Abstract
Background.
Groundwater quality can be poor in Nigeria due to indiscriminate refuse dumping. Exposed dumps serve as point source pollution that discharge potentially toxic heavy metals into the environment.
Objectives.
The present research aimed to assess the impact of metal pollution on groundwater quality in hand-dug wells around an active dumpsite and to evaluate the long-term human health effects of this pollution.
Methods.
Water samples from hand-dug wells used for drinking, irrigation and domestic purposes were collected around the dumpsite. Two samples were collected at each location for cation and anion analyses. Samples for cation analysis were acidified with concentrated hydrochloric acid to preserve the elements in the samples, while those for anion analysis were not acidified. Collected samples were analyzed using inductively coupled plasma mass spectrometry and atomic absorption spectroscopy.
Results.
Mean concentrations of metals and physical parameters were compared with the World Health Organization's standards (2012). All samples were found to be within permissible limits, except for arsenic (As) (0.13 mg/L), potassium (K) (29.94 mg/L), lead (Pb) (0.38 mg/L), cadmium (Cd) (0.003 mg/L) and average temperature (31.93°C) as a result of corroded service pipes containing Pb in the dumpsites and the reaction of leachates with various materials such as used battery, tins, and electronic wastes which later leaked into the groundwater. The geoaccumulation index revealed Pb to be moderately to highly contaminated in groundwater.
Conclusions.
Heavy metal pollution can cause deleterious health effects that can lead to short- and long-term diseases such as keratosis (skin hardening), lung cancer, bladder cancer and ultimately death if proactive steps are not taken. Disposal bags should be provided to all houses in the area, as well as guaranteed waste disposal trucks and dispose of waste at approved sites. In addition, enforcement agents should ensure compliance with rules and regulations. A centralized, deep, double-cased well should be constructed in a clean environment in the study area for drinking and domestic use.
Competing Interests.
The authors declare no competing financial interests.
Keywords: dumpsite, leachates, corroded, groundwater, indecisive, health risk
Introduction
Urbanization presents many challenges for developing countries, such as industrialization and over-population which can lead to indiscriminate dumping of waste. Haphazard dumping of domestic garbage, wood, polythene bags, plastics, broken glass, abandoned automobiles, ash, dust, demolition, agricultural, industrial, hospital, and human and animal waste can result in refuse in the environment. Refuse waste becomes a form of point source pollution if it affects the environment directly and non-point source pollution if it affects the environment indirectly. This leads to contamination of surface and groundwater and adversely impacts public health. Nigeria is a lower middle-income country which is facing a myriad of pollution and human and environmental healthissues.1–10
Diseases which may lead to increased mortality are relative to gradual increases in arsenic (As), lead (Pb) and cadmium (Cd) in the environment originating from toxic waste in solid or liquid forms.11–14 Indiscriminate dumping of refuse has consequences on the environment, such as flooding due to waste blockage of water run-off channels, and emission of contaminants that leads to slight poisoning and eventually highly toxic waters (ground and surface) and may lead to diseases such as asbestosis, respiratory problems, cancer and eventually death.5,15–17 Studies have shown that dumpsites consist of metals, nylon, cans, syringes, electronic and animal wastes, municipal, commercial, hospital and mixed industrial wastes, threaten the quality of groundwater, which is the main source of drinking water for the surrounding community.7,18–21
Many studies have been conducted on the effects of heavy metals on groundwater around dumpsites, but few have investigated the long-term health risk of metals such as Pb, As and Cd on children and adults.5,11,12,16,17 The present study will help to fill this gap with findings on health risks due to indiscriminate dumping of refuse.
The present study aimed to assess the impact of metal pollution on groundwater quality in hand-dug wells around an active dumpsite and to evaluate the long-term effects of this pollution on public health.
Methods
A geochemical survey of metals in the groundwater around the exposed dumpsite was conducted to determine the impact on the environment. The groundwater in the study area was used for drinking and domestic purposes.
Study area
The study area lies within latitude 06°51′00″ N - 06°53′00″ N and longitude 003°42′00″ E - 003°44′00″ E (Figure 1). The area has a moderately high relief; it is easily accessible with a series of interconnecting minor roads, footpaths and a few major roads. The drainage pattern is dendritic, showing irregularity in direction. The climate is characterized by a tropical climate with alternating wet and dry seasons which gives the study area typical rain forest vegetation. Ikenne is part of the southwestern Nigerian basement complex terrain; it is representative of both a migmatite gneiss complex and older granite, which shows structural disposition.22 The migmatite gneiss complex occupies the southwestern and southeastern part of the study area, while older granite occupies the north, east and a portion of the western area.23 Rock found in the area occurs directly above or is covered by a shallow mantle of superficial deposits. The complex is an extension of the Togo – Benin – Nigeria shield which occurs east of the West African craton.
Figure 1.
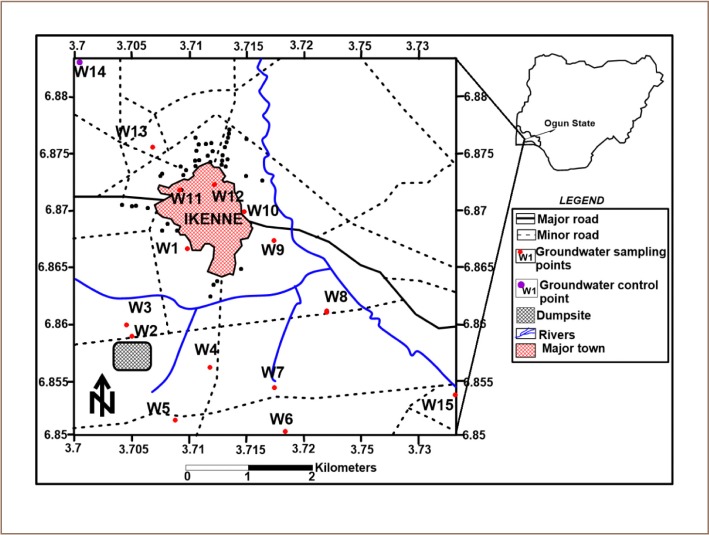
Location map with sampling points
Abbreviations
- C F
Contamination factor
- EC
Electrical conductivity
- EF
Enrichment factor
- Igeo
Geoaccumulation index
- TDS
Total dissolved solids
- WHO
World Health Organization
The study area is located at Aiyepe/Ikenne, with the headquarters in Ikenne. Ikenne is located between Sagamu and Odogbolu, and is very close to Ilisan-Remo, southwestern Nigeria. Ikenne has an area of 144 km2 and had a population of 118735 in the 2006 census.24 The climate and geographical location supports a wide range of economic activities such as agriculture, industrial and commercial activities. The town is 11.7 km from Sagamu and has a boundary between Odogbolu and Ilisan.
Ikenne harbors a 20-year-old dumpsite that is almost an acre across. The dump is situated along the Ikenne-Aiyepe road. The Sagamu-Benin Express Road is the dividing line between Ikenne and Ilisan. The dumpsite serves as the main location for waste disposal for Ikenne and the surrounding area. Presently, however, the dumpsite poses a major threat to soils and human health, as it is located within a large community with many farms and hand-dug wells. During the rainy season leachates from the dumpsite are transported and accumulated in groundwater. Various wastes such as vegetable matter, domestic wastes, human and animal wastes, organic and inorganic matter, wood, paper, cloth, glass, plastic of various types, unclassified metal scraps, empty cans of various chemicals, and other household trash are some of the refuse that is found on the dumpsite that is operated as an open dump with intermittent open-air incineration.
Sampling and analysis
Sampling was carried out in June 2016; all the instruments used in the field were cleaned with de-ionized water before use. A clean measuring tape attached to the base of a clean stone was used to determine the depth from the water surface to the wellhead and measurements were recorded at each location. A new, clean fetcher was used to draw water samples from each well and global positioning system was used to coordinate each location. A control sample was taken from groundwater in a relatively clean area. The samples were collected 20 m away from the dumpsite. Two samples were chosen at each location for cation and anion analysis, those chosen for cation analysis were acidified with concentrated hydrochloric acid to preserve the elements in the samples, while those chosen for anion analysis were not acidified. Total dissolved solids (TDS), pH, salinity and temperature were measured in situ, and all collected samples were sent for analysis at the Redeemer's University, Ede, Osun State, Nigeria.
Statistical evaluation
Assessment of contamination according to metal enrichment or the enrichment factor (EF) proposed by Sinex and Helz was employed to assess the degree of contamination and to understand the distribution of the elements of anthropogenic origin from sites via individual elements in sediments.25 Iron (Fe) was chosen as the normalizing element for determining EF values, as it is one of the widely used reference elements.26–30 Other widely used reference metals or elements included aluminum (Al) and manganese (Mn).31,32 The formula for EF and its classifications are stated in Equation 1:
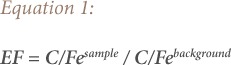 |
Where, C is the element concentration. In this case, the background value is the concentration of groundwater in the control site and is categorized as ≤ 1, background concentration; 1–2, depletion to minimal enrichment; 2–5, moderate enrichment; 5–20, significant enrichment; 20–40, very high enrichment; and ≥ 40, extremely high enrichment.28
Contamination factor
Contamination factor (CF) was used to determine the contamination status of the groundwater. The formula for the contamination factor in Equation 2:
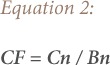 |
Where, Cn is the concentration of heavy metals in groundwater, and Bn is the background concentration at the control site.
The following categories are suggested for classifying the CF: CF<1, low contamination factor;1 ≤ CF< 3, moderate contamination;3 ≤ CF< 6, considerable contamination factor; CF ≥ 6, very high contamination factor.30,31
Geoaccumulation index
As proposed by Muller, the geoaccumulation index (Igeo) has been widely used to evaluate the degree of heavy metal contamination in terrestrial and aquatic environments.32,33 Igeo is expressed in Equation 3.
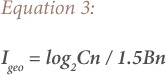 |
Where, Cn is the concentration of the examined metal in the groundwater, Bn is the background concentration of a given metal, and 1.5 is a factor accounting for possible variation in the background concentration due to lithologic differences. The geoaccumulation index is classified into seven descriptive classes as follows: ≤ 0, practically uncontaminated; 0–1, uncontaminated to slightly contaminated; 2–3, moderately to highly contaminated; 4–5, highly to very strongly contaminated; and ≥5, very strongly contaminated.
Risk assessment
Risk assessment is used to estimate the impact of carcinogens and the future rate at which an individual is affected by carcinogenic metals. The risk (Risk pathway) is the probability of an individual developing cancer resulting from exposure to the potential carcinogenic metal. A Possible pathway includes ingestion, inhalation or dermal contact (Equation 4).
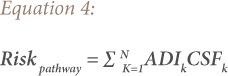 |
Where, Risk pathway is the unit less probability of an individual developing cancer over a lifetime; ADIk (mg/kg/day) is the mean daily intake; CSFk (mg/kg/day)−1 is the cancer slope factor; k is the kth metal; and n is the number of metals.
The human health risk is then calculated by the addition of all results from the three pathways which is the results of risk in inhalation, ingestion and dermal contact (Equation 5):
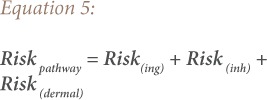 |
The results of exposure were assessed using Table 1.
Table 1.
Reference Doses (RfD) in (mg/kg-day) and Cancer Slope Factors (CSF) for the Pathways
| Heavy Metal | Oral RfD | Dermal RfD | Inhalation RfD | Oral CSF | Dermal CSF | Inhalation CSF | References |
|---|---|---|---|---|---|---|---|
| Pb | 3.60E-03 | - | - | 8.50E-03 | - | 4.20E-02 | 34,35 |
| Chromium (VI) | 3.00E-03 | - | 3.00E-05 | 5.00E-01 | - | 4.10E+01 | 36,34 |
| Cobalt | 2.00E-02 | 5.70E-06 | 5.70E-06 | - | - | 9.80E+00 | 36 |
| Nickel | 2.00E-02 | 5.60E-03 | - | - | - | - | 34 |
| Copper | 0.037 | 0.037 | - | - | - | - | 34,36 |
| Zinc | 3.00E-01 | 7.50E-02 | - | - | - | - | 34,36 |
Abbreviations: RfD, reference dose; CSF, cancer slope factor
Results
The mean concentrations of metals and physical parameters were compared with the World Health Organization (WHO) standard limits in Table 2.37 All the samples were found to be within the WHO maximum permissible limits of drinking water except for As (0.13 mg/L), potassium (K) (29.94 mg/L), Pb (0.38 mg/L), Cd (0.003 mg/L) and average temperature (31.93°C).
Table 2.
Mean Geochemical Values and Physical Parameters of the Groundwater of the Study Area (n = 15) (mg/L)
| Elements | Mean concentration | WHO (2012) | T°C | pH | EC | TDS | Depth to water table (m) |
|---|---|---|---|---|---|---|---|
| Cu | 1.22 | 2 | 32 | 8.3 | 0.5 | 0.2 | 27.6 |
| Cd | 0.003 | 0.003 | 32 | 8.7 | 0.3 | 0.1 | 37.4 |
| As | 0.13 | 0.01 | 34 | 9.2 | 0.2 | 0.1 | 42.4 |
| Pb | 0.38 | 0.01 | 33 | 7.5 | 0.4 | 0.2 | 57.7 |
| Zinc | 10.8 | 75–200 | 30 | 8.2 | 0.1 | 0.1 | 34.5 |
| Fe | 0.05 | 0.3 | 32 | 9.2 | 0.4 | 0.7 | 23.3 |
| Ca2+ | 8.87 | 75–200 | 31 | 8.7 | 0.06 | 0.3 | 36.3 |
| Mg2+ | 10.43 | 150 | 33 | 8.4 | 0.4 | 0.2 | 23.6 |
| Na+ | 10.8 | 200 | 34 | 8 | 1.3 | 0.6 | 8 |
| K+ | 29.94 | 12 | 35 | 8.1 | 1 | 0.5 | 28.9 |
| SO42− | 6.88 | 400 | 32 | 8.8 | 0.4 | 0.2 | 12 |
| NO3− | 7.15 | 50 | 34 | 8.2 | 0.3 | 0.1 | 52 |
| Cl− | 25.32 | 250 | 30 | 7.9 | 0.3 | 0.1 | 64.9 |
| CO32− | 104 | 240 | 29 | 9 | 0.1 | 0 | 51.9 |
| HCO3 | 278.57 | 500 | 28 | 9 | 0.7 | 0.3 | 18.4 |
| WHO35 | 27 | 6.5–9.2 | 300 | 500 |
Abbreviations: Ca2+, calcium(2+), Cu, copper; Mg2+, magnesium(2+); Na+, sodium(1+); SO42−, sulfate(2−); NO3− nitrate(1−); Cl−, chlorine(l-); CO32−,carbonate; HCO3,bicarbonate; T°C, temperature; EC, electrical conductivity; WHO, World Health Organization.
Contamination assessment of groundwater
The EF of background concentrations for calcium (Ca), magnesium (Mg), sodium (Na), K, copper (Cu), Cd, As, Pb, and zinc (Zn) were found in 8, 6, 1, 6, 7, 8, 5, 6 and 10 samples, respectively. Depletion to minimal enrichment for Ca, Mg, Na, K, Cu, Cd, Fe, As, Pb and Zn were found in 5, 7, 5, 4, 6, 4, 15, 8, 7 and 3 samples, respectively. Moderate enrichment for Na, K, and Cd were found in 6, 3, and 1 sample, respectively. Significant enrichment was found with regard to Ca, Mg, Na, K, Cu, Cd, As, Pb and Zn. Very high enrichment was observed for 2 samples in Na (Table 3).
Table 3.
Contamination Assessment of Groundwater
| Indices | Classification | Ca | Mg | Na | K | Cu | Cd | Fe | As | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Enrichment factor | <1: Background concentration | 8 | 6 | 1 | 6 | 7 | 8 | - | 5 | 6 | 10 |
| 1–2: Depletion to minimal enrichment | 5 | 7 | 5 | 4 | 6 | 4 | 15 | 8 | 7 | 3 | |
| 2–5: Moderate enrichment | - | - | 6 | 3 | - | 1 | - | - | - | - | |
| 5–20: Significant enrichment | 2 | 2 | 1 | 2 | 2 | 2 | - | 2 | 2 | 2 | |
| 20–40: Very high enrichment | - | - | 2 | - | - | - | - | - | - | - | |
| >40: Extreme enrichment | - | - | - | - | - | - | - | - | - | - | |
| Contamination factor | CF < 1:Low contamination factor | 11 | 1 | - | 3 | 2 | 8 | 6 | 1 | 2 | 14 |
| 1 < CF < 3: Moderate contamination factor | 4 | 14 | 12 | 11 | 13 | 7 | 9 | 14 | 11 | 1 | |
| 3 ≤ CF ≤ 6 Considerable contamination factor | - | - | 3 | 1 | - | - | - | - | - | - | |
| CF ≥ 6: Very high contamination factor | - | - | - | - | - | - | - | - | 1 | - | |
| Igeo | < 0: Practically uncontaminated | 15 | 15 | 3 | 1 | 15 | 13 | 14 | 13 | - | 15 |
| 0–1: Uncontaminated to slightly contaminated | - | - | 12 | 14 | - | 2 | 1 | 2 | 14 | - | |
| 2–3: Moderately to highly contaminated | - | - | - | - | - | - | - | - | 1 | - | |
| 4–5: Contaminated | - | - | - | - | - | - | - | - | - | - | |
| >5: Very strongly contaminated | - | - | - | - | - | - | - | - | - | - |
Table 3.
Contamination Assessment of Groundwater
| Component | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Temp. | 0.751 | 0.123 | 0.433 | −0.135 | 0.243 | −0.208 | −0.025 |
| Salinity | 0.567 | 0.504 | 0.064 | 0.488 | 0.102 | 0.082 | −0.116 |
| TDS | 0.440 | 0.616 | −0.150 | 0.092 | 0.424 | 0.266 | 0.207 |
| pH | −0.044 | 0.027 | −0.477 | 0.814 | −0.099 | −0.087 | 0.028 |
| EC | 0.590 | 0.462 | 0.137 | −0.215 | −0.100 | 0.466 | 0.214 |
| SO4 | −0.390 | 0.402 | 0.388 | 0.438 | −0.381 | 0.324 | 0.189 |
| NO3 | −0.493 | 0.437 | 0.028 | −0.225 | −0.437 | 0.371 | 0.376 |
| Cl | 0.150 | −0.522 | −0.298 | 0.132 | 0.218 | −0.055 | 0.606 |
| CO3 | −0.644 | 0.065 | 0.343 | −0.224 | 0.602 | 0.180 | −0.021 |
| HCO3 | −0.669 | 0.143 | 0.360 | −0.094 | 0.534 | 0.194 | −0.207 |
| Ca | 0.130 | 0.586 | −0.580 | −0.327 | −0.253 | 0.100 | −0.252 |
| Mg | 0.434 | 0.167 | 0.529 | −0.199 | 0.217 | −0.090 | 0.387 |
| Na | 0.612 | −0.588 | 0.422 | 0.095 | −0.168 | 0.146 | −0.092 |
| K | 0.104 | −0.629 | 0.046 | 0.314 | 0.140 | 0.408 | −0.028 |
| Cu | 0.420 | −0.150 | −0.667 | −0.502 | −0.094 | 0.109 | 0.025 |
| Cd | −0.536 | −0.675 | −0.153 | 0.195 | 0.004 | 0.146 | 0.199 |
| Fe | −0.054 | 0.618 | 0.494 | 0.450 | −0.187 | −0.157 | −0.105 |
| As | −0.250 | 0.062 | 0.462 | −0.234 | −0.331 | −0.571 | 0.287 |
| Pb | 0.128 | 0.422 | −0.473 | 0.322 | 0.418 | −0.286 | 0.200 |
| Zn | 0.612 | −0.588 | 0.422 | 0.095 | −0.168 | 0.146 | −0.092 |
| Total | 4.220 | 4.011 | 3.071 | 2.206 | 1.815 | 1.354 | 1.094 |
| % Variance | 21.10 | 20.05 | 15.35 | 11.03 | 9.08 | 6.771 | 5.472 |
Abbreviation: Temp., temperature.
Contamination factor
Results of the contamination factor analysis show that a low contamination factor was observed in Ca, Mg, Na, K, Cu, Cd, Fe, As, Pb and Zn in 11, 1, 0, 3, 2, 8, 6, 1, 2, 1and 14 samples, respectively, while a moderate contamination factor for Ca, Mg, Na, K, Cu, Cd, Fe, As, Pb and Zn was observed in 4, 14, 12, 11, 13, 7, 9, 14, 11 and 1 samples, respectively. Considerable contamination factor was found in Na and K in 3 and 1 samples, respectively.
Only Pb was found to have high degree contamination factor. The degree of contamination in groundwater in the study area was found to have a moderate degree of contamination (Table 3).
Geoaccumulation index
The Igeo results showed Ca, Mg, Cu and Zn to be practically uncontaminated, while Na, K, Cd, Fe and As ranged from practically uncontaminated to slightly contaminated. Only Pb was moderately to highly contaminated in the sampled groundwater (Table 3).
Discussion
The mean Fe+ and Cd values (0.05 and 0.003 mg/L, respectively) in the groundwater indicate that most metals did not originate from the weathering of silicate-bearing rocks underlying the area, and thus are from anthropogenic activities (dumpsites) close to the well. Zinc, Cu, Pb, As, and Cd had mean values of 10.80, 1.22, 0.38, 0.130, and 0.003 mg/L, respectively. The concentration of metals in the groundwater in the area occurred in the order of Zn =10.80 > Cu = 1.22 > Pb = 0.38 > As = 0.13 > Fe = 0.05> Cd = 0.003. All of the wells in the study area had values for Pb > 0.2 mg/L, K>12 mg/L, while some had concentrations of Cd> 0.1, which is higher than the WHO permissible limits.37 Elevated amounts of Pb may have resulted from batteries and lead pipes in the dumpsite, as when leachates becomes acidic, the acid is released into the groundwater.38 Cadmium could be linked with the dispersal of heavy metals produced from electronic wastes disposed of in the dumpsite. High values of K may be linked to leachates, which were also affirmed in the spatial distribution of metals in groundwater in the area (Figures 2a and 2b). The general trend showed higher metal pollution in the wells close to the dumpsite and decreased with distance from the dumpsite.
Figure 2a.
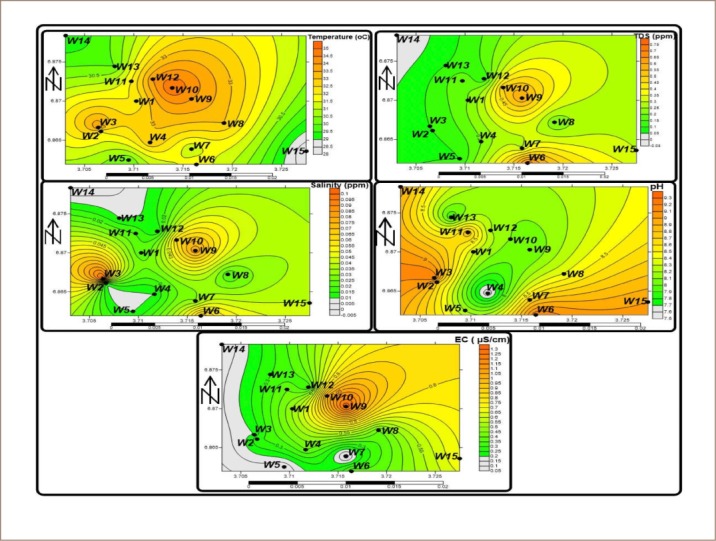
Spatial maps of physical parameters in groundwater of the study area
Abbreviations: W, water samples; EC, electrical conductivity.
Figure 2b.
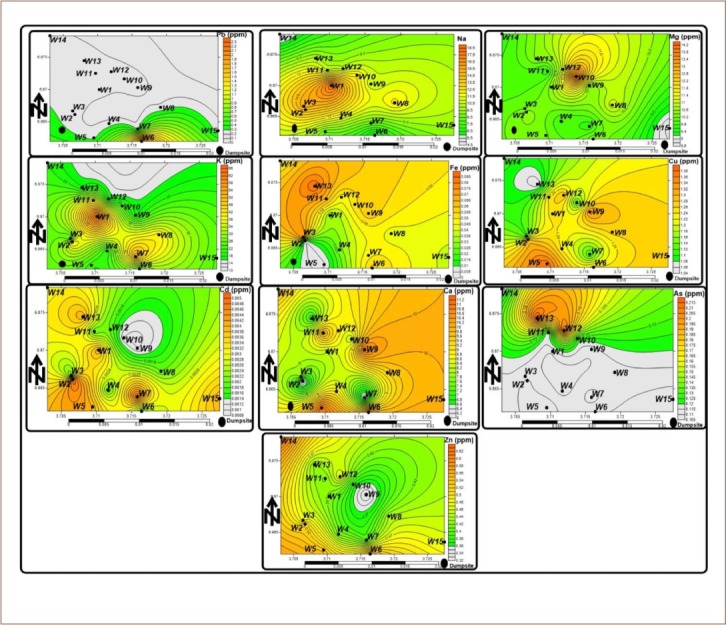
Spatial maps of metals in groundwater of the study area
Abbreviations: W, water samples.
Hydrochemical results of the anion
The high abundance of bicarbonate (HCO3) and carbonate (CO3 2−) may be caused by discarded carbonate rich products in the dumpsites and underlying rocks, where interaction between rock and water occur. The high presence of chlorine (1−) (Cl−) and sodium (1+) (Na+) was attributed to domestic effluents, fertilizers, septic tanks and leachates from sewage in the waste dumpsite.39 Low Fe+ concentrations revealed an oxidizing environment that is attributed to the oxidation of ferrous to the ferric form.
Groundwater classification
A Piper diagram (Figure 3) illustrates the hydrochemical evolution of groundwater by plotting the cations and anions. The Piper diagram consists of three major fields: the cation ternary (left), anion ternary (right) and diamond fields (center). The groundwater samples were plotted on the Piper diagram to determine water types in the area. The plot indicates that 93% of groundwater samples in the study area fall within the (HCO3, Cl + sulfate (SO4)) − (HCO3+carbon trioxide (CO3)) groundwater type, while 3% fall into (Na+K) − (calcium (Ca) + magnesium (Mg)) water type. Dumpsites, landfill leachates, and sewages are major sources of bicarbonates in groundwater.40
Figure 3.
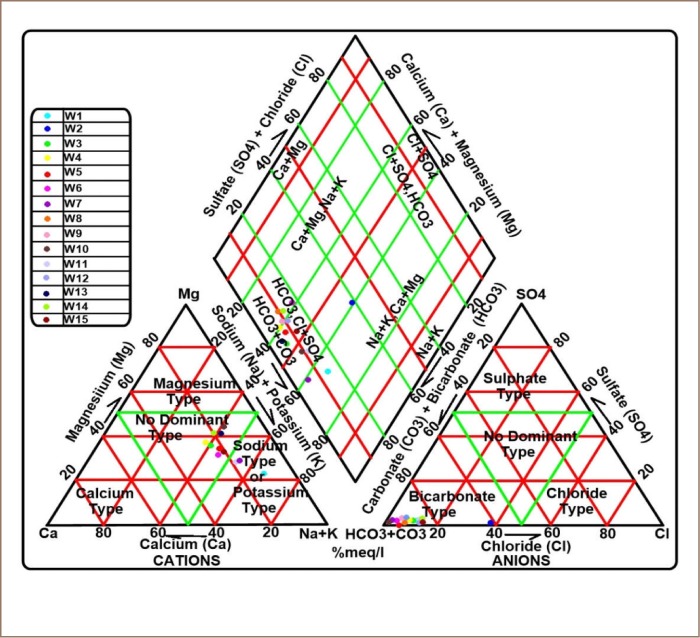
Piper diagram of the study area
Abbreviations: W, water samples.
Bivariate correlation analyses
Bivariate analysis (Supplemental Material (18.9KB, docx) ) between physicochemical parameters revealed strong a relationship (r=0.634) between TDS and salinity. This implies that the overall salinity in groundwater may have originated from the same sources. This was also reflected by the relationships between salinity and other parameters, SO4 (r = 0.516), Cl (r = 0.597), HCO3 (r = 0.501), Ca (r = 0.621), Mg (r = 0.520), Na (r = 0.603), Cu (r = 0.763) and Zn (r = 0.603). The association of salinity with SO4, Cl, HCO3, Ca and Mg reflects possible anthropogenic influence and may come from the dumpsite; it could also come from the use of agricultural nitrogen (N), phosphorus (P) and K fertilizers and recycled irrigation water. Total dissolved solvents had an extremely strong relationship with pH (r = 0.915), revealing that TDS determines the degree of acidity and alkalinity in the groundwater of the study area. Similar relationships were found with TDS and SO4 (r = 0.911), TDS and nitrate (NO3) (r = 0.994), TDS and Cl (r = 0.761), TDS and CO3 (r = 0.899), and TDS and HCO3 (r = 0.874). The relationship between TDS with NO3 indicates pollution from sewage wastes, domestic effluents, and it also suggests the presence of bicarbonate. Chloride and nitrates strongly influenced TDS and electrical conductivity (EC) in the study area. Sulfate also had a strong relationship with NO3 (r = 0.650), CO3 (r = 0.606), Ca (r = 0.838), Mg (r = 0.828), Na (r = 0.550), K (r = 0.797), Cd (r = 0.953), As (r = 0.608), Pb (r = 0.665) and Zn (r = 0.550), which reflects a possible anthropogenic source. A very strong correlation was noticed between CO3 and HCO3(r = 0.907), which indicates chemical interactions between rainwater and the aquifer as the former percolates the subsurface. Strong relationships between Pb with Cu (r = 0.721), Cd (r = 0.544), Fe (r = 0.613) and As (r = 0.633) reflect the contribution of heavy metals from the nearby dumpsite.
Factor analysis
Factor analysis is another form of correlation that helps to describe the possible source of pollution in groundwater. Seven components were extracted for analysis (Table 4). Component 1 revealed that the parameters have a common origin which is possibly anthropogenic in nature. Component 2 showed the pollution could originate from agricultural practices and activities at the dumpsite. Component 3 reflects an anthropogenic source, possibly from various wastes discarded in the dumpsites. This component shows that pH-Ca-Cu-Pb has had a negative impact and their presence in the groundwater may be hazardous to human health. In component 4, salinity-pH-SO4-Fe are strongly related and may have originated from either a natural or anthropogenic source of contamination. In component 5, associations reflect an anthropogenic source of groundwater contamination. The presence of NO3 shows a possible agricultural source, while the presence of Pb shows possible contamination from the dumpsite. In component 6, EC-K-As formed an association revealing an anthropogenic source of contamination from the dumpsite.
Hierarchical cluster analysis
The dendrogram (Figure 4) classifies parameters in the groundwater into 3 groups. In group 1, Na, Zn, pH, Ca, Mg, SO4, NO3, salinity, Fe, Cd, As, and TDS have a common source of contamination. This may be linked to anthropogenic sources which may be from agricultural practices in the area or from the dumpsite. Group 2 includes K, Cl, CO3, TDS, EC, Pb, Cu, and temperature and group 3 consists of HCO3, Cu, temperature, K, Cl, and CO3. These groupings reflect a possibly natural source of contamination arising from rock-water interactions in the area.
Figure 4.
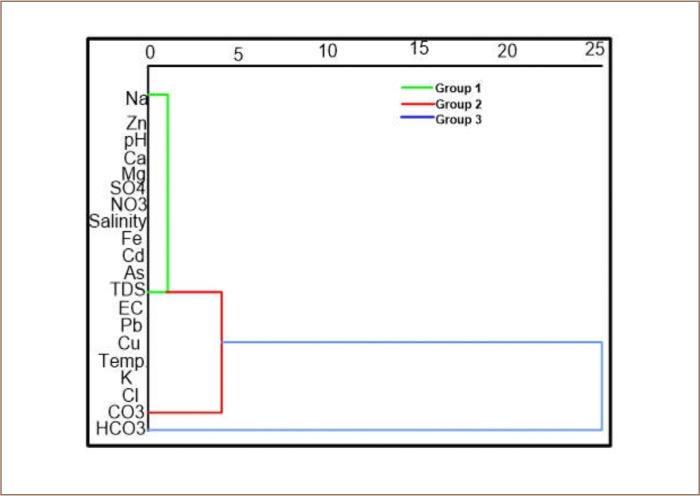
Hierarchical cluster analysis
Evaluation of groundwater for irrigation purposes
Irrigation water indices such as EC, TDS, soluble salt percentage, sodium adsorption ratio, magnesium adsorption ratio and Kelly ratio were used to assess the suitability of groundwater in the area for irrigation purposes.
Salinity
To determine the amount of dissolved ionic salts present in the groundwater, EC was used, which also measures salinity hazard to crops, as it affects the TDS in groundwater.41 The results showed that EC and TDS in all of the groundwater samples was lower than 250 and 1000 mg/l, respectively, and groundwater is therefore safe for irrigation purposes. Soluble salt percentage also evaluates the sodium hazard and was calculated using Equation 6:42
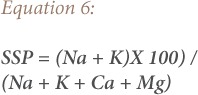 |
Where, SSP is the soluble salt percentage. The results revealed that 33% of the samples are permissible for use in irrigation, 60% are of uncertain suitability, and 7% of the groundwater samples were unsuitable for irrigation. Sodium adsorption ratio is the best parameter to determine the suitability of groundwater for irrigation, as it evaluates the alkali/sodium hazards in crops.43 Sodium adsorption ratio can be measured using Equation 7, as characterized by Karanth.44
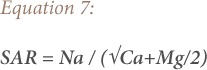 |
The results revealed that 100% of groundwater samples were suitable for irrigation. The magnesium adsorption ratio was calculated using Equation 8.45
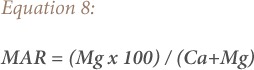 |
The findings showed that 47% of the samples were suitable for irrigation while 53% were unsuitable. The Kelly ratio values were calculated using Equation 9.46
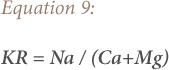 |
Where, KR is the Kelly ratio. Kelly ratio values <1 showed suitability, while KR > 1 showed unsuitability for irrigation purposes. All of the samples were safe for irrigation (Table 5).
Table 5.
Irrigation Parameters of Groundwater in the Study Area
| Parameters | Range | Class | Number of Samples | % of Samples |
|---|---|---|---|---|
| EC | <250 | Excellent | 15 | 100 |
| 250–750 | Good | 0 | 0 | |
| 750–2000 | Permissible | 0 | 0 | |
| 2000–3000 | Doubtful | 0 | 0 | |
| >3000 | Unsuitable | 0 | 0 | |
| TDS | < 1000 | Fresh | 15 | 100 |
| 1000–10000 | Brackish | 0 | 0 | |
| 10000–100000 | Saline | 0 | 0 | |
| >100000 | Brine | 0 | 0 | |
| SSP | <20 | Excellent | 0 | 0 |
| 20–40 | Good | 0 | 0 | |
| 40–60 | Permissible | 5 | 33 | |
| 60–80 | Doubtful | 9 | 60 | |
| >80 | Unsuitable | 1 | 7 | |
| SAR | 0–10 | Excellent | 15 | 100 |
| 10–18 | Good | 0 | 0 | |
| 18–26 | Permissible | 0 | 0 | |
| >26 | Doubtful | 0 | 0 | |
| MAR | ≤50 | Suitable | 7 | 47 |
| >50 | Unsuitable | 8 | 53 | |
| KR | > 1 | Unsafe | 0 | 0 |
| < 1 | Safe | 15 | 100 |
Abbreviations: SSP, soluble salt percentage; SAR, sodium adsorption ratio; MAR, magnesium adsorption ratio; KR, Kelly ratio.
Health risk assessment
Health risks in adults and children (Table 6) for Pb and As revealed that Pb represents a high carcinogenic health hazard in the area, through oral or dermal contact, as the values of the health hazards are above 10−4 and 10−8, which is the acceptable range. Use of groundwater for drinking and bathing in this area for long periods of time poses a risk for the development of carcinogenic diseases (Figure 5).
Table 6.
Carcinogenic Health Hazard for Adults and Children through Oral Ingestion and Dermal Contact with Water
| Oral Ingestion | Dermal | Hazard index | |||||
|---|---|---|---|---|---|---|---|
| HQ As | HQ Pb | HI | HQ As | HQ Pb | HI | ||
| Adults | 0.000184 | 24.11761 | 24.11779 | 0.005157 | 0.344537 | 0.349694 | 48.93497 |
| 0.000158 | 26.09586 | 26.09602 | 0.004421 | 0.372798 | 0.377219 | 52.94647 | |
| 0.00033 | 15.39639 | 15.39672 | 0.009246 | 0.219949 | 0.229195 | 31.25184 | |
| 0.000352 | 12.81906 | 12.81941 | 0.009867 | 0.183129 | 0.192996 | 26.02481 | |
| 0.000217 | 23.3136 | 23.31381 | 0.006061 | 0.333051 | 0.339112 | 47.30585 | |
| 0.000365 | 118.5775 | 118.5778 | 0.01022 | 1.693964 | 1.704184 | 240.564 | |
| 0.000139 | 37.68786 | 37.688 | 0.00389 | 0.538398 | 0.542288 | 76.46058 | |
| 0.000378 | 11.30174 | 11.30212 | 0.010582 | 0.161453 | 0.172036 | 22.9483 | |
| 0.000787 | 6.171382 | 6.172169 | 0.022038 | 0.088163 | 0.110201 | 12.56474 | |
| 0.000729 | 6.044552 | 6.045281 | 0.020399 | 0.086351 | 0.10675 | 12.30406 | |
| 0.000375 | 10.53467 | 10.53505 | 0.010499 | 0.150495 | 0.160994 | 21.39208 | |
| 0.000722 | 5.916313 | 5.917035 | 0.020215 | 0.084519 | 0.104734 | 12.04354 | |
| 0.000299 | 14.85603 | 14.85633 | 0.008371 | 0.212229 | 0.2206 | 30.15386 | |
| 0.000213 | 19.6236 | 19.62381 | 0.00596 | 0.280337 | 0.286297 | 39.82021 | |
| 0.000179 | 26.00398 | 26.00415 | 0.005006 | 0.371485 | 0.376492 | 52.76129 | |
| Children | 0.005157 | 1.464283 | 1.46944 | 0.00011 | 1.464283 | 1.464393 | 2.933833 |
| 0.004421 | 1.584391 | 1.588812 | 9.44E-05 | 1.584391 | 1.584486 | 3.173298 | |
| 0.009246 | 0.934781 | 0.944027 | 0.000197 | 0.934781 | 0.934978 | 1.879005 | |
| 0.009867 | 0.7783 | 0.788166 | 0.000211 | 0.7783 | 0.778511 | 1.566677 | |
| 0.006061 | 1.415468 | 1.421529 | 0.000129 | 1.415468 | 1.415598 | 2.837127 | |
| 0.01022 | 7.199345 | 7.209565 | 0.000218 | 7.199345 | 7.199563 | 14.40913 | |
| 0.00389 | 2.288192 | 2.292081 | 8.30E-05 | 2.288192 | 2.288275 | 4.580356 | |
| 0.010582 | 0.686177 | 0.696759 | 0.000226 | 0.686177 | 0.686403 | 1.383162 | |
| 0.022038 | 0.374691 | 0.396729 | 0.000471 | 0.374691 | 0.375162 | 0.771891 | |
| 0.020399 | 0.366991 | 0.38739 | 0.000436 | 0.366991 | 0.367426 | 0.754816 | |
| 0.010499 | 0.639605 | 0.650104 | 0.000224 | 0.639605 | 0.639829 | 1.289933 | |
| 0.020215 | 0.359205 | 0.37942 | 0.000432 | 0.359205 | 0.359636 | 0.739056 | |
| 0.008371 | 0.901973 | 0.910344 | 0.000179 | 0.901973 | 0.902152 | 1.812496 | |
| 0.00596 | 1.191433 | 1.197393 | 0.000127 | 1.191433 | 1.19156 | 2.388953 | |
| 0.005006 | 1.578813 | 1.583819 | 0.000107 | 1.578813 | 1.57892 | 3.162739 | |
Abbreviations: HQ, health hazard; HI, health index
Figure 5.
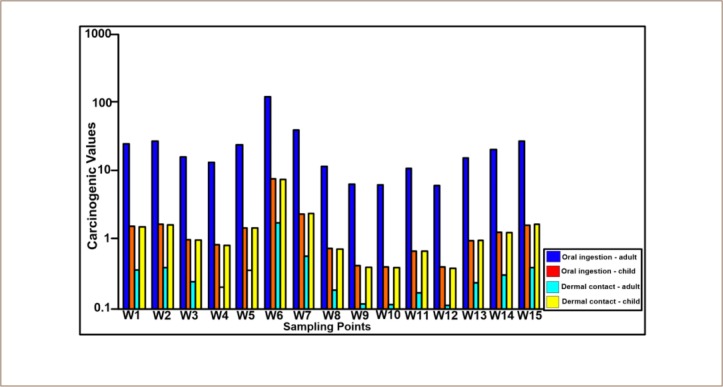
Total carcinogenic health index of heavy metals through oral and dermal contact in water samples from Ikenne
Abbreviation: W, sampling point
Cadmium, Pb and As present non-carcinogenic health risks (Table 7) in both adults and children since the values are above the threshold of 1 recommended by the United States Environmental Protection Agency (USEPA) for non-carcinogenic health diseases, but the effects are greater for children, as revealed by the results in Figure 6.3
Table 7.
Non-Carcinogenic Health Hazard for Adults and Children through Oral Ingestion and Dermal Contact with Groundwater
| Oral Ingestion | Dermal | Health Hazard | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HQ Cd | HQ As | HQ Pb | HI | HQ Cd | HQ As | HQ Pb | HI | ||
| Adults | 34.88 | 0.0009 | 73.83 | 108.7 | 69.75 | 1.00E-05 | 20.51 | 90.3 | 198.97 |
| 42.51 | 0.0008 | 79.89 | 122.4 | 85.02 | 1.00E-05 | 22.2 | 107 | 229.61 | |
| 19.63 | 0.0015 | 47.14 | 66.76 | 39.25 | 1.00E-05 | 13.1 | 52.4 | 119.11 | |
| 17.74 | 0.0016 | 39.25 | 56.98 | 35.47 | 1.00E-05 | 10.91 | 46.4 | 103.36 | |
| 31.81 | 0.001 | 71.37 | 103.2 | 63.62 | 1.00E-05 | 19.83 | 83.5 | 186.63 | |
| 17.44 | 0.0017 | 363 | 380.4 | 34.88 | 1.00E-05 | 100.8 | 136 | 516.14 | |
| 49.15 | 0.0007 | 115.4 | 164.5 | 98.3 | 1.00E-05 | 32.05 | 130 | 294.87 | |
| 17.3 | 0.0017 | 34.6 | 51.91 | 34.6 | 1.00E-05 | 9.62 | 44.2 | 96.13 | |
| 8.24 | 0.0036 | 18.9 | 27.14 | 16.47 | 1.00E-05 | 5.25 | 21.7 | 48.87 | |
| 9.77 | 0.0033 | 18.51 | 28.29 | 19.54 | 1.00E-05 | 5.14 | 24.7 | 52.98 | |
| 17.6 | 0.0017 | 32.25 | 49.85 | 35.19 | 1.00E-05 | 8.96 | 44.2 | 94 | |
| 17.07 | 0.0033 | 18.12 | 35.2 | 34.14 | 1.00E-05 | 5.04 | 39.2 | 74.39 | |
| 42 | 0.0014 | 45.48 | 87.48 | 83.99 | 1.00E-05 | 12.64 | 96.6 | 184.11 | |
| 29.36 | 0.001 | 60.08 | 89.44 | 58.72 | 1.00E-05 | 16.69 | 75.4 | 164.85 | |
| 34.63 | 0.0009 | 79.61 | 114.2 | 69.25 | 1.00E-05 | 22.12 | 91.4 | 205.6 | |
| Children | 6.98 | 9.00E-04 | 14.77 | 21.75 | 13.95 | 2.854 | 4.93 | 21.74 | 43.48 |
| 8.51 | 8.00E-04 | 15.98 | 24.49 | 17.01 | 2.447 | 5.33 | 24.79 | 49.27 | |
| 3.93 | 0.002 | 9.43 | 13.36 | 7.85 | 5.118 | 3.15 | 16.12 | 29.48 | |
| 3.55 | 0.002 | 7.85 | 11.4 | 7.1 | 5.461 | 2.62 | 15.19 | 26.59 | |
| 6.37 | 0.001 | 14.28 | 20.64 | 12.73 | 3.355 | 4.76 | 20.85 | 41.49 | |
| 3.49 | 0.002 | 72.6 | 76.09 | 6.98 | 5.657 | 24.2 | 36.84 | 112.9 | |
| 9.83 | 7.00E-04 | 23.08 | 32.91 | 19.66 | 2.153 | 7.7 | 29.51 | 62.42 | |
| 3.46 | 0.002 | 6.92 | 10.39 | 6.92 | 5.858 | 2.31 | 15.1 | 25.49 | |
| 1.65 | 0.004 | 3.78 | 5.44 | 3.3 | 12.2 | 1.26 | 16.77 | 22.21 | |
| 1.96 | 0.003 | 3.71 | 5.67 | 3.91 | 11.29 | 1.24 | 16.46 | 22.12 | |
| 3.52 | 0.002 | 6.45 | 9.98 | 7.04 | 5.811 | 2.15 | 15.01 | 24.99 | |
| 3.42 | 0.003 | 3.63 | 7.05 | 6.83 | 11.19 | 1.21 | 19.25 | 26.3 | |
| 8.4 | 0.001 | 9.1 | 17.5 | 16.8 | 4.634 | 3.04 | 24.48 | 41.98 | |
| 5.88 | 0.001 | 12.02 | 17.89 | 11.75 | 3.299 | 4.01 | 19.06 | 36.95 | |
| 6.93 | 9.00E-04 | 15.93 | 22.85 | 13.85 | 2.771 | 5.31 | 21.94 | 44.79 | |
Abbreviations: HQ, health hazard; HI, health index
Figure 6.
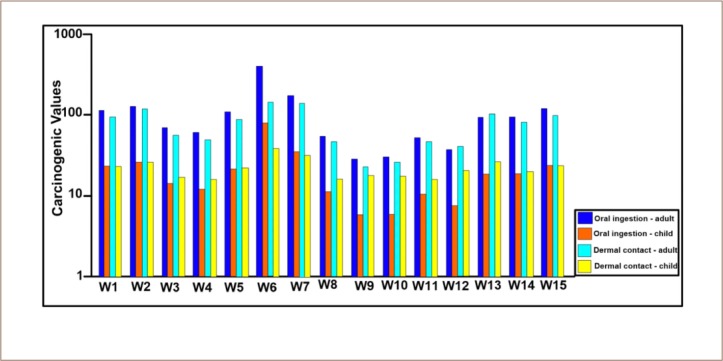
Total non-carcinogenic health index of heavy metals through oral and dermal contact in water samples from Ikenne
Abbreviation: W, sampling point
Conclusions
Analysis of water quality in the study area illustrates the nature of pollutants found in groundwater as a result of continuous random dumping of hazardous waste and discharge of effluents by agriculture and municipal waste in the community and surrounding environment. The results of this study suggest that groundwater close to the study area is no longer potable for drinking due to the moderate-abundant amounts of carbonate, which causes water hardness, and could lead to cosmetic and aesthetic effects on taste, odor and color. There is also an elevated risk for carcinogenic and non-carcinogenic diseases for adults and children in the area due to high occurrence of Pb, As and Cd found in the water. It is therefore recommended that the municipal government distribute waste disposal bags and establish a waste collection service to reduce indiscriminate dumping of refuse in the area. In addition, a task force should be formed in the community to enforce existing waste disposal and water protection laws. Construction of a centralized, deep, double-cased well is recommended to ensure an uncontaminated water supply for drinking and domestic purposes in the study area.
Supplementary Material
Acknowledgments
We acknowledge Adebisi Folasade and Adekunle Bayo for their assistance in the field. This study was funded as part of employment.
References
- 1.Bodur MN, Ergin M. Geochemical characteristics of the recent sediments from the Sea of Marmara. Chem Geol [Internet] 1994 Jul 1;115(1–2):73–101. doi: 10.1016/0009-2541(94)90146-5. [cited 2019 Oct 1] Available from: Subscription required to view. [DOI] [Google Scholar]
- 2.Vodela JK, Lenz SD, Renden JA, McElhenney WH, Kemppainen BW. Drinking water contaminants (arsenic, cadmium, lead, benzene, and trichloroethylene). 2. Effects on reproductive performance, egg quality, and embryo toxicity in broiler breeders. Poult Sci [Internet] 1997 Nov;76(11):1493–500. doi: 10.1093/ps/76.11.1493. [cited 2019 Oct 1] Available from: [DOI] [PubMed] [Google Scholar]
- 3.Annan K. On: World Water Day, Special United Nation Report. New York, NY: United Nations; 2003. Mar, [Google Scholar]
- 4.Adeniyi IF. Ife environmentalist, Official Bulletin of Nigerian Society for Environmental Management (NISEM) Ile-Ife, Nigeria: Obafemi Awolowo University; 2004. The concept of water quality; pp. 112–3. [Google Scholar]
- 5.Mor S, Ravindra K, Dahiya RP, Chandra A. Leachate characterization and assessment of groundwater pollution near municipal solid waste landfill site. Environ Monit Assess [Internet] 2006 Jul;118(1–3):435–56. doi: 10.1007/s10661-006-1505-7. [cited 2019 Oct 1] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 6.Basic information about pathogens and indicators in drinking water. Washington, DC: United States Environmental Protection Agency; 2012. [Google Scholar]
- 7.Amadi AN, Ameh MI, Jisa J. The impact of dumpsites on groundwater quality in Markurdi Metropolis, Benue State. Nat Appl Sci J. 2010;11(1):90–102. [Google Scholar]
- 8.Chopra AK, Pathak C, Prasad G. Scenario of heavy metal contamination in agricultural soil and its management. J Appl Nat Sci. 2009;1(1):99–108. [Google Scholar]
- 9.Yakubu S, Baba B. Assessment of water quality consumed by students and its health implications on their academic performance. J Stud Sci Math Educ. 2010;1(1):103–11. [Google Scholar]
- 10.Amadi AN, Olasehinde PI, Okosun EA, Okoye NO, Okunlola IA, Alkali YB, Dan-Hassan MA. A comparative study on the impact of Avu and Ihie Dumpsites on soil quality in Southeastern Nigeria. Am J Chem [Internet] 2012;2(1):17–23. doi: 10.5923/j.chemistry.20120201.05. [cited 2019 Oct 1] Available from: [DOI] [Google Scholar]
- 11.Namen AA, BrasilFda C, Abrunhosa JJ, Abrunhosa GG, Tarre RM, Marques FJ. RFID technology for hazardous waste management and tracking. Waste Manag Res [Internet] 2014 Sep;32(9 Suppl):59–66. doi: 10.1177/0734242X14536463. [cited 2019 Oct 3] Available from: [DOI] [PubMed] [Google Scholar]
- 12.Rapportoecomafia [Internet] Roma, Italy: Legambiente; 2019. Jul 5, [cited 2019 Oct 3]. [about 2 screens]. Available from: https://www.legambiente.it/rapporto-ecomafia/Italian. [Google Scholar]
- 13.Ferrara L, Iannace M, Patelli AM, Arienzo M. Geochemical survey of an illegal waste disposal site under a waste emergency scenario (Northwest Naples, Italy) Environ Monit Assess [Internet] 2013 Mar;185(3):2671–82. doi: 10.1007/s10661-012-2738-2. [cited 2019 Oct 3] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 14.Diletti G, Ceci R, Conte A, De Benedictis A, Migliorati G, Scortichini G. Milk contamination from dioxins in Italy: source identification and intervention strategies. In: Mehmetli E, Koumanova B, editors. The fate of persistent organic pollutants in the Environment. Berlin, Germany: Springer, Dordrecht; 2008. pp. 301–14. p. (NATO Science for Peace and Security Series) [Google Scholar]
- 15.Jesperson K. Public drinking water and public health: a tie that binds. On Tap. 2004 Fall;4(3):16–22. [Google Scholar]
- 16.Tijani MN, Jinno K, Hiroshiro Y. Environmental impact of heavy metals distribution in water and sediments of Ogunpa River, Ibadan area, Southwestern Nigeria. J Min Geol. 2004;40(1):73–83. [Google Scholar]
- 17.Su GL. Assessing the effect of a dumpsite to groundwater quality in Payatas, Philippines. Am J Environ Sci [Internet] 2008;4(4):276–80. doi: 10.3844/ajessp.2008.276.280. [cited 2019 Oct 3] Available from: [DOI] [Google Scholar]
- 18.Bakari A. Hydrochemical assessment of groundwater quality in the Chad Basin around Maiduguri, Nigeria. J Geol Min [Internet] 2014 Jan;6(1):1–12. doi: 10.5897/JGMR13.0196. [cited 2019 Oct 3] Available from: [DOI] [Google Scholar]
- 19.Ikem A, Osibanjo O, Sridhar MK, Sobande A. Evaluation of groundwater quality characteristics near two waste sites in Ibadan and Lagos, Nigeria. Water Air Soil Pollut [Internet] 2002 Oct;140(1–4):307–33. doi: 10.1023/A:1020165403531. [cited 2019 Oct 2] Available from: Subscription required to view. [DOI] [Google Scholar]
- 20.Tijani MN, Oke SA, Olowookere AT. Hydrogeochemical characterization of a shallow groundwater system in the weathered basement aquifer of Ilesha area, southwestern Nigeria. Evolving Water Resources Systems: Understanding, Predicting and Managing Water–Society Interactions Proceedings of ICWRS2014; June 2014; Bologna, Italy. Wallingford, UK: IAHS Press; 2014. pp. 475–80. p. [Google Scholar]
- 21.Aiyesanmi F, Imoisi OB. Understanding leaching behaviour of landfill leachate in Benin-City, Edo State, Nigeria through dumpsite monitoring. Br J Environ Clim Change. 2011;1(4):190–200. [Google Scholar]
- 22.Classification of rocks in the Nigeria Precambian Basement Complex. In: Okogbe CA, editor. Geology of Nigeria; Annual Conference of Nigerian Mining Geological and Metallurgical Society; 1971 Dec; Kaduna, Nigeria. Jos, Nigeria: Rock View (Nigeria) Ltd.; 1971. Dec, pp. 53–77. editor. p. [Google Scholar]
- 23.Olorunfemi MO, Ojo JS, Akintunde OM. Hydrogeophysical evaluation of the groundwater potentials of the Akure metropolis, southwestern Nigeria. J Min Geol. 1999;35(2):207–28. [Google Scholar]
- 24.Census 2006: results for Nigeria. Abuja, Nigeria: National Population Commission; 2006. [Google Scholar]
- 25.Sinex SA, Helz GR. Regional geochemistry of trace elements in Chesapeake Bay sediments. Environ Geol [Internet] 1981 Nov;3(6):315–23. doi: 10.1007/BF02473521. [cited 2019 Oct 3] Available from: Subscription required to view. [DOI] [Google Scholar]
- 26.Loska K, Wiechula D, Barska B, Cebula E, Chojnecka A. Assessment of arsenic enrichment of cultivated soils in Southern Poland. Pol J Environ Stud [Internet] 2003 Feb;12(2):187–92. [cited 2019 Oct 3] Available from: http://www.pjoes.com/Assessment-of-Arsenic-Enrichment-of-Cultivated-r-nSoils-in-Southern-Poland,87543,0,2.html. [Google Scholar]
- 27.Kothai P, Prathibha P, Saradhi IV, Pandit GG, Puranik VD. Characterization of atmospheric particulate matter using PIXE technique. Int J Environ Ecol Eng. 2009;3(3):39–42. [Google Scholar]
- 28.Chakravarty M, Patgiri AD. Metal pollution assessment in sediments of the Dikrong River, N.E. India. J Hum Ecol [Internet] 2009;27(1):63–7. doi: 10.1080/09709274.2009.11906193. [cited 2019 Oct 3] Available from: Subscription required to view. [DOI] [Google Scholar]
- 29.Ong MC, Kamaruzzaman BY. An assessment of metals (Pb and Cu) contamination in bottom sediment from South China Sea coastal waters, Malaysia. Am J Appl Sci. 2009;6(7):1418–23. [Google Scholar]
- 30.Seshan BR, Natesan U, Deepthi K. Geochemical and statistical approach for evaluation of heavy metal pollution in core sediments in southeast coast of India. Int J Environ Sci Tech [Internet] 2010 Mar;7(2):291–306. doi: 10.1007/BF03326139. Available online. [cited 2019 Oct 3] Available from: Subscription required to view. [DOI] [Google Scholar]
- 31.Hakanson L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res [Internet] 1980;14(8):975–1001. doi: 10.1016/0043-1354(80)90143-8. [cited 2019 Oct 3] Available from: Subscription required to view. [DOI] [Google Scholar]
- 32.Muller G. Index of geoaccumulation in sediments of theRhine River. Geol J. 1969;2(3):108–18. [Google Scholar]
- 33.Sekabira K, OryemOriga H, Basamba TA, Mutumba G, Kakudidi E. Assessment of heavy metal pollution in the urban stream sediments and its tributaries. Int J Environ Sci Technol [Internet] 2010 Jun;7(3):435–46. doi: 10.1007/BF03326153. [cited 2019 Oct 3] Available from: Subscription required to view. [DOI] [Google Scholar]
- 34.Framework for the management of contaminated land [Internet] Pretoria, South Africa: Department of Environmental Affairs; 2010. May, p. 326. [cited 2018 Feb 5] p. Available from: http://sawic.environment.gov.za/documents/562.pdf. [Google Scholar]
- 35.Kamunda C, Mathuthu M, Madhuku M. An assessment of radiological hazards from gold mine tailings in the province of Gauteng in South Africa. Int J Environ Res Public Health [Internet] 2016 Jan 18;13(1):10. doi: 10.3390/ijerph13010138. [cited 2019 Oct 29] Article 138 [ p.]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Integrated Risk Information System [Internet] Washington, DC: US Environmental Protection Agency; 1997. Mar, [cited 2019 Oct 19]. Available from: https://www.epa.gov/iris. [Google Scholar]
- 37.Guidelines for drinking-water quality. 3rd ed. Vol. 1, Recommendations: incorporating first and second addenda [Internet] Geneva, Switzerland: World Health Organization; 2008. p. 668. [cited 2019 Oct 3] p. Available from: https://www.who.int/water_sanitation_health/publications/gdwq3rev/en/ [Google Scholar]
- 38.Al Yaqout AF. Assessment and analysis of industrial liquid waste and sludge disposal at unlined landfill sites in arid climate. Waste Manag [Internet] 2003;23(9):817–24. doi: 10.1016/S0956-053X(03)00036-9. [cited 2019 Oct 3] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 39.Mor S, Ravindra K, Dahiya RP, Chandra A. Leachate characterization and assessment of groundwater pollution near municipal solid waste landfill site. Environ Monit Assess [Internet] 2006 Jul;118(1–3):435–56. doi: 10.1007/s10661-006-1505-7. [cited 2019 Oct 3] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 40.Panno SV, Hackley KC, Hwang HH, Greenberg SE, Krapac IG, Landsberger S, O'Kelly DJ. Characterization and identification of Na-Cl sources in ground water. Ground Water [Internet] 2006 Mar-Apr;44(2):176–87. doi: 10.1111/j.1745-6584.2005.00127.x. [cited 2019 Oct 3] Available from: Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 41.Subramani T, Elango L, Damodarasamy SR. Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin, Tamil Nadu, India. Environ Geol. 2005;47:1099–110. [Google Scholar]
- 42.Todd DK. Groundwater hydrology. 3rd ed. New York, NY: Wiley and Sons Inc.; 1995. [Google Scholar]
- 43.Subrahmanyam K, Yadaiah P. Assessment of the impact of industrial effluents on water quality in Patancheru and environs, Medak district, Andhra Pradesh, India. Hydrogeol J [Internet] 2001 Jul;9(3):297–312. doi: 10.1007/s100400000120. [cited 2019 Oct 3] Available from: Subscription required to view. [DOI] [Google Scholar]
- 44.Karanth KR. Groundwater assessment: development and management. New Delhi, India: Tata McGraw-Hill; 1987. p. 720. p. [Google Scholar]
- 45.Raghunath HM. Ground water. 2nd ed. New Delhi, India: Wiley Eastern Ltd; 1987. p. 456. p. [Google Scholar]
- 46.Kelly WP. Use of saline irrigation water. Soil Sci. 1963 Jun;95(6):385–91. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


