Abstract
Objectives:
The National Health Insurance Bureau of Taiwan has established a postacute care model of stroke (PAC-stroke). Patients with acute stroke occurring within the preceding 30 days and with modified Rankin scale (mRS) scores of 2–4 can be transferred to PAC hospitals for 6–12 weeks of rehabilitation. We conducted a retrospective review to explore the results of PAC-stroke.
Materials and Methods:
From April 2015 to December 2017, patients who transferred from our hospital to four PAC hospitals were reviewed. We evaluated their functional status using the mRS, Barthel index (BI), functional oral intake scale, EuroQoL-5D, Lawton–Brody instrumental activities of daily living scale, Berg balance test, usual gait speed, 6-min walk test, Fugl–Meyer sensory and motor assessments, mini-mental state examination, motor activity log quantity and quality tests, and concise Chinese aphasia test, before and after the PAC program.
Results:
A total of 53 patients with initial mRS score of 3 (6 patients) or 4 (47 patients) were enrolled, including 39 with cerebral infarction and 14 with cerebral hemorrhage, with a median age of 67 (mean: 68.3 ± 13.3) years. Seven patients had serious complications, including six cases of pneumonia and one fracture. The readmission rates within 14 days after transfer to the PAC hospital and in the overall PAC program were 3.8% and 13.2%, respectively. After exclusion of eight patients who dropped out early, 45 patients completed the PAC program. The median lengths of stay at the upstream hospital and PAC hospitals were 26 and 63 days, respectively. Improved mRS and BI scores were observed in 42% and 78% of the patients, respectively. The results of all 14 functional assessments improved significantly after the PAC program.
Conclusion:
Significant improvements in mRS and BI scores and all functional assessments within an average of 63 days of PAC hospital stay helped 73% of the patients to return home.
KEYWORDS: Acute stroke, Barthel index, Functional outcome, Modified Rankin scale, Postacute care
INTRODUCTION
With improvements in the treatment of acute stroke with intravenous thrombolytic therapy [1] and strict adherence to the stroke guidelines established by the American Stroke Association [2] and Taiwan Stroke Society [3,4], stroke has dropped from the second to the fourth highest cause of death in Taiwan [5] over the preceding two decades. However, it is still the leading cause of disability in the elderly. Early rehabilitation after treatment for acute stroke has been recommended to improve functional outcomes [6]. An efficient postacute care (PAC) program may also improve functional outcomes for patients with stroke and disabilities and hence reduce treatment costs [7,8]. Although intravenous thrombolytic therapy successfully reduces the severity of stroke after the onset of symptoms, there is no well-organized system or network for surviving patients with disabilities. In Taiwan, the public ambulance system delivers each patient with acute stroke to the closest regional hospital or medical center for immediate care. Patients may require transfer from a regional hospital to a tertiary referral center if an intra-arterial thrombectomy is required. Furthermore, patients are free to use their preferred medical center or regional hospital without referral. Under these circumstances, resources at medical centers or large regional hospitals may be scarce if lengthy hospital rehabilitation is necessary.
The two primary public health issues related to stroke in Taiwan are prolonged hospital stay and readmission after discharge. Prolonged hospital stays occur more often among patients with stroke hospitalized in medical centers, whereas readmission is more frequent among those hospitalized in district hospitals [9]. The National Health Insurance (NHI) Bureau of Taiwan established an extended rehabilitation program called the PAC model of stroke (PAC-stroke) in 2014. In this program, upstream medical centers or large regional hospitals are partnered with downstream regional or district PAC hospitals to provide ongoing patient care. A PAC hospital provides a well-organized team consisting of a physician familiar with stroke care, physical therapists, occupational therapists, a speech therapist, a nutritionist, a pharmacist, and a social worker. Redistribution of patients from overcrowded upstream hospitals to PAC hospitals promotes better utilization of medical resources. Our hospital joined the PAC-stroke system as an upstream hospital in April 2015. We conducted a retrospective review to explore the performance of the PAC-stroke program implemented at this hospital.
MATERIALS AND METHODS
We retrospectively reviewed the medical records of all inpatients with acute stroke participating in the PAC-stroke program from April 2015 to December 2017. The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of the institution (as no. 06-XD05-01). Informed written consent was waived because the study was a retrospective data analysis. According to the PAC-stroke inclusion criteria, patients with acute stroke admitted to hospital in stable condition within 30 days of symptom onset and with modified Rankin Scale (mRS; graded from 0 to 6; higher scores indicate more severe disability) [10] scores of 2–4 are candidates for transfer to PAC hospitals. Medical care and rehabilitation then continue at the PAC hospital with 1–3 h of intensive rehabilitation per workday over the following 6–12 weeks. Physicians from the upstream hospital are obliged to make follow-up visits to patients at PAC hospitals at least once within 3 weeks of transfer. We collected the following information for each patient: age, sex, length of stay at upstream and PAC hospitals and classification of ischemic stroke according to the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria [11], National Institute of Health Stroke Scale (NIHSS) scores of patients with ischemic stroke, serious complications during PAC, and final outcome.
The functional status of each patient enrolled in the PAC program was routinely assessed at the PAC hospital by PAC team members, who were specialists in relevant fields, such as physicians, physical therapists, occupational therapists, a speech therapist, and a nutritionist, before and after 6–12 weeks of rehabilitation. A total of 14 functional assessments were performed. These included the mRS, Barthel index (BI; a 10-item scale from 0 to 100 with 5-point increments; higher scores indicate higher quality of daily activities) [12], functional oral intake scale (a 7-point scale assessing the typical functional oral intake of patients with stroke and dysphagia; higher scores indicate better oral intake function) [13], the EuroQoL-5D (a measure of health status scored from 0 to 15 to provide a simple generic measure of health for clinical and economic appraisal; lower scores indicate better health status) [14], the Lawton–Brody instrumental activities of daily living (IADL) scale (domains of function scored from 0 to 8 assessing independent living skills; higher scores indicate higher quality of daily functions) [15], the Berg balance test (a performance-based measure of balance consisting of 14 observable tasks scored from 0 to 56; higher scores indicate better balance) [16], usual gait speed (5-m walking time; shorter durations indicate better gait speed) [17], the 6-min walking test (distance walked without assistance in 6 min; longer distances indicate better walking ability) [18], the Fugl–Meyer sensory assessment (a four-domain sensory test for the limbs scored from 0 to 44, with each rating denoting a specific level of ability between 0 and 2; higher scores indicate better sensory function) and motor assessment (a 33-item upper extremity motor assessment scale scored from 0 to 66, with each rating denoting a specific level of ability between 0 and 2; higher scores indicate better motor function) [19], the mini-mental state examination (an 11-item higher cortical function test scored from 0 to 30; higher scores indicate a higher cortical function) [20], motor activity log quantity and quality tests (average score on a 30-item quantity and quality assessment of affected arm movement, with each item ranked from 0 to 5; higher scores indicate better arm movement) [21], and the concise Chinese aphasia test (an average score of 9 language subtests with a 12-point multidimensional scoring system; higher scores indicate better speech function) [22].
Distributions of the measured variables were skewed, and thus, means and medians alongside 25th and 75th percentiles were used to explain the range. The Mann–Whitney U-test was used to evaluate differences in continuous variables, and the Wilcoxon signed-rank test was conducted to evaluate the changes in variables before and after the PAC program. A P < 0.05 was considered statistically significant. All statistical analyses were performed using PASW Statistics for Windows, Version 18 (SPSS Inc., Chicago, IL, USA).
RESULTS
During the study period, there were 460 inpatients with acute stroke with discharge mRS scores of 2–4. Of these 53 patients (11.5%), 36 men and 17 women with a median age of 67 years (mean: 68.3 ± 13.3 years) were transferred from this upstream hospital to four district PAC hospitals. The patients with cerebral infarction (n = 39) were transferred from the neurological department with initial mRS score of 3 in six patients and 4 in 33 patients. All patients with cerebral hemorrhage (n = 14) had an initial mRS score of 4 and were transferred from the neurosurgical department, except for one who was transferred from the rehabilitation department. Only one patient with cerebral hemorrhage received surgical intervention in the neurosurgical department during the acute stage. Cerebral infarction affected 83% of the men and 53% of the women [Table 1]. There were 21 patients with large artery atherosclerosis, 11 patients with small vessel occlusion, and seven patients with cardioembolism, according to the TOAST classification. The average NIHSS scores at admission and transfer to PAC hospitals were higher in patients with large artery atherosclerosis (12.9 ± 7.4 and 10.6 ± 7.3, respectively) and cardioembolism (14.6 ± 6.6 and 11.4 ± 5.4, respectively) than those with small vessel occlusion (6.9 ± 3.0 and 5.2 ± 1.8, respectively) (P < 0.05). Eight patients with an initial mRS score of 4 dropped out of the PAC program early. One patient was discharged 1 week after being transferred owing to dissatisfaction with the service at the PAC hospital. Seven patients were readmitted to the upstream hospital because of serious complications, including one patient who had a fall resulting in a femoral fracture and six patients with severe pneumonia after a median of 31 PAC days (range: 2–73 days); four of these six patients later died of sepsis. The mean durations of hospitalization before PAC program initiation and transfer were 17 ± 7 and 25 ± 6 days, respectively, at the upstream hospital. The average BI score before the PAC program was 25 ± 24 and was higher in men than women and in those with mRS score of 3 than those with scores of 4. The overall readmission rate related to serious complications in all patients throughout the PAC period was 13.2% (7/53), with 3.8% (2/53) occurring within 14 days after transfer. All deaths occurred in patients who had an initial mRS score of 4, with an overall mortality rate of 7.5% (4/53).
Table 1.
Comparison of patient characteristics by sex and initial modified Rankin scale score before the postacute care model of stroke program
| Characteristics | Gender | Initial mRS | Total (n=53) | ||
|---|---|---|---|---|---|
| Men (n=36) | Women (n=17) | mRS 3 (n=6) | mRS 4 (n=47) | ||
| Age (years) | 68 (60-76) | 67 (54-80) | 59 (54-71) | 68 (58-79) | 67 (57-77) |
| Men (%) | 6 (100) | 30 (64) | 36 (68) | ||
| Cerebral infarct (%) | 30 (83)* | 9 (53) | 6 (100) | 33 (70) | 39 (74) |
| LOS at upstream hospital (days) | 26 (22-27) | 28 (25-29) | 24 (20-27) | 26 (23-29) | 26 (23-29) |
| Initial mRS before PAC | 4 (4-4) | 4 (4-4) | 4 (4-4) | ||
| Initial Barthel index before PAC | 23 (9-51)* | 5 (0-30) | 68 (65-70)** | 25 (0-35) | 20 (0-40) |
| Serious complications during PAC (%) | 3 (8) | 4 (24) | 1 (17) | 6 (13) | 7 (13) |
Data are presented as n (%) or median (25th-75th percentile). *P<0.05, **P<0.001; χ2 or Mann–Whitney U-test. LOS: Length of stay, mRS: Modified Rankin scale, PAC: Postacute care
Table 2 shows the significant differences in clinical characteristics between patients with and without severe pneumonia. Patients with pneumonia were older (median age: 85 vs. 65 years) and had a longer interval from initiation of the PAC program to transfer to the PAC hospital (median interval: 15 vs. 7 days) and lower initial scores on the Lawton–Brody IADL scale, Fugl–Meyer motor assessment, mini-mental state examination, and motor activity log quality test than those without pneumonia. Severe pneumonia was more frequently observed in women than in men and those with lower initial BI scores; however, these findings did not achieve statistical significance. Five (17%) of the 29 patients with nasogastric intubation for feeding during transfer developed severe pneumonia later. Only one (4%) of 23 patients without nasogastric intubation had severe pneumonia. However, no statistical significance was observed owing to the small sample size.
Table 2.
Comparison of characteristics of patients with and without serious complications (pneumonia)
| Characteristics | Pneumonia (n=6) | No pneumonia (n=47) | P | ||
|---|---|---|---|---|---|
| Median | Mean | Median | Mean | ||
| Age (years) | 83 (73-89) | 79±13 | 65 (57-75) | 67±13 | 0.037* |
| Male gender (%) | 2 (33) | 34 (72) | 0.076 | ||
| Evaluation for PAC to transfer (days) | 15 (10-17) | 13±4 | 7 (5-11) | 8±4 | 0.009* |
| Initial Barthel index | 0 (0-15) | 9±15 | 25 (4-43) | 27±24 | 0.055 |
| Initial Lawton–Brody IADL scale | 0 (0-0) | 0±0 | 1 (0-1) | 0.77±0.84 | 0.016* |
| Initial berg balance test | 0 (0-0) | 2.2±4.9 | 4 (0-22) | 11.9±16.1 | 0.074 |
| Initial Fugl–Meyer motor assessment | 0 (0-0) | 6.0±9.1 | 33.5 (0-52.8) | 25.6±23.4 | 0.049* |
| Initial mini-mental state examination | 0 (0-0) | 2±4 | 13 (0.5-21) | 12±10 | 0.021* |
| Initial motor activity log - quality | 0 (0-0) | 0±0 | 0.1 (0-1.9) | 1.0±1.5 | 0.023* |
Data are presented as n (%), median (25th-75th percentile), or mean±SD. *P<0.05 was considered statistically significant; Mann–Whitney U-test. PAC: Postacute care, SD: Standard deviation
Forty-five patients completed the PAC program. Table 3 illustrates the initial and final functional evaluations of these 45 patients. The results indicated highly significant improvements in all 14 physical and cortical functional assessments by the end of the PAC program (P < 0.001). Improvements in mRS and BI scores were observed in 19 (42%) and 35 (78%) patients, respectively. The median BI score increased from 25 to 50. Figure 1 shows the distribution of the initial and final mRS scores of the 45 patients. Ten patients (22%) achieved an mRS score of ≤2 after the PAC program, and 33 (73%), including 100% (5/5) of those with an initial mRS score of 3 and 70% (28/40) of those with an initial mRS score of 4, were discharged home from the PAC hospital for a subsequent outpatient rehabilitation program.
Table 3.
Initial and final functional assessments of the 45 patients who completed the postacute care model of stroke program
| Functional assessments | Initial evaluation | Final evaluation | P | ||
|---|---|---|---|---|---|
| Median | Mean | Median | Mean | ||
| Modified Rankin scalea | 4 (4-4) | 3.9±0.3 | 4 (3-4) | 3.3±0.9 | <0.001 |
| Barthel index | 25 (5-40) | 27±24 | 50 (20-75) | 50±30 | <0.001 |
| Functional oral intake scale | 6 (2-7) | 4.6±2.5 | 7 (6-7) | 6.1±1.8 | <0.001 |
| EuroQoL-5Da | 11 (10-13) | 11.4±1.7 | 8 (7-10) | 8.5±2.4 | <0.001 |
| Lawton–Brody IADL scale | 1 (0-1) | 0.7±0.8 | 2 (1-3) | 2.2±1.8 | <0.001 |
| Berg balance test | 4 (0-24) | 12.4±16.3 | 29 (6-48) | 26.5±20.7 | <0.001 |
| Usual gait speed (m/s) | 0 (0-0.09) | 0.08±0.16 | 0.06 (0-0.40) | 0.31±0.62 | <0.001 |
| Six-minute walk test (m) | 0 (0-0) | 27±61 | 27 (0-138) | 81±104 | <0.001 |
| Fugl–Meyer sensory assessment | 12 (0-30) | 17±16 | 34 (10-41) | 26±17 | <0.001 |
| Fugl–Meyer motor assessment | 30 (1-46) | 27±23 | 41 (16-58) | 37±23 | <0.001 |
| Mini-mental state examination | 14 (2-21) | 13±10 | 22 (14-27) | 19±10 | <0.001 |
| Motor activity log - quantity | 0.2 (0-1.7) | 1.0±1.5 | 2.0 (0.4-4.0) | 2.3±2.5 | <0.001 |
| Motor activity log - quality | 0.2 (0-2.0) | 1.1±1.6 | 2.0 (0.4-3.8) | 2.0±1.7 | <0.001 |
| Concise Chinese aphasia test | 9.3 (4.9-11.0) | 7.9±3.5 | 11.0 (7.8-11.0) | 9.2±3.2 | <0.001 |
Data are presented as median (25th-75th percentile) or mean±SD. aLower scores indicate less severe disability, *P<0.05 was considered statistically significant; Wilcoxon signed-rank test. PAC: Postacute care, SD: Standard deviation
Figure 1.
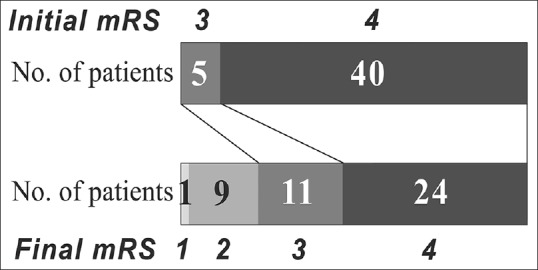
Distribution of initial and final modified Rankin Scale scores in the 45 patients who completed the postacute care program. mRS: modified Rankin Scale
The median length of PAC hospital stay among the 45 patients was 63 days (mean: 63 ± 20 days). The degree of improvement for each functional assessment did not differ greatly between men and women or between patients with initial mRS scores of 3 and 4. Women improved (by 5 points) considerably more than men (by 2 points) only in the EuroQoL-5D. Moreover, patients with an initial mRS score of 3 improved (by 3 points) more than did those with an initial mRS score of 4 (by 1 point) only on the Lawton–Brody IADL scale. No differences were observed between patients with cerebral infarction and those with cerebral hemorrhage in terms of median age, all initial functional assessments, or length of PAC hospital stay. However, the degree of improvement after the PAC program was significantly higher among patients with cerebral hemorrhage in six of the 14 functional assessments, BI, functional oral intake scale, EuroQoL-5D, usual gait speed, Fugl–Meyer sensory assessment, and concise Chinese aphasia test [Table 4].
Table 4.
Comparison of improvement in functional scores after the postacute care model of stroke program between patients with cerebral infarction and cerebral hemorrhage
| Assessments | Infarct (n=33) | Hemorrhage (n=12) | P | ||
|---|---|---|---|---|---|
| Median | Mean | Median | Mean | ||
| Length of stay at PAC hospital | 63 (42-84) | 63±21 | 61 (43-84) | 63±20 | 0.830 |
| Modified Rankin Scale | 0 (0-1) | 0.5±0.7 | 0.5 (0-1.8) | 0.8±1.0 | 0.439 |
| Barthel index | 20 (0-30) | 19±21 | 40 (16-54) | 35±21 | 0.035* |
| Functional oral intake scale | 0 (0-1.5) | 1.1±1.9 | 2.5 (0.3-4.0) | 2.4±2.1 | 0.046* |
| EuroQoL-5D | 3.0 (1.8-4.0) | 2.6±2.3 | 5 (2-5) | 4.0±1.9 | 0.046* |
| Lawton–Brody IADL scale | 1 (0-3) | 1.4±1.6 | 1.0 (0-2.8) | 1.4±1.8 | 0.950 |
| Berg balance test | 6.5 (2.0-17.5) | 11.7±13.6 | 20 (7-37) | 21±17 | 0.068 |
| Usual gait speed (m/s) | 0 (0-0.2) | 0.2±0.6 | 0.4 (0.1-0.6) | 0.4±0.3 | 0.024* |
| Six minute walk test (m) | 0 (0-90) | 46±78 | 79 (7-143) | 92±93 | 0.132 |
| Fugl–Meyer sensory assessment | 2.5 (0-12.3) | 6.8±9.1 | 17.0 (1.5-28.3) | 18.0±15.7 | 0.036* |
| Fugl–Meyer motor assessment | 8 (0-22) | 10.1±11.6 | 10.5 (2.3-26.8) | 13.9±13.7 | 0.501 |
| Mini-mental state examination | 4.5 (0-8.8) | 5.4±6.9 | 8.5 (2.5-15.5) | 10.0±8.5 | 0.125 |
| Motor activity log - quantity | 0.9 (0-2.0) | 1.4±2.3 | 0.8 (0-2.0) | 1.1±1.1 | 0.924 |
| Motor activity log - quality | 0.9 (0-1.8) | 0.9±1.2 | 1.0 (0-1.2) | 0.9±1.0 | 0.924 |
| Concise Chinese aphasia test | 0 (0-1) | 0.7±1.3 | 2 (0-6) | 3.0±2.8 | 0.012* |
Data are presented as median (25th-75th percentile) or mean±SD. *P<0.05 was considered statistically significant; Mann–Whitney U-test. PAC: Postacute care, IADL: Instrumental activities of daily living, SD: Standard deviation
DISCUSSION
An estimated 10%–23% of patients with stroke may require PAC [9]. In our study, only 11.5% of patients who had mRS scores of 2–4 participated in the PAC program. This number can be increased. The low percentage of patient transfers was a partial result of a lack of confidence in the quality of care at PAC hospitals among patients, inability to receive concurrent acupuncture treatment at the PAC hospital and the relatively sufficient number of unoccupied rehabilitation beds at the upstream hospital. Patients with a minor degree of disability (mRS 2) usually hope to return home as soon as possible after short-term rehabilitation. Therefore, the PAC-stroke enrollment criteria were adjusted by the NHI Bureau in July 2017 to exclude patients with an mRS score of 2.
The average age of the patients in this study was 68.3 years, which is 5 years older than those in the studies by Wang et al. and Lai et al. [23,24], where patients transferred from various upstream hospitals to a single regional PAC hospital after rigorous screening and selection. In contrast, the patients in the present study transferred from a single upstream regional hospital to several district PAC hospitals. Data from upstream hospitals may more accurately reflect the clinical condition of PAC patients. The average length of stay at upstream hospitals was longer in our study (25 days) than in the studies by Wang et al. (11.6 days) and Lai et al. (15.5 days after stroke). The percentage of patients with an initial mRS score of 4 was higher in this study (89%) than in Lai et al.'s study (73%; data not provided by Wang et al.). The average length of PAC hospital stay was considerably longer in our study (63 days) than in the studies by Wang et al. (28 days) and Lai et al. (44 days), and the data reported in the present study indicated higher stroke severity among patients undergoing PAC. Nevertheless, an mRS score improvement of ≥1 by the end of the PAC program was observed in 43% of the patients in this study; this result was similar to the 41% reported by Lai et al. Mortality from severe infection was higher in our study (7.5%) than in Lai et al.'s study (0.6%). To provide more opportunities for patients who exhibit potential for improvement, the PAC hospitals in this study were willing to accept older patients with an initial mRS score of 4 and multiple comorbidities. Enabling PAC hospitals to provide effective, comprehensive rehabilitation is the primary aim of the PAC-stroke program. To fulfill the requirements of the program, rehabilitation teams in PAC hospitals (mostly district hospitals) must increase their workforce and facilities to enhance their functional performance.
Data on NHI beneficiaries in Taiwan from 2000 revealed that 28.6% of all patients with stroke were readmitted within 3 months of discharge [25]. Analysis of 2005 data from the same source found that the rate of readmission within 14 days of discharge among all patients with stroke was 17.4%. There are few studies of readmission rates focused on patients with different functional status, such as mRS 3 or 4, at discharge. The readmission rate within 14 days of transfer among patients undergoing PAC with mRS 2–4 was similar in this study (3.8%) and the Lai et al.'s study (5.6%). The readmission rate throughout the PAC program was 13.2% (average: 63 days after transfer) in our study and 16.7% (average: 44 days after transfer) in the Lai et al.'s study. These results suggest that the readmission rate in patients with a moderate-to-severe degree of disability undergoing PAC tends to be lower than that reported in all patients with stroke those from NHI database.
Recurrent stroke has been reported as the most common cause of readmission for patients with stroke between 3 months and 1 year after discharge [25]. Data on recurrent stroke during the PAC program were not available in the aforementioned two studies conducted in Taiwan. Lai et al. simply stated that 16.7% of the patients were transferred to an acute ward because of infection or recurrent stroke [24]; by contrast, no patients in our study were readmitted to the upstream hospital because of recurrent stroke. A longer stay at the upstream hospital, with early rehabilitation under observation by neurologists or neurosurgeons to ensure a more stable clinical condition, may have reduced the risk of recurrent stroke after transfer compared with that of patients in those two studies. Pneumonia was the most common cause of serious complications or death among patients in the PAC program. All cases of pneumonia in this study occurred in patients with an initial mRS score of 4. The patients with severe pneumonia were considerably older (median age: 83 years), had worse scores on six of the initial functional assessments, required longer observation during PAC program initiation to transfer, and were more often women, than those without pneumonia. With awareness of these relative risks of pneumonia, appropriate nursing care and rehabilitation should be emphasized more in the PAC program.
Our study revealed that the PAC program significantly improved functional outcomes in all 14 assessments. The median BI score of all patients increased from 25 to 50. Although no significant difference was observed because of the small patient sample size, the observed initial median BI score was lower and the final median score was higher among patients with cerebral hemorrhage than those with infarcts. Degrees of improvement in six functional assessments were significantly higher in patients with cerebral hemorrhage than in those with cerebral infarction. Patients with cerebral hemorrhage are reportedly 2.5 times more likely to demonstrate a high therapeutic response on the BI than patients with ischemia [26]. The reduced compression effect from a resolving intracerebral hematoma might decrease neurological deficits and thus result in more rapid functional recovery. Thus, given a similar initial degree of stroke severity, patients with cerebral hemorrhage may benefit more from rehabilitation than those with cerebral infarction. These findings generate high expectations for the PAC program. Increasing the duration and intensity of rehabilitation in patients who can tolerate this is crucial for achieving better functional outcomes [27].
This study had some limitations. First, the sample size was relatively small. Given that PAC-stroke is a pilot model established by the NHI Bureau, physicians, patients, and PAC hospitals are still unfamiliar with this system. Through a successful learning curve with more published results, we can expect a superior clinical pathway to be developed to improve the PAC program quantitatively and qualitatively. Second, no control group of patients with mRS scores of 3 and 4 was established for comparison of functional outcomes. Moreover, follow-up data on patients not undergoing PAC after discharge were incomplete in this retrospective study. Third, this study focused on a single upstream regional hospital working alongside four district PAC hospitals, and thus, we were unable to compare results related to patients transferred from medical centers. Prospective studies from multiple upstream hospitals with a control group and longitudinal follow-up are crucial for analyzing the actual effectiveness of PAC-stroke.
CONCLUSION
This preliminary study of the NHI Bureau's newly developed PAC-stroke model demonstrated a positive effect on functional outcomes in patients with acute stroke and moderate-to-severe degree of disability. Significant improvements in mRS and BI scores and all functional assessments within an average of 63 days of PAC hospital stay helped 73% of the patients to return home.
Financial support and sponsorship
This work was supported by grants from Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation (TCRD-TPE-106-RT-12). The founder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Schwamm LH, Fonarow GC, Reeves MJ, Pan W, Frankel MR, Smith EE, et al. Get with the guidelines-stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or transient ischemic attack. Circulation. 2009;119:107–15. doi: 10.1161/CIRCULATIONAHA.108.783688. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh FI, Lien LM, Chen ST, Bai CH, Sun MC, Tseng HP, et al. Get with the guidelines-stroke performance indicators: Surveillance of stroke care in the Taiwan stroke registry: Get with the guidelines-stroke in Taiwan. Circulation. 2010;122:1116–23. doi: 10.1161/CIRCULATIONAHA.110.936526. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh FI, Jeng JS, Chern CM, Lee TH, Tang SC, Tsai LK, et al. Quality improvement in acute ischemic stroke care in Taiwan: The breakthrough collaborative in stroke. PLoS One. 2016;11:e0160426. doi: 10.1371/journal.pone.0160426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taiwan Ministry of Health and Welfare. The Ten Leading of Causes of Death in Taiwan. 2016. [Last accessed on 2018 Feb 26]. Available from: https://www.mohw.gov.tw/cp-16-33598-1.html .
- 6.Bernhardt J, Godecke E, Johnson L, Langhorne P. Early rehabilitation after stroke. Curr Opin Neurol. 2017;30:48–54. doi: 10.1097/WCO.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 7.Chen LK, Chen YM, Hwang SJ, Peng LN, Lin MH, Lee WJ, et al. Effectiveness of community hospital-based post-acute care on functional recovery and 12-month mortality in older patients: A prospective cohort study. Ann Med. 2010;42:630–6. doi: 10.3109/07853890.2010.521763. [DOI] [PubMed] [Google Scholar]
- 8.Chan L, Sandel ME, Jette AM, Appelman J, Brandt DE, Cheng P, et al. Does postacute care site matter. A longitudinal study assessing functional recovery after a stroke? Arch Phys Med Rehabil. 2013;94:622–9. doi: 10.1016/j.apmr.2012.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu KY, Wu SC, Hung YN, Wu CC, Lin LC, Hu HH. The need for post-acute care for stroke patients in Taiwan. Taiwan J Public Health. 2012;31:251–62. [Google Scholar]
- 10.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: Implications for stroke clinical trials: A literature review and synthesis. Stroke. 2007;38:1091–6. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 11.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 12.Quinn TJ, Langhorne P, Stott DJ. Barthel index for stroke trials: Development, properties, and application. Stroke. 2011;42:1146–51. doi: 10.1161/STROKEAHA.110.598540. [DOI] [PubMed] [Google Scholar]
- 13.Crary MA, Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil. 2005;86:1516–20. doi: 10.1016/j.apmr.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26:410–20. doi: 10.1177/0272989X06290495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graf C. The Lawton instrumental activities of daily living scale. Am J Nurs. 2008;108:52–62. doi: 10.1097/01.NAJ.0000314810.46029.74. [DOI] [PubMed] [Google Scholar]
- 16.Berg K, Wood-Dauphinee S, Williams JI, Gayton D. Measuring balance in the elderly: Preliminary development of an instrument. Physiother Can. 1989;41:304–11. [Google Scholar]
- 17.Fritz S, Lusardi M. White paper: “walking speed: The sixth vital sign”. J Geriatr Phys Ther. 2009;32:46–9. [PubMed] [Google Scholar]
- 18.Du H, Newton PJ, Salamonson Y, Carrieri-Kohlman VL, Davidson PM. A review of the six-minute walk test: Its implication as a self-administered assessment tool. Eur J Cardiovasc Nurs. 2009;8:2–8. doi: 10.1016/j.ejcnurse.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan KJ, Tilson JK, Cen SY, Rose DK, Hershberg J, Correa A, et al. Fugl-Meyer assessment of sensorimotor function after stroke: Standardized training procedure for clinical practice and clinical trials. Stroke. 2011;42:427–32. doi: 10.1161/STROKEAHA.110.592766. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Uswatte G, Taub E, Morris D, Light K, Thompson PA. The motor activity log-28: Assessing daily use of the hemiparetic arm after stroke. Neurology. 2006;67:1189–94. doi: 10.1212/01.wnl.0000238164.90657.c2. [DOI] [PubMed] [Google Scholar]
- 22.Chung YM, Lee SE, Chang MH, Hsu TC. The concise Chinese aphasia test and its application. J Speech Lang Hearing Assoc. 1998;13:119–37. [Google Scholar]
- 23.Wang CY, Chen YR, Hong JP, Chan CC, Chang LC, Shi HY, et al. Rehabilitative post-acute care for stroke patients delivered by per-diem payment system in different hospitalization paths: A Taiwan pilot study. Int J Qual Health Care. 2017;29:779–84. doi: 10.1093/intqhc/mzx102. [DOI] [PubMed] [Google Scholar]
- 24.Lai CL, Tsai MM, Luo JY, Liao WC, Hsu PS, Chen HY, et al. Post-acute care for stroke – A retrospective cohort study in Taiwan. Patient Prefer Adherence. 2017;11:1309–15. doi: 10.2147/PPA.S136041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng MC, Lin HJ. Readmission after hospitalization for stroke in Taiwan: Results from a national sample. J Neurol Sci. 2009;284:52–5. doi: 10.1016/j.jns.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Paolucci S, Antonucci G, Grasso MG, Bragoni M, Coiro P, De Angelis D, et al. Functional outcome of ischemic and hemorrhagic stroke patients after inpatient rehabilitation: A matched comparison. Stroke. 2003;34:2861–5. doi: 10.1161/01.STR.0000102902.39759.D3. [DOI] [PubMed] [Google Scholar]
- 27.Sonoda S, Saitoh E, Nagai S, Kawakita M, Kanada Y. Full-time integrated treatment program, a new system for stroke rehabilitation in Japan: Comparison with conventional rehabilitation. Am J Phys Med Rehabil. 2004;83:88–93. doi: 10.1097/01.PHM.0000107481.69424.E1. [DOI] [PubMed] [Google Scholar]


