Abstract
Alzheimer’s disease is a common progressive neurodegenerative disorder, pathologically characterized by the presence of β-amyloid plaques and neurofibrillary tangles. Current treatment approaches using drugs only alleviate the symptoms without curing the disease, which is a serious issue and influences the quality of life of the patients and their caregivers. In recent years, stem cell technology has provided new insights into the treatment of neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. Currently, the main sources of stem cells include neural stem cells, embryonic stem cells, mesenchymal stem cells, and induced pluripotent stem cells. In this review, we discuss the pathophysiology and general treatment of Alzheimer’s disease, and the current state of stem cell transplantation in the treatment of Alzheimer’s disease. We also assess future challenges in the clinical application and drug development of stem cell transplantation as a treatment for Alzheimer’s disease.
Keywords: Alzheimer's disease, ß-amyloid, drug development, embryonic stem cells, induced pluripotent stem cells, mesenchymal stem cells, nerve regeneration, neural regeneration, neural stem cells, neurodegenerative disorders, stem cell therapy
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder clinically characterized by loss of memory and cognitive dysfunction. It is the most common neurodegenerative form of dementia, accounting for 50–70% of these cases. The number of patients with dementia was estimated to be 46.8 million worldwide in 2015, and this number is expected to reach 131.5 million in 2050 (Prince et al., 2016). AD is classified as familial AD (fAD) or sporadic AD (sAD), with fAD mainly presenting with mutations in any of three genes: amyloid-β precursor protein (APP), presenilin 1 (PSEN1), and presenilin 2 (PSEN2), each encoding their respective proteins (Zhan et al., 2017; Filadi and Pizzo, 2019). The hallmark feature of AD is the accumulation of extracellular β-amyloid (Aβ) in senile plaques, followed by intracellular deposition of neurofibrillary tangles (NFTs) of abnormally hyperphosphorylated tau proteins (Xu, 2009). The mechanisms underlying AD remain unknown and there is no curative method or drug. With the increase in the number of the elderly individuals in society, AD is proving to be one of the greatest issues in clinical medicine. Efforts to target AD-related factors have shown promise in animal models but have failed during clinical trials (Huang and Mucke, 2012). Therefore, there is an urgent need to identify the mechanisms underlying AD and develop new therapeutic strategies for this disease.
Recently, a novel technique, stem cell therapy, has shown great potential in treating AD patients. This review will introduce the current treatment approaches for AD, and discuss the progress, challenges, and perspectives of stem cell therapy for AD. An online search of the PubMed database was performed for articles and reviews published from 1998–2018 with the terms “Alzheimer’s disease”, “stem cells therapy”, “neural stem cells”, “embryonic stem cells”, “mesenchymal stem cells”, and “induced pluripotent stem cells”. Articles relating to the mechanism of AD, and its treatment with neural stem cells (NSCs), mesenchymal stem cells (MSCs), and induced pluripotent stem cells (iPSCs) were included. These articles and reviews in the same field were published recently (1998–2018) and a total of 425 articles were retrieved, 108 of which were included according to inclusion criteria, and 317 eliminant ones were old or repeated.
Pathophysiology of Alzheimer’s Disease
With respect to onset, AD is divided into two types: late-onset AD, where the patients are usually older than 65 years; and early-onset AD, characterized by the appearance of marked AD symptoms at an early age (Rygiel, 2016). The two pathological features of AD are the deposition of Aβ and NFTs (Xu, 2009; Lin et al., 2018). Aβ is proteolytically derived from APP, which can be cleaved via two alternative pathways (Kikuchi et al., 2017): the amyloidogenic pathway and the non-amyloidogenic pathway (Figure 1). In the non-amyloidogenic pathway, βAPP is cleaved within the Aβ sequence byα-secretase, leading to the production of a membrane-anchored C-terminal fragment, CTFα. Alternatively, in the amyloidogenic pathway, APP is cleaved by beta-secretase to produce CTFβ. Both CTFα and CTFβ are subsequently cleaved by gamma-secretase to generate the short peptide p3 from CTFα, and Aβ from CTFβ (Xu, 2009). Soluble APP enhances proliferation of NSCs in vitro and in vivo and as a regulator of subventricular zone progenitor proliferation in the early central nervous system (Ohsawa et al., 1999; Caillé et al., 2004; Gakhar-Koppole et al., 2008; Li et al., 2019).
Figure 1.
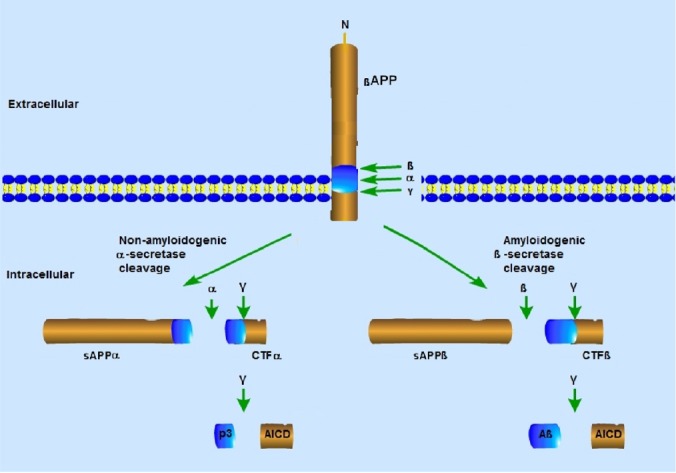
Secretase-mediated amyloid precursor protein processing pathways.
β-Amyloid (Aβ) is proteolytically derived from the amyloid-β protein precursor (APP), which can be cleaved via two alternative pathways: the amyloidogenic pathway and the non-amyloidogenic pathway. In the non-amyloid pathway, APP is first cleaved by the α-secretase ADAM metallopeptidase domain 10 leading to the release of the large N-terminal fragment (soluble APPα, sAPPα) and the generation of the C-terminal fragment (CTFα). In the amyloidogenic pathway, βAPP is first cleaved by β-secretase, leading to the release of the large N-terminal fragment (soluble APPβ, sAPPβ) and the generation of the C-terminal fragment (CTFβ). Both CTFα and CTFβ undergo further cleavage by gamma-secretase, resulting in the release of the intracellular domain (AICD) and the generation of a p3 fragment and Aβ from CTFα and CTFβ, respectively.
Another key factor, tau, is a neuronal microtubule-associated protein that plays a crucial role when phosphorylated. In the neuronal cytoplasm it can aggregate microtubules, which are major constituents of NFTs (Iqbal et al., 1998; Zhang et al., 2019). Although it is not certain whether tau pathology is dependent on Aβ aggregation in AD, tau protein is required for the toxic effects of Aβ aggregation, because no neurodegeneration is observed upon depletion of tau protein. Furthermore, tau deletion decreases intracellular Aβ clearance and increases extracellular Aβ plaques (Zhagn and Li, 2014).
As mentioned previously, three genes have been implicated in fAD: APP, PSEN1, and PSEN2, which are involved in the function of the γ-secretase complex, Aβ aggregation, and neurodegeneration (Cacquevel et al., 2012). The main risk factors for sAD include apolipoprotein E (APOE), which may affect Aβ clearance, contributing to the development of AD. The triggering receptor expressed on myeloid cells 2 (TREM2), which is selectively expressed by microglia in the brain, induces phagocytosis and influences the inflammatory response (Kanekiyo et al., 2014; Yeh et al., 2016). Further, the TREM2-APOE pathway can regulate microglial phenotypic changes in neurodegenerative diseases, and is crucial for the restoration of microglial homeostasis (Krasemann et al., 2017). To date, analyses of millions of polymorphisms in the human genome from thousands of people have revealed a number of new loci associated with AD risk, including Cluster of differentiation 33 (CD33), clusterin (CLU), Fermitin family homolog-2 (FERMT2), HLA-DRB5-DBR1, and Inositol polyphosphate-5-phosphatase (INPP5D) (Karch and Goate, 2015).
General Treatment for Alzheimer’s Disease
The research and development of anti-AD drugs or antibodies mainly focuses on three targets: 1) anti-oxidation, 2) removal of Aβ deposits in the brain, delaying cognitive impairment, and 3) regulation of the phosphorylation of tau protein and reduction in misfolding and abnormal agglomeration (Godyń et al., 2016; Wisniewski and Drummond, 2016; Ibrahim and Gabr, 2019). At present, clinical treatment using anti-AD drugs mainly involves the use of acetyl cholinesterase inhibitors to improve cognitive ability, and N-methyl-D-aspartate receptor antagonists, such as memantine, or other inhibitors to ameliorate the patient’s symptoms, but none of these agents can cure the condition (Coley et al., 2015; Zhang et al., 2019).
A human monoclonal antibody, aducanumab, from Biogen Inc. (Cambridge, MA, USA) can selectively bind aggregated Aβ, and enter the brain to reduce the Aβ level in a dose-dependent manner in a rodent AD model and in patients with AD (Budd Haeberlein et al., 2017). Although high doses of this drug may lead to brain fluid transfer and increase the risk of hematencephalon, this adverse effect can be detected in the initial stages using magnetic resonance imaging. The finding obtained with this drug is currently being validated in ongoing phase 3 clinical trials (Sevigny et al., 2017).
Another drug, ANAVEX 3-71, from Anavex Life Sciences (New York City, NY, USA) can attenuate the cognitive defect and pathological process in AD via the induction of Sigma-1 and M1 receptors. A recent study demonstrated that Sigma-1 receptor (S1R) agonists can induce oxidative stress in mitochondria and enhance complex I activity-induced by Aβ1–42. Sigma-1 receptor is highly expressed in the central nervous system, and is required for Aβ deposition and tau protein hyperphosphorylation, which occur in the late stage of AD (Goguadze et al., 2019). Goguadze et al. (2019) showed that the attenuation of AD symptoms may require vaccines targeting Aβ and tau protein simultaneously. Vaxine Pty Ltd. (Adelaide, Australia) developed a new technology, Vaxine Advax adjuvant, which could be the key point in vaccines for AD, and serves as a promising strategy for ongoing preclinical evaluation and human clinical trials (Davtyan et al., 2016).
Screening of small molecule compounds may be another focus area in AD drug development, because small molecule compounds regulate neuronal injury and enhance nerve regeneration. One study showed that allopregnanolone, an endogenous neurosteroid, improved neuronal degeneration, induced nerve differentiation both in vitro and in vivo, and restored learning and memory performance in mice (Singh et al., 2012). Apigenin and related compounds extracted from food products enhanced neuronal generation and improved learning and memory performance in rat models and adult humans (Taupin, 2009). As reported, several small molecule compounds, including daucosterol, protocatechuic acid, and fluoxetine, can induce proliferation, differentiation, and migration of neural progenitor cells (NPCs), prevent NPC apoptosis, promote nerve regeneration, and alleviate learning deficits in rodent models (Guan et al., 2008; Jiang et al., 2014; Khademi et al., 2018). However, how these small molecules affect the signaling of NSCs remains unclear. It is a complex process to regulate the biological behavior of NSCs, and further study is required to confirm the exact beneficial concentrations and treatment times.
Stem Cell Treatment for Alzheimer’s Disease
Drug treatment is a vital clinical strategy and, to date, it mainly delays neuronal degeneration and is mainly of thera-peutic benefit only in patients with early-onset AD. Stem cell therapy provides new potential in the treatment of AD due to the self-renewal ability and high differentiation potential of stem cells. Cell transplantation as a therapy for neuro-degenerative disorders was first explored in Parkinson’s disease several years ago (Lindvall et al., 1988). Based on the preclinical effect in Parkinson’s disease, the technique was applied to other neurodegenerative disorders, such as AD, amyotrophic lateral sclerosis, and Huntington’s disease. Moreover, stem cells are unspecialized cells with the capacity to differentiate into neural cells in the brain microenvironment, and can restore neuroplasticity and neurogenesis via neurotrophic factors (Enciu et al., 2011). On the basis of their ability to differentiate into various cellular types (Lee et al., 2016b), stem cells can be classified in four types: NSCs, MSCs, embryonic stem cells (ESCs), and iPSCs. Each of these cell types shows unique properties that could be utilized in stem cell therapy regimes in a variety of ways (Figure 2).
Figure 2.
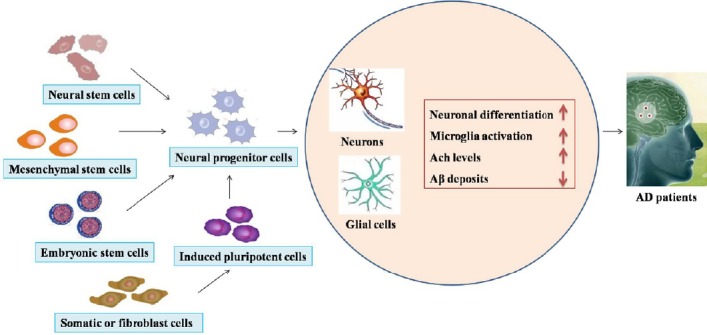
The application of stem cell therapy in Alzheimer’s disease.
Stem cells can be classified in four types: neural stem cells, mesenchymal stem cells, embryonic stem cells, and induced pluripotent stem cells. All have the potential to generate the neural progenitor cells, resulting in the generation of new neurons or the replacement of damaged neurons. Each of these cell types shows unique properties in a variety of ways, such as promoting neuronal differentiation, microglia activation, and acetylcholine (Ach) levels of neuronal cells, and decreasing β-amyloid (Aβ) deposits in Alzheimer’s disease (AD) patients.
Neural stem cells
Capable of generating all the differentiated neural cells in the central nervous system, NSCs can be sourced from primary tissues, such as the fetal, neonatal, and adult brain, or from ESCs and iPSCs (Tong et al., 2015). In AD models, a growing number of studies have suggested that NSC therapy is a promising therapeutic strategy, showing both in vitro and in vivo improvements in AD pathologies and behaviors (Zhao, 2016; Wang et al., 2017).
Blurton-Jones et al. (2009) first discovered the potential benefits of NSCs in delivering neu-rotrophic support in an AD mouse model. They demonstrated that transplantation of mouse NSCs into the hippocampus of AD mice rescued hippocampus-dependent learning and memory. They further showed that NSCs generated high levels of brain-derived neurotrophic factor in vitro and also increased brain-derived neurotrophic factor protein levels in the brain upon NSC transplantation, which was required for behavioral and synaptic responses in AD mice (Wu et al., 2016; Marsh and Blurton-Jones, 2017). Zhang et al. (2017) also demonstrated that NSC transplantation can rescue cognitive and synaptic deficits in specific regions. Recovery of neuronal function is crucial for learning and memory in AD mouse models (Marsh and Blurton-Jones, 2017; Wang et al., 2017).
Another study indicated the potential of NSCs to produce neurotrophins for treatment of AD, because modified human NPCs could rescue Aβ42-induced cell death by secreting neuronal trophic factors, including brain-derived neurotrophic factor, insulin-like growth factor 1 or glial-cell derived neurotrophic factor, which were capable of enhancing cholin-ergic functions (Kitiyanant et al., 2012).
As mentioned above, neurotrophins can promote survival and differentiation of grafted NSCs. A decrease in neuro-trophin expression and impaired neurotrophin function in axonal transport have been observed in patients with AD and animal models, indicating that neurotrophins play a significant role in neuronal death and axon degeneration (Poon et al., 2011). Modified NSC-hNGF-eGFPs can survive and integrate into the brain of AD rat models, secrete neurotrophic factors, and replace the loss of neurons. Further, transplantation of NSCs can improve cognitive performance in the presence of nerve growth factor (NGF), and offer feasible therapeutic approaches for AD (Wu et al., 2008). However, there are many limiting factors associated with the establishment of NSC lines in vitro. To overcome these problems, one approach is to generate NSCs as floating spherical aggregates, termed neurospheres, which are a mixture of NSCs and progenitors that lead to the expansion of NSCs (Kim et al., 2009). Generally, neurospheres are generated in vitro after 10 days of culturing in the presence of epidermal growth factor or basic fibroblast growth factor, which seem more sensitive to the microenvironment and improve host neural repair upon triggering the secretion of neurotrophins more than fibroblasts (Bonnamain et al., 2012). Another method involves the combination of epigenetic and genetic im-mortalization strategies, by which cells are triggered with an immortalizing gene (e.g., v-myc, c-myc, SV40T, or TERT), and the proliferation and differentiation is impaired in the presence of growth factors (Villa et al., 2009).
Grafted NSCs can also transfer potential therapeutic agents or proteins into host tissues in AD mouse models, such as neprilysin, insulin-degrading enzyme, plasmin, and cathepsin B, to reduce Aβ levels (Kim and de Vellis, 2009). Injected fibroblasts have been shown to reduce Aβ plaque production in AD mice models in the presence of neprilysin, and NSCs overexpressing the same neprilysin gene can induce greater reduction of Aβ plaques in AD mice models (Chen et al., 2012b; Choi et al., 2014).
However, transplantation of NSCs does not always yield predictable outcomes, and migration and differentiation may be significantly affected by the recipient’s brain microenvironment. Overexpression of human APP changes the cell fate of human NSCs leading to the generation of more astrocytes than neurons, indicating the negative influence of APP processing on the therapeutic effect of the grafted NSCs (Kwak et al., 2006).
Human NSCs grafted into 3xTg-AD mice differentiated into neural cell types of the central nervous system, restored recognition, and improved learning and memory defects via attenuation of Aβ accumulation and tau phosphorylation, while transplanted human NSCs grafted into NSE/APPsw mice expressing human APP only increased synaptogenesis without curtailing the other deficits (Ager et al., 2015; Li et al., 2016c). Additionally, NGF nanoparticles may be used to release NGF to enhance the generation of cholinergic neurons in comparison with that achieved by NSC transplantation in AD rat models, providing new insights into the research and development of clinical applications (Chen et al., 2015b; Corrêa-Velloso et al., 2018).
Mesenchymal stem cells
As multi-potent stem cells, MSCs can generate diverse cell types in the bone marrow, adipose tissue, lungs, liver, and umbilical cord (Phinney and Prockop, 2007). Isolated MSCs can expand and differentiate into osteoblasts, adipocytes, and pancreatic islets (Dominici et al., 2006).
Several types of MSC show beneficial effects on neurological disorders via secretion of pro-inflammatory cytokines. The mechanism of treatment using placenta-derived MSCs and human umbilical cord-derived MSCs for hypox-ia-ischemia brain damage partially involves triggering inflammatory responses including tumor necrosis factor-α, interleukin (IL)-17, interferon-γ, IL-10, and IL-8 (Zhou et al., 2015; Ding et al., 2017). Bone marrow MSCs (BMMSCs) have been shown to be involved in immune system disorders and neurodegeneration disease mediated by immune factors, including IL-1β, IL-6, IL-17, and tumor necrosis factor-α (Liu et al., 2015, 2017; Ma et al., 2015; Cui et al., 2018).
In vitro, human MSCs are able to dramatically increase hippocampal neurogenesis and trigger the differentiation of NPCs into mature neurons via the Wnt signaling pathway (Oh et al., 2015). Further, human MSCs could decrease the levels of Aβ42 by enhancing autophagy in vitro and in vivo (Shin et al., 2014). Moreover, transplantation of BMMSCs and human umbilical cord blood-derived MSCs into the lateral ventricles or hippocampi of AD mouse models improves memory and spatial learning by reducing Aβ42 deposits and increasing neuronal survival (Salem et al., 2014; Kim et al., 2015; Matchynski-Franks et al., 2016; Oron and Oron, 2016). Similarly, autologous BMMSCs have been successfully transfused into the brains of patients with ischemic disorder, and the graft of human MSCs led to a reduced infarct size and functional improvement (Honmou et al., 2011).
Adipose-derived stem cells (ADSCs) isolated from rats can trigger differentiation into neurons or astrocyte-like cells in vitro. Transplantation of ADSCs can improve neural function, demonstrating that ADSCs can facilitate beneficial neural differentiation and induce functional improvement in rats (Chen et al., 2012a). When human ADSCs were in-travenously injected into AD mouse models, strong signals in the brain were found up to 12 days after injection using a Maestro Imaging System (Ha et al., 2014). Further, in rodent models, transplantation of ADSCs can ameliorate cognitive impairment and improve learning and memory via: (1) reduction of oxidative stress; (2) prevention of Akt activity, and activation glycogen synthase kinase-3β; or (3) decreasing Aβ levels, upregulating IL-10 and vascular endothelial growth factor levels, enhancing neurogenesis, and stabilizing synapses and dendrites (Chang et al., 2014; Yan et al., 2014; Yamazaki et al., 2015).
The use of MSCs has been reported to be a promising strategy for stem cell treatments in comparison with that of NSCs. However, many types of MSCs have various drawbacks in application. Extraction and culture of BMMSCs is difficult, which limits their use in clinical trials. Despite these limitations, in recent years, ADSCs and human umbilical cord blood-derived MSCs have been shown to be novel options for AD therapy (Wang et al., 2017).
Embryonic stem cells
As self-renewing, totipotent stem cells, ESCs innately differentiate into various neuronal phenotypes in vitro, including dopaminergic neurons (Martínez-Morales et al., 2013), spinal motor neurons (Hu and Zhang, 2009), and glial cells (Krencik et al., 2011), and are extracted from the inner cell mass of blastocysts (Glat and Offen, 2013; Tong et al., 2015). It has been reported that ESC-derived NSCs can be safely transplanted without tumor formation in AD animal models; however, these results need to be validated (Borlongan, 2012; Kim et al., 2013; Tong et al., 2015).
As reported, ESCs can be induced into NPCs in vitro. When these cells are transplanted into AD animal models, a certain the MESPU35 ES cell line may show effective therapeutic activity under certain conditions. Escape latency in the Morris water maze test was found to be dramatically higher than that in control groups after ESC-derived NPC transplantation into an Aβ-injured rat model for 2 weeks. The mean time of NPC-treated group only is about 36 seconds, and control group is about 42 seconds. Sixteen weeks after transplantation, the escape latency had dramatically de-creased in comparison with that in the sham controls. Further, ESC-derived NPCs can also differentiate into astrocytic and neuron-like cells in vivo. These data demonstrate that ESC-derived NPC transplantation can improve memory impairment in AD models (Tang et al., 2008).
A few clinical trials involving grafting of human ESCs have already been initiated. The first US Food and Drug Ad-ministration-approved clinical trial using human ESC-derived cells was started in 2010 by Geron Corp. (Menlo Park, CA, USA), generating oligodendrocyte progenitors for spinal cord injury (Baker, 2011). In late 2011, because unex-pected results, and not apply for patents in Europe, Geron Corp. stopped this clinical trial. The San Francisco-based company Asterias Biotherapeutics/BioTime (Alameda, CA, USA) has purchased the shuttered stem cell program and may reinitiate the trials for the use of human ESC-to treat spinal cord injury in the next two years. Hopefully, more valuable information will be available for the treatment of patients with AD in the future (Martínez-Morales et al., 2013; Scott and Magnus, 2014).
Nevertheless, despite their many advantages, ESCs are associated with high risks of transplantation rejection and immune responses (Martínez-Morales et al., 2013; Wray and Fox, 2016). Unlike the use of NSCs and MSCs, direct transplantation of ESCs may be a concern because of the possibility of tumorigenesis and teratoma formation (Cui et al., 2013; Alonso-Alonso and Srivastava, 2015). There is insufficient information regarding the unique properties and culture conditions of ESCs, including the variability of donor cells, which have been reported for differently established ESC lines (Tong et al., 2015). Although the brain is thought to be immune-privileged, a very few donor cells will remain human leukocyte antigen haplotype-matched, necessitating a degree of immunosuppression in the recipients to avoid cell transplantation-induced immune rejection. Although a few exceptions exist, newer approaches are needed to improve donor cell and recipient compatibility and prevent immune rejection in the future (Hallett et al., 2014; Chen et al., 2015a).
Induced pluripotent stem cells
Typically, iPSCs are derived from mouse embryonic or adult fibroblasts through the introduction of reprogramming factors POU domain class 5 transcription factor 1 (POU5F1, also known as OCT3/4), sex determining region Y-box 2 (SOX2), Krüppel-like factor 4 (KLF4) and myelocytomatosis oncogene (c-MYC) (together referred to as OSKM), which facilitate maintenance of pluripotency, and lead to differentiation into neural cells (Fan et al., 2014; Takahashi and Yamanaka, 2015). In 2016, Dr. Shinya Yamanaka’s group generated the first iPSCs upon induction of these transcription factors through retrovirus (Takahashi and Yamanaka, 2006). So far, many novel methods have significantly improved the reprogramming techniques to increase success rates. A potentially powerful tool in addressing the mechanism of AD, iPSCs show the most identical properties compared to ESCs, including morphology, the ability to differentiate into any cell type, and unlimited growth (Yamanaka, 2009; Imm et al., 2017).
Human-sourced iPSCs also provide a beneficial tool for cell-replacement therapy against AD. Peripheral blood mon-onuclear cells from patients with fAD or sAD, or early or late-onset AD can be converted into iPSCs (Israel et al., 2012; Lee et al., 2016a; Zhang et al., 2017). Furthermore, iPSCs can be generated from patients with AD carrying a mutation in PSEN1 or APP (Muratore et al., 2014; Li et al., 2016a, b; Tubsuwan et al., 2016; Yang et al., 2016). Human iPSCs could be a prospective model for detecting the synaptotoxic effect of Aβ, and may indicate Aβ-induced alterations in postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors and tau protein phos-phorylation (Nieweg et al., 2015). In addition, the loss of forebrain cholinergic neurons plays a key role in AD. Thus, sAD-iPSC-derived FBCNs with a suitable APOE genotype (ε3/ε4) are more vulnerable to glutamate-mediated cell death following an increased Aβ42/Aβ40 ratio than FBCNs derived from healthy people (Duan et al., 2014). Neurons generated from iPSCs are also used for testing small molecular chemical compounds in therapeutic drug screens (Xu et al., 2013; Robbins and Price, 2017). As reported, human pluripotent stem cells exhibit enhanced assembly of the T-complex protein 1-ring complex (TRiC)/chaperonin containing T-complex protein 1 (CCT) complex, which is re-quired for the striking ability of pluripotent stem cells to maintain proteostasis of the aggregation prone huntingtin in Huntington’s disease. Likely, increased expression of CCT8, a single subunit, extends Caenorhabditis elegans lifespan in a TRiC/CCT-dependent manner (Noormohammadi et al., 2016).
AD-iPSCs can be obtained via transcription activator-like effector nuclease or clustered regulatory interspaced short palindromic repeats technology, and repaired AD-iPSCs are now being utilized for cell transplantation to control the disease process (Mungenast et al., 2016; Yang et al., 2016). However, there are several challenges in the clinical use of iPSCs for patients with AD. A report showed that iPSCs could be derived from somatic cells via reprogramming factors, and the reprogramming process reset the somatic cell to a more youthful state, elongating telomeres, and rearranging the mitochondrial network, indicating that iPSCs could not mimic ESCs (Rohani et al., 2014).
Challenges and Promises
Considering the many advantages of stem cell therapy, more and more attention has been paid to it in scientific and medical research. In 2010, the global stem cell treatment market reached 10.9 billion US dollars. In 2017, the global stem cell treatment market share increased to 51.26 billion US dollars (Figure 3). The emergence of stem cells has brought new light to the disease. Stem cells used in cellular assays and animal models have achieved certain results, but there are still many problems to be solved before they can be extended to clinical applications (Table 1).
Figure 3.
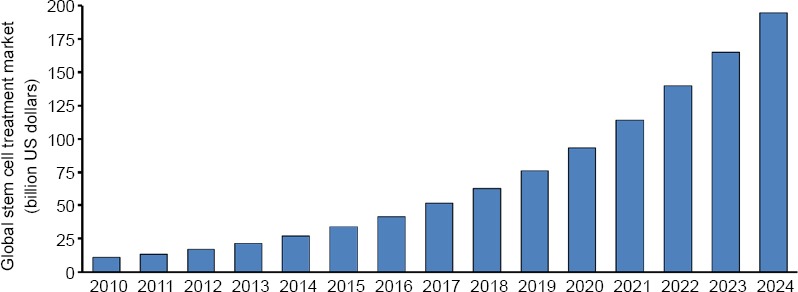
The global stem cell treatment market in 2010–2024.
Table 1.
Advantages and disadvantages of stem cells
| Cells | Source | Advantages | Disadvantages |
|---|---|---|---|
| Neural stem cells | Primary tissues, (fetal, neonatal, and adult brain) or embryonic stem cells and induced pluripotent stem cells | (1) Easy to access; | (1) Strong immunogenicity; |
| (2) No ethical issues; | (2) The mechanism of cell proliferation, differentiation, and migration is unclear | ||
| (3) No histocompatibility. | . | ||
| Mesenchymal stem cells | Bone marrow, adipose tissue, and umbilical cord | (1) Widespread sources; | (1) Bone marrow mesenchymal stem cells-limited raw materials, poor proliferation, and traumatic; |
| (2) Secrete multiple bioactive factors; | (2) No unified identification standard for umbilical cord blood mesenchymal stem cells, and the culture technology in vitro and differentiation are not yet mature. | ||
| (3) Directional migration. | |||
| Embryonic stem cells | Early embryo | (1) Strong proliferation ability; | (1) Ethical issues; |
| (2) Abundant sources; | (2) The allograft produces a great rejection reaction; | ||
| (3) Can be passed on. | (3) Unrestrained differentiation; | ||
| (4) Tumorigenicity. | |||
| Induced pluripotent stem cells | Gene recombination | (1) No ethical issues; | (1) Complex operation process; |
| (2) No histocompatibility. | (2) Low reprogramming efficiency; | ||
| (3) Mutation induction; | |||
| (4) Tumorigenicity. |
Each stem cell has a specific neurogenic potential and can achieve certain results, but there are still many problems to be solved before they can be used for clinical applications.
NSCs can self-replicate and differentiate into neurons in a certain micro-environment, and NSC grafts have obvious therapeutic effects on AD mouse models (Li et al., 2016c). However, direct transplantation of NSCs is not feasible because of their limited source and compulsive immunogenicity.
In addition to having self-renewing ability, multi-differentiating potential, rich source, and low immune rejection rates, MSCs are not susceptible to teratogenicity, and proliferate quickly in vitro. Thus, MSCs have their own advantages in clinical application. However, the differentiating potential of MSCs is less than that of ESCs, and they may have un-controllable factors upon transplantation, which influences the application of MSC in clinical treatment (Sugaya and Merchant, 2008).
Similarly, there are many unsolved problems relating to the application of iPSC in AD clinical treatment (Yagi et al., 2012). The number of iPSCs required is not clear because inducing iPSC differentiation into neurons over an extended period may trigger PSEN1 or PSEN2 mutations, affecting the morphology and function of the neurons. In addition to the grafted stem cell types, the target transplantation region is another key factor. The pathogenesis of AD is unclear and AD-related damage has been observed in all parts of the brain, which makes it difficult to determine the optimum graft region.
Freeman et al. (2017) introduced a new molecular system that can regulate different bioactive signals via orthogonal DNA handles, and discovered that NSCs derived from the murine spinal cord organize themselves as neurospheres, and can disperse and migrate upon induction by an exogenous signal and then regroup into neurospheres when the signal is withdrawn, which are an attractive model to study the important role of cell-matrix interactions in the stem cell therapy and provides new avenues for developing rationally designed dynamic regenerative biomaterials in clinical application as a treatment for AD treatment.
Conclusion
An increasing amount of attention is being paid to stem cell therapy. Recent studies have suggested that it may play a key role in the treatment of neurodegenerative disorders, including AD, Parkinson’s disease, and amyotrophic lateral sclerosis. Although, there are many unsolved problems and challenges related to the use of stem cells, the data indicate that stem cell therapy is still a prospective method for AD treatment.
Additional file: Open peer review reports 1 (106.9KB, pdf) and 2 (108KB, pdf) .
Footnotes
Conflicts of interest: There are no conflicts of interest associated with this manuscript.
Financial support: The work was supported the National Natural Science Foundation of China, No. 81701076 (to LLZ) and No. 31670795 (to XQF), 2017 Changbai Mountain Research Support Foundation, No. 440050117010 (to XQF), Opening Project of Zhejiang Provincial Top Key Discipline of Pharmaceutical Sciences, No. YKFJ2-007 (to LLZ), grants from the Science and Technology Department of Jilin Province, China, No. 20190701037GH (to FQZ), 20180520138JH (to FQZ), 20190701036GH (to LLZ). The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, or in the decision to submit the paper for publication..
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Wipawan Thangnipon, Mahidol University, Thailand; Bruno Vincent, Mahidol University, Thailand.
Funding: The work was supported by the National Natural Science Foundation of China, No. 81701076 (to LLZ) and No. 31670795 (to XQF), 2017 Changbai Mountain Research Support Foundation, No. 440050117010 (to XQF), Opening Project of Zhejiang Provincial Top Key Discipline of Pharmaceutical Sciences, No. YKFJ2-007 (to LLZ), grants from the Science and Technology Department of Jilin Province, China, No. 20190701037GH (to FQZ), 20180520138JH (to FQZ), 20190701036GH (to LLZ)..
P-Reviewers: Thangnipon W, Vincent B; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y
References
- 1.Ager RR, Davis JL, Agazaryan A, Benavente F, Poon WW, LaFerla FM, Blurton-Jones M. Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer’s disease and neuronal loss. Hippocampus. 2015;25:813–826. doi: 10.1002/hipo.22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso-Alonso ML, Srivastava GK. Current focus of stem cell application in retinal repair. World J Stem Cells. 2015;7:641–648. doi: 10.4252/wjsc.v7.i3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker M. Stem-cell pioneer bows out. Nature. 2011;479:459. doi: 10.1038/479459a. [DOI] [PubMed] [Google Scholar]
- 4.Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Muller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnamain V, Neveu I, Naveilhan P. Neural stem/progenitor cells as a promising candidate for regenerative therapy of the central nervous system. Front Cell Neurosci. 2012;6:17. doi: 10.3389/fncel.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borlongan CV. Recent preclinical evidence advancing cell therapy for Alzheimer’s disease. Exp Neurol. 2012;237:142–146. doi: 10.1016/j.expneurol.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cacquevel M, Aeschbach L, Houacine J, Fraering PC. Alzheimer’s disease-linked mutations in presenilin-1 result in a drastic loss of activity in purified gamma-secretase complexes. PLoS One. 2012;7:e35133. doi: 10.1371/journal.pone.0035133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caillé I, Allinquant B, Dupont E, Bouillot C, Langer A, Müller U, Prochiantz A. Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development. 2004;131:2173–2181. doi: 10.1242/dev.01103. [DOI] [PubMed] [Google Scholar]
- 9.Chang KA, Kim HJ, Joo Y, Ha S, Suh YH. The therapeutic effects of human adipose-derived stem cells in Alzheimer’s disease mouse models. Neurodegener Dis. 2014;13:99–102. doi: 10.1159/000355261. [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Li Y, Lin X, Cui D, Cui C, Li H, Xiao L. Functional disruption of human leukocyte antigen II in human embryonic stem cell. Biol Res. 2015a;48:59. doi: 10.1186/s40659-015-0051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Tang YX, Liu YM, Chen J, Hu XQ, Liu N, Wang SX, Zhang Y, Zeng WG, Ni HJ, Zhao B, Chen YF, Tang ZP. Transplantation of adipose-derived stem cells is associated with neural differentiation and functional improvement in a rat model of intracerebral hemorrhage. CNS Neurosci Ther. 2012a;18:847–854. doi: 10.1111/j.1755-5949.2012.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen SQ, Cai Q, Shen YY, Wang PJ, Teng GJ, Li MH, Zhang W, Zang FC. (1)H-MRS evaluation of therapeutic effect of neural stem cell transplantation on Alzheimer’s disease in AβPP/PS1 double transgenic mice. J Alzheimers Dis. 2012b;28:71–80. doi: 10.3233/JAD-2010-110893. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Pan C, Xuan A, Xu L, Bao G, Liu F, Fang J, Long D. Treatment efficacy of NGF nanoparticles combining neural stem cell transplantation on Alzheimer’s disease model rats. Med Sci Monit. 2015b;21:3608–3615. doi: 10.12659/MSM.894567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi SS, Lee SR, Kim SU, Lee HJ. Alzheimer’s disease and stem cell therapy. Exp Neurobiol. 2014;23:45–52. doi: 10.5607/en.2014.23.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coley N, Gallini A, Andrieu S. Prevention studies in Alzheimer’s disease: progress towards the development of new therapeutics. CNS Drugs. 2015;29:519–528. doi: 10.1007/s40263-015-0256-9. [DOI] [PubMed] [Google Scholar]
- 16.Corrêa-Velloso JC, Gonçalves MCB, Naaldijk Y, Oliveira-Giacomelli Á, Pillat MM, Ulrich H. Pathophysiology in the comorbidity of Bipolar Disorder and Alzheimer’s Disease: pharmacological and stem cell approaches. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80:34–53. doi: 10.1016/j.pnpbp.2017.04.033. [DOI] [PubMed] [Google Scholar]
- 17.Cui GH, Wu J, Mou FF, Xie WH, Wang FB, Wang QL, Fang J, Xu YW, Dong YR, Liu JR, Guo HD. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating in-flammatory responses in APP/PS1 mice. FASEB J. 2018;32:654–668. doi: 10.1096/fj.201700600R. [DOI] [PubMed] [Google Scholar]
- 18.Cui L, Guan Y, Qu Z, Zhang J, Liao B, Ma B, Qian J, Li D, Li W, Xu GT, Jin Y. WNT signaling determines tumorigenicity and function of ESC-derived retinal progenitors. J Clin Invest. 2013;123:1647–1661. doi: 10.1172/JCI65048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davtyan H, Zagorski K, Rajapaksha H, Hovakimyan A, Davtyan A, Petrushina I, Kazarian K, Cribbs DH, Petrovsky N, Agadjanyan MG, Ghochikyan A. Alzheimer’s disease Advax(CpG)-adjuvanted MultiTEP-based dual and single vaccines induce high-titer antibodies against various forms of tau and Abeta pathological molecules. Sci Rep. 2016;6:28912. doi: 10.1038/srep28912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding H, Zhang H, Ding H, Li D, Yi X, Ma X, Li R, Huang M, Ju X. Transplantation of placenta-derived mesenchymal stem cells reduces hypoxic-ischemic brain damage in rats by ameliorating the inflammatory response. Cell Mol Immunol. 2017;14:693–701. doi: 10.1038/cmi.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 22.Duan L, Bhattacharyya BJ, Belmadani A, Pan L, Miller RJ, Kessler JA. Stem cell derived basal forebrain cholinergic neurons from Alzheimer’s disease patients are more susceptible to cell death. Mol Neurodegener. 2014;9:3. doi: 10.1186/1750-1326-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enciu AM, Nicolescu MI, Manole CG, Muresanu DF, Popescu LM, Popescu BO. Neuroregeneration in neurodegenerative disorders. BMC Neurol. 2011;11:75. doi: 10.1186/1471-2377-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan X, Sun D, Tang X, Cai Y, Yin ZQ, Xu H. Stem-cell challenges in the treatment of Alzheimer’s disease: a long way from bench to bedside. Med Res Rev. 2014;34:957–978. doi: 10.1002/med.21309. [DOI] [PubMed] [Google Scholar]
- 25.Filadi R, Pizzo P. Defective autophagy and Alzheimer’s disease: is calcium the key? Neural Regen Res. 2019;14:2081–2082. doi: 10.4103/1673-5374.262584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freeman R, Stephanopoulos N, Álvarez Z, Lewis JA, Sur S, Serrano CM, Boekhoven J, Lee SS, Stupp SI. Instructing cells with pro-grammable peptide DNA hybrids. Nat Commun. 2017;8:15982–15982. doi: 10.1038/ncomms15982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gakhar-Koppole N, Hundeshagen P, Mandl C, Weyer SW, Allinquant B, Muller U, Ciccolini F. Activity requires soluble amyloid precursor protein alpha to promote neurite outgrowth in neural stem cell-derived neurons via activation of the MAPK pathway. Eur J Neurosci. 2008;28:871–882. doi: 10.1111/j.1460-9568.2008.06398.x. [DOI] [PubMed] [Google Scholar]
- 28.Glat MJ, Offen D. Cell and gene therapy in Alzheimer’s disease. Stem Cells Dev. 2013;22:1490–1496. doi: 10.1089/scd.2012.0633. [DOI] [PubMed] [Google Scholar]
- 29.Godyń J, Jończyk J, Panek D, Malawska B. Therapeutic strategies for Alzheimer’s disease in clinical trials. Pharmacol Rep. 2016;68:127–138. doi: 10.1016/j.pharep.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Goguadze N, Zhuravliova E, Morin D, Mikeladze D, Maurice T. Sigma-1 receptor agonists induce oxidative stress in mitochondria and enhance complex I activity in physiological condition but protect against pathological oxidative stress. Neurotox Res. 2019;35:1–18. doi: 10.1007/s12640-017-9838-2. [DOI] [PubMed] [Google Scholar]
- 31.Guan S, Ge D, Liu T, Ma X, Cui Z. Neuroprotection by protocatechuic acid against MPP+-induced cytotoxicity in cultured neural stem/progenitor cells. J Biotechnol. 2008;136:S127. [Google Scholar]
- 32.Ha S, Ahn S, Kim S, Joo Y, Chong YH, Suh YH, Chang KA. In vivo imaging of human adipose-derived stem cells in Alzheimer’s disease animal model. J Biomed Opt. 2014;19:051206. doi: 10.1117/1.JBO.19.5.051206. [DOI] [PubMed] [Google Scholar]
- 33.Hallett PJ, Cooper O, Sadi D, Robertson H, Mendez I, Isacson O. Long-term health of dopaminergic neuron transplants in Parkin-son’s disease patients. Cell Rep. 2014;7:1755–1761. doi: 10.1016/j.celrep.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, Waxman SG, Kocsis JD. Intravenous administration of auto se-rum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134:1790–1807. doi: 10.1093/brain/awr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu BY, Zhang SC. Differentiation of spinal motor neurons from pluripotent human stem cells. Nat Protoc. 2009;4:1295–1304. doi: 10.1038/nprot.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ibrahim MM, Gabr MT. Multitarget therapeutic strategies for Alzheimer’s disease. Neural Regen Res. 2019;14:437–440. doi: 10.4103/1673-5374.245463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imm J, Kerrigan TL, Jeffries A, Lunnon K. Using induced pluripotent stem cells to explore genetic and epigenetic variation associated with Alzheimer’s disease. Epigenomics. 2017;9:1455–1468. doi: 10.2217/epi-2017-0076. [DOI] [PubMed] [Google Scholar]
- 39.Iqbal K, Alonso AC, Gong CX, Khatoon S, Pei JJ, Wang JZ, Grundke-Iqbal I. Mechanisms of neurofibrillary degeneration and the formation of neurofibrillary tangles. J Neural Transm Suppl. 1998;53:169–180. doi: 10.1007/978-3-7091-6467-9_15. [DOI] [PubMed] [Google Scholar]
- 40.Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, Hefferan MP, Van Gorp S, Nazor KL, Boscolo FS, Carson CT, Laurent LC, Marsala M, Gage FH, Remes AM, Koo EH, Goldstein LS. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature. 2012;482:216–220. doi: 10.1038/nature10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang LH, Yang NY, Yuan XL, Zou YJ, Zhao FM, Chen JP, Wang MY, Lu DX. Daucosterol promotes the proliferation of neural stem cells. J Steroid Biochem Mol Biol. 2014;140:90–99. doi: 10.1016/j.jsbmb.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Kanekiyo T, Xu H, Bu G. ApoE and Aβ in Alzheimer’s disease: accidental encounters or partners? Neuron. 2014;81:740–754. doi: 10.1016/j.neuron.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karch CM, Goate AM. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry. 2015;77:43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khademi M, Taghizadeh Ghavamabadi R, Taghavi MM, Shabanizadeh A, Shariati-Kohbanani M, Hassanipour M, Taghipour Z. The effects of fluoxetine on the human adipose-derived stem cell proliferation and differentiation. Fundam Clin Pharmacol. 2018 doi: 10.1111/fcp.12426. doi: 101111/fcp12426. [DOI] [PubMed] [Google Scholar]
- 45.Kikuchi K, Kidana K, Tatebe T, Tomita T. Dysregulated metabolism of the amyloid-beta protein and therapeutic approaches in Alzheimer disease. J Cell Biochem. 2017;118:4183–4190. doi: 10.1002/jcb.26129. [DOI] [PubMed] [Google Scholar]
- 46.Kim DH, Lee D, Chang EH, Kim JH, Hwang JW, Kim JY, Kyung JW, Kim SH, Oh JS, Shim SM, Na DL, Oh W, Chang JW. GDF-15 secreted from human umbilical cord blood mesenchymal stem cells delivered through the cerebrospinal fluid promotes hippocampal neurogenesis and synaptic activity in an Alzheimer’s disease model. Stem Cells Dev. 2015;24:2378–2390. doi: 10.1089/scd.2014.0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim HJ, McMillan E, Han F, Svendsen CN. Regionally specified human neural progenitor cells derived from the mesencephalon and forebrain undergo increased neurogenesis following overexpression of ASCL1. Stem Cells. 2009;27:390–398. doi: 10.1634/stemcells.2007-1047. [DOI] [PubMed] [Google Scholar]
- 48.Kim SU, de Vellis J. Stem cell-based cell therapy in neurological diseases: a review. J Neurosci Res. 2009;87:2183–2200. doi: 10.1002/jnr.22054. [DOI] [PubMed] [Google Scholar]
- 49.Kim SU, Lee HJ, Kim YB. Neural stem cell-based treatment for neurodegenerative diseases. Neuropathology. 2013;33:491–504. doi: 10.1111/neup.12020. [DOI] [PubMed] [Google Scholar]
- 50.Kitiyanant N, Kitiyanant Y, Svendsen CN, Thangnipon W. BDNF-, IGF-1- and GDNF-secreting human neural progenitor cells rescue amyloid beta-induced toxicity in cultured rat septal neurons. Neurochem Res. 2012;37:143–152. doi: 10.1007/s11064-011-0592-1. [DOI] [PubMed] [Google Scholar]
- 51.Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, Beckers L, O’Loughlin E, Xu Y, Fanek Z, Greco DJ, Smith ST, Tweet G, Humulock Z, Zrzavy T, Conde-Sanroman P, Gacias M, Weng Z, Chen H, Tjon E, et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity. 2017;47:566–581e9. doi: 10.1016/j.immuni.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krencik R, Weick JP, Liu Y, Zhang ZJ, Zhang SC. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol. 2011;29:528–534. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwak YD, Brannen CL, Qu T, Kim HM, Dong X, Soba P, Majumdar A, Kaplan A, Beyreuther K, Sugaya K. Amyloid precursor protein regulates differentiation of human neural stem cells. Stem Cells Dev. 2006;15:381–389. doi: 10.1089/scd.2006.15.381. [DOI] [PubMed] [Google Scholar]
- 54.Lee HK, Morin P, Xia W. Peripheral blood mononuclear cell-converted induced pluripotent stem cells (iPSCs) from an early onset Alzheimer’s patient. Stem Cell Res. 2016a;16:213–215. doi: 10.1016/j.scr.2015.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee JH, Oh IH, Lim HK. Stem cell therapy: a prospective treatment for Alzheimer’s disease. Psychiatry Investig. 2016b;13:583–589. doi: 10.4306/pi.2016.13.6.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li NM, Liu KF, Qiu YJ, Zhang HH, Nakanishi H, Qing H. Mutations of beta-amyloid precursor protein alter the consequence of Alzheimer’s disease pathogenesis. Neural Regen Res. 2019;14:658–665. doi: 10.4103/1673-5374.247469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li T, Pires C, Nielsen TT, Waldemar G, Hjermind LE, Nielsen JE, Dinnyes A, Hyttel P, Freude KK. Generation of induced pluripotent stem cells (iPSCs) from an Alzheimer’s disease patient carrying an A79V mutation in PSEN1. Stem Cell Res. 2016a;16:229–232. doi: 10.1016/j.scr.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 58.Li T, Pires C, Nielsen TT, Waldemar G, Hjermind LE, Nielsen JE, Dinnyes A, Holst B, Hyttel P, Freude KK. Generation of induced pluripotent stem cells (iPSCs) from an Alzheimer’s disease patient carrying a M146I mutation in PSEN1. Stem Cell Res. 2016b;16:334–337. doi: 10.1016/j.scr.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 59.Li X, Zhu H, Sun X, Zuo F, Lei J, Wang Z, Bao X, Wang R. Human neural stem cell transplantation rescues cognitive defects in APP/PS1 model of Alzheimer’s disease by enhancing neuronal connectivity and metabolic activity. Front Aging Neurosci. 2016c;8:282. doi: 10.3389/fnagi.2016.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin W, Yang LK, Zhu J, Wang YH, Dong JR, Chen T, Wang D, Xu XM, Sun SB, Zhang L. Deep brain stimulation for the treatment of moderate-to-severe Alzheimer’s disease: Study protocol for a prospective self-controlled trial. Clin Trials Degener Dis. 2018;3:66–70. [Google Scholar]
- 61.Lindvall O, Rehncrona S, Gustavii B, Brundin P, Astedt B, Widner H, Lindholm T, Bjorklund A, Leenders KL, Rothwell JC, Frackowiak R, Marsden CD, Johnels B, Steg G, Freedman R, Hoffer BJ, Seiger L, Stromberg I, Bygdeman M, Olson L. Fetal dopamine-rich mesencephalic grafts in Parkinson’s disease. Lancet. 1988;2:1483–1484. doi: 10.1016/s0140-6736(88)90950-6. [DOI] [PubMed] [Google Scholar]
- 62.Liu X, Fu X, Dai G, Wang X, Zhang Z, Cheng H, Zheng P, An Y. Comparative analysis of curative effect of bone marrow mesenchymal stem cell and bone marrow mononuclear cell transplantation for spastic cerebral palsy. J Transl Med. 2017;15:48. doi: 10.1186/s12967-017-1149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Y, Yang R, Shi S. Systemic infusion of mesenchymal stem cells improves cell-based bone regeneration via upregulation of regulatory T cells. Tissue engineering Part A. 2015;21:498–509. doi: 10.1089/ten.tea.2013.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma L, Aijima R, Hoshino Y, Yamaza H, Tomoda E, Tanaka Y, Sonoda S, Song G, Zhao W, Nonaka K, Shi S, Yamaza T. Transplantation of mesenchymal stem cells ameliorates secondary osteoporosis through interleukin-17-impaired functions of recipient bone marrow mesenchymal stem cells in MRL/lpr mice. Stem Cell Res Ther. 2015;6:104. doi: 10.1186/s13287-015-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marsh SE, Blurton-Jones M. Neural stem cell therapy for neurodegenerative disorders: The role of neurotrophic support. Neurochem Int. 2017;106:94–100. doi: 10.1016/j.neuint.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martínez-Morales PL, Revilla A, Ocaña I, González C, Sainz P, McGuire D, Liste I. Progress in stem cell therapy for major human neurological disorders. Stem Cell Rev. 2013;9:685–699. doi: 10.1007/s12015-013-9443-6. [DOI] [PubMed] [Google Scholar]
- 67.Matchynski-Franks JJ, Pappas C, Rossignol J, Reinke T, Fink K, Crane A, Twite A, Lowrance SA, Song C, Dunbar GL. Mesenchymal stem cells as treatment for behavioral deficits and neuropathology in the 5xFAD mouse model of Alzheimer’s disease. Cell Transplant. 2016;25:687–703. doi: 10.3727/096368916X690818. [DOI] [PubMed] [Google Scholar]
- 68.Mungenast AE, Siegert S, Tsai LH. Modeling Alzheimer’s disease with human induced pluripotent stem (iPS) cells. Mol Cell Neurosci. 2016;73:13–31. doi: 10.1016/j.mcn.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muratore CR, Rice HC, Srikanth P, Callahan DG, Shin T, Benjamin LN, Walsh DM, Selkoe DJ, Young-Pearse TL. The familial Alzhei-mer’s disease APPV717I mutation alters APP processing and Tau expression in iPSC-derived neurons. Hum Mol Genet. 2014;23:3523–3536. doi: 10.1093/hmg/ddu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nieweg K, Andreyeva A, van Stegen B, Tanriover G, Gottmann K. Alzheimer’s disease-related amyloid-beta induces synaptotoxicity in human iPS cell-derived neurons. Cell Death Dis. 2015;6:e1709. doi: 10.1038/cddis.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Noormohammadi A, Khodakarami A, Gutierrez-Garcia R, Lee HJ, Koyuncu S, Konig T, Schindler C, Saez I, Fatima A, Dieterich C, Vilchez D. Somatic increase of CCT8 mimics proteostasis of human pluripotent stem cells and extends C. elegans lifespan. Nat Commun. 2016;7:13649. doi: 10.1038/ncomms13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oh SH, Kim HN, Park HJ, Shin JY, Lee PH. Mesenchymal stem cells increase hippocampal neurogenesis and neuronal differentiation by enhancing the Wnt signaling pathway in an Alzheimer’s disease model. Cell Transplant. 2015;24:1097–1109. doi: 10.3727/096368914X679237. [DOI] [PubMed] [Google Scholar]
- 73.Ohsawa I, Takamura C, Morimoto T, Ishiguro M, Kohsaka S. Amino-terminal region of secreted form of amyloid precursor protein stimulates proliferation of neural stem cells. Eur J Neurosci. 1999;11:1907–1913. doi: 10.1046/j.1460-9568.1999.00601.x. [DOI] [PubMed] [Google Scholar]
- 74.Oron A, Oron U. Low-level laser therapy to the bone marrow ameliorates neurodegenerative disease progression in a mouse model of Alzheimer’s disease: a minireview. Photomed Laser Surg. 2016;34:627–630. doi: 10.1089/pho.2015.4072. [DOI] [PubMed] [Google Scholar]
- 75.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 76.Poon WW, Blurton-Jones M, Tu CH, Feinberg LM, Chabrier MA, Harris JW, Jeon NL, Cotman CW. beta-Amyloid impairs axonal BDNF retrograde trafficking. Neurobiol Aging. 2011;32:821–833. doi: 10.1016/j.neurobiolaging.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prince M, Comas-Herrera A, Knapp M, Guerchet M, Karagiannidou M. World Alzheimer report 2016: improving healthcare for people living with dementia: coverage, quality and costs now and in the future. London, UK: Alzheimer’s Disease International (ADI); 2016. [Google Scholar]
- 78.Robbins JP, Price J. Human induced pluripotent stem cells as a research tool in Alzheimer’s disease. Psychol Med. 2017;47:2587–2592. doi: 10.1017/S0033291717002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rohani L, Johnson AA, Arnold A, Stolzing A. The aging signature: a hallmark of induced pluripotent stem cells? Aging cell. 2014;13:2–7. doi: 10.1111/acel.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rygiel K. Novel strategies for Alzheimer’s disease treatment: An overview of anti-amyloid beta monoclonal antibodies. Indian J Pharmacol. 2016;48:629–636. doi: 10.4103/0253-7613.194867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salem AM, Ahmed HH, Atta HM, Ghazy MA, Aglan HA. Potential of bone marrow mesenchymal stem cells in management of Alzheimer’s disease in female rats. Cell Biol Int. 2014;38:1367–1383. doi: 10.1002/cbin.10331. [DOI] [PubMed] [Google Scholar]
- 82.Scott CT, Magnus D. Wrongful termination: lessons from the Geron clinical trial. Stem Cells Transl Med. 2014;3:1398–1401. doi: 10.5966/sctm.2014-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, O’Gorman J, Qian F, Arastu M, Li M, Chollate S, Brennan MS, Quintero-Monzon O, Scannevin RH, Arnold HM, Engber T, et al. Addendum: The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature. 2017;546:564. doi: 10.1038/nature22809. [DOI] [PubMed] [Google Scholar]
- 84.Shin JY, Park HJ, Kim HN, Oh SH, Bae JS, Ha HJ, Lee PH. Mesenchymal stem cells enhance autophagy and increase beta-amyloid clearance in Alzheimer disease models. Autophagy. 2014;10:32–44. doi: 10.4161/auto.26508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singh C, Liu L, Wang JM, Irwin RW, Yao J, Chen S, Henry S, Thompson RF, Brinton RD. Allopregnanolone restores hippocam-pal-dependent learning and memory and neural progenitor survival in aging 3xTgAD and nonTg mice. Neurobiol Aging. 2012;33:1493–1506. doi: 10.1016/j.neurobiolaging.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sugaya K, Merchant S. How to approach Alzheimer’s disease therapy using stem cell technologies. J Alzheimers Dis. 2008;15:241–254. doi: 10.3233/jad-2008-15209. [DOI] [PubMed] [Google Scholar]
- 87.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 88.Takahashi K, Yamanaka S. A developmental framework for induced pluripotency. Development. 2015;142:3274–3285. doi: 10.1242/dev.114249. [DOI] [PubMed] [Google Scholar]
- 89.Tang J, Xu H, Fan X, Li D, Rancourt D, Zhou G, Li Z, Yang L. Embryonic stem cell-derived neural precursor cells improve memory dysfunction in Abeta(1-40) injured rats. Neurosci Res. 2008;62:86–96. doi: 10.1016/j.neures.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 90.Taupin P. Apigenin and related compounds stimulate adult neurogenesis. Mars, Inc., the Salk Institute for Biological Studies: WO2008147483. Expert Opin Ther Pat. 2009;19:523–527. doi: 10.1517/13543770902721279. [DOI] [PubMed] [Google Scholar]
- 91.Tong LM, Fong H, Huang Y. Stem cell therapy for Alzheimer’s disease and related disorders: current status and future perspectives. Exp Mol Med. 2015;47:e151. doi: 10.1038/emm.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tubsuwan A, Pires C, Rasmussen MA, Schmid B, Nielsen JE, Hjermind LE, Hall V, Nielsen TT, Waldemar G, Hyttel P, Clausen C, Kitiyanant N, Freude KK, Holst B. Generation of induced pluripotent stem cells (iPSCs) from an Alzheimer’s disease patient carrying a L150P mutation in PSEN-1. Stem Cell Res. 2016;16:110–112. doi: 10.1016/j.scr.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 93.Villa A, Liste I, Courtois ET, Seiz EG, Ramos M, Meyer M, Juliusson B, Kusk P, Martinez-Serrano A. Generation and properties of a new human ventral mesencephalic neural stem cell line. Exp Cell Res. 2009;315:1860–1874. doi: 10.1016/j.yexcr.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 94.Wang F, Jia Y, Liu J, Zhai J, Cao N, Yue W, He H, Pei X. Dental pulp stem cells promote regeneration of damaged neuron cells on the cellular model of Alzheimer’s disease. Cell Biol Int. 2017;41:639–650. doi: 10.1002/cbin.10767. [DOI] [PubMed] [Google Scholar]
- 95.Wisniewski T, Drummond E. Developing therapeutic vaccines against Alzheimer’s disease. Expert Rev Vaccines. 2016;15:401–415. doi: 10.1586/14760584.2016.1121815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wray S, Fox NC. Stem cell therapy for Alzheimer’s disease: hope or hype? Lancet Neurol. 2016;15:133–135. doi: 10.1016/S1474-4422(15)00382-8. [DOI] [PubMed] [Google Scholar]
- 97.Wu CC, Lien CC, Hou WH, Chiang PM, Tsai KJ. Gain of BDNF function in engrafted neural stem cells promotes the therapeutic potential for Alzheimer’s disease. Sci Rep. 2016;6:27358. doi: 10.1038/srep27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu S, Sasaki A, Yoshimoto R, Kawahara Y, Manabe T, Kataoka K, Asashima M, Yuge L. Neural stem cells improve learning and memory in rats with Alzheimer’s disease. Pathobiology. 2008;75:186–194. doi: 10.1159/000124979. [DOI] [PubMed] [Google Scholar]
- 99.Xu X. Gamma-secretase catalyzes sequential cleavages of the AbetaPP transmembrane domain. J Alzheimers Dis. 2009;16:211–224. doi: 10.3233/JAD-2009-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu X, Lei Y, Luo J, Wang J, Zhang S, Yang XJ, Sun M, Nuwaysir E, Fan G, Zhao J, Lei L, Zhong Z. Prevention of beta-amyloid induced toxicity in human iPS cell-derived neurons by inhibition of Cyclin-dependent kinases and associated cell cycle events. Stem Cell Res. 2013;10:213–227. doi: 10.1016/j.scr.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 101.Yagi T, Kosakai A, Ito D, Okada Y, Akamatsu W, Nihei Y, Nabetani A, Ishikawa F, Arai Y, Hirose N, Okano H, Suzuki N. Establishment of induced pluripotent stem cells from centenarians for neurodegenerative disease research. PLoS One. 2012;7:e41572. doi: 10.1371/journal.pone.0041572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamanaka S. A fresh look at iPS cells. Cell. 2009;137:13–17. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 103.Yamazaki H, Jin Y, Tsuchiya A, Kanno T, Nishizaki T. Adipose-derived stem cell-conditioned medium ameliorates antidepres-sion-related behaviors in the mouse model of Alzheimer’s disease. Neurosci Lett. 2015;609:53–57. doi: 10.1016/j.neulet.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 104.Yan YF, Ma T, Gong K, Ao Q, Zhang XF, Gong YD. Adipose-derived mesenchymal stem cell transplantation promotes adult neuro-genesis in the brains of Alzheimer’s disease mice. Neural Regen Res. 2014;9:798–805. doi: 10.4103/1673-5374.131596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang J, Li S, He XB, Cheng C, Le W. Induced pluripotent stem cells in Alzheimer’s disease: applications for disease modeling and cell-replacement therapy. Mol Neurodegener. 2016;11:39. doi: 10.1186/s13024-016-0106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yeh FL, Wang Y, Tom I, Gonzalez LC, Sheng M. TREM2 binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia. Neuron. 2016;91:328–340. doi: 10.1016/j.neuron.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 107.Zhagn L, Li Z. Alzheimer and the discovery of Alzheimer’s disease. Zhonghua Yi Shi Za Zhi. 2014;44:288–290. [PubMed] [Google Scholar]
- 108.Zhan Y, Zheng H, Wang C, Rong Z, Xiao N, Ma Q, Zhang YW. A novel presenilin 1 mutation (F388L) identified in a Chinese family with early-onset Alzheimer’s disease. Neurobiol Aging. 2017;50:168.e1–168e4. doi: 10.1016/j.neurobiolaging.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 109.Zhang L, Liu JJ, Zhao Y, Liu Y, Lin JW. N-butylphthalide affects cognitive function of APP/PS1 transgenic mice (Alzheimer’s disease model) Zhongguo Zuzhi Gongcheng Yanjiu. 2019;23:3025–3030. [Google Scholar]
- 110.Zhang S, Lv Z, Zhang S, Liu L, Li Q, Gong W, Sha H, Wu H. Characterization of human induced pluripotent stem cell (iPSC) line from a 72year old male patient with later onset Alzheimer’s disease. Stem Cell Res. 2017;19:34–36. doi: 10.1016/j.scr.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 111.Zhao H. Embryonic neural stem cell transplantation for Alzheimer’s disease. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:4805–4810. [Google Scholar]
- 112.Zhou X, Gu J, Gu Y, He M, Bi Y, Chen J, Li T. Human umbilical cord-derived mesenchymal stem cells improve learning and memory function in hypoxic-ischemic brain-damaged rats via an il-8-mediated secretion mechanism rather than differentiation pattern induction. Cell Physiol Biochem. 2015;35:2383–2401. doi: 10.1159/000374040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


