Abstract
Atopic dermatitis (AD) affects up to 20% of children worldwide and is an increasing public health problem, particularly in developed countries. Although AD in infants and young children can resolve, there is a well-recognized increased risk of sequential progression from AD to other atopic diseases, including food allergy (FA), allergic rhinitis, allergic asthma, and allergic rhinoconjunctivitis, a process referred to as the atopic march. The mechanisms underlying the development of AD and subsequent progression to other atopic comorbidities, particularly FA, are incompletely understood and the subject of intense investigation. Other major research objectives are the development of effective strategies to prevent AD and FA, as well as therapeutic interventions to inhibit the atopic march. In 2017, the Division of Allergy, Immunology, and Transplantation of the National Institute of Allergy and Infectious Diseases sponsored a workshop to discuss current understanding and important advances in these research areas and to identify gaps in knowledge and future research directions. International and national experts in the field were joined by representatives from several National Institutes of Health institutes. Summaries of workshop presentations, key conclusions, and recommendations are presented herein.
Keywords: Atopic march, atopic dermatitis, food allergy, asthma, skin barrier, skin microbiome, biomarkers, interventions
Atopic dermatitis (AD; also known as atopic eczema) is a chronic, pruritic inflammatory skin disorder with a complex etiology and heterogeneous presentation that affects up to 20% of children worldwide and is an increasing public health problem in developed countries.1–4 Aside from its significant socioeconomic effect and increased risk of serious skin infections, AD can predispose infants and children to other atopic diseases, including food allergy (FA), allergic rhinitis, allergic asthma, and allergic kerato-conjunctivitis, a process termed the atopic march.5–13 Although there is evidence from retrospective and prospective population studies to support a causal relationship between AD and other atopic diseases, other studies suggest this might be an oversimplification, and further studies are needed to clarify the connections between these atopic diseases.14–16 The incidence of FA has also increased worldwide over the past decade.17–19 Although FA has a strong association with AD, its contribution to the classically defined progressive atopic march from AD to allergic rhinitis and allergic asthma is debated.20–22
Because not all patients with early-onset AD will progress through the atopic march, the challenges are to identify those at greatest risk of progression and to develop targeted interventional therapies. Success in this endeavor will depend on a detailed understanding of pediatric AD endotypes and phenotypes, skin barrier dysfunction in patients with AD, and the innate and adaptive immune processes that normally protect barrier surfaces from inflammation, allergen sensitization, and infection.
To evaluate the current status of knowledge in these areas, the Division of Allergy, Immunology and Transplantation of the National Institute of Allergy and Infectious Diseases convened a workshop titled “Atopic dermatitis and the atopic march: mechanisms and interventions” on September 6 and 7, 2017, in Rockville, Maryland. The workshop was attended by 41 scientists and clinicians from Europe and the United States, the National Institute of Allergy and Infectious Diseases, other National Institutes of Health institutes, and the US Food and Drug Administration. The overall goals of the workshop were as follows:
- to review our current understanding and gaps in knowledge of:
- developmental relationships between AD, FA, and airway allergic diseases, even though many previous evaluations of the atopic march have not included FA in their analyses;
- abnormalities in the skin barrier and skin microbiome in patients with AD that promote inflammation and epicutaneous sensitization to allergens; and
- immune pathways involved in barrier communication, homeostasis, and defense and how these deviate in patients with AD and result in allergen sensitization and
to identify potential biomarkers that predict risk for the atopic march and opportunities for targeting interventions to prevent AD and the atopic march.
This document includes presentation summaries prepared by each speaker. Each section is followed by key conclusions and recommendations for future studies that were compiled from session discussions and a panel discussion held at the end of the workshop and chaired by Dr Mark Boguniewicz. Panel discussants included Drs Gideon Lack, Donald Leung, Kari Nadeau, Angela Simpson, Eric Simpson, and Ulrich Wahn.
1. DEVELOPMENTAL RELATIONSHIPS BETWEEN AD, FA, AND AIRWAY ALLERGIC DISEASES
1.1. How frequently does the classical atopic march from AD to allergic airway disease occur?
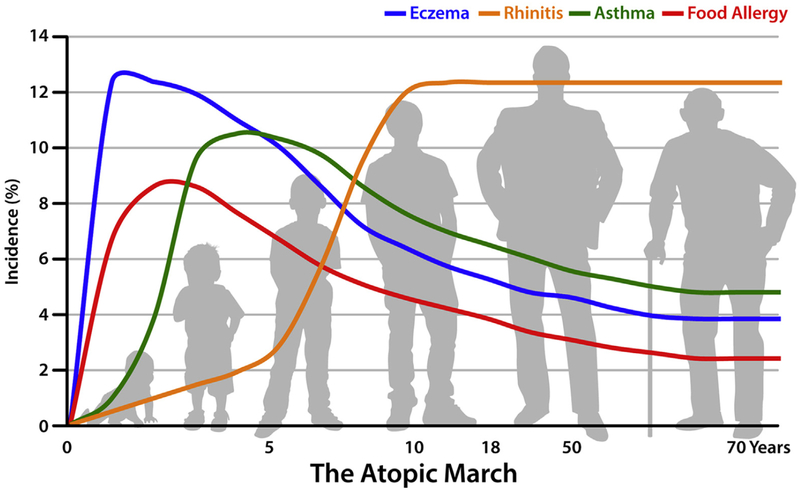
Drs Ulrich Wahn and Jonathan Spergel began the workshop with a pro/con debate on evidence for the existence of the atopic march. For the affirmative side, Dr Wahn defined the atopic march as the sequential progression of different allergic conditions frequently observed in children with IgE antibody responses against common environmental allergens. Generally, eczema or AD is the first clinical manifestation, followed by asthma, allergic rhinitis, or both (Fig 1).5,6,23
FIG 1.
A proposed model of the atopic march. AD prevalence peaks early in infancy, potentially increasing the risk for consequent development of the atopic march. Development of FA, asthma, and allergic rhinitis correlates with AD severity in infancy.23
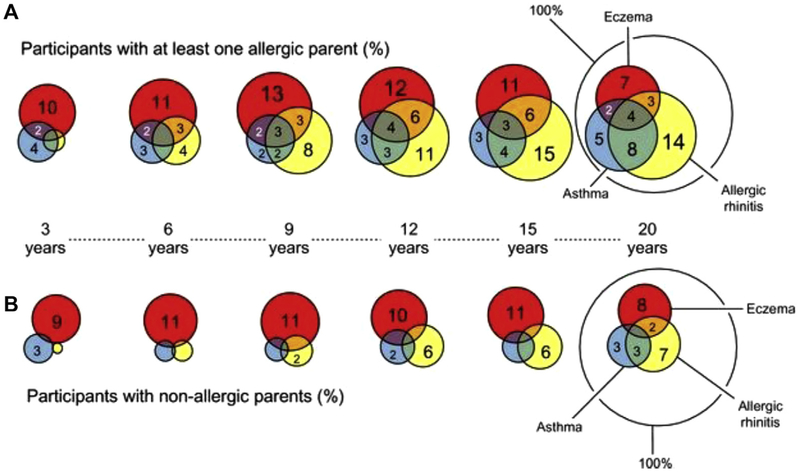
Dr Wahn summarized data from the Multicenter Allergy Study, a prospective observational birth cohort study that examined the comorbidity of AD, asthma, and rhinitis with age and the relationship with sensitization to food or environmental allergens. In this cohort AD increased the risk of asthma and rhinitis, and asthma occurred more frequently with coexisting allergies than as a single entity.8 However, not every patient with AD had asthma, and not all patients with asthma had preceding AD. Moreover, AD did not seem to be a risk factor for adult-onset asthma (Fig 2).8
FIG 2.
Allergic multimorbidity of asthma, rhinitis, and eczema over 20 years in the German birth cohort Multicenter Allergy Study. A, Percentages of all participants with allergic parents. B, Percentages of all participants with nonallergic parents. Multimorbidity of asthma, eczema, and allergic rhinitis up to 20 years of age (n = 941) by parental allergy and age is shown.8
A history of infantile AD along with a parental family history for atopy also increased the risk of subsequent allergic manifestations of the upper or lower airways, suggesting a genetic influence in the atopic march.24 Several genetic risk factors for AD have been identified that might contribute to comorbidity, including filaggrin (FLG) gene mutations.25,26 Nevertheless, among those with or without an allergic parent, only a minor proportion (<5%) had AD, asthma, and rhinitis at any age, indicating that progression from AD to asthma to allergic rhinitis is a relatively rare event (Fig 2).8 However, the caveat is that these studies were conducted on community-based populations involving milder forms of AD and asthma. It is noteworthy that patients with moderate-to-severe AD are more likely to have associated FA and respiratory allergy.9,22 Other factors that influenced comorbidity in the Multicenter Allergy Study cohort included the prevalence of IgE-mediated sensitization, degree of epitope spreading, parental atopy, and domestic allergen exposure.27–30 Domestic cat and dust mite exposure of young children significantly increased the risk of sensitization early in life, as well as the risk of persistent asthma throughout adolescence.27–30 For both grass pollen–related allergic rhinitis and dust mite sensitivity associated asthma, sensitization preceded the clinical airway manifestation.28,29 By using these parameters, a predictive risk assessment for asthma in childhood is possible, and several algorithms for asthma prediction have been published.29–31
Dr Wahn concluded that although there is no single unique pathway for the atopic march, longitudinal studies between birth and age 20 years have revealed characteristic patterns of sensitization and clinical manifestations that might be predictive of later airway disease. Furthermore, future studies might wish to broaden the definition of the atopic march to include patients who progress from AD to FA, as well as from FA or AD to respiratory allergy.
In his counterarguments Dr Spergel reiterated that adult-onset asthma is rarely associated with AD or the atopic march8 and noted that in published pediatric studies 33% to 50% of children with AD did not have atopic airway diseases.32–34 In a recent retrospective, single-site, pediatric cohort study using health care provider–diagnosed AD, asthma, allergic rhinitis, and FA, Dr Spergel and his colleagues confirmed the developmental sequence of atopic diseases from birth to age 5 years at the population level, with AD preceding FA and FA preceding airway diseases.10 Among patients with established FA, 35% had asthma and 35% had allergic rhinitis, providing further evidence that not all atopic children complete the entire atopic march. In both groups allergy to peanut, milk, and egg significantly predisposed to both airway diseases, and patients with multiple food allergies were at increased risk of disease.10,35
In a separate multisite study of infants with recent-onset AD and atopic parents who were observed for approximately 3 years, approximately 10% had asthma and 33.3% had 1 or more atopic comorbidities (asthma, FA, allergic rhinitis, and allergic conjunctivitis) by the end of the study.9 Infants with greater AD severity at baseline had a greater risk of FA or allergic rhinitis or 1 or more atopic comorbidities.9 Dr Spergel concluded that although there is evidence for the atopic march in patients with pediatric allergy, this is only observed 30% to 50% of the time.
1.2. Insights from birth cohorts: Identification of specific atopic phenotypes/endotypes
Dr Angela Simpson described developmental profiles of AD, asthma/wheeze, and rhinitis in various birth cohort studies. Population-based birth cohorts can provide a unique aspect to our understanding of the atopic march by allowing one to profile the development of atopic diseases within a subject without the effect of recall bias. A study from the European consortium of birth cohorts (MeDALL), with analysis of data from more than 17,000 children in 12 European birth cohorts, showed that the coexistence of 2 or 3 diseases (atopic eczema, rhinitis, and asthma; FA was not assessed) in the same child occurred more frequently than by chance almost half of the time.36 This effect was seen in both atopic and nonatopic children (ie, was not dependent on the presence of specific IgE to allergens).36
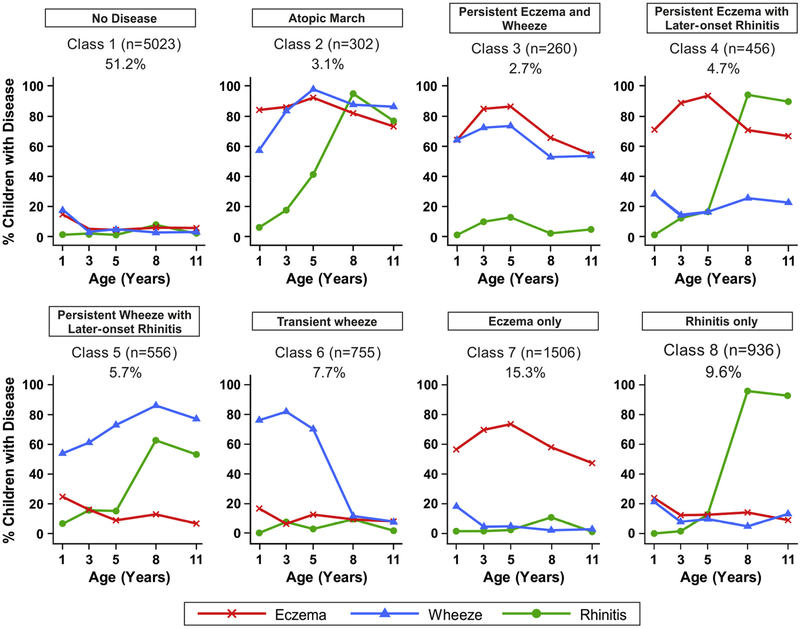
Investigators in the United Kingdom combined data from 2 birth cohorts in the Study Team for Early Life Asthma Research Network (STELAR; Manchester Asthma and Allergy Study [MAAS] and Avon Longitudinal Study of Parents and Children [ALSPAC]) to determine the prevalence of wheeze, AD, and rhinitis at ages 1, 3, 5, 8, and 11 years and compared these data with findings when a Bayesian machine learning framework was used to model the longitudinal development of eczema, wheeze, and rhinitis within subjects throughout childhood.14 The traditional approach revealed a profile plot reminiscent of the atopic march. The Bayesian machine learning approach, which was used to uncover latent structure in data, identified an 8-class solution; the largest class (approximately 50%) comprised those without disease (Fig 3).14 What has been conventionally described as the atopic march (AD followed by wheezing and rhinitis) represented a class that comprised only 3% of the population. The persistent eczema and wheeze cluster also comprised 3%, persistent eczema with late-onset rhinitis comprised 5%, and persistent wheeze and late-onset rhinitis comprised 6%; transient wheeze (8%), eczema-only (15%), and rhinitis-only (10%) classes were also seen (Fig 3). In a longitudinal analysis allergic sensitization (to any allergen) was a feature of all disease classes apart from transient wheeze, but the effect was seen most strongly for the atopic march class (odds ratio [OR] > 20). If the definition of the atopic march is broadened to include progression from AD to 1 or more comorbidities, then based on this model, approximately 10% of the population might be at risk. Although FLG-null alleles were overrepresented in the atopic march class, the majority of children carrying this variant were not in the atopic march class. Some genetic variants have been identified as associated with an atopic march phenotype in a genome-wide association study; the strongest effect was seen for a locus on chromosome 1 that was related to FLG.
FIG 3.
Bayesian machine learning methods identified 8 distinct latent disease classes based on individual profiles of eczema, wheeze, and rhinitis across 2 population-based birth cohorts: Avon Longitudinal Study of Parents and Children (ALSPAC) and Manchester Asthma and Allergy Study (MAAS). The number of children and proportion of the study population are indicated for each class. Plots indicate longitudinal trajectories of wheeze, eczema, and rhinitis within each class.14
Dr Simpson concluded that although eczema was a feature of 25% of the clusters, most eczema is not associated with the classical atopic march picture, and there is an inherent uncertainty in predicting the development of new symptoms or resolution of existing ones within any individual child. Thus when designing intervention studies to prevent the atopic march, it is important to note that most children with early-onset eczema will not follow an atopic march trajectory leading to asthma, and this needs to be considered when calculating sample size to ensure adequate power. It was noted that these studies lacked data on physician-confirmed food allergies, and therefore the link between FA and AD was not explored. In the future, the relationship of these atopic comorbidities to eosinophilic esophagitis and keratoconjunctivitis might also be of interest.10
1.3. Insights from respiratory birth cohorts: Effects of the environment on AD and the atopic march
Dr James Gern reviewed the temporal progression of allergic diseases beginning with AD followed by asthma and allergic rhinitis in respiratory birth cohorts.37 Given the large role of the environment in determining the risk for allergic diseases and asthma, Dr Gern and his colleagues evaluated this progression in 2 high-risk birth cohorts set in distinct environments: the Childhood Origins of Asthma (COAST) study set in suburban Madison, Wisconsin,38 and the Urban Environment and Childhood Asthma (URECA) study in disadvantaged urban neighborhoods of 4 US cities.39 These cohorts were used to test whether environmental exposures were associated with differences in immune development that modified the risk for early allergic phenotypes, such as AD, allergic sensitization, and wheezing illnesses.
In the COAST study dog exposure at birth was inversely related to the risk for all 3 atopic features in an age-dependent fashion. Exposure to dogs in the home beginning at birth was inversely associated with AD and allergic sensitization by age 1 year and recurrent wheezing by age 3 years.40 In addition, 3 patterns of AD (early/recurrent, late onset, or none/transient) were identified by using latent class analysis of data through age 6 years.41 Dog ownership at the time of birth was lowest in the early/recurrent group (10%, 50%, and 43%, respectively). Compared with the none/transient AD group, the early/recurrent pattern of AD was associated with high rates of progression to wheezing illnesses (45% vs 23%), food-specific IgE (48% vs 18%), and FA (15% vs 2%, all P < .01).
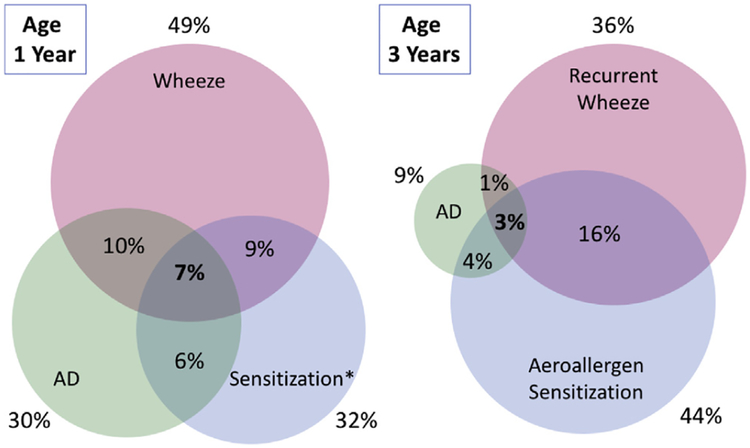
The URECA study tested relationships between environmental exposures, immune development, and the risk of atopic disease. One major finding was that early exposures to common urban allergens (cockroach, mouse, and cat) and diverse microbes in the home are inversely related to the risk of atopic wheeze at age 3 years.42 These environmental exposures were also inversely related to asthma at age 7 years.43,44 Additional analyses have identified relationships between AD, immune development, and wheeze in urban children. For example, early-onset AD was associated with a broad reduction in cord blood mononuclear responses to a variety of innate, mitogenic, and antigenic stimuli.45 Despite these findings, these cord blood responses were only weakly related to recurrent wheeze and allergic sensitization at age 3 years.46 Furthermore, although wheezing illnesses, allergic sensitization, and AD were all relatively common at age 1 year (49%, 32%, and 30%) and age 3 years (36%, 44%, and 9%), only a small number of children experienced all 3 atopic indicators (7% at age 1 year and 3% at age 3 years, Fig 4).
FIG 4.
Overlap between AD, allergic sensitization, and wheezing in urban children. Although the individual conditions are relatively common, at age 3 years, only 3% of children experienced all 3 conditions.45 Figure courtesy of Dr James Gern.
Children in the URECA study were grouped into 5 respiratory phenotypes based on a cluster analysis of wheeze, allergic sensitization, and lung function data during the first 7 years of life. Two of the phenotypes (the high-wheeze, high-atopy and high-wheeze, low-atopy phenotypes) were highly enriched for asthma.44 Eczema at some point in the first year was more common in children in the high-wheeze, high-atopy phenotype compared with either the high-wheeze, low-atopy or lowwheeze, low-atopy phenotypes (78% vs 54% and 48%, P <.01).44
Collectively, these findings provide evidence of strong relationships between individual elements of the atopic march (eg, AD and allergic sensitization or AD and wheeze), but relatively few children express all elements at once. A specific phenotype of AD that is of early onset with recurrent activity is most likely to be associated with allergic sensitization and viral wheeze. Environmental exposures, such as pet dogs, perhaps related to associated microbes,47 might be protective for this clinically important AD phenotype. Finally, AD and early allergic sensitization are linked to specific high-atopy phenotypes of childhood asthma, which might be of special clinical significance in terms of lower lung function and increased asthma morbidity.44,48
1.4. Association between AD and FA in human subjects
Dr Gideon Lack reviewed evidence for the close association between AD and FA in human subjects. There is a well-known association between AD and immediate hypersensitivity to foods. In recent years, it has become apparent that infantile AD in the first 6 months of life is associated with the development of FA and that the earlier and more severe forms of AD are associated with a higher risk of FA. Recent follow-up data from the peanut avoidance group in the Learning Early About Peanut Allergy (LEAP) study showed that among 321 children with AD as infants, 76% had at least 1 allergic disease and 48% had multiple comorbidities at age 5 years. A total of approximately 40% had FA at the ages of 5 and 6 years, 18% had asthma, and 54% had perennial rhinoconjunctivitis.22 Yet, at age 5 years, only 4% exhibited the full constellation of atopic diseases, AD, FA, asthma, and rhinoconjunctivitis.22
In regard to the mechanisms linking AD and FA, there is a growing body of evidence for cutaneous exposure to environmental peanut allergen, leading to the development of FA through a broken-down skin barrier.49 In patients with AD, the skin barrier is impaired, potentially allowing penetration of food allergens. This impaired barrier function is reflected by increased transepidermal water loss (TEWL) in infants even before they have evident AD.50
Genetic and environmental risk factors can also contribute to AD and FA. Null mutations in FLG are associated with AD and independently with peanut allergy, and FLG protein has an important role in maintaining skin barrier function.50,51 Furthermore, high-level exposure to peanut dust in the environment increases the risk of peanut sensitization and allergy in infants with null mutations in FLG, demonstrating a gene-environment interaction.50,51
Recent studies have shown that early application of emollients to the skin can prevent the development of AD.52,53 Based on these findings, Dr Lack suggested there is a rationale to consider early prevention and treatment of AD as a strategy to prevent the development of food sensitization and ultimately FAs. Given the association between AD, FA, and allergic airway disease, this intervention might also decrease the risk of allergic airway disease.
1.5. Tracing the link between AD and asthma: Clues to cellular mechanisms
Dr Judith Woodfolk reviewed cellular mechanisms that might link AD to asthma. Multiallergen sensitization is a hallmark of AD and is likely to be pivotal to the development of asthma. Among children with AD, there is a hierarchy of allergens related to age, sensitization, and asthma. High levels of specific IgE to peanut are present before 3 months of age, whereas levels of IgE antibodies to aeroallergens increase throughout childhood, particularly among patients with severe disease. Age-related progression from cat to dust mite to rye grass sensitization is evident, and sensitization to cat and dust mite, but not food allergens, predicts wheeze. Thus peanut allergy sets the stage for multisensitization, more severe disease, and asthma among a subset of patients with AD.54
Regardless of age, the majority of IgE is not accounted for by known allergens in patients with the highest total IgE levels, indicating dysregulated IgE production. Dysregulated IgE in patients with AD reflects underlying TH2-driven processes that are also perturbed and manifest systemically. Allergen exposure promotes the expansion of pathogenic TH2 effectors with the potential to traffic between the skin and respiratory tract based on coexpression of a variety of tissue-homing markers (cutaneous lymphocyte–associated antigen, CCR4, and CCR6). TH2 amplification is driven by myeloid dendritic cells of the cDC2 type through cross-talk between pathways triggered by allergen and thymic stromal lymphopoietin (TSLP). Enhanced responsiveness of dendritic cells to diverse external cues is also integral to this process.55
Recent work on peanut allergy in both human and animal models supports the skin as a site for TH2 priming that leads to asthma. In multisensitized children with AD and asthma who have high levels of IgE to peanut, those T cells responding to Ara h 2, an important predictor of peanut allergy, secrete multiple TH2 cytokines.56 Within the same patient, these T cells are more “TH2 skewed” compared with those specific for Ara h 1 or the major cat allergen Fel d 1, despite the link between cat allergen and asthma. Such cytokine heterogeneity among allergenspecific T cells likely reflects the molecular properties of the allergen combined with the anatomic site of T-cell priming. The recent identification of a unique surface TH2 signature that captures all allergen specificities could shed light on how the evolution of TH2 responses orchestrates AD and asthma in early childhood.57
Beyond allergens, asthma development reflects complex environmental exposures that culminate in “immunologic reprogramming” in the susceptible host. In healthy subjects microbes induce a robust TH1 response. However, TH1 signatures are also evident in the lower airways of children with severe asthma, including those with high IgE levels.58 Notably, CCR5+ memory T cells expressing IFN-γ constitute the dominant T-cell type in bronchoalveolar lavage fluid. Moreover, higher levels of the type III interferon IL-28A in bronchoalveolar lavage fluid are linked to sensitization to dust mite and inhalant fungal species. Recently, CCR5+ memory TH1 cells have also been implicated in the control of rhinovirus in healthy nonasthmatic subjects.59 These collective findings, coupled with known interactions between IgE and rhinovirus in wheezing children, support a role for TH1 dysregulation in asthma pathogenesis in multisensitized patients with AD. Investigating unconventional roles of IgE and the contributions of “pantissue” dendritic cell types that populate both human skin and airways60 could shed light on the development of asthma in patients with AD.
Key conclusions
Birth cohort studies, machine learning approaches, and longitudinal latent class analyses have identified multiple mixed atopic phenotypes defined by specific comorbid conditions and their time course, indicating significant heterogeneity in presentation of the atopic march.
Only a small subset of children in the general population (approximately 3%) appear to follow the complete course of what has been conventionally referred to as the atopic march. Even in high-risk cohorts, a stepwise progression from AD to FA, asthma, and rhinoconjunctivitis is not common.
The majority of children with AD do not progress to other allergic diseases, although those with severe AD more likely do. Inversely, the majority of children with asthma or rhinoconjunctivitis later in life do not have a history of early-life AD.8,10
- However, early-life AD remains a major risk factor for the development of any atopic disease:
- Early-onset persistent AD, multisensitization to allergens, and familial atopy are risk factors for the classical atopic march or development of multiple comorbidities.
- Progression from AD to FA, particularly peanut allergy, is significantly associated with multisensitization and development of allergic airway disease.
- FA in infancy is associated with an increased risk of asthma, irrespective of whether FA resolves. The risk is greater in those with multiple food allergies and those with coexistent AD.61
The strong association between AD and allergic sensitization implies that skin barrier defects in patients with AD increase the risk of epicutaneous sensitization to food allergens and aeroallergens.
FLG mutations enhance the potential for sensitization through the skin and predispose to the classical atopic march, implying skin barrier dysfunction in this process.
Workshop participants agreed that subjects who only have elements of the conventional atopic march should still be considered as part of a broader definition of the march, which is more reflective of the natural history of atopic disease. Understanding the underlying biology of the various manifestations of the atopic march is pivotal for development of endotype-targeted future intervention strategies.
Workshop recommendations
Early prevention and treatment of AD should be tested as a strategy to inhibit the development of food allergies and atopic airway disease.
A new, large, prospective birth cohort study is required to better define AD phenotypes, atopic comorbidity phenotypes, and their respective risk profiles. This longitudinal cohort should monitor not only for the development of the atopic march in its proposed broader definition but also for resolution of allergic diseases, recognizing that greater than 50% of childhood AD resolves before adulthood.
- The birth cohort study should:
- incorporate agnostic evaluations of skin, gut, airway and peripheral blood and use multiparameter approaches to further define phenotypic/endotypic subgroups of AD and to predict AD outcomes and development of other atopic conditions;
- use longitudinal latent class analysis methods and other clustering approaches in identifying patterns of atopic disease and incorporate severity and time course;
- use accepted diagnostic criteria and validated disease severity scores for all atopic conditions;
- investigate genetic, epigenetic, and environmental influences on the development of AD and atopic disease progression;
- determine mechanistically how AD severity and multiallergen sensitization influence atopic disease progression; and
- allow for reliable replication by establishing early collaborations with other cohorts with the goal of including similar clinical outcomes.
2. ROLES OF SKIN BARRIER DYSFUNCTION, CUTANEOUS INFLAMMATION AND ITCH IN THE PATHOGENESIS OF AD
2.1. How do skin barrier defects shape the immune response in patients with AD?
The relative importance of immunologic versus epithelial abnormalities in the development and perpetuation of AD, as well as all other allergic conditions, is a hotly debated topic. Dr Lisa Beck noted that murine AD models and naturally occurring human disease have demonstrated that a perturbation in one compartment typically begets the characteristic features observed in the other compartment.62 This would suggest that therapies targeting both compartments might yield the greatest benefit. In fact, recent studies showed that daily application of various emollients to the skin of high-risk infants led to a 50% reduction in AD by 6 months of age.52,63 In another study short-term application of petrolatum with occlusion affected skin barrier function and also enhanced innate immune responses in both normal skin and nonlesional AD skin, suggesting beneficial effects on both barrier and immune compartments and a potential explanation for the preventative effects of emollients in infants at high risk of AD.64 Overall, these data suggest that emollients can prevent progression to AD. This approach might also affect the development of other allergic disorders, such as FA and asthma, if skin penetration and immune activation is responsible for both food allergen and aeroallergen sensitization.
The importance of the skin barrier was also highlighted in a study of more than 1500 infants born of low-risk mothers, which demonstrated that infants who were in the upper quartile of TEWL had a high OR (OR = 3.1) for the development of AD at 12 months or FA by 2 years of age (OR = 3.5).65,66 Remarkably, measures of epidermal dysfunction are observed, even in clinically unaffected skin of patients with AD, and include TEWL, reduced stratum corneum (SC) hydration, reduced integrity of the SC, more alkaline surface pH, and a reduced irritancy threshold. This raises the possibility that the entire skin integument of patients with AD might be permissive to allergen sensitization, as well as elicitation, and this might explain why the highest levels of type 2 immune serum biomarkers (thymus and activation-regulated chemokine/CCL17, pulmonary and activation-regulated chemokine/CCL18, periostin, total serum IgE, and total eosinophils) are seen in patients with AD. This has led to the speculation that the skin might be either one of or the critical surface for systemic allergen sensitization, even in patients with allergic diseases that manifest in other organs, such as the gastrointestinal tract (FA and eosinophilic esophagitis) or the upper or lower airway (allergic rhinitis and asthma).
There are a number of abnormalities observed in the SC that could lead to a defect in epidermal barrier function in patients with AD (Fig 5). These include acquired or genetic defects in FLG or other epidermal differentiation proteins. FLG mutations increase the risk of AD, peanut allergy, AD plus asthma, and eczema herpeticum.50,51,67 Decreased expression of FLG has multiple effects, including increased TH2 cytokine production by group 2 innate lymphoid cells (ILC2s), increased production of endogenous proteases, and increased IL-1 and TSLP production.68 However, FLG mutations are neither necessary nor sufficient to induce AD. Mutations in other genes involved in the maintenance of epidermal integrity and barrier function, including DSG1, the gene encoding desmoglein, also are associated with severe dermatitis, increased expression of TSLP in keratinocytes, and multiple allergies.69 It is likely that both AD and the atopic march might require multiple genetic, immunologic, and environmental hits: (1) altered lipid composition or conformation; (2) acquired or genetic defects in proteases and/or anti-proteases; (3) products released from Staphylococcus aureus, which commonly colonizes AD skin; and/or (4) simply the consequence of the physical trauma from widespread scratching.
FIG 5.
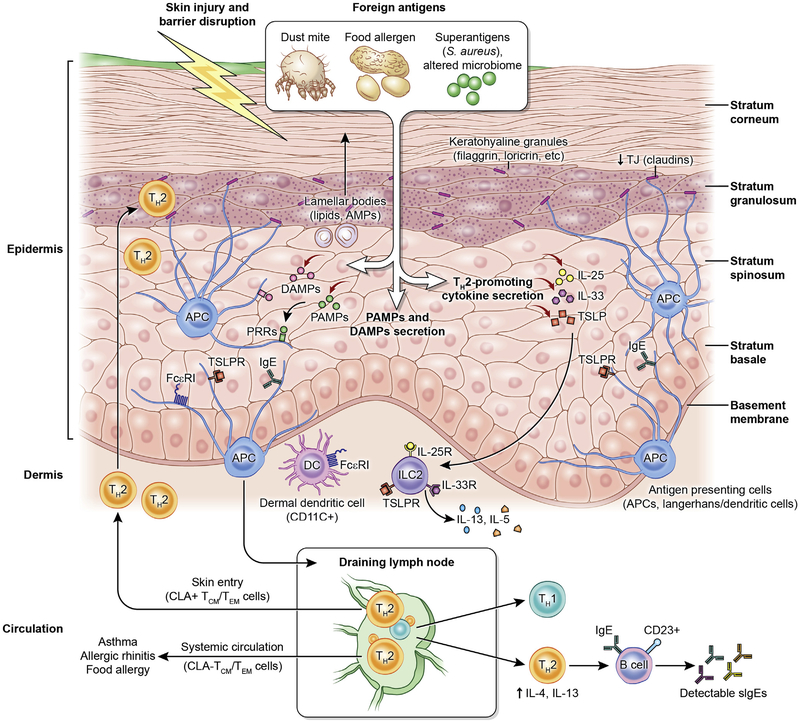
Skin structure and abnormalities associated with AD. Impaired skin barrier promotes foreign antigen (eg, dust mites and food allergens) penetration and activation of innate immune and pattern recognition receptors. Pathogen-associated molecular patterns and damage-associated molecular patterns are secreted secondary to tissue damage and/or an altered microbial profile to initiate and perpetuate tissue inflammation. Concurrently, antigen stimulation leads to TH2-promoting cytokine secretion (IL-25, IL-33, and TSLP), consequent IgE- and FcεRI-bearing Langerhans cell and dermal dendritic cell (DC) activation, and migration to regional draining lymph nodes to initiate TH2 differentiation and B-cell IgE skewing. In turn, T cells circulate back to infiltrate the skin (cutaneous lymphocyte–associated antigen [CLA]+ effector memory T [TEM]/central memory T [TCM] cells) or are distributed peripherally (CLA− TEM/TCM cells) to other end organs to initiate diverse atopic disorders. APC, Antigen-presenting cell; DAMP, damage-associated molecular pattern; PAMP, pathogen-associated molecular pattern; PRR, pattern recognition receptor; TSLPR, TSLP receptor. Figure adapted from Czarnowicki et al,23 with permission.
Only recently have we appreciated the importance of the second barrier structure in the skin called tight junctions (TJs), which reside at the level of the stratum granulosum and are the only barrier structure in all simple epithelia. These structures are also defective in patients with AD.70 Furthermore, TJs divide the epidermis into 2 compartments. The more superficial half, which is readily exposed to environmental insults but does not express pattern recognition receptors, has little to no Langerhans cell or dendritic cell processes, and is consequently immunologically inert.71 This is in contrast to the deeper epidermis, below the TJs, where all the immunologic response elements reside, and host responses are robust (Fig 5).72,73 The current paradigm postulates that patients with AD have alterations in both of these barrier structures (SC and TJs) and that this promotes their sensitization to a whole host of allergens/antigens.74
The epidermis does not merely function as a physical barrier but is likely also the critical initiator of type 2 immune responses (Fig 5). This function is in part mediated by the extensive repertoire of pattern recognition receptors expressed on barrier epithelial cells coupled with their strategic location at the interface of the microenvironments and macroenvironments.70,75 The exposure of barrier-disrupted epidermis to allergens or microbes, many of which signal through receptors relevant for innate immune responses, leads to production of chemokines and alarmins (TSLP, IL-33, and IL-25) that instruct immature dendritic cells and activate ILC2s, key drivers of a type 2 immune response.75 Importantly, enhanced type 2 immune activation downregulates the expression of epidermal differentiation complex and TJ proteins, innate immune responses, and antimicrobial peptides.
In summary, initial studies of epidermal cells from patients with AD focused on characterizing them for their “leaky” nature, but they are no longer viewed as simply “structural” cells but rather the key cells that determine the character and amplitude of the innate and adaptive immune response to environmental signals.
2.2. What is the role of type 2 immune responses in modulating the skin barrier in patients with AD?
Recent studies suggest that the atopic march is most apparent in patients with AD and allergen sensitization.11 Donald Leung noted that these observations are consistent with studies demonstrating that epicutaneous allergen sensitization associated with type 2 immune activation predisposes to FA.76 In this regard, by using a novel minimally invasive skin tape-tripping technique to examine epidermal protein profiles, TSLP can be detected in the epidermis at 2 months of age, more than 1 year before the development of clinical AD at 24 months.77 The skin-tape transcriptome in patients with nonlesional AD has revealed that 50% of patients with AD express high-level TH2 cytokines, which have been demonstrated to reduce expression of FLG and other epidermal differentiation proteins that contribute to severity of AD skin disease.78,79 The high type 2 endotype is also associated with increased expression of IL-4 receptor (IL-4R) on keratinocytes. Lipidomics studies show that AD skin has short-chain rather than long-chain fatty acids, and this might be the result of IL-13 overexpression.80 Taken together, there is increasing evidence that immune-driven abnormalities in the skin barrier can be detected in nonlesional AD skin before onset of clinical AD. This presents a potential opportunity to intervene in skin barrier dysfunction before onset of clinical skin disease and epicutaneous allergen sensitization.
2.3. Are there differences in AD phenotypes between adults and children?
The pathogenesis of AD in adults appears to be driven by TH2 and also by TH22 cytokines.81–83 These cytokines have been shown in vitro to have effects simulating the AD phenotype through downregulation of barrier differentiation proteins, such as FLG and lipid species (IL-4/IL-13 and IL-22), inhibiting antimicrobial peptides (IL-4/IL-13) and inducing epidermal hyper-plasia (IL-22). Recent data from adults with moderate-to-severe AD suggest that the spectrum of AD comorbidities expands well beyond allergic conditions (eg, allergic asthma), with increases in cardiovascular and other comorbidities likely reflecting a high level of systemic immune activation (T cells, B cells, and circulating cytokines) in the blood.83 The systemic immune abnormalities in adults with chronic moderate-to-severe AD are also reflected by widespread nonlesional skin abnormalities.83–85
However, these paradigm-shifting discoveries are based on adult AD, and the factors that initiate AD in children are not well understood. Indeed, infants and adults have a different distribution of lesions.
Emma Guttman-Yassky and colleagues have recently compared the skin and blood of patients with moderate-to-severe adult AD with that of children with early-onset AD who were less than 5 years old and within 6 months of disease initiation but with similar disease severity.86–90 They found potent and early TH2 activation in both the blood and skin compartments of children with AD, establishing that the systemic nature of new-onset disease in children is similar to adults.86 The selective activation of the TH2 axis in blood can direct B cells toward IgE class-switching, explaining the systemic atopic consequences of AD. Although in blood early AD begins mainly as TH2 polarized, it has a more complex skin phenotype than adult AD with activation of TH2, TH9, and TH17/TH22 but little TH1 polarization.86 Pediatric nonlesional AD skin is already hyperplastic, accompanied by significant inflammation and activated cytokines to levels often greater than those in adults, indicating that the clinical manifestation of AD is the tip of an iceberg that reflects abnormalities affecting the entire integument.86 Importantly, FLG deficiency of adult AD is not present in early pediatric AD, challenging the notion of FLG as central for disease elicitation and the instigator of the atopic march.86
Because AD is considered a window to the atopic march, the 2 important questions are whether AD can be prevented in high-risk children using skin barrier or other modifications and whether in children that already have moderate-to-severe AD, the atopic march can be prevented using immune (either broad or narrow T-cell targeting) manipulations. Clinical trials with targeted therapeutics against the TH2, TH22, and TH17/IL-23 pathways are needed to clarify the relative contribution of each cytokine axis to the disease phenotype in both adults and importantly also in children.23,91 In adults TH2 and TH22 targeting seem to provide therapeutic benefit.92–94 Inhibition of more than 1 cytokine axis might be needed to fully resolve the AD phenotype, particularly in children.
2.4. Immune regulation of itch in patients with AD
Itch is a central and debilitating feature of AD, and scratching in response to itch exacerbates allergic skin inflammation. Therefore understanding the pathogenesis of itch-induced epithelial injury in AD is of paramount importance in both understanding and preventing the progression of allergic disease. Classically, it was established that adaptive TH2 cells, through production of the type 2 cytokines IL-4 and IL-13, promote AD pathogenesis. Studies by Brian Kim and colleagues in 2013–2014 identified novel contributions of innate type 2 cytokine–producing immune cells, such as basophils and ILC2s, to the pathogenesis of AD in both mice and human subjects.95,96 Although these cells clearly promote skin inflammation in patients with AD, how itch arises in this context has not been well studied. Recent clinical trials with dupilumab, an anti–IL-4Rα mAb that blocks signaling of both IL-4 and IL-13, have demonstrated rapid improvement of itch in patients with AD.92,93 Anti–IL-31 mAb has also been found to reduce itch.97 However, the molecular mechanisms underlying these clinical outcomes remain unknown.
In addition to their known proinflammatory functions, IL-4, IL-13, and IL-31 contribute to chronic itch through direct effects on sensory neurons. Specifically, it was found that sensory neurons were activated by IL-4 and IL-13 involving itch-sensory pathways in mice and exhibited neural hypersensitivity to a number of pruritogens. Furthermore, IL-4 can activate human sensory neurons, suggesting that neuronal type 2 cytokine signaling contributes to itch across species. Conditional deletion of IL-4Rα from sensory neurons in mouse models has demonstrated a previously unrecognized mechanism through which neuronal IL-4Rα critically mediates chronic itch. Based on IL-4Rα signaling biology, it was hypothesized that type 2 cytokine–mediated activation of sensory neurons is dependent on neuronal Janus kinase (JAK) signaling. Consistent with this hypothesis, both pharmacologic JAK inhibition and sensory neuron–specific genetic deletion of JAK1 resulted in abatement of chronic itch in mice.98 Thus signaling mechanisms previously ascribed to the immune system might represent novel therapeutic targets (ie, JAK1) within the nervous system and might have functional implications at epithelial barrier surfaces beyond the skin. In other murine studies keratinocyte-derived TSLP acted directly on a subset of sensory neurons to trigger a robust itch response.99 Collectively, studies by Brian Kim and others reveal an evolution-arily conserved paradigm in which the sensory nervous system uses classical immune signaling pathways to influence mammalian behavior.
Key conclusions
AD is a complex heterogeneous skin disease characterized by skin barrier defects and both local and systemic inflammation that affects infants, children, and adults. The complexity of AD is further increased by environmental exposures, varying levels of severity, and racial differences in skin immune responses.
The epidermal dysfunction observed in both clinically affected and unaffected AD skin suggests that the entire skin integument of patients with AD might be permissive to local and systemic allergen sensitization.
There is mounting evidence that sensitization to allergens through inflamed AD skin might lead to allergic diseases that manifest in other organs, such as the gastrointestinal tract (FA) and upper or lower airways (asthma and allergic rhinitis, respectively); definitive evidence for this concept is needed.
Although FLG-null mutations are strongly associated with AD, FA, and the atopic march, they are only observed in a minority of subjects undergoing the atopic march. Other factors that can contribute to deficiency in skin FLG levels, including immune activation, altered gene copy number, and epigenetic modification of FLG, warrant further investigation.
Workshop recommendations
Identify endotypes and phenotypes of AD by using a longitudinal infant cohort study and determine which are predictive of atopic disease progression
Determine whether the sequence from AD to FA and to airway allergic diseases reflects a causal relationship or whether this disease pathway is based on a shared genetic background
Use minimally invasive approaches to characterize the skin of young infants, including TEWL, proteomic, lipidomic, and transcriptomic analyses of skin tape strips and skin microbiome analyses
Determine the changes in skin barrier abnormalities and lesional/nonlesional skin inflammation over time, as opposed to one snapshot in time
Determine whether barrier abnormalities are inherent or whether they can also be influenced by low-grade inflammation
Further investigate the association between neonatal TEWL levels, food sensitization, and FA at 2 to 3 years of age to determine whether TEWL can be used as a single parameter for intervention studies59
Further characterize the systemic inflammatory effects of AD and their relationships to atopic and nonatopic comorbidities
Further characterize FLG mutations and their association with AD in diverse ethnic groups
Develop individualized therapeutic approaches for AD based on well-defined disease endotypes
3. MICROBIOME DISTURBANCES IN PATIENTS WITH AD AND FA
3.1. Skin microbiome shifts in patients with AD
Dr Heidi Kong provided an overview of microbial alterations and their significance in patients with AD. In addition to the critical importance of the skin barrier, host genetics, and immune system in the pathogenesis of AD, associations between AD and the increased frequency of S aureus skin colonization and infections, as well as susceptibility to herpes simplex virus skin infections, have been well documented by many studies. In addition, there has been considerable interest in determining how micro-biota might play a role in preventing, eliciting, and/or exacerbating atopic disorders.100 In conjunction with traditional cultivation methods, microbiome sequencing has advanced how human microbiota can be studied in the context of atopy. Targeted sequencing of regions of the bacterial 16S ribosomal RNA gene has highlighted the notable changes in skin microbes in patients with AD, specifically demonstrating reduced bacterial diversity in combination with shifts in the relative abundances of both S aureus and Staphylococcus epidermidis during disease flares.101 The more complex sequencing method of shotgun metagenomics has also been used to more broadly study global microbial communities (bacteria, fungi, and viruses) in patients with AD. Shotgun metagenomics has enabled analyses of the genomic functional potential and bacterial strain differences in patients with AD compared with healthy control subjects, noting that strains derived from patients with AD can be distinct from bacterial strains obtained from other sources.102,103
Although extensive investigations seek to understand how microbes might contribute to the development or worsening of disease, other research has considered whether microbiota can have beneficial effects in preventing or ameliorating AD or other atopic disorders.104,105 In the same context the question of whether microbiome manipulations can prevent the progression of AD to other atopic diseases is becoming relevant. Of note, most of the published microbiome research findings in atopic patients remain correlative, highlighting the need for further studies to test for possible causality.
3.2. The complex relationship between S aureus and AD
Dr Alan Irvine summarized the mechanisms of colonization of AD skin with S aureus and the mechanisms through which S aureus can exacerbate AD. S aureus is frequently isolated from the skin of patients with AD, with colonization rates of 70% in patients with AD compared with 30% of unaffected subjects.106 The density of S aureus colonization is directly related to the severity of AD.107 Patients with AD and S aureus colonization often have a greater skin barrier defect than patients with AD without S aureus, decreased expression of occludin, and increased access of dendritic cell dendrites to the SC. S aureus strains isolated from patients with AD show differences to those isolated from unaffected carriers; clonal complex (CC1) strains are enriched among patients with AD, whereas the CC30 strains most frequently isolated from nasal carriers in the healthy population are less common in patients with AD.108
AD skin is permissive for S aureus colonization. The antimicrobial defensin peptides LL-37, β-defensins, and dermicidin are present at reduced levels in AD skin. One mechanism underlying this effect is the known inhibition of IL-4 and IL-13 on human β-defensin 2 and human β-defensin 3 gene expression.109 S aureus species grow poorly in acidic conditions but much better in the alkaline conditions seen in patients with AD.110 S aureus isolated from patients with AD bind more strongly to intact AD skin and also to standard binding assays than S aureus isolated from unaffected subjects.108,111 In patients with established AD, FLG deficiency, either genetic or acquired from type 2 skewing, leads to irregular or deformed corneocytes.112 S aureus isolates from patients with AD also bind more strongly to these corneocytes compared with isolates from unaffected control subjects in a clumping factor B-dependent fashion.108
Once AD skin is colonized, S aureus can drive disease severity or disease flares through several mechanisms. S aureus expresses several molecules that contribute to disease activity. These include soluble toxins, such as d-toxin, that directly stimulate mast cells (MCs) and cause increased IgE levels,113 and α-toxin, a pore-forming toxin that directly causes cellular damage in keratinocytes with a resultant effect in skin barrier function and possible effects on susceptibility to viral infection.114 When solubilized, the cell wall–bound protein A triggers inflammatory responses from keratinocytes through the TNF receptor. Staphylococcal superantigens, such as staphylococcal enterotoxin (SE) A, SEB, and SEC and toxic shock syndrome toxin 1 trigger B-cell expansion and cytokine release.115 Finally, proinflammatory staphylococcal lipoproteins induce TSLP expression in primary human keratinocytes in a Toll-like receptor 2/6–dependent manner, identifying another possible mechanism through which S aureus induces a TH2 response.116 Both of these mechanisms, barrier disruption and TH2 induction, make FA development more likely.117
3.3. Contributions of gut dysbiosis and regulatory T-cell reprogramming in patients with FA
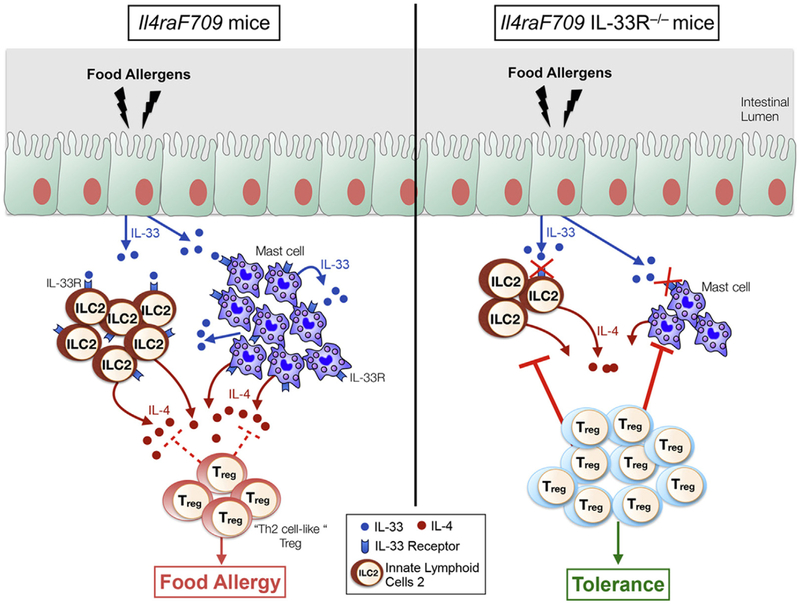
In addition to skin dysbiosis, there is increasing evidence from animal and human studies that gut dysbiosis can play a role in the etiology of atopic diseases, particularly FA.118 Dr Talal Chatila and colleagues have investigated mechanisms of oral tolerance breakdown in FA in mouse models and in human subjects with a focus on the contributions of regulatory T (Treg) cell dysfunction and gut dysbiosis. In earlier studies using a murine transgenic model (Il4raF709) of FA involving a gain-of-function mutation in the IL-4R α chain, they demonstrated that FA is associated with reprogramming of Treg cells into TH2-like cells that play an essential role in disease pathogenesis.119 Treg cell TH2 cell–like reprogramming was associated with decreased expression of TGF-β1 by Treg cells, an effect mediated by Treg cell–intrinsic IL-4/IL-4R signaling. Treg cell–specific deletion of a single Tgfb1 allele promoted FA, whereas expression of a Tgfb1 transgene protected against FA, which is consistent with a role for decreased Tgfb1 expression in mediating the pathogenic effects of TH2 cell–like reprogramming of Treg cells. More recently, using the same experimental system, they showed that IL-4 production by IL-33–stimulated ILC2s promotes FA by inducing Treg cell reprogramming to TH2-like cells. These reprogrammed Treg cells are functionally impaired and unable to control ILC2 and MC expansion and activation (Fig 6).120
FIG 6.
In the Il4raF709 model IL-33–induced IL-4 production by ILC2s plays a crucial role in enabling sensitization to food allergens by promoting production of TH2 cell–like Treg cells that have impaired Treg cell function.120
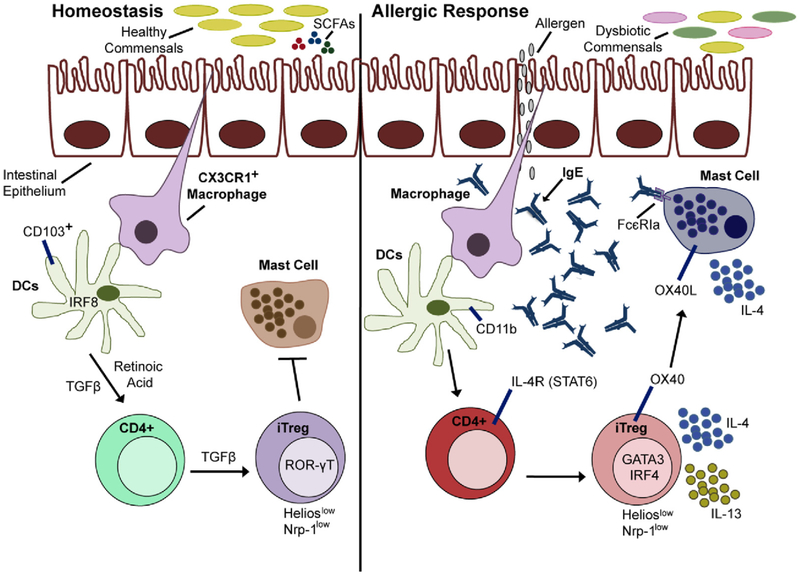
In addition to Treg cell reprogramming, Chatila and colleagues showed that dysbiosis is another factor relevant to tolerance breakdown and pathogenesis in experimental FA.118,121 They also have evidence that infants with FA exhibit dysbiosis that evolves dynamically over time. To test the hypothesis that treatment with immunomodulatory bacteria promotes tolerance in patients with FA, they designed minimal consortia of human Clostridiales and Bacteroides commensals for introduction into germ-free and conventional control and Il4raF709 mice. Both the Clostridiales and Bacteroides consortia, but not one composed of Proteo-bacteria, protected mice from having FA and cured established disease. Both consortia suppressed pathogenic TH2 cell–like reprogramming of gut Treg cells in Il4raF709 mice. These results support a link between gut dysbiosis and Treg cell impairment in patients with FA and suggest that therapy with minimal consortia of immunomodulatory bacteria could be therapeutically beneficial in a clinical setting (Fig 7). Although dysbiosis has been reported in some human studies of FA, its reproducibility and role in disease pathogenesis remains to be established.121,122
FIG 7.
Commensals can have essential functions in oral tolerance. Dysbiotic commensals can promote pathogenic TH2 cell–like reprogramming of gut Treg cells and MC dysregulation leading to FA. DCs, Dendritic cells; IRF, interferon regulatory factor; iTreg, induced Treg cell; OX40L, OX40 ligand; ROR-γT, retinoic acid–related orphan receptor γT; STAT6, signal transducer and activator of transcription 6. Figure courtesy of Dr Talal Chatila.
Recent studies in human infants and mouse models suggest that gut bacterial dysbiosis early in life might affect the development of the immune system and play a role in the subsequent development of FA.122 Further studies are needed to identify clinically beneficial bacteria for manipulation of the gut microbiota. Dietary and probiotic interventions might be part of future preventative and therapeutic interventions in patients with FA.122
Workshop recommendations
Determine optimal approaches and time points for studying the infant gut, skin, and airway microbiome: such studies should include examination of the mycome and virome.
Identify dysbiotic bacteria and determine how they contribute to atopy
Determine whether microbiome shifts are a cause or a consequence of AD, FA, or both
Define the mechanisms involved in the proallergic or protolerogenic functions of the microbiota in the context of FA
Investigate the role of S aureus in the development of FA, including the effects of SEB on tolerance in the gut and tissue homing of T cells
Determine whether gut microbial changes and Treg cell plasticity are features of AD or FA in human subjects at any age
4. USE OF MOUSE MODELS TO INVESTIGATE HOW EPICUTANEOUS SENSITIZATION TO FOOD ALLERGENS AND AEROALLERGENS LEADS TO REACTIVITY IN THE GUT AND AIRWAY
4.1. Roles of TSLP, IL-33, and IL-25 in epicutaneous sensitization
Although there is a well-recognized clinical association between AD and FA and evidence that sensitization to peanut in human subjects can occur through the skin after exposure to peanut oils and peanut-containing house dust, the immunologic mechanisms underlying epicutaneous sensitization to allergens and the communication routes between the skin and other mucosal surfaces are incompletely understood.123–126 Skin barrier defects, which are discussed in the previous sections, are thought to be important in both the initial local events that lead to AD and the systemic sensitization to allergens that predisposes to atopic diseases at other mucosal sites, such as the gastrointestinal tract and airway. Skin damage caused by scratching in response to the persistent skin itchiness characteristic of AD can also contribute to allergen entry and sensitization.127 TSLP, IL-33, and IL-25 are 3 epithelial cell–derived cytokines produced in human subjects and mice in response to a variety of stimuli that play prominent and complex roles in the innate and adaptive immune responses to allergens. The mechanisms by which these alarmins contribute to allergen sensitization in the skin and initiate crosstalk between the skin, gut, and airway are the subject of intense investigation.128 Mouse models that simulate skin inflammation and AD have been particularly useful for investigating TSLP, IL-33, and IL-25 interactions and identifying target populations and downstream signaling events.128–130
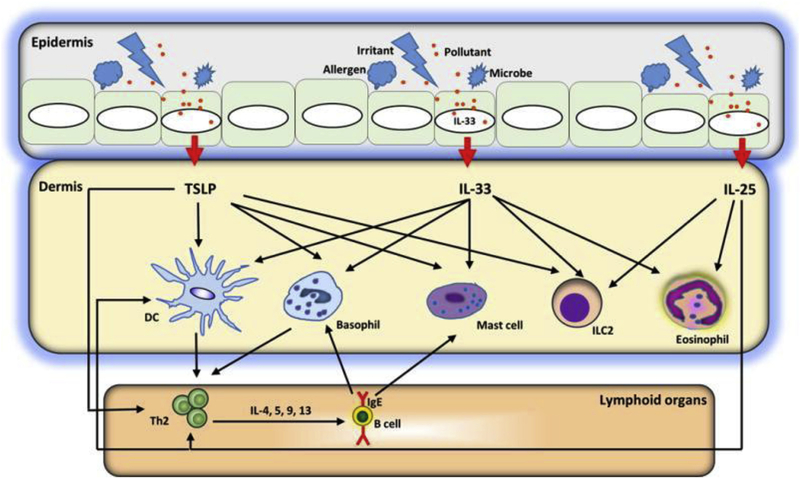
Dr Steve Ziegler and colleagues tested the functional role of IL-33 and the interplay between IL-33 and TSLP in a model of intradermal administration of ovalbumin (OVA) with either TSLP or IL-33, followed by oral allergen challenge.131,132 They found that both TSLP and IL-33 were capable of promoting sensitization to OVA, and sensitized mice had gastrointestinal inflammation and anaphylaxis after oral challenge.128 Importantly, TSLP-dependent sensitization required TSLP-responsive, skin-resident dendritic cells and IL-33 expression by keratinocytes. However, OVA sensitization mediated by IL-33 was TSLP independent. Gastrointestinal allergic responses in TSLP-sensitized mice also were TSLP independent but required IL-25 signaling. Interestingly, as was seen in the skin, IL-33 was the dominant cytokine because IL-33 blockade after sensitization alleviated gastrointestinal responses.128 Taken together, these data demonstrate that IL-33 signaling is required for allergic response to food after epicutaneous sensitization, with IL-33 being downstream of TSLP in the skin and downstream of IL-25 in the gut (Fig 8).128
FIG 8.
A model of barrier disruption and skin sensitization. Allergens, infections, and tissue damage can stimulate release of TSLP, IL-33, and IL-25 from the epithelium. These epithelial cell–derived cytokines license dendritic cells (DCs) to drive type 2 responses but also act on a variety of cell types, including basophils, eosinophils, MCs, and innate lymphoid cells, to initiate and maintain allergic inflammation.128
The same IL-33–dependent skin sensitization model was used to successfully induce an airway response after nasal allergen challenge.133 However, unlike the gut response, IL-33 was not needed for the allergen-induced inflammatory airway response. These data support the hypothesis that antigen sensitization through a disrupted skin barrier might be responsible for development of FA or airway allergy and indicate that only gut reactions are dependent on IL-33.
Dr Raif Geha also discussed the role of IL-33 in MC-dependent food anaphylaxis. In earlier studies he and his colleagues reported that oral antigen challenge of mice sensitized epicutaneously by means of application of OVA to tape-stripped skin resulted in IgE-dependent systemic anaphylaxis that was quantitated by its effects on body temperature.127,130 In subsequent studies they showed that IL-33 is produced and released locally and systemically in response to skin injury in both mice and human subjects.134 Systemic IL-33 can have downstream effects on a variety of IL-33 receptor (IL-33R)–expressing cells, including MCs, which play a critical role in food-induced anaphylaxis. By using their model system and wild-type, IL-33R–deficient, and MC-deficient mice, Dr Geha and colleagues investigated the role and mechanism of action of IL-33 in mice with MC-dependent anaphylaxis.134 These studies showed that IL-33 enhances IgE-mediated MC degranulation and cytokine production in vitro, indicating direct effects of IL-33 on MCs. IL-33R deficiency or IL-33R blockade significantly reduced the severity of the anaphylactic response after epicutaneous sensitization and oral challenge, supporting a critical role for IL-33/IL-33R signaling in oral anaphylaxis. Notably, IL-33R deficiency had no effect on production of OVA-specific IgE, TH2 cytokine production, or MC proliferation. A similar requirement for IL-33 was observed in recall anaphylactic responses to OVA, as well as in an IgE-dependent passive anaphylaxis model. The relationship between IL-33 and MC activation in vivo was explored in MC-deficient mice. Oral anaphylaxis was abrogated in these mice but could be restored by reconstitution with wild-type bone marrow–derived mast cells, but not IL-33R–deficient bone marrow–derived mast cells, demonstrating the importance of IL-33/IL-33R engagement on MCs for their activation.
In summary, these studies demonstrated that IL-33 promotes the effector phase in IgE- and MC-dependent food anaphylaxis through direct interaction with IL-33Rs on MCs and induction of MC activation and degranulation. Because patients with AD reportedly have increased levels of IL-33 in the skin,135 IL-33 might be an important link between cutaneous sensitization to food allergens and food-induced anaphylaxis and a potential therapeutic target.
In a third mouse model of epicutaneous sensitization, Dr Cecilia Berin and colleagues used peanut antigen to determine the contribution of epicutaneous allergen exposure and host factors to the generation of TH2-skewed immunity.136 C3H mice exposed topically to peanut on abdominal skin (prepared with depilatory cream) generated a robust IgE and IgG1 response to peanut and experienced anaphylaxis after oral peanut challenge. This was not observed with control legumes, such as soy or green bean, but was observed with the tree nut cashew. Soy or green bean could elicit sensitization when applied with adjuvants, such as cholera toxin or SEB, suggesting that, in contrast to some other foods, peanut has inherent adjuvant factors that could promote sensitization. This was formally shown by the ability of peanut to elicit sensitization to a bystander antigen (β-lactalbumin) that did not elicit sensitization on its own. Topical peanut induced IL-33 production by keratinocytes, which acted on skin-draining dendritic cells to drive a TH2-polarized response in the draining lymph nodes. These results demonstrate that activation of innate pathways in the skin by peanut proteins contributes to the generation of peanut allergy.136
Whether epicutaneous sensitization to peanut promotes sensitization to other food allergens or aeroallergens in human subjects remains to be determined. In support of this possibility, a study of atopic phenotypes identified by latent class analyses at age 2 years revealed 2 classes in which a high incidence of peanut sensitization was associated with sensitization to 1 or more aeroallergens.137 On the other hand, early introduction of peanut and prevention of peanut allergy in the LEAP study had no effect on the incidence of aeroallergen sensitization or allergic airway disease.21
4.2. Regulation of epicutaneous responses to food allergens
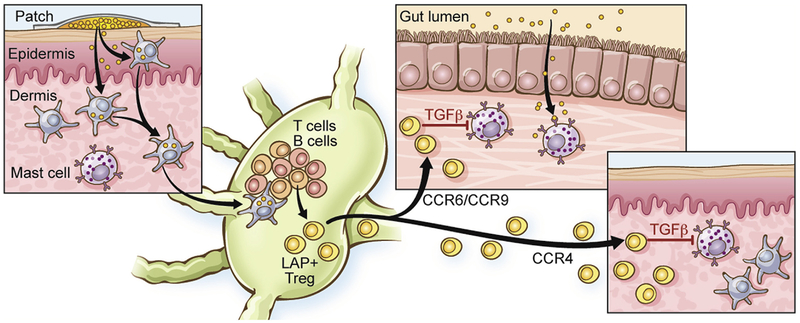
In other studies Berin and colleagues examined the capacity of the skin to generate immune tolerance to food allergens.138 In an OVA-driven model of anaphylaxis generated by epicutaneous sensitization, as described above for peanut, they observed that topical application of allergen with Viaskin patches (epicutaneous immunotherapy [EPIT]) protected mice from anaphylaxis, and mice remained protected 2 weeks after termination of treatment. In contrast, treatment of mice with oral immunotherapy resulted in transient protection that was lost after treatment was terminated. EPIT expanded OVA-specific latency-associated peptide (LAP) positive, forkhead box P3–negative Treg cells in the draining lymph nodes, and these Treg cells uniquely expressed the gut-homing receptor CCR9 in addition to CCR4 and CCR6 (Fig 9).138 Clinical protection was not associated with changes in antibody responses, but instead, the gut-homing Treg cells directly suppressed the activation of MCs through a TGF-β–dependent mechanism. This regulatory response to EPIT was also observed in peanut-sensitized mice treated with peanut EPIT.138
FIG 9.
Immune communication between the skin and gut and tolerance induction determined by using EPIT. Epicutaneous exposure to peanut results in generation of latency-associated peptide (LAP)+ Treg cells with gut- and skin-homing receptors that suppress MCs in the gut and skin through a TGF-β–dependent mechanism.138
These results indicate that topical exposure to clinically relevant food allergens can lead to sensitization or tolerance, depending on the context of allergen exposure. Important contributing factors can include site of antigen exposure and dose that might determine which antigen-presenting cells of the skin acquire and present antigen. Further studies are needed to delineate how factors present during antigen presentation control the balance between sensitization and tolerance in the skin.
In summary, the 3 animal models presented at the workshop and other published work provide compelling evidence that epicutaneous sensitization to allergens occurs and can lead to downstream hypersensitivity reactions at distant barrier sites, such as the gut and airway, after local allergen challenge. Further studies are needed to identify the cell populations and signaling pathways that link the skin and mucosal barrier surfaces.
Workshop recommendations
Further define the cell types and signaling cascades involved in cutaneous and intradermal sensitization to food and aeroallergens
- Delineate the specific pathways through which cutaneous sensitization results in gut or airway sensitization and juxtapose mechanisms of cutaneous to direct oral or airway sensitization; for example:
- determine whether tissue-resident allergen-specific memory T and B cells exist in the skin and at mucosal surfaces and whether they are clonally related and
- determine where allergen-specific IgE-producing plasma cells reside
Determine whether keratinocyte-derived IL-33 is the sole orchestrator of crosstalk between the skin, gut, and airway by using mouse models and early intervention in human allergic disease
Define normal tolerance mechanisms in the skin and determine how topical antigen exposure can induce tolerance rather than sensitization to allergens
Establish how Treg cells maintain homeostasis in the skin and at mucosal surfaces
Determine the molecular basis of the adjuvant activity of peanut and the mechanism of epitope spreading
Further investigate the potential connection between peanut sensitization, other allergies, and the atopic march
5. BIOMARKERS TO IDENTIFY SUBJECTS AT HIGH RISK FOR THE ATOPIC MARCH
Two striking messages from the workshop were the narrow interventional window for preventing AD and allergen sensitization and the paucity of reliable biomarkers to identify those at high risk of progression through the various stages of the atopic march. Although there is agreement on the importance of severity of AD, increased TEWL, and polysensitization in infancy and childhood in atopic disease progression, there is a particular need to identify biomarkers/risk factors in early infancy that predict the development of AD and FA and are upstream of multisensitization to food allergens.139
Workshop recommendations
Undertake profiling of the skin using skin tape strips and multiomics approaches to look at protein, lipid, and RNA signatures in neonates and infants before and after development of AD
Investigate the utility of profiling foreskin and cord tissue
Validate the use of TEWL measurements in neonates as a predictor of AD and FA
Perform unsupervised sequential analysis of infant blood for cell types, proteomics, transcriptomics, and metabolomics
Undertake sequential detailed immune profiling of the blood, including CyTOF analyses of adaptive and innate immune cells, cytokine profiles, serology, and evolution of allergen-specific T and B cells and Treg cells
Analyze the skin and gut microbiomes from birth onward, including consideration of the effects of mode of delivery and breast-feeding
Investigate potential maternal effects that might increase atopy in the infant, including skin barrier abnormalities
6. INTERVENTIONS TO PREVENT OR INTERVENE IN THE ATOPIC MARCH
The workshop ended with a panel discussion on primary interventions to prevent the development of AD and the atopic march and secondary interventions to modulate AD and reduce the risk of sensitization through damaged inflamed skin and to prevent disease progression once sensitization has occurred. Topics covered included the merits of local versus systemic and single versus combination interventions, choices of cells or effector molecules to be targeted, optimal times to intervene, and duration of intervention. There was general agreement that thus far the effects of emollients on AD prevention are encouraging in high-risk populations at early age time points.52,53,63 Because early-onset aggressive AD is most strongly associated with risk of FA, it was suggested that AD in infants be treated aggressively and prophylactically if there is any evidence of inflammation. It is important to assess whether this might reduce the prevalence of food allergies and, subsequently, respiratory allergy. Longitudinal birth cohort clinical trials will be needed to determine whether these early interventions affect the development of FA and atopic airway disease.
The advantages and disadvantages of future use of monoclonal therapeutics, including omalizumab, dupilumab, and antibodies to innate cytokines for AD prevention and treatment in infants, was discussed. Although cost and feasibility are major concerns, if the atopic march, or at least FA, can be prevented with omalizumab or dupilumab early in life, less expensive drugs might be developed in the future that target similar pathways. It was also suggested that reducing IgE levels and blocking IL-4 responses in sensitized but nonallergic infants might provide a window to safely introduce all the allergenic dietary foods early, induce tolerance, and prevent development of FA. This approach might also be useful for improving the safety and efficacy of oral immunotherapy in infants and older subjects who already have FA. Although there was support for using omalizumab in infants, it was agreed that once the treatment is stopped, the natural course of the FA or other allergic disease will not change.
Workshop recommendations
Prevention of AD and its atopic consequences (atopic march):
Further investigate the effectiveness of early prophylactic treatment of infants with emollients or other skin barrier protectors for the prevention of AD, FA, and respiratory allergy and determine the mechanisms of action: pilot studies suggest the efficacy of this approach.53
Investigate the effectiveness of early, topical, anti-inflammatory treatment of infants with AD in preventing FA and respiratory allergy
Using interventions that block type 2 immune skewing and inflammation, conduct trials very early in high-risk children with AD to prevent allergen sensitization and further progression of the atopic march
Determine whether allergen immunotherapy plus anti–type 2 immunity biologics offer superior results in preventing disease progression in children sensitized to aeroallergens or food allergens compared with immunotherapy alone
Develop reliable biomarkers to track and predict the effectiveness of long-term preventative and therapeutic interventions
Abbreviations used
- AD
Atopic dermatitis
- COAST
Childhood Origins of Asthma
- EPIT
Epicutaneous immunotherapy
- FA
Food allergy
- FLG
Filaggrin
- ILC2
Group 2 innate lymphoid cell
- IL-4R
IL-4 receptor
- IL-33R
IL-33 receptor
- JAK
Janus kinase
- LEAP
Learning Early About Peanut Allergy
- MC
Mast cell
- OR
Odds ratio
- OVA
Ovalbumin
- SC
Stratum corneum
- SE
Staphylococcal enterotoxin
- TEWL
Transepidermal water loss
- TJ
Tight junction
- Treg
Regulatory
- TTSLP
Thymic stromal lymphopoietin
- URECA
Urban Environment and Childhood Asthma
Footnotes
Disclosure of potential conflict of interest: D. Y. M. Leung has received personal fees from Regeneron Pharmaceuticals and Sanofi-Genzyme Pharmaceuticals and has received grants from MedImmune Pharmaceuticals, Pfizer Pharmaceuticals, and Incyte Corporation. L. A. Beck has received grants from Abbvie, Realm Therapeutics, Regeneron, and Pfizer; has received personal fees from Abbvie, Astra-Zeneca, Allakos, Boehringer Ingelheim, Celgene, Eli Lilly, GlaxoSmithKline, Leo Pharma, Novan, Novartis, Realm Therapeutics, Regeneron, and Sanofi; and has received stock from Pfizer and Medtronics. W. W. Busse has received personal fees from 3M, Boehringer Ingelheim, Boston Scientific, AstraZeneca, GlaxoSmithKline, Novartis, Sanofi/Genzyme, Teva, Genentech, Elsevier, Medscape, ICON Clinical Research, Regeneron, and PrEPBiopharm. T. A. Chatila is a member of the Scientific Advisory Board for Consortia Therapeutics, has received a grant from the National Institutes of Health (NIH; 5 R01 AI126915), and has a patent pending for Therapeutic microbiota for the treatment and/or prevention of food allergy (US20180117098A1). J. E. Gern has received a grant from the NIH/National Institute of Allergy and Infectious Diseases (NIAID); has received personal fees from PREP Biopharm, Regeneron, and MedImmune; has received stock from Melissa Vaccines; and has patents pending for Methods of Propagating Rhinovirus C in Previously Unsusceptible Cell Lines and Adapted Rhinovirus C. E. Guttman-Yassky has received grants and personal fees from Dermavant, DS Biopharma, Galderma, Glenmark, LEO Pharmaceuticals, Novartis, Pfizer, Regeneron Pharmaceuticals, and Union Therapeutics; has received grants from Dermira, Innovaderm, Novan, Ralexar, and Janssen Biotech; and has received personal fees from Eli Lilly, Escalier, Kyowa Kirin, Mitsubishi Tanabe, Sanofi, DBV, EMD Serono, and Flx Bio. A. D. Irvine has received personal fees from Sanofi/Regeneron. B. S. Kim has received grants from the NIH, the Doris Duke Charitable Foundation, LEO Pharma, and Celgene; has received personal fees from Abb-Vie, Concert, Celgene, Kiniksa, Menlo, Pfizer, Regeneron, Sanofi, Theravance, Incyte, and Nuogen Pharma; has a patent pending (US Provisional Application no. 62/295,875); and owns personal stock in Gilead and Mallinckrodt. G. Lack reports grants from the NIAID/NIH) and the UK Food Standards Agency (FSA); other support from Food Allergy Research & Education (FARE), MRC & Asthma UK Centre, the UK Department of Health through NIHR, the National Peanut Board (NPB), and Osem during the conduct of the study; and other support from DBV Technologies and Mighty Mission Me outside the submitted work. K. C. Nadeau reports grants from NIAID; other support from Novartis, personal fees from Regeneron; grants from FARE and EAT; and other support from Sanofi, Astellas, Nestle, BeforeBrands, Alladapt, ForTra, Genentech, AImmune Therapeutics, and other from DBV Technologies outside the submitted work. E. L. Simpson has received a grant from the NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS; U34 AR065739–02) and Regeneron Pharmaceuticals and has received personal fees from Regeneron Pharmaceuticals. J. M. Spergel reports grants and personal fees from DBV Technologies and Regeneron; grants and other support from the NIH; personal fees from FARE, the American Partnership for Eosinophilic Disorders, Pfizer, Kaleo, and GlaxoSmithKline; and grants from Aimmune Therapeutics. R. A. Wood reports grants from NIAID, Astellas, Sanofi, DBV, Regeneron, and HAL Allergy and royalties from UpToDate. J. A. Woodfolk reports grants from the NIH/NIAID and the NIH/NIAMS and personal fees from Boehringher Ingelheim. S. F. Ziegler has received a grant from the NIH. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest 2004;113:651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bieber T Atopic dermatitis. N Engl J Med 2008;358:1483–94. [DOI] [PubMed] [Google Scholar]
- 3.Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. ISAAC Phase Three Study Group. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol 2009;124: 1251–8.e23. [DOI] [PubMed] [Google Scholar]
- 4.Boguniewicz M, Leung DY. Recent insights into atopic dermatitis and implications for management of infectious complications. J Allergy Clin Immunol 2010;125:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wahn U What drives the allergic march? Allergy 2000;55:591–9. [DOI] [PubMed] [Google Scholar]
- 6.Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol 2003;112(suppl):S118–27. [DOI] [PubMed] [Google Scholar]
- 7.Dharmage SC, Lowe AJ, Matheson MC, Burgess JA, Allen KJ, Abramson MJ. Atopic dermatitis and the atopic march revisited. Allergy 2014;69:17–27. [DOI] [PubMed] [Google Scholar]
- 8.Gough H, Grabenhenrich L, Reich A, Eckers N, Nitsche O, Schramm D, et al. Allergic multimorbidity of asthma, rhinitis and eczema over 20 years in the German birth cohort MAS. Pediatr Allergy Immunol 2015;26:431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider L, Hanifin J, Boguniewicz M, Eichenfield LF, Spergel JM, Dakovic R, et al. Study of the atopic march: development of atopic comorbidities. Pediatr Dermatol 2016;33:388–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill DA, Spergel JM. The atopic march: critical evidence and clinical relevance. Ann Allergy Asthma Immunol 2018;120:131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran MM, Lefebvre DL, Dharma C, Dai D, Lou WYW, Subbarao P, et al. Predicting the atopic march: results from the Canadian Healthy Infant Longitudinal Development Study. J Allergy Clin Immunol 2018;141:601–7.e8. [DOI] [PubMed] [Google Scholar]
- 12.Martin PE, Matheson MC, Gurrin L, Burgess JA, Osborne N, Lowe AJ, et al. Childhood eczema and rhinitis predict atopic but not nonatopic adult asthma: a prospective cohort study over 4 decades. J Allergy Clin Immunol 2011;127: 1473–9.e1. [DOI] [PubMed] [Google Scholar]
- 13.Barnetson RS, Gawkrodger DJ. Eczema and contact dermatitis—pathophysiology In: Holgate ST, Church M, editors Allergy. London: Gower Medical Publishing; 1993. [Google Scholar]
- 14.Belgrave DC, Granell R, Simpson A, Guiver J, Bishop C, Buchan I, et al. Developmental profiles of eczema, wheeze, and rhinitis: two population-based birth cohort studies. PLoS Med 2014;11:e1001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belgrave DC, Simpson A, Buchan IE, Custovic A. Atopic dermatitis and respiratory allergy: what is the link. Curr Dermatol Rep 2015;4:221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Busse WW. The atopic march: fact or folklore? Ann Allergy Asthma Immunol 2018;120:116–8. [DOI] [PubMed] [Google Scholar]
- 17.Sicherer SH, Sampson HA. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol 2018;141:41–58. [DOI] [PubMed] [Google Scholar]
- 18.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-Sponsored Expert Panel Report. J Allergy Clin Immunol 2010;126:1105–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol 2011;127:668–76, e1–2. [DOI] [PubMed] [Google Scholar]
- 20.Malmberg LP, Saarinen KM, Pelkonen AS, Savilahti E, Makela MJ. Cow’s milk allergy as a predictor of bronchial hyperresponsiveness and airway inflammation at school age. Clin Exp Allergy 2010;40:1491–7. [DOI] [PubMed] [Google Scholar]
- 21.Alduraywish SA, Standl M, Lodge CJ, Abramson MJ, Allen KJ, Erbas B, et al. Is there a march from early food sensitization to later childhood allergic airway disease? Results from two prospective birth cohort studies. Pediatr Allergy Immunol 2017;28:30–7. [DOI] [PubMed] [Google Scholar]
- 22.du Toit G, Sayre PH, Roberts G, Lawson K, Sever ML, Bahnson HT, et al. Allergen specificity of early peanut consumption and effect on development of allergic disease in the Learning Early About Peanut Allergy study cohort. J Allergy Clin Immunol 2018;141:1343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Czarnowicki T, Krueger JG, Guttman-Yassky E. Novel concepts of prevention and treatment of atopic dermatitis through barrier and immune manipulations with implications for the atopic march. J Allergy Clin Immunol 2017;139:1723–34. [DOI] [PubMed] [Google Scholar]
- 24.Bergmann RL, Edenharter G, Bergmann KE, Forster J, Bauer CP, Wahn V, et al. Atopic dermatitis in early infancy predicts allergic airway disease at 5 years. Clin Exp Allergy 1998;28:965–70. [DOI] [PubMed] [Google Scholar]
- 25.Marenholz I, Esparza-Gordillo J, Ruschendorf F, Bauerfeind A, Strachan DP, Spycher BD, et al. Meta-analysis identifies seven susceptibility loci involved in the atopic march. Nat Commun 2015;6:8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet 2006;38:441–6. [DOI] [PubMed] [Google Scholar]
- 27.Wahn U, Lau S, Bergmann R, Kulig M, Forster J, Bergmann K, et al. Indoor allergen exposure is a risk factor for sensitization during the first three years of life. J Allergy Clin Immunol 1997;99:763–9. [DOI] [PubMed] [Google Scholar]
- 28.Illi S, von Mutius E, Lau S, Niggemann B, Gruber C, Wahn U, et al. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet 2006;368:763–70. [DOI] [PubMed] [Google Scholar]
- 29.Hatzler L, Panetta V, Lau S, Wagner P, Bergmann RL, Illi S, et al. Molecular spreading and predictive value of preclinical IgE response to Phleum pratense in children with hay fever. J Allergy Clin Immunol 2012;130:894–901.e5. [DOI] [PubMed] [Google Scholar]
- 30.Posa D, Perna S, Resch Y, Lupinek C, Panetta V, Hofmaier S, et al. Evolution and predictive value of IgE responses toward a comprehensive panel of house dust mite allergens during the first 2 decades of life. J Allergy Clin Immunol 2017; 139:541–9.e8. [DOI] [PubMed] [Google Scholar]
- 31.Matricardi PM, Illi S, Keil T, Wagner P, Wahn U, Lau S. Predicting persistence of wheezing: one algorithm does not fit all. Eur Respir J 2010;35:701–3. [DOI] [PubMed] [Google Scholar]
- 32.Kulig M, Bergmann R, Tacke U, Wahn U, Guggenmoos-Holzmann I. Long-lasting sensitization to food during the first two years precedes allergic airway disease. The MAS Study Group, Germany. Pediatr Allergy Immunol 1998;9:61–7. [DOI] [PubMed] [Google Scholar]
- 33.Kapoor R, Menon C, Hoffstad O, Bilker W, Leclerc P, Margolis DJ. The prevalence of atopic triad in children with physician-confirmed atopic dermatitis. J Am Acad Dermatol 2008;58:68–73. [DOI] [PubMed] [Google Scholar]
- 34.Pyun BY. Natural history and risk factors of atopic dermatitis in children. Allergy Asthma Immunol Res 2015;7:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill DA, Grundmeier RW, Ram G, Spergel JM. The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: a retrospective cohort study. BMC Pediatr 2016;16:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinart M, Benet M, Annesi-Maesano I, von Berg A, Berdel D, Carlsen KC, et al. Comorbidity of eczema, rhinitis, and asthma in IgE-sensitised and non-IgE-sensitised children in MeDALL: a population-based cohort study. Lancet Respir Med 2014;2:131–40. [DOI] [PubMed] [Google Scholar]
- 37.Wahn U, von Mutius E. Childhood risk factors for atopy and the importance of early intervention. J Allergy Clin Immunol 2001;107:567–74. [DOI] [PubMed] [Google Scholar]
- 38.Lemanske RF Jr. The childhood origins of asthma (COAST) study. Pediatr Allergy Immunol 2002;(suppl 15):38–43. [DOI] [PubMed] [Google Scholar]
- 39.Gern JE. The Urban Environment and Childhood Asthma study. J Allergy Clin Immunol 2010;125:545–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gern JE, Reardon CL, Hoffjan S, Nicolae D, Li Z, Roberg KA, et al. Effects of dog ownership and genotype on immune development and atopy in infancy. J Allergy Clin Immunol 2004;113:307–14. [DOI] [PubMed] [Google Scholar]
- 41.Singh AM, Evans MD, Gangnon R, Roberg KA, Tisler C, DaSilva D, et al. Expression patterns of atopic eczema and respiratory illnesses in a high-risk birth cohort. J Allergy Clin Immunol 2010;125:491–3.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lynch SV, Wood RA, Boushey H, Bacharier LB, Bloomberg GR, Kattan M, et al. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol 2014;134:593–601.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Connor GT, Lynch SV, Bloomberg GR, Kattan M, Wood RA, Gergen PJ, et al. Early-life home environment and risk of asthma among inner-city children. J Allergy Clin Immunol 2018;141:1468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bacharier LB, Beigelman A, Calatroni A, Jackson DJ, Gergen PJ, O’Connor GT, et al. Longitudinal phenotypes of respiratory health in a high-risk urban birth cohort. Am J Respir Crit Care Med 2019;199:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wood RA, Bloomberg GR, Kattan M, Conroy K, Sandel MT, Dresen A, et al. Relationships among environmental exposures, cord blood cytokine responses, allergy, and wheeze at 1 year of age in an inner-city birth cohort (Urban Environment and Childhood Asthma study). J Allergy Clin Immunol 2011;127: 913–9, e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gern JE, Calatroni A, Jaffee KF, Lynn H, Dresen A, Cruikshank WW, et al. Patterns of immune development in urban preschoolers with recurrent wheeze and/or atopy. J Allergy Clin Immunol 2017;140:836–44.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujimura KE, Johnson CC, Ownby DR, Cox MJ, Brodie EL, Havstad SL, et al. Man’s best friend? The effect of pet ownership on house dust microbial communities. J Allergy Clin Immunol 2010;126:410–2, e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Belgrave DC, Buchan I, Bishop C, Lowe L, Simpson A, Custovic A. Trajectories of lung function during childhood. Am J Respir Crit Care Med 2014;189:1101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fox AT, Sasieni P, du Toit G, Syed H, Lack G. Household peanut consumption as a risk factor for the development of peanut allergy. J Allergy Clin Immunol 2009; 123:417–23. [DOI] [PubMed] [Google Scholar]
- 50.Flohr C, England K, Radulovic S, McLean WH, Campbel LE, Barker J, et al. Filaggrin loss-of-function mutations are associated with early-onset eczema, eczema severity and transepidermal water loss at 3 months of age. Br J Dermatol 2010;163:1333–6. [DOI] [PubMed] [Google Scholar]
- 51.Brough HA, Simpson A, Makinson K, Hankinson J, Brown S, Douiri A, et al. Peanut allergy: effect of environmental peanut exposure in children with filaggrin loss-of-function mutations. J Allergy Clin Immunol 2014;134:867–75.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simpson EL, Chalmers JR, Hanifin JM, Thomas KS, Cork MJ, McLean WH, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol 2014;134:818–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lowe AJ, Su JC, Allen KJ, Abramson MJ, Cranswick N, Robertson CF, et al. A randomized trial of a barrier lipid replacement strategy for the prevention of atopic dermatitis and allergic sensitization: the PEBBLES pilot study. Br J Dermatol 2018;178:e19–21. [DOI] [PubMed] [Google Scholar]
- 54.Wisniewski JA, Agrawal R, Minnicozzi S, Xin W, Patrie J, Heymann PW, et al. Sensitization to food and inhalant allergens in relation to age and wheeze among children with atopic dermatitis. Clin Exp Allergy 2013;43:1160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romeo MJ, Agrawal R, Pomes A, Woodfolk JA. A molecular perspective on TH2-promoting cytokine receptors in patients with allergic disease. J Allergy Clin Immunol 2014;133:952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wisniewski JA, Commins SP, Agrawal R, Hulse KE, Yu MD, Cronin J, et al. Analysis of cytokine production by peanut-reactive T cells identifies residual Th2 effectors in highly allergic children who received peanut oral immuno-therapy. Clin Exp Allergy 2015;45:1201–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wambre E, Bajzik V, DeLong JH, O’Brien K, Nguyen QA, Speake C, et al. A phenotypically and functionally distinct human TH2 cell subpopulation is associated with allergic disorders. Sci Transl Med 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wisniewski JA, Muehling LM, Eccles JD, Capaldo BJ, Agrawal R, Shirley DA, et al. TH1 signatures are present in the lower airways of children with severe asthma, regardless of allergic status. J Allergy Clin Immunol 2018;141: 2048–60.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muehling LM, Turner RB, Brown KB, Wright PW, Patrie JT, Lahtinen SJ, et al. Single-cell tracking reveals a role for pre-existing CCR5+ memory Th1 cells in the control of rhinovirus-A39 after experimental challenge in humans. J Infect Dis 2018;217:381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guilliams M, Dutertre CA, Scott CL, McGovern N, Sichien D, Chakarov S, et al. Unsupervised high-dimensional analysis aligns dendritic cells across tissues and species. Immunity 2016;45:669–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vermeulen EM, Koplin JJ, Dharmage SC, Gurrin LC, Peters RL, McWilliam V, et al. Food allergy is an important risk factor for childhood asthma, irrespective of whether it resolves. J Allergy Clin Immunol Pract 2018;6:1336–41.e3. [DOI] [PubMed] [Google Scholar]
- 62.Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers 2018;4:1. [DOI] [PubMed] [Google Scholar]
- 63.Horimukai K, Morita K, Narita M, Kondo M, Kitazawa H, Nozaki M, et al. Application of moisturizer to neonates prevents development of atopic dermatitis. J Allergy Clin Immunol 2014;134:824–30.e6. [DOI] [PubMed] [Google Scholar]
- 64.Czarnowicki T, Malajian D, Khattri S, Correa da Rosa J, Dutt R, Finney R, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol 2016;137:1091–102.e7. [DOI] [PubMed] [Google Scholar]
- 65.Kelleher M, Dunn-Galvin A, Hourihane JO, Murray D, Campbell LE, McLean WH, et al. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J Allergy Clin Immunol 2015;135:930–5.e1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Kelleher MM, Dunn-Galvin A, Gray C, Murray DM, Kiely M, Kenny L, et al. Skin barrier impairment at birth predicts food allergy at 2 years of age. J Allergy Clin Immunol 2016;137:1111–6.e8. [DOI] [PubMed] [Google Scholar]
- 67.Gao PS, Rafaels NM, Hand T, Murray T, Boguniewicz M, Hata T, et al. Filaggrin mutations that confer risk of atopic dermatitis confer greater risk for eczema herpeticum. J Allergy Clin Immunol 2009;124:507–13, e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McAleer MA, Irvine AD. The multifunctional role of filaggrin in allergic skin disease. J Allergy Clin Immunol 2013;131:280–91. [DOI] [PubMed] [Google Scholar]
- 69.Samuelov L, Sarig O, Harmon RM, Rapaport D, Ishida-Yamamoto A, Isakov O, et al. Desmoglein 1 deficiency results in severe dermatitis, multiple allergies and metabolic wasting. Nat Genet 2013;45:1244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Benedetto A, Rafaels NM, McGirt LY, Ivanov AI, Georas SN, Cheadle C, et al. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol 2011;127:773–86, e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brandner JM, Zorn-Kruppa M, Yoshida T, Moll I, Beck LA, De Benedetto A. Epidermal tight junctions in health and disease. Tissue Barriers 2015;3:e974451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuo IH, Yoshida T, De Benedetto A, Beck LA. The cutaneous innate immune response in patients with atopic dermatitis. J Allergy Clin Immunol 2013;131: 266–78. [DOI] [PubMed] [Google Scholar]
- 73.Yoshida K, Kubo A, Fujita H, Yokouchi M, Ishii K, Kawasaki H, et al. Distinct behavior of human Langerhans cells and inflammatory dendritic epidermal cells at tight junctions in patients with atopic dermatitis. J Allergy Clin Immunol 2014; 134:856–64. [DOI] [PubMed] [Google Scholar]
- 74.De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol 2012;132:949–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity 2015;43:29–40. [DOI] [PubMed] [Google Scholar]
- 76.Wang YH. Developing food allergy: a potential immunologic pathway linking skin barrier to gut. F1000Res 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim J, Kim BE, Lee J, Han Y, Jun HY, Kim H, et al. Epidermal thymic stromal lymphopoietin predicts the development of atopic dermatitis during infancy. J Allergy Clin Immunol 2016;137:1282–5.e4. [DOI] [PubMed] [Google Scholar]
- 78.Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, Debenedetto A, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol 2007;120:150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dyjack N, Goleva E, Rios C, Kim BE, Bin L, Taylor P, et al. Minimally invasive skin tape strip RNA sequencing identifies novel characteristics of the type 2-high atopic dermatitis disease endotype. J Allergy Clin Immunol 2018;141: 1298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Berdyshev E, Goleva E, Bronova I, Dyjack N, Rios C, Jung J, et al. Lipid abnormalities in atopic skin are driven by type 2 cytokines. JCI Insight 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brunner PM, Leung DYM, Guttman-Yassky E. Immunologic, microbial, and epithelial interactions in atopic dermatitis. Ann Allergy Asthma Immunol 2018; 120:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gittler JK, Shemer A, Suarez-Farinas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol 2012;130:1344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brunner PM, Suarez-Farinas M, He H, Malik K, Wen HC, Gonzalez J, et al. The atopic dermatitis blood signature is characterized by increases in inflammatory and cardiovascular risk proteins. Sci Rep 2017;7:8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brunner PM, Emerson RO, Tipton C, Garcet S, Khattri S, Coats I, et al. Nonlesional atopic dermatitis skin shares similar T-cell clones with lesional tissues. Allergy 2017;72:2017–25. [DOI] [PubMed] [Google Scholar]
- 85.Suarez-Farinas M, Tintle SJ, Shemer A, Chiricozzi A, Nograles K, Cardinale I, et al. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol 2011;127:954–64, e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brunner PM, Israel A, Zhang N, Leonard A, Wen HC, Huynh T, et al. Early-onset pediatric atopic dermatitis is characterized by TH2/TH17/TH22-centered inflammation and lipid alterations. J Allergy Clin Immunol 2018;141:2094–106. [DOI] [PubMed] [Google Scholar]
- 87.Czarnowicki T, Esaki H, Gonzalez J, Malajian D, Shemer A, Noda S, et al. Early pediatric atopic dermatitis shows only a cutaneous lymphocyte antigen (CLA)(1) TH2/TH1 cell imbalance, whereas adults acquire CLA(1) TH22/TC22 cell subsets. J Allergy Clin Immunol 2015;136:941–51.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Esaki H, Brunner PM, Renert-Yuval Y, Czarnowicki T, Huynh T, Tran G, et al. Early-onset pediatric atopic dermatitis is TH2 but also TH17 polarized in skin. J Allergy Clin Immunol 2016;138:1639–51. [DOI] [PubMed] [Google Scholar]
- 89.Esaki H, Czarnowicki T, Gonzalez J, Oliva M, Talasila S, Haugh I, et al. Accelerated T-cell activation and differentiation of polar subsets characterizes early atopic dermatitis development. J Allergy Clin Immunol 2016;138:1473–7.e5. [DOI] [PubMed] [Google Scholar]
- 90.Ungar B, Garcet S, Gonzalez J, Dhingra N, Correa da Rosa J, Shemer A, et al. An integrated model of atopic dermatitis biomarkers highlights the systemic nature of the disease. J Invest Dermatol 2017;137:603–13. [DOI] [PubMed] [Google Scholar]
- 91.Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol 2017;139(suppl):S65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Beck LA, Thaci D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med 2014;371:130–9. [DOI] [PubMed] [Google Scholar]
- 93.Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016;375:2335–48. [DOI] [PubMed] [Google Scholar]
- 94.Guttman-Yassky E, Brunner PM, Neumann AU, Khattri S, Pavel AB, Malik K, et al. Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: a randomized, double-blind, phase 2a trial. J Am Acad Dermatol 2018;78:872–81.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med 2013;5:170ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim BS, Wang K, Siracusa MC, Saenz SA, Brestoff JR, Monticelli LA, et al. Basophils promote innate lymphoid cell responses in inflamed skin. J Immunol 2014;193:3717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ruzicka T, Mihara R. Anti-interleukin-31 receptor A antibody for atopic dermatitis. N Engl J Med 2017;376:2093. [DOI] [PubMed] [Google Scholar]
- 98.Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell 2017;171:217–28.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013;155:285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ong PY, Leung DY. Bacterial and viral infections in atopic dermatitis: a comprehensive review. Clin Rev Allergy Immunol 2016;51:329–37. [DOI] [PubMed] [Google Scholar]
- 101.Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 2012;22:850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Byrd AL, Deming C, Cassidy SKB, Harrison OJ, Ng WI, Conlan S, et al. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci Transl Med 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chng KR, Tay ASL, Li C, Ng AHQ, Wang J, Suri BK, et al. Whole metagenome profiling reveals skin microbiome-dependent susceptibility to atopic dermatitis flare. Nat Microbiol 2016;1:16106. [DOI] [PubMed] [Google Scholar]
- 104.Kennedy EA, Connolly J, Hourihane JO, Fallon PG, McLean WH, Murray D, et al. Skin microbiome before development of atopic dermatitis: early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year. J Allergy Clin Immunol 2017;139:166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Totte JE, van der Feltz WT, Hennekam M, van Belkum A, van Zuuren EJ, Pas-mans SG. Prevalence and odds of Staphylococcus aureus carriage in atopic dermatitis: a systematic review and meta-analysis. Br J Dermatol 2016;175: 687–95. [DOI] [PubMed] [Google Scholar]
- 107.Tauber M, Balica S, Hsu CY, Jean-Decoster C, Lauze C, Redoules D, et al. Staphylococcus aureus density on lesional and nonlesional skin is strongly associated with disease severity in atopic dermatitis. J Allergy Clin Immunol 2016;137: 1272–4.e3. [DOI] [PubMed] [Google Scholar]
- 108.Fleury OM, McAleer MA, Feuillie C, Formosa-Dague C, Sansevere E, Bennett DE, et al. Clumping factor B promotes adherence of Staphylococcus aureus to corneocytes in atopic dermatitis. Infect Immun 2017;85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hata TR, Kotol P, Boguniewicz M, Taylor P, Paik A, Jackson M, et al. History of eczema herpeticum is associated with the inability to induce human beta-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br J Dermatol 2010;163:659–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miajlovic H, Fallon PG, Irvine AD, Foster TJ. Effect of filaggrin breakdown products on growth of and protein expression by Staphylococcus aureus. J Allergy Clin Immunol 2010;126:1184–90.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cho SH, Strickland I, Boguniewicz M, Leung DY. Fibronectin and fibrinogen contribute to the enhanced binding of Staphylococcus aureus to atopic skin. J Allergy Clin Immunol 2001;108:269–74. [DOI] [PubMed] [Google Scholar]
- 112.Riethmuller C, McAleer MA, Koppes SA, Abdayem R, Franz J, Haftek M, et al. Filaggrin breakdown products determine corneocyte conformation in patients with atopic dermatitis. J Allergy Clin Immunol 2015;136:1573–80.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nakamura Y, Oscherwitz J, Cease KB, Chan SM, Munoz-Planillo R, Hasegawa M, et al. Staphylococcus delta-toxin induces allergic skin disease by activating mast cells. Nature 2013;503:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bin L, Kim BE, Brauweiler A, Goleva E, Streib J, Ji Y, et al. Staphylococcus aureus alpha-toxin modulates skin host response to viral infection. J Allergy Clin Immunol 2012;130:683–91.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Spaulding AR, Salgado-Pabon W, Kohler PL, Horswill AR, Leung DY, Schlievert PM. Staphylococcal and streptococcal superantigen exotoxins. Clin Microbiol Rev 2013;26:422–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vu AT, Baba T, Chen X, Le TA, Kinoshita H, Xie Y, et al. Staphylococcus aureus membrane and diacylated lipopeptide induce thymic stromal lymphopoietin in keratinocytes through the Toll-like receptor 2-Toll-like receptor 6 pathway. J Allergy Clin Immunol 2010;126:985–93, e1–3. [DOI] [PubMed] [Google Scholar]
- 117.Jones AL, Curran-Everett D, Leung DYM. Food allergy is associated with Staphylococcus aureus colonization in children with atopic dermatitis. J Allergy Clin Immunol 2016;137:1247–8.e3. [DOI] [PubMed] [Google Scholar]
- 118.Noval Rivas M, Burton OT, Wise P, Zhang YQ, Hobson SA, Garcia Lloret M, et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J Allergy Clin Immunol 2013; 131:201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Noval Rivas M, Burton OT, Wise P, Charbonnier LM, Georgiev P, Oettgen HC, et al. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity 2015;42:512–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Noval Rivas M, Burton OT, Oettgen HC, Chatila T. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J Allergy Clin Immunol 2016;138:801–11.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rachid R, Chatila TA. The role of the gut microbiota in food allergy. Curr Opin Pediatr 2016;28:748–53. [DOI] [PubMed] [Google Scholar]
- 122.Huang YJ, Marsland BJ, Bunyavanich S, O’Mahony L, Leung DY, Muraro A, et al. The microbiome in allergic disease: current understanding and future opportunities—2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J Allergy Clin Immunol 2017;139:1099–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lack G, Fox D, Northstone K, Golding J. Avon Longitudinal Study of Parents and Children Study Team. Factors associated with the development of peanut allergy in childhood. N Engl J Med 2003;348:977–85. [DOI] [PubMed] [Google Scholar]
- 124.Brough HA, Liu AH, Sicherer S, Makinson K, Douiri A, Brown SJ, et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J Allergy Clin Immunol 2015;135:164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Reynolds LA, Finlay BB. Early life factors that affect allergy development. Nat Rev Immunol 2017;17:518–28. [DOI] [PubMed] [Google Scholar]
- 126.Brough HA, Kull I, Richards K, Hallner E, Soderhall C, Douiri A, et al. Environmental peanut exposure increases the risk of peanut sensitization in high-risk children. Clin Exp Allergy 2018;48:586–93. [DOI] [PubMed] [Google Scholar]
- 127.Bartnikas LM, Gurish MF, Burton OT, Leisten S, Janssen E, Oettgen HC, et al. Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis. J Allergy Clin Immunol 2013;131: 451–60, e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Han H, Roan F, Ziegler SF. The atopic march: current insights into skin barrier dysfunction and epithelial cell-derived cytokines. Immunol Rev 2017;278:116–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Oyoshi MK, Oettgen HC, Chatila TA, Geha RS, Bryce PJ. Food allergy: insights into etiology, prevention, and treatment provided by murine models. J Allergy Clin Immunol 2014;133:309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Oyoshi MK, Venturelli N, Geha RS. Thymic stromal lymphopoietin and IL-33 promote skin inflammation and vaccinia virus replication in a mouse model of atopic dermatitis. J Allergy Clin Immunol 2016;138:283–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Larson RP, Comeau MR, Ziegler SF. Cutting edge: allergen-specific CD4 T cells respond indirectly to thymic stromal lymphopoietin to promote allergic responses in the skin. J Immunol 2013;190:4474–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Han H, Thelen TD, Comeau MR, Ziegler SF. Thymic stromal lymphopoietin-mediated epicutaneous inflammation promotes acute diarrhea and anaphylaxis. J Clin Invest 2014;124:5442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Han H, Ziegler SF. Intradermal administration of IL-33 induces allergic airway inflammation. Sci Rep 2017;7:1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Galand C, Leyva-Castillo JM, Yoon J, Han A, Lee MS, McKenzie ANJ, et al. IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells. J Allergy Clin Immunol 2016;138:1356–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Savinko T, Matikainen S, Saarialho-Kere U, Lehto M, Wang G, Lehtimaki S, et al. IL-33 and ST2 in atopic dermatitis: expression profiles and modulation by triggering factors. J Invest Dermatol 2012;132:1392–400. [DOI] [PubMed] [Google Scholar]
- 136.Tordesillas L, Goswami R, Benede S, Grishina G, Dunkin D, Jarvinen KM, et al. Skin exposure promotes a Th2-dependent sensitization to peanut allergens. J Clin Invest 2014;124:4965–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Havstad S, Johnson CC, Kim H, Levin AM, Zoratti EM, Joseph CL, et al. Atopic phenotypes identified with latent class analyses at age 2 years. J Allergy Clin Immunol 2014;134:722–7.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tordesillas L, Mondoulet L, Blazquez AB, Benhamou PH, Sampson HA, Berin MC. Epicutaneous immunotherapy induces gastrointestinal LAP(+) regulatory T cells and prevents food-induced anaphylaxis. J Allergy Clin Immunol 2017; 139:189–201.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McAleer MA, Jakasa I, Hurault G, Sarvari P, McLean WHI, Tanaka RJ, et al. Systemic and stratum corneum biomarkers of severity in infant atopic dermatitis include markers of innate and T helper cell-related immunity and angiogenesis. Br J Dermatol 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]