Abstract
Astrocytes, glial cells that interact extensively with neurons and other support cells throughout the central nervous system, have recently come under the spotlight for their potential contribution to, or potential regenerative role in a host of neurodegenerative disorders. It is becoming increasingly clear that astrocytes, in concert with microglial cells, activate intrinsic immunological pathways in the setting of neurodegenerative injury, although the direct and indirect consequences of such activation are still largely unknown. We review the current literature on the astrocyte’s role in several neurodegenerative diseases, as well as highlighting recent advances in genetic manipulation of astrocytes that may prove critical to modulating their response to neurological injury, potentially combatting neurodegenerative damage.
Keywords: Alzheimer's disease, amyotrophic lateral sclerosis, glia, immune system, inflammation, Parkinson's disease, reactive astrocyte, regeneration
Astrocytic Response to Neurodegeneration
Astrocyte signaling and changes in cellular behavior have been associated with a wide variety of neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS) (Pehar et al., 2017), Parkinson’s disease (PD) (Miyazaki and Asanuma, 2017), Huntington’s disease (Hsiao et al., 2013), and Alzheimer’s disease (AD) (Assefa et al., 2018). Whether these changes represent a protective/restorative response or a compounding, injurious role is still a matter of controversy. Astrocytes secrete a variety of soluble small molecules and proteins which likely contribute both to normal neural functioning and to the central nervous system (CNS) response to injury (Jha et al., 2018). However, the precise functions of many of these astrocytic-secreted factors remain unclear (Jha et al., 2018).
Astrocytes respond to neural injury or disease through complex and heterogeneous changes in gene expression and glial cell function known as reactive gliosis (Anderson et al., 2014). Reactive gliosis is necessary for proper recovery from brain injury (Anderson et al., 2014; Pekny et al., 2014; Choi et al., 2018), but can also have damaging effects (Anderson et al., 2014; Pekny et al., 2014). A wide variety of signaling pathways and mechanisms can induce astrocytes to initiate an immune response, including local hypoxia (Chavez et al., 2006; Badawi et al., 2012; Huang et al., 2014; Ramamoorthy et al., 2019), mast cell infiltration (Kempuraj et al., 2017; Kempuraj et al., 2019), microglial signaling (Kirkley et al., 2017), myelin debris (Ponath et al., 2017), and inflammation/growth factors released by damaged neurons (Cassina et al., 2005; Gontier et al., 2015). Damaged neurons themselves release microRNAs and other factors also capable of driving astrocytes toward an inflammatory response (Hoye et al., 2018).
Protein aggregation, a common feature in various neurodegenerative disorders, can mediate astrocyte activation (Kovacs et al., 2017). While astrocytes are generally more resistant to the oxidative stress of misfolded protein aggregation as compared to neurons (Zhao et al., 2017), the accumulation of such proteins can interfere with healthy astrocyte functions (Croisier and Graeber, 2006; Fellner et al., 2011; Lim et al., 2018), and lead to astrocytic-mediated neuroinflammation (Sankar et al., 2018). Reactive astrocytes cluster together around amyloid plaques and tau tangles in post-mortem tissue samples taken from AD patients (Serrano-Pozo et al., 2011). In healthy murine astrocytes, the over-accumulation of amyloid-β (Aβ) triggers an astrocytic immune response (Sankar et al., 2018). Fibrinogen plaques, a common feature in AD, multiple sclerosis, stroke, and traumatic brain injury, can also mediate astrocyte activation (Clark et al., 2018). Similarly, the aggregation of α-synuclein, a key step in the pathogenesis of PD, can be directly passed from neurons to astrocytes, triggering an astrocyte-mediated inflammatory response (Lee et al., 2010a, b). Mice that over-express a PD-associated mutant form of α-synuclein in an astrocyte-specific manner have been shown to display Parkinsonian motor symptoms (Cu et al., 2010). Analogously, expression of mutant huntingtin protein in murine astrocytes leads to an age-dependent recapitulation of certain aspects of Huntington’s disease symptomatology (Bradford et al., 2009). These data taken together suggest that astrocyte-mediated neuroinflammation, triggered by protein aggregation may be an important potential therapeutic target for treating neurodegenerative disorders. While astrocytes are not the resident immune cells of the CNS, a role canonically attributed to microglia, astrocytes do utilize immune signals in response to perceived stressors and protein aggregates in a complex fashion.
This review was compiled via PubMed and Google Scholar searches, without date restrictions. Selection criteria included articles written in or translated into English, pertaining to or including the terms astrocyte, glia, neurons and/or inflammation, and/or neuroinflammation, and/or neurodegeneration, and/or genetic manipulation.
Consequences of Astrocyte Activation
Astrocytes respond to inflammatory challenges using many of the same signaling cascades utilized by bona fide myeloid- and lymphoid-lineage cells, including microglia. In the course of responding to the insult, astrocytes may lose functions important for supporting healthy neurons and synapses, while gaining abnormal functions that can have negative consequences for neural survival and activity (Sofroniew, 2009). However, not all astrocytic transcriptional changes in response to neurodegeneration are necessarily maladaptive, for example clearing debris, and containing protein aggregates within a particular area (Pekny et al., 2007; Sofroniew, 2009; Anderson et al., 2014; Hoshi et al., 2018).
Astrocytic responses to inflammation
Astrocytes respond to inflammatory challenges with a wide variety of gene expression changes, dependent on animal age (Kluge et al., 2018), systemic immune status (Rakic et al., 2018), and the specific immune trigger (Perriot et al., 2018). One of the most common pathways activated by immune insult is the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) immune signaling cascade (Kempuraj et al., 2017, 2019). NFκB-mediated astrocyte dysfunction can initiate neurodegeneration through different mechanisms (Lattke et al., 2017; Kim et al., 2018), including astrocytic release of apoptotic factors (Kia et al., 2018), and cytokines/chemokines. These signals orchestrate bidirectional interactions between astrocytes and microglia in the context of neuroinflammation.
Immune-reactive astrocytes produce a number of cytokines and chemokines which can activate the local microglia. For instance, under conditions such as expression of mutated ALS-associated protein, reactive astrocytes can release tumor necrosis factor-α (TNF-α) (Kia et al., 2018), which can activate microglia, driving them toward a pro-inflammatory (M1) phenotype (Boche et al., 2013). Astrocytes can also synthesize lactosylceramide and interleukin-6, other pro-inflammatory drivers of microglia (Mayo et al., 2014; Savarin et al., 2015). Astrocytic production of interleukin-12 can induce interleukin-7 expression in both human and murine microglia (Jana et al., 2014), which, in turn, can amplify lymphocyte proliferation (Lin et al., 2017). T-cell lymphocytes can in turn secrete interferon-γ (Savarin et al., 2015), which enhances inflammatory microglial recruitment and proliferation (Subramaniam and Federoff, 2017). Reactive astrocytes tend to increase their nitric oxide (NO) production (Cassina et al., 2002; Xie and Yang, 2015), which is needed to prime microglial cytokine/chemokine release (Xia and Zhai, 2010), and trigger vasodilation and subsequent immune cell infiltration (Xie and Yang, 2015). Thus, astrocytes triggered by an inflammatory insult, participate actively in the recruitment of resident microglia to sites of injury.
The relationship between reactive astrocytes and microglia is far from one-sided, however, with astrocyte-activated microglia feeding back onto the astrocytes to modulate the extent of the inflammatory response. For instance, activated microglia utilize the C-C chemokine receptor-5 signaling pathway to regulate astrocytic neurotransmitter production, which may be relevant in the context of AD and possibly sporadic PD (Huerta et al., 2004; Xia and Zhai, 2010; Choi et al., 2013; Sahin-Calapoglu et al., 2016). Additionally, anti-inflammatory (M2) microglia produce interleukin-10, which upregulates astrocytic production of anti-inflammatory factors, including transforming growth factor (TGF)-β, which promotes the M2 microglial phenotype (Norden et al., 2014), as well as assisting in neuroprotection. More specifically, one of the principal roles of reactive astrocytes in the context of inflammation is to protect the integrity of synapses. For instance, astrocyte-derived TGF-β1 has been shown to protect synapses from the potentially damaging effects of Aβ aggregation in cultured murine hippocampal neurons (Diniz et al., 2017). Reactive astrocytes may also increase their release of neurotrophic factors, which have been shown to protect cortical synapses against the harmful effects of Aβ aggregation (Guo and Mattson, 2000). Astrocytic TGF-β signaling can also limit the extent of inflammation in response to cerebral infections, such as that caused by the parasite Toxoplasma gondii (Cekanaviciute et al., 2014), and following stroke (Cekanaviciute et al., 2014). The efficiency of astrocyte-microglia signaling appears to dwindle with age, however, perhaps helping to explain the onset of many neurodegenerative diseases later in life. The Figure 1 demonstrates the bidirectional relationship between astrocytes and microglia in the setting of an inflammatory/neurodegenerative insult.
Figure 1.
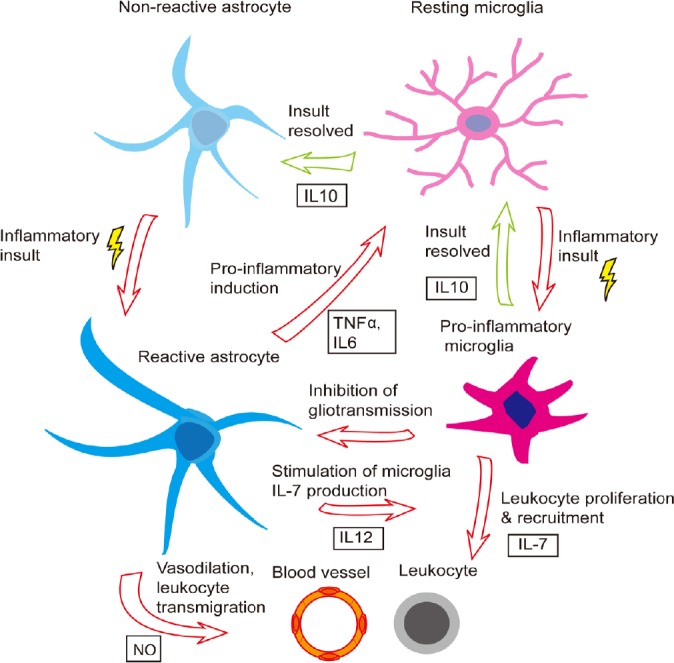
Bidirectional interaction between astrocytes and microglia in regulating neuroinflammation.
Shown is a schematic of the coordinated astrocyte-microglia response to an inflammatory insult, as well as the coordinated de-escalation and return to non-reactive or resting state, respectively. IL: Interleukin; NO: nitrous oxide; NT: neurotransmitter; TNF: tumor necrosis factor.
As evidence of this changing cell-cell interaction over time, in aged murine brains, astrocytes have decreased responsiveness to inter leukin-10, leading to prolonged microglial activation following a peripheral immune insult (Subramaniam and Federoff, 2017). TGF-β expression is also elevated in post-mortem human brain tissue samples taken from patients with AD relative to healthy controls, again implicating it as a marker of immunological stress in the brain and potentially of disease severity as well (Zhang et a., 2016). On the other hand, microglia can also increase astrocyte reactivity. In response to increases in NO production by reactive astrocytes, microglia produce interleukin-1β (Sudo et al., 2015), which can further activate astrocytes, increase astrocytic NO production (Hu et al., 1995), and promote astrocytic migration to the site of injury (Yang et al., 2015). Interleukin-1β also upregulates glial fibrillary acid protein (GFAP) expression and subsequent glial scarring (Sticozzi et al., 2013). There is evidence to suggest that this cross-talk could be maladaptive. For instance, blocking such microglial-induced astrocyte conversion has been shown to be neuroprotective in transgenic mice with the familial PD-associated A53T α-synuclein mutation (Yun et al., 2018).
Apart from their control by microglia, astrocytes exhibit several other modes of regulation. Peripheral immune cells, such as regulatory T lymphocytes, also regulate astrogliosis (the formation of a scar comprised of reactive astrocytes and other glia and inflammatory cells that borders a focus of inflammation) and the neuro-immune response (Sofroniew, 2009; Ito et al., 2019). Moreover, reactive astrocytes are capable of auto-regulation. For instance, in response to oxidative stress, astrocytes upregulate their expression of toll-like receptor-3 which upregulates neuroprotective anti-inflammatory cytokines like interleukin-10, and reduces production of pro-inflammatory cytokines like interleukin-12 (Bsibsi et al., 2006). Reactive astrocytes can also produce neuroprotective lipoxins in response to injury (Livne-Bar et al., 2017).
What factors determine whether astrocytes will assume a pro- or anti-inflammatory phenotype? Astrocyte-derived interferon-1β production appears to be important for this fate determination. Upregulating the expression of interferon regulatory factor 3, a transcription factor required for astrocyte interferon-1β production inhibits astrocyte inflammatory gene expression by suppressing pro-inflammatory microRNAs (Tarassishin et al., 2011). Astrocyte interferon-1 production is known to regulate immune responses of the endothelial cells of the blood-brain barrier, and is associated with anti-inflammatory effects in viral infections of the CNS, and in multiple sclerosis in humans (Rothhammer et al., 2016; Daniels et al., 2017). However, in post-mortem AD brains, upregulation of astrocytic interferon-1 signaling has been associated with promotion of a pro-inflammatory signaling cascade (Taylor et al., 2018). Reactive astrocytes also regulate the peripheral cytokine/chemokine responses by releasing peroxisome proliferator-activated receptor α (Dickens et al., 2017).
The chronicity of CNS inflammation appears to play an important role in determining astrocyte activation status. Chronic activation, for example in the setting of neurodegeneration, increases astrocytic responses to immune insult (Hennessy et al., 2015). In addition, some neurodegenerative diseases are associated with mutations in key astrocyte genes which interrupt astrocytic autoregulation of the neuro-immune response. For instance, fyn tyrosine kinase mutations associated with AD can facilitate persistent inflammatory responses (Lee et al., 2017), and increased microglial inflammatory activity (Panicker et al., 2015). Mutations in the gene for TGF-β have been associated with AD risk (Caraci et al., 2012, 2018), suggesting that defects in astrocytic regulation of microglia may enhance neurodegeneration. Conversely, familial-PD loss-of-function mutations in PTEN-induced putative kinase (PINK1) (Valente et al., 2004) appear to have anti-inflammatory effects (Sun et al., 2018). However, PINK1 loss also induces mitochondrial damage, increasing metabolic stress (Valente et al., 2004). Increased PINK1 expression has been reported in AD and multiple sclerosis lesions (Wilhelmus et al., 2011), where it is thought to stimulate interleukin-1β signaling (Sun et al., 2018). On the other hand, frameshift mutations of the ubiquitin gene, seen in aging and AD-brains can actually suppress inter leukin-1β/TNF-α-mediated astrocyte activation (Choi et al., 2013). Aside from their role in immune modulation, astrocytes assume fundamental functions in CNS metabolic homeostasis.
Metabolic support
A major astrocyte function is the provision of metabolic support for neurons, which is critical in limiting the extent of neuronal oxidative stress (De Miranda et al., 2018). Such support is controlled principally by neurotransmitters (especially glutamate), but also by other small molecule regulators of synaptic signaling including cytokines. Breakdown of metabolic regulation, as may occur in the context of neurodegeneration, highlights the key role of astrocytes in this critical housekeeping function.
Under physiological conditions, astrocytes sense synaptic glutamate levels and use this as a surrogate for neuronal activity, in turn recruiting nutrients and oxygen to metabolically active areas while shunting blood flow away from less metabolically active regions (Bélanger et al., 2011). On the other hand, when the CNS experiences an inflammatory stressor, astrocytes undergo a series of cellular changes to divert their resources to dealing with the stressor. Astrocytic activation causes these cells to neglect their neuro-supportive roles as they instead gear their activity toward inflammatory cell recruitment and glial scar formation (Allaman et al., 2010; Fuller et al., 2010; Steele and Robinson, 2012). For example, in the context of a superoxide dismutase 1 (SOD1) mutant mouse model of ALS, NFκB signaling regulates both the expression of oncogene astrocyte elevated gene-1 and excitatory amino acid transporter-2, decreasing astrocyte clearance of synaptic glutamate in times of oxidative stress (Vartak-Sharma et al., 2014; Yin et al., 2018). This forces neurons to slow down their metabolic activity or else suffer the consequences of oxidative stress.
Fortunately, compensatory mechanisms exist to ensure that neurons decrease their metabolic demand for the purpose of neuronal preservation in the context of oxidative stress. Anti-inflammatory cytokines such as interleukin-10 alter intracellular Ca2+ responses to ischemia, preserving neuron viability in the setting of heightened metabolic demand (Tukhovskaya et al., 2014). Following an inflammatory insult, astrocytes also upregulate expression of aquaporin-4 (AQP4), and down-regulate expression of glutamate transporter-1 to reduce neuro-excitability (Ikeshima-Kataoka, 2016; Lan et al., 2016), thereby reducing cellular metabolic activity. Additionally, interleukin-1β, which is known to activate astrocytes (Xia and Zhai, 2010), also upregulates astrocyte glutathione synthesis, protecting astrocytes (He et al., 2015) and neurons (Chowdhury et al., 2018) from oxidative stress. Mild astrocytic oxidative stress appears to be beneficial, helping to decrease astrocyte inflammatory activation and resultant blood-brain barrier hyper-permeability (Wang et al., 2018).
However, many of the inflammatory factors released as the result of astrogliosis largely have negative consequences for astrocyte metabolic support. For example, interferon-α, released as a consequence of activation, can negatively regulate astrocyte growth and glucose metabolism (Wang et al., 2012). Pro-inflammatory factors released by lymphocytes (interferon-γ) and microglia (TNF-α) can stimulate production of Aβ, potentially increasing plaque load in AD (Yamamoto et al., 2007; Zhao et al., 2011). Astrocytic activation additionally leads to increased production of new astrocytes (Hernández-Guillamon et al., 2009), but also increases astrocyte apoptosis (Saha and Biswas, 2015), potentially exacerbating oxidative/metabolic stress. Abnormal astrocyte metabolism can, in turn, modulate neuroinflammatory responses. For instance, PARK7, a regulator of astrocyte metabolism found to be mutated in some cases of familial PD (Bandopadhyay et al., 2004) is important for astrocyte mitochondrial function (Larsen et al., 2011). Loss of functional PARK7 increases intracellular protein accumulation and subsequent oxidative stress (Kumaran et al., 2007). PARK7 is also important for neural repair, as it helps to actively promote astrogliosis by stabilizing Sox9, a positive regulator of astrogliosis (Choi et al., 2018). An analogous process likely occurs in AD pathology, wherein mutations in the astrocyte protein apolipoprotein E4 can impair Aβ clearance (Liu et al., 2013; Prasad and Rao, 2018), and may increase oxidative stress by impairing the glia-neuron lactate shuttle, leading to an inflammatory response (Liu et al., 2015). In summary, astrocytes not only ensure that nutrient and oxygen supply and demand are balanced at baseline, but are also essential in mediating decreased neuronal energy expenditure in times of oxidative stress for cellular preservation. Aside from their critical role in energy budgeting under neurodegeneration-inducing stress, astrocytes have the added task of ensuring efficient synaptic transmission and are key regulators of neuroplasticity, which are also likely relevant in neurodegeneration.
Synaptic transmission and plasticity
Astrocytes contribute a great deal to the regulation of synaptic transmission and plasticity. In this regard, astrocyte contributions are multifaceted, regulating cholinergic and endocannabinoid synaptic signaling (Navarrete et al., 2012, 2014; Gómez-Gonzalo et al., 2015), setting thresholds for long-term potentiation and long-term depression (Bonansco et al., 2011), and regulating neurotransmitter release probability (Perea and Araque, 2007). In fact, the loss of appropriate synaptic and plasticity regulation due to astrocyte activation may help to explain some of the behavioral manifestations of neurodegenerative disorders, for example, the sleep disturbances seen in patients with AD (Vanderheyden et al., 2018).
Again, astrocyte regulation of synaptic transmission appears to be intimately connected to the immunological and metabolic statuses of the CNS. Astrocytes are known to release neurotransmitters such as glutamate at the synapse in a process known as gliotransmission (Halassa et al., 2007). Glutamatergic gliotransmission can be adversely affected by the increased oxidative stress frequently seen in neurodegenerative diseases (Liu et al., 2006). Additionally, inflammatory factors like histamine can trigger increased astrocytic glutamate release (Kárpáti et al., 2018), which could have a potentially negative impact on neural survival. TNF-α, an important astrocyte inflammatory mediator is also important for regulating glutamatergic gliotransmission (Santello et al., 2011). Therefore, it is possible that over-production of TNF-α by astrocytes responding to immune insult could alter gliotransmission.
Aside from neurotransmitter regulation, ion flux is also a key role of astrocytes, the dysregulation of which can exacerbate inflammatory or cytotoxic insults. Astrocytic potassium homeostasis is key for regulation of synaptic plasticity (Djukic et al., 2007), with disruptions potentially contributing to oxidative stress and neurodegeneration. For instance, decreased expression, or loss of expression of the potassium channel Kir4.1 interferes with astrocytic glutamate uptake (Djukic et al., 2007; Kucheryavykh et al., 2007). Glial Kir4.1 expression is progressively lost in mice lacking SOD1, a well-established mouse model for a subset of genetically-linked ALS (Kaiser et al., 2006; Bataveljić et al., 2012), directly implicating astrocytic synaptic dysfunction in this pathological state (Benkler et al., 2013). Activated astrocytes in the spinal cord of SOD1 mutant mice also produce excessive endothelin-1 which can induce excitotoxicity in motor neurons due to over-activation of AMPA receptors (Ranno et al., 2014).
Growth factor signaling
Astrocytes produce a wide array of growth factors, including nerve growth factor, brain-derived growth factor (BDNF), and fibroblast growth factor which are all important for neuron health and survival (Miyazaki and Asanuma, 2016). Some of these astrocyte-produced growth factors are important for maintaining healthy neuronal metabolism by activating anti-oxidative enzymes to reduce over-production of reactive oxygen species (Cabezas et al., 2019). Others help support healthy synapse function: astrocytic fibroblast growth factor supports axon and dendrite outgrowth (Le and Esquenazi, 2002) and BDNF is a well-known regulator of synaptic plasticity (Bramham and Messaoudi, 2005). Still others help to support neuron survival and neurogenesis (Palmer and Ousman, 2018).
Astrocyte production of these growth factors may decrease with age, which may partially account for age-associated increases in incidence of neurodegenerative disorders (Palmer and Ousman, 2018). Upon activation, astrocytes may fail to produce the necessary growth factors to maintain the local neuron population for which they are responsible. For example, mice with the ALS-associated G93A SOD1 mutation harbor reactive astrocytes that fail to produce sufficient nerve growth factor to allow for motor neuron survival (Pehar et al., 2004). Further, changes in serum BDNF levels have been associated with cognitive impairment in patients with AD, frontotemporal dementia, Lewy body dementia, and vascular dementia, potentially implicating these growth factors in these neurodegenerative diseases, although their source is still to be determined (Budni et al., 2015). There is evidence that loss of BDNF in the hippocampus may be associated with various cognitive and emotional symptoms of AD, presumably related to a disruption in healthy experience-driven synaptic plasticity (Budni et al., 2015). In summary, astrocyte-derived growth factors may guard against neurodegeneration by promoting neuronal survival/viability, although more direct evidence will be needed to further establish a causative role of astrocyte-specific growth factor deficiency in neurodegeneration, as well as the therapeutic potential of growth factor therapy in neuroprotection for this purpose.
Conclusions
Astrocytic response to neurodegeneration is diverse, with both positive and negative effects (Sofroniew, 2009; Pekny et al., 2014; Ferrer, 2017). In part this may be due to variability within the overall astrocyte population. There is abundant evidence from transcriptomic and morphological data to suggest that multiple astrocytic subtypes likely exist (Jha et al., 2018; Kirschen et al., 2018; Neal and Richardson, 2018). For instance, consistent with astrocytes’ known interaction with the immune system given inflammatory signals, microarray data have revealed that astrocytes assume a pro-inflammatory transcriptional profile when exposed to cytokine or other soluble molecular signals (Michelucci et al., 2016). Still, more investigation will be required before definitive subcategories of astrocytes can be created. Additionally, astrocyte response to activation can vary with age (Jiang and Cadenas, 2014; Bellaver et al., 2017; Kluge et al., 2018), systemic immune status (Rakic et al., 2018), and depending on the specific trigger for activation (Perriot et al., 2018). A full-understanding of astrocyte transcriptomic and proteomic profiles will be needed in order to determine which targets are most likely to contribute to, versus aid in recovery from neurodegeneration. Despite the many unknowns, astrocytes do appear to be a potentially viable target for precision medicine and may have immense and hitherto largely untapped therapeutic potential (Allen et al., 2017; Barreto et al., 2017).
Potential Therapeutic Targets
Given the wide variability of astrocytes’ responses to inflammation, and consequences of astrogliosis, it becomes challenging to determine which, if any of the mediators of astrogliosis might produce beneficial effects as a therapeutic target for neurodegenerative disease. For example, astrocytic release of TGF-β has been shown to have anti-inflammatory effects: dampening microglial activation (Norden et al., 2014) and potentially limiting the negative synaptic effects of protein accumulation (Diniz et al., 2017). However, constitutive over-expression of TGF-β in mice promotes AD-like microvascular degeneration, suggesting that long-term over-production of TGF-β may ultimately have detrimental effects such as neuronal apoptosis and excessive extracellular matrix protein deposition (Wyss-Coray et al., 2000; Zhang et al., 2016).
It may be more beneficial, therefore, to attempt to target the initiation of the astrogliosis response. There are several potential therapeutic targets that might help to ameliorate protein-accumulation-mediated astrocyte activation. The presence of heme and hemoglobin modulates the astrocytic inflammatory response to the over-accumulation of Aβ in otherwise healthy murine astrocytes, specifically by upregulating phosphoinositide 3-kinase/Akt pathway, ultimately increasing activity of phagocytes via cytokine-mediated recruitment (Sankar et al., 2018). By contrast, in the substantia nigra, activation of astrocytic nicotinic acetylcholine receptors can dampen astrocytic reactivity to immune insults by inhibiting release of cytokines such as TNF- α and interleukins 1β, 2 and 6 (Jurado-Coronel et al., 2016).
Toll-like receptor 2, a protein frequently over-expressed in post-mortem tissue taken from patients with PD and Lewy body dementia (Doorn et al., 2014; Dzamko et al., 2017), may mediate the transmission of α-synuclein between neurons and glia (Kim et al., 2018), and appears to trigger microglial activation through the NFκB pathway (Kim et al., 2016). Astrocytic NFκB signaling may also be modulated by estrogen receptor signaling (Mitra et al., 2016), and by the oncogene astrocyte elevated gene-1 (Vartak-Sharma et al., 2014; Yin et al., 2018). Disrupting astrocytic NFκB signaling greatly reduces astrocytic release of cytokines/chemokines, and subsequent activation (Davis et al., 2015). Thus, a number of potentially druggable pathways exist to attempt to modulate astrocyte immunological function.
It is also desirable to be able to better regulate certain consequences of astrogliosis, as such scarring can greatly limit the degree of potential regeneration after traumatic injury (Pekny et al., 2007). While astrocytes are by no means the only cell type involved in glial scarring, they are integral to glial scar formation. Astrocytic proteins vimentin (VIM) and GFAP are known to modulate transcriptional responses in astrocytes following immune insult (Kamphuis et al., 2015), and are important for glial scar creation (Wilhelmsson et al., 2004). Deleting VIM and GFAP in mice prevents astroglial process hypertrophy, greatly improving post-traumatic regeneration (Wilhelmsson et al., 2004). However, in transgenic mice expressing AD-associated mutant proteins amyloid precursor protein and presenilin-1, loss of GFAP and VIM lead to major increases in Aβ plaque load and neuron dysfunction, suggesting that VIM and GFAP-induced astrocyte activation and subsequent glial scarring may help to contain and limit Aβ plaque growth (Kraft et al., 2013). Given that, whether targeting of glial scar formation serves as a fruitful approach to combatting neurodegeneration remains unclear.
Genetic Strategies for Targeting Astrocytes
Genetic technologies are emerging as a method to target the CNS both for basic studies and for clinical intervention. Here, we will discuss how genetic strategies have been used to understand astrocytes and how they may hold promise for targeting astrocytes in neurodegenerative disease. These strategies allow for specific targeting of astrocytes without affecting other CNS cell types, although various challenges, limitations, and optimization detailed below will be required. Given astrocytes’ regulation of neurotransmitter and ion cycling, neuroplasticity, and neuronal growth and survival, it has been posited that astrocytes may be key to improving cognitive function in the setting of neurodegenerative diseases (Dallérac and Rouach, 2016). Indeed, there is evidence for example that pathological cellular damage induced by excitotoxicity in ALS may be partially attributable to astrocyte expression of GLT1 (Fumagalli et al., 2008). Thus, it stands to reason that experimental manipulation of astrocytes may, under certain circumstances, be able to reverse if not slow the molecular underpinnings of neurodegeneration with the hopes of restoring or preventing cognitive decline among other functions.
To this end, researchers have made large advances in this regard by developing viral gene constructs and transgenic mouse lines that are astrocyte-specific (Shigetomi et al., 2010, 2016; Tsai et al., 2012). The basis of these developments stand on efforts made to define what an astrocyte actually is in terms of the cell’s genetic and morphological features (Khakh and Sofroniew, 2015). Crucial transcriptomic studies have demonstrated that astrocytes express particular genes that are not highly expressed in other CNS cells such as neurons or oligodendrocytes (Gong et al., 2003; Lovatt et al., 2007; Cahoy et al., 2008; Allen and Eroglu, 2017). Combined with prior knowledge, researchers have used the genes Gfap, Aldh1I1, Slc1a3, and Gjb6 as markers for astrocytes (Srinivasan et al., 2016). Using the promoters for these genes, one is able to selectively express proteins in astrocytes that allow for the monitoring and manipulation of astrocytes. In particular relation to neurodegenerative intervention, viral strategies are emerging as a clinical candidate for targeting the CNS (Oswald et al., 2017).
Viral constructs targeting astrocyte gene expression
Viruses have been widely adopted as a way to introduce transgenes to the CNS for both preclinical and clinical studies. Compared to other methods such as nanoparticle delivery, viruses have been capitalized for their efficiency of gene transduction and long-term expression (Bouard et al., 2009). Viruses have been widely used to discover both basic and disease-oriented mechanisms regarding neurons, astrocytes, and microglia (Jahn et al., 2015; Luo et al., 2018). Inducing the expression of optogenetic and activity-based fluorescent proteins has allowed many research groups to both manipulate and monitor cells of the CNS in behaving animals. At the same time, clinical studies have attempted to use viruses to ameliorate deficits in neurological disease by modulating molecular or physiological deficits. Thus, we will review how viruses have been used to investigate astrocytes keeping in mind how these tools may be used to target astrocytes in clinical disease.
Researchers have used viruses to introduce genes implicated in neurodegenerative disease specifically into astrocytes in order to isolate astrocyte contributions to such diseases. There are a variety of viruses that have demonstrated tropism for astrocytes in the CNS. Lentiviruses, adeno-associated viruses (AAV), and canine adenovirus (CAV) have been successfully used to introduce transgenes to astrocytes. Combined with the ability to introduce astrocyte-promoter-dependent genes through viruses, a high astrocyte specificity can be achieved. While the majority of viral CNS studies have focused on neurons, recent studies demonstrate that such viral-based approaches are useful for understanding astrocyte function as well. For instance, using viral transduction strategies, astrocyte’s have been implicated in neurovascular coupling (Stobart et al., 2018), fear behavior (Martin-Fernandez et al., 2017), and feeding behavior (Chen et al., 2016) among other aspects of brain function.
Lentiviruses have been used successfully to target astrocytes in mice, and offer several advantages (Colin et al., 2009; Faideau et al., 2010; Delzor et al., 2013; Fassler et al., 2013). Lentiviral transgenes are transduced with high efficiency, with long term expression that is often required for in the context of neurodegenerative models (Park, 2007). Using a lentiviral strategy, Faideau et al., 2010 revealed that astrocyte expression of mutant Htt contributes to the mouse Huntington disease phenotypes. Aside from this study, Fassler and colleagues have developed a lentivirus construct that has preferential tropism for astrocytes (Fassler et al., 2013). Their construct expresses an anti-GLAST IgG on the lentivirus’ surface. GLAST is an amino acid transporter linked to astrocyte synapse support function and is highly expressed in astrocytes (Zhou et al., 2006), thus, preferentially targeting astrocytes
In comparison to lentiviruses, AAVs have been much more commonly used to study astrocytes in both health and disease. Several serotypes of AAVs (AAV2/1, AAV2/5, AAV2/8, AAV2/9) have been tested to have tropism for astrocytes (Foust et al., 2009; Tong et al., 2014; Hammond et al., 2017; Pignataro et al., 2017). Using AAV transduction (serotype 2/5), the Khakh group was able to show that astrocyte expression of the potassium channel Kir4.1 rescues deficits in a Huntington disease mouse model (Tong et al., 2014). This study demonstrated that astrocytes play a role in regulating the excitability of neurons in the dorsal striatum, the principle region implicated in Huntington disease. Further demonstrating the role of astrocytes in Huntington disease, Meunier et al. (2016) have also used the AAV2/5 vector to selectively express mutant forms of Huntingtin in striatal astrocytes. They demonstrated that astrocyte expression of mutant Huntingtin contributes to pathological features of the Huntington mouse model. In comparison to AAV2/5 serotypes, researchers have demonstrated that AAV2/8 may transduce astrocytes with the higher efficiency in the CNS (Aschauer et al., 2013). Martin and colleagues used the AAV2/8 vector to employ a chemogenetic strategy to manipulate astrocytes. This work demonstrated the role astrocytes play in the central amygdala for fear and anxiety behaviors. Overall, AAVs represent a feasible tool to manipulate the genes of astrocytes and will likely continue to facilitate our understanding of astrocytes in a variety of disease states.
Our laboratory has recently reported that intravenously delivered CAV2 targets perivascular astrocytes throughout the adult brain (Kirschen et al., 2018). This has translational implications as other methods described above for transgene introduction is invasively hindering. Additionally, perivascular astrocytes in particular have been implicated in neurodegenerative disease (Montagne et al., 2015). Another advantage of this viral delivery strategy is that it can systemically manipulate astrocytes. Methods of stereotactic infusion may be region-specific, but it would not allow for the analysis of astrocytes in multiple communicating brain regions. Stereotactic infusion is also well known to cause reactive astrogliosis and thus may confound studies of astrocyte-intrinsic behavior (Dashkoff et al., 2016). Overall this finding indicates that CAV2 may provide tool to investigate the role of astrocytes in disease.
In the clinical realm, gene therapy via viral transduction has emerged as a promising method for addressing diseases of the CNS (Hocquemiller et al., 2016). There are varieties of ongoing clinical trials using intraparenchymal infusion of viruses in order to treat neurodegenerative diseases by modulating gene expression. For instance, intrastriatal infusion of AAVs has been used in phase I/II clinical trials for PD (Marks et al., 2008, 2010; Christine et al., 2009). While these represent landmark efforts for neurological therapy, these viruses introduced genes under the control of ubiquitous promoters and thus may induce gene expression in neurons and astrocytes alike. As mentioned previously, a major utility of genetic strategies is the ability to target specific cell types implicated in disease. In order to consider how astrocytes may be targeted in disease, it will be important to consider what promoter elements may be more specific to astrocytes (Cahoy et al., 2008). We list astrocyte-specific promoters (Gfap, Slc1a3, and Gjb6) used previously (Dabir et al., 2006; Nomura et al., 2010; Magnusson et al., 2014; Aida et al., 2015; Benedykcinska et al., 2016), with Aldh1I1 (Chai et al., 2017; Octeau et al., 2018; Yu et al., 2018) being recently validated as the most specific astrocyte promoter in murine studies (Srinivasan et al., 2016). One major caveat of using common promoters of astrocytes is the difficulty for packaging promoters of all sizes into viral vectors. For instance, it has been reported that AAV vectors have a limited physical space that may be required for large mammalian promoters (Dong et al., 1996; de Leeuw et al., 2016). To circumvent this issue, the Simpson group has worked on creating a variety of shortened promoters, termed “MiniPromoters” in order to target specific cell populations of the brain (de Leeuw et al., 2016). This work will be essential to identifying constructs that will allow gene manipulation specifically in astrocytes of patient brains.
Transgenic mouse models for studying astrocytes in neurodegeneration
Transgenic mouse models are widely used to study astrocytes. On the other hand, in the context of neurodegenerative disease, there has traditionally been a neuron-centric bias in which neuronal protein expression is thought to be the principal if not exclusive link to pathogenesis. Transgenic lines have allowed for the determination of glial cells’ specific contributions to disease. For instance, researchers have used of the Cre/loxP system to restrict gene expression to astrocytes. Astrocyte promoters of Gfap and Ald1I1 have been used to express Cre recombinase in astrocytes (Jahn et al., 2015; Srinivasan et al., 2015, 2016). When introducing Cre-dependent genes, either through a double-transgenic cross or through viral transduction, one is able to manipulate genes specifically in astrocytes. Using a Cre-based conditional deletion scheme, the Cleveland lab showed that astrocytes play a major role in the progression of amyotrophic lateral sclerosis in the mutant SOD1 mouse model (Yamanaka et al., 2008). Researchers have used similar transgenic mouse line approaches to selectively express genes in astrocytes in the contexts of Parkinson disease, Alzheimer disease, and Huntington disease (Bradford et al., 2009; Gu et al., 2010; Lian et al., 2016; Wood et al., 2019). These studies served to dissect the contribution of astrocytes to neurodegenerative models. Recently, the Khakh lab generated an Aldh1I1 Cre line that allows for the specific expression of genes in all astrocytes of the CNS (Srinivasan et al., 2016). As opposed to previous lines based on other promoters such as GFAP and GLAST, these mice serve to restrict genetic expression to be more highly specific for a wider range of astrocytes in the CNS. This will allow for more sophisticated experiments in exploring the specific contribution of astrocytes to disease states.
Off-target effects of genetic strategies
Basic neurobiologists and developers of pharmaceuticals alike are wary of potential off-target manipulations that accompany both viral manipulation and transgenic mouse development. In terms of using viral constructs to target astrocytes in clinical contexts, immunogenic responses and oncogenic effects are principal concerns (Cotter and Muruve, 2005; Marks et al., 2008). As illustrated above, there has been an immense interest in using AAVs for therapeutic intervention for their relatively non-immunogenic and non-oncogenic profiles (Hocquemiller et al., 2016; Naso et al., 2017). However, it should be noted that patients can develop antibodies against AAVs and give rise to the risk of developing immune responses to AAVs (Louis et al., 2013). Fortunately, strategies that locally target CNS regions (intraparenchymal) benefit from being relatively immune privileged systems (Christine et al., 2009). In contrast, recent preclinical studies indicate that systemic introduction of high-dose AAVs may result in severe toxicity affecting the nervous and hepatic systems (Hinderer et al., 2018; Hordeaux et al., 2018). While local CNS delivery of AAVs appear to be clinically promising, mechanisms of intracellular transport and transynaptic spread in the CNS remains unclear (Nonnenmacher and Weber, 2012; Castle et al., 2014; Zingg et al., 2017). Thus, the specificity of CNS viral delivery may be difficult to ascertain, and future studies are needed to determine whether or not astrocytes may be targeted in a region-specific manner. Lastly, one potential way to more specifically target astrocytes is to utilize astrocyte promoters studied in transgenic models in order restrict gene expression to astrocytes. However, there are two problems with this strategy. As discussed above, astrocytes display heterogeneity and traditional promoters such as GFAP and GLAST may be neither astrocyte-specific nor pan-astrocyte targeting throughout the CNS (Sofroniew, 2009; Srinivasan et al., 2016). In addition, recent studies have remarked that there are significant differences between murine and human astrocytes, potentially confounding the contribution of astrocyte-specific genes and pathology to neurological diseases (Vasile et al., 2017). Together, these points serve as cautions for considering astrocyte gene therapy and for interpreting studies of astrocytes in general.
Conclusions and Perspective
Based on a wealth of recent investigations, the specific roles and molecular pathways implicated in astrocytes’ response to neurodegenerative injury are becoming increasingly clear. For instance, while it is traditionally held that astrocytes interact principally if not exclusively with neurons, the complex cross-talk between astrocytes and microglia especially during injury has been acknowledged and is the subject of much clinical interest. Based on the existing viral and genetic tools in the field, a number of important astrocyte-autonomous functions in neurodegenerative diseases have been uncovered, and likely more remain to be seen. Exciting new work is beginning to profile the astrocyte inflammasome in various pro-inflammatory settings (Dozio et al., 2018). The better these functions are understood, the better they can be counteracted or modulated, with the goal of controlling neuroinflammation and promoting reparative physiology rather than exacerbating injury and further compounding neurodegenerative phenotypes. Ultimately, much of this work still rests on mammalian model systems, and our genetic understanding of astrocytes, although emerging technology may allow monitoring astrocyte activity in humans (Edison et al., 2018). These emerging technologies, in tandem with clinical genetic strategies, may lead to targeted treatment of astrocyte-mediated defects in neurodegenerative disease. Overall, there is likely a lot of untapped power in astrocytes that may prove fruitful for future clinical studies in the context of currently unmodifiable neurodegenerative diseases.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: The work was supported by National Institutes of Health (NIH) Grants 1F30MH110103 (to GWK) and 1F30MH116650 (to RK).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: The work was supported by National Institutes of Health (NIH) Grants 1F30MH110103 (to GWK) and 1F30MH116650 (to RK).
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Aida T, Yoshida J, Nomura M, Tanimura A, Iino Y, Soma M, Bai N, Ito Y, Cui W, Aizawa H, Yanagisawa M, Nagai T, Takata N, Tanaka KF, Takayanagi R, Kano M, Götz M, Hirase H, Tanaka K. Astroglial glutamate transporter deficiency increases synaptic excitability and leads to pathological repetitive behaviors in mice. Neuropsychopharmacology. 2015;40:1569–1579. doi: 10.1038/npp.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allaman I, Gavillet M, Bélanger M, Laroche T, Viertl D, Lashuel HA, Magistretti PJ. Amyloid-beta aggregates cause alterations of astrocytic metabolic phenotype: impact on neuronal viability. J Neurosci. 2010;30:3326–3338. doi: 10.1523/JNEUROSCI.5098-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen CF, Shaw PJ, Ferraiuolo L. Can astrocytes be a target for precision medicine? Adv Exp Med Biol. 2017;1007:111–128. doi: 10.1007/978-3-319-60733-7_7. [DOI] [PubMed] [Google Scholar]
- 4.Allen NJ, Eroglu C. Cell biology of astrocyte-synapse interactions. Neuron. 2017;96:697–708. doi: 10.1016/j.neuron.2017.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson MA, Ao Y, Sofroniew MV. Sofroniew, heterogeneity of reactive astrocytes. Neurosci Lett. 2014;565:23–29. doi: 10.1016/j.neulet.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aschauer DF, Kreuz S, Rumpel S. Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One. 2013;8:e76310. doi: 10.1371/journal.pone.0076310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assefa BT, Gebre AK, Altaye BM. Altaye, reactive astrocytes as drug target in Alzheimer’s disease. Biomed Res Int. 2018;2018:4160247. doi: 10.1155/2018/4160247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badawi Y, Ramamoorthy P, Shi H. Hypoxia-inducible factor 1 protects hypoxic astrocytes against glutamate toxicity. ASN Neuro. 2012;4:231–241. doi: 10.1042/AN20120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bandopadhyay R, Kingsbury AE, Cookson MR, Reid AR, Evans IM, Hope AD, Pittman AM, Lashley T, Canet-Aviles R, Miller DW, McLendon C, Strand C, Leonard AJ, Abou-Sleiman PM, Healy DG, Ariga H, Wood NW, de Silva R, Revesz T, Hardy JA, et al. The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson’s disease. Brain. 2004;127:420–430. doi: 10.1093/brain/awh054. [DOI] [PubMed] [Google Scholar]
- 10.Barreto GE, Gomez RM, Bustos RH, Forero DA, Aliev G, Tarasov VV, Yarla NS, Echeverria V, Gonzalez J. Approaches of the transcriptomic analysis in astrocytes: potential pharmacological targets. Curr Pharm Des. 2017;23:4189–4197. doi: 10.2174/1381612823666170406113501. [DOI] [PubMed] [Google Scholar]
- 11.Bataveljić D, Nikolić L, Milosević M, Todorović N, Andjus PR. Changes in the astrocytic aquaporin-4 and inwardly rectifying potassium channel expression in the brain of the amyotrophic lateral sclerosis SOD1(G93A) rat model. Glia. 2012;60:1991–2003. doi: 10.1002/glia.22414. [DOI] [PubMed] [Google Scholar]
- 12.Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Bellaver B, Souza DG, Souza DO, Quincozes-Santos A. Hippocampal astrocyte cultures from adult and aged rats reproduce changes in glial functionality observed in the aging brain. Mol Neurobiol. 2017;54:2969–2985. doi: 10.1007/s12035-016-9880-8. [DOI] [PubMed] [Google Scholar]
- 14.Benedykcinska A, Ferreira A, Lau J, Broni J, Richard-Loendt A, Henriquez NV, Brandner S. Generation of brain tumours in mice by Cre-mediated recombination of neural progenitors in situ with the tamoxifen metabolite endoxifen. Dis Model Mech. 2016;9:211–220. doi: 10.1242/dmm.022715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benkler C, Ben-Zur T, Barhum Y, Offen D. Altered astrocytic response to activation in SOD1(G93A) mice and its implications on amyotrophic lateral sclerosis pathogenesis. Glia. 2013;61:312–326. doi: 10.1002/glia.22428. [DOI] [PubMed] [Google Scholar]
- 16.Boche D, Perry VH, Nicoll JA. Review: activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol. 2013;39:3–18. doi: 10.1111/nan.12011. [DOI] [PubMed] [Google Scholar]
- 17.Bonansco C, Couve A, Perea G, Ferradas CÁ, Roncagliolo M, Fuenzalida M. Glutamate released spontaneously from astrocytes sets the threshold for synaptic plasticity. Eur J Neurosci. 2011;33:1483–1492. doi: 10.1111/j.1460-9568.2011.07631.x. [DOI] [PubMed] [Google Scholar]
- 18.Bouard D, Alazard-Dany D, Cosset FL. Viral vectors: from virology to transgene expression. Br J Pharmacol. 2009;157:153–165. doi: 10.1038/bjp.2008.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradford J, Shin JY, Roberts M, Wang CE, Li XJ, Li S. Expression of mutant huntingtin in mouse brain astrocytes causes age-dependent neurological symptoms. Proc Natl Acad Sci U S A. 2009;106:22480–22485. doi: 10.1073/pnas.0911503106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Bsibsi M, Persoon-Deen C, Verwer RW, Meeuwsen S, Ravid R, Van Noort JM. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia. 2006;53:688–695. doi: 10.1002/glia.20328. [DOI] [PubMed] [Google Scholar]
- 22.Budni J, Bellettini-Santos T, Mina F, Garcez ML, Zugno AI. The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis. 2015;6:331–341. doi: 10.14336/AD.2015.0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabezas R, Baez-Jurado E, Hidalgo-Lanussa O, Echeverria V, Ashrad GM, Sahebkar A, Barreto GE. Growth factors and neuroglobin in astrocyte protection against neurodegeneration and oxidative stress. Mol Neurobiol. 2019;56:2339–2351. doi: 10.1007/s12035-018-1203-9. [DOI] [PubMed] [Google Scholar]
- 24.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caraci F, Bosco P, Signorelli M, Spada RS, Cosentino FI, Toscano G, Bonforte C, Muratore S, Prestianni G, Panerai S, Giambirtone MC, Gulotta E, Romano C, Salluzzo MG, Nicoletti F, Copani A, Drago F, Aguglia E, Ferri R. The CC genotype of transforming growth factor-beta1 increases the risk of late-onset Alzheimer’s disease and is associated with AD-related depression. Eur Neuropsychopharmacol. 2012;22:281–289. doi: 10.1016/j.euroneuro.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Caraci F, Spampinato SF, Morgese MG, Tascedda F, Salluzzo MG, Giambirtone MC, Caruso G, Munafò A, Torrisi SA, Leggio GM, Trabace L, Nicoletti F, Drago F, Sortino MA2, Copani A. Neurobiological links between depression and AD: The role of TGF-beta1 signaling as a new pharmacological target. Pharmacol Res. 2018;130:374–384. doi: 10.1016/j.phrs.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Cassina P, Pehar M, Vargas MR, Castellanos R, Barbeito AG, Estévez AG, Thompson JA, Beckman JS, Barbeito L. Astrocyte activation by fibroblast growth factor-1 and motor neuron apoptosis: implications for amyotrophic lateral sclerosis. J Neurochem. 2005;93:38–46. doi: 10.1111/j.1471-4159.2004.02984.x. [DOI] [PubMed] [Google Scholar]
- 28.Cassina P, Peluffo H, Pehar M, Martinez-Palma L, Ressia A, Beckman JS, Estévez AG, Barbeito L. Peroxynitrite triggers a phenotypic transformation in spinal cord astrocytes that induces motor neuron apoptosis. J Neurosci Res. 2002;67:21–29. doi: 10.1002/jnr.10107. [DOI] [PubMed] [Google Scholar]
- 29.Castle MJ, Gershenson ZT, Giles AR, Holzbaur EL, Wolfe JH. Adeno-associated virus serotypes 1, 8 and 9 share conserved mechanisms for anterograde and retrograde axonal. Hum Gene Ther. 2014;25:705–720. doi: 10.1089/hum.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cekanaviciute E, Dietrich HK, Axtell RC, Williams AM, Egusquiza R, Wai KM, Koshy AA, Buckwalter MS. Astrocytic TGF-beta signaling limits inflammation and reduces neuronal damage during central nervous system Toxoplasma infection. J Immunol. 2014;193:139–149. doi: 10.4049/jimmunol.1303284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cekanaviciute E, Fathali N, Doyle KP, Williams AM, Han J, Buckwalter MS. Astrocytic transforming growth factor-beta signaling reduces subacute neuroinflammation after stroke in mice. Glia. 2014;62:1227–1240. doi: 10.1002/glia.22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chai H, Diaz-Castro B, Shigetomi E, Monte E, Octeau JC, Yu X, Cohn W, Rajendran PS, Vondriska TM, Whitelegge JP4, Coppola G, Khakh BS. Neural circuit-specialized astrocytes: transcriptomic, proteomic morphological and functional, evidence. Neuron. 2017;95:531–549 e9. doi: 10.1016/j.neuron.2017.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chavez JC, Baranova O, Lin J, Pichiule P. The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J Neurosci. 2006;26:9471–9481. doi: 10.1523/JNEUROSCI.2838-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen N, Sugihara H, Kim J, Fu Z, Barak B, Sur M, Feng G, Han W. Direct modulation of GFAP-expressing glia in the arcuate nucleus bi-directionally regulates feeding. Elife. 2016 doi: 10.7554/eLife.18716. doi: 107554/eLife18716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi DJ, Eun JH, Kim BG, Jou I, Park SM, Joe EH. A Parkinson’s disease gene, DJ-1, repairs brain injury through Sox9 stabilization and astrogliosis. Glia. 2018;66:445–458. doi: 10.1002/glia.23258. [DOI] [PubMed] [Google Scholar]
- 36.Choi K, Park J, Lee J, Han EC, Choi C. Mutant ubiquitin attenuates interleukin-1beta- and tumor necrosis factor-alpha-induced pro-inflammatory signaling in human astrocytic cells. 2013 doi: 10.1371/journal.pone.0067891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chowdhury T, Allen MF, Thorn TL, He Y, Hewett SJ. Interleukin-1beta protects neurons against oxidant-induced injury via the promotion of astrocyte glutathione production. Antioxidants (Basel) 2018 doi: 10.3390/antiox7080100. doi: 103390/antiox7080100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christine CW, Starr PA, Larson PS, Eberling JL, Jagust WJ, Hawkins RA, VanBrocklin HF, Wright JF, Bankiewicz KS, Aminoff MJ. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology. 2009;73:1662–1669. doi: 10.1212/WNL.0b013e3181c29356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark VD, Layson A, Charkviani M, Muradashvili N, Lominadze D. Hyperfibrinogenemia-mediated astrocyte activation. Brain Res. 2018;1699:158–165. doi: 10.1016/j.brainres.2018.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colin A, Faideau M, Dufour N, Auregan G, Hassig R, Andrieu T, Brouillet E, Hantraye P, Bonvento G, Déglon N. Engineered lentiviral vector targeting astrocytes in vivo. Glia. 2009;57:667–679. doi: 10.1002/glia.20795. [DOI] [PubMed] [Google Scholar]
- 41.Cotter MJ, Muruve DA. The induction of inflammation by adenovirus vectors used for gene therapy. Front Biosci. 2005;10:1098–1105. doi: 10.2741/1603. [DOI] [PubMed] [Google Scholar]
- 42.Croisier E, Graeber MB. Glial degeneration and reactive gliosis in alpha-synucleinopathies: the emerging concept of primary gliodegeneration. Acta Neuropathol. 2006;112:517–530. doi: 10.1007/s00401-006-0119-z. [DOI] [PubMed] [Google Scholar]
- 43.Dabir DV, Robinson MB, Swanson E, Zhang B, Trojanowski JQ, Lee VM, Forman MS. Impaired glutamate transport in a mouse model of tau pathology in astrocytes. J Neurosci. 2006;26:644–654. doi: 10.1523/JNEUROSCI.3861-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dallérac G, Rouach N. Astrocytes as new targets to improve cognitive functions. Prog Neurobiol. 2016;144:48–67. doi: 10.1016/j.pneurobio.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Daniels BP, Jujjavarapu H, Durrant DM, Williams JL, Green RR, White JP, Lazear HM, Gale M, Jr, Diamond MS, Klein RS. Regional astrocyte IFN signaling restricts pathogenesis during neurotropic viral infection. J Clin Invest. 2017;127:843–856. doi: 10.1172/JCI88720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dashkoff J, Lerner EP, Truong N, Klickstein JA, Fan Z, Mu D, Maguire CA, Hyman BT, Hudry E. Tailored transgene expression to specific cell types in the central nervous system after peripheral injection with AAV9. Mol Ther Methods Clin Dev. 2016;3:16081. doi: 10.1038/mtm.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis RL, Das S, Thomas Curtis J, Stevens CW. The opioid antagonist, beta-funaltrexamine inhibits NF-kappaB signaling and chemokine expression in human astrocytes and in mice. Eur J Pharmacol. 2015;762:193–201. doi: 10.1016/j.ejphar.2015.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Leeuw CN, Korecki AJ, Berry GE, Hickmott JW, Lam SL, Lengyell TC, Bonaguro RJ, Borretta LJ, Chopra V, Chou AY, D’Souza CA, Kaspieva O, Laprise S, McInerny SC, Portales-Casamar E, Swanson-Newman MI, Wong K, Yang GS, Zhou M, Jones SJ, et al. rAAV-compatible MiniPromoters for restricted expression in the brain and eye. Mol Brain. 2016;9:52. doi: 10.1186/s13041-016-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Miranda BR, Rocha EM, Bai Q, El Ayadi A, Hinkle D, Burton EA, Timothy Greenamyre J. Astrocyte-specific DJ-1 overexpression protects against rotenone-induced neurotoxicity in a rat model of Parkinson’s disease. Neurobiol Dis. 2018;115:101–114. doi: 10.1016/j.nbd.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delzor A, Escartin C, Déglon N. Lentiviral vectors: a powerful tool to target astrocytes in vivo. Curr Drug Targets. 2013;14:1336–1346. doi: 10.2174/13894501113146660213. [DOI] [PubMed] [Google Scholar]
- 51.Dickens AM, Tovar-Y-Romo LB, Yoo SW, Trout AL, Bae M, Kanmogne M, Megra B, Williams DW, Witwer KW, Gacias M, Tabatadze N, Cole RN, Casaccia P, Berman JW, Anthony DC, Haughey NJ. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal. 2017 doi: 10.1126/scisignal.aai7696. doi: 101126/scisignalaai7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diniz LP, Tortelli V, Matias I, Morgado J, Bérgamo Araujo AP, Melo HM, Seixas da Silva GS, Alves-Leon SV, de Souza JM, Ferreira ST, De Felice FG, Gomes FCA. Astrocyte transforming growth factor beta 1 protects synapses against abeta oligomers in Alzheimer’s disease model. J Neurosci. 2017;37:6797–6809. doi: 10.1523/JNEUROSCI.3351-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD. Conditional knock-out of Kir4, 1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci. 2007;27:11354–11365. doi: 10.1523/JNEUROSCI.0723-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dong JY, Fan PD, Frizzell RA. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum Gene Ther. 1996;7:2101–2112. doi: 10.1089/hum.1996.7.17-2101. [DOI] [PubMed] [Google Scholar]
- 55.Doorn KJ, Moors T, Drukarch B, van de Berg WDj, Lucassen PJ, van Dam AM. Microglial phenotypes and toll-like receptor 2 in the substantia nigra and hippocampus of incidental Lewy body disease cases and Parkinson’s disease patients. Acta Neuropathol Commun. 2014;2:90. doi: 10.1186/s40478-014-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dozio V, Sanchez JC. Profiling the proteomic inflammatory state of human astrocytes using DIA mass spectrometry. J Neuroinflammation. 2018;15:331. doi: 10.1186/s12974-018-1371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dzamko N, Gysbers A, Perera G, Bahar A, Shankar A, Gao J, Fu Y, Halliday GM. Toll-like receptor 2 is increased in neurons in Parkinson’s disease brain and may contribute to alpha-synuclein pathology. Acta Neuropathol. 2017;133:303–319. doi: 10.1007/s00401-016-1648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edison P, Donat CK, Sastre M. In vivo imaging of glial activation in Alzheimer’s disease. Front Neurol. 2018;9:625. doi: 10.3389/fneur.2018.00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Faideau M, Kim J, Cormier K, Gilmore R, Welch M, Auregan G, Dufour N, Guillermier M, Brouillet E, Hantraye P, Déglon N, Ferrante RJ, Bonvento G. In vivo expression of polyglutamine-expanded huntingtin by mouse striatal astrocytes impairs glutamate transport: a correlation with Huntington’s disease subjects. Hum Mol Genet. 2010;19:3053–3067. doi: 10.1093/hmg/ddq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fassler M, Weissberg I, Levy N, Diaz-Griffero F, Monsonego A, Friedman A, Taube R. Preferential lentiviral targeting of astrocytes in the central nervous system. PLoS One. 2013;8:e76092. doi: 10.1371/journal.pone.0076092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fellner L, Jellinger KA, Wenning GK, Stefanova N. Glial dysfunction in the pathogenesis of alpha-synucleinopathies: emerging concepts. Acta NeuropathGlial dysfunction emerging concepts. Acta Neuropathol. 2011;121:675–693. doi: 10.1007/s00401-011-0833-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fuller S, Steele M, Münch G. Activated astroglia during chronic inflammation in Alzheimer’s disease--do they neglect their neurosupportive roles. Mutat Res. 2010;690:40–49. doi: 10.1016/j.mrfmmm.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 64.Fumagalli E, Funicello M, Rauen T, Gobbi M, Mennini T. Riluzole enhances the activity of glutamate transporters GLAST, GLT1 and EAAC1. Eur J Pharmacol. 2008;578:171–176. doi: 10.1016/j.ejphar.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 65.Gómez-Gonzalo M, Navarrete M, Perea G, Covelo A, Martín-Fernández M, Shigemoto R, Luján R, Araque A. Endocannabinoids induce lateral long-term potentiation of transmitter release by stimulation of gliotransmission. Cereb Cortex. 2015;25:3699–3712. doi: 10.1093/cercor/bhu231. [DOI] [PubMed] [Google Scholar]
- 66.Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 67.Gontier G, George C, Chaker Z, Holzenberger M, Aïd S. Blocking IGF signaling in adult neurons alleviates Alzheimer’s disease pathology through amyloid-beta clearance. J Neurosci. 2015;35:11500–11513. doi: 10.1523/JNEUROSCI.0343-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gosejacob D, Dublin P, Bedner P, Hüttmann K, Zhang J, Tress O, Willecke K, Pfrieger F, Steinhäuser C, Theis M. Role of astroglial connexin30 in hippocampal gap junction coupling. Glia. 2011;59:511–519. doi: 10.1002/glia.21120. [DOI] [PubMed] [Google Scholar]
- 69.Gu XL, Long CX, Sun L, Xie C, Lin X, Cai H. Astrocytic expression of Parkinson’s disease-related A53T alpha-synuclein causes neurodegeneration in mice. Mol Brain. 2010;3:12. doi: 10.1186/1756-6606-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo ZH, Mattson MP. Neurotrophic factors protect cortical synaptic terminals against amyloid and oxidative stress-induced impairment of glucose transport, glutamate transport and mitochondrial function. Cereb Cortex. 2000;101:50–57. doi: 10.1093/cercor/10.1.50. [DOI] [PubMed] [Google Scholar]
- 71.Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 72.Hammond SL, Leek AN, Richman EH, Tjalkens RB. Cellular selectivity of AAV serotypes for gene delivery in neurons and astrocytes by neonatal intracerebroventricular injection. PLoS One. 2017;12:e0188830. doi: 10.1371/journal.pone.0188830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He Y, Jackman NA, Thorn TL, Vought VE, Hewett SJ. Interleukin-1beta protects astrocytes against oxidant-induced injury via an NF-kappaB-dependent upregulation of glutathione synthesis. Glia. 2015;63:1568–1580. doi: 10.1002/glia.22828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hennessy E, Griffin ÉW, Cunningham C. Astrocytes Are primed by chronic neurodegeneration to produce exaggerated chemokine and cell infiltration responses to acute stimulation with the cytokines IL-1beta and TNF-alpha. J Neurosci. 2015;35:8411–8422. doi: 10.1523/JNEUROSCI.2745-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hernández-Guillamon M, Delgado P, Ortega L, Pares M, Rosell A, García-Bonilla L, Fernández-Cadenas I, Borrell-Pagès M, Boada M, Montaner J. Neuronal TIMP-1 release accompanies astrocytic MMP-9 secretion and enhances astrocyte proliferation induced by beta-amyloid 25-35 fragment. J Neurosci Res. 2009;87:2115–2125. doi: 10.1002/jnr.22034. [DOI] [PubMed] [Google Scholar]
- 76.Hinderer C, Katz N, Buza EL, Dyer C, Goode T, Bell P, Richman LK, Wilson JM. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum Gene Ther. 2018;29:285–298. doi: 10.1089/hum.2018.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hocquemiller M, Giersch L, Audrain M, Parker S, Cartier N. Adeno-associated virus-based gene therapy for CNS diseases. Hum Gene Ther. 2016;27:478–496. doi: 10.1089/hum.2016.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hordeaux J, Wang Q, Katz N, Buza EL, Bell P, Wilson JM. The neurotropic properties of AAV-PHP, B are limited to C57BL/6J mice. Mol Ther. 2018;26:664–668. doi: 10.1016/j.ymthe.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoshi A, Tsunoda A, Yamamoto T, Tada M, Kakita A, Ugawa Y. Altered expression of glutamate transporter-1 and water channel protein aquaporin-4 in human temporal cortex with Alzheimer’s disease. Neuropathol Appl Neurobiol. 2018;44:628–638. doi: 10.1111/nan.12475. [DOI] [PubMed] [Google Scholar]
- 80.Hoye ML, Regan MR, Jensen LA, Lake AM, Reddy LV, Vidensky S, Richard JP, Maragakis NJ, Rothstein JD, Dougherty JD, Miller TM. Motor neuron-derived microRNAs cause astrocyte dysfunction in amyotrophic lateral sclerosis. Brain. 2018;141:2561–2575. doi: 10.1093/brain/awy182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hsiao HY, Chen YC, Chen HM, Tu PH, Chern Y. A critical role of astrocyte-mediated nuclear factor-kappaB-dependent inflammation in Huntington’s disease. Hum Mol Genet. 2013;22:1826–1842. doi: 10.1093/hmg/ddt036. [DOI] [PubMed] [Google Scholar]
- 82.Hu S, Sheng WS, Peterson PK, Chao CC. Differential regulation by cytokines of human astrocyte nitric oxide production. Glia. 1995;15:491–494. doi: 10.1002/glia.440150412. [DOI] [PubMed] [Google Scholar]
- 83.Huang X, Li Y, Li J, Feng Y, Xu X. Tanshinone IIA dampens the cell proliferation induced by ischemic insult in rat astrocytes via blocking the activation of HIF-1alpha/SDF-1 signaling. Life Sci. 2014;112:59–67. doi: 10.1016/j.lfs.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 84.Huerta C, Alvarez V, Mata IF, Coto E, Ribacoba R, Martínez C, Blázquez M, Guisasola LM, Salvador C, Lahoz CH, Peña J. Chemokines (RANTES and MCP-1) and chemokine-receptors (CCR2 and CCR5) gene polymorphisms in Alzheimer’s and Parkinson’s disease. Neurosci Lett. 2004;370:151–154. doi: 10.1016/j.neulet.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 85.Ikeshima-Kataoka H. Neuroimmunological Implications of AQP4 in Astrocytes. Int J Mol Sci. 2016 doi: 10.3390/ijms17081306. doi: 103390/ijms17081306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ito M, Komai K, Mise-Omata S, Iizuka-Koga M, Noguchi Y, Kondo T, Sakai R, Matsuo K, Nakayama T, Yoshie O, Nakatsukasa H, Chikuma S, Shichita T, Yoshimura A. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature. 2019;565:246–250. doi: 10.1038/s41586-018-0824-5. [DOI] [PubMed] [Google Scholar]
- 87.Jahn HM, Scheller A, Kirchhoff F. Genetic control of astrocyte function in neural circuits. Front Cell Neurosci. 2015;9:310. doi: 10.3389/fncel.2015.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jana M, Mondal S, Jana A, Pahan K. Interleukin-12 (IL-12), but not IL-23, induces the expression of IL-7 in microglia and macrophages: implications for multiple sclerosis. Immunology. 2014;141:549–563. doi: 10.1111/imm.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jha MK, Kim JH, Song GJ, Lee WH, Lee IK, Lee HW, An SSA, Kim S, Suk K. Functional dissection of astrocyte-secreted proteins: Implications in brain health and diseases. Prog Neurobiol. 2018;162:37–69. doi: 10.1016/j.pneurobio.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 90.Jiang T, Cadenas E. Astrocytic metabolic and inflammatory changes as a function of age. Aging Cell. 2014;13:1059–1067. doi: 10.1111/acel.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jurado-Coronel JC, Avila-Rodriguez M, Capani F, Gonzalez J, Moran VE, Barreto GE. Targeting the nicotinic acetylcholine receptors (nAChRs) in astrocytes as a potential therapeutic target in parkinson’s Disease. Curr Pharm Des. 2016;22:1305–1311. doi: 10.2174/138161282210160304112133. [DOI] [PubMed] [Google Scholar]
- 92.Kaiser M, Maletzki I, Hülsmann S, Holtmann B, Schulz-Schaeffer W, Kirchhoff F, Bähr M, Neusch C. Progressive loss of a glial potassium channel (KCNJ10) in the spinal cord of the SOD1 (G93A) transgenic mouse model of amyotrophic lateral sclerosis. J Neurochem. 2006;99:900–912. doi: 10.1111/j.1471-4159.2006.04131.x. [DOI] [PubMed] [Google Scholar]
- 93.Kamphuis W, Kooijman L, Orre M, Stassen O, Pekny M, Hol EM. GFAP and vimentin deficiency alters gene expression in astrocytes and microglia in wild-type mice and changes the transcriptional response of reactive glia in mouse model for Alzheimer’s disease. Glia. 2015;63:1036–1056. doi: 10.1002/glia.22800. [DOI] [PubMed] [Google Scholar]
- 94.Kárpáti A, Yoshikawa T, Nakamura T, Iida T, Matsuzawa T, Kitano H, Harada R, Yanai K. Histamine elicits glutamate release from cultured astrocytes. J Pharmacol Sci. 2018;137:122–128. doi: 10.1016/j.jphs.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 95.Kempuraj D, Thangavel R, Selvakumar GP, Ahmed ME, Zaheer S, Raikwar SP, Zahoor H, Saeed D, Dubova I, Giler G, Herr S, Iyer SS, Zaheer A. Mast cell proteases activate astrocytes and glia-neurons and release interleukin-33 by activating p38 and ERK1/2 MAPKs and NF-kappaB. Mol Neurobiol. 2019;56:1681–1693. doi: 10.1007/s12035-018-1177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kempuraj D, Thangavel R, Selvakumar GP, Zaheer S, Ahmed ME, Raikwar SP, Zahoor H, Saeed D, Natteru PA, Iyer S, Zaheer A. Brain and peripheral atypical inflammatory mediators potentiate neuroinflammation and neurodegeneration. Front Cell Neurosci. 2017;11:216. doi: 10.3389/fncel.2017.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci. 2015;18:942–952. doi: 10.1038/nn.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kia A, McAvoy K, Krishnamurthy K, Trotti D, Pasinelli P. Astrocytes expressing ALS-linked mutant FUS induce motor neuron death through release of tumor necrosis factor-alpha. Glia. 2018;66:1016–1033. doi: 10.1002/glia.23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim C, Lee HJ, Masliah E, Lee SJ. Non-cell-autonomous neurotoxicity of alpha-synuclein through microglial toll-like receptor 2. Exp Neurobiol. 2016;25:113–119. doi: 10.5607/en.2016.25.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim C, Spencer B, Rockenstein E, Yamakado H, Mante M, Adame A, Fields JA, Masliah D, Iba M, Lee HJ, Rissman RA, Lee SJ, Masliah E. Immunotherapy targeting toll-like receptor 2 alleviates neurodegeneration in models of synucleinopathy by modulating alpha-synuclein transmission and neuroinflammation. Mol Neurodegener. 2018;13:43. doi: 10.1186/s13024-018-0276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim JH, Lukowicz A, Qu W, Johnson A, Cvetanovic M. Astroglia contribute to the pathogenesis of spinocerebellar ataxia Type 1 (SCA1) in a biphasic, stage-of-disease specific manner. Glia. 2018;66:1972–1987. doi: 10.1002/glia.23451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kirkley KS, Popichak KA, Afzali MF, Legare ME, Tjalkens RB. Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity. J Neuroinflammation. 2017;14:99. doi: 10.1186/s12974-017-0871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kirschen GW, Kéry R, Liu H, Ahamad A, Chen L, Akmentin W, Kumar R, Levine J, Xiong Q, Ge S. Genetic dissection of the neuro-glio-vascular machinery in the adult brain. Mol Brain. 2018;11:2. doi: 10.1186/s13041-017-0345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kluge MG, Jones K, Kooi Ong L, Gowing EK, Nilsson M, Clarkson AN, Walker FR. Age-dependent disturbances of neuronal and glial protein expression profiles in areas of secondary neurodegeneration post-stroke. Neuroscience. 2018;393:185–195. doi: 10.1016/j.neuroscience.2018.07.034. [DOI] [PubMed] [Google Scholar]
- 105.Kovacs GG, Lee VM, Trojanowski JQ. Trojanowski, protein astrogliopathies in human neurodegenerative diseases and aging. Brain Pathol. 2017;27:675–690. doi: 10.1111/bpa.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kraft AW, Hu X, Yoon H, Yan P, Xiao Q, Wang Y, Gil SC, Brown J, Wilhelmsson U, Restivo JL, Cirrito JR, Holtzman DM, Kim J, Pekny M, Lee JM. Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice. FASEB J. 2013;27:187–198. doi: 10.1096/fj.12-208660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kucheryavykh YV, Kucheryavykh LY, Nichols CG, Maldonado HM, Baksi K, Reichenbach A, Skatchkov SN, Eaton MJ. Downregulation of Kir4, 1 inward rectifying potassium channel subunits by RNAi impairs potassium transfer and glutamate uptake by cultured cortical astrocytes. Glia. 2007;55:274–281. doi: 10.1002/glia.20455. [DOI] [PubMed] [Google Scholar]
- 108.Kumaran R, Kingsbury A, Coulter I, Lashley T, Williams D, de Silva R, Mann D, Revesz T, Lees A, Bandopadhyay R. DJ-1 (PARK7) is associated with 3R and 4R tau neuronal and glial inclusions in neurodegenerative disorders. Neurobiol Dis. 2007;28:122–132. doi: 10.1016/j.nbd.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 109.Lan YL, Zou S, Chen JJ, Zhao J, Li S. The neuroprotective effect of the association of aquaporin-4/glutamate transporter-1 against alzheimer’s disease. Neural Plast. 2016;2016:4626593. doi: 10.1155/2016/4626593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Larsen NJ, Ambrosi G, Mullett SJ, Berman SB, Hinkle DA. DJ-1 knock-down impairs astrocyte mitochondrial function. Neuroscience. 2011;196:251–264. doi: 10.1016/j.neuroscience.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lattke M, Reichel SN, Baumann B. NF-kappaB-mediated astrocyte dysfunction initiates neurodegeneration. Oncotarget. 2017;8:50329–50330. doi: 10.18632/oncotarget.18320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Le R, Esquenazi S. Astrocytes mediate cerebral cortical neuronal axon and dendrite growth, in part, by release of fibroblast growth factor. Neurol Res. 2002;24:81–92. doi: 10.1179/016164102101199459. [DOI] [PubMed] [Google Scholar]
- 113.Lee C, Low CY, Wong SY, Lai MK, Tan MG. Selective induction of alternatively spliced FynT isoform by TNF facilitates persistent inflammatory responses in astrocytes. Sci Rep. 2017;7:43651. doi: 10.1038/srep43651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee HJ, Kim C, Lee SJ. Alpha-synuclein stimulation of astrocytes: Potential role for neuroinflammation and neuroprotection. Oxid Med Cell Longev. 2010a;3:283–287. doi: 10.4161/oxim.3.4.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E, Lee SJ. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010b;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lian H, Litvinchuk A, Chiang AC, Aithmitti N, Jankowsky JL, Zheng H. Astrocyte-microglia cross talk through complement activation modulates amyloid pathology in mouse models of Alzheimer’s disease. J Neurosci. 2016;36:577–589. doi: 10.1523/JNEUROSCI.2117-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lim S, Kim HJ, Kim DK, Lee SJ. Non-cell-autonomous actions of alpha-synuclein: Implications in glial synucleinopathies. Prog Neurobiol. 2018;169:158–171. doi: 10.1016/j.pneurobio.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 118.Lin J, Zhu Z, Xiao H, Wakefield MR, Ding VA, Bai Q, Fang Y. The role of IL-7 in Immunity and Cancer. Anticancer Res. 2017;37:963–967. doi: 10.21873/anticanres.11405. [DOI] [PubMed] [Google Scholar]
- 119.Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu HT, Tashmukhamedov BA, Inoue H, Okada Y, Sabirov RZ. Roles of two types of anion channels in glutamate release from mouse astrocytes under ischemic or osmotic stress. Glia. 2006;54:343–357. doi: 10.1002/glia.20400. [DOI] [PubMed] [Google Scholar]
- 121.Liu L, Zhang K, Sandoval H, Yamamoto S, Jaiswal M, Sanz E, Li Z, Hui J, Graham BH, Quintana A, Bellen HJ. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell. 2015;160:177–190. doi: 10.1016/j.cell.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Livne-Bar I, Wei J, Liu HH, Alqawlaq S, Won GJ, Tuccitto A, Gronert K, Flanagan JG, Sivak JM. Astrocyte-derived lipoxins A4 and B4 promote neuroprotection from acute and chronic injury. J Clin Invest. 2017;127:4403–4414. doi: 10.1172/JCI77398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Louis Jeune V, Joergensen JA, Hajjar RJ, Weber T. Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum Gene Ther Methods. 2013;24:59–67. doi: 10.1089/hgtb.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH, Han X, Takano T, Wang S, Sim FJ, Goldman SA, Nedergaard M. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci. 2007;27:12255–12266. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits: a decade of progress. Neuron. 2018;98:865. doi: 10.1016/j.neuron.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Magnusson JP, Göritz C, Tatarishvili J, Dias DO, Smith EM, Lindvall O, Kokaia Z, Frisén J. A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science. 2014;346:237–241. doi: 10.1126/science.346.6206.237. [DOI] [PubMed] [Google Scholar]
- 127.Marks WJ, Jr, Bartus RT, Siffert J, Davis CS, Lozano A, Boulis N, Vitek J, Stacy M, Turner D, Verhagen L, Bakay R, Watts R, Guthrie B, Jankovic J, Simpson R, Tagliati M, Alterman R, Stern M, Baltuch G, Starr PA, et al. Gene delivery of AAV2-neurturin for Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9:1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- 128.Marks WJ, Jr, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, Taylor R, Cahn-Weiner DA, Stoessl AJ, Olanow CW, Bartus RT. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet Neurol. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- 129.Martin-Fernandez M, Jamison S, Robin LM, Zhao Z, Martin ED, Aguilar J, Benneyworth MA, Marsicano G, Araque A. Synapse-specific astrocyte gating of amygdala-related behavior. Nat Neurosci. 2017;20:1540–1548. doi: 10.1038/nn.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mayo L, Trauger SA, Blain M, Nadeau M, Patel B, Alvarez JI, Mascanfroni ID, Yeste A1, Kivisäkk P, Kallas K, Ellezam B, Bakshi R, Prat A, Antel JP, Weiner HL, Quintana FJ. Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat Med. 2014;20:1147–1156. doi: 10.1038/nm.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Meunier C, Merienne N, Jollé C, Déglon N, Pellerin L. Astrocytes are key but indirect contributors to the development of the symptomatology and pathophysiology of Huntington’s disease. Glia. 2016;64:1841–1856. doi: 10.1002/glia.23022. [DOI] [PubMed] [Google Scholar]
- 132.Michelucci A, Bithell A, Burney MJ, Johnston CE, Wong KY, Teng SW, Desai J, Gumbleton N, Anderson G, Stanton LW, Williams BP, Buckley NJ. The neurogenic potential of astrocytes is regulated by inflammatory signals. Mol Neurobiol. 2016;53:3724–3739. doi: 10.1007/s12035-015-9296-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mitra S, Ghosh N, Sinha P, Chakrabarti N, Bhattacharyya A. Alteration of nuclear factor-kappaB pathway promote neuroinflammation depending on the functions of estrogen receptors in substantia nigra after 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine treatment. Neurosci Lett. 2016;616:86–92. doi: 10.1016/j.neulet.2016.01.046. [DOI] [PubMed] [Google Scholar]
- 134.Miyazaki I, Asanuma M. Serotonin 1A receptors on astrocytes as a potential target for the treatment of Parkinson’s disease. Curr Med Chem. 2016;23:686–700. doi: 10.2174/0929867323666160122115057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Miyazaki I, Asanuma M. Asanuma, therapeutic strategy of targeting astrocytes for neuroprotection in Parkinson’s disease. Curr Pharm Des. 2017;23:4936–4947. doi: 10.2174/1381612823666170710163731. [DOI] [PubMed] [Google Scholar]
- 136.Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Naso MF, Tomkowicz B, Perry WL, Strohl WR. Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs. 2017;31:317–334. doi: 10.1007/s40259-017-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Navarrete M, Díez A, Araque A. Astrocytes in endocannabinoid signalling. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130599. doi: 10.1098/rstb.2013.0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Navarrete M, Perea G, Fernandez de Sevilla D, Gómez-Gonzalo M, Núñez A, Martín ED, Araque A. Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS Biol. 2012;10:e1001259. doi: 10.1371/journal.pbio.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Neal M, Richardson JR. Epigenetic regulation of astrocyte function in neuroinflammation and neurodegeneration. Biochim Biophys Acta Mol Basis Dis. 2018;1864:432–443. doi: 10.1016/j.bbadis.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nomura T, Göritz C, Catchpole T, Henkemeyer M, Frisén J. EphB signaling controls lineage plasticity of adult neural stem cell niche cells. Cell Stem Cell. 2010;7:730–743. doi: 10.1016/j.stem.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nonnenmacher M, Weber T. Intracellular transport of recombinant adeno-associated virus vectors. Gene Ther. 2012;19:649–658. doi: 10.1038/gt.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Norden DM, Fenn AM, Dugan A, Godbout JP. TGFbeta produced by IL-10 redirected astrocytes attenuates microglial activation. Glia. 2014;62:881–895. doi: 10.1002/glia.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Octeau JC, Chai H, Jiang R, Bonanno SL, Martin KC, Khakh BS. An optical neuron-astrocyte proximity assay at synaptic distance scales. Neuron. 2018;98:49–66 e9. doi: 10.1016/j.neuron.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Oswald M, Geissler S, Goepferich A. Targeting the central nervous system (CNS): a review of rabies virus-targeting strategies. Mol Pharm. 2017;14:2177–2196. doi: 10.1021/acs.molpharmaceut.7b00158. [DOI] [PubMed] [Google Scholar]
- 146.Palmer AL, Ousman SS. Astrocytes and aging. Front Aging Neurosci. 2018;10:337. doi: 10.3389/fnagi.2018.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Panicker N, Saminathan H, Jin H, Neal M, Harischandra DS, Gordon R, Kanthasamy K, Lawana V, Sarkar S, Luo J, Anantharam V, Kanthasamy AG, Kanthasamy A. Fyn kinase regulates microglial neuroinflammatory responses in cell culture and animal models of parkinson’s disease. J Neurosci. 2015;35:10058–10077. doi: 10.1523/JNEUROSCI.0302-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Park F. Lentiviral vectors: are they the future of animal transgenesis? Physiol Genomics. 2007;31:159–173. doi: 10.1152/physiolgenomics.00069.2007. [DOI] [PubMed] [Google Scholar]
- 149.Pehar M, Cassina P, Vargas MR, Castellanos R, Viera L, Beckman JS, Estévez AG, Barbeito L. Astrocytic production of nerve growth factor in motor neuron apoptosis: implications for amyotrophic lateral sclerosis. J Neurochem. 2004;89:464–473. doi: 10.1111/j.1471-4159.2004.02357.x. [DOI] [PubMed] [Google Scholar]
- 150.Pehar M, Harlan BA, Killoy KM, Vargas MR. Role and therapeutic potential of astrocytes in amyotrophic lateral sclerosis. Curr Pharm Des. 2017;23:5010–5021. doi: 10.2174/1381612823666170622095802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pekny M, Wilhelmsson U, Bogestål YR, Pekna M. The role of astrocytes and complement system in neural plasticity. Int Rev Neurobiol. 2007;82:95–111. doi: 10.1016/S0074-7742(07)82005-8. [DOI] [PubMed] [Google Scholar]
- 152.Pekny M, Wilhelmsson U, Pekna M. The dual role of astrocyte activation and reactive gliosis. Neurosci Lett. 2014;565:30–38. doi: 10.1016/j.neulet.2013.12.071. [DOI] [PubMed] [Google Scholar]
- 153.Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317:1083–1086. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- 154.Perriot S, Mathias A, Perriard G, Canales M, Jonkmans N, Merienne N, Meunier C, El Kassar L, Perrier AL, Laplaud DA, Schluep M, Déglon N, Du Pasquier R. Human induced pluripotent stem cell-derived astrocytes are differentially activated by multiple sclerosis-associated cytokines. Stem Cell Reports. 2018;11:1199–1210. doi: 10.1016/j.stemcr.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pignataro D, Sucunza D, Vanrell L, Lopez-Franco E, Dopeso-Reyes IG, Vales A, Hommel M, Rico AJ, Lanciego JL, Gonzalez-Aseguinolaza G. Adeno-associated viral vectors serotype 8 for cell-specific delivery of therapeutic genes in the central nervous system. Front Neuroanat. 2017;11:2. doi: 10.3389/fnana.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ponath G, Ramanan S, Mubarak M, Housley W, Lee S, Sahinkaya FR, Vortmeyer A, Raine CS, Pitt D. Myelin phagocytosis by astrocytes after myelin damage promotes lesion pathology. Brain. 2017;140:399–413. doi: 10.1093/brain/aww298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Prasad H, Rao R. Amyloid clearance defect in ApoE4 astrocytes is reversed by epigenetic correction of endosomal pH. Proc Natl Acad Sci U S A. 2018;115:E6640–6649. doi: 10.1073/pnas.1801612115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rakic S, Hung YMA, Smith M, So D, Tayler HM, Varney W, Wild J, Harris S, Holmes C, Love S, Stewart W, Nicoll JAR, Boche D. Systemic infection modifies the neuroinflammatory response in late stage Alzheimer’s disease. Acta Neuropathol Commun. 2018;6:88. doi: 10.1186/s40478-018-0592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ramamoorthy P, Xu G, Shi H. Expression of hypoxia inducible factor 1alpha is protein kinase A-dependent in primary cortical astrocytes exposed to severe hypoxia. Neurochem Res. 2019;44:258–268. doi: 10.1007/s11064-018-2516-9. [DOI] [PubMed] [Google Scholar]
- 160.Ranno E, D’Antoni S, Spatuzza M, Berretta A, Laureanti F, Bonaccorso CM, Pellitteri R, Longone P, Spalloni A, Iyer AM, Aronica E, Catania MV. Endothelin-1 is over-expressed in amyotrophic lateral sclerosis and induces motor neuron cell death. Neurobiol Dis. 2014;65:160–171. doi: 10.1016/j.nbd.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 161.Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao CC, Patel B, Yan R, Blain M, Alvarez JI, Kébir H, Anandasabapathy N, Izquierdo G6, Jung S, Obholzer N, Pochet N, Clish CB, Prinz M, Prat A, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22:586–597. doi: 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Saha P, Biswas SC. Amyloid-beta induced astrocytosis and astrocyte death: Implication of FoxO3a-Bim-caspase3 death signaling. Mol Cell Neurosci. 2015;68:203–211. doi: 10.1016/j.mcn.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 163.Sahin-Calapoglu N, Demirci S, Calapoglu M, Yasar B. A case-control sssociation dtudy of RANTES (-28C>G) polymorphism as a risk factor for Parkinson’s disease in Isparta, Turkey. Parkinsons Dis. 2016;2016:5042604. doi: 10.1155/2016/5042604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sankar SB, Donegan RK, Shah KJ, Reddi AR, Wood LB. Heme and hemoglobin suppress amyloid beta-mediated inflammatory activation of mouse astrocytes. J Biol Chem. 2018;293:11358–11373. doi: 10.1074/jbc.RA117.001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Santello M, Bezzi P, Volterra A. TNFalpha controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron. 2011;69:988–1001. doi: 10.1016/j.neuron.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 166.Savarin C, Hinton DR, Valentin-Torres A, Chen Z, Trapp BD, Bergmann CC, Stohlman SA. Astrocyte response to IFN-gamma limits IL-6-mediated microglia activation and progressive autoimmune encephalomyelitis. J Neuroinflammation. 2015;12:79. doi: 10.1186/s12974-015-0293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Serrano-Pozo A, Mielke ML, Gómez-Isla T, Betensky RA, Growdon JH, Frosch MP, Hyman BT. Reactive glia not only associates with plaques but also parallels tangles in Alzheimer’s disease. Am J Pathol. 2011;179:1373–1384. doi: 10.1016/j.ajpath.2011.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shigetomi E, Kracun S, Sofroniew MV, Khakh BS. A genetically targeted optical sensor to monitor calcium signals in astrocyte processes. Nat Neurosci. 2010;13:759–766. doi: 10.1038/nn.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shigetomi E, Patel S, Khakh BS. Khakh, probing the complexities of astrocyte calcium signaling. Trends Cell Biol. 2016;26:300–312. doi: 10.1016/j.tcb.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Srinivasan R, Huang BS, Venugopal S, Johnston AD, Chai H, Zeng H, Golshani P, Khakh BS. Ca(2+) signaling in astrocytes from Ip3r2(-/-) mice in brain slices and during startle responses in vivo. Nat Neurosci. 2015;18:708–717. doi: 10.1038/nn.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Srinivasan R, Lu TY, Chai H, Xu J, Huang BS, Golshani P, Coppola G, Khakh BS. New Transgenic Mouse Lines for Selectively targeting astrocytes and studying calcium signals in astrocyte processes in situ and in vivo. Neuron. 2016;92:1181–1195. doi: 10.1016/j.neuron.2016.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Steele ML, Robinson SR. Reactive astrocytes give neurons less support: implications for Alzheimer’s disease. Neurobiol Aging. 2012;33:423. doi: 10.1016/j.neurobiolaging.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 174.Sticozzi C, Belmonte G, Meini A, Carbotti P, Grasso G, Palmi M. IL-1beta induces GFAP expression in vitro and in vivo and protects neurons from traumatic injury-associated apoptosis in rat brain striatum via NFkappaB/Ca(2)(+)-calmodulin/ERK mitogen-activated protein kinase signaling pathway. Neuroscience. 2013;252:367–383. doi: 10.1016/j.neuroscience.2013.07.061. [DOI] [PubMed] [Google Scholar]
- 175.Stobart JL, Ferrari KD, Barrett MJP, Stobart MJ, Looser ZJ, Saab AS, Weber B. Long-term in vivo calcium imaging of astrocytes reveals distinct cellular compartment responses to sensory stimulation. Cereb Cortex. 2018;28:184–198. doi: 10.1093/cercor/bhw366. [DOI] [PubMed] [Google Scholar]
- 176.Subramaniam SR, Federoff HJ. Targeting microglial activation states as a therapeutic avenue in Parkinson’s disease. Front Aging Neurosci. 2017;9:176. doi: 10.3389/fnagi.2017.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sudo K, Takezawa Y, Kohsaka S, Nakajima K. Involvement of nitric oxide in the induction of interleukin-1 beta in microglia. Brain Res. 2015;1625:121–134. doi: 10.1016/j.brainres.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 178.Sun L, Shen R, Agnihotri SK1, Chen Y, Huang Z, Büeler H. Lack of PINK1 alters glia innate immune responses and enhances inflammation- induced, nitric oxide-mediated neuron death. Sci Rep. 2018;8:383. doi: 10.1038/s41598-017-18786-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tarassishin L, Loudig O, Bauman A, Shafit-Zagardo B, Suh HS, Lee SC. Interferon regulatory factor 3 inhibits astrocyte inflammatory gene expression through suppression of the proinflammatory miR-155 and miR-155*. Glia. 2011;59:1911–1922. doi: 10.1002/glia.21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Taylor JM, Moore Z, Minter MR, Crack PJ. Type-I interferon pathway in neuroinflammation and neurodegeneration: focus on Alzheimer’s disease. J Neural Transm (Vienna) 2018;125:797–807. doi: 10.1007/s00702-017-1745-4. [DOI] [PubMed] [Google Scholar]
- 181.Tong X, Ao Y, Faas GC, Nwaobi SE, Xu J, Haustein MD, Anderson MA6, Mody I, Olsen ML, Sofroniew MV, Khakh BS. Astrocyte Kir4, 1 ion channel deficits contribute to neuronal dysfunction in Huntington’s disease model mice. Nat Neurosci. 2014;17:694–703. doi: 10.1038/nn.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, Tenney A, Murnen AT, Fancy SP, Merkle F, Kessaris N, Alvarez-Buylla A, Richardson WD, Rowitch DH. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337:358–362. doi: 10.1126/science.1222381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tukhovskaya EA, Turovsky EA, Turovskaya MV, Levin SG, Murashev AN, Zinchenko VP, Godukhin OV. Anti-inflammatory cytokine interleukin-10 increases resistance to brain ischemia through modulation of ischemia-induced intracellular Ca(2)(+) response. Neurosci Lett. 2014;571:55–60. doi: 10.1016/j.neulet.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 184.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, González-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 185.Vanderheyden WM, Lim MM, Musiek ES, Gerstner JR. Alzheimer’s disease and sleep-wake disturbances: amyloid, astrocytes and animal models. J Neurosci. 2018;38:2901–2910. doi: 10.1523/JNEUROSCI.1135-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Vartak-Sharma N, Gelman BB, Joshi C, Borgamann K, Ghorpade A. Astrocyte elevated gene-1 is a novel modulator of HIV-1-associated neuroinflammation via regulation of nuclear factor-kappaB signaling and excitatory amino acid transporter-2 repression. J Biol Chem. 2014;289:19599–19612. doi: 10.1074/jbc.M114.567644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Vasile F, Dossi E, Rouach N. Human astrocytes: structure and functions in the healthy brain. Brain Struct Funct. 2017;222:2017–2029. doi: 10.1007/s00429-017-1383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang T, Takikawa Y, Sawara K, Yoshida Y, Suzuki K. Negative regulation of human astrocytes by interferon (IFN) alpha in relation to growth inhibition and impaired glucose utilization. Neurochem Res. 2012;37:1898–1905. doi: 10.1007/s11064-012-0806-1. [DOI] [PubMed] [Google Scholar]
- 189.Wang Y, Chen Y, Zhou Q, Xu J, Qian Q, Ni P, Qian Y. Mild endoplasmic reticulum stress protects against lipopolysaccharide-induced astrocytic activation and blood-brain barrier hyperpermeability. Front Cell Neurosci. 2018;12:222. doi: 10.3389/fncel.2018.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wilhelmsson U, Li L, Pekna M, Berthold CH, Blom S, Eliasson C, Renner O, Bushong E, Ellisman M, Morgan TE, Pekny M. Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J Neurosci. 2004;24:5016–5021. doi: 10.1523/JNEUROSCI.0820-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wilhelmus MM, van der Pol SM, Jansen Q, Witte ME, van der Valk P, Rozemuller AJ, Drukarch B, de Vries HE, Van Horssen J. Association of Parkinson disease-related protein PINK1 with Alzheimer disease and multiple sclerosis brain lesions. Free Radic Biol Med. 2011;50:469–476. doi: 10.1016/j.freeradbiomed.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 192.Wood TE, Barry J, Yang Z, Cepeda C, Levine MS, Gray M. Mutant huntingtin reduction in astrocytes slows disease progression in the bachd conditional huntington’s disease mouse model. Hum Mol Genet. 2019;28:487–500. doi: 10.1093/hmg/ddy363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wyss-Coray T, Lin C, Sanan DA, Mucke L, Masliah E. Chronic overproduction of transforming growth factor-beta1 by astrocytes promotes Alzheimer’s disease-like microvascular degeneration in transgenic mice. Am J Pathol. 2000;156:139–150. doi: 10.1016/s0002-9440(10)64713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xia Y, Zhai Q. IL-1beta enhances the antibacterial activity of astrocytes by activation of NF-kappaB. Glia. 2010;58:244–252. doi: 10.1002/glia.20921. [DOI] [PubMed] [Google Scholar]
- 195.Xie L, Yang SH. Interaction of astrocytes and T cells in physiological and pathological conditions. Brain Res. 2015;1623:63–73. doi: 10.1016/j.brainres.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yamamoto M, Kiyota T, Horiba M, Buescher JL, Walsh SM, Gendelman HE, Ikezu T. Interferon-gamma and tumor necrosis factor-alpha regulate amyloid-beta plaque deposition and beta-secretase expression in Swedish mutant APP transgenic mice. Am J Pathol. 2007;170:680–692. doi: 10.2353/ajpath.2007.060378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yang CM, Hsieh HL, Yu PH, Lin CC, Liu SW. IL-1beta Induces MMP-9-dependent brain astrocytic migration via transactivation of PDGF receptor/NADPH oxidase 2-derived reactive oxygen species signals. Mol Neurobiol. 2015;52:303–317. doi: 10.1007/s12035-014-8838-y. [DOI] [PubMed] [Google Scholar]
- 199.Yin X, Wang S, Qi Y, Wang X, Jiang H, Wang T, Yang Y, Wang Y, Zhang C, Feng H. Astrocyte elevated gene-1 is a novel regulator of astrogliosis and excitatory amino acid transporter-2 via interplaying with nuclear factor-kappaB signaling in astrocytes from amyotrophic lateral sclerosis mouse model with hSOD1(G93A) mutation. Mol Cell Neurosci. 2018;90:1–11. doi: 10.1016/j.mcn.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 200.Yu X, Taylor AMW, Nagai J, Golshani P, Evans CJ, Coppola G, Khakh BS. Reducing astrocyte calcium signaling in vivo alters striatal microcircuits and causes repetitive behavior. Neuron. 2018;99:1170–1187e9. doi: 10.1016/j.neuron.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yun SP, Kam TI, Panicker N, Kim S, Oh Y, Park JS, Kwon SH, Park YJ, Karuppagounder SS, Park H, Kim S, Oh N, Kim NA, Lee S, Brahmachari S, Mao X, Lee JH, Kumar M, An D, Kang SU, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med. 2018;24:931–938. doi: 10.1038/s41591-018-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhang X, Huang WJ, Chen WW. TGF-beta1 factor in the cerebrovascular diseases of Alzheimer’s disease. Eur Rev Med Pharmacol Sci. 2016;20:5178–5185. [PubMed] [Google Scholar]
- 203.Zhao J, O’Connor T, Vassar R. The contribution of activated astrocytes to Abeta production: implications for Alzheimer’s disease pathogenesis. J Neuroinflammation. 2011;8:150. doi: 10.1186/1742-2094-8-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhao T, Hong Y, Yin P, Li S, Li XJ. Differential HspBP1 expression accounts for the greater vulnerability of neurons than astrocytes to misfolded proteins. Proc Natl Acad Sci U S A. 2017;114:E7803–7811. doi: 10.1073/pnas.1710549114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhou M, Schools GP, Kimelberg HK. Development of GLAST(+) astrocytes and NG2(+) glia in rat hippocampus CA1: mature astrocytes are electrophysiologically passive. J Neurophysiol. 2006;95:134–143. doi: 10.1152/jn.00570.2005. [DOI] [PubMed] [Google Scholar]
- 206.Zingg B, Chou XL, Zhang ZG, Mesik L, Liang F, Tao HW, Zhang L. AAV-mediated anterograde transsynaptic tagging: mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron. 2017;93:33–47. doi: 10.1016/j.neuron.2016.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]


