Abstract
The heart requires a high amount of energy, in the form of adenosine triphosphate (ATP), to maintain its viability and pump function. Anaerobic glycolysis and mitochondrial oxidative phosphorylation are the main metabolic pathways by which ATP is generated, utilizing fatty acids (FAs), glucose, lactate, and ketone bodies as primary substrates. Previous studies have demonstrated that, in response to stress, the heart undergoes alterations in metabolism, ranging from changes in substrate utilization to mitochondrial function, collectively called metabolic remodeling. However, the molecular mechanism mediating metabolic remodeling in the heart remains unclear. Yes-associated protein 1 (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ), which are major downstream effectors of the Hippo signaling pathway, play an important role in the regulation of heart size and cellular homeostasis of cardiomyocytes through the regulation of various transcriptional factors under both physiological and pathophysiological conditions. Recent findings in various organs and cell types have revealed that YAP and TAZ play an important role in energy metabolism. Here we summarize what is currently known about YAP/TAZ in the regulation of metabolism of various substrates and mitochondrial function in various organs and cell types and discuss the potential role of YAP/TAZ in mediating metabolic remodeling of the heart during stress and heart failure.
Keywords: the Hippo pathway, YAP, TAZ, glycolysis, metabolic remodeling, heart failure
1. Introduction
The heart has a large metabolic demand due to continuous contraction, requiring that a large amount of adenosine triphosphate (ATP) be generated (1). ATP is produced at high rates so that the heart can quickly respond to a sudden increase in hemodynamic overload. Fatty acid (FA), glucose, lactate, and ketone bodies are the primary substrates for ATP production (2, 3). There are two fundamental mechanisms for ATP synthesis: anaerobic glycolysis and aerobic oxidative phosphorylation. Under baseline conditions, more than 95% of ATP is produced from oxidative phosphorylation in mitochondria and the remaining 5% from glycolysis in the cytosol in the healthy adult heart. Under baseline conditions, 60–90% of acetyl-CoA, which is utilized in the tricarboxylic acid (TCA) cycle, comes from β-oxidation of FAs, and 10–40% from oxidation of pyruvate, derived from glycolysis and lactate oxidation (1, 4). In the fetal heart, low levels of circulating FAs and high levels of glucose and lactate allow the heart to generate approximately 50% of ATP from glucose and lactate (5, 6). Heart failure (HF) is a condition in which the pump function is insufficient for responding to systemic metabolic demand. Despite recent progress in medical treatment, HF is a major cause of mortality in developed countries (7). HF is accompanied by significant changes in metabolism in the heart, termed metabolic remodeling, ranging from changes in substrate utilization to mitochondrial dysfunction. Metabolic remodeling during HF can be either the cause or the effect of reduced cardiac contractility and either adaptive or maladaptive (3, 8, 9). Compared to glucose oxidation, FA oxidation requires more oxygen for the production of an equal amount of ATP. Together with the increased circulating level of FAs during HF, the use of FA promotes oxygen consumption, which in turn causes a proton leak from the electron transport chain and mitochondrial dysfunction due to oxidative damage. Thus, the downregulation of FA oxidation commonly observed in failing hearts may be an adaptive response. However, FA oxidation progressively declines with mitochondrial dysfunction, which in turn leads to decreases in ATP production and further decreases in cardiac contraction (10). Thus, decreases in FA oxidation can be maladaptive during HF. Intervening in the process of metabolic remodeling may allow improvement of cardiac function in HF patients. However, the signaling mechanism through which metabolic remodeling takes place in the failing heart remains poorly understood.
The Hippo signaling pathway is an evolutionarily conserved signaling pathway, regulating organ size and tumorigenesis. Yes-associated protein 1 (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ), which are the two major terminal effectors with partially overlapping functions in the Hippo signaling pathway, play a crucial role in tissue homeostasis through regulation of proliferation, hypertrophy, survival and death in the heart (11, 12). In general, activation of the upstream components of the Hippo pathway, including Mst1 and Lats2, inhibits cell proliferation and induces cell death, including apoptosis, whereas inactivation of the Hippo pathway and consequent activation of YAP/TAZ promote both proliferation and survival in many cell types. Since cell growth during tumorigenesis and organ enlargement are accompanied by changes in metabolism, an emerging hypothesis is that the Hippo pathway may also control metabolism. In fact, increasing lines of evidence suggest that YAP/TAZ plays an important role in energy metabolism in many cell types (13, 14). This review summarizes the role of YAP/TAZ in energy metabolism in various cell types and discuss the potential role of YAP/TAZ in mediating metabolic remodeling in the heart.
2. The Hippo signaling pathway
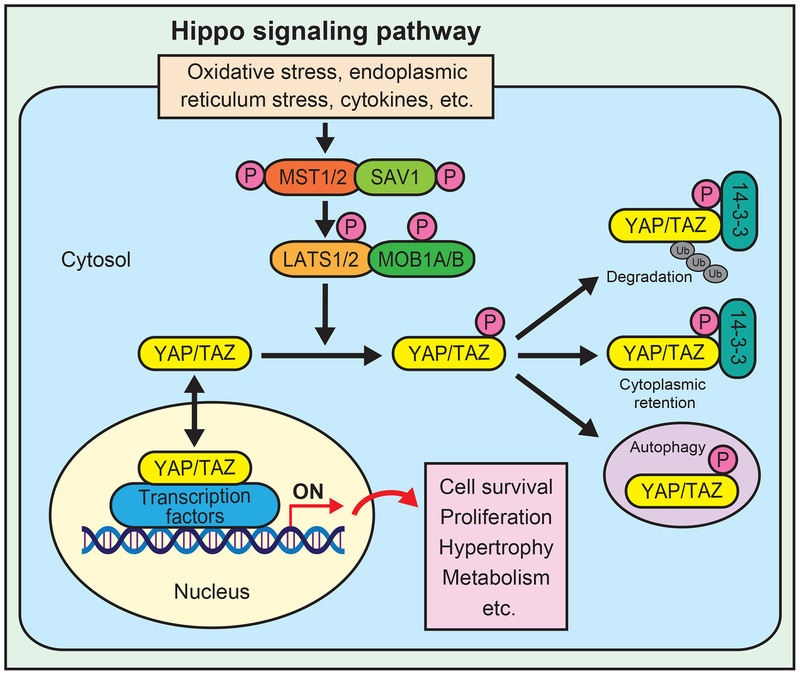
The Hippo pathway, an evolutionarily conserved signaling pathway, generally serves as a key regulator of organ size through regulation of both apoptosis and proliferation (11). Major components of the Hippo pathway include an upstream protein kinase cascade comprising mammalian sterile 20-like kinase 1 and 2 (MST1/2), large tumor suppressor 1 and 2 (LATS1/2), the scaffold protein Salvador (SAV, also known as WW domain-containing adaptor 45 (WW45)), and Mps one binder kinase activator 1A and B (MOB1A/B), and downstream effectors, including YAP and TAZ. In response to stress, such as oxidative stress, endoplasmic reticulum stress, and cytokines, MST1/2 interacts with SAV1 and activates LATS1/2, thereby phosphorylating and inhibiting YAP/TAZ (Figure 1) (15). YAP/TAZ, phosphorylated by the upstream Hippo pathway components, are bound by 14-3-3 and retained in the cytosol (16), where they undergo degradation through the ubiquitin proteasome system (17). YAP Ser127 and TAZ Ser89 are key phosphorylation sites for phosphorylation-induced inhibition by LATS. When the Hippo pathway is inactivated, active YAP/TAZ translocate to the nucleus and interact with multiple transcription factors, such as TEADs, TBX5, SMADs, p73, p63, ERBB4, EGR-1, Erb4, FOXO1, FOXOM1, HIF1α/β, KLF5, FOS, C/EBPα, CREB, OCT4, GLI, TTF-1, PAX3/8, PPARγ, MyoD, and RUNXs (12). TEAD is one of the most well characterized transcription factors mediating the function of YAP/TAZ through expression of genes promoting cell survival, proliferation, and metabolism (14, 18). YAP and TAZ exhibit 60% homology in their amino acid sequences and many of their functions are redundant. Although they also have non-redundant function, thus far, differential effects of YAP and TAZ upon metabolism have not been demonstrated.
Figure 1.
Schematic representation of the Hippo signaling pathway. In response to activation of upstream signaling, MST1/2 interacts with SAV1 and phosphorylates LATS1/2 and MOB1A/B. LATS1/2 in turn phosphorylates and inhibits YAP/TAZ by causing cytoplasmic retention and degradation. Unphosphorylated YAP/TAZ translocates into the nucleus and act as transcription coactivators by interacting with various transcription factors, resulting in cell survival, proliferation, hypertrophy, and changes in metabolism.
The Hippo signaling pathway plays an important role in regulating cardiac development (11). Mouse hearts with Nkx2.5-Cre-mediated embryonic deletion of Hippo signaling components, including Sav, Mst1 or Lats2, exhibit Yap activation, thickened ventricular walls, enlarged ventricular chambers and elevated cardiomyocyte proliferation with no effect on cell size (19). Consistent with this result, Yap gain-of-function is sufficient to promote hyperplasia with cardiomyocyte proliferation but does not induce cardiac hypertrophy in the infant mouse heart (20). In contrast, mouse hearts with Tnnt-Cre-mediated embryonic deletion of Yap exhibit hypoplasia with reduced cardiomyocyte proliferation and eventual embryonic lethality (20). Interestingly, YAP and TAZ have both distinct and overlapping functions in cardiac homeostasis before and after birth (21). Postnatal cardiac-specific knockout of Sav with Myh6-Cre does not affect mouse heart size or cardiomyocyte proliferation at baseline (12). Postnatal cardiac-specific knockout of Yap, but not Taz, with Myh6-Cre in mice induces cardiomyocyte apoptosis, fibrosis and hypertrophy, resulting in cardiomyopathy with premature death (22, 23). In addition, double cardiac-specific knockout of Yap and Taz with Myh6-Cre causes extremely severe and lethal cardiac structural abnormality at birth (23), suggesting that Yap and Taz have non-overlapping functions.
In the adult heart, the Hippo signaling pathway is activated during stress, such as myocardial infarction, ischemia/reperfusion injury, and oxidative stress, leading to suppression of YAP/TAZ (22, 24, 25). YAP promotes an anti-apoptotic effect, compensated hypertrophy, cardiomyocyte proliferation, and myocardial regeneration after cardiac injury or during HF (22, 26). In the mouse heart, YAP is activated transiently, peaking around 7 days, after transverse aortic constriction (TAC), thereby contributing to cardiomyocyte proliferation and hypertrophy, most likely compensatory mechanisms, during the acute phase of pressure overload (12, 26). The activity of the upstream kinases, including Mst1 and Lats2, is increased during the chronic phase of pathological hypertrophy, and, thus, YAP/TAZ is inactivated during cardiac remodeling after myocardial infarction and the chronic phase of HF (12). Reactivation of YAP/TAZ after myocardial infarction may allow cardiac regeneration and improve cardiac function in HF patients (27). It should be noted, however, prolonged activation of YAP in the long-term pressure-overloaded heart induces cardiomyocyte dedifferentiation and HF (12). Thus, although the Hippo pathway and its terminal effectors YAP and TAZ play an essential role in the pathogenesis of HF, their functions appear to be stress- and time-dependent. Currently, the role of the Hippo pathway in metabolic remodeling during HF is poorly understood.
3. The role of YAP/TAZ in regulating metabolism pathways in the heart under physiological and pathophysiological conditions
3.1. Carbohydrate metabolism
The glycolytic pathway starts with glucose, which is sequentially converted to two molecules of pyruvate and two molecules of nicotinamide adenine dinucleotide (NADH) and produces two molecules of ATP from each molecule of glucose. In the healthy adult heart, a relatively small amount (10–40%) of acetyl-CoA derives from pyruvate (4). The contribution of glycolysis to overall ATP production increases when mitochondrial dysfunction develops during myocardial ischemia (10, 28). In moderately ischemic hearts, anaerobic glycolysis is beneficial because glycolytically generated ATP contributes to the maintenance of intracellular Ca2+ homeostasis in cardiac excitation-contraction coupling (29). It should be noted, however, that glycolysis is also activated in the presence of normal levels of oxygen, termed aerobic glycolysis or the Warburg effect (30). Glycolytic conversion of glucose to lactate is often observed in cancer cells in the presence of oxygen. Glycolysis produces less ATP per unit of glucose than oxidative phosphorylation. However, glycolysis not only produces ATP in a low oxygen environment but also supports cell growth through production of macromolecules, such as nucleotides through the pentose phosphate pathway, properties that would benefit tumor growth and cardiac hypertrophy (31) in any oxygen environment. Recent evidence suggests that glucose promotes cardiac hypertrophy through activation of mTOR by the KLF15-branched chain amino acid catabolism pathway (32).
Since YAP/TAZ are involved in cell proliferation and tissue regeneration and that glucose metabolism is intimately involved in cell growth responses, one can speculate that YAP may be involved in both anaerobic and aerobic glycolysis. Previous studies have shown that several oncogenes, including c-Myc, hypoxia-inducible factor 1α (HIF-1α) and tumor suppressors, including p53, are involved in the transcriptional regulation of glycolysis (33). Increasing lines of evidence suggest that YAP/TAZ positively regulate glycolysis and glucose metabolism during organ growth as well as in cancer cells. For example, YAP stimulates nucleotide biosynthesis through activation of glucose uptake in zebrafish (34). Hypoxia-induced YAP activation stimulates glycolysis in hepatocellular carcinoma cells through stabilization of HIF-1α (35). YAP upregulates expression of hexokinase 2 and 6-phosphofructo-2-kinase-2,6-bisphosphatase 3 through lncRNA breast cancer anti-estrogen resistance 4 (BCAR4) and Hedgehog signaling (36). YAP-induced stimulation of glycolysis and nucleotide biosynthesis plays an essential role in mediating cell growth responses (34, 36). YAP may promote survival of tumor cells in the cancer microenvironment through activation of the aerobic glycolytic pathway and consequent activation of anti-apoptotic signaling pathways (37).
YAP and TAZ are positively regulated by phosphofructokinase 1 (38), whereas YAP is negatively regulated by AMP-activated protein kinase (AMPK) (39). Thus, both aerobic glycolysis and the availability of an energy source (glucose) positively regulate YAP/TAZ, thereby promoting a positive feedback mechanism.
We have shown previously that upregulation of YAP/TAZ occurs one week after TAC, which contributes to the development of compensatory hypertrophy and survival of cardiomyocytes (12, 26). HIF-1α is critically involved in the preservation of cardiac function after TAC without affecting cardiac hypertrophy (40). c-Myc activation in response to TAC and ischemia/reperfusion increases glucose uptake/utilization in adult cardiomyocytes, thereby contributing to preserved cardiac function (41). These findings suggest that YAP/TAZ may also promote glycolysis in cardiomyocytes in response to hypertrophic stimuli.
Which target of YAP is involved in stimulating glycolytic activity? YAP promotes glycolysis in hepatocellular carcinoma cells by upregulating pyruvate kinase muscle isozyme 2 through stabilization of HIF-1α (35). YAP upregulates expression of glut1 in the zebrafish liver (34). Glucose transport into cardiomyocytes is mediated primarily through insulin-mediated glucose transporter GLUT4 and, to a lesser degree, through GLUT1 in the healthy adult heart (3). The demand for more ATP (triggered by cell growth) accelerates glucose uptake and utilization in hypertrophied cardiomyocytes (42). Upregulation of Glut1 has been observed in hypertrophied hearts (43). GLUT1 overexpression increases basal glucose transport in the heart (44) and protects the heart against pressure overload-induced cardiac hypertrophy and dysfunction. It would be interesting to investigate whether YAP regulates glucose uptake during the development of cardiac hypertrophy.
Type II diabetes is characterized by insulin resistance and hyperglycemia, and the prevalence of hypertension is higher in diabetic patients than in non-diabetic patients (45). We have shown recently that YAP, which is activated in the mouse heart in response to high fat diet consumption and in heart failure patients with diabetes, contributes to the development of heart failure in the presence of pressure overload, such as high blood pressure (46). In pancreatic β-cells, YAP plays a key role in cell proliferation, stress adaptation and resistance to apoptosis in the presence of diabetes (47). Type II diabetes has been proposed as a major risk factor in the progression of liver cancer (38). The hexosamine biosynthesis pathway (HBP) is an important glucose metabolic pathway to generate uridine diphospho-N-acetylglucosamine (UDP-GlcNAc), which is used by O-linked β-N-acetylglucosamine (O-GlcNAc) transferase (OGT) to catalyze O-GlcNAcylation (48). Under high glucose conditions, including diabetes, O-GlcNAcylation of YAP, but not TAZ, mediates high-glucose-induced liver tumorigenesis through interaction with TEAD and CREB (49). It is possible that O-GlcNAcylation of YAP may modulate YAP-dependent responses in the heart.
3.2. FA Metabolism
The rate of FA uptake in the heart is determined by the concentration of non-esterified FAs in the plasma (50). FAs enter cardiomyocytes through either passive diffusion or protein-mediated transport across the sarcolemma assisted by FA translocase (FAT) and a plasma membrane FA binding protein (FABP). Non-esterified FAs bound to FABP are activated by esterification to fatty acyl-CoA by fatty acyl-CoA synthetase (FACS). Long-chain fatty acyl-CoA can be esterified to triglyceride, an important source of FAs in cardiomyocytes. Alternatively, long-chain fatty acyl-CoA is converted to long-chain fatty acylcarnitine by carnitine palmitoyltransferase I (CPT-I). The resultant long-chain fatty acylcarnitine is eventually transported into mitochondria, and undergoes β-oxidation, thereby producing acetyl-CoA that enters the TCA cycle.
Whether YAP affects FA metabolism remains unclear. YAP is activated in lymph node-metastatic tumors, leading to the upregulation of genes involved in FA oxidation (51). Although this appears different from the Warburg effect, this would suggest that acetyl-CoA and NADH derived from FA oxidation inhibit PDH and drive FA utilization over glucose when activation of YAP occurs. If this mechanism holds true in the heart, downregulation of YAP during HF may contribute to activation of PDH and concomitant downregulation of FA oxidation. Further investigation is required to test this hypothesis. We have proposed recently that inactivation of YAP during the chronic phase of pressure overload prevents de-differentiation of cardiomyocytes, and, thus, it may be adaptive (12, 52). Whether changes in the activity of YAP during the course of pressure overload contributes to the changes in FA metabolism remains to be elucidated.
In hepatocytes, YAP directly interacts with sterol regulatory element binding proteins (SREBP-1 and SREBP-2), thereby promoting lipogenesis and cholesterol synthesis by activating fatty acid synthase and 30-hydroxylmethyl glutaryl coenzyme A reductase (53). YAP is activated in the heart in response to high fat diet consumption in mice (46), a condition in which PPARα is activated (54). Thus far, direct interaction between YAP/TAZ and PPARα has not been demonstrated. Whether YAP contributes to changes in FA metabolism during the early phase of type II diabetes remains to be elucidated. Palmitic acid, the most common saturated FA, suppresses endothelial cell proliferation and migration through inhibition of YAP/TAZ (55).
3.3. Tricarboxylic acid (TCA) cycle
The TCA cycle acts as the biochemical hub and consists of eight sequential reactions in mitochondria (56). This cycle oxidizes acetyl-CoA derived from carbohydrates, lipids, and proteins and generates NADH, flavin adenine dinucleotide (FADH2), and ATP. NADH and FADH2 are in turn fed into the electron transport chain for ATP production. Another important task of the TCA cycle is providing precursors for the biosynthetic pathways.
In hypertrophied rat hearts, the rate of palmitate entry into oxidative metabolism was reduced 23% compared to in normal hearts despite similar TCA cycle flux rates (57). Glucose oxidation through pyruvate dehydrogenase does not increase, however, despite the fact that glycolysis is elevated. This reduced rate may be balanced by a compensatory increase in the anaplerotic flux into the TCA cycle through increased carboxylation of glycolytic pyruvate by malic enzyme (58). Several studies have confirmed this hypertrophy-associated anaplerotic change in the hearts (59, 60). Inhibition of this anaplerotic change may improve cardiac function in the failing heart by normalizing myocardial triacylglyceride content (61).
The TCA cycle intermediate succinate accumulates after ischemia/reperfusion, contributes to the production of reactive oxygen species (ROS) at Complex I through reverse electron transport, and exacerbates ischemia/reperfusion injury (62). Attenuation of succinate accumulation decreases infarct size in vivo (63). Mitochondrial structure and function, including ROS production, are controlled by YAP signaling pathways (64). However, the role of YAP/TAZ in the regulation of the TCA cycle is poorly understood.
Glutamine is one of the most abundant non-essential amino acids in the plasma. Glutaminolysis, an anaplerotic pathway, converts glutamine into α-ketoglutarate and replenishes it for use in the TCA cycle and generation of ATP. Cancer cells and stem cells often rely on glutaminolysis in order to maintain an effective TCA cycle for proliferation (65). Activation of YAP elevates the level of glutamine by inducing expression and the transcriptional activity of glutamine synthetase, resulting in liver enlargement in zebrafish (66). In pulmonary hypertension, stiffening of the extracellular matrix mechanically activates YAP/TAZ, which in turn modulates metabolic enzymes, including glutaminase, in order to coordinate glutaminolysis and glycolysis in vascular cells. Activation of glutaminolysis plays an essential role in mediating proliferation and migration of pulmonary vascular cells (67). Pulmonary hypertension activates glutaminolysis in heart cells as well, which in turn contributes to the development of cardiac hypertrophy, capillary rarefaction, and decreased cardiac contractility. Stimulation of glutaminolysis is mediated though activation of cMyc-Max as a consequence of right ventricular ischemia (68); the involvement of YAP/TAZ remains to be elucidated.
Glutamine uptake in cancer cells relies on neutral amino acid transporter solute carrier family 1 member 5 (SLCA5) (69). Receptor tyrosine kinase EphA2-dependent activation of YAP/TAZ enhances glutamine metabolism by promoting the expression of SLC1A5 (70). Cooperation between mTORC1 and YAP/TAZ mediate amino acid metabolism through upregulation of SLC38A1 and SLC7A5 expression (71). Amino acids supply TCA cycle intermediates, thereby protecting the heart against stress (72). Thus, YAP/TAZ may also protect the heart through regulation of amino acid metabolism.
3.4. Mitochondrial function
Mitochondria are central intracellular organelles that mediate oxidation of carbohydrates and FAs and produce NADH and FADH2. High-energy electrons from NADH and FADH2 are transferred through the electron transport chain (ETC) located on the mitochondrial inner membrane. Energy from these electron transfer reactions makes a proton gradient across the inner mitochondrial membrane, which is then used for ATP synthesis. Mitochondria are dynamic organelles that continuously undergo fusion and fission (73). Mitochondrial fission is controlled by dynamin-related proteins (DRP1), while mitochondrial fusion is driven by mitofusins (Mfn1 and Mfn2) and OPA1 (74). These biological processes play critical roles in maintaining mitochondrial function when cells experience metabolic or environmental stresses (75, 76). Furthermore, mitochondrial dysfunction is now recognized as one of the key mechanisms promoting HF (9). A mitochondria-specific form of autophagy (named mitophagy), by which damaged mitochondria are degraded, plays an essential role in maintaining healthy mitochondria and protecting the heart against pathological stress through multiple pathways (77–79). However, excessive mitophagy caused by YAP deficiency leads to downregulation of ETC complexes I-IV and provides insufficient ATP in hepatocellular carcinoma, suggesting that Hippo/YAP signaling potentially regulates the electron transfer reactions in mitochondria (80).
Cell growth responses are generally accompanied by appropriate mitochondrial biogenesis (81), suggesting that YAP/TAZ-induced cell proliferation and hypertrophy may also be accompanied by mitochondrial remodeling and biogenesis. Remarkably, overexpression of Yki/YAP induces larger mitochondria in human and Drosophila cells, through transcriptional upregulation of opa1 and Marf, genes mediating mitochondrial fusion (64). YAP negatively regulates mitochondrial fission in myoblasts and neuroblastoma cells (82, 83) and promotes Mfn2-mediated mitophagy in gastric cancer cells (84). In addition, YAP-mediated suppression of mitochondrial fission inhibits apoptosis and migration of human rectal cancer cells (85). Whether YAP/TAZ directly regulate genes involved in mitochondrial dynamics, and if so, which transcription factor is involved remain to be elucidated. We have shown previously that Mst1 is activated in mitochondria through a K-Ras-Rassf1A-dependent mechanism. Mst1 phosphorylates Bcl-xL, thereby inducing mitochondria-mediated apoptotic cell death in cardiomyocytes during ischemia/reperfusion (86). It is possible that other molecules in mitochondria are also phosphorylated by Mst1 and, thus, the function of mitochondria may be directly regulated by non-canonical activation of the Hippo pathway in the heart.
4. Conclusions
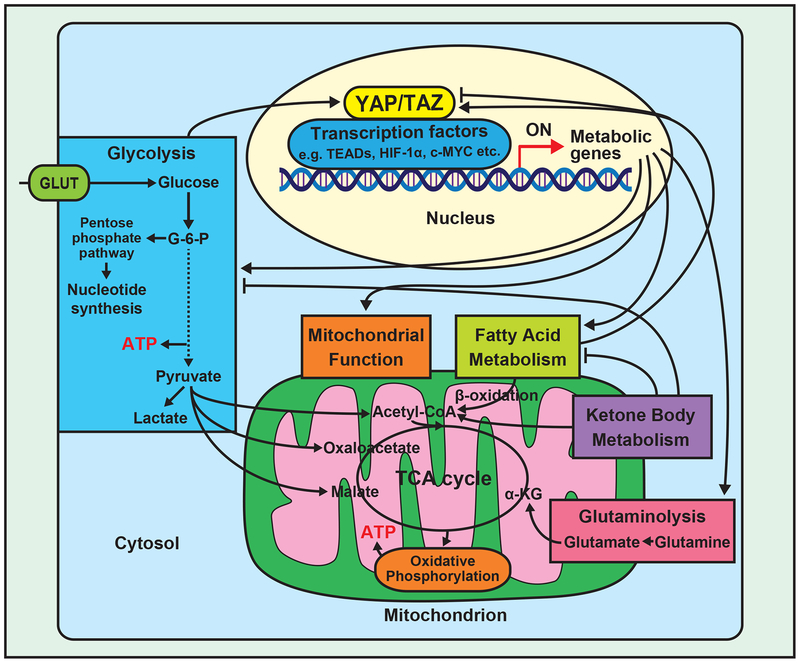
Studies regarding cardiac metabolism in normal and failing hearts have uncovered important alterations in metabolic substrates during HF; however, molecular mechanisms mediating metabolic remodeling remain unclear. As we discussed in this review, YAP/TAZ are likely to regulate metabolism during cardiac stress. Unfortunately, however, the interplay between YAP/TAZ and metabolism has been studied primarily in cancer cells (Figure 2), where YAP/TAZ transcriptionally regulate glucose metabolism, thereby conferring the ability to effectively produce ATP in a stressed environment and generating macromolecules for cell growth. Whether YAP and TAZ regulate metabolism of other substrates, including FAs and ketone bodies, remains poorly understood. Further studies are required to elucidate the role of YAP/TAZ in mediating metabolic remodeling during cardiac stress and HF.
Figure 2.
Summary of the relationship between YAP/TAZ signaling and energy metabolism pathways.
YAP and TAZ are transcription co-factors and, thus, their function is mediated through interaction with downstream transcription factors. We have shown recently that the function of YAP/TAZ is stimulus-specific since distinct transcription factors are induced in response to distinct stresses (87). Although TEAD1 is one of the most well characterized target transcription factors for YAP/TAZ, we have shown that other transcription factors, including FoxO1, can also mediate the effect of YAP/TAZ (24). In addition, YAP/TAZ may affect gene expression through epigenetic mechanisms, including chromatic remodeling (88). Identifying the specific transcription factors mediating the effect of YAP/TAZ would allow a better understanding of the effect of YAP/TAZ upon metabolism in response to cardiac stress.
Recently, specific chemical inhibitors of the components of the Hippo pathway have been developed (89, 90). These compounds may be useful for controlling cardiac metabolism, either alone or in combination with other compounds, to modulate the function of metabolic pathways for the treatment of HF.
Acknowledgements
We thank Daniela Zablocki (Rutgers New Jersey Medical School) for assistance with the manuscript.
Sources of funding:
This work was supported in part by U.S. Public Health Service Grants HL67724, HL91469, HL112330, HL138720, and AG23039 (J.S.), and by the Foundation of Leducq Transatlantic Network of Excellence 15CBD04 (J.S.). T.K. has been supported by a Postdoctoral Fellowship from the Uehara Memorial Foundation.
Abbreviations
- AMPK
AMP-activated protein kinase
- ATP
adenosine triphosphate
- BCAR4
breast cancer anti-estrogen resistance 4
- CPT-I
carnitine palmitoyltransferase I
- DRP1
dynamin-related protein
- ETC
the electron transport chain
- FA
fatty acid
- FABP
FA binding protein
- FACS
fatty acyl-CoA synthetase
- FADH2
flavin adenine dinucleotide
- FAT
FA translocase
- HBP
the hexosamine biosynthesis pathway
- HF
heart failure
- HIF-1α
hypoxia-inducible factor 1α
- LATS1/2
large tumor suppressor 1 and 2
- Mfn
mitofusin
- MOB1A/B
Mps one binder kinase activator 1A and B
- MST1/2
mammalian sterile 20-like kinase 1 and 2
- NADH
nicotinamide adenine dinucleotide
- O-GlcNAc
O-linked β-N-acetylglucosamine
- OGT
O-GlcNAc transferase
- ROS
reactive oxygen species
- SAV
the scaffold protein Salvador
- SLCA5
solute carrier family 1 member 5
- SREBP
sterol regulatory element binding protein
- TAC
transvers aortic constriction
- TAZ
transcriptional coactivator with PDZ-binding motif
- TCA cycle
the tricarboxylic acid cycle
- UDP-GlcNAc
uridine diphospho-N-acetylglucosamine
- WW45
WW domain-containing adaptor 45
- YAP
Yes-associated protein 1
Footnotes
Conflict of interests
The authors declare no conflict of interest.
References
- 1.Suga H. Ventricular energetics. Physiological reviews. 1990. April;70(2):247–77. [DOI] [PubMed] [Google Scholar]
- 2.Wisneski JA, Gertz EW, Neese RA, Gruenke LD, Craig JC. Dual carbon-labeled isotope experiments using D-[6–14C] glucose and L-[1,2,3–13C3] lactate: a new approach for investigating human myocardial metabolism during ischemia. Journal of the American College of Cardiology. 1985. May;5(5):1138–46. [DOI] [PubMed] [Google Scholar]
- 3.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005. July;85(3):1093–129. [DOI] [PubMed] [Google Scholar]
- 4.Stanley WC, Lopaschuk GD, Hall JL, McCormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions. Cardiovascular research. 1997. February;33(2):243–57. [DOI] [PubMed] [Google Scholar]
- 5.Lopaschuk GD, Spafford MA, Marsh DR. Glycolysis is predominant source of myocardial ATP production immediately after birth. The American journal of physiology. 1991. December;261(6 Pt 2):H1698–705. [DOI] [PubMed] [Google Scholar]
- 6.Makinde AO, Kantor PF, Lopaschuk GD. Maturation of fatty acid and carbohydrate metabolism in the newborn heart. Molecular and cellular biochemistry. 1998. November;188(1–2):49–56. [PubMed] [Google Scholar]
- 7.Roger VL. Epidemiology of heart failure. Circ Res. 2013. August 30;113(6):646–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagoshi T, Yoshimura M, Rosano GM, Lopaschuk GD, Mochizuki S. Optimization of cardiac metabolism in heart failure. Current pharmaceutical design. 2011. December;17(35):3846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res. 2013. August 30;113(6):709–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doenst T, Pytel G, Schrepper A, Amorim P, Farber G, Shingu Y, Mohr FW, Schwarzer M. Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovascular research. 2010. June 1;86(3):461–70. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Q, Li L, Zhao B, Guan KL. The hippo pathway in heart development, regeneration, and diseases. Circulation research. 2015. April 10;116(8):1431–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikeda S, Mizushima W, Sciarretta S, Abdellatif M, Zhai P, Mukai R, Fefelova N, Oka SI, Nakamura M, Del Re DP, Farrance I, Park JY, Tian B, Xie LH, Kumar M, Hsu CP, Sadayappan S, Shimokawa H, Lim DS, Sadoshima J. Hippo Deficiency Leads to Cardiac Dysfunction Accompanied by Cardiomyocyte Dedifferentiation During Pressure Overload. Circ Res. 2019. January 18;124(2):292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Zhao H, Li Y, Xia D, Yang L, Ma Y, Li H. The role of YAP/TAZ activity in cancer metabolic reprogramming. Molecular cancer. 2018. September 3;17(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koo JH, Guan KL. Interplay between YAP/TAZ and Metabolism. Cell metabolism. 2018. August 7;28(2):196–206. [DOI] [PubMed] [Google Scholar]
- 15.Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016. January 01;30(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007. September 21;130(6):1120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes & development. 2010. January 1;24(1):72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008. July 15;22(14):1962–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011. April 22;332(6028):458–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Gise A, Lin Z, Schlegelmilch K, Honor LB, Pan GM, Buck JN, Ma Q, Ishiwata T, Zhou B, Camargo FD, Pu WT. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc Natl Acad Sci U S A. 2012. February 14;109(7):2394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plouffe SW, Lin KC, Moore JL, Tan FE, Ma S, Ye Z, Qiu Y, Ren B, Guan KL. The Hippo pathway effector proteins YAP and TAZ have both distinct and overlapping functions in the cell. J Biol Chem. 2018. July 13;293(28):11230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Re DP, Yang Y, Nakano N, Cho J, Zhai P, Yamamoto T, Zhang N, Yabuta N, Nojima H, Pan D, Sadoshima J. Yes-associated protein isoform 1 (Yap1) promotes cardiomyocyte survival and growth to protect against myocardial ischemic injury. J Biol Chem. 2013. February 8;288(6):3977–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, Richardson JA, Sadek HA, Bassel-Duby R, Olson EN. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci U S A. 2013. August 20;110(34):13839–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shao D, Zhai P, Del Re DP, Sciarretta S, Yabuta N, Nojima H, Lim DS, Pan D, Sadoshima J. A functional interaction between Hippo-YAP signalling and FoxO1 mediates the oxidative stress response. Nature communications. 2014. February 14;5:3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuda T, Zhai P, Sciarretta S, Zhang Y, Jeong JI, Ikeda S, Park J, Hsu CP, Tian B, Pan D, Sadoshima J, Del Re DP. NF2 Activates Hippo Signaling and Promotes Ischemia/Reperfusion Injury in the Heart. Circulation research. 2016. August 19;119(5):596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byun J, Del Re DP, Zhai P, Ikeda S, Shirakabe A, Mizushima W, Miyamoto S, Brown JH, Sadoshima J. Yes-Associated Protein (YAP) mediates adaptive cardiac hypertrophy in response to pressure overload. The Journal of biological chemistry. 2019. January 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Liu S, Heallen T, Martin JF. The Hippo pathway in the heart: pivotal roles in development, disease, and regeneration. Nat Rev Cardiol. 2018. November;15(11):672–84. [DOI] [PubMed] [Google Scholar]
- 28.Rovetto MJ, Whitmer JT, Neely JR. Comparison of the effects of anoxia and whole heart ischemia on carbohydrate utilization in isolated working rat hearts. Circulation research. 1973. June;32(6):699–711. [DOI] [PubMed] [Google Scholar]
- 29.Xu KY, Zweier JL, Becker LC. Functional coupling between glycolysis and sarcoplasmic reticulum Ca2+ transport. Circulation research. 1995. July;77(1):88–97. [DOI] [PubMed] [Google Scholar]
- 30.Warburg O. On respiratory impairment in cancer cells. Science (New York, NY). 1956. August 10;124(3215):269–70. [PubMed] [Google Scholar]
- 31.Ma H, Yu S, Liu X, Zhang Y, Fakadej T, Liu Z, Yin C, Shen W, Locasale JW, Taylor JM, Qian L, Liu J. Lin28a Regulates Pathological Cardiac Hypertrophic Growth Through Pck2-Mediated Enhancement of Anabolic Synthesis. Circulation. 2019. April 2;139(14):1725–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao D, Villet O, Zhang Z, Choi SW, Yan J, Ritterhoff J, Gu H, Djukovic D, Christodoulou D, Kolwicz SC Jr., Raftery D, Tian R. Glucose promotes cell growth by suppressing branched-chain amino acid degradation. Nature communications. 2018. July 26;9(1):2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen JQ, Russo J. Dysregulation of glucose transport, glycolysis, TCA cycle and glutaminolysis by oncogenes and tumor suppressors in cancer cells. Biochimica et biophysica acta. 2012. December;1826(2):370–84. [DOI] [PubMed] [Google Scholar]
- 34.Cox AG, Tsomides A, Yimlamai D, Hwang KL, Miesfeld J, Galli GG, Fowl BH, Fort M, Ma KY, Sullivan MR, Hosios AM, Snay E, Yuan M, Brown KK, Lien EC, Chhangawala S, Steinhauser ML, Asara JM, Houvras Y, Link B, Vander Heiden MG, Camargo FD, Goessling W. Yap regulates glucose utilization and sustains nucleotide synthesis to enable organ growth. EMBO J. 2018. November 15;37(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Li Y, Ma Y, Yang L, Wang T, Meng X, Zong Z, Sun X, Hua X, Li H. Yes-associated protein (YAP) binds to HIF-1alpha and sustains HIF-1alpha protein stability to promote hepatocellular carcinoma cell glycolysis under hypoxic stress. J Exp Clin Cancer Res. 2018. September 4;37(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng X, Han H, Liu GP, Ma YX, Pan RL, Sang LJ, Li RH, Yang LJ, Marks JR, Wang W, Lin A. LncRNA wires up Hippo and Hedgehog signaling to reprogramme glucose metabolism. EMBO J. 2017. November 15;36(22):3325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen Y, Zhao S, Wang S, Pan X, Zhang Y, Xu J, Jiang Y, Li H, Zhang Q, Gao J, Yang Q, Zhou Y, Jiang S, Yang H, Zhang Z, Zhang R, Li J, Zhou D. S1P/S1PR3 axis promotes aerobic glycolysis by YAP/c-MYC/PGAM1 axis in osteosarcoma. EBioMedicine. 2019. February;40:210–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Enzo E, Santinon G, Pocaterra A, Aragona M, Bresolin S, Forcato M, Grifoni D, Pession A, Zanconato F, Guzzo G, Bicciato S, Dupont S. Aerobic glycolysis tunes YAP/TAZ transcriptional activity. EMBO J. 2015. May 12;34(10):1349–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang W, Xiao ZD, Li X, Aziz KE, Gan B, Johnson RL, Chen J. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015. April;17(4):490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silter M, Kogler H, Zieseniss A, Wilting J, Schafer K, Toischer K, Rokita AG, Breves G, Maier LS, Katschinski DM. Impaired Ca(2+)-handling in HIF-1alpha(+/−) mice as a consequence of pressure overload. Pflugers Archiv : European journal of physiology. 2010. March;459(4):569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahuja P, Zhao P, Angelis E, Ruan H, Korge P, Olson A, Wang Y, Jin ES, Jeffrey FM, Portman M, Maclellan WR. Myc controls transcriptional regulation of cardiac metabolism and mitochondrial biogenesis in response to pathological stress in mice. The Journal of clinical investigation. 2010. May;120(5):1494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, Duncker DJ, Ya X, Zhang Y, Pavek T, Wei H, Merkle H, Ugurbil K, From AH, Bache RJ. Effect of left ventricular hypertrophy secondary to chronic pressure overload on transmural myocardial 2-deoxyglucose uptake. A 31P NMR spectroscopic study. Circulation. 1995. September 1;92(5):1274–83. [DOI] [PubMed] [Google Scholar]
- 43.Tian R, Musi N, D’Agostino J, Hirshman MF, Goodyear LJ. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation. 2001. October 2;104(14):1664–9. [DOI] [PubMed] [Google Scholar]
- 44.Liao R, Jain M, Cui L, D’Agostino J, Aiello F, Luptak I, Ngoy S, Mortensen RM, Tian R. Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation. 2002. October 15;106(16):2125–31. [DOI] [PubMed] [Google Scholar]
- 45.Sowers JR. Diabetes mellitus and vascular disease. Hypertension. 2013. May;61(5):943–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ikeda S, Mukai R, Mizushima W, Zhai P, Oka SI, Nakamura M, Del Re DP, Sciarretta S, Hsu CP, Shimokawa H, Sadoshima J. Yes-associated protein (YAP) facilitates pressure-overload induced dysfunction in the diabetic heart. JACC Basic Transl Sci. 2019:(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ardestani A, Maedler K. The Hippo Signaling Pathway in Pancreatic beta-Cells: Functions and Regulations. Endocr Rev. 2018. February 1;39(1):21–35. [DOI] [PubMed] [Google Scholar]
- 48.Teo CF, Wollaston-Hayden EE, Wells L. Hexosamine flux, the O-GlcNAc modification, and the development of insulin resistance in adipocytes. Molecular and cellular endocrinology. 2010. April 29;318(1–2):44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, Qiao Y, Wu Q, Chen Y, Zou S, Liu X, Zhu G, Zhao Y, Chen Y, Yu Y, Pan Q, Wang J, Sun F. The essential role of YAP O-GlcNAcylation in high-glucose-stimulated liver tumorigenesis. Nature communications. 2017. May 5;8:15280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopaschuk GD, Belke DD, Gamble J, Itoi T, Schonekess BO. Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochimica et biophysica acta. 1994. August 4;1213(3):263–76. [DOI] [PubMed] [Google Scholar]
- 51.Lee CK, Jeong SH, Jang C, Bae H, Kim YH, Park I, Kim SK, Koh GY. Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science (New York, NY). 2019. February 8;363(6427):644–9. [DOI] [PubMed] [Google Scholar]
- 52.Sheeran FL, Angerosa J, Liaw NY, Cheung MM, Pepe S. Adaptations in Protein Expression and Regulated Activity of Pyruvate Dehydrogenase Multienzyme Complex in Human Systolic Heart Failure. Oxidative medicine and cellular longevity. 2019;2019:4532592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shu Z, Gao Y, Zhang G, Zhou Y, Cao J, Wan D, Zhu X, Xiong W. A functional interaction between Hippo-YAP signalling and SREBPs mediates hepatic steatosis in diabetic mice. J Cell Mol Med. 2019. May;23(5):3616–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakamura M, Liu T, Husain S, Zhai P, Warren JS, Hsu CP, Matsuda T, Phiel CJ, Cox JE, Tian B, Li H, Sadoshima J. Glycogen Synthase Kinase-3alpha Promotes Fatty Acid Uptake and Lipotoxic Cardiomyopathy. Cell metabolism. 2019. May 7;29(5):1119–34.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan L, Mao Y, Luo W, Wu W, Xu H, Wang XL, Shen YH. Palmitic acid dysregulates the Hippo-YAP pathway and inhibits angiogenesis by inducing mitochondrial damage and activating the cytosolic DNA sensor cGAS-STING-IRF3 signaling mechanism. The Journal of biological chemistry. 2017. September 8;292(36):15002–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krebs HA. The citric acid cycle and the Szent-Gyorgyi cycle in pigeon breast muscle. The Biochemical journal. 1940. May;34(5):775–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sorokina N, O’Donnell JM, McKinney RD, Pound KM, Woldegiorgis G, LaNoue KF, Ballal K, Taegtmeyer H, Buttrick PM, Lewandowski ED. Recruitment of compensatory pathways to sustain oxidative flux with reduced carnitine palmitoyltransferase I activity characterizes inefficiency in energy metabolism in hypertrophied hearts. Circulation. 2007. April 17;115(15):2033–41. [DOI] [PubMed] [Google Scholar]
- 58.Brunengraber H, Roe CR. Anaplerotic molecules: current and future. J Inherit Metab Dis. 2006. Apr-Jun;29(2–3):327–31. [DOI] [PubMed] [Google Scholar]
- 59.Kolwicz SC Jr., Olson DP, Marney LC, Garcia-Menendez L, Synovec RE, Tian R. Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circulation research. 2012. August 31;111(6):728–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Atherton HJ, Dodd MS, Heather LC, Schroeder MA, Griffin JL, Radda GK, Clarke K, Tyler DJ. Role of pyruvate dehydrogenase inhibition in the development of hypertrophy in the hyperthyroid rat heart: a combined magnetic resonance imaging and hyperpolarized magnetic resonance spectroscopy study. Circulation. 2011. June 7;123(22):2552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pound KM, Sorokina N, Ballal K, Berkich DA, Fasano M, Lanoue KF, Taegtmeyer H, O’Donnell JM, Lewandowski ED. Substrate-enzyme competition attenuates upregulated anaplerotic flux through malic enzyme in hypertrophied rat heart and restores triacylglyceride content: attenuating upregulated anaplerosis in hypertrophy. Circulation research. 2009. March 27;104(6):805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pell VR, Chouchani ET, Frezza C, Murphy MP, Krieg T. Succinate metabolism: a new therapeutic target for myocardial reperfusion injury. Cardiovascular research. 2016. July 15;111(2):134–41. [DOI] [PubMed] [Google Scholar]
- 63.Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord ENJ, Smith AC, Eyassu F, Shirley R, Hu CH, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa ASH, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, Murphy MP. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014. November 20;515(7527):431–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nagaraj R, Gururaja-Rao S, Jones KT, Slattery M, Negre N, Braas D, Christofk H, White KP, Mann R, Banerjee U. Control of mitochondrial structure and function by the Yorkie/YAP oncogenic pathway. Genes & development. 2012. September 15;26(18):2027–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li T, Le A. Glutamine Metabolism in Cancer. Advances in experimental medicine and biology. 2018;1063:13–32. [DOI] [PubMed] [Google Scholar]
- 66.Cox AG, Hwang KL, Brown KK, Evason K, Beltz S, Tsomides A, O’Connor K, Galli GG, Yimlamai D, Chhangawala S, Yuan M, Lien EC, Wucherpfennig J, Nissim S, Minami A, Cohen DE, Camargo FD, Asara JM, Houvras Y, Stainier DYR, Goessling W. Yap reprograms glutamine metabolism to increase nucleotide biosynthesis and enable liver growth. Nature cell biology. 2016. August;18(8):886–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, Yu Q, Zhao J, Tai Y, Tang Y, Zhang YY, Rehman S, Sugahara M, Qi Z, Gorcsan J 3rd, Vargas SO, Saggar R, Saggar R, Wallace WD, Ross DJ, Haley KJ, Waxman AB, Parikh VN, De Marco T, Hsue PY, Morris A, Simon MA, Norris KA, Gaggioli C, Loscalzo J, Fessel J, Chan SY. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. The Journal of clinical investigation. 2016. September 1;126(9):3313–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Piao L, Fang YH, Parikh K, Ryan JJ, Toth PT, Archer SL. Cardiac glutaminolysis: a maladaptive cancer metabolism pathway in the right ventricle in pulmonary hypertension. Journal of molecular medicine. 2013. October;91(10):1185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kanai Y, Hediger MA. The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pflugers Archiv : European journal of physiology. 2004. February;447(5):469–79. [DOI] [PubMed] [Google Scholar]
- 70.Edwards DN, Ngwa VM, Wang S, Shiuan E, Brantley-Sieders DM, Kim LC, Reynolds AB, Chen J. The receptor tyrosine kinase EphA2 promotes glutamine metabolism in tumors by activating the transcriptional coactivators YAP and TAZ. Science signaling. 2017. December 5;10(508). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park YY, Sohn BH, Johnson RL, Kang MH, Kim SB, Shim JJ, Mangala LS, Kim JH, Yoo JE, Rodriguez-Aguayo C, Pradeep S, Hwang JE, Jang HJ, Lee HS, Rupaimoole R, Lopez-Berestein G, Jeong W, Park IS, Park YN, Sood AK, Mills GB, Lee JS. Yes-associated protein 1 and transcriptional coactivator with PDZ-binding motif activate the mammalian target of rapamycin complex 1 pathway by regulating amino acid transporters in hepatocellular carcinoma. Hepatology (Baltimore, Md). 2016. January;63(1):159–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang Y, Zhou M, Sun H, Wang Y. Branched-chain amino acid metabolism in heart disease: an epiphenomenon or a real culprit? Cardiovascular research. 2011. May 1;90(2):220–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bereiter-Hahn J, Voth M. Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microscopy research and technique. 1994. February 15;27(3):198–219. [DOI] [PubMed] [Google Scholar]
- 74.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006. June 30;125(7):1241–52. [DOI] [PubMed] [Google Scholar]
- 75.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012. August 31;337(6098):1062–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martin OJ, Lai L, Soundarapandian MM, Leone TC, Zorzano A, Keller MP, Attie AD, Muoio DM, Kelly DP. A role for peroxisome proliferator-activated receptor gamma coactivator-1 in the control of mitochondrial dynamics during postnatal cardiac growth. Circulation research. 2014. February 14;114(4):626–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saito T, Sadoshima J. Molecular mechanisms of mitochondrial autophagy/mitophagy in the heart. Circulation research. 2015. April 10;116(8):1477–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shirakabe A, Zhai P, Ikeda Y, Saito T, Maejima Y, Hsu CP, Nomura M, Egashira K, Levine B, Sadoshima J. Drp1-Dependent Mitochondrial Autophagy Plays a Protective Role Against Pressure Overload-Induced Mitochondrial Dysfunction and Heart Failure. Circulation. 2016. March 29;133(13):1249–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saito T, Nah J, Oka SI, Mukai R, Monden Y, Maejima Y, Ikeda Y, Sciarretta S, Liu T, Li H, Baljinnyam E, Fraidenraich D, Fritzky L, Zhai P, Ichinose S, Isobe M, Hsu CP, Kundu M, Sadoshima J. An alternative mitophagy pathway mediated by Rab9 protects the heart against ischemia. The Journal of clinical investigation. 2019. February 1;129(2):802–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shi C, Cai Y, Li Y, Li Y, Hu N, Ma S, Hu S, Zhu P, Wang W, Zhou H. Yap promotes hepatocellular carcinoma metastasis and mobilization via governing cofilin/F-actin/lamellipodium axis by regulation of JNK/Bnip3/SERCA/CaMKII pathways. Redox Biol. 2018. April;14:59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luo C, Widlund HR, Puigserver P. PGC-1 Coactivators: Shepherding the Mitochondrial Biogenesis of Tumors. Trends Cancer. 2016. October;2(10):619–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Geng C, Wei J, Wu C. Yap-Hippo pathway regulates cerebral hypoxia-reoxygenation injury in neuroblastoma N2a cells via inhibiting ROCK1/F-actin/mitochondrial fission pathways. Acta Neurol Belg. 2018. May 23. [DOI] [PubMed] [Google Scholar]
- 83.Huang S, Wang X, Wu X, Yu J, Li J, Huang X, Zhu C, Ge H. Yap regulates mitochondrial structural remodeling during myoblast differentiation. Am J Physiol Cell Physiol. 2018. October 1;315(4):C474–C84. [DOI] [PubMed] [Google Scholar]
- 84.Yan H, Qiu C, Sun W, Gu M, Xiao F, Zou J, Zhang L. Yap regulates gastric cancer survival and migration via SIRT1/Mfn2/mitophagy. Oncol Rep. 2018. April;39(4):1671–81. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85.Li H, He F, Zhao X, Zhang Y, Chu X, Hua C, Qu Y, Duan Y, Ming L. YAP Inhibits the Apoptosis and Migration of Human Rectal Cancer Cells via Suppression of JNK-Drp1-Mitochondrial Fission-HtrA2/Omi Pathways. Cell Physiol Biochem. 2017;44(5):2073–89. [DOI] [PubMed] [Google Scholar]
- 86.Del Re DP, Matsuda T, Zhai P, Maejima Y, Jain MR, Liu T, Li H, Hsu CP, Sadoshima J. Mst1 promotes cardiac myocyte apoptosis through phosphorylation and inhibition of Bcl-xL. Mol Cell. 2014. May 22;54(4):639–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ikeda S, Sadoshima J. Regulation of Myocardial Cell Growth and Death by the Hippo Pathway. Circ J. 2016. June 24;80(7):1511–9. [DOI] [PubMed] [Google Scholar]
- 88.Zhu Y, Li D, Wang Y, Pei C, Liu S, Zhang L, Yuan Z, Zhang P. Brahma regulates the Hippo pathway activity through forming complex with Yki-Sd and regulating the transcription of Crumbs. Cell Signal. 2015. March;27(3):606–13. [DOI] [PubMed] [Google Scholar]
- 89.Fan F, He Z, Kong LL, Chen Q, Yuan Q, Zhang S, Ye J, Liu H, Sun X, Geng J, Yuan L, Hong L, Xiao C, Zhang W, Sun X, Li Y, Wang P, Huang L, Wu X, Ji Z, Wu Q, Xia NS, Gray NS, Chen L, Yun CH, Deng X, Zhou D. Pharmacological targeting of kinases MST1 and MST2 augments tissue repair and regeneration. Science translational medicine. 2016. August 17;8(352):352ra108. [DOI] [PubMed] [Google Scholar]
- 90.Han Y. Analysis of the role of the Hippo pathway in cancer. Journal of translational medicine. 2019. April 8;17(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]