Intron retention in aging and Alzheimer’s disease (AD): AD is an age-related neurodegenerative disorder with pathological accumulation of amyloid plaque (Masters et al., 2015), which can be classified into familial and sporadic form. In familial AD, mutations in genes encoding either amyloid precursor protein or presenilin (PS1 and PS2) cause overproduction of amyloid-42 molecules and early onset of dementia. Late-onset sporadic AD, which accounts for majority of the cases (> 95%), is characterized by high degree of genetic and pathological heterogeneity. Although aging and genetic variants are two risks factors for sporadic AD, it remains unclear how epigenetic alterations during aging may contribute to its etiology. To determine the transcriptional changes that are associated with aging, we analyzed the transcriptome of head or brain tissues isolated at different ages from adult Drosophila, mice and human (Adusumalli et al., 2019). We observed an increase in the level of intron retention (IR) in the mRNA transcripts during aging across different species. These retained introns share highly conserved features and surprisingly, do not affect the expression level of their mRNA transcripts. As many age-dependent IR mRNA transcripts overlapped with curated AD genes, we further compared the IR patterns in the frontal cortex and cerebellum isolated from large AD cohorts with their age-matched controls. Consistent with reports of aberrant splicing in AD (Tollervey et al., 2011; Bai et al., 2013; Raj et al., 2018), we found a significant increase in the rate of IR at genes that are involved in RNA processing and protein homeostasis in AD samples. Furthermore, many of these IR genes showed significant changes in their protein levels when compared to age-matched controls. Taken together, our findings suggest that increased IR is a transcriptional signature that is conserved across species during aging and may be linked to AD progression.
IR by alternative splicing has distinct biological roles in development and AD: Alternative splicing is a tightly regulated nuclear process that affects more than 95% of human genes and allows diversification of the proteome (Baralle and Giudice, 2017). Changes to the splicing patterns are prevalent during aging and its dysregulation has often been linked to neurodegenerative diseases like AD (Tollervey et al., 2011; Bai et al., 2013; Mazin et al., 2013; Raj et al., 2018). Alternative splicing can also cause specific intron(s) to be retained in the processed mRNA transcript. Indeed, many recent studies have shown that IR may play an active role in regulating gene expression and various biological processes via distinct mechanisms. In mammals, IR occur at ~75% of genes that contained multiple exons (Braunschweig et al., 2014). The mRNA transcripts with retained introns are frequently degraded by nonsense-mediated mRNA decay (NMD) to remove those mRNAs whose expression is not required for the functioning of the specific cell-types (Braunschweig et al., 2014). A similar mechanism is also in place to regulate the expression of functionally related genes during granulocyte differentiation (Wong et al., 2017). However, IR can also act as a strategy to allow rapid mobilization of selected mRNAs for protein translation in response to specific developmental cues. One example is the nuclear accumulation of specific mRNA transcripts with retained introns in mouse neocortical cells (Mauger et al., 2016). Upon N methyl-D-aspartate receptor and calmodulin-dependent kinase mediated neuronal activation, these mRNA transcripts are spliced and exported to the cytosol for protein translation (Mauger et al., 2016). Therefore, IR eliminates the time needed for de novo transcription of large genes, allowing cells to respond to the inducing neuronal signals promptly. Similar to the results in cell lines and model organisms, genome-wide analyses with human cohorts also revealed widespread changes in the splicing patterns during aging and AD pathogenesis. Transcriptome analyses of new-borns, young and old adult cerebellum and prefrontal cortex revealed age-dependent changes in splicing patterns at about 40% of the expressed genes (Mazin et al., 2013). High-resolution microarray analysis of the middle temporal gyrus in the brain cortex region from healthy individuals and AD patients revealed significant differences in the exon usage at specific genes during aging and AD pathogenesis (Tollervey et al., 2011). Splicing abnormalities and increased IR activities were also reported in the frontal and dorsolateral prefrontal cortex of AD patients (Bai et al., 2013; Raj et al., 2018). While these studies highlighted the changes in splicing patterns during aging and AD pathogenesis, the characteristics of mRNA transcripts with IR are not fully explored. As such, we used IRFinder algorithm (Wong et al., 2017) to systematically evaluate the level of IR in the heads or brain tissues isolated from Drosophila, mouse and human at different ages, as well as three independent AD cohorts (Adusumalli et al., 2019).
Compared to young adults, we observed increased IR in more than 340 genes in old flies. Surprisingly, the level of mRNA transcripts with retained introns remained unchanged, suggesting they may have evaded NMD. Genes with retained introns are enriched for distinct biological functions at different ages with approximately 15% of their human orthologues overlapped with curated AD genes from human DisGeNET database.
To address if age-dependent increase in IR is conserved in mammals, we analyzed the transcriptome of mouse frontal cortex, hippocampus and human prefrontal cortex. In both mouse and human, we saw an increase in the number of IR events in older brain tissues when compared to young adults. This suggests that elevated IR during aging is likely to be conserved across different species. Genes that exhibit differential IR with age share many common features across the three species, including shorter intron lengths, random distribution across the genome and unperturbed levels of mRNA expression. Similar to flies, approximately 15% of the differential IR genes in both mouse and human also overlapped with curated AD genes from human DisGeNET database. In aging human prefrontal cortex, the differential IR genes are enriched for the processes that regulate proteostasis. This suggests that changes in IR patterns during aging can impact protein homeostasis, which may in turn contribute to AD aetiology.
Reports of aberrant splicing in AD brain tissues (Tollervey et al., 2011; Bai et al., 2013; Raj et al., 2018) suggest the likelihood of widespread changes in IR patterns and proteomic composition across different parts of the diseased brain. To test this possibility, we analyzed the transcriptome of cerebellum and frontal cortex from AD cohorts. Our analyses revealed significant differences in the levels of IR at many genes between AD and their age matched control tissues. In cerebellum, more than 3800 differential IR events were observed between age-matched control and AD tissues. Among these differential IR events, approximately 80% showed increased IR in AD cerebellum. Genes with differentially retained introns are enriched for pathways that regulate different aspects of mRNA and protein homeostasis. This suggests a feedback mechanism where changes in IR patterns may further enhance aberrant splicing and protein aggregation in AD cerebellum. Similarly, compared to healthy frontal cortex, there are more than 1100 increased IR events occurring at ~780 genes in AD tissues. IR does not lead to NMD as mRNA expression appeared unperturbed. However, ~15% of these increased IR genes show significant changes in their protein levels between healthy control and AD frontal cortex (Ping et al., 2018). These results support the notion that translation of mRNA transcripts with retained intron may contribute to the aberrant proteomic landscape in AD frontal cortex.
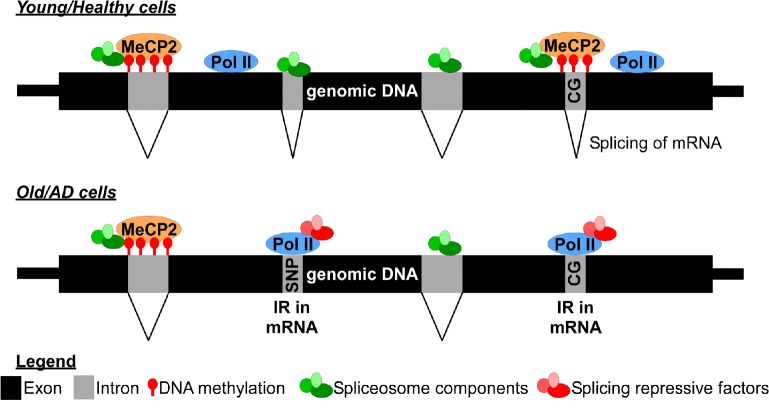
Although our study uncovered global alterations in IR patterns during aging and AD pathogenesis, the mechanisms underlying such changes remain unresolved. Recent evidence indicates that RNA splicing may be regulated through the interplay between genetic and epigenetic factors (Figure 1). Integrative analyses of transcriptomes and single nucleotide polymorphisms data from genome-wide association studies revealed that genetic variants at spliced acceptor/donor sites may impact the splicing or intron usage at some AD susceptibility loci (Raj et al., 2018). Retained introns were also shown to have higher CG content compared to the spliced introns, indicating that IR may be driven by the underlying DNA sequences (Adusumalli et al., 2019; Braunschweig et al., 2014; Wong et al., 2017). The strong correlation between RNA polymerase II stalling and higher level of retained introns at the CG-rich sequences on DNA suggests that IR may be regulated by the cross-talk between transcription and splicing machineries (Braunschweig et al., 2014). IR may also be regulated epigenetically given that CG-rich sequences are subjected to DNA methylation. Indeed, DNA methylation is required for the recruitment of MeCP2 and its interacting splicing factors to prevent IR during granulocytes differentiation (Wong et al., 2017). Insoluble aggregation of spliceosome component U1 small nuclear ribonucleoprotein into tangle-like inclusions was also correlated with aberrant splicing in the frontal cortex (Bai et al., 2013). This suggests that protein aggregation of splicing machinery may be one of the underlying causes of increased IR in AD. Interestingly, it was recently reported that dietary restriction, which extended the lifespan of Caenorhabditis elegans, could reduce the rate of IR during aging (Heintz et al., 2017). Taken together, these findings raise the intriguing possibility that the trajectory of aging and AD pathogenesis might be reverted through rectifying aberrant splicing and IR at specific genes.
Figure 1.
Epigentic regulation of intron retention during aging and AD pathogenesis.
Increased IR is observed during aging and in AD brain tissues. Retained introns are significantly shorter in length, have higher CG content and RNA polymerase II (Pol II) occupancy when compared to the spliced introns. DNA methylation can inhibit IR via recruitment of MeCP2 and its interacting spliceosome components. In AD brain tissues, aberrant IR can also be driven by genetic variants (SNPs) or by the insouble protein aggregation of splicesome components. AD: Alzheimer’s disease; IR: intron retention; MeCP2: methyl-CpG-binding protein 2.
In conclusion, our study established increased IR as one of the major outcomes of dysregulated splicing during aging and AD pathogenesis (Figure 1). It is plausible that aberrant splicing and IR at specific genes during aging may underlie the transition between healthy aging and different stages of AD pathology. To test this hypothesis, it would be necessary to analyse the IR patterns from cohorts that represent healthy, mild cognitive impairment and advanced stages of AD. The differential IR genes identified can then be tested in cellular and animal models to evaluate their roles in AD pathogenesis.
This work was supported by core funding provided by Temasek Life Sciences Laboratory.
We thanked Drs. Jun-Wei Pek, Henning Seedorf and Vinay Kumar Rao for critical reading and editing of the manuscript.
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Adusumalli S, Ngian ZK, Lin WQ, Benoukraf T, Ong CT. Increased intron retention is a post-transcriptional signature associated with progressive aging and Alzheimer’s disease. Aging Cell. 2019;18:e12928. doi: 10.1111/acel.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai B, Hales CM, Chen PC, Gozal Y, Dammer EB, Fritz JJ, Wang X, Xia Q, Duong DM, Street C, Cantero G, Cheng D, Jones DR, Wu Z, Li Y, Diner I, Heilman CJ, Rees HD, Wu H, Lin L, et al. (2013) U1 small nuclear ribonucleoprotein complex and RNA splicing alterations in Alzheimer’s disease. Proc Natl Acad Sci U S A. 110:16562–16567. doi: 10.1073/pnas.1310249110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baralle FE, Giudice J. Alternative splicing as a regulator of development and tissue identity. Nat Rev Mol Cell Biol. 2017;18:437–451. doi: 10.1038/nrm.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braunschweig U, Barbosa-Morais NL, Pan Q, Nachman EN, Alipanahi B, Gonatopoulos-Pournatzis T, Frey B, Irimia M, Blencowe BJ. Widespread intron retention in mammals functionally tunes transcriptomes. Genome Res. 2014;24:1774–1786. doi: 10.1101/gr.177790.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heintz C, Doktor TK, Lanjuin A, Escoubas C, Zhang Y, Weir HJ, Dutta S, Silva-García CG, Bruun GH, Morantte I, Hoxhaj G, Manning BD, Andresen BS, Mair WB. Splicing factor 1 modulates dietary restriction and TORC1 pathway longevity in C. elegans. Nature. 2017;541:102–106. doi: 10.1038/nature20789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer’s disease. Nat Rev Dis Primers. 2015;1:15056. doi: 10.1038/nrdp.2015.56. [DOI] [PubMed] [Google Scholar]
- 7.Mauger O, Lemoine F, Scheiffele P. Targeted intron retention and excision for rapid gene regulation in response to neuronal activity. Neuron. 2016;92:1266–1278. doi: 10.1016/j.neuron.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 8.Mazin P, Xiong J, Liu X, Yan Z, Zhang X, Li M, He L, Somel M, Yuan Y, Phoebe Chen YP, Li N, Hu Y, Fu N, Ning Z, Zeng R, Yang H, Chen W, Gelfand M, Khaitovich P. Widespread splicing changes in human brain development and aging. Mol Syst Biol. 2013;9:633. doi: 10.1038/msb.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ping L, Duong DM, Yin L, Gearing M, Lah JJ, Levey AI, Seyfried NT. Global quantitative analysis of the human brain proteome in Alzheimer’s and Parkinson’s Disease. Sci Data. 2018;5:180036. doi: 10.1038/sdata.2018.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raj T, Li YI, Wong G, Humphrey J, Wang M, Ramdhani S, Wang YC, Ng B, Gupta I, Haroutunian V, Schadt EE, Young-Pearse T, Mostafavi S, Zhang B, Sklar P, Bennett DA, De Jager PL. Integrative transcriptome analyses of the aging brain implicate altered splicing in Alzheimer’s disease susceptibility. Nat Genet. 2018;50:1584–1592. doi: 10.1038/s41588-018-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tollervey JR, Wang Z, Hortobágyi T, Witten JT, Zarnack K, Kayikci M, Clark TA, Schweitzer AC, Rot G, Curk T, Zupan B, Rogelj B, Shaw CE, Ule J. Analysis of alternative splicing associated with aging and neurodegeneration in the human brain. Genome Res. 2011;21:1572–1582. doi: 10.1101/gr.122226.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong JJ, Gao D, Nguyen TV, Kwok CT, van Geldermalsen M, Middleton R, Pinello N, Thoeng A, Nagarajah R, Holst J, Ritchie W, Rasko JEJ. Intron retention is regulated by altered MeCP2-mediated splicing factor recruitment. Nat Commun. 2017;8:15134. doi: 10.1038/ncomms15134. [DOI] [PMC free article] [PubMed] [Google Scholar]