Abstract
Background:
Data on thyroid imaging reporting and data system (TI-RADS) generally belong to studies performed in adults. Therefore, we aimed to evaluate the performance and utility of TI-RADS in the pediatric group.
Materials and Methods:
From January 2015 to 2018, 108 nodules were evaluated in 1028 thyroid ultrasound examinations. Images were retrospectively evaluated by two radiologists with 3 and 7 years of pediatric radiology experience, according to TI-RADS classification. Morphological findings of the detected nodules and their histopathological results were recorded. Histopathological findings and at least 12 months of follow-up imaging were taken as reference.
Results:
Seventy-one patients were female (67%). The mean age was 11.4 ± 4.7, and the mean nodule size was 7.4 ± 8.3 mm. According to the histopathological assessment and at least 12 months’ follow-up with clinical and sonographic stability 100 (95.2%) of the nodules were benign and 5 (4.8%) were malignant. Two nodules, nondiagnostic cytology and 1 nodule were found to be suspicious for malignancy. All malignant nodules were in the TI-RADS 5 category. The majority of benign nodules (79%) were found in low TI-RADS categories. About 80% of the malignant nodules were very hypoechoic and taller than wide in shape, also all malignant nodules had microcalcifications (P = 0.000). The sensitivity of TI-RADS was 100%, specificity was 78.8%, positive predictive value (PPV) was 19.2%, and negative predictive value (NPV) was 100%.
Conclusion:
According to our study, TI-RADS system can be used to evaluate thyroid nodules in pediatric patients similar to adults.
Keywords: Imaging, pediatric, thyroid cancer, thyroid nodule, thyroid ultrasound
INTRODUCTION
The prevalence of thyroid nodules is low in the pediatric population (0.05%–5%). On the other hand, the risk of malignancy is higher and is widely detected in the advanced stages; therefore, the detection and identification of nodules are important for the appropriate treatment approach.[1,2] All treatment strategies, particularly surgery, are shaped by the imaging findings. High-resolution ultrasonography is a very sensitive imaging method in detecting thyroid gland nodules. However, it is well known that no single ultrasonographic (US) finding allows for differentiating the malignant thyroid nodules from the benign ones.
Thyroid imaging reporting and data system (TI-RADS) are a relatively new classification system which depends on the US properties of thyroid nodules. First described in 2009, then revised in 2015, and having recently been presented by the TI-RADS committee in 2017; this is a classification system similar to breast imaging reporting and data system aiming to standardize the thyroid nodule findings and to determine risks.[3] This classification system has not been widely accepted or implemented for use in routine clinical practice yet. Furthermore, its availability for the pediatric population is unknown.[4]
Five groups of ultrasound findings are evaluated in TI-RADS, which include nodule composition, echogenicity, shape, marginal features, and echogenic focal presence. A score of 0–3 is given for each of the findings, and the total score is calculated. The total score determines the level of TI-RADS ranging from TR1 (benign) to TR5 (high suspicion of malignancy).[3] There are few publications in the literature evaluating the efficacy of TI-RADS in the pediatric population.[5,6] Therefore, assessments in this method are based on adult studies.[7,8,9] The studies in the literature, assessing the feasibility of the method for the pediatric population is not sufficient, either.
The purpose of this study was to evaluate the diagnostic performance of TI-RADS in the evaluation of thyroid nodules detected in the pediatric population.
MATERIALS AND METHODS
Patient population
This retrospective study was approved by our institution's ethics committee, which waived the requirement to obtain informed consent. From January 2015 to 2018, 108 nodules were evaluated in 1028 thyroid ultrasound examinations. Inclusion criteria were the presence of histopathologically proven thyroid nodules (n = 5) or if no histological examination reports were available (n = 100), the availability of at least a 12-month follow-up with data records of clinical and US stability of the nodule. The patients were required to be younger than 18 years old. Of the final study population of 106 patients with 108 nodules, 13 nodules received a histopathological diagnosis. Three nodules were excluded from the study due to nondiagnostic cytology. Only one patient underwent surgery without ultrasound-guided fine-needle aspiration biopsy (FNAB).
Among the exclusion criteria were the poor image quality, nondiagnostic cytology in the pathological findings, previous exposure to irradiation, or positive family history.
Imaging
Images were retrospectively evaluated by two experienced pediatric radiologists according to TI-RADS classification. Histopathological findings and imaging findings during the follow-up of at least 12 months’ duration were taken as reference. Radiologists, blinded to tissue diagnosis and follow-up results, independently evaluated the ultrasound images on the work station. The findings were evaluated using the TI-RADS classification based on the solid composition, nodule echogenicity, margins, shape, and echogenic foci. All examinations were performed with a 5–14 MHz linear array transducer (Toshiba Aplio 500, Tokyo, Japan) and a 3–12 MHz linear array transducer (RS80A with Prestige, Samsung Medison, Seoul, Korea) in longitudinal and transverse planes. Each ultrasound finding associated with the nodules was scored according to TI-RADS and the nodules were attributed to the respective TI-RADS classes based on the total score. According to TI-RADS, ultrasound findings are classified as benign (TI-RADS 1, TI-RADS 2) or mildly suspicious (TI-RADS 3), moderately suspicious (TI-RADS 4), or highly suspicious (TI-RADS 5) for malignancy. In TI-RADS, solidity, hypoechogenicity or marked hypoechogenicity, microlobulated or irregular margins, microcalcifications, and taller-than-wide shape were defined as suspicious malignant US features.[7,8]
Statistical analysis
The reference standard was surgical histopathology or cytology results or at least a 12-month follow-up data including clinical and ultrasound imaging findings with no evolution toward malignant features. Statistical analysis was performed using the SPSS software (Version 18.0, SPSS Inc., Chicago, IL, USA). Children with benign and malignant thyroid nodules were assessed, and the findings were compared using a Chi-square test. Statistical significance was accepted at a P ≤ 0.05. Descriptive statistics were presented as mean ± standard deviation (SD). The sensitivity, specificity, and the positive and negative predictive values of TI-RADS classification were calculated.
RESULTS
The study included 106 children. Of them, 71 (67%) were female and 35 (33%) were male. A total of 105 thyroid nodules were assessed. The mean age (± SD) was 11.4 ± 4.7 years (range 1–17 years). In ultrasonography, the mean diameter of the nodules was 7.4 ± 8.3 mm (2–41 mm).
Histopathological results and stability in the 12-month clinical and US follow-up period were benign for 100 (95.2%) nodules, and 5 (4.8%) nodules were found to be malignant. In 13 nodules, the tissue diagnosis was confirmed by FNAB cytologic analysis in 12 and by surgical histopathologic analysis in 1 nodule. The categorization of the nodules based on the TI-RADS system by the histopathological status is shown in Table 1.
Table 1.
Distribution of thyroid nodules according to the histopathological results and thyroid imaging reporting and data system scores on ultrasonography
| Histopathological diagnosis | ACR TI-RADS score | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Benign (n=8) | Colloid nodule | - | - | 2 | - | - |
| Degenerative cystic nodule | - | - | 1 | - | - | |
| Benign cytology | 2 | - | 1 | 2 | - | |
| Malignant (n=5) | Papillary thyroid carcinoma | - | - | - | - | 5 |
TI-RADS: Thyroid imaging reporting and data system, ACR: American College of Radiology
The histopathological distribution consisted of 2 colloid nodules, 1 degenerative cystic nodule, and 5 nodules with benign cytology. There were only 5 malignant nodules and 8 benign nodules diagnosed by the histopathological examination. All of the malignant lesions received a diagnosis of papillary thyroid carcinoma.
According to TI-RADS, 65 (65%) nodules were classified as “Score 1,” 2 (2%) nodules as “Score 2,” 12 (12%) nodules as “Score 3,” and 21 (21%) nodules as “Score 4.” A score of TI-RADS 4 was attributed to biopsied 2 nodules, which were larger than 1.5 cm and which were diagnosed as benign [Figure 1]. All of the malignant nodules were categorized as “Score 5” [Table 2].
Figure 1.

A 14-year-old woman with American College of Radiology of Radiology Thyroid Imaging Reporting and Data System category 4 nodule. Longitudinal and axial ultrasound image shows 3.9-cm right almost completely cystic nodule with thin internal septations and solid components. Cytologic analysis revealed benign cytology
Table 2.
Distribution of thyroid nodules according to the histopathological results/stable clinical and ultrasound follow-up and thyroid imaging reporting and data system scores on ultrasonography
| Histopathological results/stable clinical and ultrasound follow-up | ACR TI-RADS Scores | P | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Benign (n=100), n (%) | 65 (65) | 2 (2) | 12 (12) | 21 (21) | - | 0.000 |
| Malignant (n=5), n (%) | - | - | - | - | 5 (100) | |
TI-RADS: Thyroid imaging reporting and data system, ACR: American College of Radiology
The distribution of individual ultrasound features is shown in Table 3. Several features were well differentiated by the histopathological status. For the echogenicity feature, most (80%) malignant nodules were markedly hypoechoic; whereas, most benign nodules were hyperechoic or isoechoic (27%) or hypoechoic (35%). For the margin feature, most (83%) benign nodules were smooth; whereas, most malignant nodules displayed an extrathyroidal extension (40%) with ill-defined margins (40%). For the echogenic foci feature, most (92.9%) benign nodules had no or large comet-tail artifact; whereas, most (80%) malignant nodules had punctate echogenic foci [Figure 2]. As for shape feature, most (65%) benign nodules were wider than tall; whereas, most (60%) malignant nodules were taller than wide.
Table 3.
Distribution of thyroid nodule ultrasound features by histopathological status/stable clinical and ultrasound follow-up
| Ultrasound features in ACR TI-RADS | Benign (n=100), n (%) | Malignant (n=5), n (%) | P |
|---|---|---|---|
| Echogenicity | |||
| Hyperechoic or isoechoic | 27 (27) | - | 0.000 |
| Hypoechoic | 35 (35) | 1 (20) | |
| Markedly hypoechoic | - | 4 (80) | |
| Anechoic | 38 (39) | - | |
| Composition | |||
| Solid or almost completely solid | 31 (31) | 100 (100) | 0.005 |
| Cystic or almost completely cystic | 37 (37) | - | |
| Spongiform | 27 (27) | - | |
| Mixed cystic or solid | 5 (5) | - | |
| Echogenic foci | |||
| None or large comet-tail artifacts | 13 (92.9) | - | 0.000 |
| Macrocalcifications | 1 (7.1) | 1 (20) | |
| Peripheral (rim) calcifications | - | - | |
| Punctate echogenic foci | - | 4 (80) | |
| Margin | |||
| Smooth | 83 (83) | 1 (20) | 0.000 |
| Ill-defined | 9 (9) | 2 (40) | |
| Lobulated or irregular | 2 (2) | - | |
| Extrathyroidal extension | - | 2 (40) | |
| Shape | |||
| Wider than tall | 65 (65) | 1 (20) | 0.002 |
| Taller than wide | 6 (6) | 3 (60) |
TI-RADS: Thyroid imaging reporting and data system, ACR: American College of Radiology
Figure 2.
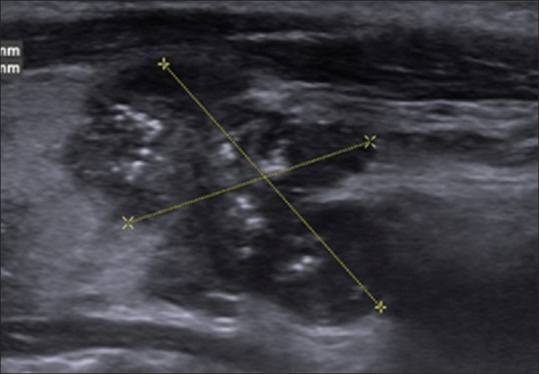
A 14-year-old boy with American College of Radiology of Radiology Thyroid Imaging Reporting and Data System category 5 nodule. Sagittal gray-scale ultrasonographic image of the left lobe of the thyroid shows a solid markedly hypoechoic mass with lobulated margins and punctate echogenic foci in the lower pole. Histopathological analysis revealed papillary thyroid cancer
Based on the results obtained, the sensitivity of TI-RADS classification in determining malignancy was found as 100%, and its specificity was 78.8%. Positive and negative predictive values were 19.2% and 100%, respectively.
DISCUSSION
This study aimed at assessing the recently developed TI-RADS classification in pediatric patients with thyroid nodules, retrospectively. This study demonstrates the utility of TI-RADS method in the differentiation between the benign and malignant thyroid nodules in the pediatric population.
In our study, all of the nodules diagnosed as malignant were in the TI-RADS 5 category, while 20% of the benign nodules were evaluated as TI-RADS 4. To the best of our knowledge, there are few studies concerning the utility of TI-RADS for the evaluation of pediatric thyroid nodules.[5,6] However, these studies employed slightly different methods. In their primary study using the TI-RADS method, Martinez-Rios et al.[5] subdivided the TI-RADS 4 category further according to the number of malignant US features. In their study, all of the malignant nodules were TI-RADS 4 or 5, and the majority of benign nodules (approximately 80%) were in the TI-RADS 4a, b, and c subgroups.
Lim-Dunham et al.[6] reported that 95% of the malignant nodules were classified under the high TI-RADS categories in their study on 62 children. In contrast with the literature, we could not detect malignant nodules in TI-RADS 4 category because most of the nodules were smaller than 10 mm and only some of them were diagnosed histopathologically. Similar to the reports in the literature, we are of the opinion that the management of nodules in TR 4 can be challenging.[5] In such cases, biopsy has to be performed depending on the size of the nodule. In our study, biopsies were performed because the nodules in TI-RADS 4 were larger than 1 cm, and they were diagnosed as benign histopathologically. In the year 2015, the American Thyroid Association Guideline, biopsy is recommended for all ≥1 cm solid or predominantly solid nodules or all nodules with high sonographic characteristics, regardless of size.[10] Due to high prevalence of malignancy in pediatric nodules compared to adults, it can be speculated that the size of the thyroid nodule is not sufficient alone in deciding whether to perform an FNAB and should be supported by additional US features of the nodule such as the shape, the presence of microcalcifications, and the characteristics of the margins.
We found that the majority of benign nodules were rated with lower TI-RADS categories, similar to the findings reported in the literature.[5,6] A low TI-RADS category was reported to show that the nodule was benign in the pediatric group similar to the adult group.[7,8,9] In our study, the most common findings in the malignant nodules were the solid composition (100%), lobulated or irregular margins and extrathyroidal extension (80%), significant hypoechogenicity (80%), microcalcifications (80%), and the taller-than-wide shape (60%), respectively.
In the recent study of Richman et al.,[11] with the largest series in the literature, a total of 404 pediatric thyroid nodules were included and it was reported that the ultrasound features of a large size, solid component, taller-than-wide shape, the presence of speckled calcifications, and the lack of smooth margins were associated with malignancy similar to our findings.
In a recent meta-analysis, Al Nofal et al.[12] emphasized that the presence of a solid component, microcalcifications, and irregular margins in nodules accompanied by the presence of abnormal lymphadenopathies (LAPs) increased the diagnostic accuracy in detecting thyroid cancers. In the literature, malignant thyroid nodules, especially in adult series, were noted to be taller than wide. Gupta et al.[13] argued that there were no significant differences in the shape of thyroid nodules in pediatric patients. However, in other studies conducted on pediatric patients similarly to our study, malignant nodules were reported to have a taller than wide appearance.[5,6,12]
Gannon et al. reported that there was not any single or combined features of ultrasound, suggestive of low risk (10% >) group for childhood thyroid cancer and thyroid biopsy was unavoidable in case of thyroid nodule.[14] However, in our study, markedly hypoechogenicity, microcalcification, and presence of solid component properties were found to be significantly associated with malignancy, which seems to be useful in determining the need for biopsy.
Limitations of our study include having a relatively small sample size, as well as the paucity of histopathological results for most of the benign nodules. Second, the ultrasound images were retrospectively screened, and also, it was not possible to investigate the interobserver compatibility.
CONCLUSION
We found a statistically significant correlation between the malignant thyroid nodules and the high TI-RADS categories, and hypoechogenicity, shape, and echogenic foci parameters. Therefore, TI-RADS classification can be applied easily and safely in the pediatric population with high sensitivity and specificity. However, large-scale, multi-centered studies are needed for further evaluation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Corrias A, Einaudi S, Chiorboli E, Weber G, Crinò A, Andreo M, et al. Accuracy of fine needle aspiration biopsy of thyroid nodules in detecting malignancy in childhood: Comparison with conventional clinical, laboratory, and imaging approaches. J Clin Endocrinol Metab. 2001;86:4644–8. doi: 10.1210/jcem.86.10.7950. [DOI] [PubMed] [Google Scholar]
- 2.Guille JT, Opoku-Boateng A, Thibeault SL, Chen H. Evaluation and management of the pediatric thyroid nodule. Oncologist. 2015;20:19–27. doi: 10.1634/theoncologist.2014-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR thyroid imaging, reporting and data system (TI-RADS): White paper of the ACR TI-RADS committee. J Am Coll Radiol. 2017;14:587–95. doi: 10.1016/j.jacr.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 4.Essenmacher AC, Joyce PH, Jr, Kao SC, Epelman M, Pesce LM, D'Alessandro MP, et al. Sonographic evaluation of pediatric thyroid nodules. Radiographics. 2017;37:1731–52. doi: 10.1148/rg.2017170059. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Rios C, Daneman A, Bajno L, van der Kaay DC, Moineddin R, Wasserman JD. Utility of adult-based ultrasound malignancy risk stratifications in pediatric thyroid nodules. Pediatr Radiol. 2018;48:74–84. doi: 10.1007/s00247-017-3974-y. [DOI] [PubMed] [Google Scholar]
- 6.Lim-Dunham JE, Toslak IE, Reiter MP, Martin B. Assessment of the American college of radiology thyroid imaging reporting and data system for thyroid nodule malignancy risk stratification in a pediatric population. AJR Am J Roentgenol. 2019;212:188–94. doi: 10.2214/AJR.18.20099. [DOI] [PubMed] [Google Scholar]
- 7.Macedo BM, Izquierdo RF, Golbert L, Meyer EL. Reliability of thyroid imaging reporting and data system (TI-RADS), and ultrasonographic classification of the American Thyroid Association (ATA) in differentiating benign from malignant thyroid nodules. Arch Endocrinol Metab. 2018;62:131–8. doi: 10.20945/2359-3997000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon HJ, Kim EK, Yoon JH, Kwak JY. Malignancy risk stratification in thyroid nodules with nondiagnostic results at cytologic examination: Combination of thyroid imaging reporting and data system and the Bethesda system. Radiology. 2015;274:287–95. doi: 10.1148/radiol.14140359. [DOI] [PubMed] [Google Scholar]
- 9.Ha EJ, Moon WJ, Na DG, Lee YH, Choi N, Kim SJ, et al. A multicenter prospective validation study for the Korean thyroid imaging reporting and data system in patients with thyroid nodules. Korean J Radiol. 2016;17:811–21. doi: 10.3348/kjr.2016.17.5.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;25:716–59. doi: 10.1089/thy.2014.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richman DM, Benson CB, Doubilet PM, Peters HE, Huang SA, Asch E, et al. Thyroid nodules in pediatric patients: Sonographic characteristics and likelihood of cancer. Radiology. 2018;288:591–9. doi: 10.1148/radiol.2018171170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al Nofal A, Gionfriddo MR, Javed A, Haydour Q, Brito JP, Prokop LJ, et al. Accuracy of thyroid nodule sonography for the detection of thyroid cancer in children: Systematic review and meta-analysis. Clin Endocrinol (Oxf) 2016;84:423–30. doi: 10.1111/cen.12786. [DOI] [PubMed] [Google Scholar]
- 13.Gupta A, Ly S, Castroneves LA, Frates MC, Benson CB, Feldman HA, et al. A standardized assessment of thyroid nodules in children confirms higher cancer prevalence than in adults. J Clin Endocrinol Metab. 2013;98:3238–45. doi: 10.1210/jc.2013-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gannon AW, Langer JE, Bellah R, Ratcliffe S, Pizza J, Mostoufi-Moab S, et al. Diagnostic accuracy of ultrasound with color flow Doppler in children with thyroid nodules. J Clin Endocrinol Metab. 2018;103:1958–65. doi: 10.1210/jc.2017-02464. [DOI] [PMC free article] [PubMed] [Google Scholar]


