Emerging evidence of critical roles for chromatin dysregulation in neuropsychiatric pathologies has added to our understanding as to how transcriptional programs in the brain may be disrupted to precipitate maladaptive behaviors. Posttranslational modifications (PTMs) on histone tails are one well-studied mechanism through which chromatin can be modulated by environmental insults over the course of a lifetime. Such perturbations (e.g., chronic stress) can increase an individual’s risk for neuropsychiatric syndromes, including major depressive disorder, posttraumatic stress disorder, and other affective illnesses. For examplee, aberrant histone acetylation and methylation states have been observed in rodent paradigms of chronic stress, in which resulting transcriptional abnormalities are associated with behavioral endophenotypes of depression. These findings are consistent with evidence in humans that a history of stress can result in a greater risk for major depressive disorder (1). Exacerbating the complexity of histone regulation in the context of stress and depression, the repertoire of chemical PTMs that can be added to histone tails has been constantly expanding over the past 15 years, such as discovery of novel acyl PTMs (crotonyl and butyryl) and sugar moieties (adenosine diphosphate ribose, O-linked N-acetylglucosamine)—yet how these modifications influence susceptibility to brain disorders remains incompletely understood. In this issue of Biological Psychiatry, Liu et al. (2) identify a novel role for histone crotonylation in regulating susceptibility to depressive-like endophenotypes using a well-characterized chronic social defeat stress model for the study of major depressive disorder.
Histone crotonylation was first identified in 2011 by Tan et al. (3), joining a growing list of short-chain acyl groups—including propionylation, butyrylation, succinylation, and malonylation—that are capable of posttranslationally modifying histones and other proteins (4). Acylation states are intricately linked with cellular metabolism. For instance, acylcoenzyme A (CoA) is an acyl group donor that is generated during short-chain fatty acid metabolism. Crotonylation is derived from the transfer of crotonyl groups via crotonyl-CoA, which is ultimately dependent on crotonate availability. Levels of histone crotonylation—a mark most abundant in the brain and the colon (5)—have been proposed to be regulated by intracellular concentrations of crotonyl-CoA (6), which can fluctuate in response to external stimuli.
Similar to histone acetylation, alternative histone acylations 1) have been observed on lysines of N-terminal histone tails, potentially competing with histone acetylation itself; 2) contain ε-amine linkages, where donor groups react with primary amines; 3) share common regulatory binding proteins, such as histone transferases; and 4) share analogous chemical structures differing only in their hydrocarbon chain length, charge, and hydrophobic properties (4). Based upon their chemical structures, acyl groups impact chromatin differently through interactions with the DNA backbone and associated regulatory enzymes. Crotonylation has a longer carbon chain than acetylation, thereby increasing its hydrophobicity to more robustly neutralize the positive charge of modified lysine residues. Crotonylation is also unique in its π-bond configuration, resulting in a planar and more rigid structure (7). Owing to these characteristics, histone crotonylation appears to result in greater transcriptional activation than histone acetylation. Two recent studies demonstrated that the histone acetyltransferase p300 and the YEATS acetyllysine reader domain of the AF9 protein preferentially bind lysine crotonylation (Kcr) versus lysine acetylation (6,7). Increased binding by AF9YEATS to Kcr results from the planar, hydrophobic chain of crotonyl groups, which sandwich between the two aromatic rings of the YEATS pocket, thus distinguishing its interactions from those associated with lysine acetylation (7). While the full catalog of histone Kcr writers, erasers, and readers is not known, especially with respect to those that may be specific to the recognition of crotonyl groups, enzymes associated with other histone PTMs have been shown to regulate histone crotonylation, including class I histone deacetylases (5), sirtuin deacetylases (4), the histone acetyltransferase P300/CBP-associated factor (8), and the transcriptional corepressor chromodomain Y-like protein (CDYL) (9). However, studies related to histone Kcr have been primarily conducted in vitro, leaving roles for histone crotonylation in brain understudied.
To begin investigating possible regulatory roles for histone Kcr in the brain, Liu et al. (2) have followed up from their previous work, which identified CDYL as a negative regulator of histone crotonylation. In addition to its chromodomain that enables reading of H3 lysine 27 trimethylation (H3K27me3), CDYL also contains C-terminal hydratase activity that acts on donor crotonyl-CoA rather than on histone proteins themselves (9). Through addition of a water molecule to the crotonyl-CoA double bond, CDYL converts crotonyl-CoA to β-hydroxybutyryl-CoA, thereby reducing the abundance of donors available for crotonylation (9). In their new study, Liu et al. (2) focus on the role of histone Kcr in the medial prefrontal cortex (mPFC), a brain region that has been heavily implicated in major depressive disorder pathophysiology and that is involved in higher-level executive functioning. Liu et al. (2) use the chronic social defeat stress model, in which adult male mice are physically “defeated” by an aggressor for 10 days and, after each interaction, are separated by a partition that allows for constant sensory contact. This model produces depressive-like behaviors in a subset of “susceptible” mice, but not in others that are termed “resilient” (1). Using this model, Liu et al. (2) identify overall reductions in histone Kcr in the mPFC (specifically in the prelimbic cortex [PL]) of susceptible, but not resilient, animals. This effect is not observed in other brain structures (e.g., the hippocampus), and alternative histone PTM states remain unperturbed. These observations suggest a possible role for mPFC histone Kcr in stress-induced vulnerability to depressive-like behaviors.
To understand how histone Kcr levels may be affected by chronic stress, Liu et al. (2) next examined the expression of a panel of known histone Kcr regulators, whereby they identified robust and specific alterations in CDYL. CDYL protein levels in the mPFC were found to be increased in susceptible, but not resilient, mice, corresponding to decreases in histone Kcr. This increase in PL CDYL expression was found to last for more than a month after two independent stress paradigms and was partially rescued by chronic antidepressant treatments. Using short hairpin RNA knockdown of CDYL, and both transgenic and viral-mediated overexpression in the PL, Liu et al. (2) observed bidirectional regulation of depressive-like behaviors in response to stress, including measures of social interaction and sucrose preference. Manipulations of CDYL in infralimbic mPFC do not affect stress behaviors, supporting CDYL’s specific role in the PL as necessary and causal in producing stress-induced susceptibility to depressive-like symptoms.
To identify downstream targets of CDYL, Liu et al. (2) next performed RNA sequencing comparing the PL of mice susceptible to chronic social defeat stress versus nave mice receiving viral-mediated overexpression of CDYL. Focusing on 41 genes downregulated in both datasets, Liu et al. (2) used functional annotation analysis that revealed alterations in ion transport and synaptic plasticity. VGF nerve growth factor inducible (Vgf) was one gene found to be negatively regulated by CDYL. In prior studies, Vgf has been shown to be reduced in patients with depression and has antidepressant effects in mice (10). Using chromatin immunoprecipitation assays on PL tissues from susceptible mice, as well as in a human neuroblastoma cell line (SH-SY5Y), Liu et al. (2) observed increased enrichment of CDYL and H3K27me at the Vgf promoter, along with reduced histone Kcr. Knockout of CDYL in SH-SY5Y cells, in turn, results in increased VGF expression, along with increased enrichment of histone Kcr and reductions in H3K27me at the Vgf promoter. In order to validate the effects of histone Kcr on VGF expression, Liu et al. (2) next used mutational assessments in which the C-terminal hydratase activity of CDYL was abolished, thereby preventing the conversion of crotonyl-CoA. In comparison to wild-type CDYL, such mutants do not repress VGF expression, as expected. Given that CDYL can also recruit EZH2, an H3K27 methyltransferase, Liu et al. (2) performed short hairpin RNA knockdown of this enzyme to demonstrate that such manipulations lead to increased expression of VGF, thus closing the mechanistic loop.
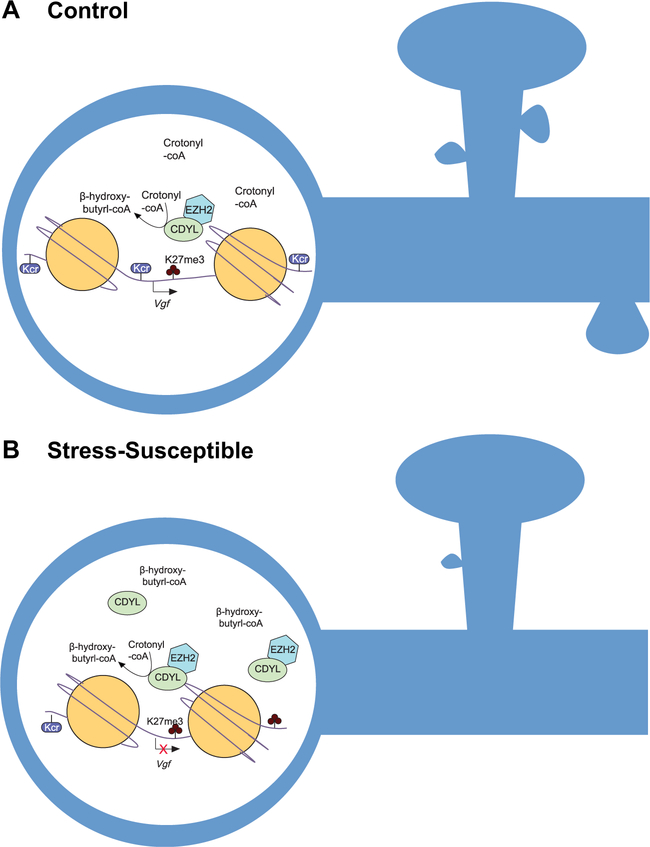
To definitively demonstrate that it is indeed the CDYL-mediated downregulation of VGF that potentiates stress-induced depressive-like behaviors, Liu et al. (2) next used combinatorial short hairpin RNAs to knockdown VGF and CDYL simultaneously, which promotes the rescue of stress-induced depressive-like behaviors that had resulted from knockdown of CDYL alone. Finally, Liu et al. (2) observed a reduction in the density of dendritic spines in the PL after overexpressing CDYL, consistent with a model in which chronic stress promotes increased CDYL expression resulting in reduced histone Kcr, repressed VGF, and subsequent inhibition of VGF-mediated spine plasticity, and resulting in the precipitation of stress susceptibility (Fig 1).
Figure 1.
Chromodomain Y-like protein (CDYL) regulation of histone crotonylation contributes to stress-induced depressive behaviors. (A) Histone lysine crotonylation (Kcr) abundance has been proposed to be dependent on donor crotonyl-coenzyme A (CoA) availability to generate a transcriptionally active state at promoter regions, such as the VGF nerve growth factor inducible (Vgf) locus. CDYL negatively regulates this availability by converting crotonyl-CoA to β-hydroxybutyryl-CoA via C-terminal hydratase activity, concurrently recruiting the H3 lysine 27 (H3K27) methyltransferase EZH2 via its N-terminal chromodomain. (B) The hypothetical model proposed by Liu et al. (2), in which chronic stress promotes increased CDYL expression, with predicted reductions in crotonyl-CoA and histone Kcr levels and increases in H3K27 trimethylation at transcriptional start sites. Transcriptional repression at the Vgf promoter results in inhibition of VGF-mediated spine plasticity and subsequently the precipitation of stress susceptibility.
These new findings add CDYL to a growing list of proteins that regulate susceptibility to stress-induced endophenotypes of depression. While these studies implicate histone Kcr as a novel chromatin regulator mediating stress-induced effects, many questions remain regarding neuronal histone Kcr. For example, few site-specific histone crotonylation antibodies are available, leaving researchers unable to specifically interrogate many of the sites where crotonylation may be functional. Moreover, studies of histone Kcr, like those of other histone PTMs, remain correlational, as direct manipulations of modifications are not yet possible without affecting associated enzymatic machineries. Nevertheless, the observation that histone Kcr may impact the brain to produce behavioral changes highlights the complexity of transcriptional regulatory mechanisms that contribute to neuropsychiatric disease. Understanding the interplay between histone modifications and other mechanisms promoting vulnerability to neuropsychiatric disorders promises to aid in the development of more targeted therapeutics and/or validation of biomarkers associated with these illnesses.
Acknowledgments and Disclosures
This work was supported by National Institutes of Health Grant Nos. DP1 DA042078, R21DA044767, and P50 MH096890 (to IM).
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Sun H, Kennedy PJ, Nestler EJ (2013): Epigenetics of the depressed brain: Role of histone acetylation and methylation. Neuropsychopharmacology 38:124137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y, Li M, Fan M, Song Y, Yu H, Zhi X, et al. (2019): Chromodomain Y-like protein–mediated histone crotonylation regulates stress-induced depressive behaviors. Biol Psychiatry 85:635–649. [DOI] [PubMed] [Google Scholar]
- 3.Tan M, Luo H, Lee S, Jin F, Yang JS, Montellier E, et al. (2011): Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell 146:1016–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabari BR, Zhang D, Allis CD, Zhao Y (2017): Metabolic regulation of gene expression through histone acylations. Nat Rev Mol Cell Biol 18:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fellows R, Denizot J, Stellato C, Cuomo A, Jain P, Stoyanova E, et al. (2018): Microbiota derived short chain fatty acids promote histone crotonylation in the colon through histone deacetylases. Nat Commun 9:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabari BR, Tang Z, Huang H, Yong-Gonzalez V, Molina H, Kong HE, et al. (2015): Intracellular crotonyl-CoA stimulates transcription through p300-catalyzed histone crotonylation. Mol Cell 58:203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Sabari BR, Panchenko T, Wen H, Zhao D, Guan H, et al. (2016): Molecular coupling of histone crotonylation and active transcription by AF9 YEATS domain. Mol Cell 62:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simithy J, Sidoli S, Yuan ZF, Coradin M, Bhanu NV, Marchione DM, et al. (2017): Characterization of histone acylations links chromatin modifications with metabolism. Nat Commun 8:1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S, Yu H, Liu Y, Liu X, Zhang Y, Bu C, et al. (2017): Chromodomain protein CDYL acts as a crotonyl-CoA hydratase to regulate histone crotonylation and spermatogenesis. Mol Cell 67:853–866. [DOI] [PubMed] [Google Scholar]
- 10.Neto FL, Borges G, Torres-Sanchez S, Mico JA, Berrocoso E (2011): Neurotrophins role in depression neurobiology: A review of basic and clinical evidence. Curr Neuropharmacol 9:530–552. [DOI] [PMC free article] [PubMed] [Google Scholar]