Abstract
We have developed a new series of glucose sensitive fluorophores that display shifts in emission wavelengths and/or intensity change upon the binding of monosaccharides. Complexation of glucose with the boronic acid moiety changes both its orbital hybridization and its ability to accept and donate electrons. This change results in distinct emission spectra for the fluorophores when free in solution or complexed with monosaccharide. The spectral changes upon saccharide binding can be modified by substitution of electron donor or acceptor group on the fluorophore allowing rational design of the spectral response.
Keywords: boronic acid, fluorescent probe, fluorescence sensing, glucose, monosaccharide, charge transfer in the excited state
1. Introduction
Fluorescence spectroscopy is a major technique used for the detection and reporting of specific analytes in heterogeneous and biological media. Fluorescence is well known for its high sensitivity and selectivity, and in addition, it is a non- or minimally invasive method that allows nondestructive measurements and sometime in vivo measurements. For example, fluorescence, spectroscopy has been found to have useful applications in analytical chemistry, DNA technologies, and clinical assays. One of the most impressive developments involving fluorescence in the past decade occurred in the field of optical microscopy. Fluorescence microscopy techniques, such as confocal, multi-photon, and Fluorescence Lifetime Imaging Microscopy (FLIM), are widely used today and are important tools for studying cellular and tissue functions and structures in real time.1–3 For this purpose, analyte sensitive fluorescence probes have proved to be useful, and today many fluorescence probes showing sensitivity for pH, oxygen, and ions (Ca2+, Na+, Cl−, etc.) are commercially available.4 Despite years of research in molecular recognition and probe chemistry, chelator groups and fluorescence probes showing sensitivity to neutral analytes are found only sporadically in the literature. We present in this review an overview of our efforts toward the development of fluorescence probes showing sensitivity to monosaccharides.
Since glucose is a central molecule of life, its noninvasive detection is important both for basic knowledge of biochemical phenomena and for medical testing. For diabetics, the development of noninvasive methods of measuring blood glucose has been a long-standing goal. To date, a wide variety of methods for glucose detection have been described in the literature, including near-infrared (NIR) spectroscopy,5–7 optical rotation,8,9 amperometric,10,11 colorimetric,12,13 and fluorescence detection.14–18 Despite some promising results, however, these methods evidence limitations. For example, the NIR technique is limited by excessive background, and the optical rotation technique results in low optical rotation and depolarization due to the tissue. At present, the most reliable method of assessing glucose concentration is based on enzymatic assays, typically using glucose oxidase. This technique has the disadvantage of consuming the glucose and generating high reactive species like hydrogen peroxide, which can be toxic and damaging to biological composites. Also, intracellular glucose imaging using enzymes is oxygen dependent, resulting in measurements controlled for oxygen diffusion. Practical blood glucose determination using enzymatic assays also evidences limitations. To date, the most reliable glucose-sensing method consists of the well-known finger stick, followed by glucose analysis using enzymatic assay.19 In addition to the fact that this method is painful, and even the most compliant individuals are not willing to stick themselves more than several times per day, this technique has not allowed the development of easy-to-use implantable devices, such as alarm systems for hypoglycemia and active materials for automatic insulin pump. For this reason, nonconsuming competitive glucose assays using fluorescence resonance energy transfer between concanavalin A and dextran have been developed.15–17,20 There also have been efforts to use the fluorescence change of inactive enzymes such as labeled apoglucose oxidase.18 Proteins and enzymes have the advantage of showing affinity constants comparable with the blood glucose level and high selectivity. Despite these advantages, however, proteins also have the drawback of low stability with heat and organic solvents, and it is difficult to modify their overall properties. Hence, the development of synthetic glucose probes remains an important goal for biomedical research. The flexibility of organic synthesis and the robustness of the organic probes provide additional possibilities in the research toward the development of nonconsuming glucose chemosensors and sensors.
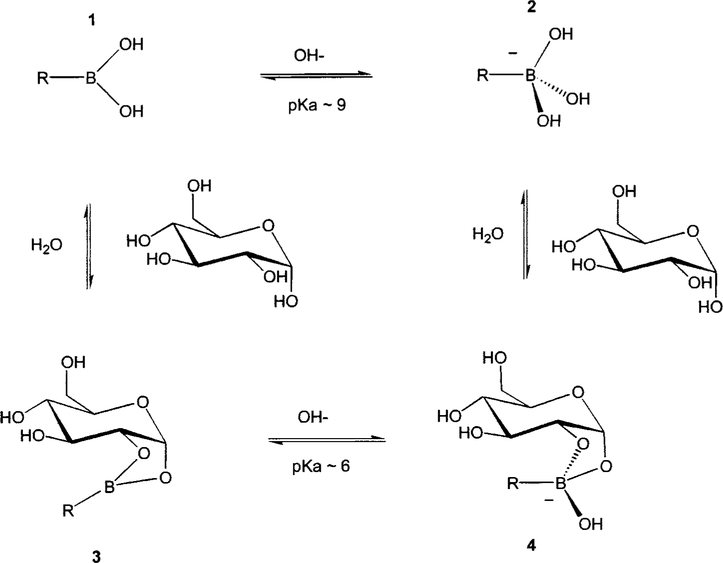
For several decades, boronic acids have been recognized for their properties to covalently bind diols21 (monosaccharides in particular). The interest in using boronic acids for the elaboration of sensing systems finds its origins in the pioneering works done by Czarnik22 and Shinkai.23 Since then, many findings on the interaction of boronic acids and sugar have been reported,24 evidencing the significant possibility of using the boronic acids as building blocks for sensing systems for sugars. Boronic acids are weak Lewis acids composed of an electron deficient boron atom and two hydroxyl groups (1 in Figure 1). The boronic acids interact with hard, strong bases like OH− to form the anionic boronate form (2 in Figure 1), and they show typical pKa of 9. Boronic acids interact with diols following a dehydration process and the formation of the boronic diester group (3 in Figure 1). The diol is linked covalently, and the interaction is fast and completely reversible in aqueous media. In comparison to the boronic acid group, the boronic ester group shows higher acidity (pKa;~6) due to a higher electrophilic boron atom. The monophenylboronic acid group shows higher affinity for D fructose, and the affinity decreases from D galactose to D glucose.21 The three sugars have binding constants (KD) of ~0.5, 6.3, and 10 mM, respectively. One of the advantages of using boronic acid groups as chelator groups for monosaccharides is that the affinity (KD) and the selectivity of the boronic acid group against the sugars both depend strongly on the molecular geometry and substitution on the phenyl and/or the aromatic system where the boronic acid group is present. Chelator groups and/or probes showing affinity for glucose from μM to hundred of mM have been reported.25–29 In the same way, selectivity for glucose over the other sugars can be enhanced.
Fig. 1.
Equilibrium involved in the boronic acid/sugar interaction.
Many fluorescence probes based on boronic acids and involving different mechanisms to induce spectral changes have been developed over the years. Photoinduced electron transfer (PET),28 molecular rigidification,30 and excimer formation31 are some examples. Despite the promising information gleaned from these interesting studies, most of the fluorescence probes developed using these mechanisms show emission in the short wavelength and/or involve a mechanism limited to few fluorophores. Excited-state charge transfer (CT) is a versatile mechanism that can be applied to a large number of different fluorophores.32 CT glucose-sensitive fluorescent probes can be developed when the boronic acid group and an electron-donor group are present on the same fluorophore.33 In this case, the boronic acid group [−B(OH)2] acts as an electron-withdrawing group. At a selected pH and in the presence of sugar, the boronic acid group is present in its anionic form [−B(OH)(sugar)]−. The anionic form of the boronic acid group is no longer an electron-withdrawing group and spectral changes are observed due to the perturbation of the charge transfer nature of the excited state. This mechanism has been first reported by Shinkai et al.33 using a stilbene derivative (see Sec. 2.1), but has never been extensively investigated and extended to different chromophores. Using this mechanism, we developed different glucose-sensitive probes showing emission covering the entire visible and near-infrared spectrum. These probes show intensity changes, wavelength shifts, and/or fluorescence lifetime changes.
2. Results and Discussion
2.1. Stilbene Derivatives
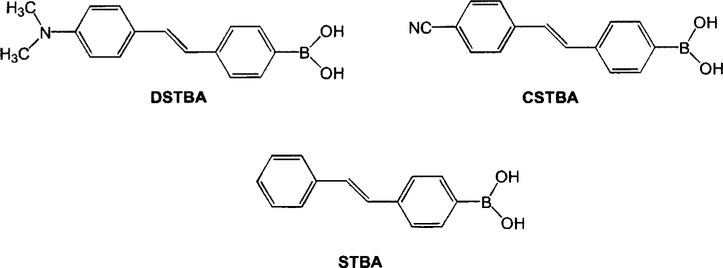
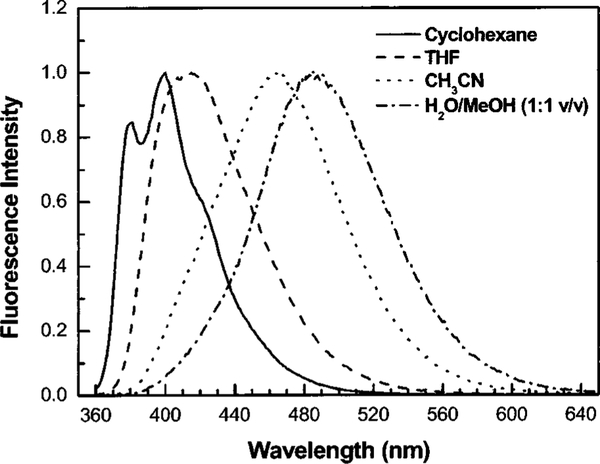
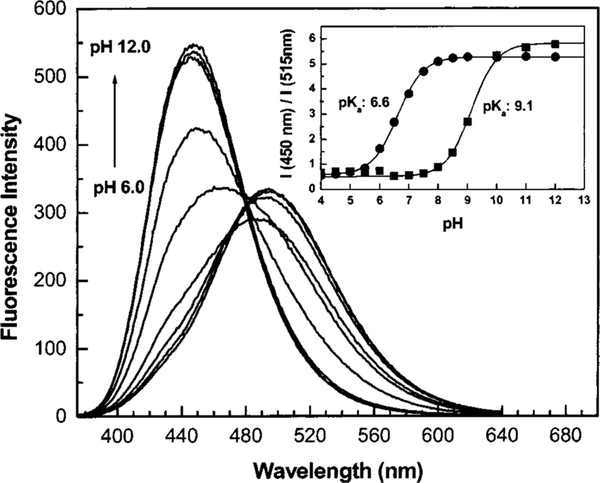
Figure 2 shows three stilebene derivatives functionalized with the boronic acid group. 4′-Dimethylaminostilbene-4-boronic acid (DSTBA) combines the electron-donating dimethylamino group with the electron-withdrawing boronic acid group. 4′-Cyanostilbene-4-boronic acid(CSTBA) combines the electron-withdrawing cyano group with the boronic acid, and STBA is a model compound used for comparison. DSTBA has been first reported by Shinmori, Takeuchi, and Shinkai33 as well as its optical responses to pH and sugar. Detail on the synthesis and complete spectroscopic characterization of these derivatives has been reported previously.33,34 Figure 3 shows the dependence of the emission spectrum of DSTBA against the polarity of the solvent. As the polarity of the solvent increases, we can observe a large redshift of the emission band. Since the absorption spectrum does not show any dependence on the polarity change of the solvent, this effect has been correlated to the formation of a charge transfer in the excited state between the dimethylamino and the boronic acid groups.32,33 As expected, CSTBA and STBA do not show significant changes in the polarity of the solvent due to the absence of the electron-donating group in these derivatives. The spectroscopic and photophysical parameters in aqueous solution of all the probes presented in this review are listed in Table 1. As the pH increases, the emission spectrum of DSTBA displays a large blueshift and an increase in the fluorescence intensity (Figure 4). These spectral changes induced by the pH are due to the formation of the anionic form of the boronic acid group (form 2 in Figure 1). As the anionic boronate species is formed, the boron group is no longer an electron-withdrawing group, resulting in the removal and/or perturbation of the charge-transfer nature of the excited state. An important feature, underlined in the inset of Figure 4, is the change of the acidity/electrophilicity of the boron group between its uncomplexed and complexed forms. This acidity change is the principal reason, and the driving force, that allows the development of a sugar sensitive fluorescent probe using the boronic acid. At a low pH (<6), the simple complexation of the boronic acid with sugar (equilibrium between species 1 and 3, Figure 1) does not result in a perturbation of the fluorophore. The same statement is true at a high pH (>9; equilibrium between species 2 and 4, Figure 1). To induce a spectral change of the fluorophore, the complexation of the probe with the analyte should result in a perturbation of the electronic properties of the fluorophore (as the charge transfer) and/or the generation of a new photophysical process (as photoinduced electron transfer). In the case of the charge transfer mechanism, the perturbation is generated by the change from the neutral (1, Figure 1) to the anionic (4, Figure 1) forms of the boronic acid in the presence of sugar at a selected pH. As discussed in the introduction, aromatic boronic acid derivatives display pKa around 9, while the complexed forms with sugar show pKa around 6. These pKa values allow the detection of sugar in the pH range of 6.5–8.5, with the maximum spectral change usually observed at pH around 7.0 and 7.5. This pH window is ideal for sugar detection in vivo and in biological media.
Fig. 2.
Molecular structures of the stilbene derivatives functionalized with the boronic acid group.
Fig. 3.
Dependence of the emission spectrum of DSTBA with the polarity of the solvent.
Table 1.
Spectroscopic and photophysical parameters of the sugar sensitive probes.
| Probe | λAbs (nm) |
ϵ (M−1 cm−1) |
λF (nm) |
ϕF |
|---|---|---|---|---|
| DSTBAa | 350 | 33000 | 485 | 0.09 |
| CSTBAa | 325 | 47000 | 385 | 0.006 |
| DDPBBAb | 368 | … | 551 | … |
| DDPHBAb | 390 | … | 580 | … |
| DAP-BAc | 350 | 20500 | 530 | 0.17 |
| Chalc1c | 440 | 22000 | 580 | 0.007 |
| Chalc2c | 445 | 19000 | 665 | 0.008 |
Water/MeOH 1:1 (v/v).
Water/MeOH 1:2 (v/v).
Water/MeOH 2:1 (v/v).
Fig. 4.
Effect of pH on the emission spectrum of DSTBA. Inset: titration curves against pH in absence (∎) and in presence (●) of D fructose (50 mM).
Figure 5 shows the effect of sugar on the emission properties of DSTBA. The emission spectrum of DSTBA shows a hypsochromic shift of about 30 nm and an increase in intensity as the concentration of sugar increases. These dramatic changes in the emission band of DSTBA are explained by the loss of the electron withdrawing property of the boronic acid group following the formation of the anionic form in presence of sugar (Figure 1), as discussed previously for the pH effect. For purposes of comparison, the spectral response of STBA to sugar is shown in Figure 6. In this case, only a small decrease is observed in the emission intensity, and this has been attributed to a PET quenching from the electron-rich boronate group.22 This later result shows that the change of the hybridization, oxidation state and charge of the boron group upon complexation with sugar does not result in a large perturbation of the fluorophore by itself. Important optical changes are observed when the boronic acid is coupled with electron-donating groups and then actively contribute to the electronic properties of the fluorophore.
Fig. 5.
Effect of D fructose on the emission spectrum of DSTBA measured in phosphate buffer pH 8.0/MeOH 2:1 (v/v). Inset: titration curves of DSTBA against D fructose (∎), D galactose (●), and D glucose (▲).
Fig. 6.
Effect of D fructose on the emission spectrum of STBA measured in phosphate buffer pH 8.0/MeOH 2:1 (v/v). Inset: titration curves of STBA against D fructose (∎), D galactose (●), and D glucose (▲).
Dissociation constants against different sugars for the probes presented in this review are listed in Table 2. As previously mentioned in the introduction, monoboronic acid derivatives show higher affinities for D fructose and affinity decreases for D galactose and D glucose. The apparent dissociation constants are pH sensitive and decrease as the pH increases. For example, the binding constant of CSTBA for D fructose shows a variation from 3.22 to 0.65 mM for pH values of 7.0–8.0, respectively. The blood glucose level is in the range of 3–8 mM for a normal person, and this range increases to between 2 and 40 mM in diabetics. The fact that boronic acids show affinities in this millimolar range is very promising and could lead to useful, noninvasive glucose sensors. Selectivity could be problematic for the usefulness of monoboronic acid probes. These probes show higher affinities for D fructose, and even if D fructose is present in small concentrations in blood (about ten times smaller than glucose), it could be a major source of interference. As mentioned previously, the selectivity of the boronic sugar can be enhanced. Usually, bis-boronic acid derivatives show higher affinities and selectivities for D glucose in comparison with other sugar.25–28 The emission properties of fluorescent probes based on a charge transfer mechanism show strong dependence on the polarity of the environment. In addition, some of these probes, especially the stilbene derivatives, can also show dependence on the viscosity of the environment. These points should be carefully evaluated in the case of their use to build sensing devices. For example, we observed that in a microenvironment composed of hydrophobic microstructures in an aqueous environment, the probes do not show CT behavior due to their hydrophobicity. In this case, the presence of sugar does not result in optical changes.
Table 2.
Apparent dissociation constants of the fluorescent probes for different sugars.
| Probe | D fructose |
KD (mM) D galactose |
D glucose |
|---|---|---|---|
| DSTBAa | 2.5 | 49 | 98 |
| CSTBAa | 0.65 | 12 | 18 |
| DDPBBAa | 1.1 | 5.9 | 17 |
| DDPHBAa | 0.84 | 5.4 | 15 |
| DAP-BAb | 1.9 | 14 | 37 |
| Chalc1c | 2.5 | 16 | 34 |
| Chalc2c | 2.1 | 14 | 30 |
Measured at pH 8.0.
Measured at pH 7.0.
Measured at pH 6.5.
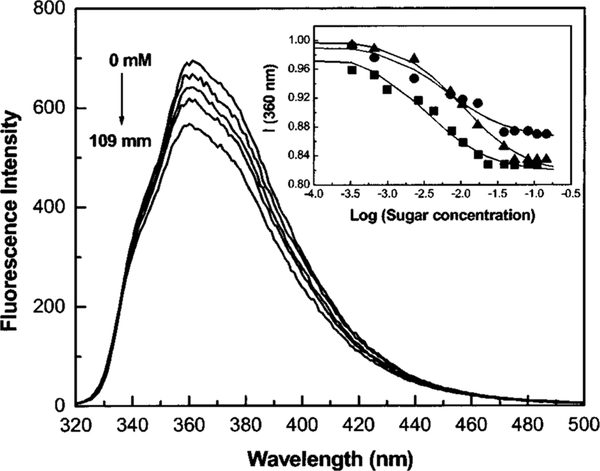
The CSTBA derivative possesses two electron-withdrawing groups, the cyano and the boronic acid groups. As expected for these kinds of compounds, no CT states are observed for the neutral form of the boronic acid group. On the other hand, we can observe a bathochromic shift, 25 nm, and a decrease of the intensity (60%) in the emission spectrum of CSTBA at high pH34 (not shown). The pKa of the uncomplexed and complexed boronic acids with D fructose are 8.2 and 5.8, respectively. The effect of D fructose on the emission band of CSTBA is shown in Figure 7. The bathochromic shift and the intensity change are very similar, but they occur in opposite directions from those observed for DSTBA. We have attributed this new, redshifted band to an excited CT state present for the anionic form of CSTBA. This suggests that the anionic form of the boronic acid group can act as an electron donor group. This later observation opens the door to the use of a more diverse group of fluorophores for the development of sugar sensitive fluorescent probes. In addition, the wavelength shift and/or the intensity change direction can be selected in function of the application.
Fig. 7.
Effect of D fructose on the emission spectrum of CSTBA measured in phosphate buffer pH 8.0/MeOH 2:1 (v/v). Inset: titration curves of CSTBA against D fructose (∎), D galactose (●), and D glucose (▲).
2.2. Polyene Derivatives
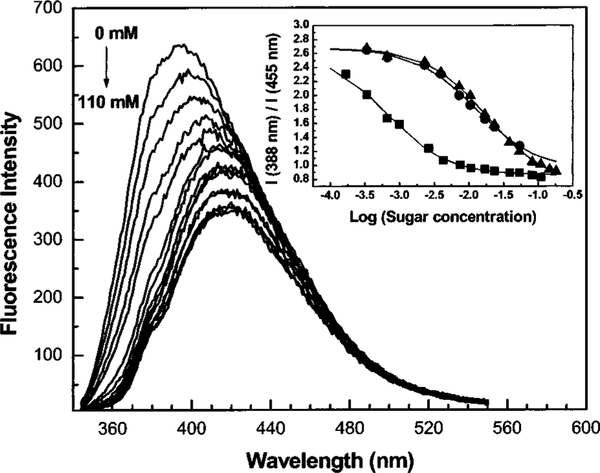
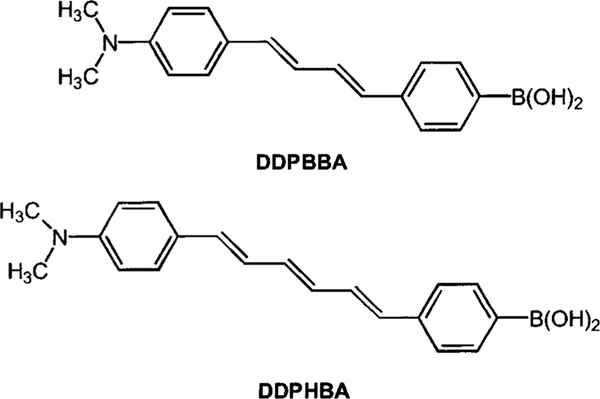
In order to test the usefulness of the CT mechanism for the development of longer-wavelength glucose sensitive fluorescent probes, we synthesized a diphenylbutadiene and a diphenylhexatriene derivative, each combining a dimethylamino group and a boronic acid group in para positions of each phenyl group (Figure 8).29 Typical spectral responses to sugar are displayed in Figure 9. As ascertained in the observation of DSTBA, we saw a blueshift of the emissive band and an increase of the emission intensity in the presence of sugar. While the 1-(p-Boronophenyl)-4-(p-dimethylaminophenyl) buta-1,2-diene (DDPBBA) produced a good spectral response, the spectral changes observed for the longer-wavelength derivative, DDPHBA, were less pronounced than in the previous compound. For the moment, it is not clear if this is due to the decrease of the energy band gap between the excited state and the ground state, or if it is due to the nature of the fluorophore.
Fig. 8.
Molecular structures of the two diphenylpolyene derivatives.
Fig. 9.
Effect of D fructose on the emission spectrum of DDPBBA measured in phosphate buffer pH 8.0/MeOH 1:2 (v/v). Inset: titration curves against D fructose (∎), D galactose (●), and D glucose (▲).
2.3. Diphenyloxazole Derivative
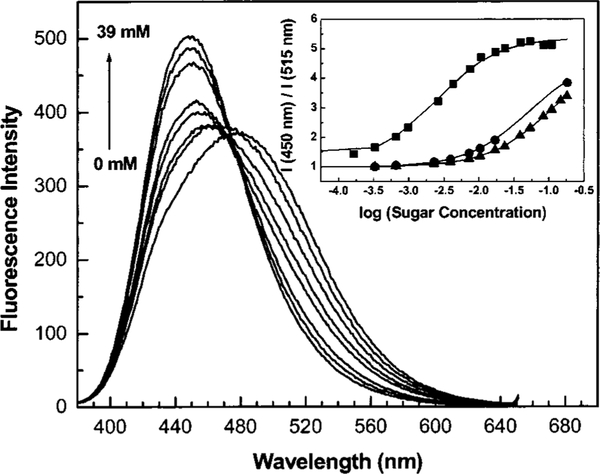
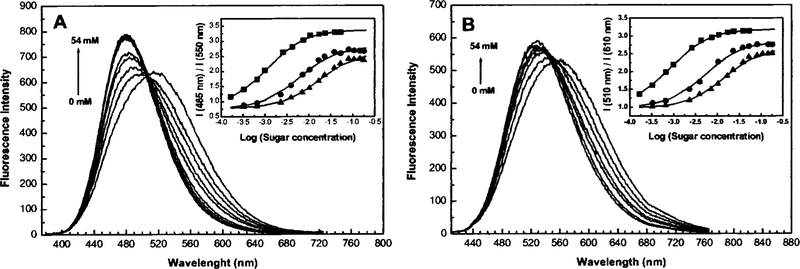
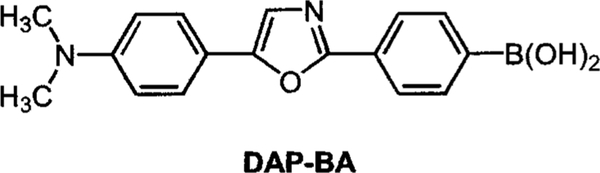
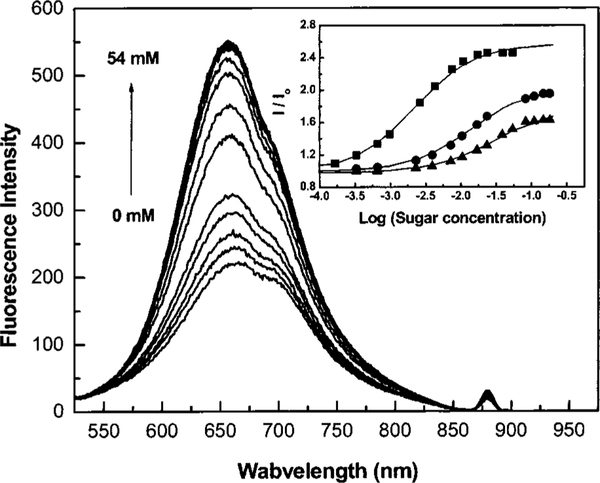
While the diphenylpolyene derivatives show interesting spectral responses to sugar, they possess small fluorescence quantum yields (1%–10%), and their chemical stability decreases as the number of double bonds increases. For these reasons, we developed a donor/acceptor diphenyloxazole derivative functionalized with the boronic acid group (Figure 10).35 This family of fluorophores is known to show high fluorescent quantum yield, long-wavelength emission and good photostability, and it is very sensitive to a small perturbation affecting the CT nature of the excited state.36 The spectral response of DAP-BA to D fructose is shown on Figure 11. Unlike the stilbene and polyene derivatives, DAP-BA shows a larger intensity change (fivefold increase) and similar blueshift (~30 nm). The fivefold intensity increase is the largest intensity change observed for a sugar sensitive fluorescent probe based on the CT mechanism.
Fig. 10.
Molecular structure of the sugar sensitive diphenyloxazole derivative.
Fig. 11.
Effect of D fructose on the emission spectrum of DAP-BA measured in phosphate buffer pH 7.0/MeOH 2:1 (v/v). Inset: titration curves of DAP-BA against D fructose (∎), D galactose (▲), and D glucose (▲).
2.4. Chalcone Derivative
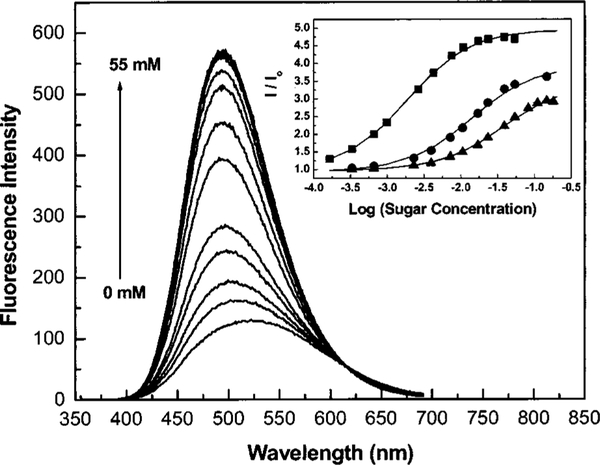
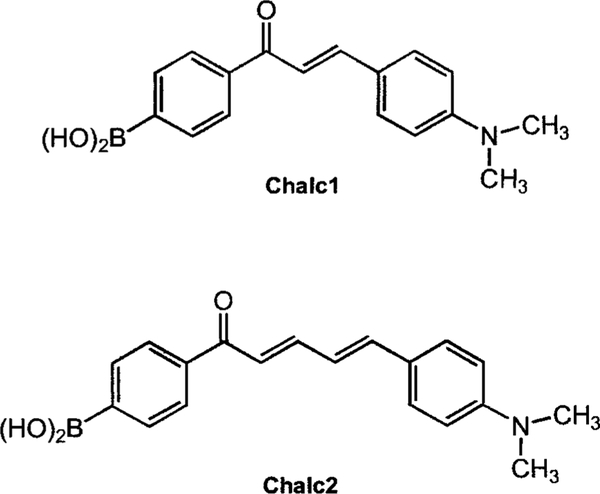
In an attempt to assess the extent of the usefulness of the CT mechanism, we synthesized two chalcone derivatives functionalized with the boronic acid group (Figure 12).37 Chalcone derivatives, unlike the corresponding polyenes, have the advantage of longer wavelength emission. In addition, for these compounds, the boronic acid group does not form resonance forms with the electron-donating amino group. In these cases, the charge transfer occurs between the dimethylamino group (electron-donating group) and the carbonyl group (electron-withdrawing group). Since the boronic group is in resonance with the carbonyl group, the change in the electronic proper ties of the boron group, both when free and when complexed with sugar, leads to a variation in the electronic density of the benzophenone moiety and then in the CT properties of the excited state of the fluorophore. Under its neutral form, the electron deficient boronic group decreases the electronic density on the carbonyl group helping the CT. On the other hand, under its anionic form, the boronate group increases the electronic density on the carbonyl group and then reduces the CT property of the excited state. Both chalcone derivatives display similar responses to pH and sugar; only their respective wavelengths of emission differ. Chalc1 shows an emission centered to 580 nm, and Chalc2 to 665 nm. Typical spectral responses to sugar are presented in Figure 13 for Chalc2. In this case, no apparent wavelength shift is observed. However, we do observe an intensity increase, typically twofold. It is interesting to note that the pH range for effective sugar sensing of the chalcone derivative, ~6.5, is shifted to lower values in comparison to the other push–pull probes described previously, which typically work at a pH of around 7.5. This is due to the higher electrophilic boron group present for the chalcone derivatives. In this case, the presence of the electron-withdrawing carbonyl group decreases the electronic density of the phenyl ring, lowering the pKa of the boronic acid group. In the other cases, in which the dimethylamino group shows resonance forms with the boronic acid group, partial charge transfers can occur in the groups’ state, first increasing the electronic density of the boron group and then increasing its pKa.
Fig. 12.
Molecular structures of the chalcone fluorescent probes.
Fig. 13.
Effect of D fructose on the emission spectrum of Chalc2 measured in phosphate buffer pH 6.5/MeOH 2:1 (v/v). Inset: titration curves of Chalc2 against D fructose (∎), D galactose (●), and D glucose (▲).
2.5. Fluorescence Lifetime
In addition to showing wavelength shift and intensity change, the sugar sensitive fluorescent probes based on CT display changes in their fluorescence lifetime in the presence of sugar. Stilbenes and diphenylpolyene derivative possess very short lifetime, in the range of a few hundred picoseconds. For example, DDPBBA show a fluorescence lifetime of 290 and 209 ps in absence and in presence of D fructose, respectively. While these short fluorescence lifetimes do not lead to useful applications, we can still see that the CT mechanism could be used as a mechanism to develop fluorescent probes for fluorescence lifetime based sensing.38 From this point of view, DAP-BA could be useful for monitoring sugar using lifetime measurements. At pH 7.0, the presence of D fructose changes the fluorescence lifetime of DAP-BA from 2.8 to 3.6 ns.
3. Concluding Remarks
The combination of an electron withdrawing and/or donating group and a boronic acid group, both of which are directly linked to a fluorophore, can lead to the formation of an excited charge transfer state. The neutral form of the boronic acid group acts as an electron-withdrawing group, while the anionic form can act as an electron-donating group. After complexation with a sugar molecule, the boronic acid changes from its neutral form to the anionic one, and a change occurs in the CT properties of the fluorophore. A shifting and a change in the intensity of the emission bands are then observed. This leads to an optical and ratiometric approach for the analysis of sugar using fluorescence probes containing the boronic acid group. This donor/acceptor combination can be applied to a large variety of fluorophores, regardless of their emission wavelength ranges.
Acknowledgments
This work was supported by the Juvenile Diabetes Foundation International, 1-2000-546, with partial support from the NIH National Center for Research Resources, RR-08119.
References
- 1.Williams RM, Zipfel WR, and Webb WW, “Multiphoton microscopy in biological research,” Curr. Opin. Chem. Biol 5, 603–608 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Truong K and Ikura M, “The use of FRET imaging microscopy to detect protein-protein interactions and protein conformational changes in vivo,” Curr. Opin. Struct. Biol 11, 573–578 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Kam Z, Zamir E, and Geiger B, “Probing molecular processes in live cells by quantitative multidimensional microscopy,” Trends Cell Biol. 11, 329–334 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Haugland RP, Handbook of Fluorescent Probes and Research Chemicals, 6th ed., Molecular Probes, Eugene, OR: (1996). [Google Scholar]
- 5.Robinson MR, Eaton RP, Haaland DM, Koepp GW, Thomas EV, Stallard BR, and Robinson PL, “Non-invasive glucose monitoring in diabetic patients: A preliminary evaluation,” Clin. Chem 38, 1618–1622 (1992). [PubMed] [Google Scholar]
- 6.Heise HM, Marbach R, Koschinsky TH, and Gries FA, “Noninvasive blood glucose sensors based on near-infrared spectroscopy,” Ann. Occup. Hyg 18, 439–447 (1994). [DOI] [PubMed] [Google Scholar]
- 7.Burmeister JJ, Chung H, and Arnold MA, “Phantoms for noninvasive blood glucose sensing with near infrared transmission spectroscopy,” Photochem. Photobiol 67, 50–55 (1998). [PubMed] [Google Scholar]
- 8.March WF, Rabinovitch B, Adams R, Wise JR, and Melton M, “Ocular glucose sensor,” Trans. Am. Soc. Artif. Intern. Organs 28, 232–235 (1982). [PubMed] [Google Scholar]
- 9.Rabinovitch B, March WF, and Adams RL, “Non-invasive glucose monitoring of the aqueous humor of the eye: Part I. Measurement of very small optical rotations,” Diabetes Care 5, 254–258 (1982). [DOI] [PubMed] [Google Scholar]
- 10.Claremont DJ, Sambrook IE, Penton C, and Pickup JC, “Sub-cutaneous implantation of a ferrocene-mediated glucose sensor in pigs,” Diabetologia 29, 817–821 (1986). [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama K, Sode K, Tamiya E, and Karube I, “Integrated biosensor for glucose and galactose,” Anal. Chim. Acta 218, 137–142 (1989). [Google Scholar]
- 12.Schier GM, Moses RG, Gan IET, and Blair SC, “An evaluation and comparison of Reflolux II and Glucometer II, two new portable reflectance meters for capillary blood glucose determination,” Diabetes Res. Clin. Pract 4, 177–181 (1988). [DOI] [PubMed] [Google Scholar]
- 13.Clarke W, Becker DJ, Cox D, Santiago JV, White NH, Betschart J, Eckenrode K, Levandoski LA, Prusinki EA, Simineiro LM, Snyder AL, Tideman AM, and Yaeger T, “Evaluation of a new system for self blood glucose monitoring,” Diabetes Res. Clin. Pract 4, 209–214 (1988). [DOI] [PubMed] [Google Scholar]
- 14.Trettnak W and Wolfbeis OS, “Fully reversible fiber-optic glucose biosensor based on the intrinsic fluorescence of glucose-oxidase,” Anal. Chim. Acta 221, 195–203 (1989). [Google Scholar]
- 15.Meadows D and Schultz JS, “Fiber-optic biosensors based on fluorescence energy transfer,” Talanta 35, 145–150 (1988). [DOI] [PubMed] [Google Scholar]
- 16.Tolosa L, Malak H, Rao G, and Lakowicz JR, “Optical assay for glucose based on the luminescence decay time of the long wavelength dye Cy5™,” Sens. Actuators B 45, 93–99 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tolosa L, Gryczynski I, Eichorn LR, Dattelbaum JD, Castellano FN, Rao G, and Lakowicz JR, “Glucose sensors for low-cost lifetime-based sensing using a genetically engineering protein,” Anal. Biochem 267, 114–120 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Auria S, DiCesare N, Gryczynski Z, Gryczynski I, Rossi M, and Lakowicz JR, “A thermophilic apoglucose dehydrogenase as nonconsuming glucose sensor,” Biochem. Biophys. Res. Commun 274, 727–731 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Kenneth ER and Ernest KJ, “Issues and implications in the selection of blood glucose monitoring techniques,” Diabetes Technol. Ther 1, 3–11 (1999). [DOI] [PubMed] [Google Scholar]
- 20.Ballerstadt JP and Schultz JS, “A fluorescence affinity hollow fiber sensor for continuous transdermal glucose monitoring,” Anal. Chem 72, 4185–4192 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Lorand JP and Edwards JO, “Polyol complexes and structure of the benzeneboronate ion,” J. Org. Chem 24, 769–774 (1959). [Google Scholar]
- 22.Yoon J and Czarnik AW, “Fluorescent chemosensors of carbohydrates. A means of chemically communicating the binding of polyols in water based on chelation-enhancement quenching,” J. Am. Chem. Soc 114, 5874–5875 (1992). [Google Scholar]
- 23.James TD, Sandanayake KRSA, and Shinkai S, “Saccharides sensing with molecular receptors based on boronic acid,” Angew. Chem. Int. Ed. Engl 35, 1910–1922 (1996). [Google Scholar]
- 24.Hartley JH, James TD, and Ward CJ, “Synthetic receptors,” J. Chem. Soc., Perkin Trans 1 19, 3155–3184 (2000). [Google Scholar]
- 25.Yang W, He H, and Drueckhammer DG, “Computer-guided design in molecular recognition: design and synthesis of a glucopyra-nose receptor,” Angew. Chem. Int. Ed. Engl 40, 1714–1718 (2001). [PubMed] [Google Scholar]
- 26.Deng G, James TD, and Shinkai S, “Allosteric interaction of metal ions with saccharides in a crowned diboronic acid,” J. Am. Chem. Soc 114, 4567–4572 (1994). [Google Scholar]
- 27.James TD, Samankumara Sandanayake KRA, and Shinkai S, “Chiral discrimination of monosaccharides using a fluorescent molecular sensor,” Nature (London) 374, 345–346 (1995). [Google Scholar]
- 28.James TD, Samankumara Sandanayake KRA, Iguchi R, and Shinkai S, “Novel saccharide-photoinduced electron-transfer sensors based on the interaction of boronic acid and amine,” J. Am. Chem. Soc 117, 8982–8987 (1995). [Google Scholar]
- 29.DiCesare N and Lakowicz JR, “Wavelength-ratiometric probes for saccharides based on donor-acceptor diphenylpolyenes,” J. Photochem. Photobiol., A 143, 39–47 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeuchi M, Mizuno H, Shinmori M, Nakashima S, and Shinkai S, “Fluorescence and CD spectroscopic sugar sensing by a cyanine-appended diboronic acid probe,” Tetrahedron 52, 1195–1204 (1996). [Google Scholar]
- 31.Sandanayake KRSA, James TD, and Shinkai S, “2-Dimensional photoinduced electron-transfer (PET) fluorescence sensor for saccharides,” Chem. Lett 7, 503–504 (1995). [Google Scholar]
- 32.Valeur B, “Principles of Fluorescent Probe Design for Ion Recognition,” Topics in Fluorescence Spectroscopy, Vol. 4, pp. 21–48, Plenum, New York: (1994). [Google Scholar]
- 33.Shinmori H, Takeuchi M, and Shinkai S, “Spectroscopic sugar sensing by a stilbene derivative with push (Me2N-)-pull ((HO)2B-)-type substituents,” Tetrahedron 51, 1893–1902 (1995). [Google Scholar]
- 34.DiCesare N and Lakowicz JR, “Spectral properties of fluorophores combining the boronic acid group with electron donor or withdrawing groups. Implication in the development of fluorescence probes for saccharides,” J. Phys. Chem. A 105, 6834–6840 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DiCesare N and Lakowicz JR, “A new highly fluorescent probe for monosaccharides based on a donor-acceptor diphenyloxazole,” J. Chem. Soc. Chem. Commun 19, 2022–2023 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diwu Z, Lu Y, Zhang C, Klaubert DH, and Haugland RP, “Fluorescent molecular probes. 2. The synthesis, spectral properties and use of fluorescent solvatochromic Dapoxyl(TM) dyes,” Photochem. Photobiol 66, 424–431 (1997). [Google Scholar]
- 37.DiCesare N and Lakowicz JR, “Chalcone-analogue fluorescent probes for saccharides signaling using the boronic acid group,” Tetrahedron Lett 43, 2615–2618 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szmacinski H and Lakowicz JR, “Lifetime-based sensing,” in Ref. 32, pp. 295–334. [Google Scholar]