Proactive therapeutic drug monitoring of infliximab is a marginally cost-effective strategy for Crohn’s disease, whereas reactive therapeutic drug monitoring is cost-effective. As the cost of infliximab decreases, a proactive strategy of dosing infliximab becomes more cost-effective.
Keywords: inflammatory bowel disease, biologics, immunosuppression, health economics
Abstract
Background
Therapeutic drug monitoring (TDM) is increasingly performed for Infliximab (IFX) in patients with Crohn’s disease (CD). Reactive TDM is a cost-effective strategy to empiric IFX dose escalation. The cost-effectiveness of proactive TDM is unknown. The aim of this study is to assess the cost-effectiveness of proactive vs reactive TDM in a simulated population of CD patients on IFX.
Methods
We developed a stochastic simulation model of CD patients on IFX and evaluated the expected health costs and outcomes of a proactive TDM strategy compared with a reactive strategy. The proactive strategy measured IFX concentration and antibody status every 6 months, or at the time of a flare, and dosed IFX to a therapeutic window. The reactive strategy only did so at the time of a flare.
Results
The proactive strategy led to fewer flares than the reactive strategy. More patients stayed on IFX in the proactive vs reactive strategy (63.4% vs 58.8% at year 5). From a health sector perspective, a proactive strategy was marginally cost-effective compared with a reactive strategy (incremental cost-effectiveness ratio of $146,494 per quality-adjusted life year), assuming a 40% of the wholesale price of IFX. The results were most sensitive to risk of flaring with a low IFX concentration and the cost of IFX.
Conclusions
Assuming 40% of the average wholesale acquisition cost of biologic therapies, proactive TDM for IFX is marginally cost-effective compared with a reactive TDM strategy. As the cost of infliximab decreases, a proactive monitoring strategy is more cost-effective.
INTRODUCTION
Infliximab (IFX) is widely used for the treatment of inflammatory bowel disease (IBD), including Crohn’s disease (CD). Unfortunately, over 50% of patients eventually lose response, with an estimated loss of response rate of 13% per year.1 Therapeutic drug monitoring (TDM) involves measuring the serum concentration of a drug and the antidrug antibody status and titrating the dose to achieve a drug concentration within a therapeutic window. Therapeutic drug monitoring can be done in a reactive setting, when a patient is having clinical recurrence of the underlying disease, or in a proactive setting, when a patient is in remission and the goal is to prevent future flares related to subtherapeutic drug concentrations or the development of antidrug antibodies.
A key limitation of TDM for IFX is the potential added cost to therapy.2, 3 Reactive TDM is a cost-effective strategy compared with empiric dose escalation of IFX.4, 5 This is due to effective triaging of patients to identify those who would benefit from a dose escalation and those who should change therapy. However, it is unknown if a proactive TDM strategy is cost-effective. In a key randomized trial to study the clinical utility of proactive TDM, the TAXIT (Trough Concentration Adapted Infliximab Treatment) trial, it was noted that both proactive TDM and a dosing strategy based on clinical features yielded similar costs.6 However in TAXIT, all participants underwent an initial proactive dose optimization, limiting the ability to truly compare proactive- and reactive-only TDM strategies. The aim of our study was to determine the cost-effectiveness of a proactive TDM strategy in managing CD patients on IFX over a 5-year time frame. We hypothesized that proactive TDM would be associated with fewer CD flares and would thus be a cost-effective strategy.
METHODS
Overview
We developed a stochastic microsimulation model of IBD progression in patients on IFX therapy (Fig. 1A). The simulation model tracks individuals’ antibody levels, IFX drug concentrations, flares, and IFX discontinuation over a 5-year period in a cohort of patients in clinical remission on IFX. Patients who discontinue IFX during the 5-year period exit the stochastic simulation model and enter a Markov model that is used to evaluate their remaining expected health utilities and costs while on subsequent therapies (Fig. 1B). These models evaluate the expected health outcomes and costs of 2 TDM strategies: “proactive TDM” and “reactive TDM.” For comparison, a “no TDM” strategy (control) was modeled, consisting of patients empirically escalated to a high dose of IFX (10 mg/kg) after a CD flare.
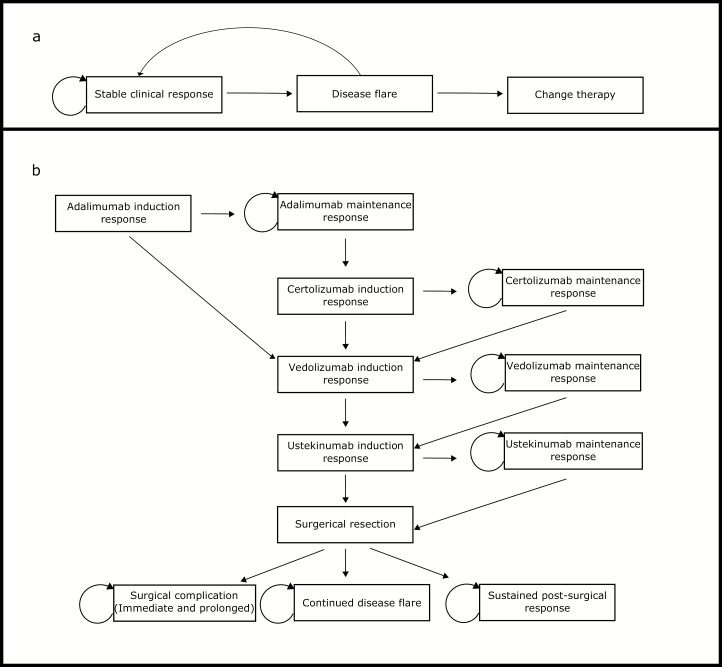
FIGURE 1.
A, Transition states for stochastic microsimulation of patients on IFX. The probability of transition to a flare was dependent on presence of a flare at prior time step, current IFX concentration, and the presence of anti-IFX antibodies. If a flare did not resolve over 2 time steps (16 weeks) without further medical escalation, the patient was transitioned off IFX to adalimumab. B, Transition states for progression of medical and surgical therapy following IFX. If subjects had an initial response, they progressed to maintenance of response. If there was no induction response, they proceeded to the subsequent medical induction. Post-surgical states were terminal states. Surgical complication included death and immediate and prolonged surgical complications.
Model Population
We simulated 100,000 average-weight (70 kg) patients with CD for 30 eight-week time increments (approximately 5 years). Patients were initialized to a stable clinical response on IFX maintenance monotherapy (ie,, no immunomodulator use), as this reflects common clinical practice.7 Initial IFX concentrations were randomly sampled such that at the start of the cohort, 15% of the patients had undetectable IFX drug concentrations, 33% had low IFX concentrations (average of 2.5 μg/mL), 29% had therapeutic drug concentrations (average of 7.5 μg/mL), and 23% had high concentrations (average of 15 μg/mL), reflective of the population from the optimization phase of TAXIT.8 Of the patients with undetectable initial IFX concentration, 75% were initialized to have low levels of antibodies present, whereas the rest were initialized with no detectable antibodies.8 Changes in IFX over time or due to a dose change were modeled using data from a previously described clinical cohort from 2 tertiary care academic centers.9 Details of the stochastic simulation model are found in the Supplemental Methods online, with full model parameters summarized in Supplemental Table 1. Table 1 shows the QALY and cost parameters for the stochastic simulation model.
TABLE 1.
Health Utilities and Medical Costs Used in the Simulation Model
| Parameter | Value | Source |
|---|---|---|
| Health utility weights (per year) | 28 | |
| Flare | 0.62 | |
| Remission | 0.89 | |
| Health care costs (per 8 week period) – 2016 USD | ||
| No flarea | 14, 15 | |
| Office Visit | $54.83 | |
| Blood work | $13.46 | |
| Total | $68.29 | |
| New flare (one-time costs) | 14, 15 | |
| Office Visit | $118.80 | |
| Blood and stool workb | $69.60 | |
| CT Scan | $265.02 | |
| Colonoscopy | $877.60 | |
| Surgical pathology | $39.70 | |
| Total | $1370.72 | |
| Continuing flare (per 8-week period) | 14, 15 | |
| Office visit (every 8 weeks) | $118.80 | |
| Blood workc | $21.46 | |
| Total | $140.26 | |
| IFX test cost (every 6 months) | $250.00 | 12 |
| IFX infusion cost | $82.22 | 15 |
| IFX drug cost (dose of 5 mg/kg for a 70 kg patient) | $1558.58 | 12 |
| IMM cost | $75.6 | 12 |
aAssuming 3 established office visits per year and routine blood work 4 times a year including complete blood count with differential, aspartate aminotransferase, alkaline phosphatase, and total bilirubin. Cost expressed as 8-week interval.
bCost of blood work to evaluate a new flare episode includes complete blood count with differential, C-reactive protein, erythrocyte sedimentation rate, and stool Clostridium difficile PCR testing.
cCosts of blood work to evaluate an ongoing flare includes complete blood count, C-reactive protein, and erythrocyte sedimentation rate
IMM: immunomodulatory—azathioprine 2 mg/kg used for costing estimates
TDM Strategies
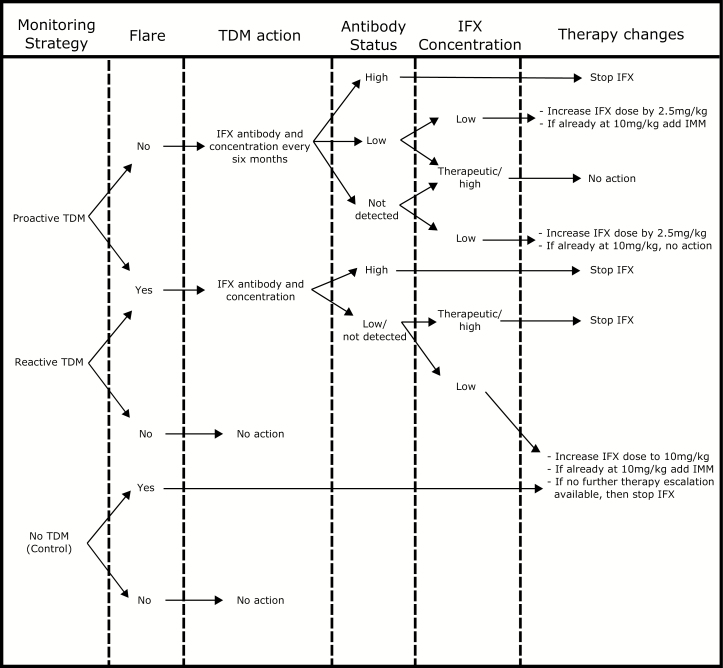
Monitoring policies and clinical decision points are summarized in Fig. 2. Under the “proactive TDM” strategy, IFX serum concentration and antibody status were assessed every 6 months. Those with low IFX drug concentrations received a dose increase of IFX by 2.5 mg/kg, or if they were already at the maximum dose of 10 mg/kg and with low antibody levels, then they received IMM. Any change in dose or addition of IMM led to retesting after 8 weeks. The addition of IMM impacted probability of anti-IFX antibody development, thus indirectly affecting the probability of a flare. Those with asymptomatic high IFX antibody concentrations and undetectable drug concentrations were transitioned off IFX and onto an alternative therapy (adalimumab). Patients also underwent reactive testing if they experienced a flare. Under the “reactive TDM” strategy, IFX serum concentration and antibody testing was only done in response to a flare. Those with high antibody status were transitioned to alternative therapy. Those with low IFX drug concentrations received a dose increase of IFX, unless already at the maximum dose of 10 mg/kg, in which case IMM was added. Those with therapeutic or high concentrations were transitioned off IFX. Under the “no TDM” strategy, no serum concentration or antibody testing was ever conducted; instead, IFX dose was increased in response to a flare to 10 mg/kg. If the flare continued, the patients were transitioned to an alternative therapy (adalimumab). Due to lack of data, none of our policies consider dose de-escalations.
FIGURE 2.
Clinical algorithm for medication change based on TDM monitoring strategy and results.
Markov Cohort Model for Patients Taken off IFX Therapy by Year 5
Patients removed from IFX therapy entered a Markov cohort model where they spent the remaining time steps until year 5 (Fig. 1B). The order of therapies in the Markov cohort was determined by the chronological approval of medical therapies for CD, with surgery reserved until after medical therapies were exhausted. Probabilities of response to subsequent therapies were based on populations who were prior anti-TNF failures. Primary nonresponders to adalimumab were switched to vedolizumab, whereas secondary loss of response to adalimumab switched first to certolizumab and then to vedolizumab if response was again lost. After vedolizumab, patients were switched to ustekinumab upon loss of response. Loss of response to ustekinumab resulted in surgery. The probabilities for initial and subsequent response to each therapeutic are summarized in Table 2. No TDM was performed for patients in the Markov model. Surgery carries a 0.15% risk of death and a 21% risk of long-term complications.10, 11 Over half of patients undergoing surgery achieved a sustained response; for those who did not, we did not model any additional changes in therapy or repeat surgeries. Health utility weights for flaring were the same as those used in the stochastic model. Drug, health care, and surgical-related costs are summarized in Table 2.
TABLE 2.
8-Week Probability and Cost Parameters for the Markov Model of Therapies for Patients Who Discontinue IFX
| Parameter | Value | Source |
|---|---|---|
| Adalimumab | ||
| Probability of initial response | 0.52 | 29 |
| Probability of maintaining response | 0.95 | 30 |
| Cost of first 8-week period | $7932.488 | 12 |
| Cost of subsequent 8-week periods | $3345.956 | 12 |
| Certolizumab | ||
| Probability of initial response | 0.64 | 31 |
| Probability of maintaining response | 0.93 | 31 |
| Cost of first 8-week period | $9801.52 | 12 |
| Cost of subsequent 8-week periods | $5684.54 | 12 |
| Vedolizumab | ||
| Probability of initial response | 0.47 | 32 |
| Probability of maintaining response | 0.88 | 33 |
| Cost of first 8-week period | $7649.416 | 12 |
| Cost of subsequent 8-week periods | $2170.762 | 12 |
| Ustekinumab | ||
| Probability of initial response | 0.38 | 34 |
| Probability of maintaining response | 0.94 | 34 |
| Cost of first 8-week period | $3470.35 | 12 |
| Cost of subsequent 8-week periods | $7140.30 | 12 |
| Surgery | ||
| Probability of surgical death | 0.0015 | 10 |
| Probability of surgical complicationsa | 0.21 | 11 |
| If no long-term complications, probability of sustained response following surgery | 0.76 | 35 |
| Cost of surgery (one-time) | $11,613.65 | 14, 15 |
| Cost of surgical complications | $27,205.22 | 14, 15 |
| Health care costs for those with a sustained response following surgery | $21.98 | 14, 15 |
| Health care costs for those without sustained response following surgery | $1954.75 | 14, 15 |
| Utility weight of surgical complications | 0.15 | 17 |
aSurgical complications combined into immediate and delayed
Costs
The average wholesale price (AWP) of medications included in our model were obtained from Red Book Online average wholesale acquisition cost package price.12 Forty percent of a medication’s listed AWP was used as an estimate of its true societal cost.13 Physician services were obtained from the centers for medicare and medicaid services (CMS) physician fee schedule online search using the national payment amount.14 Procedure, ancillary, and laboratory costs were obtained from the CMS hospital outpatient prospective payment system using the national limit for 2017.15 Costs are summarized in Table 1 and 2. Only direct health care costs were considered.
Outcomes
Our principal outcomes were the expected quality-adjusted life-years (QALYs) and direct health care costs accrued by patients initially on IFX therapy over a 5-year time horizon. Health utility weights for relevant health states were derived from Gregor et al and Kennedy et al10, 16, 17 and are listed in Tables 1 and 2. We used the expected cost and QALY outcomes, discounted at 3% per year, to evaluate the cost-effectiveness of proactive vs reactive IFX TDM strategies from a health sector perspective. A modern willingness-to-pay (WTP) threshold was used where an increment cost-effectiveness ratio less than $50,000 USD per QALY was considered extremely cost-effective, $50,001 to $100,000 USD per QALY was considered cost-effective, $100,001 to $150,000 USD per QALY was considered marginally cost-effective, and > $150,000 USD per QALY was considered not cost-effective (based on 1 to 3 times the average per capita gross domestic product in the United States in 2016).18, 19
We hypothesized that key parameters would affect the overall cost-effectiveness of various strategies and, therefore, planned 1-way sensitivity analysis based on our assumptions surrounding IFX pharmacokinetics and pharmacodynamics: the rate of flaring based on a high or low IFX concentration, the rate of change in IFX concentration depending on the presence of antibodies, and the magnitude of the uncertainty when sampling IFX concentration (ie, IFX distribution). We also tested key cost parameters that we hypothesized would be real or perceived limitations: the cost of IFX concentration testing, the cost of all biologics, and the cost of disease flaring. All statistical analyses and model simulations were performed using R v3.1.2.
RESULTS
Model Calibration/Validation
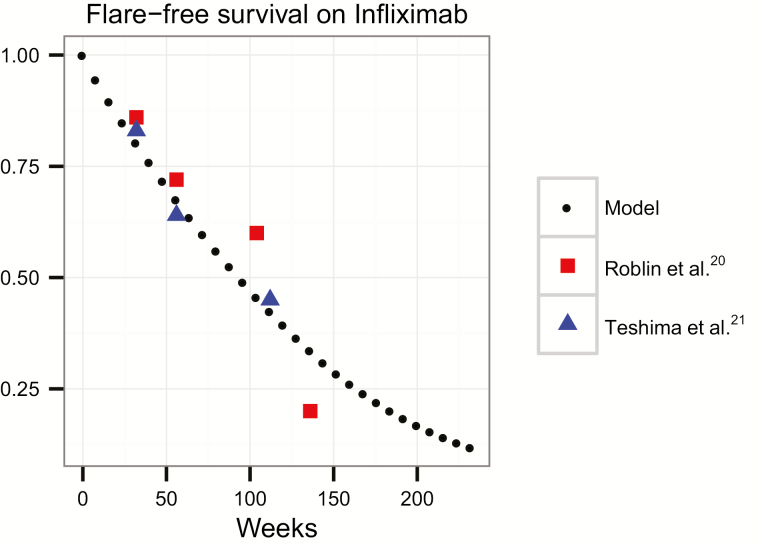
We calibrated our simulation model by optimizing for the flare rate that minimized the mean squared error between our model’s overall flare-free survival and the flare-free survival reported in Roblin et al, a prospective observational cohort that classified loss of response.20 For validation, the calibrated model’s flare-free survival was then compared with that reported in Teshima et al, an independent clinical cohort of patients on IFX.21 Flare-free survival projected by our model and the flare-free survival under no TDM strategies (ie, control) observed in the calibration and validation cohorts are shown in Fig. 3.
FIGURE 3.
Flare rate of our model in relation to the calibration model (Roblin et al)20 and validation model (Teshima et al).21
Flares
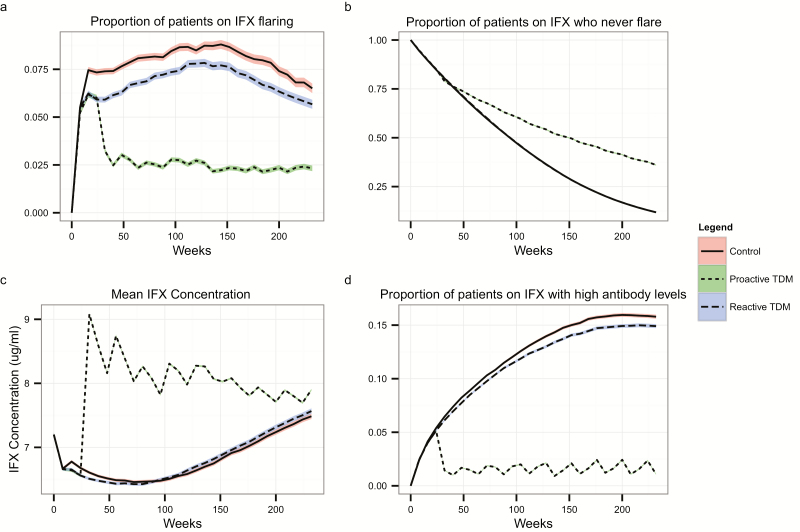
A proactive TDM strategy led to fewer flares compared with a reactive TDM strategy (Fig. 4A). One year after IFX initiation, 71.8% (95% CI, 71.5%–72.1%) of patients under the proactive strategy were flare-free, compared with 67.8% (95% CI, 67.6%–68.14%) under the reactive strategy. At 5 years, 36% (95% CI, 35.7%–36.3%) in the proactive strategy remained flare-free vs 11.8% (95% CI, 11.6%–12%) in the reactive-only strategy (Fig. 4B). Those undergoing proactive TDM were initially more likely to move to an alternative therapy due to early identification of individuals with antibodies to IFX who had not yet flared. However, by the end of our simulation (5 years), a higher proportion of patients were on an alternative therapy under a reactive TDM strategy (63.4%; 95% CI, 63.1%–63.7%) compared with proactive TDM (58.8%; 95% CI, 58.5%–59.1%).
FIGURE 4.
A, Proportion of patients currently on IFX experiencing a flare at each time step; (b) proportion of patients in the cohort who never experienced a flare; (c) Mean IFX concentration at each time step; (d) Proportion of patients who develop anti-IFX antibodies over time. Bands around lines represent the standard errors of the means.
IFX Level and Antibodies
The average IFX serum concentration was highest among patients on a proactive TDM strategy at any time in our simulation (Fig. 4C). Additionally, the proactive strategy led to a lower prevalence of antibodies to IFX compared with the reactive strategy or no TDM strategy (Fig. 4D). In our model, this is due to both decreased antibody formation under proactive TDM and earlier identification and removal of individuals who were not flaring but had developed high levels of antibodies to IFX.
Dose
Patients on the proactive TDM strategy received higher doses of IFX than those on the reactive policy (Supplemental Fig. 1). A year after start of TDM, 56.9% (95% CI, 56.6%–57.2%) of patients on the proactive TDM strategy were on a medium (7.5 mg/kg) IFX dose, and 10% (95% CI, 9.7%–10.1%) of those patients were on a high (10 mg/kg) IFX dose, compared with 0% of reactive strategy patients on a medium dose and 17.8% (95% CI, 17.5%–18%) of them on a high dose. At the end of our simulation, 76.5% (95% CI, 76.1%–77%) of patients were on a medium dose under the proactive TDM strategy, and the rest were on a high dose. Under the reactive TDM strategy, 66.7% (95% CI, 66.2%–67.1%) were on a high dose, and the rest (33.3%, 95% CI, 32.9%–33.8%) were on a low dose.
Cost-effectiveness
For our base case, a reactive TDM strategy yielded more QALYs at a lower cost than no monitoring, consistent with previous studies.4 Proactive TDM yielded on average per patient 0.025 additional QALYs at an incremental cost of $3688 compared with reactive TDM for an incremental cost-effectiveness ratio (ICER) of $146,494, which is below the cost-effective thresholds used in the United States, making proactive TDM a marginally cost-effective monitoring strategy compared with reactive testing (Table 3).18
TABLE 3.
Results of Base Case and Varying 1-Way Sensitivity Analysis
| Parameter values / Policies | Proactive | Reactive | Control | ICER Reactive-Proactive | ICER Control–Reactive | |
|---|---|---|---|---|---|---|
| Base case (*) | QALYs | 3.68 | 3.65 | 3.65 | $146,509.12 | Dominated |
| Costs | $82,927.12 | $79,238.46 | $79,268.40 | |||
| RR of flare with low IFX (2.35) | QALYs | 3.62 | 3.62 | 3.61 | $2,298,719.48 | Dominated |
| Costs | $87,156.33 | $82,165.81 | $82,208.46 | |||
| RR of flare with low IFX (9.4) | QALYs | 3.71 | 3.67 | 3.67 | $59,405.89 | 3468.88 |
| Costs | $80,076.36 | $77,576.56 | $77,559.84 | |||
| All costs reduction of 15% compared with (*) | QALYs | 3.68 | 3.65 | 3.65 | $124,532.71 | Dominated |
| Costs | $70,488.05 | $67,352.69 | $67,378.14 | |||
| All costs reduction of 40% compared with (*) | QALYs | 3.68 | 3.65 | 3.65 | $87,905.63 | Dominated |
| Costs | $49,756.27 | $47,543.07 | $47,561.04 | |||
| Flare costs -40% | QALYs | 3.68 | 3.65 | 3.65 | $156,796.68 | Dominated |
| Costs | $82,656.15 | $78,708.48 | $78,726.81 | |||
| Flare costs + 40% | QALYs | 3.68 | 3.65 | 3.65 | $136,221.55 | Dominated |
| Costs | $83,198.08 | $79,768.43 | $79,809.99 | |||
| IFX initialized to low concentration (2.5 mg/mL) | QALYs | 3.68 | 3.67 | 3.67 | $590,058.56 | Dominated |
| Costs | $87,297.51 | $83,770.73 | $83,798.13 | |||
| IFX initialized to medium concentration (7.5 mg/mL) | QALYs | 3.68 | 3.63 | 3.62 | $78,369.39 | 6992.8 |
| Costs | $81,385.67 | $77,738.28 | $77,705.26 | |||
| IFX initialized to high concentration (15 mg/kg) | QALYs | 3.69 | 3.67 | 3.66 | $165,910.83 | Dominated |
| Costs | $75,963.57 | $71, 765.86 | $71.962.54 |
Abbreviations: RR, relative rate; comparison between rates of flaring for a low < 5.5 ug/mL IFX concentration vs a high IFX concentration
We conducted a number of 1-way sensitivity analyses on key parameters, including the rate of flaring based on a high or low IFX concentration, the rate of change in IFX concentration depending on the presence of antibodies, the magnitude of the uncertainty when sampling IFX concentration, the cost of IFX concentration testing, and the cost of IFX (Table 3 and Supplemental Table 2). Our results were most sensitive to the value of the relative rate of a flare for an IFX level below vs above 5.5 μg/mL. A very low flare rate with IFX level <5.5 μg/mL (ie, similar rate of flare for high or low IFX concentrations) makes monitoring IFX levels less informative and thus not cost-effective, since flares are likely to develop even at higher IFX levels. In contrast, higher flare rates at low IFX levels make TDM more informative, as an IFX level falling below 5.5 μg/mL becomes a more accurate predictor of flares, increasing the cost-effectiveness of a proactive TDM strategy. The purchase price of IFX was the second most important factor in the overall cost-effectiveness of a proactive strategy. If the IFX dose cost is at or below 40% of the AWP, a proactive strategy was more cost-effective; however at a cost above 40% of the AWP, proactive TDM is not a cost-effective strategy. The order of medications following IFX failure was also varied, with no change in the overall results in terms of proactive TDM vs reactive TDM; although in some situations, reactive TDM was no longer cost saving compared with control, the ICER remaining below $50,000USD/QALY.
To further examine the impact of costs, specifically the cost of performing TDM testing on the cost-effectiveness of the monitoring policies, we performed a 2-way sensitivity analysis where we varied the cost of a dose of biologic drug (including IFX) from 20% to 120% of the wholesale value and the cost of an IFX test (assumed at $250) from 0% to 800% of our base case value (Supplemental Fig. 2). Our base case cost was 40% of the average wholesale price of IFX. Assuming the cost of IFX concentration testing is $250 per test, increases to IFX acquisition cost render proactive TDM not cost-effective. However, with discounted or no charge for IFX concentration testing, a proactive TDM strategy can be cost-effective at higher IFX acquisition costs and may be extremely cost-effective (ICER < $50,000/QALY) at very discounted IFX costs.
To explore how sensitive our results were to our baseline estimate of IFX concentrations (based on the optimization data from the TAXIT trial),8 we varied the initial distribution of IFX concentrations to all low concentration (2.5 ug/mL), medium (7.5 ug/mL), or high (15 ug/mL) (Supplemental Table 2). In both extremes, proactive TDM was not a cost-effective strategy compared with reactive TDM. However at the medium IFX concentration, a proactive TDM strategy was more cost-effective than in our base case estimate.
DISCUSSION
We found proactive TDM to be a marginally cost-effective strategy over a 5-year period compared with reactive TDM. Our findings confirm that using updated costs of biologics, reactive TDM is extremely cost-effective and even—under certain assumptions—cost-saving, compared with empiric dose escalation in the setting of a CD flare. The cost-effectiveness of a proactive TDM strategy was highly dependent on the effectiveness of preventing flares and antibodies when proactively dosing IFX to a higher concentration and the purchase price of IFX.
A key assumption in our model was the extent to which flare rates are reduced at therapeutic or high IFX concentrations vs low concentrations. When the rate of CD flaring is similar for low and high IFX concentrations, proactive TDM is not cost-effective. However, as the flare rate decreases with a higher IFX concentration, proactive TDM becomes increasingly cost-effective. For our base case assumption, we chose a relative flare rate of 4.7 times for a low IFX concentration compared with a high concentration.20 This was chosen as a conservative assumption based on an external prospective cohort of patients on IFX data to validate our model. However, some retrospective studies suggest that the benefit of achieving a concentration over 5 ug/mL is closer to an odds ratio of 9.4 for preventing a flare.22 Most studies suggest an odds ratio or relative risk of between 5 and 10 for response or remission with a higher drug concentration, although the exact cutoff can vary per study.23–25
Proactive TDM results in a “dose creep” phenomenon, which increases the cost of care. However, the benefits of proactive TDM of IFX are in preventing flares6, 22 and decreased hospitalizations and surgery rates.9 Under a reactive TDM strategy or no TDM strategy, the dose of IFX also increases, albeit only after a flare. However, by the end of our simulation, all strategies resulted in a similar mean IFX concentration in the patient cohort. In this sense, overall cost-effectiveness of a proactive TDM strategy can be thought of as a balance between an initial cost of IFX and the cost of disease exacerbation followed by subsequent increases in IFX cost. Central to this balance is the cost of biologics, which are escalating rapidly and may account for up to 64% of the total cost of care in IBD.26 The average wholesale price of IFX increased almost 70% from 2010 to 2016. Discounts (eg, 340b drug discount program, which can discount a drug 20%–50%)27 and pharmacy rebates often decrease the purchase price of biologics for a system. However, costs are not transparent, and the actual cost of IFX may vary widely between regions and health systems. If the cost of IFX is decreased relative to the cost of other biologics (eg, due to competition from IFX biosimilars), then proactive TDM is even more cost-effective than in our base case analysis.
The cost of the IFX concentration assay also impacted the cost-effectiveness of a proactive TDM strategy. Under certain conditions with a substantially reduced purchase price of IFX and free IFX concentration testing, proactive TDM was extremely cost-effective. A proactive TDM strategy resulted in a 3.5-fold increase in number of IFX concentration tests; thus the price of concentration testing can substantially impact the cost-effectiveness of the strategy. However, similar to the purchase price of IFX, the importance of the cost of IFX concentration testing is lessened when the odds of a flare with a high serum IFX concentration are much lower than the odds of a flare with a low serum IFX concentration.
Our results were sensitive to the population’s initial distribution of IFX concentration. We based the distribution of the sample population on the optimization phase of TAXIT. This provided a real-world sample of IFX concentrations in patients on a stable clinical response in maintenance IFX dosing. If instead, a patient population starts with low IFX concentrations, there is little difference between proactive and reactive monitoring as most people experience a flare early on and undergo reactive testing. If the population is initialized to have high IFX concentrations, there is also little value in proactively measuring concentrations to identify those with subtherapeutic concentrations or antidrug antibodies. When the patient cohort was initialized to have a medium IFX concentration, proactive TDM was more cost-effective than our baseline population assumption. In this situation, there was a beneficial mix of those flaring from low drug concentrations or antibodies such that proactively identifying and preventing those flares by escalating the dose or changing therapy was cost-effective.
There remain potential benefits of a proactive TDM strategy that we did not effectively capture in our model due to lack of data. First, the ability to dose de-escalate IFX is a key benefit of proactive TDM estimated to occur between 15% and 30% of the time.6, 22 Second, we limited our dose escalations to a medium dose (IFX at 7.5 mg/kg) and a high dose (IFX at 10 mg/kg). In actuality, many dose escalations occur with only 50 to 100 mg of IFX or an interval shortening of 1 to 2 weeks.22 Smaller dose increases would limit the overall cost of the drug while still attaining a therapeutic drug concentration and, therefore, the benefits of proactive TDM.
Our study has several important limitations. First, as noted previously, we did not account for IFX dose de-escalations and smaller dose increases or dose interval changes. Although this may limit the generalizability, this also likely biased our results against proactive TDM being cost-effective. Indeed, by the end of our simulation, no one in the proactive TDM strategy was on a standard dose due to the strict design assumptions that governed decision-making and the inability to de-escalate the dose. Second, we did not consider indirect costs associated with flaring, which would again likely benefit a proactive TDM strategy. Third, we initialized our population to mimic the optimization phase of TAXIT, in which the median time on IFX was 4.5 years. Antibodies to IFX frequently occur in the first year, and thus proactive TDM from the start of maintenance IFX may be more cost-effective than in established patients. Fourth, we transitioned all patients from IFX to adalimumab, regardless of the mechanism of IFX failure. This was done to standardize the time in the Markov model prior to surgery. Choosing subsequent therapy based on how a patient failed IFX would improve the chance of response, reducing costs and increasing benefits to patients post-IFX. Fifth, we did not model adverse events associated with IFX. Infusion reactions may have been prevented in a proactive TDM strategy. Lastly, due to the opacity of costs in our health care system, we are subject to making our best assumptions regarding the purchase price of biologics. Transparency in costing would allow for more accurate assessment of optimal strategies.
CONCLUSION
We found that for patients with CD on IFX, proactive TDM is a marginally cost-effective strategy compared with reactive TDM. A proactive strategy prevented CD flares but brought a higher cost of IFX therapy. At higher purchase prices of IFX or when the risk of a CD flare is similar regardless of the IFX concentration, proactive TDM is not cost-effective compared with reactive TDM. As the cost of IFX or associated costs of testing are decreased, proactive TDM becomes a more cost-effective strategy. Societal interventions to limit pharmaceutical cost increases and competition from biosimilars will likely create a more favorable environment for proactive TDM. Incorporating a proactive TDM strategy for IFX should be based on a given health care system’s cost of biologic therapy and the underlying cost of care for patients with CD.
Supplementary Material
Author contributions: DMN contributed to the study content and design, analysis and interpretation, statistical analysis, and manuscript writing. EAE contributed to the study content and design, analysis and interpretation, statistical analysis, and critical review of the manuscript. BS and BB contributed to the study design, statistical analysis, critical review of the manuscript. JC, MO, KP, and ASC contributed to the data acquisition and interpretation and critical review of the manuscript. BPV contributed to the study content and design, analysis and interpretation, and manuscript writing.
Supported by: KP is supported by Ruth L. Kirschstein NRSA Institutional Research Training Grant 5T32DK007760-18. EAE is supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number K25AI118476. BPV was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number KL2TR000113. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This project was supported by the University of Minnesota’s Undergraduate Research Opportunities Program.
Conflicts of Interest: EAE received compensation from ViiV Health care for participation in an advisory board meeting. MTO received consultancy fees from Janssen, AbbVie, UCB, Takeda, Pfizer, Merck, and Lycera and research support from UCB. ASC received consultancy fees from AbbVie, Janssen, Takeda, Ferring, Miraca, AMAG, Arena, Samsung, Alfasigma, and Pfizer and research support from Miraca. BPV received research support from Takeda, Genentech, and Celgene and has received compensation from Janssen and AbbVie for speaking and advisory boards. DMN, BS, BB, JC, MO, and KP have no conflicts of interest to declare.
REFERENCES
- 1. Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol. 2009;104:760–767. [DOI] [PubMed] [Google Scholar]
- 2. Campbell JP, Burton E, Wymer S, et al. Out-of-pocket cost is a barrier to therapeutic drug monitoring in inflammatory bowel disease. Dig Dis Sci. 2017;62:3336–3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grossberg LB, Papamichael K, Feuerstein JD, et al. A survey study of gastroenterologists’ attitudes and barriers toward therapeutic drug monitoring of anti-TNF therapy in inflammatory bowel disease. Inflamm Bowel Dis. 2017;24:191–197. [DOI] [PubMed] [Google Scholar]
- 4. Velayos FS, Kahn JG, Sandborn WJ, et al. A test-based strategy is more cost effective than empiric dose escalation for patients with Crohn’s disease who lose responsiveness to infliximab. Clin Gastroenterol Hepatol. 2013;11:654–666. [DOI] [PubMed] [Google Scholar]
- 5. Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut. 2014;63:919–927. [DOI] [PubMed] [Google Scholar]
- 6. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148:1320–1329.e3. [DOI] [PubMed] [Google Scholar]
- 7. Peyrin-Biroulet L, Salleron J, Filippi J, et al. Anti-TNF monotherapy for Crohn’s disease: a 13-year multicentre experience. J Crohns Colitis. 2016;10:516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vande Casteele N, Compernolle G,Ballet V, et al. Results on the optimisation phase of the prospective controlled trough level adapted infliximab treatment (TAXIT) trial. Gastroenterology. 2012;142:S211–S212. [Google Scholar]
- 9. Papamichael K, Chachu KA, Vajravelu RK, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol. 2017;15:1580–1588.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gregor JC, McDonald JW, Klar N, et al. An evaluation of utility measurement in Crohn’s disease. Inflamm Bowel Dis. 1997;3:265–276. [PubMed] [Google Scholar]
- 11. Fumery M, Seksik P, Auzolle C, et al. ; REMIND study group investigators Postoperative complications after ileocecal resection in Crohn’s disease: a prospective study from the REMIND group. Am J Gastroenterol. 2017;112:337–345. [DOI] [PubMed] [Google Scholar]
- 12. IBM Micromedex® Red Book ® (2018). Greenwood Village, Colorado, USA: Truven Health Analytics; http://www.micromedexsolutions.com.ezp3.lib.umn.edu/. Accessed April 17, 2017. [Google Scholar]
- 13. Hay JW, Smeeding J, Carroll NV, et al. Good research practices for measuring drug costs in cost-effectiveness analyses: issues and recommendations: the ISPOR drug cost task force report–part I. Value Health. 2010;13:3–7. [DOI] [PubMed] [Google Scholar]
- 14. Center for Medicare & Medicaid Services. Physician Fee Schedule Search https://www.cms.gov/apps/physician-fee-schedule/. Accessed April 17, 2017.
- 15. Center for Medicare & Medicaid Services. Hospital Outpatient Prospective Payment System https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/index.html?redirect=/HospitalOutpatientPPS. Accessed April 17, 2017.
- 16. Kennedy AP, Nelson E, Reeves D, et al. A randomised controlled trial to assess the effectiveness and cost of a patient orientated self management approach to chronic inflammatory bowel disease. Gut. 2004;53:1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kennedy ED, Urbach DR, Krahn MD, et al. Azathioprine or ileocolic resection for steroid-dependent terminal ileal Crohn’s disease? A Markov analysis. Dis Colon Rectum. 2004;47:2120–2130. [DOI] [PubMed] [Google Scholar]
- 18. Neumann PJ, Sanders GD, Russell LB, et al. Cost-Effectiveness in Health and Medicine. 2nd ed. New York, NY: Oxford University Press; 2016. [Google Scholar]
- 19. Marseille E, Larson B, Kazi DS, et al. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roblin X, Marotte H, Leclerc M, et al. Combination of C-reactive protein, infliximab trough levels, and stable but not transient antibodies to infliximab are associated with loss of response to infliximab in inflammatory bowel disease. J Crohns Colitis. 2015;9:525–531. [DOI] [PubMed] [Google Scholar]
- 21. Teshima CW, Thompson A, Dhanoa L, et al. Long-term response rates to infliximab therapy for Crohn’s disease in an outpatient cohort. Can J Gastroenterol. 2009;23:348–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vaughn BP, Martinez-Vazquez M, Patwardhan VR, et al. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis. 2014;20:1996–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Steenholdt C, Bendtzen K, Brynskov J, et al. Cut-off levels and diagnostic accuracy of infliximab trough levels and anti-infliximab antibodies in Crohn’s disease. Scand J Gastroenterol. 2011;46:310–318. [DOI] [PubMed] [Google Scholar]
- 24. Maser EA, Villela R, Silverberg MS, et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:1248–1254. [DOI] [PubMed] [Google Scholar]
- 25. Papamichael K, Vajravelu RK, Vaughn BP, et al. Proactive infliximab monitoring following reactive testing is associated with better clinical outcomes than reactive testing alone in patients with inflammatory bowel disease. J Crohns Colitis. 2018;12:804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van der Valk ME, Mangen MJ, Leenders M, et al. ; COIN study group and the Dutch Initiative on Crohn and Colitis Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COIN study. Gut. 2014;63:72–79. [DOI] [PubMed] [Google Scholar]
- 27. Lee J. HHS Fights Back to Keep Expanded 340B Drug Discounts, Vol. 2018 Modern Healthcare; 2014. https://www.modernhealthcare.com/article/20140612/NEWS/306129940/hhs-fights-back-to-keep-expanded-340b-drug-discounts. [PubMed] [Google Scholar]
- 28. Silverstein MD, Loftus EV, Sandborn WJ, et al. Clinical course and costs of care for Crohn’s disease: markov model analysis of a population-based cohort. Gastroenterology. 1999;117:49–57. [DOI] [PubMed] [Google Scholar]
- 29. Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146:829–838. [DOI] [PubMed] [Google Scholar]
- 30. Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65. [DOI] [PubMed] [Google Scholar]
- 31. Sandborn WJ, Abreu MT, D’Haens G, et al. Certolizumab pegol in patients with moderate to severe Crohn’s disease and secondary failure to infliximab. Clin Gastroenterol Hepatol. 2010;8:688–695.e2. [DOI] [PubMed] [Google Scholar]
- 32. Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618–627.e3. [DOI] [PubMed] [Google Scholar]
- 33. Sandborn WJ, Feagan BG, Rutgeerts P, et al. ; GEMINI 2 Study Group Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–721. [DOI] [PubMed] [Google Scholar]
- 34. Feagan BG, Sandborn WJ, Gasink C, et al. ; UNITI–IM-UNITI Study Group Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–1960. [DOI] [PubMed] [Google Scholar]
- 35. Renna S, Cammà C, Modesto I, et al. Meta-analysis of the placebo rates of clinical relapse and severe endoscopic recurrence in postoperative Crohn’s disease. Gastroenterology. 2008;135:1500–1509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.