Abstract
The technology for fluorescence protein-sensing is advancing rapidly owing to the continued introduction of new concepts, new fluorophores, and proteins engineered for sensing-specific analytes. Concerns about the reversibility and selectivity of engineered proteins are being addressed by developing biosensors that are based on the utilisation of coenzyme-depleted enzymes. Such biomolecules do not consume the substrate and can exhibit conformational changes upon the binding of the analyte, which can be easily detected as fluorescence change. In addition, concerns about the stability of biosensors can be overcome by using thermostable enzymes isolated from thermophilic microorganisms. Finally, the development of new techniques such as polarization-based sensing, anisotropy-based sensing and lifetime-based sensing, all of which can be accomplished with light-emitting diodes as the light source, is prompting the design of a new class of specific and stable biosensors, as has occurred with blood glucose measurement. These biosensors represent a valid alternative to the conventional clinical chemistry diagnostics.
Introduction
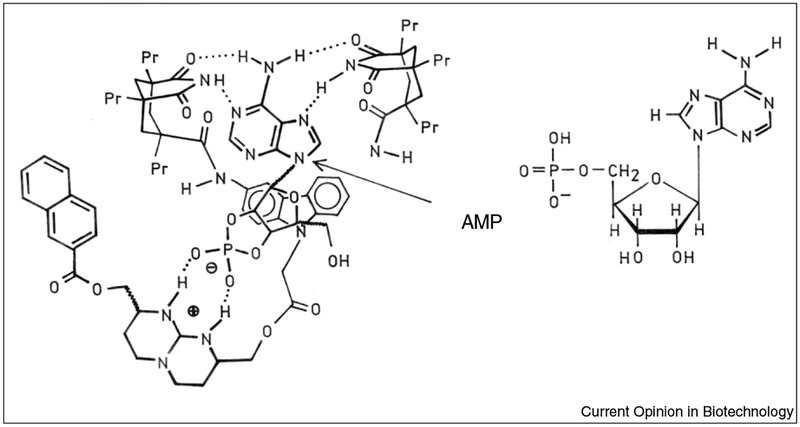
Fluorescence detection is the dominant analytical tool in medical testing, biotechnology and drug discovery. In the 1980s fluorescence probes for specific analytes became available [1–3]. Some of these sensing fluorophores are relatively simple, as illustrated by quinoline probes that are collisionally quenched by chloride [4,5]; however, the molecular complexity of the sensors quickly increases if analyte binding is required to cause a spectral change. For example, the fluorophores specific for calcium are structurally complex and only a few display spectral shifts upon binding calcium [6]. As a consequence, the development of specific sensors for biochemically relevant analytes is even more challenging. Indeed, it is difficult to imagine how a fluorescent probe could be designed that specifically binds pyruvate, lactate or creatinine. Even if a suitable structure could be designed and synthesized, there is no guarantee that the final molecule will display a spectral change, adequate water solubility and a suitable affinity constant. The sensor for AMP illustrates the complexity of fluorescent probes for biochemical analytes (Figure 1).
Figure 1.
Fluorescent sensor for AMP. The arrow shows the AMP-binding site.
To circumvent these difficulties, the use of proteins and enzymes as specific sensors for biochemical analytes is becoming a priority in modern biotechnology [7–9]. There are a large number of enzymes known that bind specific ligands, perhaps a greater number than non-reactive binding proteins. This widespread use of proteins as sensors depends on protocols to enhance stability such as the introduction of changes in the amino acid composition leading to enhanced protein structural stability [10]. An alternative method is to use naturally thermostable enzymes or proteins isolated from thermophilic microorganisms. These macromolecules have intrinsically stable structural features [11••,12–15,16•] and they can be considered as ideal biosensors. The use of enzymes as biosensors, however, presents the disadvantage of the substrate consumption, which is a disadvantage in the design of implantable clinical sensors or in biotechnology assays.
In this article we briefly review the most recent and advanced applications of fluorescence-protein sensors and, in particular, we show the use of coenzyme-depleted enzymes (apo-enzymes) as non-consuming substrate sensors.
Apo-enzymes are still able to bind the substrate, but not transform it. Additionally, the binding of substrates may result in enzymatic conformational changes that can be easily detected by fluorescence and/or polarization measurements. There is a wealth of knowledge on enzymes that transform numerous substances of biochemical interest. Hence, the use of the possibility of inactive apo-enzymes for reversible sensors greatly expands the range of biochemically relevant analytes that can be measured using proteins as sensors.
Glucose-sensing proteins
There is considerable medical interest in measurements of blood glucose [17]. Close control of blood glucose is necessary to avoid the long-term health effects of diabetes that include blindness and neuropathies. As a consequence, there is a substantial worldwide effort to develop non-invasive and minimally invasive methods for frequent and/or continuous monitoring of blood glucose [18,19]. A wide variety of methods have been tested: optical rotation; near-infrared absorbance; Raman scattering; the design and synthesis of glucose-specific fluorescence probes; and resonance energy transfer between proteins that bind glucose and acceptor-labelled glucose analogues [20–24]. Proteins that bind glucose have also been used, for example hexokinases, glucose oxidase and glucose–galactose-binding protein, as probes to monitor the concentrations of glucose [25,26,27••,28]. In this last example we introduced the concept of using an inactive form of an enzyme as a non-consuming sensor and found how to monitor the substrate-induced enzyme conformational changes by fluorescence spectroscopy.
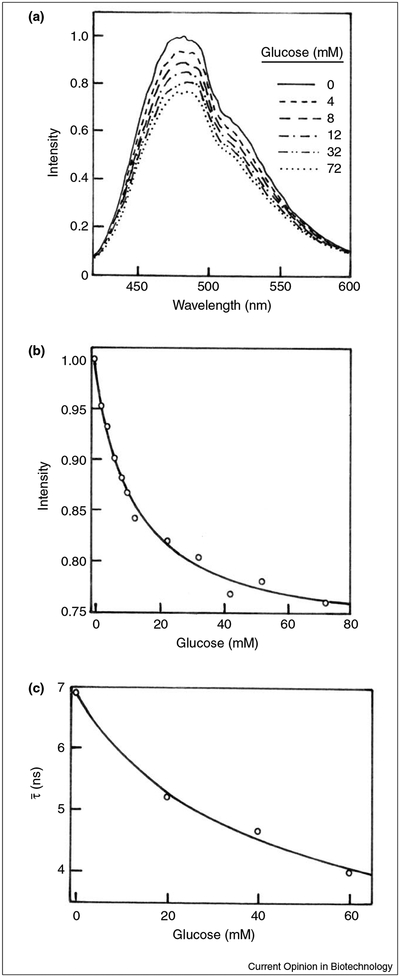
Glucose oxidase (GO) from Aspergillus niger catalyses the conversion of b-D-glucose and oxygen to D-glucono-1,5-lactone and hydrogen peroxide. GO is a flavoprotein, highly specific to b-D-glucose, and is widely used to estimate glucose concentration in blood or urine samples through the formation of coloured dyes. Because glucose is consumed, this enzyme cannot be used as a reversible sensor. In a recent report [27••] we extended the use of GO under conditions where no reaction occurs. In particular, in order to prevent glucose oxidation, we removed the FAD cofactor that is required for the reaction. The coenzyme-depleted enzyme can still bind glucose with an affinity comparable to the holo-enzyme. Additionally, the tryptophan fluorescence of the apo-GO was sensitive to glucose binding, resulting in a decrease of approximately 25%. The intrinsic fluorescence intensity from proteins, however, is usually not useful for clinical sensing because of the need for complex or bulky light sources as well as for the presence of numerous proteins in most biological samples. In an attempt to obtain a glucose sensor with longer excitation and emission wavelengths, we labelled GO with 8-anilino-1-naphthalene sulfonic acid (ANS). Figure 2a shows the effect of the addition of glucose on emission intensity spectra of ANS-labelled GO. Figures 2b and c are depicted as the glucose effect on the maximum emission intensity and on mean lifetime decay of the ANS-labelled enzyme, respectively. The results show that the glucose affects the fluorescence features of the ANS-labelled GO. In particular, the glucose addition results in a decrease of the intensity and the mean lifetime of 25% and over 40%, respectively, indicating the potential usefulness of the apo-GO as a glucose sensor.
Figure 2.
Effect of glucose on the fluorescence properties of the apo-glucose oxidase from A. niger. (a) Emission spectra of ANS-labelled apo-GO in the absence and in the presence of glucose. (b) Glucose-dependent emission intensity of ANS-labelled apo-GO. (c) Effect of glucose on the mean lifetime of ANS-labelled apo-GO. For details see [27••].
Recently, it has also been shown that the apo-glucose dehydrogenase from the thermophilic microorganism Thermoplasma acidophilum can be used as a non-consuming glucose sensor. This protein is stable in extreme environmental conditions and for extended periods of time [28].
Pyruvate kinase as a sodium sensor
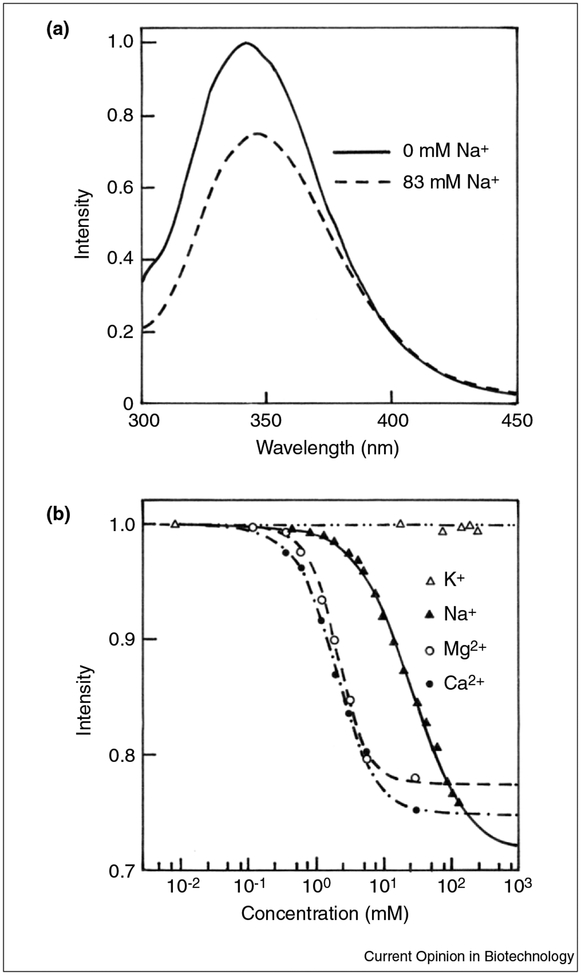
Measurements of sodium and potassium in blood are a routine part of clinical blood analysis. Fluorescent probes for sodium are known [29–31]. It would be valuable to have simple optical methods for rapid point-of-care testing especially for potassium, which is measured during hypertensive screening. A variety of fluorescence probes have been developed that respond to sodium and/or potassium. Most of these responses are based on partially selective binding of these cations to crown ethers [32]. These probes typically display association constants suitable for intracellular measurements when sodium and potassium concentrations are near 6 mM and 120 mM, respectively. In blood the extracellular concentrations of sodium and potassium are near 140 mM and 4 mM, respectively. Of consequence, measurements of these ions in blood are particularly difficult given the 25-fold excess of chemically similar sodium ions. Because of this difficulty, we are only aware of one report describing the use of fluorescent probes to measure potassium at the concentration present in blood [33]; however, absolute specificity for either cation seems unlikely, so that additional methods to determine sodium or potassium in blood are useful for correction for non-specific responses of the sodium or potassium sensors. In this context, we have recently isolated a thermostable pyruvate kinase (PK) from the thermophilic eubacterium Bacillus acidocaldarius [34]. The enzyme, which is very stable in respect to the temperature, exhibited the interesting ability to bind sodium, magnesium and calcium, but the binding of calcium and magnesium appears to occur at cation concentrations that are higher than that present in whole blood. The enzyme titration by potassium ions, however, did not result in any variation of its fluorescence features. Figure 3a shows the fluorescence emission spectra of PK in the absence and in the presence of sodium chloride. Figure 3b shows the fluorescence titration curves of the enzyme as a function of potassium, sodium, magnesium and calcium. Additionally, frequency-domain experiments (data not shown) confirmed that the fluorescence properties of PK are sensitive to sodium, but not to potassium [34]. These results are in agreement with the probable substitution of a lysine at position 117 that serves as an internal cation [35].
Figure 3.
Effect of the addition of ions on the fluorescence emission of the pyruvate kinase from B. acidocaldarius. (a) Steady-state fluorescence spectra of PK from B. acidocaldarius in the absence and in the presence of sodium chloride. (b) Fluorescence titration curves of PK from B. acidocaldarius.
It seems possible that with cloning and insertion of cysteine residues for labelling, a protein sensor could be developed for blood sodium; however, it is worth pointing out that the more important clinical need is for a potassium sensor. Blood potassium is routinely measured for hypertensive screening. Present technology does not provide rapid point-of-care measurements of potassium and the measurements are performed in a central clinical laboratory. Site-directed mutagenesis experiments should allow us to change the specificity of our thermostable PK. It has been shown [35,36] that the PK from Corynebacterium glutaminum, in which the Glu117 has been replaced by Lys117, does not require potassium for its activation, indicating the importance of this amino acid residue in the PK cation-binding.
A protein biosensor for lactate
Measurements of blood lactate are available in predicting multiple organ failure and death in patients with septic shock. Lactate acidosis is also known to accompany decreased tissue oxygenation, hypovolemic left-ventricular failure and drug toxicity. Although blood lactate is a useful diagnostic indicator, its use is hindered by the time required for a lactate determination [37–39]. Even under favourable conditions a lactate measurement takes 30 minutes or longer, which is too long for many clinical decisions. Lactate determinations are typically performed by enzymatic oxidation to pyruvate by lactate dehydrogenase (LDH) or lactate oxidase, followed by detection of NADH or H2O2, respectively [40,41].
Recently, an alternative way for measuring lactate using the LDH from beef heart has been reported [42]. The tryptophan fluorescence of LDH was sensitive to micro-molar additions of lactate, resulting in a fluorescence decrease of approximately 30%; however, a clinically useful sensor requires that the wavelength be long enough to allow the use of simple excitation sources. In the past few years, ultraviolet output near 370 nm has become available from light-emitting diodes, and laser diodes as short as 399 nm have been reported [43,44]. Light-emitting diodes are also known to be useful for nanosecond lifetime measurements because of their capability of high-frequency modulation. Hence, we labelled the LDH with a fluorophore suitable for light-emitting diode excitation. LDH, when non-covalently labelled with ANS, displayed a decrease in the ANS emission intensity upon binding lactate. This decrease occurs without the consumption of lactate.
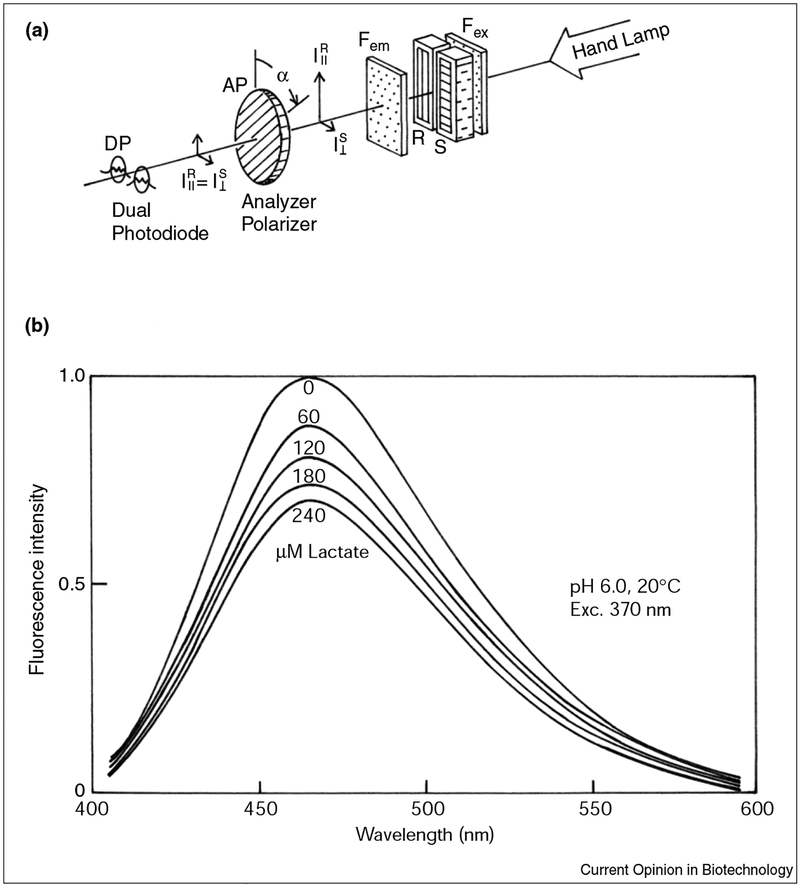
Figure 4a shows a simple apparatus used for measuring polarization changes. The device, composed of a dual-photo diode, an analyser polarizer and a hand lamp, can measure change in polarization of the sample respect to the reference. The lactate concentration is determined by rotating the polarizer until the signals from both photodiodes are equal. Figure 4b shows the effect of lactate on the emission spectra of ANS-labelled LDH. The addition of lactate results in a 40% decrease of the ANS–LDH fluorescence emission. The use of the above-described device for measuring the effect of the lactate on the ANS-labelled LDH results in a compensation angle change of about 6°. Although the range seems small, the compensation angles are readily measured to approximately 0.1° so that a 6° change corresponds to an accuracy of 2% in the lactate concentration. The paper by D’Auria et al. [42] gives a detailed description of the polarization sensing method. Despite the obtained results, we believe that the ANS–LDH system only represents the first step toward the development of a useful point-of-care lactate sensor. Indeed, the LDH used in these experiments was only marginally stable. The utilisation of LDH from thermophilic sources should result in a long-term stable sensor.
Figure 4.
Effect of lactate on the fluorescence emission of the LDH from beef heart. (a) Simple apparatus for polarization sensing. (b) Emission spectra of ANS-labelled LDH (from bovine heart in 10 mM phosphate) in the presence of increasing concentrations of lactate.
Conclusions
In conclusion, the use of coenzyme-depleted enzymes, when labelled with suitable fluorophores, has a great potential in biotechnology. This opens up new perspectives in clinical diagnosis as well as in all the applications requiring use of sensors with high specificity and/or non-consuming substrate.
Acknowledgements
This paper is dedicated to Professor Mose’ Rossi on the occasion of his 63rd birthday.
Abbreviations
- ANS
8-anilino-1-naphthalene sulfonic acid
- GO
glucose oxidase
- LDH
lactate dehydrogenase
- PK
pyruvate kinase
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
••of outstanding interest
- 1.Wolfbeis OS: Fiber-optic chemical sensors and biosensors. Anal Chem 2000, 72:81R–89R. [DOI] [PubMed] [Google Scholar]
- 2.Lakowicz JR: Advances in fluorescence sensing technology II. Proc SPIE 1995, 2388:159–170. [Google Scholar]
- 3.Spichiger-Keller UE: Chemical Sensors and Biosensors for Medical and Biological Applications. New York: Wiley-VCH, 1998:313–328. [Google Scholar]
- 4.Verkman AS, Sellers MC, Chao AC, Leung T, Ketcham R: Synthesis and characterization of improved chloride-sensitive fluorescent indicators for biological applications. Anal Biochem 1989, 178:355–361. [DOI] [PubMed] [Google Scholar]
- 5.Biwersi J, Tulk B, Verkman AS: Long-wavelength chloride-sensitive fluorescent indicators. Anal Biochem 1994, 219:139–143. [DOI] [PubMed] [Google Scholar]
- 6.Tsien RY, Rink TJ, Poenie M: Measurements of cytosolic free Ca++ in individual small cells using fluorescence microscopy with dual excitation wavelengths. Cell Calcium 1985, 6:145–157. [DOI] [PubMed] [Google Scholar]
- 7.Gilardi G, Mei G, Rosato N, Finazzi-Agro’ AF, Cass AEG: Spectroscopic properties of an engineered maltose-binding protein. Prot Eng 1997, 10:479–486. [DOI] [PubMed] [Google Scholar]
- 8.Romoser VA, Hinkle PM, Persechini A: Detection of living cells of Ca++ dependent changes in the fluorescence emission of an indicator composed of two green fluorescent protein variants linked by a calmodulin-binding sequence. J Biol Chem 1997, 272:13270–13274. [DOI] [PubMed] [Google Scholar]
- 9.Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY: Fluorescent indicators for Ca++ based on green fluorescent proteins and calmodulin. Nature 1997, 388:882–887. [DOI] [PubMed] [Google Scholar]
- 10.Colacino F, Crichton RR: Enzyme stabilization: the state of the art. Biotechnol Genet Eng Rev 1997, 14:211–277. [DOI] [PubMed] [Google Scholar]
- 11.••.D’Auria S, Nucci R, Rossi M, Gryczynski I, Gryczynski Z, Lakowicz JR:The β-glycosidase from the hyperthermophilic archaeon Sulfolobus solfataricus: enzyme activity and conformational dynamics at temperatures above 100°C. Biophys Chem 1999, 81:23–31. [DOI] [PubMed] [Google Scholar]; This paper shows how to perform direct measurements of enzyme activity as well as protein fluorescence spectra above 100°C without boiling the sample. It also shows protein stability measurements up to 160°C.
- 12.D’Auria S, Herman P, Lakowicz JR, Bertoli E, Tanfani F, Rossi M, Manco G: The thermophilic esterase from Archaeoglobus fulgidus: structure and conformational dynamics at high temperature. Proteins 2000, 38:351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Auria S, Nucci R, Rossi M, Bertoli E, Tanfani F, Gryczynski I, Malak H, Lakowicz JR: b-Glycosidase from the hyperthermophilic archaeon Sulfolobus solfataricus: structure and activity in the presence of alcohols. J Biochem 1999, 126:545–552. [DOI] [PubMed] [Google Scholar]
- 14.D’Auria S, Moracci M, Febbraio F, Tanfani F, Nucci R, Rossi M: Structure-function studies on b-glycosidase from Sulfolobus solfataricus. Molecular bases of thermostability. Biochimie 1998, 80:949–957. [DOI] [PubMed] [Google Scholar]
- 15.D’Auria S, Barone R, Rossi M, Nucci R, Barone G, Fessas D, Bertoli E, Tanfani F: Effects of temperature and SDS on the structure of b-glycosidase from the thermophilic archaeon Sulfolobus solfataricus. Biochem J 1997, 323:833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.•.Sun MC, Tolliday N, Vetriani C, Robb FT, Clark DS: Pressure-induced thermostabilization of glutamate dehydrogenase from the hyperthermophile Pyrococcus furiosus. Protein Sci 1999, 8:1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this paper the authors show the effect of elevated pressures on the activity and structure of a highly thermostable glutamate dehydrogenase.
- 17.Amato I: Race quickens for non-stick blood monitoring technology. Science 1992, 258:892. [DOI] [PubMed] [Google Scholar]
- 18.Tamada JA, Bohannon NJV, Potts RO: Measurements of glucose in diabetic subjects using non-invasive transdermal extraction. Nat Med 1995, 2:1198–1201. [DOI] [PubMed] [Google Scholar]
- 19.Marvin JS, Hellinga HW: Engineering biosensors by introducing fluorescent allosteric signal transducer: construction of a novel glucose sensor. J Am Chem Soc 1998, 120:7–11. [Google Scholar]
- 20.Szmacinski H, Lakowicz JR: Lifetime-based sensing In Topics in Fluorescence Spectroscopy, vol 4 Edited by Lakowicz JR. New York: Plenum Press; 1994:295–334. [Google Scholar]
- 21.Thompson RB: Red and near infrared fluorometry. J Am Chem Soc 1998, 120:151–181. [Google Scholar]
- 22.Casay GA, Shealy DB, Patonay G: Near infrared fluorescence probes. J Am Chem Soc 1998, 120:183–222. [Google Scholar]
- 23.Lakowicz JR, Maliwal B: Optical sensing of glucose using phase- modulation fluorometry. Anal Chem Acta 1993, 271:155–164. [Google Scholar]
- 24.Tamada JA, Bohannon NJV, Potts RO: Measurements of glucose in diabetic subjects using noninvasive transdermal extraction. Nat Med 1995, 11:1198–1201. [DOI] [PubMed] [Google Scholar]
- 25.Tolosa L, Gryczynski I, Eichorn LR, Dattelbaum JD, Castellano FN, Rao G, Lakowicz JR: Glucose sensor for low-cost lifetime based sensing using a genetically engineered protein. Anal Biochem 1999, 267:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomita K, Kamei S, Nagata K, Okuno H, Shiraishi T, Motoyama A, Ohkubo A, Yamanaka M: A stable glucokinase from a thermophilic bacterium II. Application to glucose determination. J Clin Biochem Nutr 1987, 3:11–16. [Google Scholar]
- 27.••.D’Auria S, Herman P, Rossi M, Lakowicz JR: The fluorescence emission of apo-glucose oxidase from Aspergillus niger as probe to estimate glucose concentrations. Biochem Biophys Res Commum 1999, 263:550–553. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first paper that shows the possibility of using coenzyme-depleted enzymes as non-consuming substrate sensors.
- 28.D’Auria S, Di Cesare N, Gryczniski Z, Gryczniski I, Rossi M, Lakowicz JR: A thermophilic apo-glucose dehydrogenase as non- consuming glucose sensor. Biochem Biophys Res Commun 2000, 274:727–731. [DOI] [PubMed] [Google Scholar]
- 29.Oguz U, Akkaya EU: A squaraine-based sodium selective fluorescent chemosensor. Tetrahedron Letts 1998, 39:5857–5860. [Google Scholar]
- 30.Smith GA, Hesketh TR, Metcalfe JC: Design and properties of a fluorescent indicator of intracellular free Na+ concentrations. Biochem J 1988, 250:227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minta A, Tsien RY: Fluorescent indicator for cytosolic sodium. J Biol Chem 1989, 264:19449–19457. [PubMed] [Google Scholar]
- 32.Pedersen CJ: The discovery of crown ethers. Science 1988, 241:536–540. [DOI] [PubMed] [Google Scholar]
- 33.Szmacinski H, Lakowicz JR: Potassium and sodium measurements at clinical concentrations using phase-modulation fluorometry. Sensor Actuator B — Chem 1999, 60:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Auria S, Rossi M, Herman P, Lakowicz JR: Pyruvate kinase from the thermophilic eubacterium Bacillus acidocaldarius as probe to monitor the sodium concentration in the blood. Biophys Chem 2000, 84:167–176. [DOI] [PubMed] [Google Scholar]
- 35.Larsen TM, Benning MM, Esenberg GE, Rayment I, Reed GH: Ligandinduced domain movement in pyruvate kinase: structure of the enzyme from rabbit muscle with Mg++, K+, and L-phospholactate at 2.7 Å resolution. Arch Biochem Biophys 1997, 348:262–267. [DOI] [PubMed] [Google Scholar]
- 36.Jetten MSM, Gubler ME, Lee SH, Sinksey AJ: Structural and functional analysis of pyruvate kinase from Corynebacterium glutaminum. Appl Environ Microbiol 1994, 60:2501–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siegel LB, Dalton HJ, Hertzog JH, Hopkins RA, Hannan RL, Hauser GJ: Initial postoperative serum lactate levels predict survival in children after open heart surgery. Intensive Care Med 1996, 22:1418–1423. [DOI] [PubMed] [Google Scholar]
- 38.Deshpande SA, Ward-Platt MP: Association between blood lactate and acid-base status and mortality in ventilated babies. Dis Childhood 1997, 76:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bakker J, Gris P, Coffernils M, Kahn RJ, Vincent JL: Serial blood lactate levels can predict the development of multiple organ failure following septic shock. Am J Surg 1996, 171:221–226. [DOI] [PubMed] [Google Scholar]
- 40.Palmisano F, Centonze D, Quinto M, Zambonin PG: A microdialysis fibre based sampler for flow injection analysis: determination ofL-lactate in biofluids by an electrochemically synthesized bilayer membrane based biosensor Biosens Bioelect 1996, 11:419–425. [DOI] [PubMed] [Google Scholar]
- 41.Marzouk SA, Cosofret VV, Buck RP, Yang H, Cascio WE, Hassan SM: A conducting salt-based amperometric biosensor for measurement of extracellular lactate accumulation in ischemic myocardium. Anal Chem 1997, 69:2646–2652. [DOI] [PubMed] [Google Scholar]
- 42.D’Auria S, Gryczniski Z, Gryczniski I, Rossi M, Lakowicz JR: A protein biosensor for lactate. Anal Biochem 2000, 283:83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Landgraf S, Grampp G: Application of laser diodes and ultrabright ligh emitting diodes for the determination of fluorescence lifetime in the nano- and subnanosecond region. J Inf Rec 1998, 24:141–148. [Google Scholar]
- 44.Someya T, Werner R, Forchel A, Catalano M, Cingolani R, Arakawa Y: Room temperature lasing at blue wavelengths in gallium nitride microcavities. Science 1999, 285:1905–1906. [DOI] [PubMed] [Google Scholar]