Keywords: bilateral carotid artery ligation, lycopene, hippocampus, learning and memory, malondialdehyde, neuron, neuron-restrictive silencer factor, reactive oxygen species, superoxide dismutase
Abstract
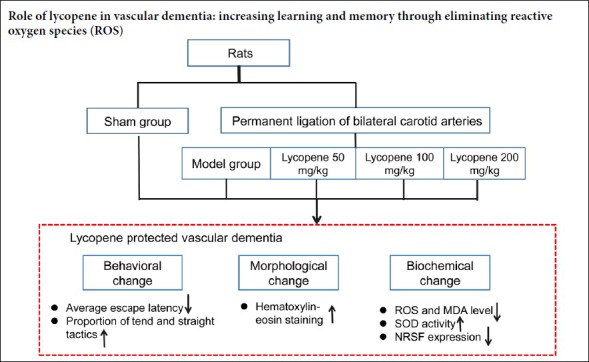
Oxidative stress is involved in the pathogenesis of vascular dementia. Studies have shown that lycopene can significantly inhibit oxidative stress; therefore, we hypothesized that lycopene can reduce the level of oxidative stress in vascular dementia. A vascular dementia model was established by permanent bilateral ligation of common carotid arteries. The dosage groups were treated with lycopene (50, 100 and 200 mg/kg) every other day for 2 months. Rats without bilateral carotid artery ligation were prepared as a sham group. To test the ability of learning and memory, the Morris water maze was used to detect the average escape latency and the change of search strategy. Hematoxylin-eosin staining was used to observe changes of hippocampal neurons. The levels of oxidative stress factors, superoxide dismutase and malondialdehyde, were measured in the hippocampus by biochemical detection. The levels of reactive oxygen species in the hippocampus were observed by dihydroethidium staining. The distribution and expression of oxidative stress related protein, neuron-restrictive silencer factor, in hippocampal neurons were detected by immunofluorescence histochemistry and western blot assays. After 2 months of drug administration, (1) in the model group, the average escape latency was longer than that of the sham group, and the proportion of straight and tend tactics was lower than that of the sham group, and the hippocampal neurons were irregularly arranged and the cytoplasm was hyperchromatic. (2) The levels of reactive oxygen species and malondialdehyde in the hippocampus of the model group rats were increased, and the activity of superoxide dismutase was decreased. (3) Lycopene (50, 100 and 200 mg/kg) intervention improved the above changes, and the lycopene 100 mg/kg group showed the most significant improvement effect. (4) Neuron-restrictive silencer factor expression in the hippocampus was lower in the sham group and the lycopene 100 mg/kg group than in the model group. (5) The above data indicate that lycopene 100 mg/kg could protect against the learning-memory ability impairment of vascular dementia rats. The protective mechanism was achieved by inhibiting oxidative stress in the hippocampus. The experiment was approved by the Animal Ethics Committee of Fujian Medical University, China (approval No. 2014-025) in June 2014.
Chinese Library Classification No. R453; R364; R363
Introduction
Vascular dementia is a progressive disease caused by reduced blood flow to the brain and it affects a person’s ability to perform everyday activities (Zhang et al., 2018). Vascular dementia is the second most common type of dementia after Alzheimer’s disease (Alzheimer’s Association, 2017). There are no licensed treatments for vascular dementia because of uncertainty over the exact nature of the relationship between cerebrovascular pathology and cognitive impairment. Therefore, there is an urgent need to advance our understanding of vascular dementia pathobiology and to explore effective management approaches.
Vascular dementia pathogenesis involves a series of factors, including imbalanced cholinergic signaling, damage from oxidative stress, over-expression of inflammatory cytokines, disrupted lipid metabolism and abnormal gene expression (Li et al., 2013; Huang et al., 2016; Mei et al., 2017; Jin and Liu, 2019). Among these factors, oxidative stress is considered as a major contributing factor to vascular dementia (Baskys and Cheng, 2012).
Oxidative stress can increase neuron-restrictive silencer factor (NRSF) expression in the ageing brain (Lu et al., 2014). NRSF, a RE1-silencing transcription factor, acts as a transcriptional regulator (Chong et al., 1995; Song et al., 2016) of genes encoding apoptosis and synaptic plasticity proteins (Rodenas-Ruano et al., 2012; Lu et al., 2014; Song et al., 2018). NRSF is widely expressed during embryogenesis and causes neuronal differentiation (Chong et al., 1995; Thompson and Chan, 2018). NRSF exerts a neuroprotective effect by reducing oxidative damage during ageing. NRSF plays a key role in the repression of neuronal genes during the early development of the nervous system (Meyer et al., 2019). Dysregulation of NRSF and its target genes is involved in many neurodegenerative disorders and diseases, such as Alzheimer’s disease (Lu et al., 2014), Huntington’s disease (Zuccato and Cattaneo, 2007), Parkinson’s disease (Kawamura et al., 2019) and epilepsy (Patterson et al., 2017). In addition, NRSF can be induced in vulnerable hippocampal neurons in response to ischemic insult (Calderone et al., 2003; Noh et al., 2012). However, few studies have investigated NRSF in vascular disease.
Lycopene is an acyclic carotenoid endowed with a wide spectrum of biological properties, including antioxidation, anticancer, anti-inflammation, suppression of cell proliferation and nerve protection (Prakash and Kumar, 2013). Lycopene exhibits a memory enhancing effect in different animal models (Wang et al., 2018; Zhao et al., 2018).
Although, lycopene has been mostly studied for its effects on protecting neuronal systems, the potential effect of lycopene on vascular dementia has been seldom reported. Therefore, the present study investigated the potential effect and mechanism of lycopene in a vascular dementia model. A permanent bilateral common carotid artery occlusion (also called two-vessel occlusion) rat model was selected, because this model can mimic the reduced blood flow to the brain of vascular dementia (Venkat et al., 2015). The appropriate concentration of lycopene for this rat model was also investigated.
Materials and Methods
Animals
Sixty female Sprague-Dawley rats weighting 190–210 g and aged 2 mouths were obtained from the Laboratory Animal Center of Fujian Medical University, China (license No. SCXK (Min) 2012-0001). All rats were maintained in a steady temperature (24 ± 2°C) and light-controlled room with a 14-hour light/10-hour dark cycle. The animal care and the experimental procedures were in accordance with the principles of the Animal Ethics Committee of Fujian Medical University, China (approval No. 2014-025) in June 2014. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Treatment schedule
The rats were randomly allocated into five groups (n = 10). In the sham group, rats were subjected to the operation without bilateral carotid artery occlusion and were then treated with vehicle (double distilled water + 0.1% Tween-80). In the model group, rats underwent bilateral ligation of common carotid arteries and were then treated with vehicle. In lycopene-treated groups, rats underwent permanent ligation of bilateral carotid arteries and were then administrated lycopene (50, 100, or 200 mg/kg; Changsha Huirui Biotechnology Co., Ltd., Changsha, China). The experimental design is summarized in Figure 1.
Figure 1.
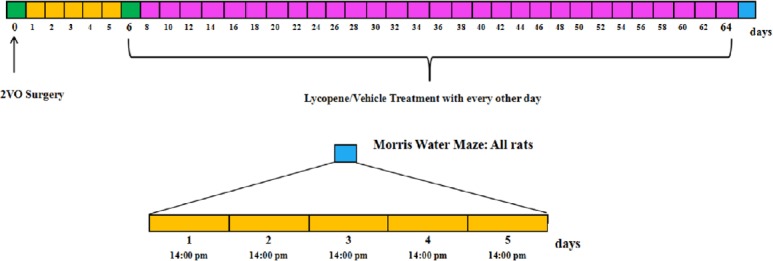
Timeline showing experimental design.
Five days after surgery, intragastric administration of lycopene or vehicle was conducted every other day and continued for 2 months. Each animal performed the Morris water maze task for 5 consecutive days after drug administration. 2VO: Two-vessel occlusion.
Five days after surgery, lycopene or vehicle were intragastrically administered every other day for two months. All animal were trialed in the Morris water maze task for five consecutive days after drug administration for two months. When behavioral testing was completed, rats were anesthetized with 10% chloral hydrate. The brains of three rats from each group were rapidly removed from the skull after perfusion with cold 0.9% NaCl solution and fixed in 4% paraformaldehyde. Paraffin embedding was performed and paraffin sections were prepared for hematoxylin-eosin staining and immunofluorescence. The brains of three rats from each group were rapidly dissected from the skull and cut in frozen artificial cerebrospinal fluid for dihydroethidium staining. Hippocampal tissues used for western blot assays and biochemical detection were directly collected from the remaining four rats of each group after perfusion with 0.9% NaCl.
Vascular dementia model
The following procedures were performed to establish the vascular dementia model (Liu et al., 2019; Zong et al., 2019). Under anesthesia induced by intraperitoneal injection of 10% chloral hydrate, rats were fixed in a supine position and a median incision of the cervical skin was performed to expose bilateral carotid arteries. After these arteries were ligated, the incision was routinely sutured. Immediately after the operation, rats were maintained in groups in cages. Operative mortality of this model was about 8% (five mice died during the operation).
Lycopene solution preparation and intragastric administration
Different doses of lycopene (5, 10 and 20 mg/mL) were prepared by grinding lycopene and Tween-80 together, and gradually dissolving in double distilled water. The mixture was eventually suspended by a vortex mixer for 20 minutes at room temperature away from light.
During administration, the rats of low-, moderate- and high-dose groups were given 50, 100 and 200 mg/kg of lycopene, respectively. The rats in the sham and model groups were given double distilled water containing 0.1% Tween-80. Double distilled water was added to fill the stomach (stomach volume was 3 mL) when the required solution was less than 3 mL.
Morris water maze test
After 2 months of drug administration, all rats were tested in the Morris water maze according to a previously published study (Barnhart et al., 2015). The apparatus consisted of a circular, plastic pool (160 cm diameter, 55 cm high) filled with opaque water (24 ± 2°C). A circular platform (12 cm diameter) was submerged 2 cm below the water in one of four quadrants. The laboratory was kept dark and quiet during the test.
In acquisition trials, each rat was trained to escape from the water by swimming to the hidden platform. Once the platform was found, the rat was allowed to sit on the platform for 10 seconds. If the rat failed to locate the platform within 60 seconds, it would be guided to the platform and assigned a latency of 60 seconds. Over the course of five days, each rat underwent four trials per day (inter-trial interval: 20 seconds). The platform was located in the same place relative to the distal cues in the room. For each trial, the rat was placed in the water at different start locations (east, south, west, and north). Data of average escape latency, average speed and search strategy were collected by an online video tracking device (RD1101-MWM-G, Moblie Datum Inc., Shanghai, China).
The search strategy of rats can be divided into the following four types: (a) straight tactic, (b) tend tactic, (c) margin tactic, and (d) random tactic.
Straight tactic: the rat can accurately swim to the location of the platform. Tend tactic: judgment of location and direction of the target position is basically correct, but not as accurate as the straight tactic. Margin type: the rat finds the target position according to distal clues, and the reference point is itself. This strategy can be seen as an instinctive behavior in rats. Random type: This is a blind search strategy. The rat has poor accuracy in judging target position.
Hematoxylin-eosin staining
After the Morris water maze test, morphological changes in the hippocampus were observed by hematoxylin-eosin staining. The brains of three rats from each group were paraffin embedded and cut on a microtome into 5 µm sections from the optic chiasm to the cerebral transverse fissure. The tissues were stained using a hematoxylin-eosin staining kit (Beyotime, Shanghai, China). Sections were observed under an optical microscope (400 ×; ERc5S, Carl Zeiss Company, Oberkochen, Germany).
Measurement of reactive oxygen species (ROS) generation by the fluorescent probe, dihydroethidium
Intracellular superoxide was detected using the fluorescent probe, dihydroethidium (Beyotime, Shanghai, China). Dihydroethidium can be oxidized by superoxide to ethidium bromide, which can intercalate with chromosomal DNA to yield red fluorescence. Brains were cut into 400 µm slices on a vibratome (Vibrotome 1000Plus, New Jersey, USA) filled with frozen artificial cerebrospinal fluid. After immersion in gassed (95% O2 and 5% CO2) artificial cerebrospinal fluid for 15 minutes, brain slices were incubated with dihydroethidium (10 nM) at 37°C for 90 minutes, and then washed with artificial cerebrospinal fluid to remove free dihydroethidium molecules. Fluorescence was monitored via a fluorescence microscope (SP5; Leica, Wetzlar, Germany). Thickness of the projection was 25 µm and eight photographs with a 3.125 µm interval were taken from every brain slice. The value of ROS fluorescence intensity was acquired by LAS AF Lite software (Leica).
Detection of superoxide dismutase (SOD) and malondialdehyde (MDA) in the hippocampus
After the Morris water maze test, hippocampal tissues were obtained after normal perfusion (0.9% NaCl). Ten milligrams of hippocampus were lysed in 100 mL SOD sample preparation fluid and homogenized in an ice bath. The homogenate was then centrifuged at 12,000 × g and 4°C for 3 to 5 minutes. The levels of SOD and MDA in the supernatants were determined on a microplate reader (Multiskan FC, Thermo, Waltham, MA, USA) using corresponding diagnostic reagent kits (Beyotime) according to the kit instructions. The protein content of the lysate was determined using a Bicinchoninic Acid Protein Assay Kit (Beyotime).
Observation of NRSF protein distribution in the hippocampus by fluorescent immunohistochemistry
After paraffin embedding, the hippocampus was cut into sections. These sections were deparaffinized in xylene, rehydrated in a graded ethanol series, and antigens were retrieved by microwaving in citric acid for 15 minutes. Sections were then then blocked with 5% bovine serum albumin at room temperature. Sections were incubated with primary polyclonal rabbit anti-NRSF antibody at a dilution of 1:100 (Bioss, Beijing, China) for 24 hours at 4°C. After washing three times with phosphate-buffered saline, the slices were further incubated with Alexa Fluor 647-labeled goat anti-rabbit IgG (H+L) (Beyotime) for 2 hours at a dilution of 1:500 at room temperature for 2 hours. Nuclei were stained with DAPI. After washing in phosphate-buffered saline, sections were mounted and images were captured using a laser scanning confocal microscope (SP5; Leica, Solms, Germany).
Measurement of NRSF by western blotting
NRSF was measured after SOD and MDA assays. Total proteins in the collected hippocampal tissues were isolated using cell lysis solution (Beyotime). Protein concentrations were determined by Bicinchoninic Acid assays (Beyotime). Equal amounts of protein were separated on 10% sodium dodecyl sulfonate-polyacrylamide gels and transferred to polyvinylidene fluoride membranes. The membranes were blocked in Tris buffered saline containing nonfat milk (10%) and 0.1% Tween-20 for 1 hour. The following primary antibodies were used: polyclonal rabbit anti-NRSF (Bioss) diluted 1:500, and mouse anti-β-actin (Beyotime) diluted 1:1000. After incubation with primary antibodies for 24 hours at 4°, the membranes were washed three times with Tris buffered saline containing 0.1% Tween-20 for 5 minutes and then incubated with goat anti-rabbit and goat anti-mouse IgG horseradish peroxidase antibody (Beyotime) at a dilution of 1:1000 for 1 hour at room temperature. The chemiluminescence signals were captured using a LAS 4000 mini (General Electric Company, Japan), and the bands were quantified by densitometry using the ImageJ system (National Institutes of Health, Maryland, USA). Grayscale values were measured and normalized to β-actin in the same sample.
Statistical analysis
All data were statistically analyzed using SPSS 19.0 software (International Business Machines Corporation, IBM, USA). Data are presented as the mean ± SEM. For two-way analysis of variance in the Morris water maze test, the groups and the training days were taken as between-group factors; the least-significant difference post hoc test was used for comparison within groups. Search strategy of different groups was compared by the chi-square test. For one-way analysis of variance, Dunnett’s test was used for multiple comparisons to determine whether the means differed significantly between two groups. A value of P < 0.05 was considered statistically significant.
Results
Effect of lycopene in the Morris water maze test
In the Morris water maze test, the average speed of rats on the 5 consecutive days showed no significant difference for all groups (P > 0.05, data not shown), and there was also no difference in the average daily speed among the five groups (P > 0.05; Figure 2).
Figure 2.
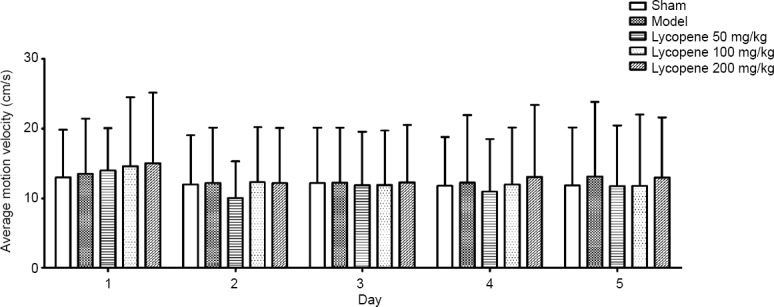
Effect of lycopene on the average motion velocity of rats in each group.
Data are presented as the mean ± SEM (n = 10; two-way analysis of variance followed by the least significant difference post hoc test). The main factor of group and the secondary factor of day did not show statistical difference.
The average escape latency of rats was compared among the five days and also among the five groups. As illustrated in Table 1, the average escape latency tended to gradually reduce from day 1 to day 5. Similar to the sham group, the lycopene 100 mg/kg group had a relatively short average escape latency compared with the model group (F(4,50) = 3.041, P < 0.05). Exact escape latency values are summarized in Table 1. Among the three lycopene dose groups, the latency to reach the platform was significantly shorter in the lycopene 100 mg/kg group than in the lycopene 50 mg/kg and the lycopene 200 mg/kg groups. Exact values of path lengths of the five groups over the five days are summarized in Table 1.
Table 1.
Average escape latency (s) in the Morris water maze test in each group
| Group | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
|---|---|---|---|---|---|
| Sham | 48.37±17.95 | 49.47±16.34 | 36.94±19.69 | 43.32±17.16 | 38.89±16.02 |
| Model | 52.87±15.17 | 51.40±15.41 | 49.15±16.71** | 48.68±16.44 | 43.12±16.94 |
| Lycopene 50 mg/kg | 52.25±13.27 | 49.00±16.21 | 54.20±12.24*** | 46.85±15.85 | 44.17±17.26 |
| Lycopene 100 mg/kg | 46.47±14.49 | 41.72±16.54 | 37.32±18.10##†† | 38.02±16.19 | 34.68±17.34#† |
| Lycopene 200 mg/kg | 47.72±16.09 | 42.94±17.64* | 44.42±18.19 | 46.77±15.96 | 45.39±16.70¦¦ |
| F-value | 1.259 | 2.556 | 7.42 | 2.552 | 2.715 |
| P-value | 0.288 | 0.04 | 0 | 0.0741 | 0.031 |
*P < 0.05, **P < 0.01, ***P < 0.001, vs. sham group; #P < 0.05, ##P < 0.01, vs. model group; †P < 0.05, †††P < 0.001, vs. lycopene 50 mg/kg group; §§P < 0.01, vs. lycopene 100 mg/kg group. Data are presented as the mean ± SEM (n = 10; two-way analysis of variance followed by the least-significant difference post hoc test). The main factor of group and the secondary factor of day had statistical difference.
The path lengths of the five groups tested over the 5 days are presented in Figure 3. From day 3 to day 5, the path lengths of the five groups showed significant difference [F(4,50) = 2.53, P ≤ 0.05 for day 3; F(4,50) = 2.946, P < 0.05 for day 4; F(4,50) = 2.471, P < 0.05 for day 5]. Path lengths gradually reduced in the sham group and the lycopene 100 mg/kg group, but there was no obvious change in the model group. Among three dose groups, path length was lowest in the lycopene 100 mg/kg group.
Figure 3.
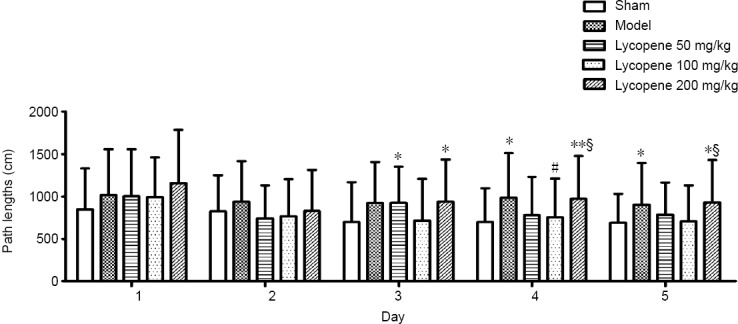
Effect of lycopene on the path lengths of rats from each group over 5 days.
Data are presented as the mean ± SEM (n = 10; two-way analysis of variance followed by the least significant difference post hoc test). The main factor of group and the secondary factor of day had statistical difference. *P < 0.05, **P < 0.01, vs. sham group; #P < 0.05, vs. model group; §P < 0.05, vs. lycopene 100 mg/kg group.
Search strategy was also evaluated. During the 5 days, the proportion of tend and straight tactics of the sham and lycopene 100 mg/kg groups rapidly increased from day 3. In addition, from day 3 to day 5, the proportion of tend and straight tactics in these two groups stayed relatively high compared with the other three groups (Figure 4 and Table 2).
Figure 4.
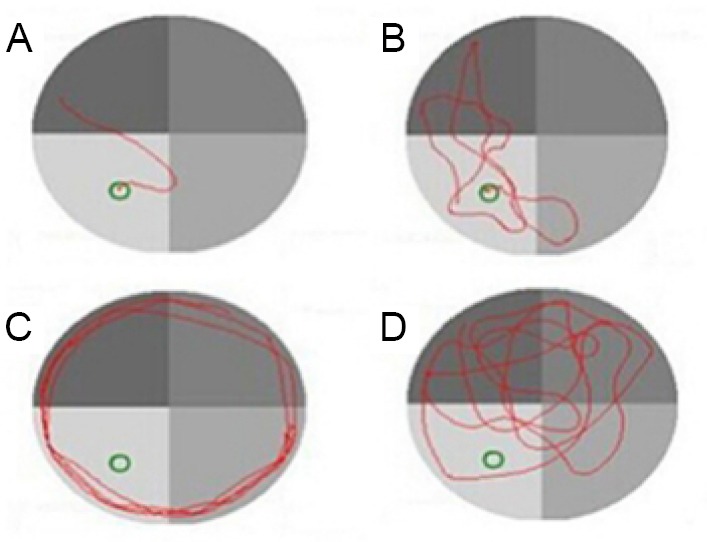
Four search strategies in the Morris water maze.
(A–D) Straight tactic, tend tactic, margin tactic and random tactic, respectively.
Table 2.
Proportion of tend and straight tactics in the Morris water maze test in each group
| Group | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Missing (n) | Valid (n) | n (%) | Missing (n) | Valid (n) | n (%) | Missing (n) | Valid (n) | n (%) | Missing (n) | Valid (n) | n (%) | Missing (n) | Valid (n) | n (%) | |
| Sham | 31 | 9 | 22.5 | 31 | 9 | 22.5 | 21 | 19 | 47.5## | 27 | 13 | 32.5 | 20 | 20 | 50## |
| Model | 35 | 5 | 12.5 | 34 | 6 | 15 | 34 | 5 | 12.5 | 34 | 6 | 15 | 32 | 8 | 20 |
| Lycopene 50 mg/kg | 30 | 10 | 25 | 32 | 8 | 20 | 31 | 9 | 22.5 | 29 | 11 | 27.5 | 30 | 10 | 25 |
| Lycopene 100 mg/kg | 30 | 10 | 25 | 26 | 14 | 35 | 25 | 15 | 37.5# | 21 | 19 | 47.5# | 20 | 20 | 50## |
| Lycopene 200 mg/kg | 32 | 8 | 20 | 32 | 8 | 20 | 31 | 9 | 22.5 | 31 | 9 | 22.5 | 29 | 11 | 27.5 |
| χ2-value | 3.293 | 4.117 | 11.120 | 8.638 | 9.524 | ||||||||||
| P-value | 0.628 | 0.271 | 0.005 | 0.021 | 0.005 | ||||||||||
Missing: The number of margin tactic and random tactic; Valid: the number of straight tactic and tend tactic. Data are presented as the mean ± SEM (n = 10; chi-square test). #P < 0.05, ##P < 0.01, vs. model group.
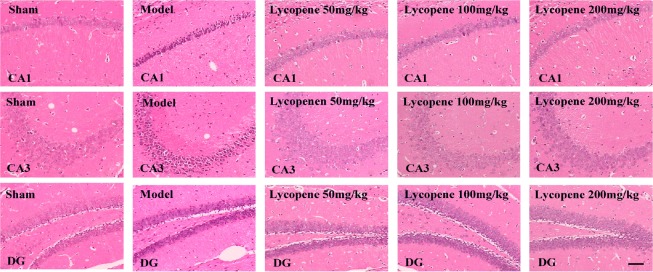
Effect of lycopene on cellular morphology in the hippocampus
Hematoxylin-eosin staining of hippocampal sections is illustrated in Figure 5. The hippocampal nerve cells in the sham group were orderly arranged, with clear nuclei and distinct nucleoli. In the model group, the main features of the nerve cells were irregular arrangement and ambiguous morphology. Swollen neurons with loose structure and deeply-stained, pyknotic nuclei were numerous, and some cells had lost their nucleus, forming a vacuole-like structure. Of the three lycopene-treated groups, the lycopene 100 mg/kg group presented regularly arranged neurons, with distinct layers and integrated structure and clear nuclei. The other two dose groups exhibited closely and irregularly arranged neurons with loose structure, especially in the high-dose group, where most of the neurons had lost their nuclei, forming a vacuole-like structure.
Figure 5.
Effect of lycopene on neuronal morphology in the hippocampus detected by hematoxylin-eosin staining.
Scale bar: 200 µm. CA1: Cornu ammonis region 1; CA3: cornu ammonis region 3; DG: dentate gyrus.
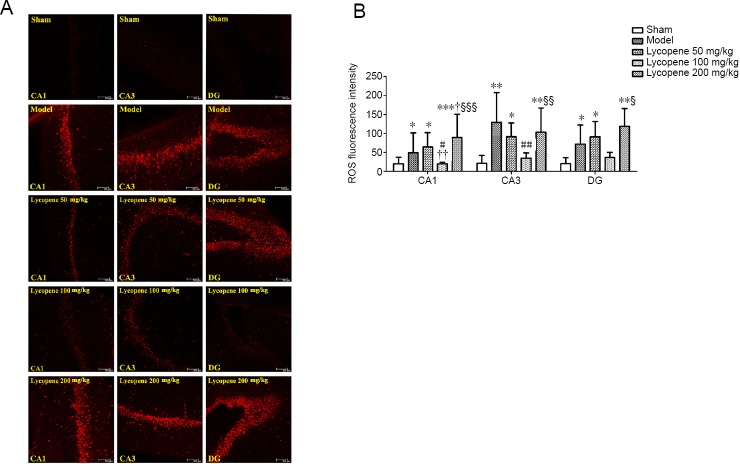
Effect of lycopene on ROS generation in the hippocampus
To characterize and localize ROS content within the hippocampus, red ethidium fluorescence was analyzed in CA1, CA3 and dentate gyrus (DG) areas of the hippocampus incubated with dihydroethidium. As depicted in Figure 6, ROS fluorescence intensity was very weak in the hippocampus of the sham group. In contrast, abnormally bright fluorescence was found in the model groups. ROS fluorescence intensity in the lycopene 100 mg/kg group was the weakest among the three lycopene-treated groups, approaching the intensity of the sham group. ROS fluorescence intensity among hippocampal CA1, CA3 and DG areas of each group was significantly different (F(4, 15) = 9.468, P < 0.001 for CA1; F(4, 15) = 5.686, P < 0.01 for CA3; F(4, 15) = 3.601, P < 0.05 for DG). ROS generation was mainly concentrated in CA3 and DG areas, especially in the DG.
Figure 6.
Effect of lycopene on the level of ROS in the hippocampus of rats from each group.
(A) ROS fluorescence in the hippocampus of rats in different groups determined by dihydroethidium staining (Red: ROS; Scale bars: 100 µm). (B) Mean values of ROS fluorescence intensity in the hippocampus (mean ± SEM, n = 3; one-way analysis of variance followed by Dunnett’s post hoc test). *P < 0.05, **P < 0.01, ***P ≤ 0.001, vs. sham group; #P < 0.05, ##P < 0.01, vs. model group; †P < 0.05, ††P < 0.01, vs. lycopene 50 mg/kg group; §P≤ 0.05, §§P ≤ 0.01, §§§P ≤ 0.001, vs. lycopene 100 mg/kg group. CA1: Cornu ammonis region 1; CA3: cornu ammonis region 3; DG: dentate gyrus; ROS: reactive oxygen species.
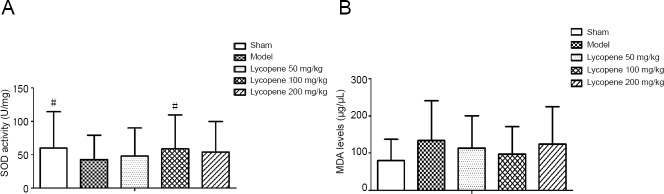
Expression of SOD and MDA in the hippocampus
To determine how lycopene influences the oxidation system, we analyzed SOD activity and MDA content in the hippocampus of each group. The results showed that SOD activity was decreased in the hippocampus of vascular dementia rats (F(4, 20) = 4.368, P < 0.05). Treatment with lycopene (50, 100, and 200 mg/kg) increased SOD activity in the hippocampus, and SOD activity achieved a peak in the lycopene 100 mg/kg group (F(4, 20) = 3.421, P < 0.05). MDA expression was increased in the hippocampus of vascular dementia rats, but no significant difference was determined. MDA expression was hardly changed in the three-dose groups (Figure 7A and B).
Figure 7.
Effect of lycopene on SOD activity and MDA levels in the rat hippocampus of each group by biochemical detection.
(A) SOD activity. (B) MDA level (mean ± SEM, n = 4; one-way analysis of variance followed by Dunnett's post hoc test). #P < 0.05, vs. model group. MDA: Malondialdehyde; SOD: superoxide dismutase.
Expression of NRSF in the hippocampus tested by immunofluorescence
The distribution of NRSF in CA1, CA3 and DG areas of the hippocampus was observed by immunofluorescence labeling and laser confocal scanning microscopy. DAPI was used to label nuclei.
The immunoreactivity of NRSF in the three hippocampal areas was stronger in the model group than in the sham group and the lycopene 100 mg/kg group. Compared with the NRSF fluorescence intensity of the lycopene 100 mg/kg group, the fluorescence of the hippocampus in the other two dose groups was much brighter (Figure 8).
Figure 8.
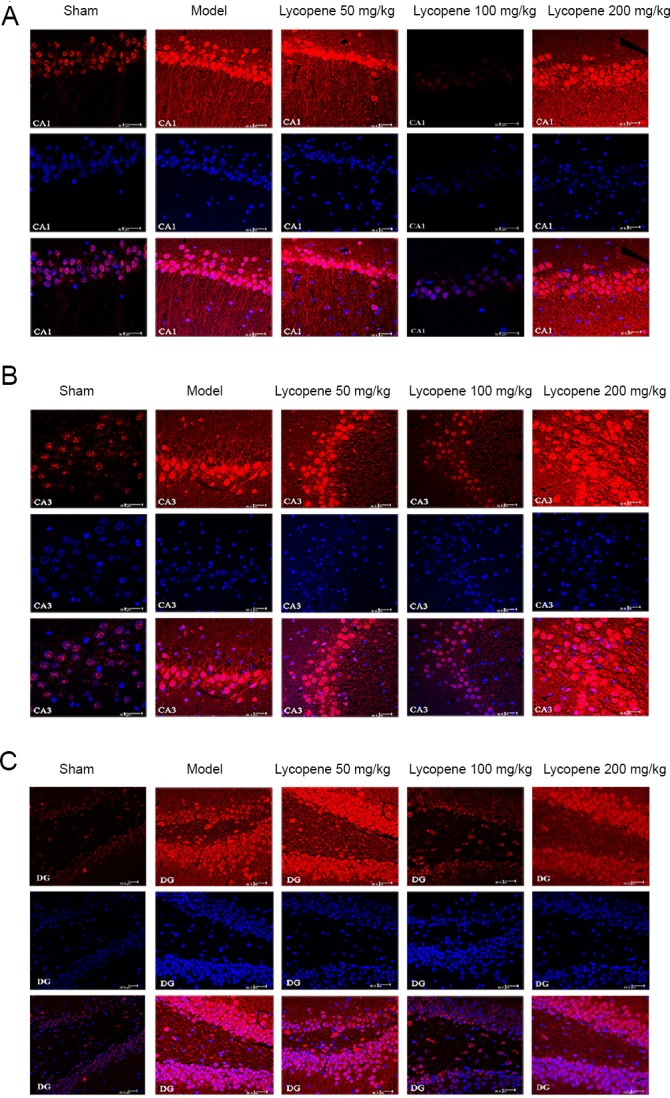
Immunohistofluorescence detection of NRSF in the rat hippocampus.
(A) NRSF distribution in the CA1 region. (B) NRSF distribution in the CA3 region. (C) NRSF distribution in the DG region. Scale bars: 30 µm. Red: NRSF; blue: DAPI; CA1: cornu ammonis region 1; CA3: cornu ammonis region 3; DG: dentate gyrus; NRSF: neuron-restrictive silencer factor.
Expression of NRSF in the hippocampus assessed by western blotting
NRSF levels in the hippocampus were also measured by western blotting. NRSF content was remarkably higher in the model group than in the sham group and the lycopene 100 mg/kg group (F(4, 20) = 3.094, P < 0.05). NRSF content was the lowest in the lycopene 100 mg/kg group among the three-dose groups. These results were consistent with the immunofluorescence results (Figure 9).
Figure 9.
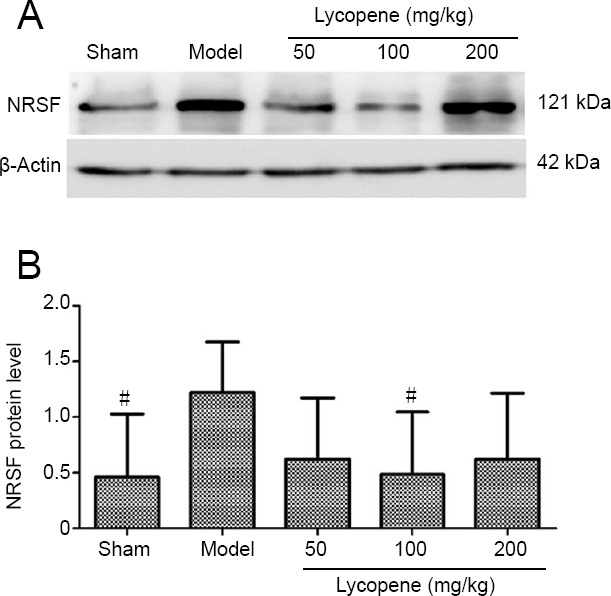
Effect of lycopene on NRSF levels in the rat hippocampus.
(A) Western blot assay of NRSF in the hippocampus of the five groups. Top panel shows the NRSF protein band and the bottom panel shows the β-actin loading control. (B) NRSF protein level (mean ± SEM, n = 4; one-way analysis of variance followed by Dunnett’s post hoc test). #P < 0.05, vs. model group. NRSF: Neuron-restrictive silencer factor.
Discussion
The main findings of this study are: bilateral ligation of common carotid arteries results in learning and memory impairment in rats. Lycopene can prevent this brain injury by suppressing oxidative stress. The optimal dosage of lycopene to protect vascular dementia model rats was 100 mg/kg; NRSF expression may be induced by ROS generation.
Vascular dementia is a cerebrovascular disease that causes intellectual impairment (O’Brien and Thomas, 2015; Wu et al., 2016; Jia et al., 2018). Bilateral ligation of common carotid arteries in rats causes low perfusion and hypoxic conditions in the brain leading to learning and memory impairment, and this pathogenesis is similar to that of vascular dementia (Nanri and Watanabe, 1999). The two-vessel occlusion model is a classical animal model used in studying vascular dementia (Venkat et al., 2015). Chronic cerebral hypoperfusion induces mitochondrial dysfunction and oxidative stress (Gironi et al., 2011). In our study, we observed significant memory deficits in the model group, i.e., long average escape latency and a low proportion of tend and straight tactics. In addition, we found that hippocampal neurons in the vascular dementia rats were seriously damaged. These results are in agreement with previously obtained results (Wang et al., 2014) and demonstrated that the vascular dementia model was successfully established.
Oxidative stress is an important factor in the development of cognitive impairment caused by vascular hypoperfusion-induced pathology (Bennett et al., 2009; Liu and Zhang, 2012). Oxidative stress can cause irreversible cellular injury because reactive oxygen species can be generated that exceed antioxidant defenses (Bennett et al., 2009; Choi et al., 2009). ROS are a group of highly reactive molecules that damage proteins, nucleic acids, and membrane polyunsaturated fatty acids, causing lipid peroxidation and loss of membrane integrity, reduction of mitochondrial membrane potential, and increased permeability of the plasma membrane to Ca2+ (Wassmann et al., 2004; Choi et al., 2009; Di Meo et al., 2016). Previous evidence has suggested that ROS-induced oxidative stress plays a vital role in the pathogenesis of brain injuries (Wang et al., 2007). Our findings here show that ROS levels in the hippocampus of vascular dementia rats was lower than that in the sham group. We also tested SOD (a common enzyme of the endogenous antioxidant system) (Warner et al., 2004; Yun et al., 2013) and MDA (an end product of lipid peroxidation) levels in the hippocampus. The activity of SOD was lower in the model group than in the sham group, but MDA levels were the opposite. Additionally, hematoxylin-eosin staining showed the hippocampal neurons of the model group were severely impaired compared with the sham group, which was likely to cause cognitive impairment. All our results showed that hypoperfusion rats had a ROS surge.
Many compounds from fruits and vegetables have antioxidant properties and have thus been considered as therapeutic agents. Lycopene is a compound extracted from tomato and has strong antioxidant activity (Holzapfel et al., 2013). Moreover, lycopene can protect the nervous system by preventing oxidative stress, because its lipophilic nature enables it to traverse the blood-brain barrier (Khachik et al., 2002). Lycopene possesses the ability to protect learning and memory impairment (Prakash and Kumar, 2013). The results of the present study are consistent with previous results; the average escape latency and path length were decreased; and the proportion of tend and straight tactics was increased when vascular dementia rats were treated with lycopene, indicating that the learning and memory impairment of vascular dementia rats was ameliorated by lycopene. Of the three dosage groups, learning and memory ability was most significantly improved in the 100 mg/kg group. Hematoxylin-eosin staining demonstrated that the vascular dementia injury was alleviated in the lycopene-treated groups. These findings indicate that lycopene protected hippocampal neurons against damage from bilateral ligation of common carotid arteries. Lycopene can protect against learning and memory impairment in rats by blocking ROS generation (Maycotte and Thorburn, 2011). This function benefits from the large system of conjugated double bonds in the structure of lycopene. In several experimental models of oxidative damage, lycopene also exerts its neuroprotective role by inhibiting free radicals (Hsiao et al., 2004; Qu et al., 2011). Lycopene is not only a scavenger of ROS, but is also a scavenger of other free radicals (Prakash and Kumar, 2013; Müller et al., 2016) and is also an anti-inflammatory agent (Sachdeva and Chopra, 2015)
To explore the underlying mechanism of lycopene neuroprotection in vascular dementia rats, we measured the ROS levels in the hippocampus. We found that ROS fluorescence intensity was weaker in the three lycopene-treated groups than in the model group; ROS content was lowest in the lycopene 100 mg/kg group among the three dose groups. Furthermore, SOD activity was high and MDA levels were low in the hippocampus in the three dose groups. SOD activity showed the maximum reversion in the lycopene 100 mg/kg group. MDA content was low in the model group. These results indicate that lycopene protects against memory impairment by suppressing ROS levels. Of the three dose groups, the lycopene 100 mg/kg group showed the strongest effect of ROS elimination, suggesting that lycopene 100 mg/kg was the optimal dose in this rat model. The low (50 mg/kg) and high (200 mg/kg) dose groups were less effective at scavenging ROS, implying that 50 mg/kg was not a sufficient dose while 200 mg/kg was an excess dose with a toxic effect. Adverse effects were observed in Wistar rats intragastrically administrated ultrahigh doses of lycopene (Jonker et al., 2003). The pathobiology of vascular dementia is very complex; therefore, whether lycopene played its neuroprotective role by directly eliminating ROS or via ROS-activated downstream pathways remains unknown.
We also detected the expression of another factor, NRSF, which is implicated in the pathogenesis and therapeutic mechanism of various neurodegenerative diseases (Song et al., 2015). NRSF, as a transcription factor, can silence the expression of target genes containing the canonical RE1-NRSE element (Bruce et al., 2004; Mortazavi et al., 2006). Previous studies have found that NRSF plays diverse roles in multiple cellular processes in the nervous system (Zuccato and Cattaneo, 2007; Hu et al., 2011; Noh et al., 2012; Warburton et al., 2015). In aging and Alzheimer’s disease, NRSF is markedly induced under stress-related conditions and has a neuroprotective role in oxidative stress-damaged neurons. Cell death induced by hydrogen peroxide was also significantly reduced when NRSF was overexpressed (Lu et al., 2014). However, higher levels of NRSF overexpression result in cell death, because NRSF represses expression of many target genes, such as miR-132, the NMDAR subunit NR1, and AMPA receptor subunit GluA2 (Calderone et al., 2003; Hwang et al., 2014; Hwang and Zukin, 2018). In addition to these genes, NRSF regulates synaptic plasticity and apoptosis-related genes (Lu et al., 2014; Hwang and Zukin, 2018). Therefore, the role of NRSF in neurons depends not only on the cell type and tissue specificity, but also on the concentration of NRSF. NRSF was induced in vulnerable hippocampal neurons, and can silence GluA2 expression in neurons destined to die (Calderone et al., 2003). Our findings indicate that NRSF levels were significantly higher in the model group than in the sham group, indicating ischemia-induced NRSF expression. Of the three dose groups, NRSF expression in the lycopene 100 mg/kg group approached that of the sham group. These results showed that NRSF expression was consistent with ROS levels in this rat model. Based on previously reported theories, it is reasonable to infer that NRSF expression was induced by ROS in this vascular dementia model, which is similar to the conditions of aging and Alzheimer’s disease. Of course, the exact pathobiology mechanism of vascular dementia needs to be further clarified in future studies.
In summary, lycopene plays a neuroprotective role in vascular dementia rats by decreasing ROS generation, which provides a clue for potential therapeutic intervention in vascular dementia. Future studies should focus on determining the detailed mechanism of the lycopene antioxidant effect in vascular dementia models, including changes to receptors and signal pathways. The exact role of NRSF in vascular dementia pathogenesis also needs to be defined.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This work was financially supported by the National Innovation and Entrepreneurship Training Project of China in 2013, No. 201310392009 (to XZZ); the Innovation and Entrepreneurship Training Project of Fujian Province of China in 2014, No. 201410392058 (to XZZ). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication..
Institutional review board statement: The animal care and the experimental procedures were approved by the Animal Ethics Committee of Fujian Medical University, China (approval No. 2014-025) in June 2014.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was financially supported by the National Innovation and Entrepreneurship Training Project of China in 2013, No. 201310392009 (to XZZ); the Innovation and Entrepreneurship Training Project of Fujian Province of China in 2014, No. 201410392058 (to XZZ)..
C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Allen J, Yajima W, Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Alzheimer’s Association (2017) 2017 Alzheimer’s disease facts and figures. Alzheimers Dement. 13:325–373. [Google Scholar]
- 2.Barnhart CD, Yang D, Lein PJ. Using the Morris water maze to assess spatial learning and memory in weanling mice. PLoS One. 2015;10:e0124521. doi: 10.1371/journal.pone.0124521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baskys A, Cheng JX. Pharmacological prevention and treatment of vascular dementia: approaches and perspectives. Exp Gerontol. 2012;47:887–891. doi: 10.1016/j.exger.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Bennett S, Grant MM, Aldred S. Oxidative stress in vascular dementia and Alzheimer’s disease: a common pathology. J Alzheimers Dis. 2009;17:245–257. doi: 10.3233/JAD-2009-1041. [DOI] [PubMed] [Google Scholar]
- 5.Bruce AW, Donaldson IJ, Wood IC, Yerbury SA, Sadowski MI, Chapman M, Göttgens B, Buckley NJ. Genome-wide analysis of repressor element 1 silencing transcription factor neuron-restrictive silencing factor (RESTNRSF) target genes. Proc Natl Acad Sci U S A. 2004;101:10458–10463. doi: 10.1073/pnas.0401827101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calderone A, Jover T, Noh KM, Tanaka H, Yokota H, Lin Y, Grooms SY, Regis R, Bennett MV, Zukin RS. Ischemic insults derepress the gene silencer rest in neurons destined to die. J Neurosci. 2003;23:2112–2121. doi: 10.1523/JNEUROSCI.23-06-02112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi K, Kim J, Kim GW, Choi C. Oxidative stress-induced necrotic cell death via mitochondira-dependent burst of reactive oxygen species. Curr Neurovasc Res. 2009;6:213–222. doi: 10.2174/156720209789630375. [DOI] [PubMed] [Google Scholar]
- 8.Chong JA, Tapia-Ramírez J, Kim S, Toledo-Aral JJ, Zheng Y, Boutros MC, Altshuller YM, Frohman MA, Kraner SD, Mandel G. REST: a mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell. 1995;80:949–957. doi: 10.1016/0092-8674(95)90298-8. [DOI] [PubMed] [Google Scholar]
- 9.Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS sources in physiological and pathological conditions. Oxid Med Cell Longev. 2016;2016:1245049. doi: 10.1155/2016/1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gironi M, Bianchi A, Russo A, Alberoni M, Ceresa L, Angelini A, Cursano C, Mariani E, Nemni R, Kullmann C, Farina E, Martinelli Boneschi F. Oxidative imbalance in different neurodegenerative diseases with memory impairment. Neurodegener Dis. 2011;8:129–137. doi: 10.1159/000319452. [DOI] [PubMed] [Google Scholar]
- 11.Holzapfel NP, Holzapfel BM, Champ S, Feldthusen J, Clements J, Hutmacher DW. The potential role of lycopene for the prevention and therapy of prostate cancer: from molecular mechanisms to clinical evidence. Int J Mol Sci. 2013;14:14620–14646. doi: 10.3390/ijms140714620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsiao G, Fong TH, Tzu NH, Lin KH, Chou DS, Sheu JR. A Potent antioxidant, lycopene affords neuroprotection against microglia activation and focal cerebral ischemia in rats. In Vivo. 2004;18:351–356. [PubMed] [Google Scholar]
- 13.Huang J, Wu FF, He X, Li JP, Yan XX, Pan AH, Li ZY. Effects of enriched environment on immature neurons in piriform cortex of a rat model of vascular dementia. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:4006–4012. [Google Scholar]
- 14.Hu XL, Cheng X, Cai L, Tan GH, Xu L, Feng XY, Lu TJ, Xiong H, Fei J, Xiong ZQ. Conditional deletion of NRSF in forebrain neurons accelerates epileptogenesis in the kindling model. Cereb Cortex. 2011;21:2158–2165. doi: 10.1093/cercor/bhq284. [DOI] [PubMed] [Google Scholar]
- 15.Hwang JY, Kaneko N, Noh KM, Pontarelli F, Zukin RS. The gene silencing transcription factor REST represses miR-132 expression in hippocampal neurons destined to die. J Mol Biol. 2014;426:3454–3466. doi: 10.1016/j.jmb.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang JY, Zukin RS. REST a master transcriptional regulator in neurodegenerative disease. Curr Opin Neurobiol. 2018;48:193–200. doi: 10.1016/j.conb.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia XY, Yuan X, Zhou XM, Jiao R, Xie HL, Wang D, Yin L, Tan TT, Liu QQ, Chen SJ. The effect of moxibustion on brain functional connectivity and effective brain networks in patients with amnestic mild cognitive impairment: study protocol for a randomized controlled trial and preliminary results. Asia Pac J Clin Trials Nerv Syst Dis. 2018;3:112–119. [Google Scholar]
- 18.Jin BR, Liu HY. Comparative efficacy and safety of cognitive enhancers for treating vascular cognitive impairment: systematic review and Bayesian network meta-analysis. Neural Regen Res. 2019;14:805–816. doi: 10.4103/1673-5374.249228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonker D, Kuper CF, Fraile N, Estrella A, Rodríguez Otero C. Ninety-day oral toxicity study of lycopene from Blakeslea trispora in rats. Regul Toxicol Pharmacol. 2003;37:396–406. doi: 10.1016/s0273-2300(03)00013-8. [DOI] [PubMed] [Google Scholar]
- 20.Kawamura M, Sato S, Matsumoto G, Fukuda T, Shiba-Fukushima K, Noda S, Takanashi M, Mori N, Hattori N. Loss of nuclear REST/NRSF in aged-dopaminergic neurons in Parkinson’s disease patients. Neurosci Lett. 2019;699:59–63. doi: 10.1016/j.neulet.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 21.Khachik F, Carvalho L, Bernstein PS, Muir GJ, Zhao DY, Katz NB. Chemistry distribution and metabolism of tomato carotenoids and their impact on human health. Exp Biol Med (Maywood) 2002;227:845–851. doi: 10.1177/153537020222701002. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Xu WP. Meta-analysis of statins for treatment of vascular cognitive impairment. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;8:8769–8774. [Google Scholar]
- 23.Liu DD, Yuan X, Chu SF, Chen C, Ren Q, Luo P, Lin MY, Wang SS, Zhu TB, Ai QD, Zang YD, Zhang DM, He X, Huang ZH, Sun HS, Feng ZP, Chen NH. CZ-7 a new derivative of Claulansine F, ameliorates 2VO-induced vascular dementia in rats through a Nrf2-mediated antioxidant responses. Acta Pharmacol Sin. 2019;40:425–440. doi: 10.1038/s41401-018-0078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu H, Zhang JJ. Cerebral hypoperfusion and cognitive impairment: the pathogenic role of vascular oxidative stress. Int J Neurosci. 2012;122:494–499. doi: 10.3109/00207454.2012.686543. [DOI] [PubMed] [Google Scholar]
- 25.Lu T, Aron L, Zullo J, Pan Y, Kim H, Chen Y, Yang TH, Kim HM, Drake D, Liu XS, Bennett DA, Colaiácovo MP, Yankner BA. REST and stress resistance in ageing and Alzheimer’s disease. Nature. 2014;507:448–454. doi: 10.1038/nature13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maycotte P, Thorburn A. Autophagy and cancer therapy. Cancer Biol Ther. 2011;11:127–137. doi: 10.4161/cbt.11.2.14627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mei ZG, Tan LJ, Wang JF, Li XL, Huang WF, Zhou HJ. Fermented Chinese formula Shuan-Tong-Ling attenuates ischemic stroke by inhibiting inflammation and apoptosis. Neural Regen Res. 2017;12:425–432. doi: 10.4103/1673-5374.202946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer K, Feldman HM, Lu T, Drake D, Lim ET, Ling KH, Bishop NA, Pan Y, Seo J, Lin YT, Su SC, 4, Church GM, Tsai LH, Yankner BA. REST and neural gene network dysregulation in ipsc models of alzheimer’s disease. Cell Rep. 2019;26:1112–1127. doi: 10.1016/j.celrep.2019.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mortazavi A, Leeper Thompson EC, Garcia ST, Myers RM, Wold B. Comparative genomics modeling of the NRSF/REST repressor network: from single conserved sites to genome-wide repertoire. Genome Res. 2006;16:1208–1221. doi: 10.1101/gr.4997306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Müller L, Caris-Veyrat C, Lowe G, Böhm V. Lycopene and its antioxidant role in the prevention of cardiovascular diseases-a critical review. Crit Rev Food Sci Nutr. 2016;56:1868–1879. doi: 10.1080/10408398.2013.801827. [DOI] [PubMed] [Google Scholar]
- 31.Nanri M, Watanabe H. Availability of 2VO rats as a model for chronic cerebrovascular disease. Nihon Yakurigaku Zasshi. 1999;113:85–95. doi: 10.1254/fpj.113.85. [DOI] [PubMed] [Google Scholar]
- 32.Noh KM, Hwang JY, Follenzi A, Athanasiadou R, Miyawaki T, Greally JM, Bennett MV, Zukin RS. Repressor element-1 silencing transcription factor (REST)-dependent epigenetic remodeling is critical to ischemia-induced neuronal death. Proc Natl Acad Sci U S A. 2012;109:E962–E971. doi: 10.1073/pnas.1121568109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Brien JT, Thomas A. Vascular dementia. Lancet. 2015;386:1698–1706. doi: 10.1016/S0140-6736(15)00463-8. [DOI] [PubMed] [Google Scholar]
- 34.Patterson KP, Barry JM, Curran MM, Singh-Taylor A, Brennan G, Rismanchi N, Page M, Noam Y, Holmes GL, Baram TZ. Enduring memory impairments provoked by developmental febrile seizures are mediated by functional and structural effects of neuronal restrictive silencing factor. J Neurosci. 2017;37:3799–3812. doi: 10.1523/JNEUROSCI.3748-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prakash A, Kumar A. Lycopene protects against memory impairment and mito-oxidative damage induced by colchicine in rats: an evidence of nitric oxide signaling. Eur J Pharmacol. 2013;721:373–381. doi: 10.1016/j.ejphar.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Qu M, Zhou Z, Chen C, Li M, Pei L, Chu F, Yang J, Wang Y, Li L, Liu C, Zhang L, Zhang G, Yu Z, Wang D. Lycopene protects against trimethyltin-induced neurotoxicity in primary cultured rat hippocampal neurons by inhibiting the mitochondrial apoptotic pathway. Neurochem Int. 2011;59:1095–1103. doi: 10.1016/j.neuint.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Rodenas-Ruano A, Chávez AE, Cossio MJ, Castillo PE, Zukin RS. REST-dependent epigenetic remodeling promotes the developmental switch in synaptic NMDA receptors. Nat Neurosci. 2012;15:1382–1390. doi: 10.1038/nn.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sachdeva AK, Chopra K. Lycopene abrogates Abeta(1-42)-mediated neuroinflammatory cascade in an experimental model of Alzheimer’s disease. J Nutr Biochem. 2015;26:736–744. doi: 10.1016/j.jnutbio.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Song Z, Yang W, Cheng G, Zhou X, Yang L, Zhao D. Prion protein is essential for the RE1 silencing transcription factor (REST)-dependent developmental switch in synaptic NMDA receptors. Cell Death Dis. 2018;9:541. doi: 10.1038/s41419-018-0576-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song Z, Zhao D, Zhao H, Yang L. NRSF: an angel or a devil in neurogenesis and neurological diseases. J Mol Neurosci. 2015;56:131–144. doi: 10.1007/s12031-014-0474-5. [DOI] [PubMed] [Google Scholar]
- 41.Song Z, Zhu T, Zhou X, Barrow P, Yang W, Cui Y, Yang L, Zhao D. REST alleviates neurotoxic prion peptide-induced synaptic abnormalities, neurofibrillary degeneration and neuronal death partially via LRP6-mediated Wnt-β-catenin signaling. Oncotarget. 2016;7:12035–12052. doi: 10.18632/oncotarget.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson R, Chan C. NRSF and its epigenetic effectors: new treatments or neurological disease. Brain Sci. 2018;8:E226. doi: 10.3390/brainsci8120226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venkat P, Chopp M, Chen J. Models and mechanisms of vascular dementia. Exp Neurol. 2015;272:97–108. doi: 10.1016/j.expneurol.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Fu X, Jiang C, Yu L, Wang M, Han W, Liu L, Wang J. Bone marrow mononuclear cell transplantation promotes therapeutic angiogenesis via upregulation of the VEGF-VEGFR2 signaling pathway in a rat model of vascular dementia. Behav Brain Res. 2014;265:171–180. doi: 10.1016/j.bbr.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Li L, Wang Z, Cui Y, Tan X, Yuan T, Liu Q, Liu Z, Liu X. Supplementation of lycopene attenuates lipopolysaccharide-induced amyloidogenesis and cognitive impairments via mediating neuroinflammation and oxidative stress. J Nutr Biochem. 2018;56:16–25. doi: 10.1016/j.jnutbio.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 46.Wang Q, Sun AY, Simonyi A, Kalogeris TJ, Miller DK, Sun GY, Korthuis RJ. Ethanol preconditioning protects against ischemia/reperfusion-induced brain damage: role of NADPH oxidase-derived ROS. Free Radic Biol Med. 2007;43:1048–1060. doi: 10.1016/j.freeradbiomed.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Warburton A, Breen G, Rujescu D, Bubb VJ, Quinn JP. Characterization of a REST-regulated internal promoter in the schizophrenia genome-wide associated gene MIR137. Schizophr Bull. 2015;41:698–707. doi: 10.1093/schbul/sbu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warner DS, Sheng H, Batinić-Haberle I. Oxidants antioxidants and the ischemic brain. J Exp Biol. 2004;207:3221–3231. doi: 10.1242/jeb.01022. [DOI] [PubMed] [Google Scholar]
- 49.Wassmann S, Wassmann K, Nickenig G. Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension. 2004;44:381–386. doi: 10.1161/01.HYP.0000142232.29764.a7. [DOI] [PubMed] [Google Scholar]
- 50.Wu P, Luo Y, Zhen L, Hu X, Shang Y, Liao Y, Xue H, Huang F, Xiao W. Rannasangpei is a therapeutic agent in the treatment of vascular dementia. Evid Based Complement Alternat Med. 2016;2016:2530105. doi: 10.1155/2016/2530105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yun X, Maximov VD, Yu J, Zhu H, Vertegel AA, Kindy MS. Nanoparticles for targeted delivery of antioxidant enzymes to the brain after cerebral ischemia and reperfusion injury. J Cereb Blood Flow Metab. 2013;33:583–592. doi: 10.1038/jcbfm.2012.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang XQ, Li L, Huo JT, Cheng M, Li LH. Effects of repetitive transcranial magnetic stimulation on cognitive function and cholinergic activity in the rat hippocampus after vascular dementia. Neural Regen Res. 2018;13:1384–1389. doi: 10.4103/1673-5374.235251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao B, Liu H, Wang J, Liu P, Tan X, Ren B, Liu Z, Liu X. Lycopene supplementation attenuates oxidative stress, neuroinflammation and cognitiveimpairment in aged CD-1 mice. J Agric Food Chem. 2018;66:3127–3136. doi: 10.1021/acs.jafc.7b05770. [DOI] [PubMed] [Google Scholar]
- 54.Zong W, Zeng X, Chen S, Chen L, Zhou L, Wang X, Gao Q, Zeng G, Hu K, Ouyang D. Ginsenoside compound K attenuates cognitive deficits in vascular dementia rats by reducing the Aβ deposition. J Pharmacol Sci. 2019;139:223–230. doi: 10.1016/j.jphs.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 55.Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington’s disease. Prog Neurobiol. 2007;81:294–330. doi: 10.1016/j.pneurobio.2007.01.003. [DOI] [PubMed] [Google Scholar]