, van der Gast CJ, Ciric L, Singer AC. Bioaugmentation for bioremediation: the challenge of strain selection. Environ Microbiol [Internet] 2005 Jul;7(7):909–15. doi: 10.1111/j.1462-2920.2005.00804.x. [cited 2019 Aug 21] Available from: [DOI] [PubMed] [Google Scholar]
- 55.Perfumo A, Banat IM, Marchant R, Vezzulli L. Thermally enhanced approaches for bioremediation of hydrocarbon-contaminated soils. Chemosphere [Internet] 2007 Jan;66(1):179–84. doi: 10.1016/j.chemosphere.2006.05.006. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 56.Morley GF, Gadd GM. Sorption of toxic metals by fungi and clay minerals. Mycol Res [Internet] 1995 Dec;99(12):1429–38. doi: 10.1016/S0953-7562(09)80789-2. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [Google Scholar]
- 57.Satyapal GK, Mishra SK, Srivastava A, Ranjan RK, Prakash K, Haque R, Kumar N. Possible bioremediation of arsenic toxicity by isolating indigenous bacteria from the middle Gangetic plain of Bihar, India. Biotechnol Rep (Amst) 2018 Feb 8;17:117–25. doi: 10.1016/j.btre.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tak IR. Role of biotools in restoration of freshwater ecosystems. In: Qadri H, Bhat RA, Mehmood MA, Dar GH, editors. Fresh water pollution dynamics and remediation. Singapore: Springer, Singapore; 2020. pp. 123–42. p. [Google Scholar]
- 59.Fomina M, Gadd GM. Biosorption: current perspectives on concept, definition and application. Bioresour Technol [Internet] 2014 May;160:3–14. doi: 10.1016/j.biortech.2013.12.102. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 60.Sinha A, Pant KK, Khare SK. Studies on mercury bioremediation by alginate immobilized mercury tolerant Bacillus cereus cells. Int Biodeterior Biodegrad [Internet] 2012 Jul;71:1–8. doi: 10.1016/j.ibiod.2011.12.014. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [Google Scholar]
- 61.Esposito A, Pagnanelli F, Lodi A, Solisio C, Veglio F. Biosorption of heavy metals by Sphaerotilus natans: an equilibrium study at different pH and biomass concentrations. Hydrometall [Internet] 2001 Apr;60(2):129–41. doi: 10.1016/S0304-386X(00)00195-X. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [Google Scholar]
- 62.Gupta R, Ahuja P, Khan S, Saxena RK, Mohapatra M. Microbial biosorbents: meeting challenges of heavy metal pollution in aqueous solutions. Curr Sci. 2000 Apr;78(8):967–73. [Google Scholar]
- 63.Wang J, Chen C. Biosorbents for heavy metals removal and their future. Biotechnol Adv [Internet] 2009 Mar-Apr;27(2):195–226. doi: 10.1016/j.biotechadv.2008.11.002. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 64.Tiwari S, Hasan A, Pandey LM. A novel biosorbent comprising encapsulated Agrobacterium fabrum (SLAJ731) and iron oxide nanoparticles for removal of crude oil co-contaminant, lead Pb(II) J Environ Chem Eng [Internet] 2017 Feb;5(1):442–52. doi: 10.1016/j.jece.2016.12.017. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [Google Scholar]
- 65.Grujic S, Vasic S, Radojevic I, Comic L, Ostojic A. Comparison of the Rhodotorula mucilaginosa biofilm and planktonic culture on heavy metal susceptibility and removal potential. Water Air Soil Pollut [Internet] 2017 Feb;228(2):8. doi: 10.1007/s11270-017-3259-y. [cited 2019 Aug 21] Article 73 [ p.]. Available from: Subscription required to view. [DOI] [Google Scholar]
- 66.Volesky B, Holan ZR. Biosorption of heavy metals. Biotechnol Prog [Internet] 1995 May-Jun;11(3):235–50. doi: 10.1021/bp00033a001. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 67.Veglio F, Beolchini F. Removal of metals by biosorption: a review. Hydrometall [Internet] 1997 Mar;44(3):301–16. doi: 10.1016/S0304-386X(96)00059-X. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [Google Scholar]
- 68.Demirbas A. Heavy metal adsorption onto agro-based waste materials: a review. J Hazard Mater [Internet] 2008 Sep 15;157(2–3):220–9. doi: 10.1016/j.jhazmat.2008.01.024. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 69.Vijayaraghavan K, Yun YS. Biosorption of C.I. Reactive Black 5 from aqueous solution using acid-treated biomass of brown seaweed Laminaria sp. Dyes Pigment [Internet] 2008;76(3):726–32. doi: 10.1016/j.dyepig.2007.01.013. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [Google Scholar]
- 70.Romera E, Gonzalez F, Ballester A, Blazquez ML, Munoz JA. Biosorption with algae: a statistical review. Crit Rev Biotechnol [Internet] 2006 Oct-Dec;26(4):223–35. doi: 10.1080/07388550600972153. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 71.Lovley DR, Coates JD. Bioremediation of metal contamination. Curr Opin Biotechnol [Internet] 1997 Jun;8(3):285–9. doi: 10.1016/S0958-1669(97)80005-5. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 72.Davis TA, Volesky B, Mucci A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res [Internet] 2003 Nov;37(18):4311–30. doi: 10.1016/S0043-1354(03)00293-8. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 73.Das N, Vimala R, Karthika P. Biosorption of heavy metals - an overview. Indian J Biotechnol. 2008 Apr;7:159–69. [Google Scholar]
- 74.Morin E, Kohler A, Baker AR, Foulongne-Oriol M, Lombard V, Nagy LG, Ohm RA, Patyshakuliyeva A, Brun A, Aerts AL, Bailey AM, Billette C, Coutinho PM, Deakin G, Doddapaneni H, Floudas D, Grimwood J, Hilden K, Kues U, Labutti KM, Lapidus A, Lindquist EA, Lucas SM, Murat C, Riley RW, Salamov AA, Schmutz J, Subramanian V, Wosten HA, Xu J, Eastwood DC, Foster GD, Sonnenberg AS, Cullen D, de Vries RP, Lundell T, Hibbett DS, Henrissat B, Burton KS, Kerrigan RW, Challen MP, Grigoriev IV, Martin F. Genome sequence of the button mushroom Agaricus bisporus reveals mechanisms governing adaptation to a humic-rich ecological niche. Proc Natl Acad Sci U S A [Internet] 2012 Oct 23;109(43):17501–6. doi: 10.1073/pnas.1206847109. [cited 2019 Aug 21] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ohm RA, de Jong JF, Lugones LG, Aerts A, Kothe E, Stajich JE, de Vries RP, Record E, Levasseur A, Baker SE, Bartholomew KA, Coutinho PM, Erdmann S, Fowler TJ, Gathman AC, Lombard V, Henrissat B, Knabe N, Kues U, Lilly WW, Lindquist E, Lucas S, Magnuson JK, Piumi F, Raudaskoski M, Salamov A, Schmutz J, Schwarze FW, vanKuyk PA, Horton JS, Grigoriev IV, Wosten HA. Genome sequence of the model mushroom Schizophyllum commune. Nat Biotechnol [Internet] 2010 Sep;28(9):957–63. doi: 10.1038/nbt.1643. [cited 2019 Aug 21] Available from: [DOI] [PubMed] [Google Scholar]
- 76.Pinedo-Rivilla C, Aleu J, Collado IG. Pollutants biodegradation by fungi. Curr Org Chem [Internet] 2009;13(12):1194–214. doi: 10.2174/138527209788921774. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [Google Scholar]
- 77.D'Annibale A, Leonardi V, Federici E, Baldi F, Zecchini F, Petruccioli M. Leaching and microbial treatment of a soil contaminated by sulphide ore ashes and aromatic hydrocarbons. Appl Microbiol Biotechnol [Internet] 2007 Apr;74(5):1135–44. doi: 10.1007/s00253-006-0749-z. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 78.Tunali S, Akar T, Ozcan AS, Kiran I, Ozcan A. Equilibrium and kinetics of biosorption of lead(II) from aqueous solutions by Cephalosporium aphidicola. Sep Purif Technol [Internet] 2006 Jan;47(3):105–12. doi: 10.1016/j.seppur.2005.06.009. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [Google Scholar]
- 79.Abbas SH, Ismail IM, Mostafa TM, Sulaymon AH. Biosorption of heavy metals: a review. J Chem Sci Technol. 2014 Oct;3(4):74–102. [Google Scholar]
- 80.Comte S, Guibaud G, Baudu M. Biosorption properties of extracellular polymeric substances (EPS) towards Cd, Cu and Pb for different pH values. J Hazard Mater [Internet] 2008 Feb 28;151(1):185–93. doi: 10.1016/j.jhazmat.2007.05.070. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 81.Wiatrowski HA, Ward PM, Barkay T. Novel reduction of mercury(II) by mercury-sensitive dissimilatory metal reducing bacteria. Environ Sci Technol [Internet] 2006 Nov;40(21):6690–6. doi: 10.1021/es061046g. [cited 2019 Aug 21] Available from: [DOI] [PubMed] [Google Scholar]
- 82.Bachate SP, Khapare RM, Kodam KM. Oxidation of arsenite by two β-proteobacteria isolated from soil. Appl Microbiol Biotechnol [Internet] 2012 Mar;93(5):2135–45. doi: 10.1007/s00253-011-3606-7. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 83.Battaglia-Brunet F, Dictor MC, Garrido F, Crouzet C, Morin D, Dekeyser K, Clarens M, Baranger P. An arsenic(III)-oxidizing bacterial population: selection, characterization, and performance in reactors. J Appl Microbiol [Internet] 2002 Oct;93(4):656–67. doi: 10.1046/j.1365-2672.2002.01726.x. [cited 2019 Aug 21] Available from: [DOI] [PubMed] [Google Scholar]
- 84.Chang JS, Yoon IH, Lee JH, Kim KR, An J, Kim KW. Arsenic detoxification potential of aox genes in arsenite-oxidizing bacteria isolated from natural and constructed wetlands in the Republic of Korea. Environ Geochem Health [Internet] 2010 Apr;32(2):95–105. doi: 10.1007/s10653-009-9268-z. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 85.Kinegam S, Yingprasertchai T, Tanasupawat S, Leepipatpiboon N, Akaracharanya A, Kim KW. Isolation and characterization of arsenite-oxidizing bacteria from arsenic-contaminated soils in Thailand. World J Microbiol Biotechnol [Internet] 2008 Dec;24(12):3091–6. doi: 10.1007/s11274-008-9821-4. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [Google Scholar]
- 86.Santini JM, Sly LI, Wen A, Comrie D, Wulf-Durand P, Macy JM. New arsenite-oxidizing bacteria isolated from Australian gold mining environments--phylogenetic relationships. Geomicrobiol J [Internet] 2002;19(1):67–76. doi: 10.1080/014904502317246174. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [Google Scholar]
- 87.Pokhrel D, Viraraghavan T. Arsenic removal from an aqueous solution by a modified fungal biomass. Water Res [Internet] 2006 Feb;40(3):549–52. doi: 10.1016/j.watres.2005.11.040. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 88.Bolan N, Kunhikrishnan A, Thangarajan R, Kumpiene J, Park J, Makino T, Kirkham MB, Scheckel K. Remediation of heavy metal(loid)s contaminated soils – to mobilize or to immobilize? J Hazard Mater [Internet] 2014 Feb 15;266:141–66. doi: 10.1016/j.jhazmat.2013.12.018. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 89.Wengel M, Kothe E, Schmidt CM, Heide K, Gleixner G. Degradation of organic matter from black shales and charcoal by the wood-rotting fungus Schizophyllum commune and release of DOC and heavy metals in the aqueous phase. Sci Total Environ [Internet] 2006 Aug 15;367(1):383–93. doi: 10.1016/j.scitotenv.2005.12.012. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 90.Mosa KA, Saadoun I, Kumar K, Helmy M, Dhankher OP. Potential biotechnological strategies for the cleanup of heavy metals and metalloids. Front Plant Sci. 2016;7:14. doi: 10.3389/fpls.2016.00303. Article 303 [ p.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gadd GM. Bioremedial potential of microbial mechanisms of metal mobilization and immobilization. Curr Opin Biotechnol [Internet] 2000 Jun;11(3):271–9. doi: 10.1016/S0958-1669(00)00095-1. [cited 2019 Aug 21] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 92.Azubuike CC, Chikere CB, Okpokwasili GC. Bioremediation techniques-classification based on site of application: principles, advantages, limitations and prospects. World J Microbiol Biotechnol [Internet] 2016 Nov;32(11):180. doi: 10.1007/s11274-016-2137-x. [cited 2019 Aug 22] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Singh R, Dong H, Liu D, Zhao L, Marts AR, Farquhar E, Tierney DL, Almquist CB, Briggs BR. Reduction of hexavalent chromium by the thermophilic methanogen Methanothermobacter thermautotrophicus. Geochim Cosmochim Acta. 2015 Jan 1;148:442–456. doi: 10.1016/j.gca.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen Z, Huang Z, Cheng Y, Pan D, Pan X, Yu M, Pan Z, Lin Z, Guan X, Wu Z. Cr(VI) uptake mechanism of Bacillus cereus. Chemosphere [Internet] 2012 Apr;87(3):211–6. doi: 10.1016/j.chemosphere.2011.12.050. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 95.Middleton SS, Latmani RB, Mackey MR, Ellisman MH, Tebo BM, Criddle CS. Cometabolism of Cr(VI) by Shewanella oneidensis MR-1 produces cell-associated reduced chromium and inhibits growth. Biotechnol Bioeng [Internet] 2003 Sep 20;83(6):627–37. doi: 10.1002/bit.10725. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 96.Igiri BE, Okoduwa SI, Idoko GO, Akabuogu EP, Adeyi AO, Ejiogu IK. Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: a review. J Toxicol [Internet] 2018;2018:16. doi: 10.1155/2018/2568038. [cited 2019 Aug 22] Article 2568038 [ p.]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Velasquez L, Dussan J. Biosorption and bioaccumulation of heavy metals on dead and living biomass of Bacillus sphaericus. J Hazard Mater [Internet] 2009 Aug 15;167(1–3):713–6. doi: 10.1016/j.jhazmat.2009.01.044. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 98.Ahluwalia SS, Goyal D. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour Technol [Internet] 2007 Sep;98(12):2243–57. doi: 10.1016/j.biortech.2005.12.006. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 99.Klaus-Joerger T, Joerger R, Olsson E, Granqvist C. Bacteria as workers in the living factory: metalaccumulating bacteria and their potential for materials science. Trends Biotechnol [Internet] 2001 Jan;19(1):15–20. doi: 10.1016/S0167-7799(00)01514-6. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 100.Poirier I, Hammann P, Kuhn L, Bertrand M. Strategies developed by the marine bacterium Pseudomonas fluorescens BA3SM1 to resist metals: a proteome analysis. Aquat Toxicol [Internet] 2013 Mar 15;128–129:215–32. doi: 10.1016/j.aquatox.2012.12.006. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 101.Paliwal V, Puranik S, Purohit HJ. Integrated perspective for effective bioremediation. Appl Biochem Biotechnol [Internet] 2012 Feb;166(4):903–24. doi: 10.1007/s12010-011-9479-5. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 102.Wang CL, Ozuna SC, Clark DS, Keasling JD. A deep-sea hydrothermal vent isolate, Pseudomonas aeruginosa CW961, requires thiosulfate for Cd 2+ tolerance and precipitation. Biotechnol Lett [Internet] 2002 Apr;24(8):637–41. doi: 10.1023/A:1015043324584. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sharma PK, Balkwill DL, Frenkel A, Vairavamurthy MA. A new Klebsiella planticola strain (Cd-1) grows anaerobically at high cadmium concentrations and precipitates cadmium sulfide. Appl Environ Microbiol [Internet] 2000 Jul;66(7):3083–7. doi: 10.1128/AEM.66.7.3083-3087.2000. [cited 2019 Aug 22] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mustapha MU, Halimoon N. Microorganisms and biosorption of heavy metals in the environment: a review paper. J Microb Biochem Technol. 2015;7(5):253–6. [Google Scholar]
- 105.Akhtar K, Akhtar MW, Khalid AM. Removal and recovery of uranium from aqueous solutions by Trichoderma harzianum. Water Res [Internet] 2007 Mar;41(6):1366–78. doi: 10.1016/j.watres.2006.12.009. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 106.Hassan SH, Kim SJ, Jung AY, Joo JH, Eun Oh S, Yang JE. Biosorptive capacity of Cd(II) and Cu(II) by lyophilized cells of Pseudomonas stutzeri. J Gen Appl Microbiol [Internet] 2009 Feb;55(1):27–34. doi: 10.2323/jgam.55.27. [cited 2019 Aug 22] Available from: [DOI] [PubMed] [Google Scholar]
- 107.Kao WC, Huang CC, Chang JS. Biosorption of nickel, chromium and zinc by MerP-expressing recombinant Escherichia coli. J Hazard Mater [Internet] 2008 Oct 1;158(1):100–6. doi: 10.1016/j.jhazmat.2008.01.032. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 108.Tuzen M, Saygi KO, Usta C, Soylak M. Pseudomonas aeruginosa immobilized multiwalled carbon nanotubes as biosorbent for heavy metal ions. Bioresour Technol [Internet] 2008 Apr;99(6):1563–70. doi: 10.1016/j.biortech.2007.04.013. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 109.Leung WC, Chua H, Lo WH. Biosorption of heavy metals by bacteria isolated from activated sludge. Appl Biochem Biotechnol [Internet] 2001 Mar;91(1–9):171–84. doi: 10.1385/ABAB:91-93:1-9:171. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 110.Ozer A, Ozer D. Comparative study of the biosorption of Pb(II), Ni(II) and Cr(VI) ions onto S. cerevisiae: determination of biosorption heats. J Hazard Mater [Internet] 2003 Jun 27;100(1–3):219–29. doi: 10.1016/S0304-3894(03)00109-2. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 111.Sherbet GV. The Biophysical characterisation of the cell surface. London: Academic press; 1978. p. 414. p. [Google Scholar]
- 112.Doyle RJ, Matthews TH, Streips UN. Chemical basis for selectivity of metal ions by the Bacillus subtilis cell wall. J Bacteriol [Internet] 1980 Jul;143(1):471–80. doi: 10.1128/jb.143.1.471-480.1980. [cited 2019 Aug 22] Available from: https://jb.asm.org/content/143/1/471.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van der Wal A, Norde W, Zehnder AJ, Lyklema J. Determination of the total charge in the cell walls of Gram-positive bacteria. Colloids Surf B Biointerfaces [Internet] 1997 Jun;9(1–2):81–100. doi: 10.1016/S0927-7765(96)01340-9. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [Google Scholar]
- 114.Yee N, Fein J. Cd adsorption onto bacterial surfaces: a universal adsorption edge? Geochim Cosmochim Acta [Internet] 2001 Jul;65(13):2037–42. doi: 10.1016/S0016-7037(01)00587-7. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [Google Scholar]
- 115.Kang SY, Lee JU, Kim KM. Biosorption of Cr(III) and Cr(VI) onto the cell surface of Pseudomonas aeruginosa. Biochem Eng J [Internet] 2007 Aug;36(1):54–8. doi: 10.1016/j.bej.2006.06.005. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [Google Scholar]
- 116.Adenipekun CO, Lawal R. Uses of mushrooms in bioremediation: a review. Biotechnol Mol Biol Rev. 2012 Sep;7(3):62–8. [Google Scholar]
- 117.Hasan SH, Srivastava P. Batch and continuous biosorption of Cu(2+) by immobilized biomass of Arthrobacter sp. J Environ Manage [Internet] 2009 Aug;90(11):3313–21. doi: 10.1016/j.jenvman.2009.05.005. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 118.Tunali S, Cabuk A, Akar T. Removal of lead and copper ions from aqueous solutions by bacterial strain isolated from soil. Chem Eng J [Internet] 2006 Jan;115(3):203–11. doi: 10.1016/j.cej.2005.09.023. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [Google Scholar]
- 119.Dey U, Chatterjee S, Mondal NK. Isolation and characterization of arsenic-resistant bacteria and possible application in bioremediation. Biotechnol Rep [Internet] 2016 Jun;10:1–7. doi: 10.1016/j.btre.2016.02.002. [cited 2019 Aug 22] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zouboulis AI, Loukidou MX, Matis KA. Biosorption of toxic metals from aqueous solutions by bacteria strains isolated from metal-polluted soils. Process Biochem [Internet] 2004 Apr;39(8):909–16. doi: 10.1016/S0032-9592(03)00200-0. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [Google Scholar]
- 121.Choi SB, Yun YS. Lead biosorption by waste biomass of Corynebacterium glutamicum generated from lysine fermentation process. Biotechnol Lett [Internet] 2004 Feb;26(4):331–6. doi: 10.1023/B:BILE.0000015453.20708.fc. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 122.Kim IH, Choi JH, Joo JO, Kim YK, Choi JW, Oh BK. Development of a microbe-zeolite carrier for the effective elimination of heavy metals from seawater. J Microbiol Biotechnol [Internet] 2015 Sep;25(9):1542–6. doi: 10.4014/jmb.1504.04067. [cited 2019 Aug 22] Available from: http://www.jmb.or.kr/journal/viewJournal.html?doi=10.4014/jmb.1504.04067. [DOI] [PubMed] [Google Scholar]
- 123.Rani MJ, Hemambika B, Hemapriya J, Rajeshkannan V. Comparative assessment of heavy metal removal by immobilized and dead bacterial cells: a biosorption approach. Glob J Environ Res. 2010;4(1):23–30. [Google Scholar]
- 124.Zhou M, Liu Y, Zeng G, Li X, Xu W, Fan T. Kinetic and equilibrium studies of Cr(VI) biosorption by dead Bacillus licheniformis biomass. World J Microbiol Biotechnol [Internet] 2007 Jan;23(1):43–8. doi: 10.1007/s11274-006-9191-8. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [Google Scholar]
- 125.Lee KY, Bosch J, Meckenstock RU. Use of metal-reducing bacteria for bioremediation of soil contaminated with mixed organic and inorganic pollutants. Environ Geochem Health [Internet] 2012 Jan;34(Suppl 1):135–42. doi: 10.1007/s10653-011-9406-2. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 126.He Y, Gong Y, Su Y, Zhang Y, Zhou X. Bioremediation of Cr (VI) contaminated groundwater by Geobacter sulfurreducens: environmental factors and electron transfer flow studies. Chemosphere [Internet] 2019 Apr;221:793–801. doi: 10.1016/j.chemosphere.2019.01.039. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 127.Samarth DP, Chandekar CJ, Bhadekar RK. Biosorption of heavy metals from aqueous solution using Bacillus licheniformis. Int J Pure Appl Sci Technol. 2012;10(2):12–9. [Google Scholar]
- 128.Puyen ZM, Villagrasa E, Maldonado J, Diestra E, Esteve I, Sole A. Biosorption of lead and copper by heavy-metal tolerant Micrococcus luteus de2008. Boresour Technol [Internet] 2012 Dec;126:233–7. doi: 10.1016/j.biortech.2012.09.036. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 129.Chellaiah E. Cadmium (heavy metals) bioremediation by Pseudomonas aeruginosa: a minireview. Appl Water Sci [Internet] 2018 Oct;8(6):10. doi: 10.1007/s13201-018-0796-5. [cited 2019 Aug 22] Article 154 [ p.]. Available from: [DOI] [Google Scholar]
- 130.Lopez A, Lazaro N, Priego J, Marques A. Effect of pH on the biosorption of nickel and other heavy metals by Pseudomonas fluorescens 4F39. J Ind Microbiol Biotechnol [Internet] 2000 Feb;24(2):146–51. doi: 10.1038/sj.jim.2900793. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [Google Scholar]
- 131.Pardo R, Herguedas M, Barrado E, Vega M. Biosorption of cadmium, copper, lead and zinc by inactive biomass of Pseudomonas putida. Anal Bioanal Chem [Internet] 2003 May;376(1):26–32. doi: 10.1007/s00216-003-1843-z. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 132.Chen XC, Wang YP, Lin Q, Shi JY, Wu WX, Chen YX. Biosorption of copper(II) and zinc(II) from aqueous solution by Pseudomonas putida CZ1. Colloids Surf B Biointerfaces [Internet] 2005 Dec 10;46(2):101–7. doi: 10.1016/j.colsurfb.2005.10.003. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 133.Tariq A, Ullah U, Asif M, Sadiq I. Biosorption of arsenic through bacteria isolated from Pakistan. Int Microbiol [Internet] 2019 Mar;22(1):59–68. doi: 10.1007/s10123-018-0028-8. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 134.Preetha B, Viruthagiri T. Batch and continuous biosorption of chromium(VI) by Rhizopus arrhizus. Sep Purif Technol [Internet] 2007 Oct;57(1):126–33. doi: 10.1016/j.seppur.2007.03.015. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [Google Scholar]
- 135.Gao R, Wang Y, Zhang Y, Tong J, Dai W. Cobalt(II) bioaccumulation and distribution in Rhodopseudomonas palustris. Biotechnol Biotechnol Equip [Internet] 2017;31(3):527–34. doi: 10.1080/13102818.2017.1292148. [cited 2019 Aug 22] Available from: [DOI] [Google Scholar]
- 136.Goyal N, Jain SC, Banerjee UC. Comparative studies on the microbial adsorption of heavy metals. Adv Environ Res [Internet] 2003 Jan;7(2):311–9. doi: 10.1016/S1093-0191(02)00004-7. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [Google Scholar]
- 137.Aryal M, Ziagova M, Liakopoulou-Kyriakides M. Study on arsenic biosorption using Fe(III)-treated biomass of Staphylococcus xylosus. Chem Eng J [Internet] 2010 Aug;162(1):178–85. doi: 10.1016/j.cej.2010.05.026. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [Google Scholar]
- 138.Mire CE, Tourjee JA, O'Brien WF, Ramanujachary KV, Hecht GB. Lead precipitation by Vibrio harveyi: evidence for novel quorum-sensing interactions. Appl Environ Microbiol [Internet] 2004 Feb;70(2):855–64. doi: 10.1128/AEM.70.2.855-864.2004. [cited 2019 Aug 22] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brinza L, Dring M, Gavrilescu M. Marine micro and macro algal species as biosorbents for heavy metals. Environ Eng Manag J. 2007 May-Jun;6(3):237–51. [Google Scholar]
- 140.Oyedepo TA. Biosorption of lead (II) and copper (II) metal ions on Calotropis procera (Ait.) Sci J Pure Appl Chem [Internet] 2011:7. [cited 2019 Aug 22]: [ p.]. Available from: https://www.sjpub.org/sjpac.html. [Google Scholar]
- 141.Romera E, Gonzalez F, Ballester A, Blazquez ML, Munoz JA. Comparative study of biosorption of heavy metals using different types of algae. Bioresour Technol [Internet] 2007 Dec;98(17):3344–53. doi: 10.1016/j.biortech.2006.09.026. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 142.Sari A, Tuzen M. Biosorption of cadmium(II) from aqueous solution by red algae (Ceramium virgatum): equilibrium, kinetic and thermodynamic studies. J Hazard Mater [Internet] 2008 Sep 15;157(2–3):448–54. doi: 10.1016/j.jhazmat.2008.01.008. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 143.Tuzun I, Bayramoglu G, Yalcin E, Basaran G, Celik G, Arica MY. Equilibrium and kinetic studies on biosorption of Hg(II), Cd(II) and Pb(II) ions onto microalgae Chlamydomonas reinhardtii. J Environ Manage [Internet] 2005 Oct;77(2):85–92. doi: 10.1016/j.jenvman.2005.01.028. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 144.Klimmek S, Stan HJ, Wilke A, Bunke G, Buchholz R. Comparative analysis of the biosorption of cadmium, lead, nickel, and zinc by algae. Environ Sci Technol [Internet] 2001 Oct;35(21):4283–8. doi: 10.1021/es010063x. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 145.Goher ME, El-Monem AM, Abdel-Satar AM, Ali MH, Hussian AM, Napiorkowska-Krzebietke A. Biosorption of some toxic metals from aqueous solution using non-living algal cells of Chlorella vulgaris. J Elementology. 2016;21(3):703–14. [Google Scholar]
- 146.Kwarciak-Kozlowska A, Slawik-Dembiczak L, Banka B. Phycoremediation of wastewater: heavy metal and nutrient removal processes. Environ Prot Nat Resour [Internet] 2014;25(4):51–4. doi: 10.2478/oszn-2014-0026. [cited 2019 Aug 22] Available from: [DOI] [Google Scholar]
- 147.Horvathova H, Kadukova J, Stofko M. Biosorption of Cu2+ and Zn2+ by immobilized algae biomass of Chlorella kessleri. Acta Metall Slovaca. 2009;15(4):255–63. [Google Scholar]
- 148.Ibrahim WM. Biosorption of heavy metal ions from aqueous solution by red macroalgae. J Hazard Mater [Internet] 2011 Sep 15;192(3):1827–35. doi: 10.1016/j.jhazmat.2011.07.019. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 149.Deng L, Su Y, Su H, Wang X, Zhu X. Sorption and desorption of lead (II) from wastewater by green algae Cladophora fascicularis. J Hazard Mater [Internet] 2007 May 8;143(1–2):220–5. doi: 10.1016/j.jhazmat.2006.09.009. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 150.Sarada B, Prasad MK, Kumar KK, Murthy CV. Cadmium removal by macro algae Caulerpa fastigiata: characterization, kinetic, isotherm and thermodynamic studies. J Environ Chem Eng [Internet] 2014 Sep;2(3):1533–42. doi: 10.1016/j.jece.2014.07.016. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 151.Apiratikul R, Pavasant P. Batch and column studies of biosorption of heavy metals by Caulerpa lentillifera. Bioresour Technol [Internet] 2008 May;99(8):2766–77. doi: 10.1016/j.biortech.2007.06.036. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 152.Kumar JI, Oommen C. Removal of heavy metals by biosorption using freshwater alga Spirogyra hyalina. J Environ Biol. 2012 Jan;33(1):27–31. [PubMed] [Google Scholar]
- 153.Mata YN, Blazquez ML, Ballester A, Gonzalez F, Munoz JA. Biosorption of cadmium, lead and copper with calcium alginate xerogels and immobilized Fucus vesiculosus. J Hazard Mater [Internet] 2009 Apr 30;163(2–3):555–62. doi: 10.1016/j.jhazmat.2008.07.015. [cited 2019 Aug 22] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 154.Ahmady-Asbchin S, Mohammadi M. Biosorption of copper ions by marine brown alga Fucus vesiculosus. J Biol Environ Sci. 2011;5(15):121–7. [Google Scholar]
- 155.Kadimpati KK, Mondithoka KP, Bheemaraju S, Challa VR. Entrapment of marine microalga, Isochrysis galbana, for biosorption of Cr(III) from aqueous solution: isotherms and spectroscopic characterization. Appl Water Sci [Internet] 2013 Mar;3(1):85–92. doi: 10.1007/s13201-012-0062-1. [cited 2019 Aug 23] Available from: [DOI] [Google Scholar]
- 156.Kumar D, Singh A, Gaur JP. Mono-component versus binary isotherm models for Cu(II) and Pb(II) sorption from binary metal solution by the green alga Pithophora oedogonia. Bioresour Technol [Internet] 2008 Nov;99(17):8280–7. doi: 10.1016/j.biortech.2008.03.008. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 157.Senthilkumar R, Vijayaraghavan K, Jegan J, Velan M. Batch and column removal of total chromium from aqueous solution using Sargassum polycystum. Environ Prog Sust Energy [Internet] 2010 Oct;29(3):334–41. doi: 10.1002/ep.10416. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [Google Scholar]
- 158.Sheng PX, Ting YP, Chen JP, Hong L. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mechanisms. J Colloid Interface Sci [Internet] 2004 Jul 1;275(1):131–41. doi: 10.1016/j.jcis.2004.01.036. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 159.Bayramoglu G, Arica Y. Construction a hybrid biosorbent using Scenedesmus quadricauda and Ca-alginate for biosorption of Cu(II), Zn(II) and Ni(II): kinetics and equilibrium studies. Bioresour Technol [Internet] 2009 Jan;100(1):186–93. doi: 10.1016/j.biortech.2008.05.050. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 160.Subhashini S, Kaliappan S, Velan M. Removal of heavy metal from aqueous solution using Schizosaccharomyces pombe in free and alginate immobilized cells. 2nd International Conference on Environmental Science and Technology (ICEST); 2011 Feb 26–28; Singapore. Singapore: IACSIT Press; 2011. pp. 107–11. p. [Google Scholar]
- 161.Kumar JI, Oommen C. Removal of heavy metals by biosorption using freshwater alga Spirogyra hyalina. J Environ Biol. 2012 Jan;33(1):27–31. [PubMed] [Google Scholar]
- 162.Rajfur M, Kłos A, Wacławek M. Sorption properties of algae Spirogyra sp. and their use for determination of heavy metal ions concentrations in surface water. Bioelectrochem [Internet] 2010 Nov;80(1):81–6. doi: 10.1016/j.bioelechem.2010.03.005. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 163.Rangsayatorn N, Pokethitiyook P, Upatham ES, Lanza GR. Cadmium biosorption by cells of Spirulina platensis TISTR 8217 Immobilized in alginate and silica gel. Environ Int [Internet] 2004 Mar;30(1):57–63. doi: 10.1016/S0160-4120(03)00146-6. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 164.Schiewer S, Wong MH. Ionic strength effects in biosorption of metals by marine algae. Chemosphere [Internet] 2000 Jul;41(1–2):271–82. doi: 10.1016/S0045-6535(99)00421-X. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 165.Hamdy AA. Removal of Pb2+ by biomass of marine algae. Curr Microbiol [Internet] 2000 Oct;41(4):239–45. doi: 10.1007/s002840010127. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 166.Abdi O, Kazemi M. A review study of biosorption of heavy metals and comparison between different biosorbents. J Mater Environ Sci. 2015;6(5):1386–99. [Google Scholar]
- 167.Archana, Jaitly AK. Mycoremediation: utilization of fungi for reclamation of heavy metals at their optimum remediation conditions. Biolife. 2015;3(1):77–106. [Google Scholar]
- 168.Hamba Y, Tamiru M. Mycoremediation of heavy metals and hydrocarbons contaminated environment. Asian J Nat Appl Sci. 2016 Jun;5(2):48–58. [Google Scholar]
- 169.Esterhuizen-Londt M, Schwartz K, Pflugmacher S. Using aquatic fungi for pharmaceutical bioremediation: uptake of acetaminophen by Mucor hiemalis does not result in an enzymatic oxidative stress response. Fungal Biol [Internet] 2016 Oct;120(10):1249–57. doi: 10.1016/j.funbio.2016.07.009. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 170.Thenmozhi R, Arumugam K, Nagasathya A, Thajuddin N, Paneerselvam A. Studies on mycoremediation of used engine oil contaminated soil samples. Adv Appl Sci Res. 2013;4(2):110–8. [Google Scholar]
- 171.Ogbo EM, Okhuoya JA. Bio-absorption of some heavy metals by Pleurotus tuber-regium Fr. Singer (an edible mushroom) from crude oil polluted soils amended with fertilizers and cellulosic wastes. Int J Soil Sci. 2011;6(1):34–48. [Google Scholar]
- 172.Damodaran D, Suresh G, Mohan R. Bioremediation of soil by removing heavy metals using Saccharomyces cerevisiae. 2nd International Conference on Environmental Science and Technology (ICEST); 2011 Feb 26–28; Singapore. Singapore: IACSIT Press; 2011. pp. 22–7. p. [Google Scholar]
- 173.Ismail IM, Moustafa TM. Biosorption of heavy metals. In: Pathania D, editor. Heavy metals: sources, toxicity and remediation techniques. Hauppauge, NY: Nova Science Publishers; 2016. May, editor. Chapter 5. [Google Scholar]
- 174.Gadd GM, Mowll JL. Copper uptake by yeast-like cells, hyphae, and chlamydospores of Aureobasidium pullulans. Exp Mycol [Internet] 1985 Sep;9(3):0–40. doi: 10.1016/0147-5975(85)90019-2. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [Google Scholar]
- 175.Remacle J. The cell wall and metal binding. In: Volesky B, editor. Biosorption of heavy metals. Boca Raton, FL: CRC Press; 1990. pp. 83–92. editor. p. [Google Scholar]
- 176.Srivastava S, Thakur IS. Isolation and process parameter optimization of Aspergillus sp. for removal of chromium from tannery effluent. Bioresour Technol [Internet] 2006 Jul;97(10):1167–73. doi: 10.1016/j.biortech.2005.05.012. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 177.Raj DD, Mohan B, Vidya Shetty BM. Mushrooms in the remediation of heavy metals from soil. Int J Environ Pollut Control Manag. 2011;3(1):89–101. [Google Scholar]
- 178.Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, Martinez AT, Otillar R, Spatafora JW, Yadav JS, Aerts A, Benoit I, Boyd A, Carlson A, Copeland A, Coutinho PM, de Vries RP, Ferreira P, Findley K, Foster B, Gaskell J, Glotzer D, Gorecki P, Heitman J, Hesse C, Hori C, Igarashi K, Jurgens JA, Kallen N, Kersten P, Kohler A, Kues U, Kumar TK, Kuo A, LaButti K, Larrondo LF, Lindquist E, Ling A, Lombard V, Lucas S, Lundell T, Martin R, McLaughlin DJ, Morgenstern I, Morin E, Murat C, Nagy LG, Nolan M, Ohm RA, Patyshakuliyeva A, Rokas A, Ruiz-Duenas FJ, Sabat G, Salamov A, Samejima M, Schmutz J, Slot JC, St John F, Stenlid J, Sun H, Sun S, Syed K, Tsang A, Wiebenga A, Young D, Pisabarro A, Eastwood DC, Martin F, Cullen D, Grigoriev IV, Hibbett DS. The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Sci [Internet] 2012 Jun 29;336(6089):1715–9. doi: 10.1126/science.1221748. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 179.Falandysz J, Kawano M, Swieczkowski A, Brzostowski A, Dadej M. Total mercury in wild-grown higher mushrooms and underlying soil from Wdzydze Landscape Park, Northern Poland. Food Chem [Internet] 2003 May;8(1):21–6. doi: 10.1016/S0308-8146(02)00344-8. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [Google Scholar]
- 180.Demirbas A. Metal ion uptake by mushrooms from natural and artificially enriched soils. Food Chem [Internet] 2002 Jul;78(1):89–93. doi: 10.1016/S0308-8146(01)00389-2. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [Google Scholar]
- 181.Isildak O, Turkekul I, Elmastas M, Tuzen M. Analysis of heavy metals in some wild-grown edible mushrooms from the Middle Black Sea Region, Turkey. Food Chem [Internet] 2004 Aug;86(4):547–52. doi: 10.1016/j.foodchem.2003.09.007. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [Google Scholar]
- 182.Singh H. Mycoremediation: fungal bioremediation. Hoboken, NJ: Wiley; 2006. Nov, p. 592. p. [Google Scholar]
- 183.Frutos I, Garcia-Delgado C, Garate A, Eymar E. Biosorption of heavy metals by organic carbon from spent mushroom substrates and their raw materials. Int J Environ Sci Technol [Internet] 2016 Nov;13(11):2713–20. doi: 10.1007/s13762-016-1100-6. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [Google Scholar]
- 184.Al-Hares HS. Single and binary biosorption isotherms of different heavy metal ions using fungal waste biomass. Al-Nahrain J Eng Sci [Internet] 2017;20(3):673–84. [cited 2019 Aug 23] Available from: https://nahje.com/index.php/main/article/view/260. [Google Scholar]
- 185.Bahobil A, Bayoumi RA, Atta HM, El-Sehrawey MM. Fungal biosorption for cadmium and mercury heavy metal ions isolated from some polluted localities in KSA. Int J Curr Microbiol App Sci. 2017;6(6):2138–54. [Google Scholar]
- 186.Amini M, Younesi H, Bahramifar N. Biosorption of nickel(II) from aqueous solution by Aspergillus niger: Response surface methodology and isotherm study. Chemosphere [Internet] 2009 Jun;75(11):1483–91. doi: 10.1016/j.chemosphere.2009.02.025. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 187.Cardenas-Gonzalez JF, Acosta-Rodriguez I, Teran-Figueroa Y, Rodriguez-Perez AS. Bioremoval of arsenic (V) from aqueous solutions by chemically modified fungal biomass. 3 Biotech [Internet] 2017 Jul;7(3) doi: 10.1007/s13205-017-0868-5. [cited 2019 Aug 23] Article 226. Available from: Subscription required to view. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bano A, Hussain J, Akbar A, Mehmood K, Anwar M, Hasni MS, Ullah S, Sajid S, Ali I. Biosorption of heavy metals by obligate halophilic fungi. Chemosphere [Internet] 2018 May;199:218–22. doi: 10.1016/j.chemosphere.2018.02.043. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 189.Ahmad I, Zafar S, Ahmad F. Heavy metal biosorption potential of Aspergillus and Rhizopus sp. isolated from wastewater treated soil. J Appl Sci Environ Manag. 2005;9(1):123–6. [Google Scholar]
- 190.Manguilimotan LC, Bitacura JG. Biosorption of cadmium by filamentous fungi isolated from coastal water and sediments. J Toxicol [Internet] 20182018:6. doi: 10.1155/2018/7170510. [cited 2019 Aug 23] Article 7170510 [ p.]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Javaid A, Bajwa R. Biosorption of electroplating heavy metals by some basidiomycetes. Mycopathol. 2008;6(1&2):1–6. [Google Scholar]
- 192.Arbanah M, Najwa MR, Halim KH. Biosorption of Cr(III), Fe(II), Cu(II), Zn(II) ions from liquid laboratory chemical waste by Pleurotus ostreatus. Int J Biotechnol Wellness Ind. 2012;1(3):152–62. [Google Scholar]
- 193.Adebayo AO. Investigation on Pleurotus ferulae potential for the sorption of Pb(II) from aqueous solution. Bull Chem Soc Ethiop [Internet] 2013;27(1):25–34. doi: 10.4314/bcse.v27i1.3. [cited 2019 Aug 23] Available from: [DOI] [Google Scholar]
- 194.Jiang Y, Hao R, Yang S. Natural bioaccumulation of heavy metals onto common edible macrofungi and equilibrium and kinetic studies on biosorption of Pb(II) to them. Acta Sci Nat Univ Pekin. 2017;53(1):125–34. [Google Scholar]
- 195.Prasad AS, Varatharaju G, Anushri C, Dhivyasree S. Biosorption of lead by Pleurotus florida and Trichoderma viride. Br Biotechnol J. 2013;3(1):66–78. [Google Scholar]
- 196.Kogej A, Likozar B, Pavko A. Lead biosorption by self-immobilized Rhizopus nigricans pellets in a laboratory scale packed bed column: mathematical model and experiment. Food Technol Biotechnol. 2010;48(3):344–51. [Google Scholar]
- 197.Shoaib A, Aslam N, Athar MM, Akhtar S, Nafisa, Khurshid S. Removal of Cr(III) through bread mold fungus. Pol J Environ Stud [Internet] 2013;22(4):1171–6. [cited 2019 Aug 23] Available from: http://www.pjoes.com/Removal-of-Cr-III-through-Bread-Mold-Fungus,89076,0,2.html. [Google Scholar]
- 198.Bhargavi SD, Savitha J. Arsenate resistant Penicillium coffeae: a potential fungus for soil bioremediation. Bull Environ Contam Toxicol [Internet] 2014 Mar;92(3):369–73. doi: 10.1007/s00128-014-1212-y. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 199.Martinez-Juarez VM, Cardenas-Gonzalez JF, Torre-Bouscoulet ME, Acosta-Rodriguez I. Biosorption of mercury (II) from aqueous solutions onto fungal biomass. Bioinorg Chem Appl [Internet] 2012;2012:5. doi: 10.1155/2012/156190. [cited 2019 Aug 23] Article 156190 [ p.]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mamisahebei S, Khaniki JG, Torabian A, Nasseri S, Naddafi K. Removal of arsenic from an aqueous solution by pretreated waste tea fungal biomass. Iran J Environ Health Sci Eng. 2007;4(2):85–92. [Google Scholar]
- 201.Ramrakhiani L, Majumder R, Khowala S. Removal of hexavalent chromium by heat inactivated fungal biomass of Termitomyces clypeatus: surface characterization and mechanism of biosorption. Chem Eng J [Internet] 2011 Jul;171(3):1060–8. doi: 10.1016/j.cej.2011.05.002. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [Google Scholar]
- 202.Romero MC, Reinoso EH, Urrutia MI, Kiernan AM. Biosorption of heavy metals by Talaromyces helicus: a trained fungus for copper and biphenyl detoxification. Electron J Biotechnol [Internet] 2006;9(3):221–6. [cited 2019 Aug 23] Available from: http://www.ejbiotechnology.info/content/vol9/issue3/full/11/index.html. [Google Scholar]
- 203.El-Kassas HY, El-Taher EM. Optimization of batch process parameters by response surface methodology for mycoremediation of chrome-VI by a chromium resistant strain of marine Trichoderma viride. American-Eurasian J Agric Environ Sci. 2009;5(5):676–81. [Google Scholar]
- 204.Adams GO, Fufeyin PT, Okoro SE, Ehinomen I. Bioremediation, biostimulation and bioaugmention: a review. Int J Environ Bioremed Biodegradation. 2015;3(1):28–39. [Google Scholar]
- 205.Kumar A, Bisht BS, Joshi VD, Dhewa T. Review on bioremediation of polluted environment: a management tool. Int J Environ Sci. 2011;1(6):1079–93. [Google Scholar]
- 206.Abatenh E, Gizaw B, Tsegaye Z, Wassie M. Application of microorganisms in bioremediationreview. J Environ Microbiol. 2017;1(1):2–9. [Google Scholar]
- 207.Coulon F, Al Awadi M, Cowie W, Mardlin D, Pollard S, Cunningham C, Risdon G, Arthur P, Semple KT, Paton GI. When is a soil remediated? Comparison of biopiled and windrowed soils contaminated with bunker-fuel in a full-scale trial. Environ Pollut [Internet] 2010 Oct;158(10):3032–40. doi: 10.1016/j.envpol.2010.06.001. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 208.Murray KJ, Tebo BM. Cr(III) is indirectly oxidized by the Mn(II)-oxidizing bacterium Bacillus sp. strain SG-1. Environ Sci Technol [Internet] 2007;41(2):528–33. doi: 10.1021/es0615167. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hochella MF. Sustaining Earth: thoughts on the present and future roles of mineralogy in environmental science. Mineral Mag. 2002;66:627–52. [Google Scholar]
- 210.Brown GE, Trainor TP, Chaka AM. Geochemistry of mineral surfaces and factors affecting their chemical reactivity. In: Nilsson S, Pettersson LG, Norskov JK, editors. Chemical bonding at surfaces and interfaces. Amsterdam, Netherlands: Elsevier; 2008. Chapter 7. [Google Scholar]
- 211.Wan Ngah WS, Hanafiah MA. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: a review. Bioresour Technol [Internet] 2008 Jul;99(10):3935–48. doi: 10.1016/j.biortech.2007.06.011. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 212.Babel S, Kurniawan TA. Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere [Internet] 2004 Feb;54(7):951–67. doi: 10.1016/j.chemosphere.2003.10.001. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 213.Chuah TG, Jumasiah A, Azni I, Katayon S, Choong ST. Rice husk as a potentially low-cost biosorbent for heavy metal and dye removal: an overview. Desalination [Internet] 2005 May;175(3):305–16. doi: 10.1016/j.desal.2004.10.014. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [Google Scholar]
- 214.Han R, Li H, Li Y, Zhang J, Xiao H, Shi J. Biosorption of copper and lead ions by waste beer yeast. J Hazard Mater [Internet] 2006 Oct 11;137(3):1569–76. doi: 10.1016/j.jhazmat.2006.04.045. [cited 2019 Aug 23] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 215.Sodango TH, Li X, Sha J, Bao Z. Review of the spatial distribution, source and extent of heavy metal pollution of soil in China: impacts and mitigation approaches. J Health Poll [Internet] 2018 Mar;8(17):53–70. doi: 10.5696/2156-9614-8.17.53. [cited 2019 Aug 23] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]


