Abstract
Background:
Acoustic radiation force impulse (ARFI) imaging is a popular modality to measure liver fibrosis. ARFI selects optimal locations for measurement under imaging guiding. However, there are concerns on study locations and observers bias. To decrease the variations, ARFI at two locations was measured with standardized protocol. This study attempted to establish its cutoff values according to Metavir fibrosis score in different etiologies.
Methods:
A consecutive series of patients who received liver histology study were prospectively enrolled. All cases had hemogram, liver biochemistry, viral markers, and ARFI two-location measurements within 4 weeks of histology study. A standardized protocol was performed by single technologist. We excluded patients with alanine aminotransferase >5x upper limit normal.
Results:
Five hundred and ten patients that included 153 seronegative for both HBsAg and anti-HCV Non-B non-C (NBNC), 33 autoimmune liver diseases (AILD), 261 chronic hepatitis B (CHB), and 63 chronic hepatitis C (CHC) were enrolled. About 83% of NBNC patients had fat cell >5%. For diagnosis of liver cirrhosis, the area under receiver operating characteristic curve of NBNC, AILD, CHB, and CHC groups was 0.937, 0.929, 0.784, and 0.937; the cutoff values for mean ARFI were 1.788, 2.095, 1.455, and 1.710 m/s, respectively. The sensitivity and specificity are both over 0.818 for patients with nonalcoholic fatty liver diseases, CHC, and AILD, but the corresponding data are only 0.727–0.756 in CHB. The Fibrosis-4 Score is as good as ARFI on fibrosis assessment in NBNC.
Conclusion:
The performance of ARFI two-location measurement is excellent in NBNC, AILD, and CHC, but is only satisfactory in CHB.
Keywords: Acoustic radiation force impulse, chronic hepatitis B, chronic hepatitis C, hepatic steatosis, liver fibrosis
INTRODUCTION
FibroScan and Acoustic radiation force impulse (ARFI) imaging is two most popular ultrasound-based noninvasive modalities to measure liver fibrosis.[1,2,3] The area under receiver operating characteristic curve (AUROC) for diagnosis of liver cirrhosis (range 0.723–0.97) had been well-reported.[4,5,6,7,8] ARFI may select an optimal location for measurement under imaging guiding. This is beneficial in a patient who will undergo surgical resection for the right lobe liver cancer. However, the presence of an image information will have a greater potential for the operator to influence the measurement. An ARFI-operator may try to confirm an impression from the two-dimensional (2D) ultrasound real-time imaging.[9] In addition, the area used for detection of the fibrosis is smaller in ARFI than in FibroScan,[1,2] and the quality criteria form the manufacturer is better in FibroScan than in ARFI.[9] Intra- and inter-operator reproducibility of ARFI had been an issue of concern.[10] Our previous ARFI study in patients with viral hepatitis had shown a difference in liver stiffness between two locations.[11] The ARFI value differences were >0.2 m/s between two locations in 27% of patients. Such measurements may provide a variation information instantly. The mean data from two locations will decrease the variation and make the ARFI assessment more objective.[11,12] In this ARFI two-location measurements study, we would like to establish its cutoff values according to histology fibrosis score in different etiologies.
Patients
We enrolled a series of consecutive patients who received liver histology study from March 2011 to March 2018. This study was approved by the Institute Review Board of Chang Gung Memorial Hospital (IRB:100-2029B and 104-2353C). Written informed consent was obtained from all participants before the study.
All patients are older than 18-year-old. They received ARFI measurements within 4 weeks of liver histology study. All the participants had received hemogram, liver biochemistry, and viral markers studies. Antinuclear antibody, anti-mitochondria antibody, and anti-smooth muscle antibody were examined when autoimmune diseases were suspected.
The diagnosis of autoimmune hepatitis was based on Simplified criteria for the diagnosis of autoimmune hepatitis.[13] The diagnosis of primary biliary cirrhosis was according to Lindor et al.[14]
Those patients with alcoholism, dual infection of hepatitis B and C, incomplete data and refused to sign the inform consent were excluded.
Inflammation plays a role on liver stiffness.[11,15] Therefore, patients with alanine aminotransferase (ALT) level >5x ULN were excluded from this study.
Most of the patients receive specific therapy after the diagnosis of liver biopsy. In chronic hepatitis B (CHB), some of the patients have been receiving nucleos(t)ide analogs therapy at the time of liver biopsy. They had liver histology study because of tumor resection, or they would like to establish the diagnosis of liver cirrhosis for continuing long-term therapy of NA. Since therapy may reduce inflammation. All of the patients who were receiving therapy at the time of liver biopsy were excluded from the study.
METHODS
The severity of liver fibrosis was according to Metavir fibrosis grading system.[16] For patients with nonalcoholic fatty liver diseases (NAFLD), the diagnosis and fibrosis grading was according to Kleiner et al.[17]
A standardized protocol for ARFI study was used according to the guidelines[18] with some modification.[11,12] Most of the measurements were done by a well-trained technologist. In brief, two locations form right intercostal space, one at the right lower liver (LocationA), and the other at the right upper liver (LocationB) with optimal 2D imaging were select for assessments. For each location, 10 measurements were done with the area of interest perpendicular to the skin. All patients were keep on spontaneous slow breathing during the measurements. The mean of two median data (meanAB) becomes the result. Those patients with interquartile range (IQR) over median (IQR/med) value >0.30 were considered failed to the measurement and were excluded from this study.[18]
The Fibrosis-4 Score (FIB4) was calculated and compared with ARFI.[19]
Statistics
The patient characteristics are expressed in the number and percentage or mean ± standard deviation. Continuous variables of four independent groups were compared with ANOVA. Categorical variables were tested using the Chi-square test or Fisher's exact test. The receiver-operating characteristic curves and AUROC were calculated for the evaluation of the best prediction tests according to histology Metavir score. All statistical analyses were performed using SPSS (version 22 SPSS Inc., Chicago, IL, USA) and a P < 0.05 was considered to indicate statistical significance.
The case numbers needed for AUROC analysis to reach type I error 0.05 and type II error (1-power) 0.20 was calculated by MedCalc-version 16.8 (MedCalc Software bvba, Belgium). When the AUROC curve is expected to be 0.8, and null hypothesis value is 0.5, the minimal case numbers required to achieve statistic power of 0.8 will be around 27 cases.
RESULTS
A total of 606 patients were enrolled in the study period. All of the patients complete the measurements. However, 24 patients had IQR over median values >0.30 in one of the two location ARFI measurements and 72 patients with ALT level >5x ULN were excluded from this study. Among the rest of the 510 patients, 153 were seronegative for both HBsAg and anti-CHC (NBNC), 33 were autoimmune liver diseases (AILD, 6 autoimmune hepatitis and 27 primary biliary cirrhosis), 261 were CHB and 63 were chronic hepatitis C (CHC). Among patients with NBNC, 127 patients without alcohol drinking habit and with fat-containing cells >5% were classified as NAFLD.[20]
There is more male (196 or 75.1%, P < 0.001) in CHB group and more female (27 or 81.8%, P < 0.001) in AILD group than other groups [Table 1]. More patients associated with hepatocellular carcinoma (23.8%, P < 0.001) in CHB group than those in NBNC (3.3%) or ALID (0%) group. More patients (83.0%, P < 0.001) in NBNC group were associated with liver steatosis (Fat cell > 5%) than the other groups (21.2%–55.6%). Patients in AILD group tended to have higher aspartate aminotransferase levels than the other groups (P < 0.001); higher alkaline phosphatase, gamma-glutamyl transpeptidase, and platelet levels than viral hepatitis groups. International normalization ratio of prothrombin time was lower in NBNC than in viral hepatitis group. The mean age was younger (P < 0.01) and histology fibrosis score was lower (P < 0.001) in NBNC group than the other groups.
Table 1.
Demography of study patients
| Group | NBNC | AILD | CHB | CHC | Multiple comparison | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total | (n=153) | (Percentage or SD) | (n=33) | (Percentage or SD) | (n=261) | (Percentage or SD) | (n=63) | (Percentage or SD) | |
| Male | 90 | 58.8% | 6 | 18.2% | 196 | 75.1% | 37 | 58.7% | CHB≠(NBNC=CHC)≠AILD |
| HCC | 5 | 3.3% | 0 | 0.0% | 62 | 23.8% | 6 | 9.5% | CHB≠(NBNC=AILD) |
| Fat cell fraction >5% | 127 | 83.0% | 7 | 21.2% | 145 | 55.6% | 31 | 49.2% | NBNC≠AILD≠(CHB=CHC) |
| Age (year) | 47.81 | ±11.71 | 53.99 | ±13.73 | 51.35 | ±11.06 | 52.47 | ±11.05 | NBNC≠(AILD=CHB=CHC) |
| Bilirubin (mg/dL) | 0.82 | ±0.61 | 1.43 | ±1.07 | 0.94 | ±1.37 | 0.79 | ±0.24 | AILD≠(NBNC=CHC) |
| AST (U/L) | 58.09 | ±31.26 | 94.41 | ±52.50 | 50.17 | ±41.63 | 54.73 | ±39.64 | AILD≠(NBNC=CHB=CHC) |
| ALT (U/L) | 98.17 | ±44.94 | 90.94 | ±41.06 | 60.34 | ±40.35 | 63.93 | ±49.91 | AILD≠(CHB=CHC); NBNC≠CHB |
| ALP (U/L) | 83.54 | ±49.76 | 267.85 | ±186.79 | 77.55 | ±35.49 | 81.05 | ±26.91 | AILD≠(NBNC=CHB=CHC) |
| GGT (U/L) | 104.64 | ±194.33 | 468.40 | ±447.37 | 44.94 | ±47.85 | 59.0 | ±47.92 | AILD≠(CHB=CHC); NBNC≠CHB |
| PLT (109/L) | 226.45 | ±68.31 | 250.23 | ±120.31 | 184.73 | ±57.44 | 186.48 | ±61.05 | AILD≠(CHB=CHC); NBNC≠CHB |
| INR | 1.04 | ±0.09 | 1.03 | ±0.10 | 1.07 | ±0.09 | 1.07 | ±0.08 | NBNC≠(CHB=CHC) |
| BMI | 26.80 | ±4.48 | 24.01 | ±3.66 | 25.11 | ±3.9 | 25.67 | ±3.84 | NBNC≠(AILD=CHB) |
| Spleen index | 16.77 | ±6.46 | 21.49 | ±11.39 | 15.82 | ±6.44 | 15.77 | ±6.15 | AILD≠(CHB=CHC=NBNC) |
| Fibrosis score 0 |
28 | 18.3% | 0 | 0.0% | 8 | 3.1% | 2 | 3.2% | NBNC≠(CHB=CHC=AILD) |
| 1 | 80 | 52.3% | 11 | 33.3% | 50 | 19.23% | 21 | 33.3% | |
| 2 | 14 | 9.2% | 6 | 18.2% | 81 | 31.0% | 4 | 22.2% | |
| 3 | 20 | 13.1% | 11 | 33.3% | 78 | 39.9% | 15 | 23.8% | |
| 4 | 11 | 7.2% | 5 | 15.2% | 44 | 16.9% | 11 | 17.5% | |
| Fat cell fraction (%) | 50.46 | ±33.38 | 5.45 | ±14.64 | 18.27 | ±23.70 | 19.29 | ±26.66 | NBNC≠(AILD=CHB=CHC) |
| FIB4 | 1.596 | ±1.418 | 3.066 | ±3.156 | 2.172 | ±2.209 | 2.472 | ±2.015 | NBNC≠(AILD=CHB=CHC) |
| ARFI meanAB (m/s) | 1.328 | ±0.524 | 1.722 | ±0.508 | 1.425 | ±0.461 | 1.557 | ±0.660 | NBNC≠(AILD=CHC); CHB≠AILD |
PLT: Platelet, AILD: Autoimmune liver diseases, CHB: Chronic hepatitis B, CHC: chronic hepatitis C, AST: Aspartate aminotransferase, HCC: Hepatocellular carcinoma, ALT: Alanine aminotransferase, GGT: Gamma-glutamyl transpeptidase, INR: International normalization ratio, FIB4: Fibrosis-4 score, ARFI: Acoustic radiation force impulse, MeanAB: Mean of ARFI measured from location A and B, ALP: Alkaline phosphatase, BMI: Body mass index, SD: Standard deviation, NBNC: Non-B non-C
There were a significantly higher fat cell fraction and a lower FIB4 level in NBNC than other groups [Table 1]. The different between two-location ARFI measurements was <0.1 m/s in 62.4%, between 0.1 and 0.2 m/s in 20% and >0.2 m/s in 17.6% of patients. The meanAB level was lower in NBNC than in AILD and CHC. The meanAB level of CHB was lower than AILD [Table 1].
Only 33 patients were classified into AILD group. These case numbers were greater than the minimal case numbers (n = 27) required to achieve statistic power of 0.8. The AUROC for fibrosis score 4 was 0.929, and the cutoff value was 2.095 m/s [Figure 1 and Table 2].
Figure 1.
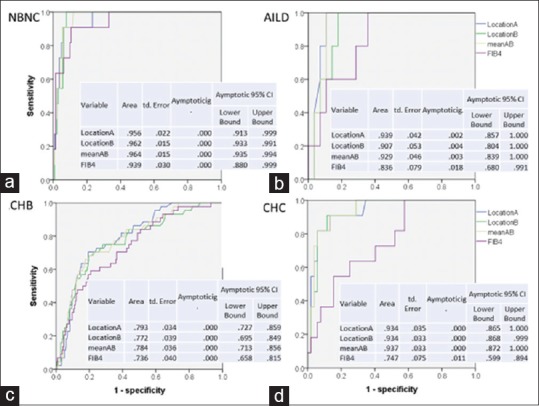
The areas under the receiver-operating characteristic of acoustic radiation force impulse values and fibrosis-4 score for prediction of liver cirrhosis. (a) Areas under the receiver-operating characteristic in patients with nonalcoholic fatty liver diseases. (b) Areas under the receiver-operating characteristic in patients with AILD. (c) Areas under the receiver-operating characteristic in patients with chronic hepatitis B. (d) Areas under the receiver-operating characteristic in patients with chronic hepatitis C. Acoustic radiation force impulse showed higher areas under the receiver-operating characteristic than fibrosis-4 score in most etiologies. The fibrosis-4 score showed similar areas under the receiver-operating characteristic with acoustic radiation force impulse in nonalcoholic fatty liver diseases
Table 2.
The acoustic radiation force impulse areas under receiver-operating characteristic, cutoff values, sensitivity, and specificity in different fibrosis grades and etiology groups
| Group | Metavir | Case number | AUROC | MeanAB | ||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | No | CI | Cutoff (m/s) | Sensitivity | Specificity | ||
| NBNC (n=154) | F=4 | 11 | 142 | 0.937 | 0.932-0.994 | 1.788 | 0.909 | 0.937 |
| F ≥3 | 31 | 122 | 0.873 | 0.805-0.941 | 1.320 | 0.774 | 0.836 | |
| F ≥2 | 46 | 107 | 0.854 | 0.784-0.924 | 1.245 | 0.804 | 0.813 | |
| AILD (n=33) | F=4 | 5 | 28 | 0.929 | 0.839-1.000 | 2.095 | 1.000 | 0.893 |
| F ≥3 | 16 | 17 | 0.897 | 0.790-1.000 | 1.720 | 0.750 | 0.824 | |
| F ≥2 | 22 | 11 | 0.938 | 0.853-1.000 | 1.490 | 0.864 | 0.818 | |
| CHB (n=261) | F=4 | 44 | 217 | 0.784 | 0.713-0.856 | 1.455 | 0.727 | 0.756 |
| F ≥3 | 122 | 139 | 0.762 | 0.705-0.820 | 1.320 | 0.705 | 0.712 | |
| F ≥2 | 203 | 58 | 0.758 | 0.688-0.828 | 1.220 | 0.704 | 0.655 | |
| CHC (n=63) | F=4 | 11 | 52 | 0.937 | 0.850-0.989 | 1.710 | 0.818 | 0.865 |
| F ≥3 | 26 | 37 | 0.857 | 0.759-0.955 | 1.370 | 0.731 | 0.784 | |
| F ≥2 | 40 | 23 | 0.786 | 0.675-0.898 | 1.225 | 0.650 | 0.709 | |
AUROC: Areas under ROC, ROC: Receiver-operating characteristic, AILD: Autoimmune liver diseases, CHB: Chronic hepatitis B, CHC: Chronic hepatitis C, MeanAB: Mean of ARFI measured from location A and B, ARFI: Acoustic radiation force impulse, CI: Confidence interval, NBNC: Non-B non-C
Four fibrosis measurement data that included ARFI LocationA, LocationB, meanAB, and FIB4 were evaluated by AUROC analysis. The data of ARFIs were quite similar, although the LocationA showed a higher AUROC than the LocationB in most situations [Figure 1]. To decrease the complexity, we use meanAB as a result.
The AUROC for diagnosis of fibrosis score 4 in NBNC, AILD, CHB, and CHC group was 0.937, 0.929, 0.784, and 0.937; the cutoff values were 1.788, 2.095, 1.455, and 1.710 m/s respectively. The sensitivity and specificity are both over 0.818 for patients with NAFLD, CHC, and AILD, but the corresponding data are only 0.727–0.756 in CHB [Table 2].
For diagnosis of liver fibrosis greater than F3, the AUROC was 0.873, 0.897, 0.762, and 0.857; the cutoff values were 1.320, 1.720, 1.320, and 1.370 m/s for NBNC, AILD, CHB, and CHC groups, respectively [Table 2].
For diagnosis of liver fibrosis greater than F2, the AUROC was 0.854, 0.938, 0.758, and 0.786 and 0.900; the cutoff values were 1.245, 1.490, 1.220, and 1.225 and 1.581 m/s for NBNC, AILD, CHB, and CHC groups [Table 2], respectively.
DISCUSSION
This study established the cutoff values of ARFI two-location measurements of fibrosis in different etiologies of liver diseases. These ARFI measurements are superior to FIB4 in most etiologies of liver diseases [Figure 1]. The FIB4 is as good as ARFI in the diagnosis of liver fibrosis in patients with NBNC group.
The correlation between ARFI with histology fibrosis score is excellent in patients of NBNC, AILD, and CHC. The AUROC, sensitivity, and specificity in the diagnosis of F4 liver cirrhosis were generally above 0.8 [Table 2]. On the other hand, the AUROC in CHB groups were all below 0.79. The sensitivity and specificity were also lower in CHB groups (0.727–0.756) than in other groups (0.818–0.937). Similar situation can be found in patients with F3 severe fibrosis [Table 2]. The correlation between ARFI and liver histology is relatively poorer in CHB group than in other etiologies.
The poor diagnosis performance may point out different pathogenesis between CHB and other groups. CHB is characterized by intermittent acute exacerbation during immune clearance stage.[11] The unstable or unpredictable liver inflammation and injury may also bring different degree of liver stiffness.[11,21,22] This makes the assessment of liver fibrosis complicated. In addition, advanced fibrosis stage (F3-4) and body mass index were also reported to affect the accuracy of ARFI in CHB.[23]
Patients with repeated acute exacerbation were more likely to develop liver cirrhosis.[24,25,26] Such inflammation could be controlled by nucleos (t) ide analogs therapy. This study excluded patient receiving therapy at enrolment. The best timing for measurement of liver fibrosis in CHB with active inflammation will be at the 2nd year of nucleos (t) ide analogs therapy when the inflammation is controlled.[12] At that time, liver fibrosis will be the main factor responsible for liver stiffness.
There is a wide range of cutoff values reported in the literatures.[4,5,6,7,8,9,10,11] For example, the cutoff value for F4 has a range between 1.41 and 2 m/s. The range of sensitivity (0.67–1.0) and specificity (0.65–0.95) showed a similar situation.[8] These variations could be due to the different study protocol, population, and coexisting different etiologies. The cutoff values in this study were relatively lower than those in other studies.[4,5,6,7,8,9,10,11] This study allowed patients to keep on spontaneous slow breathing during measurements. Repeatedly asking patients to stop breathing may increase liver stiffness due to emotional stress and elevation of blood pressure.[27] Therefore, persistent spontaneous breathing may be one of the reasons for relatively low cutoff values in this study.
The AUROC, sensitivity, and specificity for ARFI to measure liver fibrosis in patients with AILD are good, but the case numbers are small. We will need more cases to confirm these results.
CONCLUSION
That diagnostic performance of two-location ARFI measurements is excellent in patients with NAFLD, AILD, or CHC, but is only satisfactory in CHB.
Financial support and sponsorship
This research was supported by the grant from Chang Gung Memorial Hospital (CMRPG3F0331 and CMRPG3E1121).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, et al. Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–13. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Palmeri ML, Wang MH, Dahl JJ, Frinkley KD, Nightingale KR. Quantifying hepatic shear modulus in vivo using acoustic radiation force. Ultrasound Med Biol. 2008;34:546–58. doi: 10.1016/j.ultrasmedbio.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, et al. Liver fibrosis in viral hepatitis: Noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595–604. doi: 10.1148/radiol.2523081928. [DOI] [PubMed] [Google Scholar]
- 4.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835–47. doi: 10.1016/j.jhep.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, et al. Performance of acoustic radiation force impulse imaging for the staging of liver fibrosis: A pooled meta-analysis. J Viral Hepat. 2012;19:e212–9. doi: 10.1111/j.1365-2893.2011.01537.x. [DOI] [PubMed] [Google Scholar]
- 6.Liu H, Fu J, Hong R, Liu L, Li F. Acoustic radiation force impulse elastography for the non-invasive evaluation of hepatic fibrosis in non-alcoholic fatty liver disease patients: A systematic review and meta-analysis. PLoS One. 2015;10:e0127782. doi: 10.1371/journal.pone.0127782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bota S, Herkner H, Sporea I, Salzl P, Sirli R, Neghina AM, et al. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013;33:1138–47. doi: 10.1111/liv.12240. [DOI] [PubMed] [Google Scholar]
- 8.Hu X, Qiu L, Liu D, Qian L. Acoustic radiation force impulse (ARFI) elastography for noninvasive evaluation of hepatic fibrosis in chronic hepatitis B and C patients: A systematic review and meta-analysis. Med Ultrason. 2017;19:23–31. doi: 10.11152/mu-942. [DOI] [PubMed] [Google Scholar]
- 9.Piscaglia F, Salvatore V, Mulazzani L, Cantisani V, Schiavone C. Ultrasound shear wave elastography for liver disease. A Critical appraisal of the many actors on the stage. Ultraschall Med. 2016;37:1–5. doi: 10.1055/s-0035-1567037. [DOI] [PubMed] [Google Scholar]
- 10.Bota S, Sporea I, Sirli R, Popescu A, Danila M, Costachescu D, et al. Intra- and interoperator reproducibility of acoustic radiation force impulse (ARFI) elastography – preliminary results. Ultrasound Med Biol. 2012;38:1103–8. doi: 10.1016/j.ultrasmedbio.2012.02.032. [DOI] [PubMed] [Google Scholar]
- 11.Tai DI, Tsay PK, Jeng WJ, Weng CC, Huang SF, Huang CH, et al. Differences in liver fibrosis between patients with chronic hepatitis B and C: Evaluation by acoustic radiation force impulse measurements at 2 locations. J Ultrasound Med. 2015;34:813–21. doi: 10.7863/ultra.34.5.813. [DOI] [PubMed] [Google Scholar]
- 12.Lee CH, Wan YL, Hsu TH, Huang SF, Yu MC, Lee WC, et al. Interpretation US Elastography in chronic hepatitis B with or without anti-CHB therapy. Appl Sci. 2017;7:1164. [Google Scholar]
- 13.Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–76. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 14.Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ, et al. Primary biliary cirrhosis. Hepatology. 2009;50:291–308. doi: 10.1002/hep.22906. [DOI] [PubMed] [Google Scholar]
- 15.Chen YP, Liang XE, Dai L, Zhang Q, Peng J, Zhu YF, et al. Improving transient elastography performance for detecting hepatitis B cirrhosis. Dig Liver Dis. 2012;44:61–6. doi: 10.1016/j.dld.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR cooperative study group. Hepatology. 1996;24:289–93. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 17.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 18.Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, et al. EFSUMB guidelines and recommendations on the clinical use of liver ultrasound elastography, update 2017 (Long version) Ultraschall Med. 2017;38:e16–47. doi: 10.1055/s-0043-103952. [DOI] [PubMed] [Google Scholar]
- 19.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–6. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 20.Bedossa P. Pathology of non-alcoholic fatty liver disease. Liver Int. 2017;37(Suppl 1):85–9. doi: 10.1111/liv.13301. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Jin Q, Zhang H, Jing X, Ding Z, Zhou H, et al. Changes in liver stiffness and its associated factors during oral antiviral therapy in Chinese patients with chronic hepatitis B. Exp Ther Med. 2017;13:1169–75. doi: 10.3892/etm.2017.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong MZ, Ye L, Jin LX, Ren YD, Yu XF, Liu XB, et al. Noninvasive scoring system for significant inflammation related to chronic hepatitis B. Sci Rep. 2017;7:43752. doi: 10.1038/srep43752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park MS, Kim SW, Yoon KT, Kim SU, Park SY, Tak WY, et al. Factors influencing the diagnostic accuracy of acoustic radiation force impulse elastography in patients with chronic hepatitis B. Gut Liver. 2016;10:275–82. doi: 10.5009/gnl14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin DY, Sheen IS, Chiu CT, Lin SM, Kuo YC, Liaw YF, et al. Ultrasonographic changes of early liver cirrhosis in chronic hepatitis B: A longitudinal study. J Clin Ultrasound. 1993;21:303–8. doi: 10.1002/jcu.1870210502. [DOI] [PubMed] [Google Scholar]
- 25.Tai DI, Lin SM, Sheen IS, Chu CM, Lin DY, Liaw YF, et al. Long-term outcome of hepatitis B e antigen-negative hepatitis B surface antigen carriers in relation to changes of alanine aminotransferase levels over time. Hepatology. 2009;49:1859–67. doi: 10.1002/hep.22878. [DOI] [PubMed] [Google Scholar]
- 26.Chang ML, Liaw YF. Hepatitis B flares in chronic hepatitis B: Pathogenesis, natural course, and management. J Hepatol. 2014;61:1407–17. doi: 10.1016/j.jhep.2014.08.033. [DOI] [PubMed] [Google Scholar]
- 27.Piecha F, Peccerella T, Bruckner T, Seitz HK, Rausch V, Mueller S, et al. Arterial pressure suffices to increase liver stiffness. Am J Physiol Gastrointest Liver Physiol. 2016;311:G945–53. doi: 10.1152/ajpgi.00399.2015. [DOI] [PubMed] [Google Scholar]


