INTRODUCTION
Parkinson disease (PD) is a complex progressive neurodegenerative disease described by James Parkinson in his 1817 publication, “Essay on the Shaking Palsy”(1). In that essay Dr. Parkinson optimistically declared that “there appears to be sufficient reason for hoping that some remedial process may ere long be discovered, by which, at least, the progress of the disease may be stopped.” More than 200 years later we have yet to definitively achieve neuroprotective therapy for PD. However, there has been great progress in recent decades in understanding the molecular basis for neurodegeneration in PD, hopefully bringing us steadily getting closer to achieving truly disease-modifying therapies for PD.
Pathologically, PD is defined by loss of dopaminergic neurons in the substantia nigra pars compacta (SN) located in the midbrain and associated with Lewy bodies, which are cytoplasmic inclusions that include insoluble alpha-synuclein aggregates. However, PD is characterized by more widespread pathology in other brain regions and involves non-dopaminergic neurons as well. The clinical diagnosis of PD is based primarily on motor features, such as a slowly progressive asymmetric resting tremor, cogwheel rigidity and bradykinesia, although non-motor features, which include anosmia, constipation, depression and REM sleep behavior disorder, can develop years before motor deficits. During later stages of the disease, additional non-motor features, such as autonomic dysfunction, pain and cognitive decline, can appear(2).
NEUROPATHOLOGY
The neuropathological hallmarks of PD are the degeneration of dopaminergic neurons in the SN and intraneuronal protein aggregates called Lewy bodies and Lewy neurites(3). It was long considered that 50–70% of SN dopaminergic neurons have died by the time that clinical motor symptoms become evident(4). However, more recent work suggests that the loss of dopaminergic terminals in the basal ganglia, as opposed to loss of the neurons in the SN, is crucial for onset of motor symptoms (6).
EPIDEMIOLOGY
Distribution of disease
In estimates based on health care utilization, PD incidence ranges from 5/100,000 to over 35/100,000 new cases yearly(5). Incidence increases 5 to 10 fold from the sixth to the ninth decades of life. PD prevalence also increases with age. In a meta-analysis of four North American populations, prevalence increased from less than 1 percent of men and women aged 45–54 years to 4 percent of men and 2 percent of women aged 85 or older(6). Mortality is not increased, compared to non-affected individuals, in the first decade after PD is diagnosed, but increases thereafter (7). As the global population ages, PD prevalence is expected to increase dramatically, doubling in the next two decades(8). Accompanying this increase, the societal and economic burden of PD will escalate, unless more effective treatments, cures or means of prevention are identified(9).
Determinants of disease
Most PD cases likely have a multifactorial etiology, resulting from the combined effects of environmental and genetic factors. Exposure to toxicant chemicals and head injury may increase the risk of PD, while certain lifestyle factors may lower risk. Genetic susceptibility factors may modify the effects of environmental exposures. Although identifiable mutations in certain genes cause PD in around 5–10% of cases, these mutations are absent in most people with PD. Moreover, the most common PD-associated genetic mutations have incomplete penetrance, indicating that other environmental or genetic factors are involved. A study comparing concordance rates in monozygotic and dizygotic twins estimated the heritability of PD to be only 30%, suggesting that the majority of PD risk is related to environmental and behavioral factors(10).
Toxicant chemical exposure
In studies spanning many decades in numerous populations worldwide, pesticide exposure, farm work or rural residence have been associated with an increased PD risk(11). Occupational exposure as well as “passive” exposure due to residence near to pesticide treated fields is associated with a greater risk of PD. Pesticides associated with PD, including paraquat, rotenone, 2,4-D and several dithiocarbamates and organochlorines, cause experimental parkinsonism in laboratory studies, supporting the possibility that these associations reflect causal effects(11, 12). Genetically determined impairment in toxicant handling can amplify the effect of pesticide exposure on PD risk, an example of gene-environment interaction. Conversely, behaviors such as good hygiene practices or eating a healthy diet may protect against the adverse effects of pesticide exposure(13, 14). Chlorinated solvents (trichloroethylene, perchloroethylene, carbon tetrachloride), used in dry cleaning, degreasing, as an anesthetic and viscose rayon manufacturing and polychlorinated biphenyls, formerly used as coolants and lubricants, have also been associated with increased PD risk in humans and cause parkinsonism-associated toxicity in animal models(12, 15). While some of these pesticides and toxicant chemicals are no longer in use, they are environmentally persistent, and remain common contaminants of soil and water. Others, such as trichloroethylene, have continuing applications and can be found in nearly one third of US drinking water supplies, as well as in air, soil, food and human breast milk.(16) Working as a welder also has been associated with greater risk of PD, possibly as the result of manganese in welding fumes(17, 18). Manganese exposure also can cause PD-like pathology in mice (19). However, data on the association of welding with PD are mixed(20). Exposure to other metals such as iron, and lead, has been suggested to increase risk of PD, based on experimental in vitro and in vivo studies, but human evidence remains inconclusive. Ambient total suspended particles (TSP) from traffic has also been associated with an increased risk of PD(21), possibly due to metal exposure or to induction of inflammatory processes, but these findings have been inconsistent.
Head injury
Mild to moderate head injury occurring decades before PD onset is associated with higher risk of PD in most but not all studies(12, 22). Risk increases with the number of head injuries, and genetic susceptibility factors such as certain variants in or near the gene encoding alpha-synuclein may increase risk 2 – 5 fold.
Lifestyle Factors
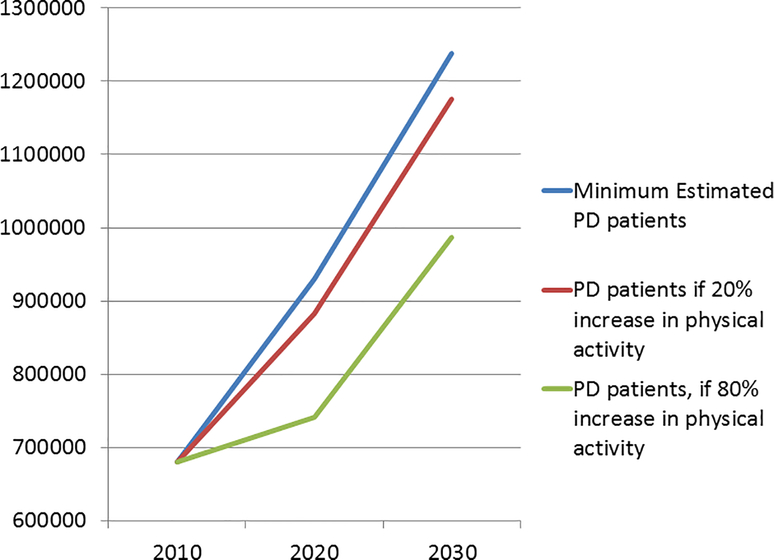
A number of life-style factors have been associated with reduced risk of developing PD. The most consistent association is a reduced risk of PD in cigarette smokers and, in a few studies, other tobacco users(23, 24). Longer duration and greater frequency of tobacco use confer lower risk and there is some evidence of genetic modification. Nicotine has been suggested to play a central role in this association, although a recently completed clinical study failed to detect a disease modifying effect of the nicotine patch in PD patients. Coffee drinking and caffeine use are also associated with a lower risk of PD(25, 26), particularly in men. The effect is greatest in men with the highest levels of coffee use, and may be further modified by genetic factors. Similarly, reports have shown reduced risk of PD in heavy tea drinkers in some, but not all populations studied. Conversely, higher dietary intake of dairy products has been associated with a higher risk of PD, possibly due to the concentration of toxicants in milk(27). Other dietary associations generally support a reduced risk of PD in those eating “healthy” diets higher in fruits, vegetables and grains(28). Physical activity has been associated with a lower risk of PD, especially in men and particularly at higher intensities of physical activity, although even modest levels reduce risk(29) (Fig. 1). The combined effects of these lifestyle factors appear to be additive, suggesting an approach to disease prevention(30).
FIGURE 1:
Estimated number of people with PD in the U.S. (blue line) & the projected reduction in PD if physical activity in adults increases by 20% (red line) or 80% (green line). Estimates based on Marras et al, 2018(6).
GENETICS AND PATHOPHYSIOLOGY
Specific genetic factors that play a major role in PD risk can be identified in a subset of PD patients (Fig. 2). Polymeropoulos et al identified a mutation in the alpha-synuclein gene, SNCA, in association with rare families with autosomal dominant PD in 1997(31). Families with these high-penetrant mutations are quite rare, but this seminal discovery led to the recognition that alpha-synuclein comprises a major component of Lewy bodies even in sporadic PD patients. The later discovery of autosomal dominant PD families with alpha-synuclein gene duplications or triplications added to other data indicating that high levels of alpha-synuclein contribute to the pathogenesis of PD(32).
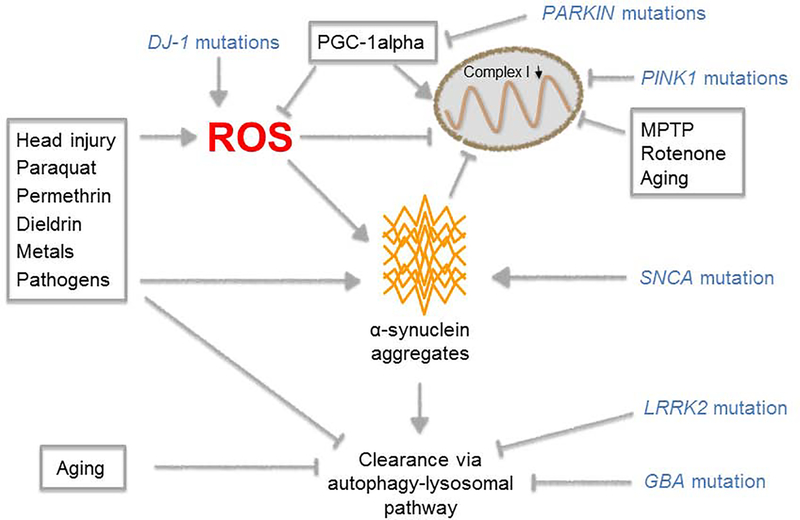
FIGURE 2:
Environmental and genetic factors influence PD pathogenesis by impacting similar pathways, including mitochondrial function, oxidative stress, alpha-synuclein aggregation, and clearance pathways for abnormal proteins.
Mutations in the PARKIN(33) and PINK1(34) genes are causes of early-onset autosomal recessive PD. Both PARKIN and PINK1 have been linked to a cellular pathway involving the preferential degradation in lysosomes of dysfunctional mitochondria through macro-autophagy, a process termed “mitophagy”. Loss of function of these genes leads to impaired mitophagy, resulting in the accumulation of dysfunctional mitochondria. PARKIN also indirectly regulates levels of an important transcriptional regulator, PGC-1alpha, which coordinately regulates the expression of genes required for mitochondrial biogenesis as well as multiple antioxidant defenses(35). PGC-1alpha levels also are low in sporadic PD(36), suggesting that these data are relevant beyond rare genetic forms of PD. These genetic links to both mitochondrial degradation and to mitochondrial biogenesis implicate dysfunction of mitochondrial turnover in PD.
These genetic data are complemented by many other lines of data implicating mitochondrial dysfunction in the pathogenesis of PD. For example, exposure to a toxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), causes a rapid onset parkinsonian phenotype and death of dopaminergic neurons in the SN, likely due to inhibition of mitochondrial complex I activity. Chronic exposure of rodents to rotenone, also a potent mitochondrial complex I inhibitor, also causes preferential degeneration of dopaminergic neurons(37), and exposure to pesticides (including rotenone) is a risk factor for PD(38).
Mutations in the DJ-1 gene also cause autosomal recessive early-onset PD(39). DJ-1 has antioxidant effects through multiple mechanisms, including regulation of NRF2, a transcription factor the upregulates multiple antioxidant defenses, and by stimulating glutathione synthesis(40).
Mutations in the LRRK2 gene are associated with autosomal dominant PD with incomplete penetrance (about 25% for the G2019S mutation, but much higher for the R144G mutation), and are present in about 1 to 2 percent of all PD patients and 5% in familial PD, but higher in some populations such as patients of Ashkenazi Jewish ancestry and in North African Berbers(41). Prior studies suggest that mutations in LRRK2 lead to increased kinase activity(42), and that LRRK2 kinase inhibitors may be protective(43), although the possibility of a role for loss of LRRK2 function has been raised(44).
Another common genetic factor contributing to PD risk relates to mutations in the GBA gene associated with autosomal recessive Gaucher’s disease(45). Carriers of a GBA mutation have an approximately 4-fold increased risk of PD, although the risk varies with different GBA mutations. Some studies suggest an increased risk of dementia in GBA mutation associated PD(46). PD-linked GBA mutations cause a loss of activity of the lysosomal enzyme glucocerebrosidase (GCase), and agents that upregulate GCase activity and an agent targeting “substrate reduction” have shown promise in animal models and are now moving forward in clinical trials(47).
Neuroinflammation previously was often viewed only as a response to ongoing neurodegeneration. More recent studies suggest that neuroinflammation might be a significant and essential upstream contributor to alpha-synuclein aggregation and to the neurodegenerative process(48), and epidemiological studies have provided evidence of associations between diseases with peripheral inflammation (e.g. type 2 diabetes and inflammatory bowel disease) and elevated PD risk(49, 50). Genetic studies also have linked HLA gene variants with the risk of late-onset PD(51).
PD genetics are complex. Common variants may contribute to PD risk, and can interact with other genetic factors and with environmental factors. The most recent large genome wide association study (GWAS) identified 70 loci that affect PD risk(52). Several of these loci are close to genes involved in the lysosomal-autophagy system and in immunity, which are both functions expected to play important roles in the handling of misfolded alpha-synuclein. Acquired (somatic) mitochondrial DNA mutations are increased in SN neurons in early PD and also may play a role(53). Epigenetic factors also may contribute to PD pathogenesis(54).
Given the strong genetic and experimental data linking alpha-synuclein toxicity to PD, many potential neuroprotective strategies have focused on mechanisms for clearing away alpha-synuclein aggregates(55, 56). Clinical studies currently are underway using infusion of monoclonal antibodies to target oligomeric alpha-synuclein, or using an active vaccine strategy. Other strategies use more indirect approaches, such as ongoing studies of nilotinib, a c-abl inhibitor, that may reduce inflammation and promote clearance of alpha-synuclein(57). Additional strategies that specifically target genetically defined subpopulations, such as LRRK2 kinase inhibitors or GCase activators, are moving forward in clinical trials. Although currently genetic testing has a limited role clinically, already it is an important research tool, and one can envision a not-too-distant future when genetic testing will be routine for all PD patients to guide the selection of targeted therapies.
ALPHA-SYNUCLEIN AGGREGATION AND SPREAD OF PATHOLOGY
Alpha-synuclein aggregation
The presence of Lewy pathology is pathognomonic for sporadic PD, although some rare inherited genetic forms of PD that exhibit loss of SN dopaminergic neurons do not display these protein aggregates(58). Alpha-synuclein is normally enriched in synapses where it is thought to participate in synaptic vesicle function(59). Alpha-synuclein is also present in non-neuronal cells, e.g. liver, muscle, lymphocytes and red blood cells(59), and its normal functional roles are not fully elucidated. In a series of seminal studies, Heiko Braak and colleagues proposed that there are six stages of Lewy pathology in PD(60). They suggested that in the first stages the Lewy pathology is limited to the dorsal motor nucleus of the vagal nerve, located on the medulla oblongata and the origin of the nerve fibers innervating the gut and other visceral organs, and the olfactory bulb and closely associated olfactory nucleus. They also suggested that the pathology subsequently spreads in a stereotypic fashion along neural pathways throughout the brain, not reaching the SN until the third neuropathological stage, and eventually involving the cerebral hemispheres in the terminal sixth stage (60). The Braak staging model is based on post-mortem observations, and it has not been possible to obtain definitive proof that pathology spreads in accordance with the Braak stages(61). Indeed, some subsequent reports indicate that the anatomical pattern of Lewy pathology in some people clinically diagnosed with PD is not consistent with the Braak staging(62). Still, this model has gained significant traction over the past two decades, and it has been proposed that the earliest stages of Lewy pathology (before the SN is engaged) are coupled to the symptoms and signs of pre-motor PD (discussed above)(63). Notably, alpha-synuclein aggregates have been reported in the gut of neurologically normal people(64) and around 10% of people who die without a diagnosis of PD still display alpha-synuclein aggregates in the brain (so-called Incidental Lewy Body Disease) and show mild levels of SN dopaminergic neuronal loss(65). These observations suggest that these people had elevated risk of developing PD or a related synucleinopathy if they had lived longer.
A prion-like role for alpha-synuclein assemblies?
Different alpha-synuclein assemblies have been shown to be secreted by neurons, via a process that is elevated if the lysosomal-autophagy system is inhibited, and then be taken up by neighboring neurons where they are capable of seeding monomeric alpha-synuclein into Lewy-like aggregates(66). The realization that misfolded alpha-synuclein exhibit such prion-like properties has increased the spotlight on the Braak staging system. Because these pathogenic alpha-synuclein assemblies can be transported intra-axonally to interconnected nuclei, prion-like behavior of alpha-synuclein assemblies might explain how Lewy pathology propagates from one brain region to another(66) and could be consistent with the spread of alpha-synuclein pathology proposed by Braak and colleagues.
An important question is what might be the initial trigger of alpha-synuclein aggregation. One model proposes that numerous factors are involved, including some of the environmental risk factors we discussed earlier, i.e. pesticides and environmental pollutants, as well as common pathogens (e.g. viruses, bacteria and fungi) that all can gain access to alpha-synuclein containing cells initially in the olfactory system and gut(67). For the most part, these aggregates are handled by normal cellular proteostatic mechanisms and do not lead to spreading Lewy pathology. In the simultaneous presence of facilitating factors, e.g. aging, genetic predisposition and peripheral inflammation, the model proposes that alpha-synuclein aggregates can bypass normal clearance and cause synucleinopathy in the brain(67). Although a role for alpha-synuclein is well-established in PD, it is worth noting that it remains controversial whether or not the aggregates themselves are pathogenic(68) (see Table 1).
TABLE 1:
KEY GENETIC FACTORS ASSOCIATED WITH PD (partial list; see Schulte and Gasser 2011(69), and Lin and Farrer, 2014(70) for a more comprehensive reviews)
| GENE | Gene product | Inheritance | mutation types | penetrance | Age of onset | Frequency | notes |
|---|---|---|---|---|---|---|---|
| SNCA | alpha-synuclein | AD | point mutations; duplications; triplications | high | late (earlier onset with triplications) | Very rare | earlier onset for triplications compared to duplications |
| PRKN | Parkin | AR | multiple, including point mutations; deletions, duplication,… (loss of function) | high | early, often teens or 20s | Rare, 3 to 7% with onset 30 – 45; up to 50% with onset <25 | role in protein degradation; mitophagy |
| PINK1 | PINK1 | AR | high | early | Rare (2 to 4% of early-onset cases) | role in mitophagy | |
| LRRK2 | Leucine rich repeat kinase 2 | AD | G2019S; many other point mutations | moderate (∼25%) | late | ∼1 to 2% of all PD cases; higher in Ashkenazi Jews, North African Berbers, and Basques | Mutations cause increased kinase activity |
| DJ-1 | DJ-1 | AR | point mutations | high | early | Rare (1% of early-onset cases) | protects against oxidative stress |
| GBA | Glucocerebrosidase | mixed | point mutations (loss of function) | low (∼4-fold increased risk) | late | 5 – 10% of all PD patients; higher in Ashkenazi Jews | Lysosomal enzyme |
Summary
PD is a complex disorder, with both environmental and genetic factors converging on a common set of pathways including mitochondrial dysfunction, oxidative stress, protein aggregation, impaired autophagy and neuroinflammation. Reducing the burden of PD can be approached with a two-pronged strategy: the implementation of interventions to reduce modifiable factors such as behavioral or environmental risk factors and the development of drugs targeting the mechanisms of genes or environmental exposures associated with PD. PD incidence is increased in persons with prodromal symptoms such as impaired olfaction, sleep disorders and constipation(71). Targeting people in this prodromal stage of disease may be an effective strategy for reducing the burden of PD in future decades. Clinical testing of interventions aimed at identifying disease-modifying therapies thus far has yielded mainly negative results, perhaps in part because the pathophysiological factors contributing to PD differ between patients. The lack of clinically proven success at slowing disease progression highlights the need for more research to better understand the molecular pathophysiology of subtypes of PD.
Key points.
Parkinson disease (PD) is a complex age-related neurodegenerative disease associated with dopamine deficiency and both motor and nonmotor deficits.
Many environmental and genetic factors influence PD risk, with different factors predominating in different patients.
These factors converge on specific pathways, including mitochondrial dysfunction, oxidative stress, protein aggregation, impaired autophagy and neuroinflammation.
Ultimately, treatment of PD may focus on targeted therapies for pathophysiologically defined subtypes of PD patients.
SYNOPSIS.
Parkinson’s disease (PD) is a complex age-related neurodegenerative disease associated with dopamine deficiency and both motor and nonmotor deficits. Many environmental and genetic factors influence PD risk, with different factors predominating in different patients. These factors converge on specific pathways, including mitochondrial dysfunction, oxidative stress, protein aggregation, impaired autophagy and neuroinflammation. Ultimately, treatment of PD may focus on targeted therapies for pathophysiologically defined subtypes of PD patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David K. Simon, Beth Israel Deaconess Medical Center and Harvard Medical School, 330 Brookline Avenue, Boston, MA 02215.
Caroline M. Tanner, University of California-San Francisco, Director, PADRECC, San Francisco Veterans Affairs Health Care System, 675 Nelson Rising Lane, San Francisco CA 94158.
Patrik Brundin, Van Andel Research Institute, Director, Center for Neurodegenerative Science, Van Andel Institute, 333 Bostwick Ave. N.E., Grand Rapids, Michigan 49503-2518.
REFERENCES
- 1.Parkinson J An Essay on the Shaking Palsy. London: Sherwood, Neely, and Jones; 1817. [Google Scholar]
- 2.Sung VW, Nicholas AP. Nonmotor symptoms in Parkinson’s disease: expanding the view of Parkinson’s disease beyond a pure motor, pure dopaminergic problem. Neurologic clinics. 2013. August;31(3 Suppl):S1–16. [DOI] [PubMed] [Google Scholar]
- 3.Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nature reviews Disease primers. 2017. March 23;3:17013. [DOI] [PubMed] [Google Scholar]
- 4.Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain : a journal of neurology. 1991;114(Pt 5):2283–301. [DOI] [PubMed] [Google Scholar]
- 5.Twelves D, Perkins KS, Counsell C. Systematic review of incidence studies of Parkinson’s disease. Mov Disord. 2003. January;18(1):19–31. [DOI] [PubMed] [Google Scholar]
- 6.Marras C, Beck JC, Bower JH, et al. Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis. 2018;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinter B, Diem-Zangerl A, Wenning GK, et al. Mortality in Parkinson’s disease: a 38-year follow-up study. Mov Disord. 2015. February;30(2):266–9. [DOI] [PubMed] [Google Scholar]
- 8.Dorsey ER, Sherer T, Okun MS, Bloem BR. The Emerging Evidence of the Parkinson Pandemic. Journal of Parkinson’s disease. 2018;8(s1):S3–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaltenboeck A, Johnson SJ, Davis MR, et al. Direct costs and survival of medicare beneficiaries with early and advanced Parkinson’s disease. Parkinsonism & related disorders. 2012. May;18(4):321–6. [DOI] [PubMed] [Google Scholar]
- 10.Goldman SM, Marek K, Ottman R, et al. Concordance for Parkinson’s disease in twins: A 20-year update. Ann Neurol. 2019. April;85(4):600–5. [DOI] [PubMed] [Google Scholar]
- 11.Tanner CM, Goldman SM, Ross GW, Grate SJ. The disease intersection of susceptibility and exposure: chemical exposures and neurodegenerative disease risk. Alzheimers Dement. 2014. June;10(3 Suppl):S213–25. [DOI] [PubMed] [Google Scholar]
- 12.Goldman SM. Environmental toxins and Parkinson’s disease. Annu Rev Pharmacol Toxicol. 2014;54:141–64. [DOI] [PubMed] [Google Scholar]
- 13.Furlong M, Tanner CM, Goldman SM, et al. Protective glove use and hygiene habits modify the associations of specific pesticides with Parkinson’s disease. Environ Int. 2015. February;75:144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamel F, Goldman SM, Umbach DM, et al. Dietary fat intake, pesticide use, and Parkinson’s disease. Parkinsonism & related disorders. 2014. January;20(1):82–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weisskopf MG, Knekt P, O’Reilly EJ, et al. Polychlorinated biphenyls in prospectively collected serum and Parkinson’s disease risk. Mov Dis. 2012. October 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman SM, Quinlan PJ, Ross GW, et al. Solvent exposures and Parkinson disease risk in twins. Ann Neurol. 2012. June;71(6):776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Criswell SR, Nielsen SS, Warden M, et al. [(18)F]FDOPA positron emission tomography in manganese-exposed workers. Neurotoxicology. 2018. January;64:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Racette BA, Searles Nielsen S, Criswell SR, et al. Dose-dependent progression of parkinsonism in manganese-exposed welders. Neurology. 2017. January 24;88(4):344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harischandra DS, Rokad D, Neal ML, et al. Manganese promotes the aggregation and prion-like cell-to-cell exosomal transmission of alpha-synuclein. Science signaling. 2019. March 12;12(572). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenborg L, Lassen CF, Hansen J, Olsen JH. Parkinson’s disease and other neurodegenerative disorders among welders: a Danish cohort study. Mov Disord. 2012. September 1;27(10):1283–9. [DOI] [PubMed] [Google Scholar]
- 21.Finkelstein MM, Jerrett M. A study of the relationships between Parkinson’s disease and markers of traffic-derived and environmental manganese air pollution in two Canadian cities. Environ Res. 2007. July;104(3):420–32. [DOI] [PubMed] [Google Scholar]
- 22.Kenborg L, Rugbjerg K, Lee PC, et al. Head injury and risk for Parkinson disease: results from a Danish case-control study. Neurology. 2015. March 17;84(11):1098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morens DM, Davis JW, Grandinetti A, Ross GW, Popper JS, White LR. Epidemiologic observations on Parkinson’s disease: incidence and mortality in a prospective study of middle-aged men. Neurology. 1996. April;46(4):1044–50. [DOI] [PubMed] [Google Scholar]
- 24.Ritz B, Ascherio A, Checkoway H, et al. Pooled analysis of tobacco use and risk of Parkinson disease. Archives of neurology. 2007. July;64(7):990–7. [DOI] [PubMed] [Google Scholar]
- 25.Ross GW, Abbott RD, Petrovitch H, et al. Association of coffee and caffeine intake with the risk of Parkinson disease. Jama. 2000;283(20):2674–9. [DOI] [PubMed] [Google Scholar]
- 26.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. The Lancet Neurology. 2016. November;15(12):1257–72. [DOI] [PubMed] [Google Scholar]
- 27.Park M, Ross GW, Petrovitch H, et al. Consumption of milk and calcium in midlife and the future risk of Parkinson disease. Neurology. 2005. March 22;64(6):1047–51. [DOI] [PubMed] [Google Scholar]
- 28.Gao X, Chen H, Fung TT, et al. Prospective study of dietary pattern and risk of Parkinson disease. Am J Clin Nutr. 2007. November;86(5):1486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang F, Trolle Lagerros Y, Bellocco R, et al. Physical activity and risk of Parkinson’s disease in the Swedish National March Cohort. Brain : a journal of neurology. 2015. February;138(Pt 2):269–75. [DOI] [PubMed] [Google Scholar]
- 30.Kim IY, O’Reilly EJ, Hughes KC, et al. Integration of risk factors for Parkinson disease in 2 large longitudinal cohorts. Neurology. 2018. May 8;90(19):e1646–e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–7. [DOI] [PubMed] [Google Scholar]
- 32.Singleton AB, Farrer M, Johnson J, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. [DOI] [PubMed] [Google Scholar]
- 33.Kitada T, Asakawa S, Hattori N, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392(6676):605–8. [DOI] [PubMed] [Google Scholar]
- 34.Valente EM, Abou-Sleiman PM, Caputo V, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004. May 21;304(5674):1158–60. [DOI] [PubMed] [Google Scholar]
- 35.Shin JH, Ko HS, Kang H, et al. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson’s disease. Cell. 2011. March 4;144(5):689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng B, Liao Z, Locascio JJ, et al. PGC-1alpha, a potential therapeutic target for early intervention in Parkinson’s disease. Science translational medicine. 2010. October 6;2(52):52ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenamyre JT, Betarbet R, Sherer TB. The rotenone model of Parkinson’s disease: genes, environment and mitochondria. Parkinsonism & related disorders. 2003. August;9 Suppl 2:S59–64. [DOI] [PubMed] [Google Scholar]
- 38.Tanner CM, Kamel F, Ross GW, et al. Rotenone, paraquat, and Parkinson’s disease. Environmental health perspectives. 2011. June;119(6):866–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonifati V, Rizzu P, van Baren MJ, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003. January 10;299(5604):256–9. [DOI] [PubMed] [Google Scholar]
- 40.Raninga PV, Di Trapani G, Tonissen KF. The Multifaceted Roles of DJ-1 as an Antioxidant. Advances in experimental medicine and biology. 2017;1037:67–87. [DOI] [PubMed] [Google Scholar]
- 41.Alessi DR, Sammler E. LRRK2 kinase in Parkinson’s disease. Science. 2018. April 6;360(6384):36–7. [DOI] [PubMed] [Google Scholar]
- 42.West AB, Moore DJ, Choi C, et al. Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Human molecular genetics. 2007. January 15;16(2):223–32. [DOI] [PubMed] [Google Scholar]
- 43.Hatcher JM, Choi HG, Alessi DR, Gray NS. Small-Molecule Inhibitors of LRRK2. Advances in neurobiology. 2017;14:241–64. [DOI] [PubMed] [Google Scholar]
- 44.Giaime E, Tong Y, Wagner LK, Yuan Y, Huang G, Shen J. Age-Dependent Dopaminergic Neurodegeneration and Impairment of the Autophagy-Lysosomal Pathway in LRRK-Deficient Mice. Neuron. 2017. November 15;96(4):796–807 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clark LN, Nicolai A, Afridi S, et al. Pilot association study of the beta-glucocerebrosidase N370S allele and Parkinson’s disease in subjects of Jewish ethnicity. Mov Disord. 2005. January;20(1):100–3. [DOI] [PubMed] [Google Scholar]
- 46.Riboldi GM, Di Fonzo AB. GBA, Gaucher Disease, and Parkinson’s Disease: From Genetic to Clinic to New Therapeutic Approaches. Cells. 2019. April 19;8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balestrino R, Schapira AHV. Glucocerebrosidase and Parkinson Disease: Molecular, Clinical, and Therapeutic Implications. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2018. October;24(5):540–59. [DOI] [PubMed] [Google Scholar]
- 48.Kannarkat GT, Boss JM, Tansey MG. The role of innate and adaptive immunity in Parkinson’s disease. Journal of Parkinson’s disease. 2013;3(4):493–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rolli-Derkinderen M, Leclair-Visonneau L, Bourreille A, Coron E, Neunlist M, Derkinderen P. Is Parkinson’s disease a chronic low-grade inflammatory bowel disease? J Neurol. 2019. April 12. [DOI] [PubMed] [Google Scholar]
- 50.De Pablo-Fernandez E, Goldacre R, Pakpoor J, Noyce AJ, Warner TT. Association between diabetes and subsequent Parkinson disease: A record-linkage cohort study. Neurology. 2018. July 10;91(2):e139–e42. [DOI] [PubMed] [Google Scholar]
- 51.Hamza TH, Zabetian CP, Tenesa A, et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genet. 2010. September;42(9):781–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nalls MA, Blauwendraat C, Vallerga CL, et al. Expanding Parkinson’s disease genetics: novel risk loci, genomic context, causal insights and heritable risk. bioRxiv. 2019:388165. [Google Scholar]
- 53.Lin MT, Cantuti-Castelvetri I, Zheng K, et al. Somatic mitochondrial DNA mutations in early parkinson and incidental lewy body disease. Ann Neurol. 2012. June;71(6):850–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Heesbeen HJ, Smidt MP. Entanglement of Genetics and Epigenetics in Parkinson’s Disease. Frontiers in neuroscience. 2019;13:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalia LV, Kalia SK, Lang AE. Disease-modifying strategies for Parkinson’s disease. Mov Disord. 2015. September 15;30(11):1442–50. [DOI] [PubMed] [Google Scholar]
- 56.Savitt D, Jankovic J. Targeting alpha-Synuclein in Parkinson’s Disease: Progress Towards the Development of Disease-Modifying Therapeutics. Drugs. 2019. April 13. [DOI] [PubMed] [Google Scholar]
- 57.Wyse RK, Brundin P, Sherer TB. Nilotinib - Differentiating the Hope from the Hype. Journal of Parkinson’s disease. 2016. July 12;6(3):519–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schneider SA, Alcalay RN. Neuropathology of genetic synucleinopathies with parkinsonism: Review of the literature. Mov Disord. 2017. November;32(11):1504–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burre J, Sharma M, Sudhof TC. Cell Biology and Pathophysiology of alpha-Synuclein. Cold Spring Harbor perspectives in medicine. 2018. March 1;8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of aging. 2003. Mar-Apr;24(2):197–211. [DOI] [PubMed] [Google Scholar]
- 61.Braak H, Del Tredici K. Neuropathological Staging of Brain Pathology in Sporadic Parkinson’s disease: Separating the Wheat from the Chaff. Journal of Parkinson’s disease. 2017;7(s1):S71–S85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beach TG, Adler CH, Lue L, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta neuropathologica. 2009. June;117(6):613–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Postuma RB, Berg D. Advances in markers of prodromal Parkinson disease. Nature reviews Neurology. 2016. October 27;12(11):622–34. [DOI] [PubMed] [Google Scholar]
- 64.Lionnet A, Leclair-Visonneau L, Neunlist M, et al. Does Parkinson’s disease start in the gut? Acta neuropathologica. 2018. January;135(1):1–12. [DOI] [PubMed] [Google Scholar]
- 65.van de Berg WD, Hepp DH, Dijkstra AA, Rozemuller JA, Berendse HW, Foncke E. Patterns of alpha-synuclein pathology in incidental cases and clinical subtypes of Parkinson’s disease. Parkinsonism & related disorders. 2012. January;18 Suppl 1:S28–30. [DOI] [PubMed] [Google Scholar]
- 66.Brundin P, Melki R. Prying into the Prion Hypothesis for Parkinson’s Disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2017. October 11;37(41):9808–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnson ME, Stecher B, Labrie V, Brundin L, Brundin P. Triggers, Facilitators, and Aggravators: Redefining Parkinson’s Disease Pathogenesis. Trends in neurosciences. 2019. January;42(1):4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Espay AJ, Vizcarra JA, Marsili L, et al. Revisiting protein aggregation as pathogenic in sporadic Parkinson and Alzheimer diseases. Neurology. 2019. February 12;92(7):329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schulte C, Gasser T. Genetic basis of Parkinson’s disease: inheritance, penetrance, and expression. The application of clinical genetics. 2011;4:67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin MK, Farrer MJ. Genetics and genomics of Parkinson’s disease. Genome medicine. 2014;6(6):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ross GW, Abbott RD, Petrovitch H, Tanner CM, White LR. Pre-motor features of Parkinson’s disease: the Honolulu-Asia Aging Study experience. Parkinsonism & related disorders. 2012. January;18 Suppl 1:S199–202. [DOI] [PubMed] [Google Scholar]