Abstract
Background
Organophosphate esters (OPEs) are widely detected among U.S. pregnant women. OPEs, some of which are present in nail polish, have been associated with adverse reproductive health outcomes. More research is needed to investigate associations with OPEs and personal care products (PCP) use.
Methods
Pregnant women (18–40 years) were recruited from two hospitals and five prenatal clinics in Northern Puerto Rico (n=148 women) between 2011 and 2015. Concentrations of bis(2-chloroethyl) phosphate (BCEtP), bis(1-chloro-2-propyl) phosphate (BCPP), bis(1,3-dichloro-2-propyl) phosphate (BDCPP), di-n-butyl phosphate (DNBP), di-benzyl phosphate (DBzP), di-cresyl phosphate (DCP), DPHP, and 2,3,4,5-tetrabromobenzoic acid (TBBA) were measured twice during pregnancy. Participants completed questionnaires on PCP and household products (HP) use. Associations among products and metabolite concentrations (n=296 observations) were assessed using linear mixed models.
Results
BCEtP, BCPP, BDCPP and DPHP were detected frequently (≥77%). Correlations among metabolites (0.16≤r≤0.35) and Intraclass correlation coefficients (ICCs) (0.03≤ICC≤0.34) were weak-to-moderate. Suntan lotion was associated with a 110% increase in BDCPP. DPHP increased with perfume (51%) and nail polish (49%) use. BCPP increased 46% with pesticide use in home.
Conclusion
Biomarkers of OPEs were often detected among pregnant women. Associations with PCP and HP use suggest OPEs may be used in such products, specifically in perfume and nail polish. Further investigation into these products is warranted.
Keywords: organophosphate esters, exposure, diphenyl phosphate, personal care products, household products
Graphical Abstract
Introduction
Organophosphate esters (OPEs) are commonly used in consumer products including: furniture foams, baby products, cars, electronics, and carpeting (Castorina et al., 2017; Hoffman et al., 2015a; Kademoglou et al., 2016; Stapleton et al., 2009; Sugeng et al., 2018). Although OPEs have been used as flame retardants (FRs) for over a century, their prevalence has risen drastically over the past decade as polybrominated diphenyl ethers have been phased out of production because of their toxicity (ATSDR, 2015; van der Veen and De Boer, 2012). U.S. production of certain OPEs is projected to reach 50,000 tons by 2020 (Schreder et al., 2016). Many OPEs are used to comply with flammability standards, yet some non-chlorinated alkyl and aryl phosphates are also used as plasticizers and antifoaming agents (Marklund et al., 2003; Mendelsohn et al., 2016; Sühring et al., 2016). Exposure to OPEs has been associated with disrupting endocrine, immune, developmental, metabolic, and reproductive systems (Canbaz et al., 2017; Carignan et al., 2017; He et al., 2018; Lipscomb et al., 2017; Meeker and Stapleton, 2009). Tris(2-chloroethyl) phosphate (TCEP) and tris(1,3-dichloroisopropyl) phosphate (TDCIPP) are also known carcinogens (State of California Environmental Protection Agency Office of Environmental Health Hazard Assessment, 2017).
Physically mixed or added during manufacturing, OPEs are classified as ‘additive’ chemicals (Hoffman et al., 2015b; Marklund et al., 2003). The relatively weak bonds of these semi-volatile compounds allow for leaching, abrasion, or volatilization into indoor environments and exposure via ingestion, inhalation, or absorption (Cequier et al., 2015; He et al., 2018; Stapleton et al., 2008). However, exposure to OPEs is dependent upon their respective physicochemical properties and specific application. TCEP and TDCIPP are used as flame retardants, plasticizers, and in lacquers, paints and glues, whereas triphenyl phosphate (TPHP) is also used as hydraulic fluid (He et al., 2018; Wei et al., 2015). While dust ingestion is likely a main route of exposure, recent studies using air samplers, silicone wrist bands, and hand wipes suggest inhalation and dermal absorption routes may also contribute to internal exposure (Hammel et al., 2016; Meeker and Stapleton, 2009; Schreder et al., 2016; Sühring et al., 2016).
Despite the relatively short half-lives of OPEs that range from a few hours to days, metabolites have been detected in nearly 100% of urine samples of adults and children in the United States, Europe, and Australia (Cequier et al., 2015; He et al., 2018; Hoffman et al., 2017a; Ospina et al., 2018), while slightly lower in China (Sun et al., 2018). While many OPEs have been detected in the dusts of homes, cars, and offices (Ali et al., 2012; Brommer and Harrad, 2015; Kademoglou et al., 2016; Meeker and Stapleton, 2009) studies have found OPEs in dust to be weakly correlated to their respective metabolites in urine, thus suggesting alternate pathways of exposure (Castorina et al., 2017; Dodson et al., 2014; Hoffman et al., 2015b; Phillips et al., 2018). OPEs have also been detected in foodstuffs and suggest that meat and dairy contribute to OPE internal exposure (Wang and Kannan, 2018). Many studies to date have focused on the application of OPEs in polyurethane foams in furniture, baby products, and electronics; yet, TCEP, tris(2-chloroisopropyl) phosphate (TCIPP), TDCPP, and TPHP are also used as plasticizers. Plasticizers are used for numerous reasons in consumer products. They increase flexibility and durability of personal care products (PCP) like nail polish, yet are also added to lotions and soaps as surfactants to enhance absorption to skin. OPEs are also used in PCPs and household products (HP). However, few studies have assessed the relationship between these products and OPEs. TPHP has been detected in nail polish and concentrations of its metabolite, diphenyl phosphate (DPHP) have increased after application (Mendelsohn et al., 2016). A previous study found increased associations with DPHP and 2-isopropylphenyl phenyl phosphate (ip-PPP) with self-reported use of PCPs including nail polish, face moisturizer, colored cosmetics and deodorant among women seeking fertility treatment in Boston (Ingle et al., 2019). In this work, we set out to characterize self-reported PCP and HP use within the 48 hours prior to collection of a urine sample where we measured the concentration of eight FR metabolites from commonly used OPEs: bis(2-chloroethyl) phosphate (BCEtP), bis(1-chloro-2-propyl) phosphate (BCPP), bis(1,3-dichloro-2-propyl) phosphate (BDCPP), di-n-butyl phosphate (DNBP), di-benzyl phosphate (DBzP), di-cresyl phosphate (DCP), DPHP, and 2,3,4,5-tetrabromobenzoic acid (TBBA) among pregnant women in the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) study.
Methods
Participant Recruitment
Participant recruitment for the PROTECT cohort has been reported previously (Cantonwine et al., 2014; Meeker et al., 2013a). Briefly, pregnant women of 18–40 years were recruited between 2011–2015 from two hospitals and five prenatal clinics in Northern Puerto Rico. Women must have resided in the Northern karst region of Puerto Rico, abstained from using oral contraceptives three months prior to pregnancy or used in vitro fertilization as a method of assisted reproductive technology to achieve pregnancy, and no known medical or obstetric conditions. Research protocols were approved by the ethics and Research Committees of the University of Puerto Rico, participating clinics, the University of Michigan School of Public Health, and Northwestern University. The role of the Centers for Disease Control and Prevention (CDC) did not constitute engagement with human subject research. The study was described in detail to all participants and informed consent was obtained from all participants.
Personal Care Products (PCP) and Household Products (HP) Questionnaires
At the first and third visits (approximately 18 and 26 weeks gestation, respectively), women competed a questionnaire on PCP (n=14 products) and HP (n=20 products) use within the 48 hours prior to urine sample collection. Products with n<5 participants reporting use for both visits were excluded from the analysis.
Urine Collection and Quantification of Flame Retardant Biomarkers
Participants provided two spot urine samples at approximately 18, and 26 weeks gestation in polypropylene containers. Specific Gravity (SG) was measured at the University of Puerto Rico Medical Sciences campus using a hand-held digital refractometer (Atago Co., Ltd., Tokyo, Japan). Samples were separated into aliquots, frozen at −80°C, and shipped overnight to the CDC, Atlanta, Georgia. Urine samples were analyzed at the CDC’s National Center for Environmental Health for eight FR metabolites: BCEtP, BCPP, BDCPP, DNBP, DBzP, DCP, DPHP, and TBBA. The analytical approach is described elsewhere (Jayatilaka et al., 2017; Ospina et al., 2018). Briefly, metabolites were extracted, isolated, and quantified using automated off-line solid phase extraction, reversed phase high-performance liquid chromatography, and isotope dilution-electrospray ionization tandem mass spectrometry, respectively. Accuracy and precision were monitored using reagent blanks, and quality control materials of low (4 μg/L) and high (15 μg/L) concentrations (Jayatilaka et al., 2017). The limits of detection (LOD) were 0.05 μg/L (DBzP and TBBA), 0.50 μg/L (DCP), and 0.10 μg/L (all other metabolites). FR concentrations below LOD were imputed a value equal to the LOD divided by the square root of 2.
Statistical Analysis
Descriptive statistics for the demographic characteristics of the women were calculated. Because of the high percentage of samples with TBBA below LOD for both visits (n<1% detected, data not shown), TBBA was excluded from further analysis. Geometric means (GM) and percentiles were calculated for the OPE metabolites. Metabolite concentrations were presented as uncorrected and corrected for SG as: CSG = C*[(SGM-1)/(SGi −1)], where CSG = SG-corrected urinary metabolite concentration, C = urinary metabolite concentration, SGM = mean SG for the population, and SGi = SG for an individual sample (Boeniger et al., 1993). Spearman correlation coefficients among metabolites as well as between visits one and three were calculated (Tables S1–S2). Intraclass correlation coefficients (ICC) and 95% confidence intervals for metabolites (uncorrected and SG corrected) were calculated to assess variability within and between women. All metabolites presented as right-skewed and were transformed by the natural logarithm for further statistical testing. Covariates for models were selected a priori and from bivariate testing (data not shown) (Hoffman et al., 2017b; Ospina et al., 2018). Final covariates for modeling were SG, age, body mass index (BMI), income in U.S. $ (≤ 19,999/ 20,000–39,999/ ≥40,000), and season (winter/spring/summer/fall). Missing covariates for BMI and income were imputed with the median for continuous variables (BMI=26.47; n=1) and the category with the highest frequency (income ≤ 19,999; n=1). Linear mixed models (LMM) were used to evaluate associations with PCP and HP use (independent-variable) and OPE metabolites concentrations (dependent variable) (Tables S3–S4). Heat maps were created from LMM results to reflect the adjusted percent change in OPE metabolite concentrations with self-reported PCP and HP use in the 48 hrs. leading up to urine sample collection [statistical significance (p<0.05) is indicated by an asterisks on maps]. Statistical analyses were carried out using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Demographic characteristics of our sample of pregnant women (n=148) can be found in Table 1. This subset of women from the PROTECT cohort are demographically similar to previous reports from the cohort (Aker et al., 2016; Cantonwine et al., 2014). Women from our sample identified as white (55.8%), in their mid-to-late 20s (Mean=27 years), and non-smokers (ever smoker n=26). Most women had either some college or a two-year degree (34.7%), while over half reported a household income less than or equal to $19,999. The majority of urine samples from both visit one and three were collected in the fall (visit 1=36.5%, visit 3=29.7%), while the fewest samples were collected in the spring months (visit 1=16.9%, visit 3= 15.5%).
Table 1.
Demographic characteristics among 148 women from the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) cohort (2011–2015)
| Characteristic | Median or n | (IQR or %) |
|---|---|---|
| Age | 27.0 | 23, 31 |
| BMI a | 27.6 | 23.8, 29.8 |
| Race | ||
| White | 82 | 55.8 |
| Mestiza | 58 | 39.5 |
| Other | 11 | 7.5 |
| Smoking Status | ||
| Ever smokerb | 26 | 18.8 |
| Education a | ||
| ≤ High school diploma or GED | 32 | 21.8 |
| Some college/ 2 yr. degree | 51 | 34.7 |
| Bachelor degree | 42 | 28.6 |
| Graduate or doctoral degree | 22 | 15.0 |
| Household Income c | ||
| ≤ 19,999 | 52 | 41.9 |
| 20,000–39,999 | 41 | 33.1 |
| ≥ 40,000 | 31 | 25.0 |
| Season of sample Visit 1 | ||
| Winter | 28 | 18.9 |
| Spring | 25 | 16.9 |
| Summer | 41 | 27.7 |
| Fall | 54 | 36.5 |
| Visit 3 | ||
| Winter | 44 | 29.7 |
| Spring | 23 | 15.5 |
| Summer | 37 | 25.0 |
| Fall | 44 | 29.7 |
IQR: Interquartile range; More than one possible choice for race
n= 1 missing
n=10 missing
n=24 missing
Season: Winter: Dec.-Feb., Spring: March-May, Summer: June-Aug., Fall: Sept-Nov
BCEtP, BCPP, BDCPP, and DPHP were detected frequently (77–99% samples had concentrations above LOD) for both visits (Table 2). Concentrations of metabolites were similar for both visits, yet GMs were higher in visit one for all metabolites except BCEtP, which had a higher GM concentration for visit two, and DNBP which had similar concentrations for both visits. All concentrations of DBzP were below LOD as well as third visits concentrations of DCP. All metabolites except for DCP were correlated, yet associations were weak to moderate (0.16≤r≤0.35; p≤0.01) (Table S1). Correlations between visit one and three were moderate for BCEtP, BCPP, BDCPP, DNBP, and DPHP (0.20≤r≤0.43; p≤0.01) (Table S2). First visit concentrations of DNBP were also associated with third visit DPHP concentrations (r=0.20, p=0.01). ICCs for metabolite concentrations were primarily weak (0.03≤ICC≤0.35) (Table 3).
Table 2.
Distribution of organophosphate ester (OPE) metabolites (μ/L) among 148 women from the PROTECT cohort (n= 296 samples)
| Uncorrected | n > LOD (%) | GM | (95% CI) | Percentiles | |||||
|---|---|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | 90th | 95th | Max | ||||
| Visit 1 | |||||||||
| BCEtP | 143 (96.6) | 0.88 | (0.74, 1.05) | 0.43 | 0.84 | 1.78 | 3.70 | 5.64 | 17.70 |
| BCPP | 117 (79.1) | 0.26 | (0.22, 0.31) | 0.12 | 0.26 | 0.50 | 0.98 | 1.66 | 4.35 |
| BDCPP | 140 (94.6) | 1.24 | (1.01, 1.52) | 0.71 | 1.41 | 2.60 | 5.29 | 8.25 | 20.50 |
| DCP | 2 (1.4) | <LOD | (<LOD, <LOD) | <LOD | <LOD | <LOD | <LOD | <LOD | 0.92 |
| DNBP | 48 (32.4) | 0.11 | (0.10, 0.12) | <LOD | <LOD | 0.14 | 0.37 | 0.54 | 3.46 |
| DPHP | 146 (98.7) | 1.65 | (1.37, 2.00) | 0.80 | 1.55 | 3.46 | 7.20 | 12.30 | 90.20 |
| SG Adjusted a | |||||||||
| BCEtP | 144 (98.0) | 0.96 | (0.82, 1.13) | 0.52 | 0.92 | 1.91 | 3.26 | 5.64 | 11.42 |
| BCPP | 120 (81.6) | 0.28 | (0.24, 0.33) | 0.14 | 0.28 | 0.56 | 1.02 | 1.51 | 5.80 |
| BDCPP | 142 (96.6) | 1.34 | (1.10, 1.61) | 0.76 | 1.49 | 2.73 | 5.49 | 7.48 | 21.63 |
| DCP | 28 (19.1) | <LOD | (<LOD, <LOD) | <LOD | <LOD | <LOD | <LOD | <LOD | 1.41 |
| DNBP | 63 (42.9) | 0.12 | (0.10, 0.13) | <LOD | <LOD | 0.18 | 0.38 | 0.72 | 4.61 |
| DPHP | 146 (99.3) | 1.78 | (1.49, 2.13) | 0.83 | 1.66 | 2.81 | 7.93 | 12.60 | 90.20 |
| SG | - | 1.02 | (1.02, 1.02) | 1.02 | 1.02 | 1.02 | 1.03 | 1.03 | 1.03 |
| Uncorrected | |||||||||
| Visit 3 | |||||||||
| BCEtP | 145 (98.0) | 0.91 | (0.75, 1.09) | 0.40 | 0.89 | 1.83 | 3.51 | 7.16 | 65.20 |
| BCPP | 114 (77.0) | 0.24 | (0.21, 0.28) | 0.12 | 0.26 | 0.44 | 0.90 | 1.23 | 3.57 |
| BDCPP | 146 (98.7) | 1.11 | (0.94, 1.33) | 0.47 | 1.22 | 2.26 | 4.65 | 8.19 | 20.10 |
| DNBP | 45 (30.4) | 0.11 | (0.09, 0.12) | <LOD | <LOD | 0.12 | 0.27 | 0.52 | 6.44 |
| DPHP | 147 (99.3) | 1.56 | (1.30, 1.88) | 0.82 | 1.34 | 2.98 | 6.28 | 13.10 | 39.40 |
| SG Adjusted a | |||||||||
| BCEtP | 146 (99.3) | 0.98 | (0.81, 1.18) | 0.45 | 0.85 | 2.12 | 4.76 | 7.82 | 86.93 |
| BCPP | 118 (80.3) | 0.26 | (0.22, 0.31) | 0.12 | 0.27 | 0.50 | 0.94 | 1.35 | 3.48 |
| BDCPP | 145 (98.6) | 1.18 | (0.99, 1.41) | 0.54 | 1.18 | 2.59 | 4.86 | 7.25 | 17.50 |
| DNBP | 61 (41.5) | 0.11 | (0.10, 0.13) | <LOD | <LOD | 0.14 | 0.39 | 0.66 | 6.20 |
| DPHP | 146 (99.3) | 1.67 | (1.39, 2.00) | 0.79 | 1.48 | 3.26 | 7.28 | 11.90 | 35.82 |
| SG | - | 1.02 | (1.02, 1.02) | 1.02 | 1.02 | 1.02 | 1.03 | 1.03 | 1.03 |
LOD: Limit of detection; GM: Geometric mean; LOD=0.10 (ng/mL) for all metabolites except DCP: LOD=0.50 ng/mL; SG: Specific gravity
n=147; DBzP (visit 1 & 3), DCP (visit 3): All concentrations <LOD
Table 3.
Intraclass correlation coefficients (95% CI) for uncorrected and SG corrected repeated urinary OPE metabolites
| All samples |
||||
|---|---|---|---|---|
| Metabolite | Uncorrected a | SG Adjusted b | ||
| BCEtP | 0.03 | (0.00, 0.86) | 0.03 | (0.00, 0.86) |
| BCPP | 0.27 | (0.14, 0.44) | 0.34 | (0.21, 0.49) |
| BDCPP | 0.26 | (0.14, 0.44) | 0.23 | (0.11, 0.41) |
| DPHP | 0.34 | (0.22, 0.49) | 0.25 | (0.13, 0.43) |
n=148 women, n=296 urine samples
n=147 women, n=294 samples
Self-reported PCP use within 48 hr. of urine collection can be found in Table 4. Deodorant, bar soap, and perfume were the most frequently used products for visit one (84–100%), while liquid soap replaced perfume use for the most frequently used products for visit three (93–100%). However, at least 75% of women also reported using cosmetics and lotion. Shaving cream and suntan lotion were less frequently used among women (≤8%).
Table 4.
Reported personal care product use within 48 hrs. of urine collection among 148 women from the PROTECT cohort
| Personal care product | Visit 1 | Visit 3 | ||
|---|---|---|---|---|
| Yes (n, %) | No (n, %) | Yes (n, %) | No (n, %) | |
| Deodorant | (148, 100) | (0, 0) | (148, 100) | (0, 0) |
| Bar soap | (134, 90.5) | (14, 9.5) | (137, 92.6) | (11,7.4) |
| Perfume | (124, 83.8) | (24, 16.2) | (125, 84.5) | (23, 15.5) |
| Liquid soap | (119, 80.4) | (28, 18.9) | (140, 94.6) | (8, 5.4) |
| Cosmetics | (111,75.0) | (37, 25.0) | (114, 77.0) | (34, 23.0) |
| Lotion | (111,75.0) | (37, 25.0) | (122, 82.4) | (26, 17.8) |
| Shampoo | (98, 66.2) | (50, 35.1) | (110, 74.3) | (38, 25.7) |
| Conditioner | (96, 64.9) | (52, 35.1) | (106, 71.6) | (42, 28.4) |
| Mouthwash | (68, 46.0) | (80, 54.1) | (85, 57.4) | (63, 42.6) |
| Nail polish | (47, 31.8) | (101,68.2) | (38, 25.7) | (110, 74.3) |
| Hair spray | (41,27.7) | (107, 72.3) | (58, 39.2) | (90, 60.8) |
| Other hair products a | (35, 25.2) | (104, 74.8) | (1, 100) | (0, 0) |
| Shaving cream | (12, 8.1) | (136, 91.9) | (13, 8.8) | (135, 91.22) |
| Suntan lotion | (5, 3.4) | (143, 96.6) | (6, 4.1) | (142, 96.0) |
Other hair products include mousse, hair bleach & relaxers; n: number of women who reported using product; n=9 missing responses (visit 1); n=147 missing responses (visit 3)
Self-reported HP use was less frequent compared to PCP (Table 5). However, over half of women reported using a vinyl shower curtain, cleaners, detergents, and storing pesticides inside the home. Few women (<3%) reported using paint or having their homes treated by an exterminator for visit one, yet for visit three, fewer women used furniture polish and pet grooming products (≤2%).
Table 5.
Reported household product use within 48 hrs. of urine collection among 148 women from the PROTECT cohort
| Household product | Visit 1 | Visit 3 | ||
|---|---|---|---|---|
| Yes (n, %) | No (n, %) | Yes (n, %) | No (n, %) | |
| Used bath with vinyl curtain | (98, 66.2) | (50, 33.8) | (98, 66.2) | (50, 33.8) |
| Store pesticides inside home | (89, 60.1) | (59, 39.9) | (90, 60.8) | (57, 38.5) |
| Cleaners | (79, 53.4) | (69, 46.6) | (93, 62.8) | (55, 37.2) |
| Detergent | (78, 52.7) | (70, 47.3) | (84, 56.7) | (64, 43.2) |
| Fabric softener | (66, 44.6) | (82, 55.4) | (71,45.0) | (77, 52.0) |
| Applied pesticides inside home | (24, 16.2) | (124, 83.8) | (18, 12.2) | (130, 87.8) |
| Vinyl gloves | (13, 8.8) | (135, 91.2) | (10, 6.8) | (138, 93.2) |
| Used pesticides inside home | (8, 5.4) | (140, 94.6) | (26, 17.6) | (120, 81.1) |
| Applied pesticides outdoors | (6, 4.1) | (142, 96.0) | (7, 4.7) | (141, 95.3) |
| Furniture polish | (6, 4.1) | (142, 96.0) | (3, 2.0) | (145, 9.0) |
| Pet grooming | (6, 4.1) | (142, 96.0) | (2, 1.4) | (146, 98.7) |
| Paint | (4, 2.7) | (144, 97.3) | (10, 6.8) | (138, 93.2) |
| Home treated by exterminator | (3, 2.0) | (145, 98.0) | (9, 6.1) | (139, 93.9) |
Products with n<5 reporting 'yes' for both visits are not listed; n: number of women who reported using product
Women who 'used pesticides in the home' did not necessarily apply them compared to women who 'applied pesticides'
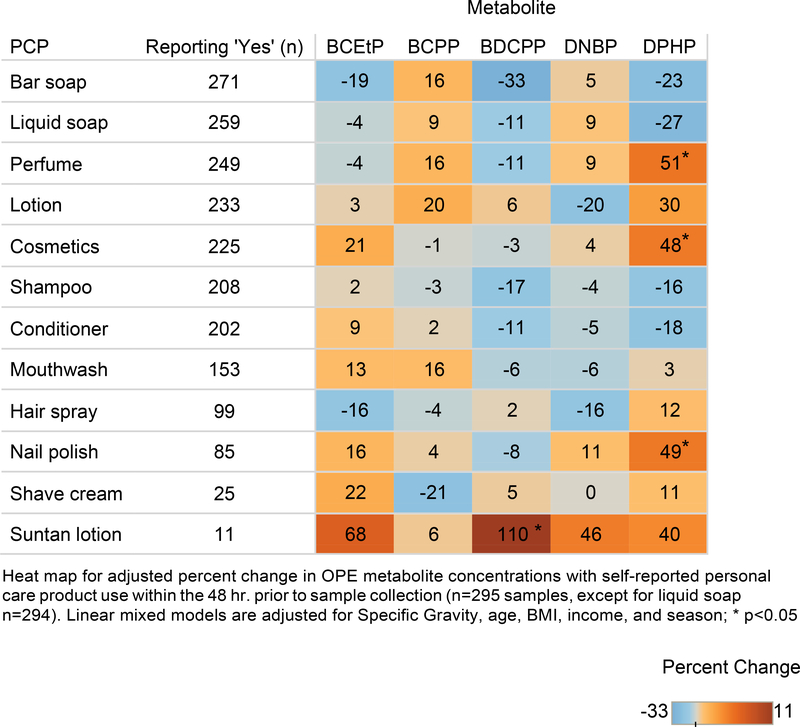
A heat map depicting the adjusted percent change in OPE urinary concentrations with reported PCP use can be found in Figure 1. Perfume use was associated with a 51% increase in DPHP concentrations (p=0.04). DPHP concentrations were also positively associated with nail polish use (49%, p=0.01). Cosmetics use was associated with a 48% increase in DPHP concentrations (p=0.02). However, the strongest association identified was a 110% increase in BDCPP concentrations with reported suntan lotion use (p=0.04). Suntan lotion use was also associated with elevated BCEtP (68%), DNBP (46%), and DPHP (40%) concentrations, although none of these were statistically significant (0.15≤p≤0.35).
Figure 1.
Adjusted percent change in urinary OPE concentrations with self-reported personal care product use for 148 women in the PROTECT cohort
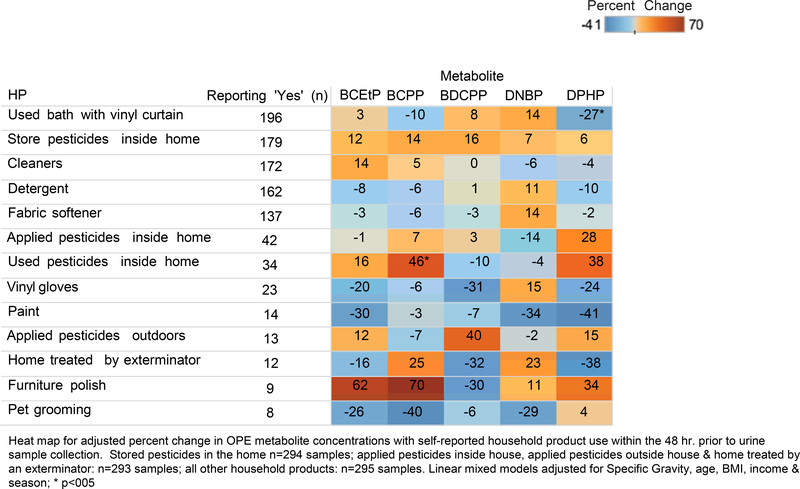
Figure 2 depicts the associations for adjusted percent change in OPE urine concentrations with self-reported HP use. The largest percent change involved furniture polish use in association with BCPP (70%) and BCEtP (62%), though not significant (p=0.08, p=0.19, respectively). However, elevated BCPP concentrations (46%) were associated with using pesticides in the home (p=0.04). Increased DPHP (38%; p=0.16) concentrations were associated with pesticides used in the home while BDCPP concentrations increased (40%; p=0.26) with pesticides applied outside the home. Several HP were associated with a decrease in OPE concentrations. Reported use of a vinyl shower curtain was associated with a 27% decrease in DPHP concentrations (p=0.02). Reported use of paint and pet grooming products were also associated with a decrease in DPHP (41%; p=0.08) and BCPP (40%; p=0.90), respectively.
Figure 2.
Adjusted percent change in urinary OPE metabolite concentrations with self-reported household product use among 148 women from the PROTECT cohort
Discussion
BCEtP, BCPP, BDCPP, and DPHP were frequently detected for both visits (77%≥LOD). Similar detection frequencies for these metabolites have also been observed in other pregnant cohorts in North Carolina (NC), California, Rhode Island, and Canada (Castorina et al., 2017; Hoffman et al., 2017b; Kosarac et al., 2016; Romano et al., 2017), as well as females from a fertility clinic in Boston (Carignan et al., 2017), and in adults (20–59 years) from the National Health and Nutritional Examination Survey (NHANES) (Ospina et al., 2018). Similar to NHANES, we also observed relatively low detection for DCP (1%) and no detectable concentrations for DBzP and TBBA. Overall, OPE metabolite concentrations in our sample were higher than previous studies. Distributions of BCEtP were nearly twice as high compared to NHANES (GM=0.39 μg/L) while concentrations were below the limit of quantification for a Canadian sample (Kosarac et al., 2016; Ospina et al., 2018). However, BCPP GMs in the present study were similar to NHANES (GM=0.19 μg/L), while median concentrations were almost three times higher (0.70 ng/mL) among pregnant women in NC. Concentrations of BDCPP were also lower in our sample (GM=1.24 ng/mL) compared to NC (GM=1.80 ng/mL), yet higher than pregnant women in Canada (GM=0.27 ng/mL) and California (GM=0.28 ng/mL), as well as women in Boston (GM=0.66 μg/L) and NHANES (GM=0.82 μg/L). DCP and DNBP concentrations were lower compared to other OPE metabolites in our sample (GM=<LOD and 0.11 ng/mL, respectively) and were also lower compared to other studies (Kosarac et al., 2016; Ospina et al., 2018). The highest OPE concentrations in our sample were for DPHP (GM=1.56–1.65 ng/mL, depending on the visit) and concentrations were similar to those in NC (GM=1.40 ng/mL) (Hoffman et al., 2017b), yet were more than double those from other previous cohorts of women (GM=0.76–1.4 ng/mL) (Carignan et al., 2017; Castorina et al., 2017; Ospina et al., 2018). However, a small sample (n=24) of Canadian women did report higher urine concentrations of DPHP (GM=2.88 ng/mL) (Kosarac et al., 2016).
Correlations among OPE metabolites in our sample were weak to moderate (0.16≤r≤0.35). The correlation of BDCPP and DPHP in our sample (r=0.20) was similar for a sample of subfertile women in Boston (r=0.21) (Carignan et al., 2017), slightly higher compared to pregnant women in California (r=0.16) (Castorina et al., 2017), yet lower compared to women in NC (r=0.31) and NHANES (r=0.45) (Hoffman et al., 2017b; Ospina et al., 2018). BCEtP and BDCPP significantly correlated with all metabolites except for DCP and TBBA, with similar moderately strong correlation coefficients found in NHANES. Few studies to date have captured repeated OPE measurements as we did in our present work. Reproducibility of OPE concentrations over two visits were primarily weak over time (0.03≤ICC≤0.34). Similar temporal variability was observed in a cohort of subfertile females for DPHP (ICC=0.34), yet coefficients were higher for BDCPP (ICC=0.56) (Carignan et al., 2017). A small study (n=59) of pregnant women in Rhode Island observed more stability of OPE concentrations throughout pregnancy (pooled samples, one urine sample per trimester) for BDCPP (ICC=0.60) and DPHP (ICC=0.43) (Romano et al., 2017). A smaller study (n=19) of males and females in New York state also observed increased reproducibility with BCEtP, DNBP, and DPHP (0.49≤ICC≤0.64) with urine measurements for three consecutive days over a five week period (Wang et al., 2018). It is possible the differences in ICCs are due to different exposure levels, activities, or behaviors from the different study populations.
Organophosphates are used in a wide variety of consumer products including: polyurethane foams, electronics, baby products, hydraulic fluids, and glues (Brandsma et al., 2014; Stapleton et al., 2011; van der Veen and De Boer, 2012; Wei et al., 2015). However, their specific use is typically dependent upon the physicochemical properties of each specific compound. Many parent OPEs from this analysis including: TCEP, TCIPP, tricresyl phosphate (TCP), TDCPP, tri-n-butyl phosphate (TBP), and TPHP can be used as plasticizers and in paints, polyvinylchloride (PVC), lacquers, and floor polishes (Wei et al., 2015). We observed several associations with self-reported PCP use and OPE concentrations. Consistent with our results, previous studies have also reported similar findings of increased DPHP concentrations after nail polish application. Although we found a significant 49% increase with nail polish use and DPHP concentrations, this relationship is somewhat weaker than observed in other studies. A small study (n=26) in NC reported a 7-fold increase in DPHP concentrations after nail polish application (Mendelsohn et al., 2016). A larger study of (n=230) women in Boston also found a large (134%) increase in DPHP concentrations with reported nail polish use ≤24 hrs. prior to urine sample collection (Ingle et al., 2019). Upon further investigation, we observed a particular brand of nail polish frequently reported among PROTECT participants and performed a secondary analysis. Using this brand was associated with a 75% increase in DPHP concentrations (p=0.01) although TPHP is not listed as an ingredient. In a study of 10 nail polishes, Mendelsohn et al., 2016 reported detection of TPHP in two out of three nail polishes that also did not have TPHP listed as an ingredient. Similarly, a recent study detected TPHP in 60% of nail polished sampled (n=40) and 13 of those did not list TPHP as an ingredient (Young et al., n.d.).
We also observed a significant association with perfume use and a 49% increase in DPHP, yet our largest association was with suntan lotion and BDCPP metabolite concentrations (110% increase). Our findings are considerably larger compared to the association from a prior study of women in Boston which found a 30% increase in BDCPP with suntan lotion (Ingle et al., 2019). Differences in results of our study and the previous work could possibly relate to the variation of PCP use among different demographic groups as well as the differences in climate (Park et al., 2015). There is also a large degree of variation of formulations among products by brand (Braun et al., 2014). The time frame of product use (48 hours in our study vs. 24 hours)) in relation to urine sample collection could also explain the observed differences among studies (Ingle et al., 2019; Nassan et al., 2017).
Associations with self-reported HP use and OPE metabolites were mixed. The use of pesticides in the home was associated with a 46% increase in BCPP, while using a vinyl shower curtain was associated with a 27% decrease in DPHP concentrations. We observed a significant association with those reporting using pesticides inside the home, yet not for those who use pesticides outside the home, which may indicate OPEs are added to certain types of pesticides commonly use in the home (i.e. insecticides) compared to those used outside the home (e.g., herbicides, fungicides). However, the largest percent increases we observed for HP were with reported furniture polish use and BCEtP (62%) and BCPP (70%), although the associations were not statistically significant. These results could reflect OPEs directly added to the furniture polish, dust ingestion from volatized OPEs added to PUF in furniture, or from glues used in making furniture, or products (lacquers or paints) applied to furniture which may contain TCEP and/or TCPP (Marklund et al., 2003; Wei et al., 2015).
Our observation of a decrease in DPHP concentrations with use of a vinyl shower curtain was unexpected as the parent compound TPHP is commonly used in PVC and vinyl for its plasticizing properties and unsaturated polyester resins (Marklund et al., 2003; Wei et al., 2015). However, the decrease in concentrations could be a result of showering as washing with soap and water has been associated with a 67% decrease in TPHP in skin wipes among a sample of 30 adults in China (Liu et al., 2016). A similar decrease (27%) was observed among women in Boston with hand dishwashing liquid use (my paper under review). If washing does limit the absorption of TPHP, it possibly explains our associations with a decrease in concentrations of the metabolite DPHP with items used while showering: bar soap (−23%), liquid soap (−27), shampoo (−16%), and conditioner (−18%), although none were statistically significant. We observed negligible percent increases or decreases with OPE concentrations and cleaners which was also observed in a previous study (Ingle et al., 2019). Cleaning agents could also be a source of OPE exposure, but active cleaning may be removing OPEs from indoor environments. A Dutch study found that more frequent vacuuming was associated with a 44–43% decrease in the parent compounds of BCEtP (TCEP) and BCPP (TCIPP), while frequent dusting was also associated with a 37% decrease in TCIPP (Sugeng et al., 2018).
Exposure to OPEs via dust ingestion had been the presumed primary exposure route/pathway. However, recent studies suggest that volatized OPEs transfer to lipids on the epidermis where they are absorbed (Phillips et al., 2018; Weschler and Nazaroff, 2008). In skin samples exposed to various OPEs, some chlorinated OPEs such as TCIPP & TCEP were absorbed into the skin more rapidly compared to others (e.g., TPHP), which could explain our largest association (110% increase) with BDCPP and suntan lotion (Frederiksen et al., 2018). However, we are unaware of any other studies to date that have assessed the relationship between BDCPP (TDCIPP) and suntan lotion. Among children in a NC study (n=202), the strongest predictor of OPE metabolite urine concentrations was the amount of OPEs measured on hand wipes (Phillips et al., 2018). OPE levels were also moderately correlated (0.16≤r≤0.41) for parent compounds and BCPP, BDCPP, and DPHP metabolites. A study of 53 adults in NC also found moderate (significant) correlation to levels of TPHP measured in hand wipes and concentrations of DPHP in urine (Hoffman et al., 2015b).
Our study was subject to several limitations. We did not obtain information on frequency, duration, and time of PCP or HP use in relation to urine collection. While previous studies have shown a high variability in the amount of products used among people, the addition of all of these factors would likely overfit our models and possibly mask identified associations (Loretz et al., 2006, 2005; Nassan et al., 2017). The half-lives of OPEs range from hours to days and our questionnaire design asked about product use within the last 48 hours, which may have resulted in some measurement error. However, previous studies have reported OPE concentrations and PCP use within person to remain moderately stable over time (Carignan et al., 2017; Loretz et al., 2006; Meeker et al., 2013b). Some of our findings are also based on a low number of women who report using these products and should be interpreted with caution. Our present cohort is comprised solely of pregnant women and it remains unclear if pregnancy could affect the pharmacokinetics of OPE absorption, metabolism, or excretion, thus our results may not be generalizable to non-pregnant populations. Finally, the objective of this study was to identify the relationships between PCP and HP use and OPE metabolite concentrations, however, OPE internal exposure is likely a result of a mixture of product use and exposure from other OPE sources.
However, our study also had several strengths. To the best of our knowledge, we are the first to examine the relationship between PCP and HP use and OPE metabolites among pregnant women. Urine collection and questionnaire data were obtained simultaneously to minimize the likelihood of systematic error. Finally, multiple urine samples and questionnaire responses for each participant increased precision of measurements and effect estimates.
Conclusions
To the best of our knowledge, only one other study has investigated the association of self-reported PCP and HP use and OPE metabolite concentrations. However, we are the first to assess this relationship among pregnant women. We observed high detection frequencies for BCEtP, BCPP, BDCPP, and DPHP. Concentrations of several metabolites in our sample were slightly higher compared to previous studies. Similar to prior studies, we also found a positive association between nail polish use and DPHP concentrations, which was even greater when only using a specific nail polish brand. However, our largest association was a 110% increase in BDCPP concentrations with reported suntan lotion use. Pesticide use in the home resulted in elevated (46%) BCPP concentrations while vinyl shower curtain use was associated with a decrease (−27%) in DPHP concentrations. Our results suggest that OPEs may also be used in some PCP and HP, yet washing may decrease absorption. Future studies are necessary to target specific OPE compounds, which may be used in suntan lotion, nail polish, perfume, and pesticides highlighted by our findings.
Supplementary Material
Highlights.
OPEs were widely detected among pregnant women.
Nail polish, perfume, and suntan lotion use was associated with increased OPE metabolite concentrations.
Using pesticides in the home was associated with elevated OPE metabolite concentrations.
OPEs may be used in personal care and household products.
The association of urinary phosphorous-containing flame retardant metabolites and self-reported personal care and household product use among pregnant women in Puerto Rico
Acknowledgements
Funding for this work was supported by the National Institute of Environmental Health Sciences, National Institutes of Health (Grants P42ES017198, P50ES026049, and P30ES17885)
We also gratefully acknowledge the effort provided by our research participants. Last, we acknowledge Dr. Nayana Jayatilaka, Paula Restrepo, and Zachary Davis for technical assistance in measuring the urinary concentrations of the FR metabolites.
Funding for this work was supported by the National Institute of Environmental Health Sciences, National Institutes of Health (Grants P42ES017198, P50ES026049, P30ES017885, and T32ES007062)
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
Conflict of Interest
The authors declare no conflict of interest
CRediT author statement
Mary E. Ingle: Methodology, Formal Analysis, Writing - Original Draft, Visualization. Deborah Watkins: Methodology, Formal Analysis, Data Curation, Writing - Review & Editing, Visualization. Zaira Rosario: Investigation, Resources, Data Curation, Project Administration. Carmen M. Vélez-Vega: Investigation, Resources, Data Curation, Project Administration. Gredia Huerta-Montanez: Conceptualization, Supervision. Antonia M. Calafat: Resources, Data Curation, Writing - Review & Editing, Visualization. Maria Ospina: Resources, Data Curation, Writing - Review & Editing, Visualization. José F Cordero: Conceptualization, Writing - Review & Editing, Supervision, Funding Acquisition. Akram Alshawabkeh: Conceptualization, Writing - Review & Editing, Supervision Project Administration, Funding Acquisition. John D. Meeker: Conceptualization, Methodology, Formal Analysis, Writing - Review & Editing, Visualization, Supervision, Project Administration, Funding Acquisition.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aker AM, Watkins DJ, Johns LE, Ferguson KK, Soldin OP, Anzalota Del Toro LV, Alshawabkeh AN, Cordero JF, Meeker JD, 2016. Phenols and parabens in relation to reproductive and thyroid hormones in pregnant women. Environ. Res 151, 30–37. 10.1016/j.envres.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N, Van den Eede N, Dirtu AC, Neels H, Covaci A, 2012. Assessment of human exposure to indoor organic contaminants via dust ingestion in Pakistan. Indoor Air 22, 200–211. 10.1111/j.1600-0668.2011.00757.x [DOI] [PubMed] [Google Scholar]
- ATSDR, 2015. Toxicological Profile for Polybrominated Diphenyl Ethers (PBDEs). Atlanta. [PubMed] [Google Scholar]
- Boeniger MF, Lowry LK, Rosenberg J, 1993. Interpretation of Urine Results Used to Assess Chemical Exposure with Emphasis on Creatinine Adjustments: A Review. Am. Ind. Hyg. Assoc. Journal; October 54, 615–627. [DOI] [PubMed] [Google Scholar]
- Brandsma SH, De Boer J, van Velzen MJMM, Leonards PEGG, 2014. Organophosphorus flame retardants (PFRs) and plasticizers in house and car dust and the influence of electronic equipment. Chemosphere 116, 3–9. [DOI] [PubMed] [Google Scholar]
- Braun JM, Just AC, Williams PL, Smith KW, Calafat AM, Hauser R, 2014. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J. Expo. Sci. Environ. Epidemiol 24, 459–466. 10.1038/jes.2013.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brommer S, Harrad S, 2015. Sources and human exposure implications of concentrations of organophosphate flame retardants in dust from UK cars, classrooms, living rooms, and offices. Environ. Int 83, 202–207. https://doi.org/10.1016Zj.envint.2015.07.002 [DOI] [PubMed] [Google Scholar]
- Canbaz D, Logiantara A, van Ree R, van Rijt LS, 2017. Immunotoxicity of organophosphate flame retardants TPHP and TDCIPP on murine dendritic cells in vitro. Chemosphere 177, 56–64. 10.1016/j.chemosphere.2017.02.149 [DOI] [PubMed] [Google Scholar]
- Cantonwine DE, Cordero JF, Rivera-González LO, Anzalota Del Toro LV, Ferguson KK, Mukherjee B, Calafat AM, Crespo N, Jimenez-Velez B, Padilla IY, Alshawabkeh AN, Meeker JD, 2014. Urinary phthalate metabolite concentrations among pregnant women in Northern Puerto Rico: Distribution, temporal variability, and predictors. Environ. Int 62, 1–11. 10.1016/j.envint.2013.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carignan CC, Mínguez-Alarcón L, Butt CM, Williams PL, Meeker JD, Stapleton HM, Toth TL, Ford JB, Hauser R, 2017. Urinary Concentrations of Organophosphate Flame Retardant Metabolites and Pregnancy Outcomes among Women Undergoing in Vitro Fertilization for the EARTH Study Team. Environ. Health Perspect 125, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castorina R, Butt C, Stapleton HM, Avery D, Harley KG, Holland N, Eskenazi B, Bradman A, 2017. Flame retardants and their metabolites in the homes and urine of pregnant women residing in California (the CHAMACOS cohort). Chemosphere 179, 159–166. 10.1016/j.chemosphere.2017.03.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cequier E, Sakhi AK, Marcé RM, Becher G, Thomsen C, 2015. Human exposure pathways to organophosphate triesters — A biomonitoring study of mother-child pairs. Environ. Int 75, 159–165. 10.1016/j.envint.2014.11.009 [DOI] [PubMed] [Google Scholar]
- Dodson RE, Van Den Eede N, Covaci A, Perovich LJ, Brody JG, Rudel RA, 2014. Urinary biomonitoring of phosphate flame retardants: Levels in California adults and recommendations for future studies. Environ. Sci. Technol 48, 13625–13633. 10.1021/es503445c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen M, Stapleton HM, Vorkamp K, Webster TF, Jensen NM, Sorensen JA, Nielsen F, Knudsen LE, Sørensen LS, Clausen PA, Nielsen JB, Clausen A, Nielsen JB, 2018. Dermal uptake and percutaneous penetration of organophosphate esters in a human skin ex vivo model. Chemosphere 197, 185–192. 10.1016/j.chemosphere.2018.01.032 [DOI] [PubMed] [Google Scholar]
- Hammel SC, Hoffman K, Webster TF, Anderson KA, Stapleton HM, 2016. Measuring Personal Exposure to Organophosphate Flame Retardants Using Silicone Wristbands and Hand Wipes. Environ. Sci. Technol 50, 4483–4491. 10.1021/acs.est.6b00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, English K, Baduel C, Thai P, Jagals P, Ware RS, Li Y, Wang X, Sly PD, Mueller JF, 2018. Concentrations of organophosphate flame retardants and plasticizers in urine from young children in Queensland, Australia and associations with environmental and behavioural factors. Environ. Res 164 https://doi.org/10.1016Zj.envres.2018.02.040 [DOI] [PubMed] [Google Scholar]
- Hoffman K, Butt CM, Chen A, Limkakeng AT, Stapleton HM, 2015a. High Exposure to Organophosphate Flame Retardants in Infants: Associations with Baby Products. Environ. Sci. Technol 49, 14554–14559. 10.1021/acs.est.5b03577 [DOI] [PubMed] [Google Scholar]
- Hoffman K, Butt CM, Webster TF, Preston EV, Hammel SC, Makey C, Lorenzo AM, Cooper EM, Carignan C, Meeker JD, Hauser R, Soubry A, Murphy SK, Price TM, Hoyo C, Mendelsohn E, Congleton J, Daniels JL, Stapleton HM, 2017a. Temporal Trends in Exposure to Organophosphate Flame Retardants in the United States. Environ. Sci. Technol. Lett 4, 112–118. 10.1021/acs.estlett.6b00475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Garantziotis S, Birnbaum LS, Stapleton HM, 2015b. Monitoring indoor exposure to organophosphate flame retardants: Hand wipes and house dust. Environ. Health Perspect 123, 160–165. 10.1289/ehp.1408669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Lorenzo A, Butt CM, Adair L, Herring AH, Stapleton HM, Daniels JL, 2017b. Predictors of urinary flame retardant concentration among pregnant women. Environ. Int 98, 96–101. 10.1016/j.envint.2016.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle ME, Mínguez-Alarcon Lidia, •, Courtney, •, Carignan C, Butt CM, Stapleton HM, Paige, •, Williams L, Ford JB, Hauser, • Russ, Meeker JD, 2019. The association of urinary phosphorous-containing flame retardant metabolites and self-reported personal care and household product use among couples seeking fertility treatment. J. Expo. Sci. Environ. Epidemiol 10.1038/s41370-019-0122-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayatilaka NK, Restrepo P, Williams LT, Ospina M, Valentin-Blasini L, Calafat AM, 2017. Quantification of three chlorinated dialkyl phosphates, diphenyl phosphate, 2,3,4,5-tetrabromobenzoic acid, and four other organophosphates in human urine by solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem 409, 1323–1332. 10.1007/s00216-016-0061-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kademoglou K, Xu F, Padilla-Sanchez JA, Haug S, Covaci A, Collins CD, 2016. Legacy and alternative flame retardants in Norwegian and UK indoor environment: Implications of human exposure via dust ingestion. Environ. Int 102, 48–56. 10.1016/j.envint.2016.12.012 [DOI] [PubMed] [Google Scholar]
- Kosarac I, Kubwabo C, Foster WG, 2016. Quantitative determination of nine urinary metabolites of organophosphate flame retardants using solid phase extraction and ultra performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS). J. Chromatogr. B 1014, 24–30. 10.1016/jjchromb.2016.01.035 [DOI] [PubMed] [Google Scholar]
- Lipscomb ST, Mcclelland MM, Macdonald M, Cardenas A, Anderson KA, Kile ML, 2017. Cross-sectional study of social behaviors in preschool children and exposure to flame retardants. 10.1186/s12940-017-0224-6 [DOI] [PMC free article] [PubMed]
- Liu X, Yu G, Cao Z, Wang B, Huang J, Deng S, Wang Y, 2016. Occurrence of organophosphorus flame retardants on skin wipes: Insight into human exposure from dermal absorption. Environ. Int 98, 113–119. https://doi.org/10.1016Zj.envint.2016.10.021 [DOI] [PubMed] [Google Scholar]
- Loretz L, Api AM, Barraj L, Burdick J, Davis DA, Dressler W, Gilberti E, Jarrett G, Mann S, Laurie Pan YH, Re T, Renskers K, Scrafford C, Vater S, 2006. Exposure data for personal care products: Hairspray, spray perfume, liquid foundation, shampoo, body wash, and solid antiperspirant. Food Chem. Toxicol 44, 2008–2018. 10.1016/j.fct.2006.06.029 [DOI] [PubMed] [Google Scholar]
- Loretz LJ, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, Rothenstein A, Scrafford CG, Sewall C, 2005. Exposure data for cosmetic products: Lipstick, body lotion, and face cream. Food Chem. Toxicol 43, 279–291. 10.1016/j.fct.2004.09.016 [DOI] [PubMed] [Google Scholar]
- Marklund A, Andersson B, Haglund P, 2003. Screening of organophosphorus compounds and their distribution in various indoor environments. Chemosphere 53, 1137–1146. 10.1016/S0045-6535(03)00666-0 [DOI] [PubMed] [Google Scholar]
- Meeker JD, Cantonwine DE, Rivera-González LO, Ferguson KK, Mukherjee B, Calafat AM, Ye X, Anzalota Del Toro LV, Crespo-Hernandez N, Jiménez-Vélez B, Alshawabkeh AN, Cordero JF, 2013a. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in puerto rico. Environ. Sci. Technol 47, 3439–3447. 10.1021/es400510g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Cooper EM, Stapleton HM, Hauser R, 2013b. Exploratory analysis of urinary metabolites of phosphorus-containing flame retardants in relation to markers of male reproductive health. Endocr. Disruptors 1, e26306 10.4161/endo.26306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Stapleton HM, 2009. House Dust Concentrations of Organophosphate Flame Retardants in Relation to Hormone Levels and Semen Quality Parameters. Environ. Health Perspect 118, 318–323. 10.1289/ehp.0901332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn E, Hagopian A, Hoffman K, Butt CM, Lorenzo A, Congleton J, Webster TF, Stapleton HM, 2016. Nail polish as a source of exposure to triphenyl phosphate. Environ. Int 86, 45–51. 10.1016/j.envint.2015.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassan FL, Coull BA, Gaskins AJ, Williams MA, Skakkebaek NE, Ford JB, Ye X, Calafat AM, Braun JM, Hauser R, 2017. Personal Care Product Use in Men and Urinary Concentrations of Select Phthalate Metabolites and Parabens: Results from the Environment And Reproductive Health (EARTH) Study. Environ. Health Perspect 125, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ospina M, Jayatilaka NK, Wong LY, Restrepo P, Calafat AM, 2018. Exposure to organophosphate flame retardant chemicals in the U.S. general population: Data from the 2013–2014 National Health and Nutrition Examination Survey. Environ. Int 110, 32–41. https://doi.Org/10.1016/j.envint.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Lee K, Hwang Y, Kim jin H., 2015. Determining the exposure factors of personal and home care products for exposure assessment. Food Chem. Toxicol 77, 105–110. [DOI] [PubMed] [Google Scholar]
- Phillips AL, Hammel SC, Hoffman K, Lorenzo AM, Chen A, Webster TF, Stapleton HM, 2018. Children’s residential exposure to organophosphate ester flame retardants and plasticizers: Investigating exposure pathways in the TESIE study. Environ. Int 116, 176–185. 10.1016/j.envint.2018.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano ME, Hawley NL, Eliot M, Calafat AM, Jayatilaka NK, Kelsey K, McGarvey S, Phipps MG, Savitz DA, Werner EF, Braun JM, 2017. Variability and predictors of urinary concentrations of organophosphate flame retardant metabolites among pregnant women in Rhode Island. Environ. Heal 16, 40 10.1186/s12940-017-0247-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreder ED, Uding N, La Guardia, M.J., 2016. Inhalation a significant exposure route for chlorinated organophosphate flame retardants. Chemosphere 150, 499–504. 10.1016/j.chemosphere.2015.11.084 [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, Mcclean MD, Webster TF, 2008. Alternate and new brominated flame retardants detected in U.S. house dust. Environ. Sci. Technol 42, 6910–6916. 10.1021/es801070p [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, Webster TF, 2009. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ. Sci. Technol 43, 7490–7495. 10.1021/es9014019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, Webster TF, Blum A, 2011. Identification of Flame Retardants in Polyurethane Foam Collected from Baby Products 45, 5323–5331. 10.1021/es2007462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- State of California Environmental Protection Agency Office of Environmental Health Hazard Assessment, 2017. Chemicals Known to the State to Cause Cancer or Reproductive Toxicity; January 27, 2017 [WWW Document]. URL https://oehha.ca.gov/media/downloads/proposition-65//p65single01272017.pdf (accessed 4.1.17). [Google Scholar]
- Sugeng EJ, de Cock M, Leonards PEG, van de Bor M, 2018. Electronics, interior decoration and cleaning patterns affect flame retardant levels in the dust from Dutch residences. Sci. Total Environ 645, 1144–1152. 10.1016/j.scitotenv.2018.07.127 [DOI] [PubMed] [Google Scholar]
- Sühring R, Diamond ML, Scheringer M, Wong F, Pućko M, Stern G, Burt A, Hung H, Fellin P, Li H, Jantunen LM, 2016. Organophosphate esters in Canadian Arctic air: Occurrence, levels and trends. Environ. Sci. Technol 50, 7409–7415. 10.1021/acs.est.6b00365 [DOI] [PubMed] [Google Scholar]
- Sun Y, Gong X, Lin W, Liu Y, Wang Y, Wu M, Kannan K, Ma J, 2018. Metabolites of organophosphate ester flame retardants in urine from Shanghai, China. 10.1016/j.envres.2018.03.031 [DOI] [PubMed]
- van der Veen I, De Boer J, 2012. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88, 1119–1153. https://doi.org/10.1016d.chemosphere.2012.03.067 [DOI] [PubMed] [Google Scholar]
- Wang Y, Kannan K, 2018. Concentrations and Dietary Exposure to Organophosphate Esters in Foodstuffs from Albany, New York, United States. 10.1021/acs.jafc.8b06114 [DOI] [PubMed] [Google Scholar]
- Wang Y, Li W, Pilar Martínez-Moral M, Sun H, Kannan K, 2018. Metabolites of organophosphate esters in urine from the United States: Concentrations, temporal variability, and exposure assessment. 10.1016/j.envint.2018.11.007 [DOI] [PMC free article] [PubMed]
- Wei GL, Li DQ, Zhuo MN, Liao YS, Xie ZY, Guo TL, Li JJ, Zhang SY, Liang ZQ, 2015. Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity and human exposure. Environ. Pollut 10.1016/j.envpol.2014.09.012 [DOI] [PubMed] [Google Scholar]
- Weschler CJ, Nazaroff WW, 2008. Semivolatile organic compounds in indoor environments. Atmos. Environ. 42, 9018–9040. 10.1016/j.atmosenv.2008.09.052 [DOI] [Google Scholar]
- Young AS, Allen JG, Kim U-J, Seller S, Webster TF, Kannan K, Ceballos DM, n.d. Phthalate and Organophosphate Plasticizers in Nail Polish: Evaluation of Labels and Ingredients. 10.1021/acs.est.8b04495 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.