Abstract
Background:
Sharing needles/syringes and sexual transmission are widely appreciated as means of HIV transmission among persons who inject drugs (PWIDs). London, Canada, is experiencing an outbreak of HIV among PWIDs, despite a large needle/syringe distribution program and low rates of needle/syringe sharing.
Objective:
To determine whether sharing of injection drug preparation equipment (IDPE) is associated with HIV infection.
Methods:
Between August 2016 and June 2017, individuals with a history of injection drug use and residence in London were recruited to complete a comprehensive questionnaire and HIV testing.
Results:
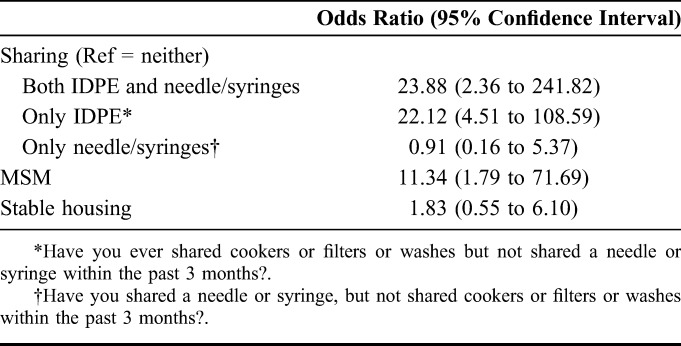
A total of 127 participants were recruited; 8 were excluded because of failure to complete HIV testing. The remaining 35 HIV-infected (cases) and 84 HIV-uninfected (controls) participants were assessed. Regression analysis found that sharing IDPE, without sharing needles/syringes, was strongly associated with HIV infection (adjusted odds ratio: 22.1, 95% confidence interval: 4.51 to 108.6, P < 0.001).
Conclusions:
Sharing of IDPE is a risk factor for HIV infection among PWIDs, even in the absence of needle/syringe sharing. Harm reduction interventions to reduce HIV transmission associated with this practice are urgently needed.
Key Words: HIV transmission, injection drug use, prescription opioid injection
INTRODUCTION
HIV transmission among persons who inject drugs (PWID) accounts for 9% of all new HIV infections globally and thus remains a major public health challenge.1,2 The control of HIV outbreaks among PWID generally depends on a multipronged approach of needle/syringe distribution,3,4 opiate substitution therapy (with methadone or buprenorphine),5 and provision of antiretroviral therapy.6,7 Such multipronged interventions were effective in mitigating large HIV outbreaks among PWID in New York City, United States; Vancouver, Canada; and France.6 However, despite aggressive outbreak control measures, HIV transmission among PWIDs has continued across Europe and North America in recent years.8 A critical component to outbreak control is an understanding of all mechanisms of transmission, so that appropriate control measures can be implemented. Sharing of needles and/or syringes has been recognized for many years as a major means of HIV transmission,9 while the role of sexual transmission has also been appreciated.10 Whether other means of transmission may be occurring among PWIDs is unknown.
London, Canada, has experienced an HIV outbreak among PWIDs in recent years.11 This HIV outbreak is unusual because it is occurring in a setting with the second largest needle/syringe distribution program in Canada; it is the largest program in the country on a per-capita basis.12 More than 2.5 million needles/syringes were distributed across London in 2015, among a total population of 370,000, followed by more than 3 million in 2016.12 Thus, needle/syringe sharing was believed to be less common in London, which provided an opportunity to explore the impact of other factors associated with injection drug use on HIV transmission.
In some centers, sharing of injection drug preparation equipment (IDPE; ie, cookers and filters) has been identified as the primary risk factor for hepatitis C virus transmission among PWID.13–16 Recent studies have shown that sharing of IDPE is more common than sharing of needles/syringes,13,17–20 and that HIV has been detected in used IDPE.21,22 Heating of IDPE with a lighter to sterilize it before sharing has been proposed as a harm reduction approach.23
The 3 most commonly used drugs injected in London were hydromorphone controlled-release, hydromorphone immediate-release, and crystal methamphetamine.24 Hydromorphone controlled-release use is associated with performing multiple drug washes. A wash refers to a process performed to extract residual drug after the initial aspiration. PWID add water to a previously used cooker and filter with residual drug and then place a needle into the filter to aspirate the solution. Performing multiple drug washes from the same IDPE increases the opportunities for sharing. The objectives of this study were to investigate the hypothesis that HIV transmission could occur among PWIDs through IDPE sharing.
METHODS
Design and Recruitment
A cross-sectional study was conducted between August 15, 2016, and June 1, 2017. Cases were defined as HIV-infected individuals (HIV+), and controls were defined as individuals who were HIV uninfected (HIV−). The primary exposure of interest was IDPE sharing. Inclusion criteria were age >17 years, residence in London-Middlesex county, and reported injection drug use within the last 3 months. Eligible participants were recruited from multiple sites: (1) local needle exchange clinic; or (2) consecutive patients admitted to 1 of 2 local hospitals with a diagnosis of injection drug use–associated endocarditis. Participants at the needle exchange clinic were identified by the harm reduction material distribution staff. Consecutive patients admitted with injection drug–associated endocarditis were identified by the attending infectious disease specialist (all 5 attending ID specialists who worked at these sites participated).
Data Collection
Participants completed a detailed questionnaire regarding injection behaviors. Routine HIV testing was performed using rapid diagnostic tests at the safe-injection site and fourth generation testing at the hospital sites. Race was based on self-reported status. Participants received a $10 CAN ($7.50 US) coffee gift card for participating. Written informed consent was obtained from all participants. The study was approved by the Research Ethics Board at Western University. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.25
Sample Size Calculation
Based on a previous study that found 40% prevalence of sharing IDPE among PWIDs,15 we estimated an exposure prevalence of 20% in controls and 50% in cases, corresponding to an odds ratio of 4.0. A minimum of 33 cases and 66 controls were needed to detect the association between sharing IDPE and HIV status, with 80% power, a 2-tailed alpha of 5%, and the continuity correction.26
Statistical Analysis
Analyses were conducted using SAS 9.4 (SAS Institute Inc. Cary, NC). Univariable logistic regressions were used to compare characteristics of HIV-infected and HIV-uninfected patients. A multivariable logistic regression was conducted to estimate the independent effect of the primary exposure of interest (ie, IDPE sharing). If the addition of any sociodemographic variable (listed in Table 1) demonstrated a confounding influence (>10% change in the IDPE sharing parameter estimate) or interaction with the IDPE sharing variable, or if it was a significant independent predictor itself, it was retained in the multivariable model. Variables that met these criteria included employment; housing; lesbian, gay, bisexual, transgender, and queer (LGBTQ); and men who have sex with men (MSM). Only housing and MSM were retained in the multivariable model as a result of multicollinearity with employment and LGBTQ variables, respectively. Two-tailed tests were used, and significance was defined as P < 0.05.
TABLE 1.
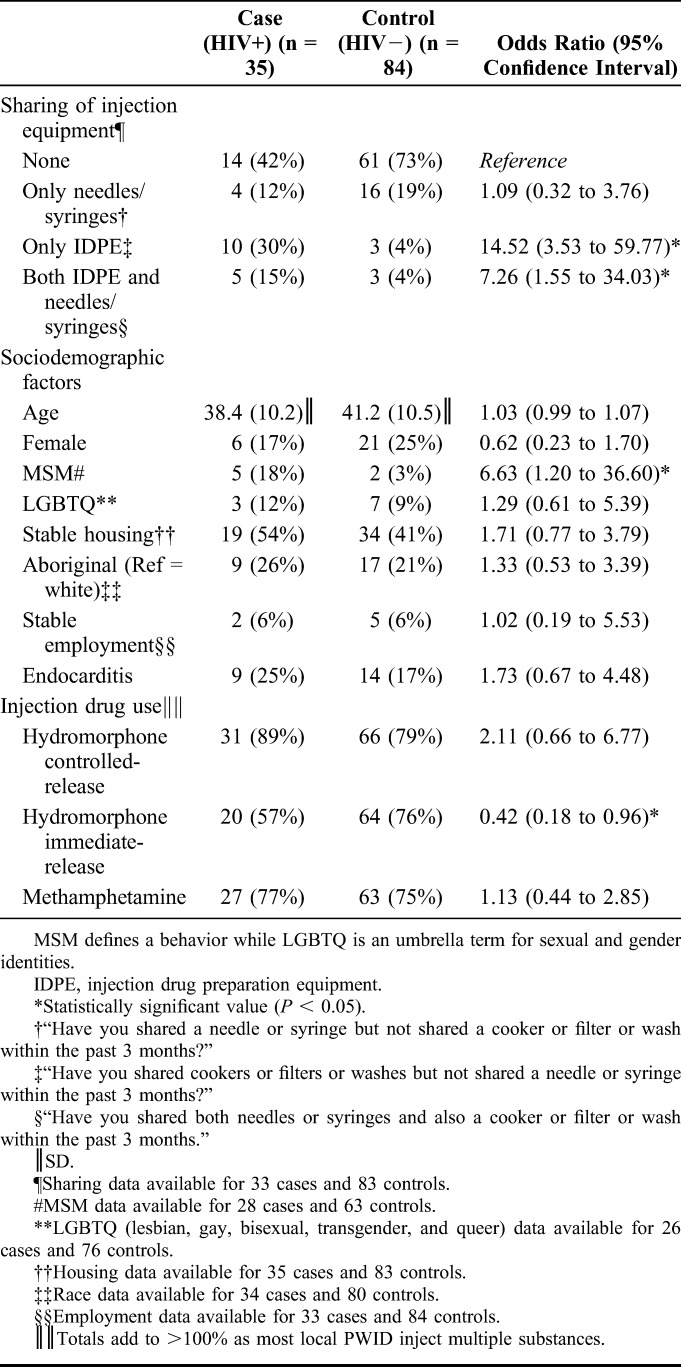
Characteristics of HIV-Infected and HIV-Uninfected Participants
RESULTS
One hundred forty-seven individuals were approached, and 127 agreed to participate in the study. Of the 27 patients approached at the hospital to participate, 4/27 (15%) declined and 23/27 (85%) participated. Similarly, 16/120 (13%) of the patients approached at the safe-injection site declined and 104/120 (87%) participated. Eight participants were excluded because of failure to perform the HIV test. The remainder were divided into cases (HIV+; n = 35) and controls (HIV−; n = 84). A total of 9/35 (26%) HIV cases were new diagnoses. Cases and controls were similar with regard to age, sex, race, housing, employment, and recent endocarditis (Table 1). There was no difference in the proportion of participants recruited from the hospital sites and those recruited at the safe-injection site in the case and control groups.
Unadjusted (Table 1) and adjusted (Table 2) models showed that HIV infection was strongly and consistently associated with sharing IDPE among PWIDs. HIV infection was associated with MSM status, although the association was weaker than sharing IDPE. Sharing needles/syringes in the absence of IDPE sharing was not associated with HIV status. Removing either MSM status or housing status from the regression model did not change the results. Barriers and negative beliefs about heating IDPE preparation before use were reported by 80% (20/25) of cases, compared with only 56% (36/64) of controls (χ2 = 4.346; P = 0.04).
TABLE 2.
Association of HIV Status With Injecting Behavior and Sociodemographic Variables (Adjusted Model)
Participants reported performing multiple drug washes more frequently when injecting hydromorphone controlled-release compared with other locally used drugs. Multiple drug washes were reported when using hydromorphone controlled-release [93/97 (96%); 31/31 HIV+ and 62/66 HIV−] in comparison with hydromorphone immediate-release [41/84 (49%); 12/20 HIV+ and 29/64 HIV−; P < 0.001] and crystal methamphetamine [19/92 (21%); 9/29 HIV+ and 10/63 HIV−; P < 0.001].
DISCUSSION
We found that sharing of IDPE among PWID was a strong risk factor for HIV infection. To the best of our knowledge, this is the first study that has demonstrated an association of IDPE sharing with HIV infection in the absence of needle/syringe sharing.
Sharing of needles/syringes has been well established as a risk for HIV infection.6 We found that sharing both IDPE and needles/syringes was associated with the greatest risk of HIV transmission. However, this finding was likely driven by the significant risk associated with sharing IDPE, as sharing needles/syringes alone was not a significant independent risk factor for HIV infection. Our study occurred in an area with a large and well-used needle and syringe distribution program, which enabled assessment of the impact of IDPE sharing when needle/syringe sharing is relatively infrequent. The low rates of needle/syringe sharing likely allowed the impact of IDPE sharing to be detected. In contrast to our study, a recent HIV outbreak in Indiana demonstrated a 90% rate of needle/syringe sharing among cases and 66% among controls during their outbreak.27 Therefore, as needle/syringe distribution programs are established, interventions to prevent HIV transmission associated with IDPE sharing will become increasingly important to eliminate further HIV transmission.
As in previous studies,13,17–20 we found that sharing of IDPE is a regular occurrence. This behavior is likely due to the risk of HIV transmission with IDPE not being widely recognized among PWID. In fact, participants who avoided needle/syringe sharing frequently reported IDPE sharing several times per day. Those who share needle/syringes likely do so very rarely, due to the known significant associated risk of HIV and hepatitis C virus transmission.8,9,15,28–30 Although the absolute risk of HIV infection associated with a single episode of needle/syringe sharing is higher than that of a single episode of IDPE sharing, the high frequency of IDPE sharing may contribute to a greater cumulative risk. This greater cumulative risk associated with IDPE sharing occurs in populations where needle/syringe sharing is infrequent and sharing IDPE is frequent. We suspect that due to awareness of the risks associated with needle/syringe sharing, individuals may only do so with partners whom they believe are seroconcordant. Given the lack of awareness regarding the risk associated with IDPE sharing, we also suspect that individuals do not avoid this behavior nor do they attempt to serosort with IDPE sharing. Further studies are necessary to confirm these hypotheses.
Counseling regarding the risk of IDPE sharing is potentially an important harm reduction intervention. Free IDPE are routinely distributed with clean syringes/needles in our region and elsewhere. However, sharing of IDPE is still widespread due to the perception of valued drug remaining in the used IDPE.31 Participants frequently reuse their own syringe/needle, placing it into the shared IDPE and thus contaminating the IDPE for the next user. Using a new syringe/needle with each use to prevent IDPE contamination has therefore been suggested for harm reduction. Heating the IDPE before use has been shown to inactivate residual HIV.23 Our finding that negative attitudes toward heating IDPE before use were associated with HIV infection raise the prospect that encouraging IDPE heating may be an effective harm reduction technique. Studies of a corresponding harm reduction campaign are ongoing.
Study Limitations
This study is subject to limitations that should be noted. First, as the focus of this study was on HIV transmission associated with injection drug use, some sexual risk factors for HIV were not fully investigated (eg, condomless sex, multiple partners, sex with a known HIV-positive patient, and commercial sex work). No patients were from an HIV-endemic country. Race in our cohort was reported as white or aboriginal because none of our participants were of African or Asian descent. This reflects the local epidemiology of our PWID community. Second, the exposure of interest (ie, IDPE sharing) was identified retrospectively, and so, the possibility of recall bias cannot be ruled out. However, it is notable that cases were no more likely to recall sharing of needles/syringes than controls, and thus, we do not believe that recall bias was leading cases to nonspecifically report more risky behaviors. Third, we did not differentiate between receptive sharing of IDPE (ie, using IDPE after another PWID had used it) and used IDPE donation (ie, giving used IDPE to another PWID). As risk of IDPE sharing was not widely recognized, most respondents in our study could not reliably remember the order of use, and those who reported IDPE sharing generally reported that both activities occurred. Finally, our study question related to “shared IDPE within 3 months” or “shared needles/syringes within 3 months,” to improve the reliability of reporting. We therefore cannot rule out that remote needle/syringe or IDPE sharing behaviors may have been different from recent patterns.
As 74% of HIV-positive cases were aware of their status before the study, it is possible that knowledge of HIV status may have influenced injection practices. However, given that individuals were largely unaware that sharing IDPE is a risk factor for HIV transmission, it is unlikely that HIV status influenced this practice. We demonstrated that percentage of participants reporting sharing of IDPE either alone or along with needle/syringes was increased in the HIV+ cohort. Although it is possible that knowledge of HIV status may have reduced needle syringe sharing, it would not be expected to have increased IDPE sharing, and so, we do not feel that reverse causality could have led to this effect. Furthermore, there is biological plausibility as noted in our accompanying article (Ball et al “Heating IDPE Used for Opioid Injection May Reduce HIV Transmission Associated with Sharing Equipment”), where we demonstrated that hydromorphone controlled-release (which was injected by 89% of our HIV+ patients) prolonged HIV survival within IDPE. Moreover, institution of an intervention to “cook your wash” (ie, heat the contents of the IDPE) was associated with a reduction in HIV incidence (Ball et al), suggesting that sharing the IDPE was important in HIV transmission in our context.
Public Health Implications
We demonstrated that sharing of IDPE is strongly associated with HIV infection in the absence of needle/syringe sharing. HIV transmission associated with sharing IDPE may represent a novel mechanism of HIV transmission, which has not previously been described. As the popularity of injecting prescription opioids continues to increase,31,32 the implementation of public health interventions that target IDPE sharing as a potential mechanism of HIV transmission may help to prevent and control future outbreaks.
ACKNOWLEDGMENTS
We would like to thank the Middlesex-London Health Unit for sharing data on the HIV incidence and sociodemographic characteristics and who along with the Regional HIV/AIDS Connection conducted the “cook your wash campaign”; Dr. Gayane Hovhannisyan for her input; and Drs. Susana Pearl and David Hill for editing.
Footnotes
Supported by unrestricted grants from the Ontario HIV Treatment Network, and St Joseph's Hospital Foundation.
Presented at the Canadian Society of Addiction Medicine Conference; October 27, 2018; Vancouver, BC, Canada.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Mathers BM, Degenhardt L, Phillips B, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372:1733–1745. [DOI] [PubMed] [Google Scholar]
- 2.Joint United Nations, Programme on HIV/AIDS. UNAIDS Data 2018. Switzerland: UNAIDS; 2018:376. 978-92-9173-945-5. [Google Scholar]
- 3.Abdul-Quader AS, Feelemyer J, Modi S, et al. Effectiveness of structural-level needle/syringe programs to reduce HCV and HIV infection among people who inject drugs: a systematic review. AIDS Behav. 2013;17:2878–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aspinall EJ, Nambiar D, Goldberg DJ, et al. Are needle and syringe programmes associated with a reduction in HIV transmission among people who inject drugs: a systematic review and meta-analysis. Int J Epidemiol. 2014;43:235–248. [DOI] [PubMed] [Google Scholar]
- 5.MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ. 2012;345:e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Des Jarlais DC, Kerr T, Carrieri P, et al. HIV infection among persons who inject drugs. AIDS. 2016;30:815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood E, Kerr T, Marshall BDL, et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ. 2009;338:1191–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Des Jarlais D, Sypsa V, Feelemyer J, et al. Complacency is the new problem: comparative analysis of recent outbreaks of HIV among persons who inject drugs in Europe and North America. In: 22nd International AIDS Conference (AIDS 2018). Amsterdam, Netherlands; 2018. Available at: http://www.natap.org/2018/IAC/IAC_103.htm. Accessed April 24, 2019.
- 9.Chaisson RE, Bacchetti P, Osmond D, et al. Cocaine use and HIV infection in intravenous drug users in San Francisco. JAMA. 1989;261:561–565. [PubMed] [Google Scholar]
- 10.Strathdee SA, Sherman SG. The role of sexual transmission of HIV infection among injection and non-injection drug users. J Urban Health. 2003;80(4 suppl 3):iii7–iii14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackie C. Persons Who Inject Drugs in Middlesex-London: An Update. Report No. 040-16. London, ON; 2016. Available at: https://www.healthunit.com/uploads/2016-06-16-report-040-16.pdf. Accessed July 15, 2018. [Google Scholar]
- 12.Campanella E. More than 2.5 Million Needles Distributed in London Last Year. London Free Press; 2015. Available at: www.lfpress.com/2015/08/13/more-than-25-million-needles-distributed-in-london-last-year. Accessed April 24, 2019. [Google Scholar]
- 13.Zibbell JE, Hart-Malloy R, Barry J, et al. Risk factors for HCV infection among young adults in rural New York who inject prescription opioid analgesics. Am J Public Health. 2014;104:2226–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corson S, Greenhalgh D, Taylor A, et al. Modelling the prevalence of HCV amongst people who inject drugs: an investigation into the risks associated with injecting paraphernalia sharing. Drug Alcohol Depend. 2013;133:172–179. [DOI] [PubMed] [Google Scholar]
- 15.Roy É, Arruda N, Leclerc P, et al. Injection of drug residue as a potential risk factor for HCV acquisition among Montréal young injection drug users. Drug Alcohol Depend. 2012;126:246–250. [DOI] [PubMed] [Google Scholar]
- 16.Hagan H, Thiede H, Weiss NS, et al. Sharing of drug preparation equipment as a risk factor for hepatitis C. Am J Public Health. 2001;91:42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abadie R, Welch-Lazoritz M, Gelpi-Acosta C, et al. Understanding differences in HIV/HCV prevalence according to differentiated risk behaviors in a sample of PWID in rural Puerto Rico. Harm Reduct J. 2016;13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broz D, Wejnert C, Pham HT, et al. HIV infection and risk, prevention, and testing behaviors among injecting drug users—National HIV Behavioral Surveillance System, 20 U.S. cities, 2009. MMWR Surveill Summ. 2014;63:1–51. [PubMed] [Google Scholar]
- 19.McCoy CB, Metsch LR, Chitwood DD, et al. Parenteral transmission of HIV among injection drug users: assessing the frequency of multiperson use of needles, syringes, cookers, cotton, and water. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(suppl 1):S25–S29. [DOI] [PubMed] [Google Scholar]
- 20.Koester S, Glanz J, Barón A. Drug sharing among heroin networks: implications for HIV and hepatitis B and C prevention. AIDS Behav. 2005;9:27–39. [DOI] [PubMed] [Google Scholar]
- 21.Shah SM, Shapshak P, Rivers JE, et al. Detection of HIV-1 DNA in needle/syringes, paraphernalia, and washes from shooting galleries in Miami: a preliminary laboratory report. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11:301–306. [DOI] [PubMed] [Google Scholar]
- 22.Page JB, Shapshak P, Duran EM, et al. Detection of HIV-1 in injection paraphernalia: risk in an era of heightened awareness. AIDS Patient Care STDS. 2006;20:576–585. [DOI] [PubMed] [Google Scholar]
- 23.Clatts MC, Heimer R, Abdala N, et al. HIV-1 transmission in injection paraphernalia: heating drug solutions may inactivate HIV-1. J Acquir Immune Defic Syndr. 1999;22:194–199. [DOI] [PubMed] [Google Scholar]
- 24.Caldarelli H, Locker A, Warshawsky B. A Profile of People Who Inject Drugs in London, Ontario Report on the Public Health Agency of Canada. London, United Kingdom; 2013. Available at: https://bit.ly/2OcTxmw. Accessed April 24, 2019. [Google Scholar]
- 25.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Prev Med. 2007;45:247–251. [DOI] [PubMed] [Google Scholar]
- 26.Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions . Hoboken, NJ: John Wiley & Sons; 2013. [Google Scholar]
- 27.Dasgupta S, Halleck B, Tanner M, et al. Reported Injection Behaviours before and after an HIV outbreak—Indiana, 2016. Presented at CROI; March 6, 2018 Boston, MA. ABS 967.
- 28.Zibbell JE, Asher AK, Patel RC, et al. Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. Am J Public Health. 2018;108:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jordan AE, Des Jarlais DC, Arasteh K, et al. Incidence and prevalence of hepatitis c virus infection among persons who inject drugs in New York City: 2006–2013. Drug Alcohol Depend. 2015;152:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Des Jarlais DC, Friedman SR. HIV infection among persons who inject illicit drugs: problems and prospects. J Acquir Immune Defic Syndr. 1988;1:267–273. [PubMed] [Google Scholar]
- 31.Roy É, Arruda N, Bourgois P. The growing popularity of prescription opioid injection in downtown montréal: new challenges for harm reduction. Subst Use Misuse. 2011;46:1142–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones CM, Christensen A, Gladden RM. Increases in prescription opioid injection abuse among treatment admissions in the United States, 2004–2013. Drug Alcohol Depend. 2017;176:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]


