Abstract
The impact of the immune response in malignancy is poorly understood. While immune cells can destroy transformed cells, the targeting and accumulation of monocytes and macrophages at tumor sites may promote tumor metastases. The growth factor M-CSF is important in promoting monocyte survival. Since M-CSF−/− mice are protected against tumor metastases, we hypothesized that M-CSF induced monocytes to produce angiogenic factors that facilitate metastases. In this study we demonstrate that recombinant human M-CSF induces freshly isolated normal human monocytes to produce and release the growth factor vascular endothelial growth factor (VEGF) in a dose-dependent manner, which peaked at 5 days in culture. VEGF released by these monocytes is biologically active, as cell-free supernatants from these M-CSF-stimulated monocytes induced tube formation in HUVEC. Network formation by these HUVECs after treatment with supernatants from monocytes stimulated with M-CSF were inhibited by anti-VEGF, but not by the isogenic control, Abs. Collectively, these data support an important role for M-CSF and monocytes in VEGF production and angiogenesis.
Vascular endothelial growth factor (VEGF)4 was first defined as a soluble factor inducing vascular permeability (1). Subsequently, VEGF was recognized to be a potent angiogenic molecule with a number of pathophysiological as well as physiological functions (2). The important physiological role of VEGF is illustrated by the finding that VEGF-deficient animals do not survive embryogenesis (3). In contrast, in human disease VEGF plays an important role in the genesis of retinopathy in the eyes of diabetics (4), in wound repair (4), in cancer (5), and in organ repair and regeneration (6). However, it is in human cancer that VEGF plays its most publicized role. VEGF promotes endothelial cell proliferation and new blood vessel formation that appears to be critical in regulating the growth and spread of cancer (5). In fact, recent therapeutic approaches have focused on neutralizing VEGF to reduce angiogenesis in the treatment of cancer (7).
Antiangiogenic strategies include the use of naturally occurring molecules such as angiostatin and endostatin (7, 8). These approaches have led to exciting reductions in tumor mass and metastatic disease in murine models of cancer (9, 10). Similar to these naturally occurring antiangiogenic molecules, a recent paper suggested that animals deficient in M-CSF had fewer tumor metastases than animals producing normal levels of M-CSF in an animal model of breast cancer (11). Local expression of M-CSF in the primary breast tumor in these M-CSF-deficient mice led to the accumulation of monocytes and macrophages in the tumor and promoted metastases equivalent to those seen in wild-type mice with normal levels of M-CSF (11). Based on this observation, we speculated that M-CSF would influence myeloid cells to produce proangiogenic factors to promote tumor metastases. While the specific mechanism of the role of M-CSF in cancer metastases is not known, M-CSF is an important growth factor in tumor growth (12), but has not been identified to directly promote angiogenesis.
There are a number of proangiogenic growth factors that promote blood vessel formation in human cancer (13–15), including molecules of the fibroblast growth factor family, IL-8, and VEGF. Of these factors, VEGF appears to play the dominant role in human health and disease. The VEGF family currently includes VEGF-A, -B, -C, -D, and -E and placental growth factor (16). However, in human disease it appears that VEGF-A is the most important, as it appears to be the most readily induced form of VEGF in humans during episodes of hypoxemia (17). Alternative splicing events in the mRNA of VEGF-A produce nine different isoforms of the protein (121, 145, 148, 162, 165, 165b, 183, 189, and 206), the most common being the 121- and 165-aa variants (18). VEGF-A signals through the VEGF receptor (VEGFR)-1 and VEFGR-2 (16). These receptors are expressed on endothelial cells in adults and in most cells during embryogenesis (19, 20). In the adult, VEGFR-1 is expressed on monocytes, renal mesangial cells, vascular smooth muscle cells, and endothelial cells, while VEGFR-2 is found in pancreatic duct cells, retinal progenitor cells, and hemopoietic cells (16). VEGF receptors do not signal alone, but rely on accessory proteins and coreceptors to regulate their signaling. These accessory proteins include cadherins, integrins, neuropilins, heparin, and heparan-sulfated proteoglycans (16). Signaling through VEGFR appears to follow classical receptor tyrosine kinase signaling pathways (21). As such, tyrosine phosphatases such as Src homology protein tyrosine phosphatase 1 bind to selected VEGFR and may serve to regulate signal transduction events (16). Inducing tyrosine kinase activity in the receptor activates signaling pathways that include phosphoinositol 3-kinase, mitogen-activated protein kinase/extracellular signal-regulated kinase, phospholipase C-γ, and Nck (16, 22–24).
Using an ELISA specific for VEGF-A (which we will address as VEGF for this study), we found that M-CSF induced VEGF production from normal human monocytes. VEGF production was maximal over 5 days in culture, and VEGF produced by these monocytes was biologically active, as indicated by HUVEC tube formation using Matrigel matrix. Neutralizing Abs to VEGF were sufficient to inhibit the ability of these supernatants to promote tube formation. This suggests that M-CSF promotes monocytes to secrete biologically active VEGF, thus providing a new molecular target for angiogenesis in human disease.
Materials and Methods
Materials
Blood donors were obtained from the American Red Cross (Columbus, OH). RPMI 1640 was purchased from BioWhittaker (Walkersville, MD). FBS (certified <0.06 endotoxin units/ml endotoxin levels) was obtained from HyClone (Logan, UT). Recombinant human M-CSF, recombinant human VEGF, and VEGF Duoset ELISA Development Kit were purchased from R&D Systems (Minneapolis, MN). Anti-human VEGF neutralizing Ab and anti-human M-CSF Ab were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). Growth Factor-Reduced Matrigel Matrix was purchased from BD Biosciences (Bedford, MA). HUVECs and endothelial basal medium were purchased from BioWhittaker. Absolutely RNA RT-PCR Miniprep Kit for total RNA purification was purchased from Stratagene (La Jolla, CA). SuperScript First-Strand Synthesis System for RT-PCR Kit for cDNA synthesis was purchased from Life Technologies (Carlsbad, CA). TaqMan Universal PCR Master Mix was obtained from PE Applied Biosystems (Branchburg, NJ). VEGF probe (5′−6FAM-AGCCCTGGCGCTGAGCCTCTC-TAMRA-3′), VEGF forward primer (5′-CAGTTTTGGGAACACCGACAA-3′), and VEGF reverse primer (5′-TCTGTCCGTCTGACCTGGG-3′) were purchased from PE Applied Biosystems (Foster City, CA). All tissue culture reagents contained <10 pg/ml endotoxin levels.
Purification of peripheral blood monocytes
Single-donor monocytes were isolated from source leukocyte packs obtained from the American Red Cross according to a method described previously (25). For all experiments, monocytes were resuspended in 5 × 106 cells/condition in RPMI 1640, 0.1% human serum albumin (HSA; used as a carrier protein for VEGF), and 10 μg/ml polymyxin B and were left unstimulated or were stimulated with 1, 10, or 100 ng/ml of recombinant human M-CSF. Polymyxin B was added as further protection against endotoxin contamination in cell cultures. For VEGF measurement by ELISA, Matrigel assays, or endothelial cell proliferation assays, monocytes were stimulated under the indicated conditions immediately after monocyte isolation.
VEGF production measured by ELISA
Isolated monocytes were cultured for the indicated time (1, 2, 3, 4, or 5 days) in RPMI 1640 containing 0.1% HSA and 10 μg/ml polymyxin B in either polypropylene tubes or 48-well culture plates. The monocytes were immediately stimulated after isolation by 1, 10, or 100 ng/ml recombinant human M-CSF, then incubated for the indicated times at 37°C in 5% CO2, and cell-free supernatants were harvested. The cultured monocytes were spun down at 5000 rpm for 5 min at 4°C, and supernatants were collected and immediately frozen at −80°C until measurement by VEGF ELISA. Statistical analyses were made using one-way ANOVA with post hoc testing when appropriate.
VEGF measurement in monocytic whole cell lysates vs supernatants
Isolated monocytes were cultured with 0.1% HSA and 10 μg/ml polymyxin B, then stimulated by 1, 10, or 100 ng/ml of recombinant human M-CSF and incubated in 37°C for 24 h in polypropylene tubes. Supernatants were collected and frozen at −80°C until an ELISA was performed. The monocytes were collected by centrifugation (5,000 rpm for 5 min) and then washed with KPM buffer (50 mM PIPES, 10 mM EGTA, 1.92 mM MgCl2, 1 mM DTT, 0.1 mM PMSF, 10 mg/ml of cytochalasin B, and 2 mg/ml of the following protease inhibitors: chymostatin, pepstatin, leupeptin, and antipain). The cell suspension was lysed by four cycles of freeze-thawing using liquid nitrogen and water at room temperature. The lysates were vigorously vortexed between each freeze-thaw cycle. To collect the final cytosolic protein, the lysates were centrifuged at high speed (14,000 rpm) for 20 min at 4°C. The cellular pellet was discarded, and the desired aqueous protein portion was collected and stored at −80°C until an ELISA was performed to assess total VEGF production. Statistical analyses were made using one-way ANOVA when appropriate.
In vitro Matrigel assays to assess the biological activity of VEGF produced by M-CSF stimulation
Isolated monocytes (5 × 106 cells/condition) were cultured with 0.1% HSA and 10 μg/ml polymyxin B and were left unstimulated or were stimulated with recombinant human M-CSF (100 ng/ml). The monocytes were plated in 24-well plates and incubated at 37°C in 5% CO2 for 72 h. Cell-free supernatants were harvested and frozen at −80°C until a VEGF ELISA was performed. To determine the biological activity of VEGF in these supernatants, HUVECs were cultured in supernatants from monocytes left unstimulated or stimulated with M-CSF using growth factor-depleted Matrigel. Biological activity was assessed by the formation of capillary-like networking structures by the endothelial cells. Growth factor-depleted Matrigel was thawed at 4°C overnight. Using precooled pipette tips and plates, the Matrigel was then distributed in 24-well plates (200 μl/well) and allowed to solidify at 37°C for at least 1 h. Within that time, all samples and controls were prepared. Meanwhile, HUVECs were serum-starved in RPMI 1640 for 1.5 h. All controls and samples were resuspended in RPMI 1640 and had 150,000 HUVECs/well.
HUVECs were incubated in the following conditions: alone, with recombinant human (rh) M-CSF (100 ng/ml), with rhVEGF (10 ng/ml), with rhVEGF (10 ng/ml) and normal goat IgG Ab control (0.6 μg/ml), with rhVEGF (10 ng/ml) and anti-VEGF IgG Ab (0.6 μg/ml), with unstimulated monocyte supernatants, with M-CSF-stimulated monocyte supernatants, with M-CSF-stimulated monocyte supernatants and normal goat IgG control Ab (0.6 μg/ml), or with M-CSF-stimulated monocyte supernatants and anti-VEGF IgG Ab (0.6 μg/ml).
Supernatants from monocytes that had been incubated in M-CSF for 72 h were used these studies, since quantification of VEGF in these samples demonstrated physiologically achievable VEGF levels (at least 1 ng/ml) (26). In experiments assessing the role of M-CSF-stimulated supernatants, each study was performed from a single donor, so that the amount of VEGF in each supernatant was controlled in the same experiment. Reagents for conditions outlined above were rotated at 4°C for 1 h before addition to HUVECs. Additionally, all conditions were supplemented with fresh RPMI 1640 to bring the total volume in each well to 2 ml. The plate was incubated at 37°C for 20 h. Tube formation was observed, and digital pictures were captured using an Olympus digital camera (New Hyde Park, NY). Quantification of biological activity of the VEGF was measured by counting completely enclosed endothelial networks in each well. A total of three blinded, high powered fields were counted per well, and the total enclosed networks were graphed onto an Excel spreadsheet (Microsoft, Redmond, WA).
Total RNA isolation from monocytes
After time points were reached, monocytes were centrifuged at 5000 rpm for 5 min at 4°C. Supernatants were discarded, and the remaining cell pellet was treated with 600 μl of lysis buffer and 4.2 μl of 2-ME (from Absolutely RNA RT-PCR Miniprep Kit, Stratagene). Purification of total cellular RNA was conducted using the Miniprep kit, and RNA concentrations were determined using UV spectroscopy (GeneQuant, Amersham Pharmacia Biotech, Arlington Heights, IL); 1 OD at 260 nm equals 40 μg of RNA/ml. 260/280 ratios were between 1.9 and 2.1. RNA integrity was verified on a 1.5% agarose gel to detect 18S and 28S ribosomal RNA bands at 0.7 and 1.5 kDa, respectively. cDNA was synthesized from monocyte total cellular RNA using the SuperScript First-Strand Synthesis System for RT-PCR Kit (Life Technologies).
Real-time PCR
The VEGF forward primer, VEGF reverse primer, and the VEGF probe with TAMRA quencher (PE Applied Biosystems) for VEGF mRNA were designed using Primer Express version 1.0 software (ABI PRISM, PerkinElmer, Branchburg, NJ) based on the human VEGF sequence obtained from PubMed (accession no. NM 003376). 2X Universal Master Mix (PE Applied Biosystems) was used in the reaction mixture containing 0.83 μl of 12 μM of each forward and reverse VEGF primer, 200 nM VEGF probe (FAM), 0.25 μl of 20X 18S internal control probe (VIC), 17.3 μl of diethylpyrocarbonate-treated water, and 4 μl of cDNA from each sample for a 50- μl total reaction volume. The mixture was added to MicroAmp Optical PCR tubes (PE Applied Biosystems) inside a 96-well tube stand where the cDNA for each sample was added. The tubes were capped using MicroAmp Optical Caps (PE Applied Biosystems) and centrifuged at 1000 rpm for 1 min. The real-time PCR was completed on the ABI PRISM Sequence Detector 7700 (PerkinElmer) using Sequence Detector version 1.7 software. Reaction conditions were as follows: 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min.
Fold induction or reduction calculations of VEGF mRNA
After completion of the PCR, the baselines and thresholds were set for both FAM and VIC probes. Using the Ct values (cycle number where sample crosses the threshold value) for FAM and VIC, the ΔCt was calculated: ΔCt = CtFAM − CtVIC. Then the values for each sample (sam) were compared with the control (con): ΔΔCt = ΔCtsam − ΔCtcon.
The fold change of mRNA level from control to sample is: fold induction or reduction = 2−ΔΔCt.
Statistical analyses
Minitab statistical software, SigmaPlot, and Microsoft Excel software were used for all analyses. For ELISA analyses, a nonparametric ANOVA with Tukey’s post hoc test was performed to determine differences between groups. Groups were considered significantly different at p < 0.05.
Results
M-CSF induces human monocytes to produce VEGF
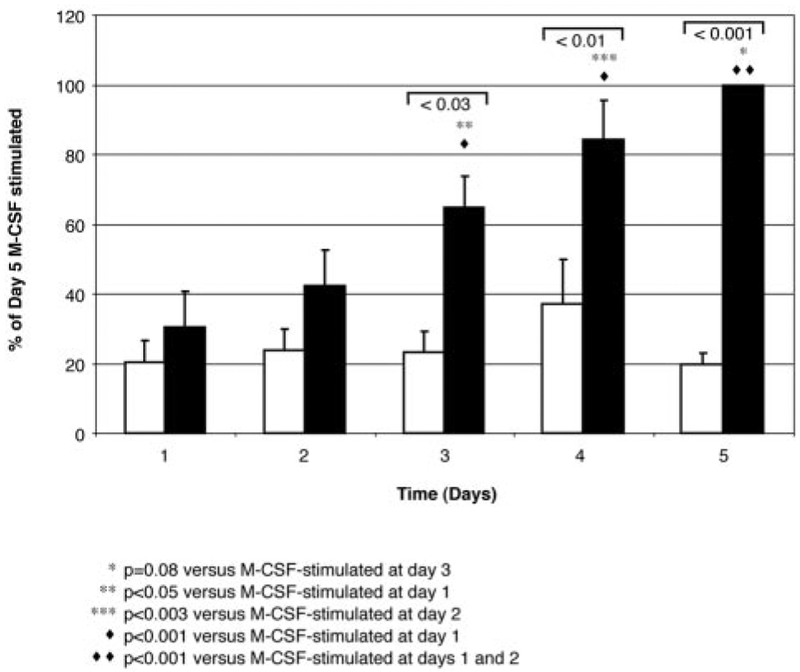
Previous studies demonstrated that animals deficient in M-CSF have fewer tumor metastases than wild-type littermate mice (11). Because new blood vessel formation is critical in tumor metastases, we hypothesized that M-CSF induced monocytes to produce proangiogenic factors. We found that M-CSF induced monocytes to produce VEGF in a dose-dependent manner that was measured at 24 h (100 ng/ml M-CSF-stimulated sample, 100%; 10 ng/ml M-CSF-stimulated, 90 ± 9%; 1 ng/ml M-CSF-stimulated, 52 ± 6.9%; unstimulated, 41 ± 1.7%). The release of VEGF induced by M-CSF stimulation peaked at 5 days of incubation (Fig. 1; day 1, 446 ± 90 pg/ml; day 2, 743 ± 197 pg/ml; day 3, 1178 ± 235 pg/ml; day 4, 1533 ± 385 pg/ml; day 5, 1802 ± 272 pg/ml; p < 0.03, comparing VEGF production in unstimulated monocyte supernatants to M-CSF-stimulated monocyte supernatants on days 3, 4, and 5; p = 0.08 between VEGF production from M-CSF-stimulated supernatants from day 3 vs M-CSF-stimulated supernatants from day 5).
FIGURE 1.
Kinetic analysis of VEGF production by M-CSF-stimulated monocytes. VEGF detected in supernatants over 5 days. Monocytes (5 × 106/condition) were left unstimulated (☐) or were stimulated with M-CSF (100 ng/ml; ■) for the indicated time points, and VEGF in the cell-free supernatants was assayed by ELISA (picograms per milliliter). M-CSF induced more VEGF production from monocytes at 5 days than that in unstimulated, time-matched cells (*, p = 0.08 vs VEGF released from M-CSF-stimulated monocytes on day 3;**, p < 0.05 vs VEGF released from M-CSF-stimulated monocytes on day 1; ***, p < 0.003 vs VEGF released from M-CSF-stimulated monocytes on day 2; ♦, p < 0.001 vs VEGF released from M-CSF-stimulated monocytes on day 1; ♦ ♦, p < 0.001 vs VEGF released from M-CSF-stimulated monocytes on days 1 and 2). Data represent the mean ± SEM from six independent experiments.
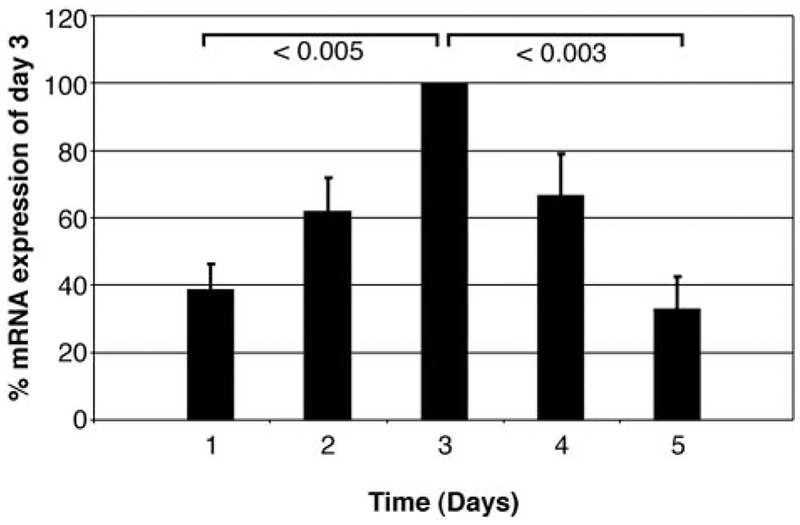
To determine whether M-CSF regulated VEGF production by monocytes at the transcriptional level, we concomitantly assayed cellular VEGF mRNA levels using real-time PCR and also measured the cell supernatants for VEGF production by ELISA in these same monocytes that were left unstimulated or were stimulated with M-CSF. Using real-time PCR, we found that VEGF mRNA produced by these monocytes was enhanced by M-CSF stimulation and peaked at 3 days (Fig. 2; p < 0.05 for day 3 VEGF mRNA level vs unstimulated monocytes at the same time; p < 0.005 for day 3 VEGF mRNA compared with M-CSF-stimulated monocytes on days 1 and 5). The data were presented as a percentage of maximal VEGF mRNA production to account for interindividual monocyte donor variability in the data.
FIGURE 2.
M-CSF induces the expression of VEGF mRNA in human monocytes. Total RNA was isolated from monocytes (5 × 106/condition) stimulated by M-CSF (100 ng/ml) for 1, 2, 3, 4, and 5 days (■) as detected by real-time PCR. M-CSF induced more VEGF mRNA at each time point and peaked on day 3 as assessed by ANOVA with post hoc testing vs unstimulated control samples at the same time (p < 0.05 for VEGF mRNA level vs unstimulated monocytes on day 3; p < 0.005 for day 3 VEGF mRNA compared with M-CSF-stimulated monocytes on day 1; p < 0.003 for day 3 VEGF mRNA compared with M-CSF-stimulated monocytes on day 5). Data represent the mean ± SEM from six independent studies.
Of note, the probe and primer sequences designed for use in the real-time PCR amplification of VEGF mRNA did not distinguish between any of the nine reported isoforms of VEGF-A due to the binding sites of the probe and primers residing outside the open reading frame. In contrast, even though each of the VEGF variants, 121, 145, 165, and 189, has been reported to be highly secreted, isoform 145 is highly uncommon, and isoform 189 binds avidly to the extracellular matrix after secretion. VEGF detected in the cell-free supernatants in this study using ELISAs represents VEGF-A isoforms 165 and 121 as specified by the manufacturer.
One possible interpretation of these data is that VEGF production was a byproduct of cellular survival by M-CSF as opposed to selective induction of VEGF. To address this concern, we also incubated cells in the monocyte survival factor GM-CSF for an equal number of days. Although cellular viability was similar to the M-CSF-stimulated monocyte supernatants, there was no increase in detectable VEGF compared with unstimulated monocytes on each day from days 1–5 (data not shown). We are currently investigating the mechanism for these differences in VEGF production from monocytes stimulated with either M-CSF or GM-CSF.
There is no evidence of paracrine factors inducing VEGF in M-CSF-stimulated monocyte supernatants
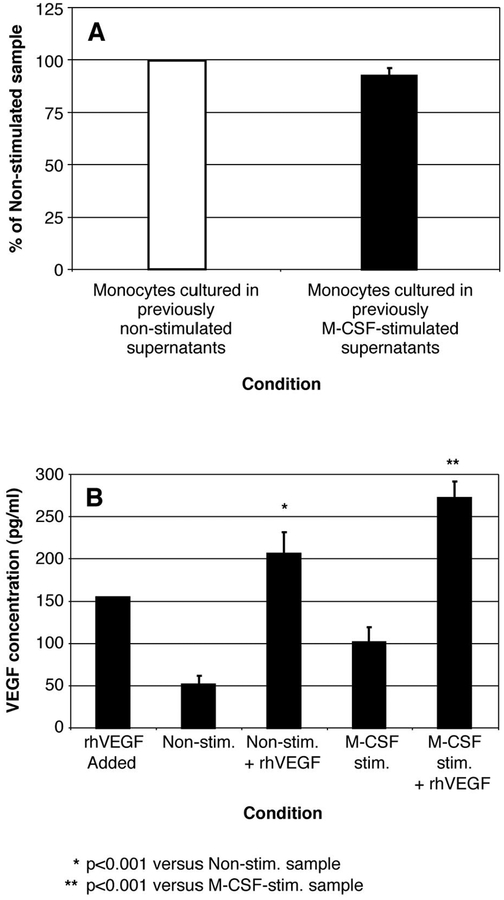
Because of the difference in the kinetics in the peak of VEGF mRNA production (day 3) and peak of VEGF protein production (day 5), we wanted to explore the possibility that paracrine factors were produced by these M-CSF-stimulated human monocytes on day 3 that resulted in delayed VEGF protein production. To evaluate this hypothesis, we depleted VEGF and M-CSF from 72-h M-CSF-stimulated or unstimulated monocyte supernatants and added these depleted supernatants to freshly isolated monocytes for an additional 3 days. Assaying these cells for VEGF disclosed no discernable difference (p = 0.405) in VEGF production from these depleted supernatants (Fig. 3A). Moreover, this finding was not the result of persistence of anti-VEGF Abs in the supernatant, as we were able to detect a known amount of rhVEGF that was added to these depleted supernatants (Fig. 3B; p < 0.001 for depleted, unstimulated supernatants vs depleted, unstimulated supernatants supplemented with rhVEGF (150 pg/ml); p < 0.001 for depleted, M-CSF-stimulated supernatants vs depleted, M-CSF-stimulated supernatants supplemented with rhVEGF (150 pg/ml)). As expected, there was no significant difference (p = 0.3) between the depleted, unstimulated supernatants and the depleted, M-CSF-stimulated supernatants.
FIGURE 3.
There is no evidence of paracrine factors inducing VEGF in M-CSF-stimulated monocyte supernatants. A, Monocytes (5 × 106/condition) were either left unstimulated (☐) or were stimulated with M-CSF (100 ng/ml; ■) for 72 h. The cell-free supernatants of these monocytes were subsequently immunodepleted of VEGF and M-CSF using specific neutralizing Abs, and the Abs were then removed by incubation with protein G agarose. The resulting supernatants were directly added to freshly isolated monocytes for an additional 72 h. Afterward, the supernatants from these monocytes were assayed for VEGF by ELISA. There was no significant difference in VEGF levels produced by the two samples after depletion (p = 0.405). Data represent the mean ± SEM calculated from four independent studies. B, Recombinant human VEGF (150 pg/ml) was supplemented back into the monocyte supernatants that were either left unstimulated (Non-stim.+ rhVEGF) or were stimulated with M-CSF (100 ng/ml; M-CSF-stim.+ rhVEGF) from A that were previously immunodepleted of VEGF and M-CSF using either anti-VEGF or anti-M-CSF neutralizing Abs (*, p < 0.001 for samples containing recombinant human VEGF added to depleted, unstimulated supernatants vs depleted, unstimulated supernatants alone; **, p < 0.001 for samples containing rhVEGF added to depleted, M-CSF-stimulated supernatants vs depleted, M-CSF-stimulated supernatants alone). These data represent the mean ± SEM calculated from four independent studies.
Supernatants from M-CSF-stimulated monocytes and recombinant human VEGF induce monocytes to produce biologically active angiogenic factors
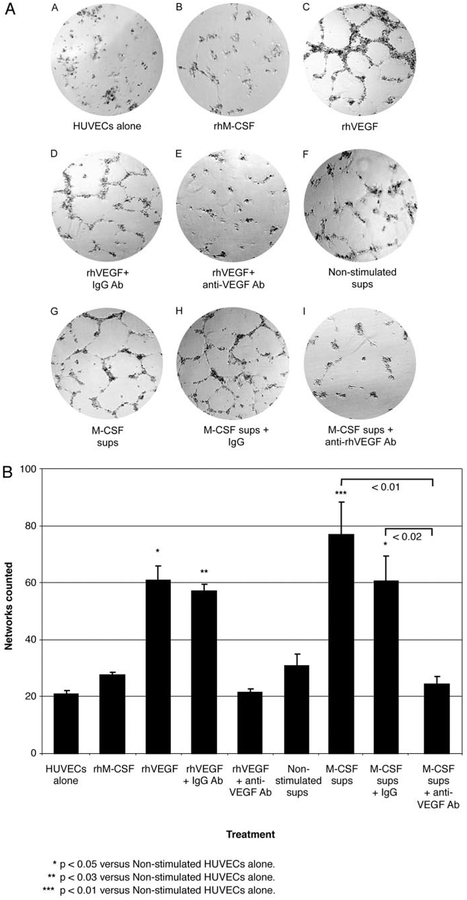
Next, we hypothesized that supernatants from M-CSF-stimulated monocytes would stimulate endothelial cells to form new blood vessel networks. Using Matrigel matrix, we found that rhVEGF, alone or with isogenic IgG control, induced endothelial cell tube formation in HUVECs (Fig. 4A). Similarly, supernatants from monocytes stimulated with M-CSF induced endothelial cells to form tubes (Fig. 4A). In contrast, supernatants from monocytes incubated without M-CSF did not induce these endothelial cell events (Fig. 4A). Statistical comparisons between samples were gained by blinded counting of the numbers of completely enclosed cell networks in these samples, and these data are depicted as a graph that shows that rhVEGF- and M-CSF-stimulated monocyte supernatants both enhanced tube formation in HUVECs vs HUVECs that were incubated with unstimulated monocyte supernatants (p < 0.05 and p < 0.01 compared with supernatants from unstimulated monocytes, respectively; Fig. 4B).
FIGURE 4.
VEGF within the supernatants of M-CSF-stimulated monocytes induces network tube formation by HUVECs. A, HUVEC (1.5 × 105) were grown on Matrigel as follows: A) HUVECs with RPMI medium (1 ml) alone for 20 h (HUVECs alone); B) HUVECs plus rhMCSF (100 ng/ml); C) HUVECs plus rhVEGF (10 ng/ml); D) HUVECs, rhVEGF (10 ng/ml), and isogenic IgG Ab (0.6 μg/ml; rhVEGF + IgG Ab); E) HUVECs, rhVEGF (10 ng/ml), and anti-VEGF neutralizing IgG Ab (0.6 μg/ml; rhVEGF + anti-VEGF Ab); F) HUVECs and 1 ml of supernatants from monocytes left unstimulated for 72 h (Non-stimulated sups); G) HUVECs and supernatants from 72-h M-CSF (100 ng/ml)-stimulated monocytes (1 ml; M-CSF sups); H) HUVECs, M-CSF-stimulated supernatants (1 ml), and isogenic IgG Ab (1 μg/ml; M-CSF sups + IgG); and I) HUVECs, M-CSF-stimulated supernatants (1 ml), and anti-VEGF neutralizing IgG Ab (0.6 μg/ml; M-CSF sups + anti-VEGF Ab). Pictures were taken after 20-h incubation. B, Networks of tube formation from HUVECs (1 × 105/condition) that were stimulated as described above were counted in a blinded manner by adding the sum of three different fields in the well for each condition and counting completely enclosed networks. Error bars represent the mean ± SEM calculated from three independent studies. Recombinant human VEGF with or without isogenic IgG control induced endothelial tube formation (*, p < 0.05 vs unstimulated HUVECs). Similarly, supernatants from monocytes stimulated with M-CSF induced endothelial cells to form tubes (***, p < 0.01 vs unstimulated HUVECs) that were reduced by anti-VEGF, but not by isogenic control Abs (p < 0.02).
Anti-VEGF Abs inhibit tube formation induced by M-CSF-stimulated monocyte supernatants
To make certain that VEGF was responsible for HUVEC tube formation when cultured in the supernatants of M-CSF-stimulated monocytes, neutralizing Abs to VEGF blocked tube formation in HUVEC cells induced by supernatants from M-CSF-stimulated monocytes, while isogenic IgG Abs did not (Fig. 4, A and B; p < 0.02 comparing HUVEC tube formation from M-CSF-stimulated monocyte supernatants with anti-VEGF IgG to M-CSF-stimulated monocyte supernatants with isogenic IgG control).
Discussion
To our knowledge this is the first report detailing that monocytes can promote angiogenesis via M-CSF-induced VEGF production. Our data suggest that M-CSF regulates VEGF at the level of transcription and enhances VEGF production by these monocytes. Of interest, the peak in VEGF protein levels was seen at 5 days of incubation in M-CSF, while the peak in VEGF mRNA was at 3 days of incubation. While the peak of VEGF protein production was on day 5, there was no significant difference between the VEGF produced in M-CSF-stimulated monocytes on day 4 vs day 5. However, to evaluate the possibility that the difference between peak VEGF mRNA and protein production was a reflection of paracrine factors released by the cells in the supernatants, we next performed depletion experiments on these 3-day supernatants from M-CSF-stimulated monocytes or supernatants from matched monocytes that were not stimulated during this same period. After depleting both M-CSF and VEGF from these supernatants and removing the Abs, we stimulated fresh monocytes. Importantly, there was no evidence for the production of paracrine factors in these supernatants that were responsible for VEGF production. Thus, it appeared that M-CSF directly induced VEGF production through transcriptional regulation.
It is interesting that despite adding a complicated biological supernatant to HUVEC cells, VEGF contained in these supernatants appeared to play a dominant role in promoting endothelial tube formation. This relative selectivity for VEGF rests in part in the cells that we chose to analyze. HUVEC cells lack CXCR2 receptors and thus will not respond to the angiogenic factor, IL-8 (27). Thus, it is possible that if we used alternate types of endothelial cells, we may have seen that other factors contained in these supernatants were also biologically active. In future studies it may be interesting to determine whether endothelial cells from different sized vessels respond to angiogenic factors produced by M-CSF-stimulated monocyte supernatants differently, giving a more global understanding of the roles of VEGF, M-CSF, and monocytes in regulating endothelial function.
VEGF is an important biological activator of angiogenesis. First coined vascular permeability factor, VEGF has a number of biological properties (5, 28–31). As its initial name suggests, VEGF enhances vascular permeability through effects on endothelial cells. In addition, VEGF is recognized as a potent proangiogenic growth factor and is an important target for novel cancer therapies (32, 33). The important role of VEGF in tumor growth is based on the discovery of significant tumor regression and the interruption of metastases in animals in which VEGF is neutralized (34, 35). Interestingly, as previously referenced, a recent article demonstrated that animals deficient in M-CSF were protected against metastases in a breast cancer model (11). Local expression of M-CSF in the primary tumor promoted monocyte and macrophage targeting in the primary breast cancer and enhanced metastases (11). Since M-CSF-deficient animals have fewer monocytes and macrophages than wild-type animals, we speculated that M-CSF and/or monocytes and macrophages were critical in enhancing tumor metastases in this model.
We considered other possible mechanisms that may be regulated by M-CSF to increase the susceptibility of tumors toward metastasis. These may include enhancement of protease production by these monocytes and macrophages, which could directly cleave and liberate cancer cells from the primary tumor. However, since angiogenesis is an important contributing factor to metastases, we focused on the role of M-CSF in stimulating monocytes/macrophages to promote angiogenesis. The finding that M-CSF and monocytes/macrophages are important in VEGF production and angiogenesis is interesting from a number of perspectives. While monocytes and macrophages are recognized as important in wound repair and chronic inflammation, the mechanisms of this function are unclear. Furthermore, M-CSF-deficient animals are protected from developing coronary artery disease (36), transplant-associated organ rejection (37), and inflammatory arthritis (38). This manuscript suggests that one contributing factor may be the production of VEGF, which may influence organ function as well as new blood vessel formation.
The production of VEGF by monocytes stimulated with M-CSF may extend past tissue repair, as the original function ascribed to VEGF was to increase vascular permeability (2). Thus, in tissue injury, monocytes and macrophages exposed to M-CSF may lead to enhanced vascular permeability, which may be important to promote the repair process. Understanding the regulation of VEGF production by monocytes and macrophages could lead to targeted strategies to modify these processes when they become dysregulated.
Although this paper presents a mechanism to explain the roles of M-CSF and monocyte/macrophages in angiogenesis and tumor metastases, we did not correlate this to in vivo studies as proof of concept. However, in vivo studies have found that animals deficient in M-CSF have fewer breast cancer metastases than littermate wild-type mice (11). This correlates with clinical observations in patients with breast cancer, in whom the presence of M-CSF or M-CSF receptors in the tumor predicts a poorer outcome (39). While the exact mechanism of this effect or whether VEGF is responsible is not known, we speculate that M-CSF in the tumor promotes angiogenesis and may be at least part of the explanation.
In summary, this paper describes a mechanism by which M-CSF promotes VEGF production and angiogenic activity by normal human monocytes. Neutralizing VEGF in these supernatants blocks endothelial tube formation of HUVECs cultured in these stimulated supernatants, providing an explanation for the observed effects.
Acknowledgments
This work was supported by National Institutes of Health Grants RO1HL63800, RO1HL6108, RO1HL67176, and PO1 HL70294; the American Lung Association Johnie Walker Murphy Career Investigator Award; and The Kelly Clark Fund.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Abbreviations used in this paper: VEGF, vascular endothelial growth factor; HSA, human serum albumin; rh, recombinant human; VEGFR, VEGF receptor.
References
- 1.Dvorak HF, Sioussat TM, Brown LF, Berse B, Nagy JA, Sotrel A, Manseau EJ, Van De WL, and Senger DR. 1991. Distribution of vascular permeability factor (vascular endothelial growth factor) in tumors: concentration in tumor blood vessels. J. Exp. Med 174:1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dvorak HF 2000. VPF/VEGF and the angiogenic response. Semin. Perinatol 24:75. [DOI] [PubMed] [Google Scholar]
- 3.Hamada K, Oike Y, Takakura N, Ito Y, Jussila L, Dumont DJ, Alitalo K, and Suda T. 2000. VEGF-C signaling pathways through VEGFR-2 and VEGFR-3 in vasculoangiogenesis and hematopoiesis. Blood 96:3793. [PubMed] [Google Scholar]
- 4.Romano DP, Mangoni A, Zambruno G, Spinetti G, Melillo G, Napolitano M, and Capogrossi MC. 2002. Adenovirus-mediated VEGF(165) gene transfer enhances wound healing by promoting angiogenesis in CD1 diabetic mice. Gene Ther. 9:1271. [DOI] [PubMed] [Google Scholar]
- 5.Senger DR, Van De WL, Brown LF, Nagy JA, Yeo KT, Yeo TK, Berse B, Jackman RW, Dvorak AM, and Dvorak HF. 1993. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev. 12:303. [DOI] [PubMed] [Google Scholar]
- 6.Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, Hong K, Marsters JC, and Ferrara N. 2002. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature 417:954. [DOI] [PubMed] [Google Scholar]
- 7.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, and Folkman J. 1997. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88:277. [DOI] [PubMed] [Google Scholar]
- 8.O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, and Folkman J. 1994. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79:315. [DOI] [PubMed] [Google Scholar]
- 9.Perletti G, Concari P, Giardini R, Marras E, Piccinini F, Folkman J, and Chen L. 2000. Antitumor activity of endostatin against carcinogen-induced rat primary mammary tumors. Cancer Res. 60:1793. [PubMed] [Google Scholar]
- 10.Cao Y, O’Reilly MS, Marshall B, Flynn E, Ji RW, and Folkman J. 1998. Expression of angiostatin cDNA in a murine fibrosarcoma suppresses primary tumor growth and produces long-term dormancy of metastases. J. Clin. Invest 101:1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin EY, Nguyen AV, Russell RG, and Pollard JW. 2001. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J. Exp. Med 193:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aharinejad S, Abraham D, Paulus P, Abri H, Hofmann M, Grossschmidt K, Schafer R, Stanley ER, and Hofbauer R. 2002. Colony-stimulating factor-1 antisense treatment suppresses growth of human tumor xenografts in mice. Cancer Res. 62:5317. [PubMed] [Google Scholar]
- 13.Achen MG, and Stacker SA. 1998. The vascular endothelial growth factor family; proteins which guide the development of the vasculature. Int. J. Exp. Pathol 79:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawighorst T, Velasco P, Streit M, Hong YK, Kyriakides TR, Brown LF, Bornstein P, and Detmar M. 2001. Thrombospondin-2 plays a protective role in multistep carcinogenesis: a novel host anti-tumor defense mechanism. EMBO J. 20:2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiraoka N, Allen E, Apel IJ, Gyetko MR, and Weiss SJ. 1998. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell 95:365. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto T, and Claesson-Welsh L. 2001. VEGF receptor signal transduction. Sci. STKE 2001:RE21. [DOI] [PubMed] [Google Scholar]
- 17.Hudlicka O, Milkiewicz M, Cotter MA, and Brown MD. 2002. Hypoxia and expression of VEGF-A protein in relation to capillary growth in electrically stimulated rat and rabbit skeletal muscles. Exp. Physiol 87:373. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N 1999. Vascular endothelial growth factor: molecular and biological aspects. Curr. Top. Microbiol. Immunol 237:1. [DOI] [PubMed] [Google Scholar]
- 19.Jakeman LB, Winer J, Bennett GL, Altar CA, and Ferrara N. 1992. Binding sites for vascular endothelial growth factor are localized on endothelial cells in adult rat tissues. J. Clin. Invest 89:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jakeman LB, Armanini M, Phillips HS, and Ferrara N. 1993. Developmental expression of binding sites and messenger ribonucleic acid for vascular endothelial growth factor suggests a role for this protein in vasculogenesis and angiogenesis. Endocrinology 133:848. [DOI] [PubMed] [Google Scholar]
- 21.Kliche S, and Waltenberger J. 2001. VEGF receptor signaling and endothelial function. IUBMB Life 52:61. [DOI] [PubMed] [Google Scholar]
- 22.Shibuya M 2001. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct. Funct 26:25. [DOI] [PubMed] [Google Scholar]
- 23.Sato Y, Kanno S, Oda N, Abe M, Ito M, Shitara K, and Shibuya M. 2000. Properties of two VEGF receptors, Flt-1 and KDR, in signal transduction. Ann. NY Acad. Sci 902:201. [DOI] [PubMed] [Google Scholar]
- 24.Ortega N, Hutchings H, and Plouet J. 1999. Signal relays in the VEGF system. Front. Biosci 4:D141. [DOI] [PubMed] [Google Scholar]
- 25.Marsh CB, Pomerantz RJ, Parker JM, Winnard AV, Mazzaferri ELJ, Moldovan N, Kelley TW, Beck E and Wewers MD. 1999. Regulation of monocyte survival in vitro by deposited IgG: role of MCSF. J. Immunol 162:6217. [PubMed] [Google Scholar]
- 26.Feldman AL, Alexander HR Jr., Yang JC, Linehan WM, Eyler RA, Miller MS, Steinberg SM, and Libutti SK. 2002. Prospective analysis of circulating endostatin levels in patients with renal cell carcinoma. Cancer 95:1637. [DOI] [PubMed] [Google Scholar]
- 27.Salcedo R, Resau JH, Halverson D, Hudson EA, Dambach M, Powell D, Wasserman K, and Oppenheim JJ. 2000. Differential expression and responsiveness of chemokine receptors (CXCR1–3) by human microvascular endothelial cells and umbilical vein endothelial cells. FASEB J. 14:2055. [DOI] [PubMed] [Google Scholar]
- 28.Zelzer E, Glotzer DJ, Hartmann C, Thomas D, Fukai N, Soker S, and Olsen BR. 2001. Tissue specific regulation of VEGF expression during bone development requires Cbfa1/Runx2. Mech. Dev 106:97. [DOI] [PubMed] [Google Scholar]
- 29.Clauss M 2000. Molecular biology of the VEGF and the VEGF receptor family. Semin. Thromb. Hemost 26:561. [DOI] [PubMed] [Google Scholar]
- 30.Ferrara N, and Keyt B. 1997. Vascular endothelial growth factor: basic biology and clinical implications. EXS 79:209. [DOI] [PubMed] [Google Scholar]
- 31.Ferrara N, and Davis-Smyth T. 1997. The biology of vascular endothelial growth factor. Endocr. Rev 18:4. [DOI] [PubMed] [Google Scholar]
- 32.Martiny-Baron G, and Marme D. 1995. VEGF-mediated tumour angiogenesis: a new target for cancer therapy. Curr. Opin. Biotechnol 6:675. [DOI] [PubMed] [Google Scholar]
- 33.Ferrara N 2002. Timeline: VEGF and the quest for tumour angiogenesis factors. Nat. Rev. Cancer 2:795. [DOI] [PubMed] [Google Scholar]
- 34.Verheul HM, and Pinedo HM. 2000. The role of vascular endothelial growth factor (VEGF) in tumor angiogenesis and early clinical development of VEGF-receptor kinase inhibitors. Clin. Breast Cancer 1(Suppl. 1):S80. [DOI] [PubMed] [Google Scholar]
- 35.Im SA, Kim JS, Gomez-Manzano C, Fueyo J, Liu TJ, Cho MS, Seong CM, Lee SN, Hong YK, and Yung WK. 2001. Inhibition of breast cancer growth in vivo by antiangiogenesis gene therapy with adenovirus-mediated antisense-VEGF. Br. J. Cancer 84:1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, and Miyata M. 1995. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc. Natl. Acad. Sci. USA 92:8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi CW, Lee WS, He Q, Zhang D, Fletcher DL Jr., Newell JB, and Haber E. 1996. Immunologic basis of transplant-associated arteriosclerosis. Proc. Natl. Acad. Sci. USA 93:4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang YH, and Hamilton JA. 2001. Dependence of interleukin-1-induced arthritis on granulocyte-macrophage colony-stimulating factor. Arthritis Rheum. 44:111. [DOI] [PubMed] [Google Scholar]
- 39.Sapi E, and Kacinski BM. 1999. The role of CSF-1 in normal and neoplastic breast physiology. Proc. Soc. Exp. Biol. Med 220:1. [DOI] [PubMed] [Google Scholar]