Abstract
Peripheral nerve injury leads to morphological, molecular and gene expression changes in the spinal cord and dorsal root ganglia, some of which have positive impact on the survival of neurons and nerve regeneration, while the effect of others is the opposite. It is crucial to take prompt measures to capitalize on the positive effects of these reactions and counteract the negative impact after peripheral nerve injury at the level of spinal cord, especially for peripheral nerve injuries that are severe, located close to the cell body, involve long distance for axons to regrow and happen in immature individuals. Early nerve repair, exogenous supply of neurotrophic factors and Schwann cells can sustain the regeneration inductive environment and enhance the positive changes in neurons. Administration of neurotrophic factors, acetyl-L-carnitine, N-acetyl-cysteine, and N-methyl-D-aspartate receptor antagonist MK-801 can help counteract axotomy-induced neuronal loss and promote regeneration, which are all time-dependent. Sustaining and reactivation of Schwann cells after denervation provides another effective strategy. FK506 can be used to accelerate axonal regeneration of neurons, especially after chronic axotomy. Exploring the axotomy-induced changes after peripheral nerve injury and applying protective and promotional measures in the spinal cord which help to retain a positive functional status for neuron cell bodies will inevitably benefit regeneration of the peripheral nerve and improve functional outcomes.
Keywords: axotomy, dorsal root ganglion, neural regeneration, neurotrophic factors, outcomes, peripheral nerve injury, repair, spinal cord
Introduction
Peripheral nerve injuries (PNI) cause partial or complete motor, sensory and autonomic function disabilities in patients and impose enormous socioeconomic burden. The incidence of PNI is steadily growing over the last few decades, accounting for several hundred thousand cases each year around the world. Despite the development and advances in treatment modalities, the functional clinical outcomes often remain unsatisfactory. Most of the research in nerve regeneration so far has focused on strategies that are directed at peripheral nerves. Compared to the plethora of studies concerning the repair methods at the peripheral nerve injury site, there are few studies on the changes in and strategies directed at the spinal cord after PNI. Given that cell bodies of the peripheral nerves reside in the spinal cord and dorsal root ganglia (DRG), and the integral relationship between the peripheral nerves and the spinal cord, more emphasis should be given to research in this direction.
The phenotype of the spinal cord neurons is regulated by an intrinsic genetic program, extrinsic environmental signals and specically targeted molecular signals. PNI trigger a phenotype switch to a state required for axonal re-growth. Previous studies reported that in the cases with spinal cord lesions whose peripheral nerves were damaged 1 week earlier, the corresponding central axons in the dorsal roots of primary sensory neurons could be enhanced to regenerate (Richardson et al., 1987). Although the underlying mechanisms of this phenomenon are not fully understood, the increase in the level of intracellular cyclic adenosine monophosphate (Neumann et al., 2002), upregulation of the growth associated protein 43 (Neumann et al., 1999), increased expression of endogenous brain-derived neurotrophic factor (BDNF) in the sensory neurons induced by axotomy (Song et al., 2008), and manipulation of multiple pathways providing additive or synergistic effects on axon elongation (Norsworthy et al., 2017) may have all played a role in the strengthened regeneration of ascending sensory neurons. Taking advantage of the central changes induced by PNI to enhance spinal cord regeneration and promote functional recovery after the spinal cord lesion is a new potential therapeutic direction. In the opposite direction, exploring axotomy-induced changes in the spinal cord after PNI and applying protective and promotional measures aiming at the spinal cord to retain a positive functional status for neuron cell bodies will likely benefit regeneration of the peripheral nerve and accelerate the improvements in functional outcomes.
This article reviews recent findings related to studying PNI induced changes in the spinal cord. Of major interest is the possibility of therapeutically influencing the changes in spinal cord to promote peripheral nerve regeneration and improve functional outcomes. The progress in this direction is focused on what happens after PNI and how we can counteract the detrimental changes in the spinal cord or capitalize on the beneficial ones. Although the changes are different in their locations and extent and depend on many influencing factors such as the types of peripheral nerves, severity of the injury, and age of the patient, here we summarize the changes that are categorized as promoting or inhibiting nerve regeneration, followed by corresponding therapeutic strategies to enhance/counteract them.
Search Strategy and Selection Criteria
The articles reviewed in this manuscript were retrieved by electronic search in the Medline database in the time frame from 1970 to April 2019 for literatures focused on neuron regeneration in the dorsal root ganglia or spinal cord following peripheral nerve injuries in rodents and humans. The following terms were searched: “peripheral nerve injury” AND “neuron regeneration”. Additionally, the following keywords were used to retrieve further literature: “dorsal root ganglion”, “spinal cord”, “neurotrophic factors”, “axotomy”, “apoptosis”, “Schwann cells”. All the search results were manually screened for relevance by reading the titles and abstracts.
Changes in the Spinal Cord after Peripheral Nerve Injury
Post-injury, the axons distal to the site of lesion lose their connection with neuronal bodies located in the spinal cord or DRG and begin to degenerate. The body of axotomized neurons is inevitably induced to undergo a series of functional and structural alterations (cell body reaction). Overall, the initial neuronal reaction is an adaptive change to survive and compensate for the axon loss and the broken connection between the central nervous system (CNS) and the denervated targets.
Changes inductive for nerve regeneration
After PNI, surviving neurons are triggered to establish a switch program at the cellular and molecular levels in order to achieve axonal elongation and reinnervation of the target tissue. Switching from a ‘‘transmitting’’ to a ‘‘regenerative’’ state is necessary for survival and axonal regeneration (Fu and Gordon, 1997; Yu et al., 2013), which includes morphological changes, up-regulation and down-regulation of numerous cellular factors, as well as de novo synthesis of some molecules that are not normally produced by the adult neurons.
Morphological changes
As early as few hours after injury, a series of morphological alterations take place in the neurons, including dissolution of the Nissl bodies (chromatolysis), nuclear and nucleolar enlargement, nuclear eccentricity, swelling of the cells and retraction of dendrites (Sun et al., 2018). Among them, chromatolysis is the most characterized and the earliest change observed after axotomy. It is shown to be associated with anabolic responses in the injured neuron soma, as evident from the fact that disorganization of the clusters of ribosomes is linked to the increase in protein synthesis (Fu and Gordon, 1997), cellular protein content, elevated level of RNA synthesis, transfer of RNA to the cytoplasm, and reduced DNA repression (Watson, 1974).
Simultaneously with the neuronal body reaction, there is a brisk proliferative response in perineuronal glial cells, which is characterized by the dendritic tree retraction and a reduction in the number of synapses. Most likely this response is by the process of chromatolysis, i.e. morphological changes leading to functional isolation of the injured, nonfunctional neurons from the rest of neural circuits. Several days after axotomy, the number of synaptic terminals and the area of their coverage in chromatolyzed motoneurons get reduced. This is followed by further decrease by one third at 1–3 months post-axotomy.
The time course and intensity of the neuronal response is affected by several factors, such as severity of the injury, age of the patient, proximity of injury to the cell body, the neuron type, and whether the interaction of CNS with the target is restored. Chromatolysis can start as soon as 8 hours following sciatic nerve transection (Groves et al., 1999). After severance of the sciatic nerve in rats, restoration of normal morphological properties along with axon regeneration takes 5 months. The reaction is more intense and lasts longer after axotomy without reinnervation, as compared to axotomy with reinnervation (Guntinas-Lichius et al.,1996). The reaction is more pronounced in sensory neurons compared to motoneurons (Valero-Cabré et al., 2001), in small sensory neurons compared to large ones (Arvidsson et al., 1986), after avulsion or transection in ventral roots compared to sciatic nerve injury (Hoang et al., 2003). Moreover, neurons in adults are less susceptible to this reaction compared to neurons in young individuals (Snider et al., 1992).
Changes in molecular and gene expression
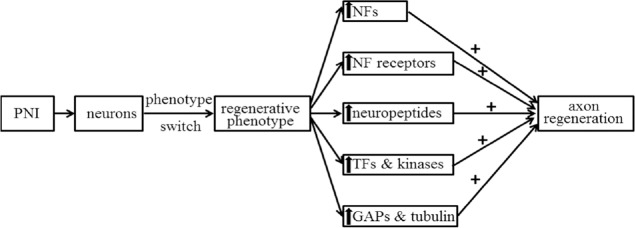
After PNI, injury-induced electrical excitation signals (first set of signals) and subsequent signals are conveyed from the lesioned axons and non-neuronal cells retrogradely to their own injured neuronal body. Hundreds of molecular responses in the spinal cord have been identified post-axotomy. Some of these responses are up-regulated, including neurotrophic factors (NGF, BDNF, etc.) and neurotrophic receptors (Ret, Trk, etc.) (Table 1), growth associated proteins (growth associated protein 43 and others) (Dubovy et al., 2019), and tubulin (Smriti et al., 2012), neuropeptides (VIP, NPY, CGRP, etc.) (Table 2), transcription factors (c-fos, c-jun, NFkB, ATF3, STAT, CREB) (Qin wt al., 2018) and relevant kinases, and some ion channels (Kang et al., 2018) in the axotomized neurons of the spinal cord and DRG. These up-regulations may contribute to the creation of more supportive phenotype that directly or indirectly affects neuronal survival and growth (Figure 1). Some other responses are down-regulated, including a number of neurotransmitters, transmitter-related proteins, postsynaptic receptors, neurofilaments, and proteins involved in the molecular apparatus of neurotransmission (Jankowski et al., 2009). These signals do not have a direct effect on nerve regeneration. The molecular responses are accompanied by gene expression changes in the injured and regenerating neurons induced by the PNI signals. Transcription factors regulate gene transcription, playing a critical role in multiple biological processes (Palazon et al., 2014; Bhagwat and Vakoc, 2015). Many of these transcription factors have been identified using the RNA sequencing technique (Gong et al., 2016; Wu et al., 2016; Qin et al., 2018).
Table 1.
Summary of increased expression of neurotrophic factors/receptors in axotomized neurons after PNI
| Neurotrophic factors/receptors | Mechanism of injury | Location of measurement | References |
|---|---|---|---|
| GFRα1 (GDNF receptor) | SN transection | DRG | Keast et al., 2010 |
| FN transection/crush | MN | Burazin and Gundlach, 1998 | |
| SN transection in adult/neonatal rats and avulsion in adult rats | VH | Hammaberg et al., 2000 | |
| GFRα3 | SN transection | DRG | Keast et al., 2010 |
| Ret (GDNF receptor) | SN crush | DRG | Naveilhan et al., 1997 |
| SN transection in adult/neonatal rats and avulsion in adult rats | VH | Hammaberg et al., 2000 | |
| BDNF | SN transection | DRG | Tonra et al., 1998 |
| SN crush | DRG | Tonra et al., 1998 | |
| SN CCI | DRG | Obata et al., 2003 | |
| Spinal nerve ligation | DRG | Fukuoka et al., 2001 | |
| Ventral root avulsion/SN transection | VH | Hammaberg et al., 2000 | |
| SN transection | MN | Gu et al., 1997 | |
| NGF | SN crush | DRG | Sebert et al., 1993 |
| Spinal nerve transection | DRG | Zhou et al., 1999 | |
| Spinal nerve ligation | DRG | Shen et al., 1999 | |
| NT-3 | Spinal nerve transection | DRG | Zhou et al., 1999 |
| SN transection in adult/neonatal rats and avulsion in adult rats | VH | Hammaberg et al., 2000 | |
| TrkB (BDNF and NT-4/5 receptor) | SN crush/dorsal root crush | DRG | Ernfors et al., 1993 |
| SN transection in adult/neonatal rats and avulsion in adult rats | VH | Hammaberg et al., 2000 | |
| Trk C (NT-3 receptor) | SN crush/dorsal root crush | DRG | Ernfors et al., 1993 |
| SN transection in adult/neonatal rats | VH | Hammaberg et al., 2000 | |
| LNR (p75) | median and ulnar nerve transection | DRG/DH | Murray et al., 2003 |
| SN crush | DRG | Ernfors et al., 1993 | |
| SN transection/spinal nerve root avulsion in aged rats | MN | Xie et al., 2003 | |
| SN crush/transection | MN | Rende et al., 1992 |
All injuries were performed in adult rats unless stated otherwise. The changes indicated are mainly based on the studies on mRNA expression or immunoreactivity. Measurements were performed in sensory neurons of the dorsal root ganglia (DRG) or motoneurons (MN) in the spinal cord. In some cases, determinations were described as expression in the dorsal horn (DH) or ventral horn (VH). Note that for each molecule, references are grouped according to the type of injury, as in some cases the expression varies depending on the injury model or cell type. BDNF: Brain derived neurotrophic factor; CCI: chronic constriction injury; FN: facial nerve; GDNF: glial cell line-derived neurotrophic factor; LNR: low-affinity NGF receptor; NGF: nerve growth factor; NT-3: neurotrophin-3; SN: sciatic nerve.
Table 2.
Increase of neuropeptides in neurons after axotomy
| Neuropeptide | Mechanism of injury | Location of measurement | References |
|---|---|---|---|
| CGRP | Spinal nerve transection | DRG | Nitzan-Luques et al., 2013 |
| SN transection | DRG | Fu et al., 2013 | |
| SN crush/transection | MN | Calderó et al., 1992 | |
| SP | Spinal nerve transection | DRG | Nitzan-Luques et al., 2013 |
| SN CCI | DRG | Da et al., 2017 | |
| SN transection | VH | Zhang et al., 1993 | |
| VIP | SN transection | DRG | Villar et al., 1989 |
| SN transection | DRG/DH | Shehab et al., 1986 | |
| SN CCI | DRG | Nahin et al., 1994 | |
| SN transection | MN | Zignond et al., 1996 | |
| SST | SN ligation and dorsal rhizotomy | DRG | Shi et al., 2014 |
| SN transection | MN | Zhang et al., 1993 | |
| GAL | SN transection | DRG/DH | Villar et al., 1989 |
| SN CCI | DRG | Nahin et al., 1994 | |
| SN transection | MN | Zhang et al., 1993 | |
| NPY | SN transection | DRG | Wakisaka et al., 1991 |
| SN transection | DH | Wakisaka et al., 1991 | |
| SN CCI | DRG | Nahin et al., 1994 | |
| SN ligation/crush | DRG | Wakisaka et al., 1991 | |
| Spinal nerve ligation | DRG | Fukuoka et al., 2015 |
All injuries were performed in adult rats. The changes indicated are mainly based on the studies of mRNA expression or immunoreactivity. Measurements were performed in sensory neurons of the dorsal root ganglia (DRG) or motoneurons (MN) in the spinal cord; in some cases, determinations were described as expression in the dorsal horn (DH) or ventral horn (VH). Note that for each molecule, the references are grouped according to the type of injury, as in some cases the expression varies depending on the injury model or cell type. CCI: Chronic constriction injury; CGRP: calcitonin gene related peptide; FN: facial nerve; GAL: galanin; NPY: neuropeptide tyrosine; SN: sciatic nerve; SP: substance P; SST: somatostatin; VIP: vasoactive intestinal peptide.
Figure 1.
Illustrative summary of changes in molecular expression in axotomized neurons of the spinal cord/ dorsal root ganglia after PNI that are conducive for nerve regeneration.
The “↑” represents increase in molecular or gene expression; “+” represents enhancement of a function. GAPs: Growth associated proteins; NFs: neurotrophic factors; PNI: peripheral nerve injuries; TFs: transcription factors.
It is generally believed that the majority of molecular responses is regulated by phenotypic changes in the injured and regenerating neurons, which down-regulate the neurotransmitters and the genes encoding the proteins related to neurotransmission, while up-regulating the proteins associated with the growth and the structural components of membrane. This implies that neurons’ reprogrammed shift from a ‘‘transmitting’’ to a ‘‘regenerative’’ state is essential and beneficial for survival and axonal regeneration.
The extent of responses to injury is variable and determined by the type of neurons, the extent of the lesion, and the distance from the lesion to the cell body of neuron. These factors are critical for cell survival and the capability of renewed axons to find appropriate targets. For example, after sciatic nerve transection, little change in Ret (Glial cell line-derived neurotrophic factor (GDNF) receptor) expression in DRG was observed, while its expression was greatly increased in motoneurons (Hammarberg et al., 2000). NPY, Somatostatin, Substance P were markedly up-regulated in small axotomized DRG neurons and motoneurons but declined in sympathetic neurons (Wakisaka et al., 1991). Similarly, following ligation of the spinal nerve where the lesion site is closer to the cell body, the level of NGF mRNA increased 4 times compared to the normal level. This elevated level was maintained for 3 weeks (Shen et al., 1999; Terada et al., 2018; Martin et al., 2019). On the other hand, after transection of the sciatic nerve where the lesion site is not as close to the cell body, the expression of NGF mRNA remained unchanged (Gu et al., 1997). Evidence suggests that the neurons’ peak of regenerative capacity occurs immediately after an injury, but the window of opportunity for axonal regeneration is narrow and limited in time. Spinal neurons that regenerate their axons and effectively reinnervate their target organs slowly return to the normal distribution, morphological and molecular properties a few months after PNI, although some long lasting morphological alterations persist. If no successful regeneration has occurred, the ‘‘regenerative’’ state of spinal neurons slowly switches off as time goes by, and the capacity for regeneration gradually declines (Fu and Gordon, 1995, 1997).
Changes detrimental to nerve regeneration
Subsequent to a peripheral nerve lesion, a broad range of events crucial for neuronal survival and regeneration is initiated in the spinal cord. However, axotomy and retrograde degeneration may also damage neuronal cell body and even result in the death of neurons by apoptosis or necrosis. Degenerative morphological changes after PNI have been shown mainly in the superficial dorsal horn (laminae I and II). They include cavitation of dendrites, formation of dark (pyknotic) nucleus, loss of γ-aminobutyric acid-containing cell profiles, TUNEL-labeled nuclei, and death of glial cells. Sometimes these changes also take place in motoneurons, especially in severe proximal or chronic injuries of the peripheral nerve. Molecular changes linked to down-regulation of some neuropeptides hinder neuron regeneration. Moreover, several studies have suggested that the increased activity of transcription factors and relevant kinases such as Erk and JUK not only benefits neuron regeneration but also mediates the death of injured neurons.
Neuronal death and apoptosis
Peripheral nerve injuries lead to partial or total loss of sensory, motor and autonomic functions conveyed by the damaged nerves to the target tissues and organs. This loss is caused by the interruption of axon continuity, degradation of nerve fibers distal to the lesion and consequent death of injured neurons. The processes involved in the death of injured neurons are still poorly understood, but it is generally agreed that it is related to the apoptosis of neuronal cell body (Martin et al., 1999). Trophic factors and their receptors, as well as retrograde neuronal injury and target deprivation may contribute to the development of abnormal neuronal death (Oliveira, 2001). A growing number of findings indicate that the death of adult motor neurons after nerve avulsion is an apoptosis induced by DNA damage and is dependent on p53 and Bax. Mitochondria have been shown to take part in the key effector stage of this process (Martin et al., 1999). After axotomy and deprivation in DRG, the death of neurons commences within 24 hours. It, however, does not lead to a substantial neuronal loss for 1 week. The neurons’ death rate peaks at 2 weeks post-injury. By this time, 21% of neurons have died. Two months after axotomy, the loss of neurons reaches a plateau at approximately 35% (McKay et al., 2002). If an early repair is achieved to obtain normal trafficking of mitochondria into axonal and dendritic compartments, the first stage will last longer, for about a few weeks to a few months (Martin et al., 1999). The fact that some timely procedures can make the changes partly reversible in the first process (Aszmann et al., 2004) suggest that effective strategies to rescue the neurons should be applied early before the second stage commences.
The progression of neuronal injury induced by axotomy and the probability of neuronal death is influenced by multiple factors, which include the neuron type, the lesion severity, the injury location in relation to neuron cell body, the continuing presence or absence of target innervation, the distance for axons to regrow, the age, and many others. Following axonotomesis, the probability of apoptosis-related cell death in DRG neurons ranges from 10 to 50%, with small neurons affected more often compared to the large ones (Arvidsson et al., 1986). Only a 0–10% loss of motoneurons was reported after sciatic nerve injury in adult rats (Valero-Cabré, et al., 2001). However, avulsion or transection of the ventral roots in adult rats results in retrograde cell death in 50–80% of motoneurons within several weeks (Hoang et al., 2003). In an immature nervous system, axotomized neurons often die rapidly. After transection of the sciatic nerve in neonatal rats, the majority of motoneuron cells die, while the rate of cell death reduces to 50% when transections are performed in 1-week-old rats, and becomes undetectable when the rats are 1 month old (Sendtner et al., 1992). The death of neurons may become more relevant if the nerve injury is chronic, with the sequential insults leading to the death of oligodendrocytes (Ma et al., 2001).
Low regeneration capacity of neurons
Although neurons, in most conditions, can escape the death, some of them will always lose a part of the capacity to regenerate (Oliveira, 2001). This loss of capacity is even more prominent in chronic axotomy. Simultaneous and sequential double retrograde labeling has shown that the number of motoneurons that regenerated their axons following a prolonged and chronic axotomy and delayed nerve repair was reduced to 66% in comparison to the regenerative capacity that was observed after immediate nerve repair (Boyd and Gordon, 2002; Furey et al., 2007). Only 10–50% of the surviving motoneurons remain capable of regeneration after chronic axotomy (Fu and Gordon, 1995).
The mechanism behind this phenomenon is not yet fully understood. After PNI, the damage caused by axotomy and retrograde degeneration of the neuronal cell body have an effect on the possibility of regeneration. It is likely that a gradual reduction of neurotrophic support and substrates from the denervated distal nerve stumps to the regenerating axons is at least partially responsible for the reduction in the regenerative capacity of motoneurons when axotomy is accompanied by the absence of target connections (Furey et al., 2007). During this period, the initial proliferation of Schwann cells (SCs) is not maintained. As a result, the number of these cells may reduce to a level that does not provide an adequate substrate and trophic support. Therefore, the protection of the injured neurons and maintenance of the growth-supportive phenotype of the SCs, or reactivation of these cells to support axonal regeneration, are decisive factors that can contribute to counteract the low regeneration capacity of neurons.
Strategies Targeting the Spinal Cord to Promote Nerve Regeneration
As reviewed above, multiple responses take place in the spinal cord after PNI. Apart from refining surgical repair techniques, there is much more we can do to achieve more satisfactory functional recovery. Many efforts have been made to develop different strategies to enhance nerve regeneration. Considering the complexity of the neuronal responses, targeted treatment approaches should be used. Strategies should be applied according to the timing of neuronal changes, severity of neuronal injury, neuron type, location of axonal trauma relative to neuron cell body, patient’s age, and so on.
Sustaining the regeneration inductive environment
Reestablishing the connection between neurons and target organs
After PNI-induced loss of connectivity between proximal and distal nerve parts, the distal part undergoes Wallerian degeneration. Also, a change in the phenotype of Schwann cells (SCs) from supportive to proliferative and secretory occurs. These cells become a neurotrophic factory, creating a permissive environment for the growth of axons and the maintenance of motor, sensory and autonomic peripheral neurons. The best way to make these favorable conditions available to the neurons is to reestablish the connection between neurons and target organs, regardless of the reconstruction methods, which may include primary repair, and repair with vascularised or non-vascularised nerve grafting (Ma et al., 2001).
However, nerve repair is only partially effective (Ma et al., 2001; McKay et al., 2002) and must take place soon after PNI. Studies have pointed to the failure of SCs to uphold a high level of trophic factors 2 months after chronic denervation. These findings emphasize the importance of early repair for regeneration: this is the time window when the early responses in spinal cord and the peripheral changes can be fully taken advantage of. In DRG, the retrograde neuronal degeneration becomes apparent 2 weeks after the injury, and delayed nerve repairs will inevitably lead to a reduced sensory recovery and the loss of larger number of sensory neurons (Ma et al., 2001).
Additional supply of exogenous neurotrophic factors
As an adaptive response to the injury, expression of neurotrophic factors increases sharply. However this response is transient. Insufficient expression of neurotrophic factors may be linked to the extended period of time, inadequte axonal regeneration and apoptotic death of chronically axotomized neurons (Fu and Gordon, 1995, 1997). It has been demonstrated that exogenous application of these neurotrophic factors helps to maintain the regeneration of axons after PNI. This is one of the most promising therapeutic approaches to aid functional recovery (Table 3).
Table 3.
Application and functions of neurotropic factors in vivo
| Neurotropic factors | Injury type | Target neurons | Function | Administration | References | |||
|---|---|---|---|---|---|---|---|---|
| Neuron survival | Axon growth | Location | Method | Time/Duration | ||||
| NGF | SN transection | DRG | (=) | NPS | OMP | Acute (d0)/12 d | Mohiuddin et al., 1999 | |
| SN crush | DRG | (=) | Spinal cord | OMP | Acute (d0)/7–10 d | Gold, 1997 | ||
| Dorsal root transection | DRG | (=) | Spinal cord | Nitrocellulose strips | Acute (d0) | Houle, 1992 | ||
| SN transection | DRG | (+) | NPS | Capsules containing NGF | Chronic (d2 or 18 wk)/2 wk | Siri et al., 2001 | ||
| SN transection | DRG | (+) | NPS | Local application | Acute (d0)/6 wk | Rich et al., 1987 | ||
| Dorsal rhizotomy and/or SN crush (N) | DRG | (+) | Systemic medication | Injection | Acute (d0)/5 d | Yip and Johnson, 1984 | ||
| SN transection (D) | MN | (+) | NPS | Gelfoam | Acute (d0) | Li et al., 1994 | ||
| SN transection | MN | (=) | (=) | NPS | Connective tissue chamber | Acute (d0) | Marcol et al., 2004 | |
| FN transection | MN | (=) | NPS | Gelfoam | Acute (d0) | Vassilis et al., 1993 | ||
| FN transection (N) | MN | (=) | NPS | Gelfoam | Acute (d0) | Sendtner et al., 1992 | ||
| SN transection (N) | MN | (=) | Systemic medication | Injection | Acute (d0)/1 wk | Yan et al., 1988 | ||
| SN crush (N) | MN | (=) | Systemic medication | Injection | Acute (d0)/2 wk | Yan et al., 1988 | ||
| BDNF | SN transection (N) | MN | (+) | Systemic medication | Injection | Acute (d0)/1–2 wk | Vejsada et al., 1998 | |
| SN transection (N) | MN | (+) | PT | Polymer rods | Acute (d0) | Vejsada et al., 1998 | ||
| SN transection (N) | MN | (+) | NPS | Local application | Acute (d0) | Vejsada et al., 1998 | ||
| SN transection (D) | MN | (+) | NPS | Gelfoam | Acute (d0) | Li et al., 1994 | ||
| FN transection | MN | (+) | NPS | Gelfoam | Acute (d0) | Vassilis et al., 1993 | ||
| FN transection (N) | MN | (+) | NPS | Gelfoam | Acute (d0) | Sendtner et al., 1992 | ||
| Spinal root avulsion (N) | MN | (=) | Spinal cord | Local application | Acute (d0) | Yuan et al., 2000 | ||
| Spinal nerve transection (N) | MN | (+) | NPS | Gelfoam | Acute (d0) | Yuan et al., 2000 | ||
| Ventral root avulsion | MN | (+) | (+) | Spinal cord | OMP | Acute (d0)/4 wk | Novikov et al., 1997 | |
| Spinal root avulsion | MN | (+) | (+) | Spinal cord | OMP | Acute (d0)/2 wk | Kishino et al., 1997 | |
| BP crush (N) | MN | (=) | Injury site | Polymer rods | Acute (d0) | Aszmann et al., 2001 | ||
| TIB-CP transection | MN | (+) | Suture site | OMP/low dose | Chronic (2 mon)/4 wk | Boyd and Gordon, 2002 | ||
| TIB-CP transection | MN | (=) | Suture site | OMP/low dose | Acute (d0)/4 wk | Boyd and Gordon, 2002 | ||
| TIB-CP transection | MN | (–) | Suture site | OMP/high dose | Acute (d0)/chronic (2 mon)/4 wk | Boyd and Gordon, 2002 | ||
| SN transection | DRG | (+) | NPS | OMP | Acute (d0)/7 d | Song et al., 2008 | ||
| Spinal nerve transection | DRG | (–) | PT | Injection | Acute (d0) | Ljungberg et al., 1999 | ||
| GDNF | SN transection (N) | MN | (+) | NPS | Local application | Acute (d0) | Vejsada et al., 1998 | |
| SN transection (N) | MN | (+) | PT | Viruses | Acute (d0) | Baumgartner and Shine, 1998a | ||
| SN transection (D) | MN | (+) | NPS | Gelfoam | Acute (d0) | Li et al., 1994 | ||
| SN transection (N) | MN | (+) | NPS | Encapsulated cells | Acute (d0) | Vejsada et al., 1998 | ||
| FN transection | MN | (+) | Spinal cord | Viruses | Acute (–1 mon) | Hottinger et al., 2000 | ||
| FN crush or transection (N) | MN | (+) | (+) | PT | Viruses | Acute (–2 d) | Baumgartner and Shine, 1998b | |
| BP crush (N) | MN | (=) | NPS | Polymer rods | Acute (d0) | Aszmann et al., 2001 | ||
| Spinal root avulsion (N) | MN | (+) | Spinal cord | Gelfoam | Acute (d0) | Yuan et al., 2000 | ||
| TIB-CP cross suture | MN | (+) | Suture site | OMP | Chronic (2 mon)/4 wk | Boyd and Gordon, 2003 | ||
| BDNF+ GDNF | TIB-CP cross suture | MN | (++) | Suture site | OMP | Chronic (2 mon)/4 wk | Boyd and Gordon, 2003 | |
| TIB-CP cross suture | MN | (–) | Suture site | OMP | Acute (d0) | Boyd and Gordon, 2003 | ||
| SN transection (N) | MN | (++) | NPS | Local application | Acute (d0) | Vejsada et al., 1998 | ||
| SN transection (N) | MN | (++) | PT | BDNF -polymer rods/GDNF-encapsulated cells | Acute (d0) | Vejsada et al., 1998 | ||
| BP crush (N) | MN | (++) | Injury site | Polymer rods | Acute (d0) | Aszmann et al., 2001 | ||
| NT-3 | SN transection | DRG | (+) | NPS | Local application | Acute (d0) | Groves et al., 1999 | |
| SN transection (D) | MN | (+) | NPS | Gelfoam | Acute (d0) | Li et al., 1994 | ||
| FN transection (N) | MN | (+) | NPS | Gelfoam | Acute (d0) | Sendtner et al., 1992 | ||
| Spinal nerve transection | DRG | (+) | PT | Injection | Acute (d0) | Ljungberg et al., 1999 | ||
Except for (N) – injury in newborn animals and (D) – injury in developing animals, all injuries were in adult animals. (=) – no direct effect; (+) – positive effect; (++) – enhanced positive effect; (–) – inhibiting effect. BDNF: Brain derived neurotrophic factor; BP: brachial plexus; CP: common peroneal nerve; DRG: sensory neurons in the dorsal root ganglia; FN: facial nerve; GDNF: glial cell line-derived neurotrophic factor; MN: motoneurons in the spinal cord; NGF: nerve growth factor; NPS: nerve proximal stump; NT-3: neurotrophin-3; OMP: osmotic mini-pump; PT: peripheral tissues; SN: sciatic nerve; TIB: tibial nerve. The (–) in front of d or mon denotes that the administration of neurotropic factors happened before nerve conditional lesion.
NGF is the first and the most studied one. NGF was shown to act via activation of the Ras-MAP kinase cascade specifically on a subpopulation of small primary sensory and sympathetic neurons which are in charge of sprouting of nociceptive and sympathetic axons into denervated skin. Continuous infusion of NGF via catheters placed in the dorsal spinal cord, or using grafts of primary fibroblasts that can deliver NGF to the sites of spinal cord injury has been proven to be a potent stimulus for regrowth of sensory axons after injury (Tuszynski et al., 1996). Motor axons can also be induced into sprouting after the transplatation of graft that expresses or secretes NGF (Tuszynski et al., 1996). However, function of NGF in promoting regeneration is deduced to be indirect, as NGF works through supporting non-neuron cells, such as SCs (Houle, 1992). Even continuous intrathecal infusion of NGF on the axotomized spinal cord will not promote sprouting. Instead, it will delay the onset of regeneration without affecting the regeneration rate (Gold, 1997), which points to the importance of choosing the right administration method (Kemp et al., 2011). Retrograde delivery of NGF from proximal peripheral nerve stump to axotomized neurons was proven to have a protective effect on sensory neurons in DRG (Rich et al., 1989). Also, the timing of NGF administration, which should be 2 to 18 weeks after the sciatic nerve lesion but not intermediately after injury, is crucial for the efficiency of reversing the axotomy-induced changes in the spinal cord (Siri et al., 2001). Worth mentioning is that hyperalgesia, the side effect of NGF administration, reduces its potential value as a therapeutic agent.
BDNF plays a crucial role in protecting neurons from degeneration and inducing axonal regeneration, especially for motoneurons. Locally administered BDNF markedly enhances the lesion-induced immunoreactivity of low-affinity NGF receptors in motoneurons. Due to the complexity of its functions, the administration of BDNF for therapeutic purposes has many specific issues to be considered, such as administration dose, method, time, and the duration of injury. In acutely injured and repaired peripheral nerve models, BDNF continuously applied to the sciatic nerve suture site at low doses has no clear effect on neuron protection and axonal regeneration. Its effect on the improved regeneration was demonstrated in chronic injury models (Boyd and Gordon, 2002). High levels of exogenous BDNF can affect axonal regeneration, possibly through signaling via p75 receptors (Boyd and Gordon, 2003). Furthermore, direct administration of BDNF to the injured parts of the CNS may have no effect on preventing neuronal death and regenerating the nerves, since the truncated trkB receptor in astroglia functions as a negative regulator and prevents the diffusion of BDNF into the CNS. Strong dependency on the dose and administration method may limit the effectiveness of BDNF as a therapeutic tool in promoting motor axonal regeneration. This, once again, underlines the importance of achieving the localized delivery of growth factors at optimal dose.
GDNF is one of the most potent survival factors for motoneurons, as tested by several experimental techniques. In neonatal rodents local administration of GDNF to a transected nerve stump or motoneuron cell bodies completely or partially prevented axotomy-induced motoneuron death (Vejsada et al., 1998). Similar findings were reported for adult mice with more severe injuries such as facial nerve or spinal root avulsion. In addition, continuous administration of exogenous GDNF has been shown to fully reverse the negative consequences of chronic axotomy in that it significantly increases the number of spinal cord motoneurons which regenerate their aoxn. GDNF’s motor axon regeneration effect was not as evident in animals that had immediate nerve repair. It is more potent than BDNF, although it does not stimulate the regeneration of motor axons (Boyd and Gordon, 2003). Unlike BDNF, the effects of GDNF on the regeneration of motor axons are not dose-dependent. Moreover, it has greater synergistic effects when applied in combination with BDNF. Long-term (28 days), continuous treatment with GDNF and BDNF can be utilized as a viable long-term strategy for sustaining the regeneration of motor axons in chronically axotomized motoneurons (Boyd and Gordon, 2003).
The role of neurotrophin-3 (NT-3) and neurotrophin-4/5 (NT-4/5) in the promotion of axonal regeneration is less studied. Based on the changes in their expression levels after nerve injuries they probably play a less significant role in comparison to BDNF, GDNF and NGF. Other neurotrophic factors, such as insulin-like growth factors-I and -II, ciliary neurotrophic factor (CNTF), basic fibroblast growth factor, neuregulins, osteopontin and pleiotrophin were also reported to stimulate axonal regeneration. However, they appear to influence the local responses of axons and SCs at the nerve repair site.
The efficacy of neurotrophic factors depends on the neuronal subpopulation, injury type (acute or chronic) and the route of administration. Specific neurotrophic factors stimulate regrowth in specific axonal populations of injured spinal cord. For example, NGF and NT-3 stimulate sensory neurons, whereas BDNF and GDNF stimulate motor neurons. Combined administration of neurotrophic factors can target multiple injured neuron types, but i which combination is the best remains unknown. Enhancement of specific functions has been achieved by simultaneous application of GDNF and BDNF, or CNTF and BDNF (Vejsada et al., 1998). Most neurotrophic factors are only effective in acute axotomy. Exception is GDNF or GDNF combined with BDNF, which have been shown to work on chronically axotomized neurons (Boyd and Gordon, 2003). This indicates that time window also exists for effective therapeutic application of neurotrophic factors.
Although BDNF, NGF, GDNF and NT-3 can be retrogradely transported, most neurotrophins applied systemically cannot reach the spinal cord due to the blood-brain barrier. Thus, their therapeutic applications in the peripheral nerve injury site are limited. Administration methods such as direct injection, continuous infusion, placement of growth-factor saturated Gelfoam or infusion into channels containing SCs did not achieve a long-term, localized, high dose delivery of neurotrophic factors. Ex vivo gene therapy and viral-mediated delivery of neurotrophic factors which can continuously supply low doses of recombinant neurotrophic factors have been proven useful for promoting localized and robust regrowth of axons through long-term and site-specific delivery of neurotrophic factors to the injured cells (Hottinger et al., 2000). These methods provide a potential reliable alternative approach for future applications.
Additional supply of Schwann cells or stem cells
The close interaction with SCs promotes stability and survival of neurons, particularly via myelin metabolism and protein binding. Denervated SCs are a rich source of neurotrophic factors including BDNF, GDNF and NGF (Madduri and Gander, 2010). SCs can be isolated from patient’s peripheral nerve and cultured. They can also be derived from stem cells, such as adipose-derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) (Yousefi et al., 2019). These alternative SC sources reduce the limitations of donor site morbidity caused by harvesting a nerve and the difficulties in generating a sufficiently large number of cells in a relatively short turnaround time. Various administration approaches have been suggested to utilize the properties of SCs to promote nerve regeneration, either by direct application to injured nerve sheath (Guo et al., 2014), filling SCs into silicone chamber (Timmer et al., 2003), using semipermeable polyacrylonitrile/polyvinylchloride (PAN/PVC) polymer tube, microtube array sheet (Wang et al., 2015), or DuraGen® wrap (Gersey et al., 2017). The extent and scope of neuron responses to SCs can be greatly enhanced by combined infusion of glucocorticosteroid, methylprednisolone, neurotrophins BDNF and NT-3. Such approaches hold greater potential for promoting nerve regeneration. However, most of the studies focus on the functions at the level of peripheral nerve rather than neuron body, or are done in the field of spinal cord injury rather than PNI.
Counteracting the detrimental changes
Inhibiting neuron death
Peripheral nerve injury inevitably causes neuronal death. Effective therapy should include efforts for protecting and improving the survival of injured neurons.
The time course of neuronal loss varies depending on the neuron type, extent of injury, proximity of injury to cell body, age and so on. In adult animal models, the loss of sensory neurons reaches a plateau at 2 months after sciatic nerve transection at the mid-thigh level in rats (McKay et al., 2002). For adult motor neurons, the cell loss plateaus 4 months after the injury (Ma et al., 2001). However, this plateau is reached faster in neonates (within 10 days of brachial plexus trunk division (Aszmann et al., 2004)) and in injuries at a more proximal level (within 2 months after ventral rhizotomy and 1 month after ventral root avulsion (Zhang et al., 2005)). Knowledge of the time course of neuronal loss can inform the optimal timing for various interventions.
Nerve repair appears to be a realistic and effective neuroprotective strategy. Following C7 spinal nerve root transection 1 cm distal to the dorsal root ganglion, the immediate repair reduced the loss of sensory neurons by half and the loss of motor neurons by > 70% (Ma et al., 2001). As the site of repair in these experiments was far from target muscles, this suggests that SCs around the distal nerve and the cell bodies of the injured motoneurons themselves provide sufficient trophic support. This effect is even more pronounced in distal nerve injuries (Boyd and Gordon, 2003). The neuroprotective effectiveness of nerve repair relates to how early the surgery is performed (McKay et al., 2002). Whenever possible, it should be done immediately after the injury. However, when nerve injuries are severe, located proximal to the cell body, occur in immature individuals, or do not receive adequate early repair, additional interventions are needed to protect the neurons. Therefore the availability of nonsurgical neuroprotection remains essential for clinical practice.
Neurotrophic factors such as NGF, BDNF, NT-3 and NT-4, as well as GDNF, basic fibroblast growth factor, leukaemia inhibitory factor, CNTF and small organic molecule CEP-1347 can protect axotomized neuron cells and partly or completely rescue neuron cells destined to die (Novikov et al., 1997). Their neuron protection function equals or outweights their value of promoting axonal regeneration. Similar to surgical neuroprotection, there is a time window for effective neurotrophic factor application, based on the fact that most neurotrophic factors have positive effect on acutely axotomized neurons.
There are explorations on other therapeutic strategies. Acetyl-L-carnitine and N-acetyl-cysteine (NAC) have been shown, both in experiments and in clinical applications, to be highly effective in protecting axotomized neuron cells. Therapeutic targets of these molecules are involved in preservation of bio-energetic function, preventing disruptions of the electron transport chain caused by nitric oxide and reactive oxygen species, and strengthening the protective effects of apoptosis regulator Bcl-2 and its counterparts. Acetyl-L-carnitine (50 mg/kg per day) can arrest the death of sensory neurons and speed up regeneration (Wilson et al., 2007), while NAC (150 mg/kg per day) can provide similarly effective sensory and motor neuron protection without the loss of neuroprotective effect even when the treatment is delayed by 24 hours and 1 week after the injury, respectively (Zhang et al., 2005). This provides a valuable time for clinical diagnosis and surgical preparations. N-methyl-D-aspartate receptor antagonist MK-801 applied by intraperitoneal administration once prior to neurontomy can significantly reduce the incidence of cellular degeneration 7 days later. An almost complete rescuing of neurons was achieved through the combination of acute injection and continuous infusion of MK-801 for 1 week (Azkue et al., 1998).
However, the neuronal protection function of therapeutics is transient. Ultimately, axotomy induced death of motoneurons can only be delayed but not avoided (Vejsada et al., 1998; Hottinger et al., 2000). Inhibitors of neuronal death can help in sustaining the viability of neurons until reconstructive surgery gives them a chance for regeneration. Timely nerve repair is required to maintain the axotomized neurons alive.
Increasing neuron regenerative capacity
Apart from using neurotrophic factors to promote neuronal regeneration, there are other strategies to increase the regenerative capacity of neurons.
The immunophilin ligand FK506 has been shown to enhance the neurite outgrowth (Labroo et al., 2016, 2017). The underlying mechanism involves FK506-binding protein-52, a chaperone component of mature steroid receptor complexes. FK506 was demonstrated to be effective in rat models of sciatic nerve crush (1 mg/kg, subcutaneous injection), immediate nerve repair (1 mg/kg, subcutaneous injection), nerve graft (1 mg/kg, intraperitoneally), and chronic axotomy (5 mg/kg, subcutaneous injection). The ligand not only increases the number of injured motoneurons that regenerated their axons after chronic axotomy, but also accelerates the rate of axonal regeneration (Sulaiman and Gordon, 2009). Unlike other factors that improve the decreased ability of SCs to support axonal regeneration, FK506 acts directly on the neurons whose capacity for regeneration was significantly diminished by chronic axotomy (Sulaiman and Gordon, 2009). Elevated production of GAP-43 may contribute to the ability of FK506 to accelerate nerve regeneration.
The ability of denervated SCs to support axonal regeneration in the distal nerve stumps starts to decline around 4 weeks post-injury (Ridley et al., 1989). Eventually this results in low regenerative capacity of neurons. Sometimes, timely reinnervation is not achievable, which is especially true for nerve injuries that occurred at a long distance from the denervated target. Thus, maintaining the growth-supportive phenotype of SCs or reactivation of SCs after denervation is one of the effective strategies to promote neuronal regeneration (Sulaiman and Gordon, 2009). One approach to maintain the growth support by SCs is to reinnervate the distal nerve stumps by end-to-side neurorrhaphy. This approach allows to sustain the growth-supportive phenotype of SCs after denervation till the proximal regenerating nerve can reach the distal target. This surgical method is called a “baby-sitting” technique: it encourages more motoneurons to regenerate axons into the “baby-sat” distal nerve stumps (Placheta et al., 2015; Györi et al., 2018). This is a practical strategy to enhance axonal regeneration over long distances in peripheral nerve injuries. Another method to reverse the incapacity of chronically denervated SCs is the administration of cytokines, such as transforming growth factor (Ridley et al., 1989), which are proven to help reactivating SCs into growth-supportive phenotype to support axonal regeneration.
Another strategy is to enhance the mechanisms of intrinsic injury signaling and achieve a better regenerative response by stimulating neuronal activity soon after axonal damage. Various systemic physical treatments have been shown to stimulate the activity of neurons soon after axotomy. A brief one-hour low-frequency electrical stimulation (Chan et al., 2016; Gordon, 2016), and treadmill walking/running are some of the most commonly used and safe treatments. Sometimes, better outcomes can be achieved by combination of different approaches. It is important to emphasize the therapy time window, which should be controlled strictly within the early period after PNI, even immediately after axotomy, as stimulations attempted 2 weeks post-injury do not lead to additional positive effects in axonal regeneration.
Conclusions
As spinal cord and DRG are the sites where the cell bodies of the peripheral nerve reside, their axotomy-induced changes are destined to affect the survival of neurons and regeneration of axons. The strategies to counteract and/or enhance various changes that take place after PNI can provide directions for therapy and improve functional outcomes (Figure 2). These interventions are only effective within a limited time frame. Immediate or early nerve repair is one preferable approach to partially reverse the detrimental changes. After reestablishment of the connection between neuron bodies and their target organs, the neurons can derive sufficient trophic support to protect themselves from death. Moreover, nerve repair creates a favorable milieu for axonal outgrowth. It is crucial to take additional neuroprotection measures for peripheral nerve injuries that are severe, proximal to the cell body, in immature individuals, and when immediate repair is impossible (e.g., in obstetric brachial plexus injuries). The administration of neurotrophic factors is one of the most commonly used methods for neuronal cell protection. Acetyl-L-carnitine and N-acetyl-cysteine are good choices of pharmaceutical interventions, as they can prevent sensory neuron death and accelerate regeneration, gaining additional time for implementation of further therapies. NMDA receptor antagonist MK-801 can also be used to rescue neurons that are destined to die. In terms of promoting axonal regeneration, exogenous supply of neurotrophic factors has been tried, but none of them on its own has longlasting effect. Questions such as which neurotrophic factor(s) to choose, how to combine them and how to establish an effective and simple administration method to recruit the growth of multiple injured axonal populations warrant further investigation. Sustaining and reactivation of Schwann cells after denervation is another effective strategy to promote regeneration. In addition, physical treatments and FK506 administration have been proven to accelerate and promote axonal re-growth.
Figure 2.
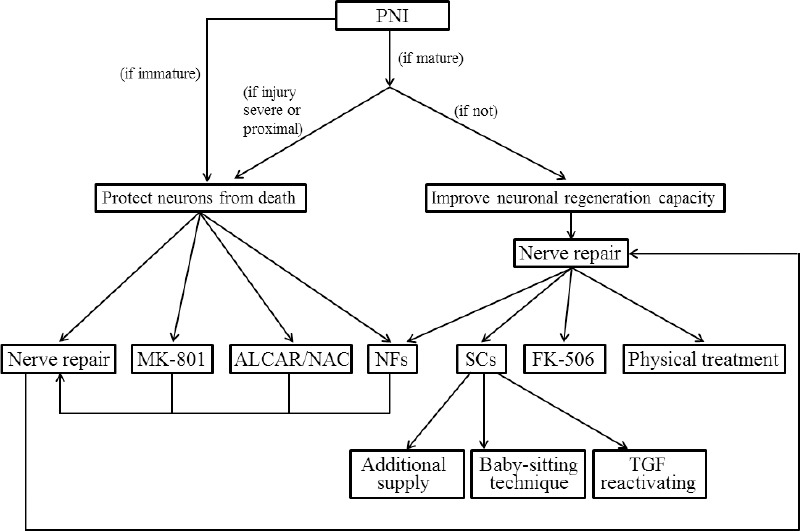
The strateges to sustain the regeneration inductive environment and counteract the detrimental changes after PNI.
ALCAR: Acetyl-L-carnitine; NAC: N-acetyl-cysteine; NFs: neurotrophic factors; PNI: peripheral nerve injuries; SCs: Schwann cells; TGF: transforming growth factor.
Footnotes
Conflicts of interest: We declare no conflicts of interest.
Financial support: YL was supported by Chinese Scholar Council; HW was supported by Mayo Clinic Center for Regenerative Medicine and Fund for the Center for Regenerative Medicine Program Director, Neuroregenerative Medicine.
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: YL was supported by Chinese Scholar Council; HW was supported by Mayo Clinic Center for Regenerative Medicine and Fund for the Center for Regenerative Medicine Program Director, Neuroregenerative Medicine.
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Arvidsson J, Ygge J, Grant G. Cell loss in lumbar dorsal root ganglia and transganglionic degeneration after sciatic nerve resection in the rat. Brain Res. 1986;373:15–21. doi: 10.1016/0006-8993(86)90310-0. [DOI] [PubMed] [Google Scholar]
- 2.Aszmann OC, Korak KJ, Kropf N, Fine E, Aebischer P, Frey M. Simultaneous GDNF and BDNF application leads to increased motoneuron survival and improved functional outcome in an experimental model for obstetric brachial plexus lesions. Plast Reconstr Surg. 2001;110:1066–1072. doi: 10.1097/01.PRS.0000020990.82332.43. [DOI] [PubMed] [Google Scholar]
- 3.Aszmann OC, Winkler T, Korak K, Lassmann H, Frey M. The influence of GDNF on the timecourse and extent of motoneuron loss in the cervical spinal cord after brachial plexus injury in the neonate. Neurol Res. 2004;26:211–217. doi: 10.1179/016164104225013789. [DOI] [PubMed] [Google Scholar]
- 4.Azkue JJ, Zimmermann M, Hsieh TF, Herdegen T. Peripheral nerve insult induces NMDA receptor-mediated, delayed degeneration in spinal neurons. Eur J Neurosci. 1998;10:2204–2206. doi: 10.1046/j.1460-9568.1998.00260.x. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner BJ, Shine HD. Neuroprotection of spinal motoneurons following targeted transduction with an adenoviral vector carrying the gene for glial cell line-derived neurotrophic factor. Exp Neurol. 1998a;153:102–112. doi: 10.1006/exnr.1998.6878. [DOI] [PubMed] [Google Scholar]
- 6.Baumgartner BJ, Shine HD. Permanent rescue of lesioned neonatal motoneurons and enhanced axonal regeneration by adenovirus-mediated expression of glial cell-line-derived neurotrophic factor. J Neurosci Res. 1998b;54:766–777. doi: 10.1002/(SICI)1097-4547(19981215)54:6<766::AID-JNR4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 7.Bhagwat AS, Vakoc CR. Targeting transcription factors in cancer. Trends Cancer. 2015;1:53–65. doi: 10.1016/j.trecan.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyd JG, Gordon T. Dose-dependent facilitation and inhibition of peripheral nerve regeneration by exogenous brain-derived neurotrophic factor. Eur J Neurosci. 2002;15:613–626. doi: 10.1046/j.1460-9568.2002.01891.x. [DOI] [PubMed] [Google Scholar]
- 9.Boyd JG, Gordon T. Glial cell line-derived neurotrophic factor and brain derived neurotrophic factor sustain the axonal regeneration of chronically axotomized motoneurons in vivo. Exp Neurol. 2003;183:610–619. doi: 10.1016/s0014-4886(03)00183-3. [DOI] [PubMed] [Google Scholar]
- 10.Burazin TC, Gundlach AL. Up-regulation of GDNFR-alpha and c-ret mRNA in facial motor neurons following facial nerve injury in the rat. Brain Res Mol Brain Res. 1998;55:331–336. doi: 10.1016/s0169-328x(98)00017-5. [DOI] [PubMed] [Google Scholar]
- 11.Calderó J, Casanovas A, Sorribas A, Esquerda JE. Calcitonin generelated peptide in rat spinal cord motoneurons: subcellular distribution and changes induced by axotomy. Neuroscience. 1992;48:449–461. doi: 10.1016/0306-4522(92)90504-u. [DOI] [PubMed] [Google Scholar]
- 12.Chan KM, Curran MW, Gordon T. The use of brief post-surgical low frequency electrical stimulation to enhance nerve regeneration in clinical practice. J Physiol. 2016;594:3553–3559. doi: 10.1113/JP270892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Da Silva JT, Evangelista BG, Venega RAG, Oliveira ME, Chacur M. Early and late behavioral changes in sciatic nerve injury may be modulated by nerve growth factor and substanceP in rats: a chronic constriction injury long-term evaluation. J Biol Reg Homeos Ag. 2017;31:309–319. [PubMed] [Google Scholar]
- 14.Dubový P, Klusáková I, Hradilová-Svíženská I, Brázda V, Kohoutková M, Joukal M. A Conditioning sciatic nerve lesion triggers a pro-regenerative state in primary sensory neurons also of dorsal root ganglia non-associated with the damaged nerve. Front Cell Neurosci. 2019;13:11. doi: 10.3389/fncel.2019.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernfors P, Rosario CM, Merlio JP, Grant G, Aldskogius H, Persson H. Expression of mRNAs for neurotrophin receptors in the dorsal root ganglion and spinal cord during development. Brain Res Mol Brain Res. 1993;17:217–226. doi: 10.1016/0169-328x(93)90005-a. [DOI] [PubMed] [Google Scholar]
- 16.Fu C, Yin Z, Yu D, Yang Z. Substance P and calcitonin gene-related peptide expression in dorsal root ganglia in sciatic nerve injury rats. Neural Regen Res. 2013;33:3124–3130. doi: 10.3969/j.issn.1673-5374.2013.33.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J Neurosci. 1995;15:3886–3895. doi: 10.1523/JNEUROSCI.15-05-03886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- 19.Fukuoka T, Kondo E, Dai Y. Brain-derived neurotrophic factor increase in the uninjured dorsal root ganglion neurons in selective spinal nerve ligation model. J Neurosci. 2001;21:4891–4900. doi: 10.1523/JNEUROSCI.21-13-04891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuoka T, Noguchi K. A potential anti-allodynic mechanism of GDNF following L5 spinal nerve ligation; Mitigation of NPY up-regulation in the touch sense pathway. Neuroscience. 2015;304:240–249. doi: 10.1016/j.neuroscience.2015.07.059. [DOI] [PubMed] [Google Scholar]
- 21.Furey MJ, Midha R, Xu QG, Belkas J, Gordon T. Prolonged target deprivation reduces the capacity of injured motoneurons to regenerate. Neurosurgery. 2007;60:723–732. doi: 10.1227/01.NEU.0000255412.63184.CC. [DOI] [PubMed] [Google Scholar]
- 22.Gersey ZC, Burks SS, Anderson KD, Dididze M, Khan A, Dietrich WD, Levi AD. First human experience with autologous Schwann cells to supplement sciatic nerve repair: report of 2 cases with long-term follow-up. Neurosurg Focus. 2017;42:E2. doi: 10.3171/2016.12.FOCUS16474. [DOI] [PubMed] [Google Scholar]
- 23.Gold B. Axonal regeneration of sensory nerves is delayed by continuous intrathecal infusion of nerve growth factor. Neuroscience. 1997;76:1153–1158. doi: 10.1016/s0306-4522(96)00416-2. [DOI] [PubMed] [Google Scholar]
- 24.Gong L, Wu J, Zhou S, Wang Y, Qin J, Yu B, Gu X, Yao C. Global analysis of transcriptome in dorsal root ganglia following peripheral nerve injury in rats. Biochem Biophys Res Commun. 2016;478:206–212. doi: 10.1016/j.bbrc.2016.07.067. [DOI] [PubMed] [Google Scholar]
- 25.Gordon T. Electrical stimulation to enhance axon regeneration after peripheral nerve injuries in animal models and humans. Neurotherapeutics. 2016;13:295–310. doi: 10.1007/s13311-015-0415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groves MJ, An SF, Giornetto B, Scaravilli F. Inhibition of sensory neuron apoptosis and prevention of loss by NT-3 administration following axotomy. Exp Neurol. 1999;155:284–294. doi: 10.1006/exnr.1998.6985. [DOI] [PubMed] [Google Scholar]
- 27.Gu ZZ, Pan YC, Cui JK, Klebuc MJ, Shenaq S, Liu PK. Gene expression and apoptosis in the spinal cord neurons after sciatic nerve injury. Neurochem Int. 1997;20:417–426. doi: 10.1016/s0197-0186(96)00077-0. [DOI] [PubMed] [Google Scholar]
- 28.Guntinas-Lichius O, Neiss WF, Schulte E, Stennert E. Quantitative image analysis of the chromatolysis in rat facial and hypoglossal motoneurons following axotomy with and without reinnervation. Cell Tissue Res. 1996;286:537–541. doi: 10.1007/s004410050723. [DOI] [PubMed] [Google Scholar]
- 29.Guo L, Davis B, Nizari S, Normando EM, Shi H, Galvao J, Turner L, Shi J, Clements M, Parrinello S, Cordeiro MF. Direct optic nerve sheath (DONS) application of Schwann cells prolongs retinal ganglion cell survival in vivo. Cell Death Dis. 2014;5:1460. doi: 10.1038/cddis.2014.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Györi E, Radtke C, Gordon T, Borschel GH. “Pathway protection” - enhanced motoneuron regeneration by end-to-side coaptation of sensory axons. Handchir Mikrochir Plast Chir. 2018;50:341–347. doi: 10.1055/a-0746-3557. [DOI] [PubMed] [Google Scholar]
- 31.Hammarberg H, Piehl F, Risling M, Cullheim S. Differential regulation of trophic factor receptor mRNAs in spinal motoneurons after sciatic nerve transection and ventral root avulsion in the rat. J Comp Neurol. 2000;426:587–601. doi: 10.1002/1096-9861(20001030)426:4<587::aid-cne7>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 32.Hoang TX, Nieto JH, Tillakaratne NJ, Havton LA. Autonomic and motor neuron death is progressive and parallel in a lumbosacral ventral root avulsion model of cauda equina injury. J Comp Neurol. 2003;467:477–486. doi: 10.1002/cne.10928. [DOI] [PubMed] [Google Scholar]
- 33.Hottinger AF, Azzouz M, Deglon N, Aebischer P, Zurn AD. Complete and long-term rescue of lesioned adult motoneurons by lentiviral-mediated expression of glial cell line-derived neurotrophic factor in the facial nucleus. J Neurosci. 2000;20:5587–5593. doi: 10.1523/JNEUROSCI.20-15-05587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Houle JD. Regeneration of dorsal root axons is related to specific non-neuronal cells lining NGF-treated intraspinal nitrocellulose implants. Expl Neurol. 1992;118:133–142. doi: 10.1016/0014-4886(92)90030-t. [DOI] [PubMed] [Google Scholar]
- 35.Jankowski MP, McIlwrath SL, Jing X, Cornuet PK, Salerno KM, Koerber HR, Albers KM. Sox11 transcription factor modulates peripheral nerve regeneration in adult mice. Brain Res. 2009;1256:43–54. doi: 10.1016/j.brainres.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang XJ, Chi YN, Chen W, Liu FY, Cui S, Liao FF, Cai J, Wan Y. Increased expression of CaV3, 2 T-type calcium channels in damaged DRG neurons contributes to neuropathic pain in rats with spared nerve injury. Mol Pain. 2018;14 doi: 10.1177/1744806918765808. 1744806918765808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keast JR, Forrest SL, Osborne PB. Sciaticnerveinjuryin adult rats causes distinct changes in the central projections of sensory neurons expressing differentglial cell line-derived neurotrophic factor family receptors. J Comp Neurol. 2010;518:3024–3045. doi: 10.1002/cne.22378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kemp SW, Webb AA, Dhaliwal S, Syed S, Walsh SK, Midha R. Dose and duration of nerve growth factor (NGF) administration determine the extent of behavioral recovery following peripheralnerve injury in the rat. Exp Neurol. 2011;229:460–470. doi: 10.1016/j.expneurol.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 39.Kishino A, Ishige Y, Tatsuno T, Nakayama C, Noguchi H. BDNF prevents and reverses adult rat motor neuron degeneration and induces axonal outgrowth. Exp Neurol. 1997;144:273–286. doi: 10.1006/exnr.1996.6367. [DOI] [PubMed] [Google Scholar]
- 40.Labroo P, Ho S, Sant H, Shea J, Gale BK, Agarwal J. Controlled delivery of FK506 to improve nerve regeneration. Shock. 2016;46:154–159. doi: 10.1097/SHK.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 41.Labroo P, Shea J, Sant H, Gale B, Agarwal J. Effect of combining FK506 and neurotrophins on neurite branching and elongation. Muscle Nerve. 2017;55:570–581. doi: 10.1002/mus.25370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li L, Oppenheim RW, Lei M, Houenou LJ. Neurotrophic agents prevent motoneuron death following sciatic nerve section in the neonatal mouse. J Neurobiol. 1994;25:759–766. doi: 10.1002/neu.480250702. [DOI] [PubMed] [Google Scholar]
- 43.Ljungberg C, Novikov L, Kellerth JO, Ebendal T, Wiberg M. The neurotrophins NGF and NT-3 reduce sensory neuronal loss in adult rat after peripheral nerve lesion. Neurosci Lett. 1999;262:29–32. doi: 10.1016/s0304-3940(99)00040-3. [DOI] [PubMed] [Google Scholar]
- 44.Ma J, Novikov LN, Wiberg M, Kellerth JO. Delayed loss of spinal motoneurons after peripheral nerve injury in adult rats: a quantitative morphological study. Exp Brain Res. 2001;139:216–223. doi: 10.1007/s002210100769. [DOI] [PubMed] [Google Scholar]
- 45.Madduri S, Gander B. Schwann cell delivery of neurotrophic factors for peripheral nerve regeneration. J Peripher Nerv Syst. 2010;15:93–103. doi: 10.1111/j.1529-8027.2010.00257.x. [DOI] [PubMed] [Google Scholar]
- 46.Marcol W, Kotulska K, Larysz-Brysz M, Pietrucha-Dutczak M, Olakowska E, Malinowska I, Lewin-Kowalik J. Influence of nerve growth factor upon the injured peripheral nerve in the absence of its distal part. Ital J Anat Embryol. 2004;4:199–208. [PubMed] [Google Scholar]
- 47.Martin LJ, Kaiser A, Price AC. Motor neuron degeneration after sciatic nerve avulsion in adult rat evolves with oxidative stress and is apoptosis. J Neurobiol. 1999;40:185–201. [PubMed] [Google Scholar]
- 48.Martin SL, Reid AJ, Verkhratsky A, Magnaghi V, Faroni A. Gene expression changes in dorsal root ganglia following peripheral nerve injury: roles in inflammation, cell death and nociception. Neural Regen Res. 2019;14:939–947. doi: 10.4103/1673-5374.250566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKay Hart A, Brannstrom T, Wiberg M, Terenghi G. Primary sensory neurons and satellite cells after peripheral axotomy in the adult rat: time course of cell death and elimination. Exp Brain Res. 2002;142:308–318. doi: 10.1007/s00221-001-0929-0. [DOI] [PubMed] [Google Scholar]
- 50.Mohiuddin L, Delcroix JD, Fernyhough P, Tomlinson DR. Focally administered nerve growth factor suppresses molecular regenerative responses of axotomized peripheral afferents in rats. Neuroscience. 1999;91:265–271. doi: 10.1016/s0306-4522(98)00582-x. [DOI] [PubMed] [Google Scholar]
- 51.Nahin RL, Ren K, De Leon M, Ruda M. Primary sensory neurons exhibit altered gene expression in a rat model of neuropathic pain. Pain. 1994;58:95–108. doi: 10.1016/0304-3959(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 52.Naveilhan P, ElShamy WM, Ernfors P. Differential regulation of mRNAs for GDNF and its receptors Ret and GDNFR alpha after sciatic nerve lesion in the mouse. Eur J Neurosci. 1997;9:1450–1460. doi: 10.1111/j.1460-9568.1997.tb01499.x. [DOI] [PubMed] [Google Scholar]
- 53.Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- 54.Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- 55.Nitzan-Luques A, Minert A, Devor M, Tal M. Dynamic genotype-selective “phenotypic switching” of CGRP expression contributes to differential neuropathic pain phenotype. Exp Neurol. 2013;250:194–204. doi: 10.1016/j.expneurol.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 56.Norsworthy MW, Bei F, Kawaguchi R, Wang Q, Tran NM, Li Y, Brommer B, Zhang Y, Wang C, Sanes JR, Coppola G, He Z. Author informationSox11 Expression promotes regeneration of some retinal ganglion cell types but kills others. Neuron. 2017;94:1112–1120.e4. doi: 10.1016/j.neuron.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Novikov L, Novikova L, Kellerth JO. Brain derived neurotrophic factor promotes axonal regeneration and long-term survival of adult rat spinal motoneurons in vivo. Neuroscience. 1997;79:765–774. doi: 10.1016/s0306-4522(96)00665-3. [DOI] [PubMed] [Google Scholar]
- 58.Obata K, Yamanaka H, Fukuoka T, Yi D, Tokunaga A, Hashimoto N, Yoshikawa H, Noguch K. Contribution of injured and uninjured dorsal root ganglion neurons to pain behavior and the changes in gene expression following chronic constriction injury of the sciatic nerve in rats. Pain. 2003;101:65–77. doi: 10.1016/s0304-3959(02)00296-8. [DOI] [PubMed] [Google Scholar]
- 59.Oliveira AL. Apoptosis of sensory neurons and satellite cells after sciatic nerve transection in C57BL/6J mice. Braz J Med Biol Res. 2001;34:375–380. doi: 10.1590/s0100-879x2001000300012. [DOI] [PubMed] [Google Scholar]
- 60.Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF transcription factors, and immunity. Immunity. 2014;41:518–528. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Placheta E, Wood MD, Lafontaine C, Liu EH, Hendry JM, Angelov DN, Frey M, Gordon T, Borschel GH. Enhancement of facial nerve motoneuron regeneration through cross-face nerve grafts by adding end-to-side sensory axons. Plast Reconstr Surg. 2015;135:460–471. doi: 10.1097/PRS.0000000000000893. [DOI] [PubMed] [Google Scholar]
- 62.Qin J, Wu JC, Wang QH, Zhou SL, Mao SS, Yao C. Transcription factor networks involved in cell death in the dorsal root ganglia following peripheral nerve injury. Neural Regen Res. 2018;13:1622–1627. doi: 10.4103/1673-5374.237183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rende M, Hagg T, Manthorpe M, Varon S. Nerve growth factor receptor immunoreactivity in neurons of the normal adult rat spinal cord and its modulation after peripheral nerve lesions. J Comp Neurol. 1992;319:285–298. doi: 10.1002/cne.903190208. [DOI] [PubMed] [Google Scholar]
- 64.Rich KM, Disch SP, Eichler ME. The influence of regeneration and nerve growth factor on the neuronal cell body reaction to injury. J Neurocytol. 1989;18:569–576. doi: 10.1007/BF01187077. [DOI] [PubMed] [Google Scholar]
- 65.Rich KM, Luszczynski JR, Osborne PA, Johnson EM., Jr Nerve growth factor protects adult sensory neurons from cell death and atrophy caused by nerve injury. J Neurocytol. 1987;16:261–268. doi: 10.1007/BF01795309. [DOI] [PubMed] [Google Scholar]
- 66.Richardson PM, Verge VM. Axonal regeneration in dorsal spinal roots is accelerated by peripheral axonal transection. Brain Res. 1987;411:406–408. doi: 10.1016/0006-8993(87)91096-1. [DOI] [PubMed] [Google Scholar]
- 67.Ridley AJ, Davis JB, Stroobant P, Land H. Transforming growth factors-beta 1 and beta 2 are mitogens for rat Schwann cells. J Cell Biol. 1989;109:3419–3424. doi: 10.1083/jcb.109.6.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sebert ME, Shooter EM. Expression of mRNA for neurotrophic factors and their receptors in the rat dorsal root ganglion and sciatic nerve following nerve injury. J Neurosci Res. 1993;36:357–367. doi: 10.1002/jnr.490360402. [DOI] [PubMed] [Google Scholar]
- 69.Sendtner M, Holtmann B, Kolbeck R, Thoenen H, Barde YA. Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature. 1992;360:757–759. doi: 10.1038/360757a0. [DOI] [PubMed] [Google Scholar]
- 70.Shehab SA, Atkinson ME. Vasoactive intestinal polypeptide (VIP) increases in the spinal cord after peripheral axotomy of the sciatic nerve originate from primary afferent neurons. Brain Res. 1986;372:37–44. doi: 10.1016/0006-8993(86)91456-3. [DOI] [PubMed] [Google Scholar]
- 71.Shen H, Chung JM, Chung K. Expression of neurotrophin mRNAs in the dorsal root ganglion after spinal nerve injury. Brain Res Mol Brain Res. 1999;64:186–192. doi: 10.1016/s0169-328x(98)00314-3. [DOI] [PubMed] [Google Scholar]
- 72.Shi TJ, Xiang Q, Zhang MD, Barde S, Kai-Larsen Y, Fried K, Josephson A, Glück L, Deyev SM, Zvyagin AV, Schulz S, Hökfelt T. Somatostatin and its 2A receptor in dorsal root ganglia and dorsal horn of mouse and human: expression, trafficking and possible role in pain. Mol Pain. 2014;10:12. doi: 10.1186/1744-8069-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siri CR, Shortland PJ, Grant G, Olivius NP. Delayed administration of NGF reverses nerve injury induced central alterations of primary afferents. Neuroreport. 2001;12:1899–1902. doi: 10.1097/00001756-200107030-00026. [DOI] [PubMed] [Google Scholar]
- 74.Smriti P, Gennadij R. Downstream effector molecules in successful peripheral nerve regeneration. Cell Tissue Res. 2012;349:15–26. doi: 10.1007/s00441-012-1416-6. [DOI] [PubMed] [Google Scholar]
- 75.Snider WD, Elliott JL, Yan Q. Axotomy-induced neuronal death during development. J Neurobiol. 1992;23:1231–1246. doi: 10.1002/neu.480230913. [DOI] [PubMed] [Google Scholar]
- 76.Song XY, Li F, Zhang FH, Zhong JH, Zhou XF. Peripherally-derived BDNF promotes regeneration of ascending sensory neurons after spinal cord injury. PLoS One. 2008;3:e1707. doi: 10.1371/journal.pone.0001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sulaiman OA, Gordon T. Role of chronic Schwann cell denervation in poor functional recovery after nerve injuries and experimental strategies to combat it. Neurosurgery. 2009;65:A105–114. doi: 10.1227/01.NEU.0000358537.30354.63. [DOI] [PubMed] [Google Scholar]
- 78.Sun ZG, Wei W, Liu HW, Ma JT, Huang HT. Acute Response of Neurons: An early event of neuronal cell death after facial nerve injury. World Neurosurg. 2018;109:252–257. doi: 10.1016/j.wneu.2017.09.157. [DOI] [PubMed] [Google Scholar]
- 79.Terada Y, Morita-Takemura S, Isonishi A, Tanaka T, Okuda H, Tatsumi K, Shinjo T, Kawaguchi M, Wanaka A. NGF and BDNF expression in mouse DRG after spared nerve injury. Neurosci Lett. 2018;686:67–73. doi: 10.1016/j.neulet.2018.08.051. [DOI] [PubMed] [Google Scholar]
- 80.Timmer M, Robben S, Müller-Ostermeyer F, Nikkhah G, Grothe C. Axonal regeneration across long gaps in silicone chambers filled with Schwann cells overexpressing high molecular weight FGF-2. Cell Transplant. 2003;12:265–277. doi: 10.3727/000000003108746821. [DOI] [PubMed] [Google Scholar]
- 81.Tonra JR, Curtis R, Wong V, Cliffer KD, Park JS, Timmes A, Nguyen T, Lindsay RM, Acheson A, DiStefano PS. Axotomy upregulates the anterograde transport and expression of brain-derived neurotrophic factor by sensory neurons. J Neurosci. 1998;18:4374–4383. doi: 10.1523/JNEUROSCI.18-11-04374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tuszynski MH, Mafong E, Meyer S. Central infusions of brain-derived neurotrophic factor and neurotrophin-4/5, but not nerve growth factor and neurotrophin-3, prevent loss of the cholinergic phenotype in injured adult motor neurons. Neuroscience. 1996;71:761–771. doi: 10.1016/0306-4522(95)00440-8. [DOI] [PubMed] [Google Scholar]
- 83.Valero-Cabré A, Navarro X. H reflex restitution and facilitation after different types of peripheral nerve injury and repair. Brain Res. 2001;919:302–312. doi: 10.1016/s0006-8993(01)03052-9. [DOI] [PubMed] [Google Scholar]
- 84.Vassilis E, Koliatsos Richard E, Clatterbuck John W, Winslow Michelle H, Cayouette S, Donald Price. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron. 1993;10:359–367. doi: 10.1016/0896-6273(93)90326-m. [DOI] [PubMed] [Google Scholar]
- 85.Vejsada R, Tseng J, Lindsay RM, Acheson A, Aebischer P, Kato AC. Synergistic but transient rescue effects of BDNF and GDNF on axotomized neonatal motoneurons. Neuroscience. 1998;84:129–139. doi: 10.1016/s0306-4522(97)00497-1. [DOI] [PubMed] [Google Scholar]
- 86.Villar MJ, Corte´s R, Theodorsson E, Wiesenfeld-Hallin Z, Schalling M, Fahrenkrug J, Emson PC, Ho¨kfelt T. Neuropeptide expression in rat dorsal root ganglion cells and spinal cord after peripheral nerve injury with special reference to galanin. Neuroscience. 1989;33:587–604. doi: 10.1016/0306-4522(89)90411-9. [DOI] [PubMed] [Google Scholar]
- 87.Wakisaka S, Kajander KC, Bennett GJ. Increased neuropeptide Y (NPY)-like immunoreactivity in rat sensory neurons following peripheral axotomy. Neurosci Lett. 1991;124:200–203. doi: 10.1016/0304-3940(91)90093-9. [DOI] [PubMed] [Google Scholar]
- 88.Wang Y, Wang W, Wo Y, Gui T, Zhu H, Mo X, Chen CC, Li Q, Ding W. Orientated guidance of peripheral nerve regeneration using conduits with a microtube array sheet (MTAS) ACS Appl Mater Interfaces. 2015;7:8437–8450. doi: 10.1021/acsami.5b00215. [DOI] [PubMed] [Google Scholar]
- 89.Watson WE. The binding of actinomycin D to the nuclei of axotomized neurones. Brain Res. 1974;65:317–322. doi: 10.1016/0006-8993(74)90043-2. [DOI] [PubMed] [Google Scholar]
- 90.Wilson AD, Hart A, Brännström T, Wiberg M, Terenghi G. Delayed acetyl-L-carnitine administration and its effect on sensory neuronal rescue after peripheral nerve injury. J Plast Reconstr Aesthet Surg. 2007;60:114–118. doi: 10.1016/j.bjps.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 91.Xie Y, Yao Z, Chai H, Wong WM, Wu W. Potential roles of Alzheimer precursor protein A4 and beta-amyloid in survival and function of aged spinal motor neurons after axonal injury. J Neurosci Res. 2003;73:557–564. doi: 10.1002/jnr.10667. [DOI] [PubMed] [Google Scholar]
- 92.Xu XF, Zhang DD, Liao JC, Xiao L, Wang Q, Qiu W. Galanin and its receptor system promote the repair of injured sciatic nerves in diabetic rats. Neural Regen Res. 2016;11:1517–1526. doi: 10.4103/1673-5374.191228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yan Q, Snider WD, Pinzone JJ, Johnson EM. Retrograde transport of nerve growth factor (NGF) in motoneurons of developing rats: assessment of potential neurotrophic effects. Neuron. 1988;4:335–343. doi: 10.1016/0896-6273(88)90082-7. [DOI] [PubMed] [Google Scholar]
- 94.Yip HK, Johnson EM., Jr Developing dorsal root ganglion neurons require trophic support from their central processes: evidence for a role of retrogradely transported nerve growth factor from the central nervous system to the periphery. Proc Natl Acad Sci U S A. 1984;81:6245–6249. doi: 10.1073/pnas.81.19.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yousefi F, Lavi Arab F, Nikkhah K, Amiri H, Mahmoudi M. Novel approaches using mesenchymal stem cells for curing peripheral nerve injuries. Life Sci. 2019;221:99–108. doi: 10.1016/j.lfs.2019.01.052. [DOI] [PubMed] [Google Scholar]
- 96.Yu B, Zhou S, Hu W, Qian T, Gao R, Ding G, Ding F, Gu X. Altered long noncoding RNA expressions in dorsal root ganglion after rat sciatic nerve injury. Neurosci Lett. 2013;534:117–122. doi: 10.1016/j.neulet.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 97.Yuan Q, Wu W, So KF, Cheung AL, Prevette DM, Oppenheim RW. Effects of neurotrophic factors on motoneuron survival following axonal injury in newborn rats. NeuroReport. 2000;11:2237–2241. doi: 10.1097/00001756-200007140-00035. [DOI] [PubMed] [Google Scholar]
- 98.Zhang CG, Welin D, Novikov L, Kellerth JO, Wiberg M, Hart AM. Motorneuron protection by N-acetyl-cysteine after ventral root avulsion and ventral rhizotomy. Br J Plast Surg. 2005;58:765–773. doi: 10.1016/j.bjps.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 99.Zhang X, Verge VM, Wiesenfeld-Hallin Z, Piehl F, Hökfelt T. Expression of neuropeptides and neuropeptide mRNAs in spinal cord after axotomy in the rat, with special reference to motoneurons and galanin. Exp Brain Res. 1993;93:450–461. doi: 10.1007/BF00229360. [DOI] [PubMed] [Google Scholar]
- 100.Zhou XF, Deng YS, Chie E, Xue Q, Zhong JH, McLachlan EM, Rush RA, Xian CJ. Satellite-cell-derived nerve growth factor and neurotrophin-3 are involved in noradrenergic sprouting in the dorsal root ganglia following peripheral nerve injury in the rat. Eur J Neurosci. 1999;11:1711–1722. doi: 10.1046/j.1460-9568.1999.00589.x. [DOI] [PubMed] [Google Scholar]