Abstract
Introduction:
iRoot BP Plus, also known as EndoSequence root repair material (EERM) is a premixed bioceramic thick/putty. According to its instruction manual, iRoot BP Plus is composed of tricalcium silicate, zirconium oxide, tantalum pentoxide, dicalcium silicate, calcium sulfate, calcium phosphate monobasic, and filler agents. This systematic review was carried out to evaluate and present the iRoot BP Plus material as a pulp-capping agent.
Materials and Methods:
A systematic search for articles with the scope of the selection criteria undergoing for data extraction was conducted through electronic databases. Studies on evaluation of the cytotoxicity, bioactivity, and dentinal bridge formation of iRoot BP, iRoot BP Plus, ERRM putty, or ERRM paste (ERRM) on variant human cells were selected for in vitro models, and dentinal bridge formation on human and animals teeth for in vivo models were selected.
Results:
A total of 22 articles were discussed in the review, 14 in vitro studies, five in vivo studies, and three articles with both studies. Methyl thiazol tetrazolium was the most used method for evaluating cytotoxicity. As for dentinal bridge formation, histological assessment and micro-Computed tomography were used. Human dental pulp cells (hDPCs) were the most investigated for in vitro models and rats for in vivo models. Except for one study, all studies involved in this review were primarily examining the material and comparing it to different types of mineral trioxide aggregate.
Conclusion:
iRoot BP, iRoot BP Plus, and ERRM are biocompatible materials that enhance hDPCs and other variant human cells proliferation, migration, attachment adhesion, mineralization, and dentinal bridge formation.
KEYWORDS: EndoSequence root repair material, iRoot BP, iRoot BP Plus, pulp capping
INTRODUCTION
Pulp capping is defined as the management of an exposed vital pulp in which the pulpal wound is sealed with a dental material to facilitate the formation of a reparative dentin and the maintenance of a vital pulp.[1] The capping material plays a principal role in the treatment success. Therefore, it should possess specific characteristics such as biocompatibility, enhancement of reparative dentin formation, preservation of pulp vitality, having a high PH, and attachment to dentin and to filling material. Also, it should be bacteriostatic/bactericidal, sterile, radiopaque, have a short-setting time, low porosity and solubility, and moderate flow.[2,3] To this day, no material exists that possesses all these properties.
Mineral trioxide aggregate (MTA) was launched in 1993 by Mahmoud Taorabinejad. It is composed of a mixture of dicalcium silicate, tricalcium silicate, tricalcium aluminate, gypsum, tetracalcium aluminoferrite, and bismuth oxide.[3] MTA has overtaken calcium hydroxide by its superior properties, which include having no or low solubility, high pH, releasing calcium hydroxide when exposed to water, biocompatibility, and promoting cell viability, as well as dentin bridge formation that forms faster and has better structural integrity than calcium hydroxide. On the contrary, MTA drawbacks include difficult manipulation, delayed setting time, tooth discoloration, and expensiveness.[4,5,6]
iRoot BP Plus (Innovative BioCeramix, Vancouver, BC) and iRoot BP (Innovative BioCeramix), also known as EndoSequence root repair material putty (ERRM putty), and ERRM paste are different formulations, similar composition, laboratory-synthesized, ready-to-use, premixed bioceramic thick/putty white pastes, developed for permanent root canal repair and surgical use. According to its instruction manual, ERRM is composed of tricalcium silicate, zirconium oxide, tantalum pentoxide, dicalcium silicate, calcium sulfate, calcium phosphate monobasic, and filler agent. Setting time is a minimum of 2h, which requires the existence of water to set and harden.
Setting may prolong if the application site on the tooth is arid. The moisture needed for setting depends on the moisture present within the dentin, which reaches the root canal through dentinal tubules, therefore eliminating the need to add moisture before placing the material. As iRoot BP Plus is a premixed paste, it can be provided in a jar or a syringe and must be stored in a dry area at room temperature. Sterile plastic instruments should be used to introduce the material to the desired site on a tooth. It is essential to ensure that the site is completely filled to prevent procedural delay from occurring. It is also crucial to control bleeding in case the material washes out. The setting reaction starts as soon as the material comes in contact with the moisture in the tooth. iRoot BP Plus and ERRM are biocompatible,[7,8,9,10] insoluble, produce caustic calcium hydroxide when coming into contact with water, and do not shrink during setting. Their pH is more than 12; they have an antimicrobial effect,[11] are radiopaque, have an excellent sealing ability when used as root-end fillings,[12,13] and are known to be aluminum free. This review aimed to answer the specific research question: Are iRoot BP (Plus) and ERRM suitable as pulp-capping agent?
MATERIALS AND METHODS
SEARCH STRATEGY AND STUDY SELECTION
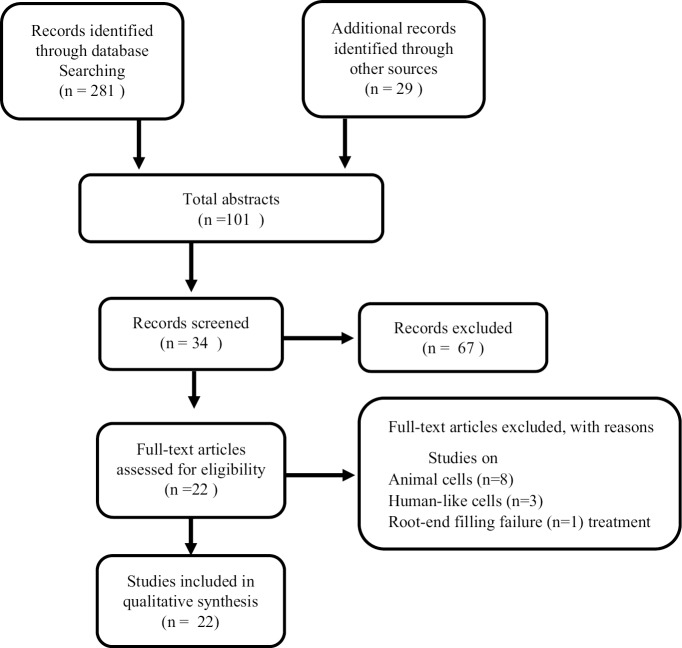
A systematic search was conducted through the electronic database MEDLINE (PubMed), Google Scholar, and other sources by two reviewers individually. A total of 310 articles were recognized using the following search terms: iRoot BP, iRoot BP Plus, ERRM, and pulp capping. The search was limited to the English language, and 209 articles were removed after the duplicate record was checked, leaving 101 articles. Following the abstract screening, 67 articles were eliminated leaving 34 articles in total. All articles met the inclusion criteria which were as follows: Material: iRoot BP, iRoot BP Plus, ERRM. Studies: In vitro models on human cells (human dental pulp cells [hDPCs], human dental pulp stem cells [DPSCs], human osteoclast, human fibroblast, human periodontal ligament fibroblasts and osteoclast, and human bone marrow-derived stem cells [hBMSCs]) and in vivo models on animals or human teeth were assessed leading to the exclusion of 12 articles. The reason for this is that (n = 8) articles were conducted on animal cells, (n = 3) on humanlike cells, and (n = 1) root-end filling treatment failure; therefore, 22 articles advanced for full-text assessment and were included in this review [Figure 1]. Any disagreements were set on by consent with a third party. The reference lists of selected articles were hand screened, and the search was regularly updated from November 2016 to April 17, 2018. Because of the lack of required statistical data provided by the articles, only a qualitative summary of the studies was attained.
Figure 1.
A process graphic representing study selection
QUALITY ASSESSMENT
The following 11 parameters were used for quality assessment: teeth free of caries, teeth randomized, appropriate comparator material is chosen, reproduced the results with different models, control group inclusion, examiner blinding, samples with similar dimensions, failure mode evaluation, analysis by a single observer, description of the coefficient of variation, and sample size calculation. The studies were classified as high (0–4), moderate (5–8), or low (9–11) risk of bias.[14,15]
DATA EXTRACTION
A comprehensive data extraction is presented in Table 1 containing authors’ names, country, year of publication, type of cells, source of cells, type of tests, material used, control group, duration of the study, and risk of bias for in vitro studies.
Table 1.
List of in vitro studies and tested parameters
| Year/country | Author | Cells | Cells’s source | Test | Materials | Control | Duration | Risk of bias | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2011 China[16] | Ma et al. | Human gingival fibroblasts | From healthy patients who underwent oral surgery | MTT assay- SEM -EDAX | ERRM Putty -ERRM Paste -GMTA -IRM -Cavit G |
DMEM incubated for 24 h (negative control) | 1, 3, and 7 days | Moderate | |||
| 2011 USA/ Michigan[17] | Damas et al. | Human dermal fibroblasts | Adult human dermal fibroblasts | MTT assay | RRM -RRP Putty -White MTA- Angelus -ProRoot MTA |
-Culture medium (negative control) -culture medium +cholorhexidine (positive control) |
1 day | Low | |||
| 2012 USA/ Michigan[18] | Hirschman et al. | Human dermal fibroblasts | Adult human dermal fibroblasts | MTT assay | MTA Angelus -ERRM -Ultra-blend Plus -Dycal |
-Culture medium (negative control) -Culture medium +chlorhexidine (positive control) |
2, 5, and 8 days | Low | |||
| 2012 Brazil[7] | De-Deus et al. | Human osteoblast | 36 human maxillary incisor teeth | XTT -NR -CVDE |
iRoot BP Pus -White ProRoot MTA |
-Zinc oxide eugenol positive control -Medium not exposed to material negative control |
24 and 48 h | Low | |||
| 2012 Iran[19] | Shokouhinejad et al. | Cement/ dentin-cement | 30 extracted single-rooted human teeth | SEM -EDX assay |
ERRM -Bioaggregate -ProRoot MTA |
1 week and 2 months | Moderate | ||||
| 2012 China[10] | Zhang et al. | hDPCs | Molars of patients who needed orthodontic treatment | -CCK-8 assay -ALP activity -qRT- PCR assays |
iRoot BP Plus - Bioaggregate -MTA |
Cells without material | 1, 3, 5, and 7 days 3, 5, and 7 days | Low | |||
| 2013 Germany[20] | Willershausen et al. | Human periodontal ligament fibroblasts and human osteoblasts | Promocell, Heidelberg, Germany | ERRM -ProRoot MTA -MTA-Angelus GMTA -WMTA |
Almar blue-fluorescence microscope | Cells without material | 6 h, 1, 3, and 4 days 24 h | Low | |||
| 2014 India[21] | Samyuktha et al. | Human periodontal ligament fibroblasts | Healthy premolar extracted for orthodontic treatment | ERRM -MTA -Biodentine |
Trypan blue dye assay -inverted phase contrast microscope |
Alpha MEM culture medium | 1 and 2 days | Low | |||
| 2014 Turkey[8] | Öncel Torun et al. | hDPCs | Molars of 5 patients undergoing orthodontic treatment | iRoot BP -MTA |
XTT assay -qRT-PCR |
Untreated cell cultures | 1, 2, and 3 days | Moderate | |||
| 2014 China[22] | Zhu et al. | Human dental pulp stem cells (DPSCs) | Extracted 3rd molars | iRoot BP Plus ProRoo t MTA | CCK-8 assay -EDU incorporation assay -Scratch wound healing assay -Transwell assay -SEM |
Cells without material | 1,2,3 and 7 days | Low | |||
| 2015 China[23] | Zhang et al. | DPCs | - | -iRoot BP Plus t MTA | -Scratch wound healing -proRoo Transwell assay -Annexin-V propidium iodide |
- | - | - | |||
| 2015 China[9] | Liu et al. | hDPCs | Impacted 3rd molars or premolars extracted for orthodontic treatment | iRoot BP Plus -MTA |
MTT assay | Cells without material | 1, 3, 5, and 7 days | Low | |||
| 2016 Turkey[24] | Torun et al. | Human dental pulp cells (hDPCs) | Extracted teeth of patients who undergoing orthodontic treatment | iRoot BP | Microarray analysis -qRT-PCR |
Cells without material | 1 and 3 days | Moderate | |||
| 2016 Pennsylvania[25] | Chen et al. | -DPSCs -PDLSCs -hBMSCs |
Center of Craniofacial Molecular Biology, University of Southern California, Los Angeles, CA -Thermo Scientific (Rockford, IL) |
RRM -ProRoot MTA |
MTT assay-SEM- immunoblot analysis | Wells with non-coated cover glass | 1, 3, and 5 days -3 days |
Moderate | |||
| 2016 Peru[26] | Coaguila- Llerena et al. | Human periodontal ligament fibroblasts | Erupted premolars extracted for orthodontic treatment | ERRM -MTA Angelus -Super EBA |
MTT assay | Culture medium Negative control |
1, 3, and 7 days | Low | |||
| 2017 Mexico[27] | Martínez- Cortés et al. | Human gingival fibroblasts | Gingival tissues donated from healthy patients | -ERRM -MTA Angelus -IRM |
MTT assay | Culture media | 1 h, 1 and 3 days | Low | |||
| 2017 India[28] | Sultana et al. | Human bone marrow-derived mesenchymal stem cells (hBMSCs) | _ | ERRM -ProRoot MTA -ALP activity -Biodentine -CLSM -GIC |
MTT assay | -GIC negative control hBMSCs positive control | 1, 3, 5, and 7 days -7 and 21 days |
Low | |||
ERRM = EndoSequence root repair material, MTA = mineral trioxide aggregate, GMTA = gray mineral trioxide aggregate, WMTA = white mineral trioxide aggregate, UBP = ultra- blend Plus, GIC = glass–ionomer cement, IRM = intermediate restoration material, hDPCs = human dental pulp cells, DPSCs = dental pulp stem cells, hBMSCs = human bone marrow- derived mesenchymal stem cells, DPCs = dental pulp cells, PDLSCs = periodontal ligament stem cells, MTT = methyl thiazol tetrazolium, NR = neutral red dye, CVDE = Crystal violet dye elution, CCK = cell counting kit, EDU = Scratch wound healing assay Transwell assay Annexin V propidium iodine, ALP = alkaline phosphatase activity, qRT-PCR = quantitative reverse transcriptase polymerase chain reaction, CLSM = confocal laser scanning, EDAX = energy dispersive analysis by X-ray, SEM = scanning electron microscopy
In addition, Table 2 for in vivo studies contains authors’ names, year of publication, species, age/weight, sample size, type of teeth, type of cavity, material, treatment, duration of the study, and risk of bias.
Table 2.
List of in vivo studies and tested parameters
| Year/author | Species | Age/weight | Sample size | Teeth | Cavity | Material | Treatment | Duration | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| 2013 Azimi et al.[29] | Human | 12–16 years | 12 | 24 upper and lower premolars | Class 1 | `iRoot BP -ProRoot MTA |
Partial pulpotomy | 6 weeks | Low |
| 2014 Zhu et al.[22] | Wistar rats | 220 g | 20 | Upper 1st molars | Class V mesial surface | iRoot BP Plus -MTA |
DPC | 28 days | Low |
| 2014 Nicholson[30] | Human | 7–58 years | 41 | According to the case | According to the case | -EERM -ProRoot MTA -Biodentine |
Vital pulp therapy | 160– 1000 days | Moderate |
| 2015 Liu et al.[9] | Wistar rats | 180–200 g | 20 | 24 upper 1st molars | Class V Mesial surface | iRoot BP Plus MTA |
DPC/pulpotomy | 1 and 4 weeks | Low |
| 2015 Zhang et al.[23] | Rats | - | - | Maxillary segment | _ | iRoot BP Plus -ProRoot MTA |
_ | 28 days | Low |
| 2016 Shi et al.[31] | Beagle dogs | 8 months | 3 | 36 upper and lower incisors | Class V Labial surfaces |
iRoot BP Plus -MTA |
DPC | 3 months | Moderate |
| 2016 Anujalkhur[32] | Human | 19–28 years | 5 | 15 upper and lower premolars | Class 1 | ERRM -MTA -GIC |
DPC | 3 weeks | Low |
| 2018 Okamoto et al.[33] | Wistar rats | 180–200 g | 10 | 20 upper first molars | Class 1 | -iRoot BP Plus -ProRoot MTA |
DPC | 2–4 weeks | Low |
ERRM = EndoSequence root repair material, MTA = mineral trioxide aggregate, GMTA = gray mineral trioxide aggregate, WMTA = white mineral trioxide aggregate, UBP = ultra-blend Plus, GIC = glass–ionomer cement, IRM = intermediate restoration material, DPC = direct pulp capping
RESULTS
DESCRIPTION OF INCLUDED ARTICLES
Most of the studies were conducted in China (n = 6), the earliest article was published in 2010 and the latest on 2018, (n = 15) and (n = 7) has low and moderate risk of bias respectively, and 27% of the articles were published in the Journal of Endodontics. There were 17 in vitro models, seven of which were performed on dental pulp cells, (hDPCs five studies,[8,9,10,20,22] DPSCs two studies),[23,33] human gingival fibroblasts two studies,[24,25] human dermal fibroblasts two studies,[16,18] human osteoblasts one study, human periodontal ligament fibroblasts three studies,[17,19,26] human periodontal ligament stem cells (PDLSCs) one study,[33] human periodontal ligament osteoblasts one study,[17] and hBMSCs two studies.[21,33] For cytotoxicity evaluation, methyl thiazol tetrazolium (MTT) assay was the most used test (47%),[9,16,21,26,33] followed by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) (17%),[8,10,22] then cell proliferation kit II assay (11%),[7,8] cell counting kit (CCK)-8 assay (11%),[10,23] alkaline phosphatase (ALP) activity (11%),[10,21] scratch wound healing and transwell assay (11%),[20,23] neutral red (NR) assay and crystal violet dye elution (CVDE) (5%),[7] Almar blue (5%),[17] and Trypan blue (5%),[19] through cell viability (proliferation, differentiation, migration, adhesion, and gene expression).
Also, there were eight in vivo models, three of which were conducted on humans, and the remaining five were on animals, four used rats and one used dogs. These studies evaluated dentinal bridge formation, mineralization, and the presence of inflammatory cells using histological evaluation and micro-computed tomography. Three studies performed pulpotomy[9,27,28] and seven performed direct pulp capping.[9,20,23,28,29,30,31] Among these, only one study had long-term follow-up, including clinical and radiographic evaluation after vital pulp therapy treatment.[28] Except for one study,[22] all studies compared iRoot BP, iRoot BP Plus, or ERRM to different types of MTA [Table 2].
DESCRIPTION OF RESULTS
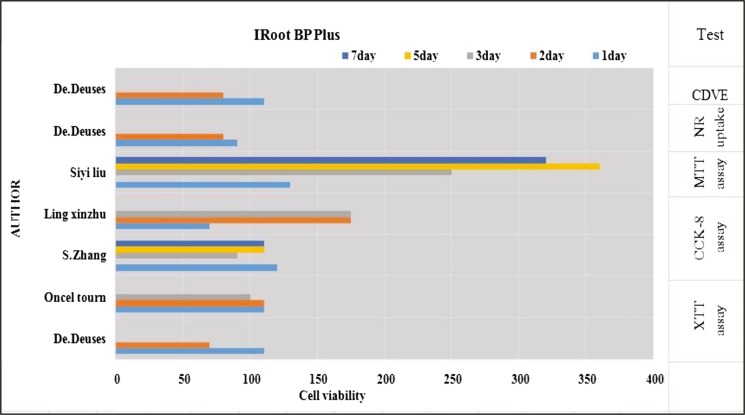
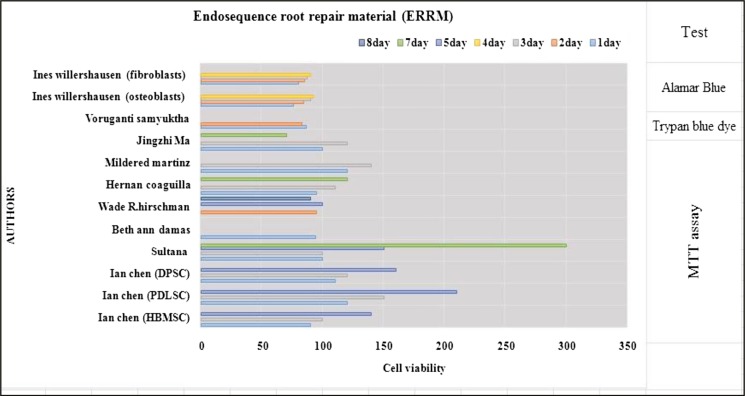
All in vitro studies showed that ERRM and iRoot BP Plus was a biocompatible material that was comparable to MTA. MTT assay showed that the material manifests some degree of cytotoxicity within the first two days as the cell viability decreases, but this increases at day seven as the material sets.[7,9,16,18,21,24,25,26,33] In contrast, one study showed that iRoot BP has a high cell viability at 24 and 48h[8] [Tables 1 and 3]. Trypan dye assay also revealed that the material manifests less cell viability in day one before increasing in day two.[33] CCK-8 assay shows that the viability slightly decreases at day three with the same result at one study.[23] In another study, it showed that iRoot BP Plus increases cell proliferation in day one and then decreases on days three, five, and seven.[10] Almar blue assay showed that ERRM manifests low cell proliferation at days three and four[17] [Figures 2 and 3].
Table 3.
Results of cytotoxicity tests of included studies
| Author | Materials | Cells | Significant results | Significant level (P value) |
|---|---|---|---|---|
| Ma et al.[16] | ERRM putty ERRM paste GMTA IRM Cavit G |
Human gingival fibroblast | IRM, Cavit G > ERRM Putty, MTA, ERRM paste |
P < 0.001 |
| Damas et al.[17] | RRM ERRM putty ProRoot MTA MTA Angelus |
Human dermal fibroblast | RRP > ProRoot MTA, MTA Angelus, RRM |
P < 0.01 |
| Hirschman et al.[18] | ERRM Putty MTA Angelus UBP Dycal |
Human dermal fibroblast | Materials > Control (8 days) | P < 0.05 |
| De-Deus et al.[7] | iRoot BP Plus ProRoot MTA |
Human osteoblast | IRoot BP Plus > ProRoot MTA (48 h) |
P < 0.001 |
| Liu et al.[9] | IRoot BP Plus MTA | Human dental pulp cells | MTA > iRoot BP Plus (1 and 3 days) | P < 0.05 |
| Chen et al.[25] | ERRM ProRoot MTA | -Human bone marrow- derived mesenchymal stem cells -Periodontal ligament stem cells -Dental pulp stem cells |
Control, MTA > ERRM (day 3) | P < 0.05 |
| Coaguila- Llerena et al.[26] |
ERRM Putty MTA Angelus Super EBA |
Human periodontal ligament fibroblast |
Super EBA> ERRM > MTA (whole time) |
P < 0.05 |
| Martínez- Cortés et al.[27] | ERRM White MTA Angelus IRM |
Human gingival fibroblast | IRM > MTA > ERRM | |
| Sultana et al.[28] | ERRM ProRoot MTA Biodentine |
Human bone marrow- derived mesenchymal cells | Biodentine > ProRoot MTA > ERRM (day 7) |
P = 0.0003 |
| Zhang et al.[10] | iRoot BP Plus MTA Bioaggregate |
Human dental pulp cells | -MTA > IRoot BP Plus, Bioaggregate (day 1) -iRoot BP Plus, Bioaggregate > MTA (day 3, 5, 7) |
P < 0.01 |
| Willershausen et al.[20] | ERRM GMTA White MTA Angelus ProRoot MTA |
Human periodontal ligament fibroblasts and osteoblast | MTAs > ERRM (whole time osteoblast) and 24 for human periodontal ligament fibroblasts | P < 0.001 |
| Zhu et al.[22] | iRoot BP Plus ProRoot MTA |
Human dental pulp stem cells | No significant difference between the two materials | _ |
| Samyuktha et al.[21] | ERRM MTA Biodentine |
Human periodontal ligament fibroblasts | Control > materials | P < 0.05 |
| Öncel Torun et al.[8] | iRoot BP white MTA |
Human dental pulp cells | White MTA > IRoot BP (after 48 h) | P < 0.05 |
ERRM = EndoSequence root repair material, MTA = mineral trioxide aggregate, GMTA = gray mineral trioxide aggregate, WMTA = white mineral trioxide aggregate, UBP = ultra-blend Plus, GIC = glass–ionomer cement, IRM = intermediate restoration material, DPC = direct pulp capping
Figure 2.
Description of cytotoxicity of the material iRoot BP Plus
Figure 3.
Description of cytotoxicity of the material ERRM
Scanning electron microscopy (SEM) analysis and confocal laser scanning microscopy (CLSM) were used to evaluate cells grown over the surface of the material when tested on different types of human cells, and these showed proper attachment and adhesion, similar to MTA.[21,23,24,33] Also, the material enhanced mineralization as the results of ALP assay showed[10,21] and expressed genes that were related to mineralization (collagen type 1, bone sialoprotein, bone morphogenetic protein [BMP], heme oxygenase 1, osteopontin (OPN), osteonectin, dentin sialophosphoprotein, dentin matrix protein-1, secreted protein acidic cysteine-rich [SPARC])[8,10,22] and apatite crystal formation.[23,19]
In vivo studies showed that the material enhanced reparative dentin bridge formation. In animal teeth,[29] the bridge formed after the application of iRoot BP Plus was slightly thicker than the one developed with MTA, and it continued with primary dentin and had well distinguishable dentin tubules.[9,20,23,29,31]
Two studies were conducted on humans undergoing orthodontic treatment. The first study showed that of the five teeth treated with ERRM as pulp-capping material, three teeth showed complete bridge formation with few chronic mild inflammatory cells in two specimens after three weeks.[32] In the other, partial Cvek pulpotomy was performed, and it showed either complete dentin bridge formation six weeks later (58%) or incomplete bridge formation (42%) with mild and moderate inflammation in all teeth treated with iRoot BP.[27] Another study was conducted on humans for long-term follow-up to evaluate the success of the treatment with ERRM, ProRoot MTA, and Biodentine. The result of this study showed that MTA has a double success than ERRM.[28]
DISCUSSION
The acquired data, a comprehensive discussion of the experimental models, procedures, cells type, activated pathways, the gene expressed, and capping materials in every covered study was out of the range of this review. The authors outlined the typical results obtained from the included studies.
Several factors affected the success of pulp capping: diagnosis, case selection, the hemostatic agent, coronal seal, and the capping material.[34] Several materials had been introduced as pulp-capping agent, each having its properties and drawbacks. This systematic review evaluated iRoot BP (Plus) and ERRM as a capping agent through its biocompatibility and its ability to form a calcified dentinal bridge.
Approximately 68% of the articles in this review studied the effect of the material on pulp either in vitro or in vivo or on both. Dental pulp is a highly vascular and innervated loose connective tissue that contains various cells such as fibroblast, undifferentiated mesenchymal cell, odontoblasts, and other cells.[35] DPSCs are self-renewal cells that can be transformed into odontoblasts, osteoblasts neural cells, and adipocytes.[36] The cytotoxicity of the material is an important factor due to the fact that it can affect the healing and general health of the pulp.
Most of the in vitro studies used MTT assay, which estimates mitochondrial dehydrogenase activity in living metabolically active cells,[24] and which displayed that iRoot BP Plus showed approving biocompatibility to the pulp tissue, encouraged hDPCs proliferation, and was similar or even higher than MTA. This could be explained by the fact that MTA has bismuth oxide as one of its components, giving the material its radiopacity property, which can increase the toxicity of the material, whereas iRoot BP Plus has a tantalum oxide in place of bismuth oxide. In addition, it could be due to the superior hydrophilic calcium silicate component of iRoot BP Plus,[8,9,23] whereas another study showed that the material can discourage the proliferation of hDPCs equivalent to MTA.[10] One study tested ERRM and MTA on DPSCs and PDLSCs which had quick reaction to the material and displayed a rise in the number of cells on days three and five, which was more than HBMSCs cell number and this may be due to higher basal proliferation of DPSCs and PDLSCs.[33]
Other in vitro studies conducted on different human cells to evaluate the cytotoxicity of the same composition material ERRM revealed that the material showed a little cytotoxicity in the first two days [Tables 1 and 3],[16,18,24,26] however, it showed high cell viability at days five, seven, and eight. This may be attributed to the lack of toxicant leaking out from the set material.[24] On the contrary, another study on ERRM putty showed a slightly less cell viability in day seven than day two,[26] and another one displayed ERRM putty as of the lowest cell viability among the tested materials.[18] The different results might be contributed to the different cell types and their responses to the material as well as to the various toxicity tests and the culture medium.
Through the beginning stage of dental pulp repair, adhesion and migration play a significant role.[37] Scratch wound healing assay is used to measure basic cell migration parameters such as persistence, speed, and polarity.[38] Transwell assay is another test for measuring cell migration. Results obtained showed that iRoot BP Plus encouraged the adhesion ability of DPSCs and contributed in wound closure and were similar to MTA.[23] SEM showed that ERRM- and MTA-treated DPSCs, PDLSCs, and hBMSCs spread well and were quite same,[33] as well as it showed that DPSCs treated with iRoot BP Plus displayed finer spreading than those treated with MTA.[23] Also, human gingival fibroblast manifested good adhesion when treated with ERRM and MTA.[24] The similarity in activity between ERRM and MTA might be due to their similar surface characteristics.[33] Another study used CLSM on hBMSCs treated with ERRM and Biodentine. Both materials expressed lower adherence to the cells compared to MTA.[21] The dissimilarity in the results can be accredited to the bioactivity of the specimen samples, the particle size of the material, or to their differences in constituent components.[23] When the material displays adhesion, attachment, migration, and proliferation, this means it can promote healing and it is biocompatible.
When a material gets involved with living tissue and forms biomineralization, an apatite layer at the material tissue interfaces and is considered a bioactive material.[19] The studies showed that iRoot BP Plus has apatite-forming capacity potential, which was better than MTA when tested on DPSCs.[23] ALP assay is used to determine the mineralization potential of the materials. ALP is secreted from osteoblasts and is known to be a membrane-bound biochemical identifying marker of bone turnover. The result exposed that iRoot BP Plus had a mineralization capacity potential more than MTA, same results were obtained from ERRM when tested on hBMSCs.[10,21] Besides, quantitative reverse transcriptase analysis (real-time PCR) showed that hDPCs treated with iRoot BP expressed gene associated with mineralization BMP-2, osteopontin (OPN), osteonectin (ON) dentin sialoprotein, SPARC.[8,10,22]
Dentinal bridge formation plays a vital role in the success of pulp capping. In vivo studies, which were 32% of this review, evaluated the bridge formation in human (n = 2) two studies, and in animals (n = 5) five studies. In human studies, one study concluded that there was no difference between iRoot BP and MTA in the appearance of the dentinal bridge and pulp inflammation, and there was less sensitivity to cold in patients treated with MTA. Partial Cvek pulpotomy was the choice of treatment on teeth planned for orthodontic extraction.[27] The other study performed direct pulp capping using ERRM and MTA as capping agents also in teeth scheduled for removal for orthodontic treatment reasons. The materials resulted in dentinal bridge formation with ERRM showing chronic mild inflammation cells in two sample specimens of five.[32] One clinical study with 41 participants and 730 average days follow-up period performed vital pulp therapies for patients treated with ERRM, MTA, and Biodentine. The results showed that the failure of patients who get ERRM was double the odds of failure when compared to patients who get MTA.[28]
Animal studies showed that the dentinal bridge formed after iRoot BP Plus was slightly thicker than the one created with MTA treatment after one month,[20,23] and it was in all iRoot BP Plus group without inflammatory cells, whereas one specimen in MTA group has incomplete bridge, containing inflammatory cells.[9] One study also revealed no difference between the two materials, as both manifested complete dentinal bridge without inflammatory cells.[29] Although one study showed that iRoot BP Plus developed tertiary dentin faster and had better quality than MTA,[31] all these results can be partially due to the apatite-forming property of the material and somewhat related to its suitability in an acidic environment.[20,31] It should be taken into consideration that these studies were under controlled and ideal conditions, caries free (healthy pulp tissue), synthetically made with limited time, and small sample size. For these reasons, further clinical studies should be performed on pulpitis cases for more investigations and accurate results related to clinical conditions.
CONCLUSION
The iRoot BP Plus, also known as ERRM putty, seems to be a biocompatible material, with apatite-forming ability to enhance dentinal bridge formation on hDPCs and other variant human cells in vitro and in vivo studies without any severe complications. However, this review strongly recommends more clinical studies to evaluate the prognosis and outcomes for long-term follow-up to assess the material as pulp-capping agent.
FINANCIAL DISCLOSURE
Nil.
CONFLICTS OF INTEREST
There are no conflicts of interest.
ACKNOWLEDGEMENT
We would like to thank Professor Mohmoud M. Mohmed Ali for his support; Dr. Nuha I. Mirghani for providing language help; Assistant Professor Yasir Satti for reviewing; Mr. Ahmed Ramsess for quality assessment; Dr. Sara Abdu, Dr. Osama Sayed Ahmed, and Mr. Mark Song for all their help during the course of the research.
REFERENCES
- 1.Walton RE, Torabinejad M. Philadelphia, itd: WB Saunders Co; 1996. Principles and Practice of Endodontics; pp. 201–33. [Google Scholar]
- 2.Cohen B, Combe E. Development of new adhesive pulp capping materials. Dental Update. 1994;21:57–62. [PubMed] [Google Scholar]
- 3.Wang Z. Bioceramic materials in endodontics. Endodontic Topics. 2015;32:3–30. [Google Scholar]
- 4.Budig CG, Eleazer PD. In vitro comparison of the setting of dry ProRoot MTA by moisture absorbed through the root. J Endodontics. 2008;34:712–4. doi: 10.1016/j.joen.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Souza LCd, Yadlapati M, Dorn SO, Silva R, Letra A. Analysis of radiopacity, pH and cytotoxicity of a new bioceramic material. J Appl Oral Sci. 2015;23:383–9. doi: 10.1590/1678-775720150065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faraco IM, Holland R. Response of the pulp of dogs to capping with mineral trioxide aggregate or a calcium hydroxide cement. Dent Traumatol. 2001;17:163–6. doi: 10.1034/j.1600-9657.2001.170405.x. [DOI] [PubMed] [Google Scholar]
- 7.De‐Deus G, Canabarro A, Alves GG, Marins JR, Linhares ABR, Granjeiro JM. Cytocompatibility of the ready-to-use bioceramic putty repair cement iRoot BP Plus with primary human osteoblasts. Int Endodontic J. 2012;45:508–13. doi: 10.1111/j.1365-2591.2011.02003.x. [DOI] [PubMed] [Google Scholar]
- 8.Öncel Torun Z, Torun D, Demirkaya K, Yavuz ST, Elçi MP, Sarper M, et al. Effects of iRoot BP and white mineral trioxide aggregate on cell viability and the expression of genes associated with mineralization. Int Endodontic J. 2015;48:986–93. doi: 10.1111/iej.12393. [DOI] [PubMed] [Google Scholar]
- 9.Liu S, Wang S, Dong Y. Evaluation of a bioceramic as a pulp capping agent in vitro and in vivo . J Endodontics. 2015;41:652–7. doi: 10.1016/j.joen.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S, Yang X, Fan M. BioAggregate and iRoot BP Plus optimize the proliferation and mineralization ability of human dental pulp cells. Int Endodontic J. 2013;46:923–9. doi: 10.1111/iej.12082. [DOI] [PubMed] [Google Scholar]
- 11.Damlar I, Ozcan E, Yula E, Yalcin M, Celik S. Antimicrobial effects of several calcium silicate-based root-end filling materials. Dent Mater J. 2014;33:453–7. doi: 10.4012/dmj.2013-250. [DOI] [PubMed] [Google Scholar]
- 12.El Sayed M, Saeed M. In vitro comparative study of sealing ability of Diadent BioAggregate and other root-end filling materials. J Conserv Dent. 2012;15:249. doi: 10.4103/0972-0707.97950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leal F, De-Deus G, Brandao C, Luna A, Souza E, Fidel S. Similar sealability between bioceramic putty ready-to-use repair cement and white MTA. Braz Dent J. 2013;24:362–6. doi: 10.1590/0103-6440201302051. [DOI] [PubMed] [Google Scholar]
- 14.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Commun Health. 1998;52:377–84. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Możyńska J, Metlerski M, Lipski M, Nowicka A. Tooth discoloration induced by different calcium silicate–based cements: A systematic review of in vitro studies. J Endodontics. 2017;43:1593–601. doi: 10.1016/j.joen.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Ma J, Shen Y, Stojicic S, Haapasalo M. Biocompatibility of two novel root repair materials. J Endodontics. 2011;37:793–8. doi: 10.1016/j.joen.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 17.Damas BA, Wheater MA, Bringas JS, Hoen MM. Cytotoxicity comparison of mineral trioxide aggregates and EndoSequence bioceramic root repair materials. J Endodontics. 2011;37:372–5. doi: 10.1016/j.joen.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 18.Hirschman WR, Wheater MA, Bringas JS, Hoen MM. Cytotoxicity comparison of three current direct pulp-capping agents with a new bioceramic root repair putty. J Endodontics. 2012;38:385–8. doi: 10.1016/j.joen.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Shokouhinejad N, Nekoofar MH, Razmi H, Sajadi S, Davies TE, Saghiri MA, et al. Bioactivity of EndoSequence root repair material and bioaggregate. Int Endodontic J. 2012;45:1127–34. doi: 10.1111/j.1365-2591.2012.02083.x. [DOI] [PubMed] [Google Scholar]
- 20.Willershausen I, Wolf T, Kasaj A, Weyer V, Willershausen B, Marroquin BB. Influence of a bioceramic root end material and mineral trioxide aggregates on fibroblasts and osteoblasts. Archiv Oral Biol. 2013;58:1232–7. doi: 10.1016/j.archoralbio.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Samyuktha V, Ravikumar P, Nagesh B, Ranganathan K, Jayaprakash T, Sayesh V. Cytotoxicity evaluation of root repair materials in human-cultured periodontal ligament fibroblasts. J Conservative Dent. 2014;17:467. doi: 10.4103/0972-0707.139844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu L, Yang J, Zhang J, Lan DL, Ying XXC, Peng LB. In vitro and in vivo evaluation of a nanoparticulate bioceramic paste for dental pulp repair. Acta Biomaterialia. 2014;10:5156–68. doi: 10.1016/j.actbio.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Zhu LX, Cheng X, Lin Y, Yan P, Peng B. Promotion of dental pulp cell migration and pulp repair by a bioceramic putty involving FGFR-mediated signaling pathways. J Dent Res. 2015;94:853–62. doi: 10.1177/0022034515572020. [DOI] [PubMed] [Google Scholar]
- 24.Torun D, Torun ZÖ, Demirkaya K, Sarper M, Elçi MP, Avcu F. Gene expression changes in bioceramic paste-treated human dental pulp cells. J Oral Sci. 2016;58:307–15. doi: 10.2334/josnusd.15-0600. [DOI] [PubMed] [Google Scholar]
- 25.Chen I, Salhab I, Setzer Syngcuk Kim FC, Nah H-D. A new calcium silicate–based bioceramic material promotes human osteo- and odontogenic stem cell proliferation and survival via the extracellular signal-regulated kinase signaling pathway. J Endodontics. 2016;42:480–6. doi: 10.1016/j.joen.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Coaguila-Llerena H, Vaisberg A, Velásquez-Huamán Z. In vitro cytotoxicity evaluation of three root-end filling materials in human periodontal ligament fibroblasts. Braz Dent J. 2016;27:187–91. doi: 10.1590/0103-6440201600447. [DOI] [PubMed] [Google Scholar]
- 27.Martínez-Cortés M, Tinajero-Morales C, Rosales C, Uribe-Querol E. Cytotoxicity assessment of three endodontic sealing cements used in periapical surgery. In vitro study. Revista Odontol Mexicana. 2017;21:e40–8. [Google Scholar]
- 28.Sultana N, Singh M, Nawal RR, Chaudhry S, Yadav S, Mohanty S, et al. Evaluation of biocompatibility and osteogenic potential of tricalcium silicate–based cements using human bone marrow–derived mesenchymal stem cells. J Endodontics. 2018;44:446–451. doi: 10.1016/j.joen.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 29.Azimi S, Fazlyab M, Sadri D, Saghiri MA, Khosravanifard B, Asgary S. Comparison of pulp response to mineral trioxide aggregate and a bioceramic paste in partial pulpotomy of sound human premolars: A randomized controlled trial. Int Endodontic J. 2014;47:873–81. doi: 10.1111/iej.12231. [DOI] [PubMed] [Google Scholar]
- 30.Nicholson NT. A retrospective clinical study to evaluate treatment outcomes of vital pulp therapy with ProRoot® mineral trioxide aggregate, EndoSequence® root repair material, and Biodentine®. West Virginia University. 2014:1565515. [Google Scholar]
- 31.Shi S, Bao ZF, Liu Y, Zhang DD, Chen X, Jiang LM, et al. Comparison of in vivo dental pulp responses to capping with iRoot BP Plus and mineral trioxide aggregate. Int Endodontic J. 2016;49:154–60. doi: 10.1111/iej.12439. [DOI] [PubMed] [Google Scholar]
- 32.Anujalkhur Comparative evaluation of response of human dental pulp on direct pulp capping with MTA. ERRM. 2016 [Google Scholar]
- 33.Okamoto M, Takahashi Y, Komichi S, Ali M, Yoned N, Ishimot T, et al. Novel evaluation method of dentin repair by direct pulp capping using high-resolution micro-computed tomography. Clin Oral Invest. 2018;22:2879–2887. doi: 10.1007/s00784-018-2374-5. [DOI] [PubMed] [Google Scholar]
- 34.Cho SY, Seo DG, Lee SJ, Lee J, Lee SJ, Jung IY. Prognostic factors for clinical outcomes according to time after direct pulp capping. J Endodontics. 2013;39:327–31. doi: 10.1016/j.joen.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg M, Farges JC, Lacerda-Pinheiro S, Six N, Jegat N, Decup F, et al. Inflammatory and immunological aspects of dental pulp repair. Pharm Res. 2008;58:137–47. doi: 10.1016/j.phrs.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–5. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 37.Lin L, Rosenberg P. Repair and regeneration in endodontics. Int Endodontic J. 2011;44:889–906. doi: 10.1111/j.1365-2591.2011.01915.x. [DOI] [PubMed] [Google Scholar]
- 38.Cory G. Cell Migration. Humana Press, Springer; 2011. Scratch-wound assay; pp. 25–30. [DOI] [PubMed] [Google Scholar]