Abstract
OBJECTIVE
This multicenter, open-label, randomized trial examined the safety and efficacy of exenatide alone or in combination with basal insulin in non–critically ill patients with type 2 diabetes (T2D).
RESEARCH DESIGN AND METHODS
A total of 150 patients with blood glucose (BG) between 140 and 400 mg/dL, treated at home with diet, oral agents, or insulin at a total daily dose <0.5 units/kg, were randomized to exenatide alone (5 μg twice daily), exenatide plus basal insulin, or a basal-bolus insulin regimen. The primary end point was difference in mean daily BG concentration among groups.
RESULTS
Mean daily BG was similar between patients treated with exenatide plus basal and a basal-bolus regimen (154 ± 39 vs. 166 ± 40 mg/dL, P = 0.31), and exenatide plus basal resulted in lower daily BG than did exenatide alone (177 ± 41 mg/dL, P = 0.02). Exenatide plus basal resulted in a higher proportion of BG levels in target range between 70 and 180 mg/dL compared with exenatide and basal-bolus (78% vs. 62% vs. 63%, respectively, P = 0.023). More patients in the exenatide and exenatide plus basal groups experienced nausea or vomiting than in the basal-bolus group (10% vs. 11% vs. 2%, P = 0.17), with three patients (6%) discontinued exenatide owing to adverse events. There were no differences in hypoglycemia <54 mg/dL (2% vs. 0% vs. 4%, P = 0.77) or length of stay (5 vs. 4 vs. 4 days, P = 0.23) among basal plus exenatide, exenatide, and basal-bolus groups.
CONCLUSIONS
The results of this pilot study indicate that exenatide alone or in combination with basal insulin is safe and effective for the management of hospitalized general medical and surgical patients with T2D.
Introduction
The association between hyperglycemia and poor clinical outcomes among hospitalized patients with and without diabetes is well established (1–4). Data from observational and prospective randomized controlled trials in hospitalized patients have reported that hyperglycemia is associated with prolonged hospital stay (length of stay [LOS]), increased rate of wound and systemic infections, disability after hospital discharge, and mortality (1,2,5). Improvement in glycemic control with insulin therapy has been shown to reduce hospital complications and infections in critically ill patients, as well as in general medicine and surgery patients with type 2 diabetes (T2D) (3,6,7). Despite these benefits, there is great controversy on the optimal blood glucose (BG) target and management strategy in hospitalized patients. The debate has been fueled by the risk of inpatient hypoglycemia, which has been reported in 10–30% of insulin-treated non–critically ill patients with T2D (8,9). The development of hypoglycemia, like hyperglycemia, has been associated with higher rates of hospital complications, longer LOS, more health care resource utilization, and hospital mortality (10–13).
Clinical guidelines recommend the use of basal-bolus insulin as the preferred regimen to manage hospitalized patients with T2D (13,14). This approach involves the administration of basal insulin once or twice daily in combination with rapid-acting insulin before meals—usually three times per day. Several observational and randomized controlled trials have shown that this is an effective regimen in achieving glycemic control and in reducing hospital complications (9,15). However, the requirement of multiple insulin injections and associated risk of hypoglycemia (16,17) has triggered the study of alternative approaches for the management of patients with diabetes in the hospital (18,19) and after discharge (20).
Based on their mechanism of action and safety profiles, there has been a great interest in using incretin-based therapies instead of, or complementary to, an insulin-based approach to improve glycemic control in hospitalized patients with diabetes (19,21,22). Use of these agents is attractive in hospitalized patients owing to their metabolic effects, such as glucose-dependent stimulation of insulin secretion and inhibition of glucagon secretion, which leads to improved glycemic control with low rates of hypoglycemia (23). Few studies have reported on the efficacy of intravenous administration of native glucagon-like peptide 1 (GLP-1) and exenatide in patients in the intensive care unit (ICU) (24–26). The efficacy and safety of subcutaneous administration of GLP-1 receptor agonists (GLP1-RAs), however, have not previously been studied for the management of hospitalized patients with T2D in general medicine and surgery wards. Therefore, we conducted a randomized control study to compare the safety and efficacy of short-acting exenatide alone or in combination with basal insulin to a basal-bolus insulin regimen (standard of care) in hospitalized patients with T2D.
Research Design and Methods
Patient Population
This pilot, multicenter, prospective, open-label, randomized trial enrolled 150 non–critically ill hospitalized adults with T2D at four academic institutions: Emory University Hospital, Grady Memorial Hospital, Temple University Hospital, and Vanderbilt University Medical Center. Enrolled subjects met the following inclusion criteria: age 18–80 years; known history of T2D treated with diet, oral agents, and/or low-dose insulin at a total daily dose (TDD) of <0.5 units/kg; admission or randomization BG >140 and <400 mg/dL, and BMI ≥25 and ≤50 kg/m2. The initial version of the protocol had inclusion criteria of HbA1c range 6.5–11% (48–97 mmol/mol). After recruiting of 36 patients (24%), this inclusion was removed to allow for broader and more generalizable inclusion based only on randomization BG. Patients were excluded if they had a history of type 1 diabetes, they had a history of diabetic ketoacidosis or hyperosmolar hyperglycemic state, were treated with GLP1-RAs during the 3 months prior to admission, or had recurrent severe hypoglycemia or clinically relevant liver disease or estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2. In addition, we excluded patients with a history of pancreatitis or gastrointestinal obstruction and those requiring gastrointestinal suction or with active nausea or vomiting, parenteral nutrition, or alcoholism. We also excluded patients receiving treatment with steroids at a dose equal to a prednisone dose >5 mg/day, ICU admission, or immunosuppressive treatment.
The investigational review board at each participating institution approved the study.
Study Procedures
Subjects were randomized using a computer-generated block randomization table, stratified by randomization BG levels ≤200 or >200 mg/dL to ensure equal inclusion of patients in both groups. Subjects were randomized to treatment in the hospital with exenatide alone, exenatide plus basal insulin, or basal-bolus insulin therapy. Exenatide 5 μg was administered twice daily for the two groups receiving exenatide. For the exenatide plus basal group, the initial TDD of basal insulin was based on randomization BG; patients with randomization BG between 140 and 200 mg/dL received an initial dose of glargine or detemir at 0.2 units/kg, and patients with BG between 201 and 400 mg/dL were started at 0.25 units/kg. Patients in the basal-bolus group were started at a TDD of 0.4 IU/kg or 0.5 IU/kg for a randomization BG between 140 and 200 mg/dL or 201 and 400 mg/dL, respectively. TDD of insulin was administered half as basal and half as rapid-acting insulin lispro or aspart in three equally divided doses before each meal. The dose of insulin was adjusted daily based on glycemic control with goal BG of 70–180 mg/dL (Supplementary Table 1). The insulin dose was reduced in patients ≥70 years of age and for those with an eGFR <60 mL/min/1.73 m2 with a starting dose of 0.15 units/kg for the basal plus exenatide group and 0.3 units/kg for those in the basal-bolus therapy group. Both treatment groups also received correction or supplemental rapid-acting insulin, which was given before meals to correct hyperglycemia for BG >140 mg/dL and at bedtime for BG >220 mg/dL (Supplementary Table 2). Capillary BG was measured by point-of-care testing using a hospital-calibrated glucometer. BG testing was ordered before meals and bedtime. In addition, most patients had BG measured by laboratory tests daily.
The study team collected daily information on the presence of gastrointestinal adverse events including nausea, vomiting, abdominal pain, diarrhea, constipation, or other adverse events and complications during the hospital stay. In surgical patients who were expected to remain NPO after surgery, the dose of exenatide prior to surgery was held and patients received only correctional insulin using a sliding scale (Supplementary Table 2). For patients in the basal plus exenatide group, we continued basal insulin, held exenatide if they were expected to remain NPO after surgery, and used a correctional sliding insulin scale. For the basal-bolus group, we continued basal insulin, held the premeal standard order of prandial insulin, and covered with a correctional sliding insulin scale as needed.
Outcome Measures
The primary outcome was to determine differences among groups in glycemic control as measured by mean daily BG concentration during the hospital stay. Secondary outcomes included number of hypoglycemic events <70 mg/dL, clinically significant hypoglycemia <54 mg/dL, and severe hypoglycemia <40 mg/dL; number of hyperglycemic episodes ≥240 mg/dL after the 1st day of treatment; TDD of insulin; LOS; and composite of hospital complications including nosocomial pneumonia, bacteremia, respiratory failure, acute renal failure, and wound infections (surgery patients), and death. In addition, we estimated rates of BG within target range of 70–180 mg/dL and the number of treatment failures, defined as 3 consecutive days with fasting BG >240 mg/dL or with average daily BG of >240 mg/dL.
Statistical Analysis
In this pilot study, per-protocol analysis included 150 patients who received at least one dose of study medication. We compared baseline and clinical characteristics and outcomes, such as mean daily BG after day 1 (days 2–10) and rates of hypoglycemia and complications among treatment groups. Based on the normality of the data, continuous variables were compared using ANOVA or Kruskal-Wallis test for three-group comparisons. Tukey or Wilcoxon tests were used for multiple comparisons (post hoc analysis). Categorical data were compared using χ2 or Fisher exact test. Multivariate regression analysis was performed to assess for the difference in the mean daily BG from day 2 to 10 between the three treatment groups after controlling for a variety of factors, including admission HbA1c, duration of diabetes, BMI, and race, which were selected through stepwise selection procedure. A P value <0.05 was considered significant. Statistical analyses were performed using SAS (version 9.3; Cary, NC). The data were generally presented as mean ± SD for continuous variables and count (percentage) for discrete variables.
Results
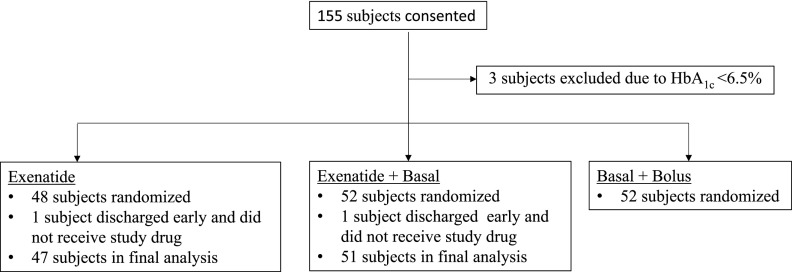
A total of 155 eligible subjects admitted to general medicine and surgery services consented. Of them, five patients who were screen failures, withdrew consent, or stayed in the hospital for <24 h were excluded from the analysis. We randomly assigned 48 patients to treatment with exenatide, 52 to exenatide and basal insulin, and 52 patients to the basal-bolus regimen (Fig. 1). One subject randomized to exenatide alone voluntarily withdrew, and one subject randomized to exenatide plus basal was discharged <24 h after admission. Neither received any study drug, and both were excluded from the per-protocol analysis. Demographic and clinical characteristics at baseline are shown in Table 1. There were no significant differences in age, sex, race, body weight, BMI, duration of diabetes, admission HbA1c, randomization BG, or admission service among treatment groups.
Figure 1.
Randomization scheme. Three subjects were withdrawn owing to HbA1c being <6.5% (48 mmol/mol) prior to removal of HbA1c cutoff from the inclusion criteria. Patients who did not receive any study drug were excluded from per-protocol analysis.
Table 1.
Baseline characteristics
| Variable | Exenatide (N = 47) | Exe + basal (N = 51) | Basal bolus (N = 52) | P |
|---|---|---|---|---|
| Age, years | 55 ± 12 | 55 ± 12 | 57 ± 11 | 0.68 |
| Weight, kg | 102 ± 21 | 104 ± 22 | 98 ± 21 | 0.44 |
| BMI, kg/m2 | 34 ± 6 | 34 ± 7 | 33 ± 6 | 0.78 |
| Sex, n (%) | 0.99 | |||
| Female | 23 (49) | 25 (49) | 26 (50) | |
| Male | 24 (51) | 26 (51) | 26 (50) | |
| Race, n (%) | 0.67 | |||
| Black | 35 (74) | 33 (65) | 35 (67) | |
| White | 10 (21) | 15 (29) | 16 (31) | |
| Other | 2 (4) | 3 (6) | 1 (2) | |
| Admission HbA1c, % (mmol/mol) | 8.9 ± 2.2 (74) | 8.3 ± 2.0 (67) | 8.5 ± 1.7 (69) | 0.22 |
| Admission BG, mg/dL | 220.7 ± 82 | 186.9 ± 64 | 195.1 ± 82 | 0.15 |
| Randomization BG, mg/dL | 196.5 ± 61 | 194.8 ± 51 | 200.6 ± 58 | 0.91 |
| Hospital service, n (%) | 0.88 | |||
| Medicine | 33 (70) | 35 (69) | 38 (73) | |
| Surgery | 14 (30) | 16 (31) | 14 (27) | |
| Surgical interventions | 17 (36) | 16 (31) | 17 (33) | 0.87 |
| Diabetes duration, years | 8.6 ± 5.8 | 9.8 ± 8.7 | 12 ± 9.0 | 0.13 |
| Home diabetes therapy, n (%) | 0.57 | |||
| Diet alone | 6 (13) | 8 (16) | 7 (13) | |
| Oral agents | 24 (51) | 26 (51) | 20 (39) | |
| Insulin | 7 (15) | 11 (22) | 12 (23) | |
| Oral agent and insulin | 10 (21) | 6 (12) | 13 (25) | |
| Comorbidities, n (%) | ||||
| CAD | 2 (4) | 6 (12) | 3 (6) | 0.36 |
| CHF | 3 (6) | 3 (6) | 3 (6) | >0.99 |
| eGFR <60 mL/min/1.73 m2 | 13 (28) | 15 (29) | 14 (27) | 0.97 |
| HTN | 20 (43) | 19 (37) | 23 (44) | 0.76 |
Data are means ± SD unless otherwise indicated; for HbA1c, data are means ± SD in % followed by mean in mmol/L in parentheses. CAD, coronary artery disease; CHF, congestive heart failure; Exe, exenatide; HTN, hypertension.
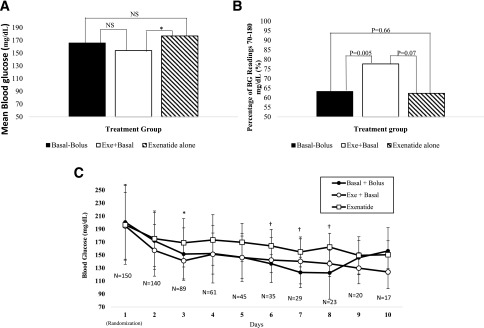
Hospital glycemic control and complications are shown in Table 2. Patients treated with exenatide plus basal insulin had the lowest overall mean hospital BG (154.1 ± 39 mg/dL), followed by basal-bolus insulin (166.1 ± 40 mg/dL) and exenatide alone (177.1 ± 41 mg/dL) (P = 0.03) (Table 2). In multiple comparisons of mean BG between treatment groups (Fig. 2A), treatment with exenatide plus basal was associated with significantly lower mean BG than exenatide alone, with a difference in mean BG of −23 mg/dL (P = 0.02). In addition to having a lower mean daily BG, patients treated with exenatide plus basal had the highest proportion of BG readings within target range 70–180 mg/dL (77.7%) compared with basal bolus (63.3%, P = 0.005) and exenatide alone (62.3%, P = 0.07) (Fig. 2B). Patients treated with exenatide plus basal and basal-bolus insulin tended to have lower daily BG than those treated with exenatide alone (Fig. 2C). In multivariate analysis, we found that only exenatide alone versus exenatide plus basal had a significant difference in mean daily BG (21.2 mg/dL [95% CI 6.2; 36.2]), which was consistent with the unadjusted results.
Table 2.
Primary and secondary outcomes for patients in the hospital
| Variable | Exenatide (N = 47) | Exe + basal (N = 51) | Basal bolus (N = 52) | P |
|---|---|---|---|---|
| Overall BG, mg/dL* | 177.1 ± 41 | 154.1 ± 39 | 166.1 ± 40 | 0.03 |
| Glucose readings within range, mg/dL | ||||
| % BG readings <70* | 0.0 ± 0.0 | 0.4 ± 1.5 | 1.1 ± 4.1 | 0.06 |
| % BG readings 70–139*† | 23.9 ± 31 | 39.6 ± 33 | 36.4 ± 32 | 0.05 |
| % BG readings 70–180*† | 62.3 ± 39 | 77.7 ± 31 | 63.3 ± 31 | 0.02 |
| % BG readings 180–240*† | 27.4 ± 31.9 | 16.8 ± 24.8 | 24.4 ± 27.5 | 0.19 |
| % BG readings >240* | 10.4 ± 24 | 5.1 ± 16 | 11.2 ± 23 | 0.08 |
| Subjects with hypoglycemia, n (%)* | ||||
| BG <70 mg/dL | 0 (0) | 3 (6) | 6 (12) | 0.06 |
| BG <54 mg/dL | 0 (0) | 1 (2) | 2 (4) | 0.77 |
| BG <40 mg/dL | 0 (0) | 0 (0) | 0 (0) | NA |
| Glucose checks, number/day* | 2.7 ± 1.0 | 2.9 ± 0.9 | 2.7 ± 1.0 | 0.46 |
| Insulin administration* | ||||
| Total insulin, units/day | 8.1 ± 5 | 17.7 ± 11 | 28.0 ± 15 | <0.001 |
| Total basal insulin, units/day | — | 18.5 ± 7 | 22.0 ± 8 | 0.12 |
| Total corrective insulin, units/day | 8.1 ± 5 | 5.2 ± 3 | 6.3 ± 3 | 0.01 |
| Total number of injections/day‡ | 2.8 ± 1 | 3.1 ± 1 | 2.6 ± 1 | 0.04 |
| LOS, days, median (IQR) | 4.0 (2.0, 8.0) | 5.0 (3.0, 7.0) | 4.0 (2.0, 5.0) | 0.23 |
| Adverse events, n (%) | ||||
| Nausea | 5 (11) | 5 (10) | 1 (2) | 0.17 |
| Vomiting | 1 (2) | 3 (6) | 0 (0) | 0.17 |
| Nausea or vomiting | 5 (11) | 5 (10) | 1 (2) | 0.17 |
| Medication discontinued owing to AE, n (%) | 3 (6) | 0 (0) | 0 (0) | 0.03 |
| Treatment failure‖ | 2 (4) | 0 (0) | 0 (0) | 0.10 |
| Hospital complications, n (%) | ||||
| Composite complications | 3 (6) | 6 (12) | 4 (8) | 0.63 |
| Myocardial infarction | 0 (0) | 0 (0) | 0 (0) | NA |
| Congestive heart failure | 0 (0) | 0 (0) | 1 (2) | >0.99 |
| Acute renal failure | 3 (6) | 6 (12) | 3 (6) | 0.52 |
| Respiratory failure | 1 (2) | 0 (0) | 0 (0) | 0.31 |
Data are means ± SD unless otherwise indicated. AE, adverse event; Exe, exenatide; IQR, interquartile range.
*Values included from day 2 to day 10.
†Glycemic targets included in post hoc analysis.
‡Total number of injections includes insulin and exenatide injections.
‖Treatment failure defined by 3 consecutive days with fasting BG >240 mg/dL or average daily BG >240 mg/dL.
Figure 2.
A: Mean hospital BG with direct comparison of each treatment group: multiple comparisons done using post hoc Tukey test. Difference in means between exenatide alone and basal bolus: 11 mg/dL (P = 0.39). Difference in means between exenatide alone and exenatide plus basal: 23 mg/dL (P = 0.02). Difference in means between basal bolus and exenatide plus basal: 12 mg/dL (0.31). NS, not significant (P > 0.05). B: Hospital BG within target range 70–180 mg/dL. Differences in number of BGs within target of 70–180 mg/dL reported as percentage of all BG readings. Multiple comparisons of each pair were performed using Wilcoxon tests. C: Daily mean hospital BG. Significant differences in treatment groups: *P = 0.01; †P ≤ 0.05. Exe, exenatide; N, number of patients.
As expected, subjects treated with the basal-bolus regimen received the highest total daily insulin dose (28.0 ± 15 units) followed by exenatide plus basal (17.7 ± 11 units) and exenatide alone (8.1 ± 5 units) (P < 0.01). However, patients treated with exenatide alone received more supplemental insulin (8.1 ± 5 units) than exenatide plus basal (5.2 ± 3 units) or basal bolus (6.3 ± 3) (P = 0.01).
Hypoglycemia (BG <70 mg/dL) occurred more frequently in patients treated with the basal bolus regimen (12%), followed by exenatide plus basal (6%) and exenatide alone (0%), but did not reach statistical significance (P = 0.06) (Table 2). There were no significant differences in the frequency of BG <54 mg/dL or BG <40 mg/dL. Only one subject in the exenatide plus basal and two in the basal-bolus group developed BG <54 mg/dL, and none had BG <40 mg/dL.
The overall number of patients with gastrointestinal side effects was low, with no significant differences among the three groups (Table 2). In the exenatide plus basal group, five patients experienced nausea and three patients had vomiting compared with five patients with nausea and one patient vomiting in the exenatide group and only one patient with nausea in the basal-bolus group (P = 0.17). A total of three subjects in the exenatide group had to discontinue the drug owing to gastrointestinal side effects, while no subjects in either the exenatide plus basal or basal-bolus group had to discontinue study medications (P = 0.029).
We found no significant differences in the hospital length of stay (exenatide plus basal 5.0 vs. exenatide alone 4.0 vs. basal bolus 4.0 days, P = 0.23) or in the composite of complications during the hospital stay among patients in the exenatide plus basal, basal-bolus, and exenatide treatment groups (Table 2).
Conclusions
This pilot multicenter randomized controlled trial explored the efficacy and safety of exenatide, a short-acting GLP1-RA, in the management of general medicine and surgery patients with T2D. Our study demonstrates that the inpatient use of exenatide alone or in combination with basal insulin is effective for the management of general medicine and surgery patients with T2D. Treatment with exenatide plus basal insulin resulted in similar mean daily BG and in a higher proportion of BG values in target range between 70 and 180 mg/dL than did treatment with the basal-bolus insulin regimen, and both regimens resulted in better glycemic control compared with exenatide alone. Treatment with exenatide alone was associated with lower rates of inpatient hypoglycemia and with a higher rate of gastrointestinal adverse events compared with the basal-bolus insulin regimen.
The results of observational and randomized controlled trials have shown that improvement in glycemic control with insulin therapy in critically ill, general medicine, and surgery patients reduces hospital complications (5,9). Recent trials and meta-analyses, however, have shown that regimens with multiple-dose insulin injections increase the risk for hypoglycemia (28–29), which has been associated with increased hospital complications and mortality (12). Thus, while a multiple–insulin dose approach is the current standard of care for achieving glycemic control in hospitalized patients (1,30), the risk of hypoglycemia and its associated potential for adverse cardiovascular events prompted a search for alternative treatment options, such as incretin-based therapy (19,21,22). Incretin-based agents are known to stimulate insulin secretion in a glucose-dependent fashion and do not cause hypoglycemia when used as monotherapy (23,31). In addition, increasing evidence indicates that incretin therapies have cardiovascular benefits, including reduced inflammation and oxidative stress (30,32) and improved endothelial function (24), which could benefit hospitalized patients with diabetes.
Previous studies have reported on the use of native GLP-1 and GLP1-RA infusions in critically ill and surgical patients (33–35). Besch et al. (34) compared the use of intravenous exenatide to insulin infusion in cardiac surgery patients and reported similar glycemic control and reduced insulin use in the ICU with exenatide therapy. Perioperative treatment with a GLP1-RA, liraglutide, given subcutaneously prior to noncardiac surgery was studied by Polderman et al. (35), who demonstrated improved glycemic control with liraglutide lowering insulin requirements. However, GLP-1 was associated with increased rates of nausea. Only two studies from Japan have reported on the use of GLP-1, liraglutide, in hospitalized patients outside of the ICU (36,37). Unlike the current study, these trials enrolled elective subjects hospitalized primarily for management of diabetes. During an approximate month-long admission, they demonstrated that use of liraglutide was associated with similar improvement in glycemic control and greater weight loss compared with insulin therapy (36).
In agreement with previous studies, we observed an increase in gastrointestinal adverse events among patients treated with exenatide either with or without basal insulin (35). The presence of gastrointestinal adverse events represents a significant limitation of the use of GLP1-RA therapy in the hospital setting. In this study, we observed that 10% and 11% of patients on exenatide alone and patients on exenatide plus basal experienced nausea or vomiting vs. 2% of patients on the basal-bolus regimen. These differences were not statistically significantly different, likely due to the small number of patients in the study.
To our knowledge, this is the first study to compare the administration of subcutaneous exenatide alone or in combination with basal insulin and basal-bolus insulin (standard of care) in general medicine and surgery patients with T2D. Our results indicate that exenatide is well tolerated and can be used safely in non-ICU hospital settings. Although patients treated with exenatide experienced lower rates of hypoglycemia, its use as monotherapy was associated with higher mean daily BG and lower rates of BG within target range 70–180 mg/dL compared with the combination of exenatide and basal insulin or the basal-bolus regimen. The use of low doses of a short-acting GLP1-RA (exenatide) was associated with nonsignificantly higher number of gastrointestinal adverse events, which may represent a limitation for widespread utilization for the management of hospitalized patients with T2D.
There are several limitations to this pilot study including relatively few patients randomized to each treatment group. Given the nature of the intervention with insulin and GLP1-RA, participants and investigators were not masked to treatment in this trial. The study results cannot be generalized to patients receiving a high insulin dose >0.5 units/kg/day, those admitted to the ICU or undergoing gastrointestinal surgery, those with gastrointestinal obstruction or active nausea and vomiting, those requiring corticosteroid therapy, those with history of pancreatitis or relevant hepatic disease, or those with an eGFR <30 mL/min/1.73 m2. In such patients, a standard basal-bolus approach might be preferred for glycemic control. Additionally, we used a single short-acting GLP1-RA, exenatide; thus, our results cannot be generalized to other GLP1-RAs. These limitations, however, are balanced by a number of strengths including the novelty of testing a subcutaneous GLP1-RA in this population, a multicenter participation, inclusion of both medical and surgical patients, and a racially diverse sample of men and women.
These preliminary results suggest that incretin agents may help reduce the incidence of iatrogenic hypoglycemia in hospitalized patients with T2D. A larger study using newer GLP1-RAs associated with lower incidence of gastrointestinal side effects is needed to confirm our findings and expand on patient satisfaction measures, patient-centered outcomes, and cost-effectiveness.
In summary, the inpatient use of short-acting exenatide alone or in combination with basal insulin was shown to be safe and to improve glycemic control in general medicine and surgery patients with T2D. The use of exenatide in combination with basal insulin resulted in similar improvement in glycemic control compared with a basal-bolus insulin regimen. As expected, treatment with exenatide was associated with lower rates of hypoglycemia but higher rates of gastrointestinal adverse events.
Supplementary Material
Article Information
Funding. This study was supported in part by research grants from the U.S. Public Health Service through the National Institutes of Health (NIH) Clinical and Translational Science Awards Program (grant UL1-TR-002378) and the National Center for Research Resources (grant 1P30-DK-111024-01). D.J.R. was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of NIH under award number K23-DK-102963. P.V. was supported in part by the NIH (grant K12HD085850).
The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Duality of Interest. This investigator-initiated study was supported by an unrestricted grant from AstraZeneca. R.J.G. has received research grants from Novo Nordisk (to Emory University) and consulting fees from Novo Nordisk and Abbott Diabetes Care. D.J.R. has received research support for an outpatient clinical trial by Boehringer Ingelheim. F.J.P. has received consulting fees from Boehringer Ingelheim, Sanofi, and Merck. P.V. has received consulting fees from Boehringer Ingelheim and Merck. G.E.U. has received unrestricted research support for inpatient studies (to Emory University) from Merck, Novo Nordisk, AstraZeneca, and Sanofi. AstraZeneca was not involved in protocol development, study implementation, or writing of the manuscript. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.F. wrote the initial draft of the manuscript. M.F., R.J.G., D.J.R., D.L.M., F.J.P., J.S.H., and P.V. were to co-investigators of the trial, helped with data collection, and critically revised and edited the manuscript. I.A., M.A.U., C.R., B.S.A., R.W., and S.C. enrolled study subjects, coordinated their follow-up, and collected data. H.W. performed statistical analysis. G.E.U. conceived and designed the study. G.E.U. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Footnotes
Clinical trial reg. no. NCT02455076, clinicaltrials.gov
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-1760/-/DC1.
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002;87:978–982 [DOI] [PubMed] [Google Scholar]
- 2.Finney SJ, Zekveld C, Elia A, Evans TW. Glucose control and mortality in critically ill patients. JAMA 2003;290:2041–2047 [DOI] [PubMed] [Google Scholar]
- 3.Van den Berghe G, Wouters PJ, Bouillon R, et al. Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemic control. Crit Care Med 2003;31:359–366 [DOI] [PubMed] [Google Scholar]
- 4.Pomposelli JJ, Baxter JK III, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr 1998;22:77–81 [DOI] [PubMed] [Google Scholar]
- 5.Kosiborod M, Inzucchi SE, Krumholz HM, et al. Glucose normalization and outcomes in patients with acute myocardial infarction. Arch Intern Med 2009;169:438–446 [DOI] [PubMed] [Google Scholar]
- 6.Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg 2003;125:1007–1021 [DOI] [PubMed] [Google Scholar]
- 7.Clement S, Braithwaite SS, Magee MF, et al.; American Diabetes Association Diabetes in Hospitals Writing Committee . Management of diabetes and hyperglycemia in hospitals [published correction appears in Diabetes Care 2004;27:856; 1255]. Diabetes Care 2004;27:553–591 [DOI] [PubMed] [Google Scholar]
- 8.Moghissi ES, Korytkowski MT, DiNardo M, et al.; American Association of Clinical Endocrinologists; American Diabetes Association . American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 2009;15:353–369 [DOI] [PubMed] [Google Scholar]
- 9.Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care 2011;34:256–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Ancona G, Bertuzzi F, Sacchi L, et al. Iatrogenic hypoglycemia secondary to tight glucose control is an independent determinant for mortality and cardiac morbidity. Eur J Cardiothorac Surg 2011;40:360–366 [DOI] [PubMed] [Google Scholar]
- 11.Egi M, Bellomo R, Stachowski E, et al. Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc 2010;85:217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosiborod M, Inzucchi SE, Goyal A, et al. Relationship between spontaneous and iatrogenic hypoglycemia and mortality in patients hospitalized with acute myocardial infarction. JAMA 2009;301:1556–1564 [DOI] [PubMed] [Google Scholar]
- 13.Umpierrez GE, Hellman R, Korytkowski MT, et al.; Endocrine Society . Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2012;97:16–38 [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association 14. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S144–S151 [DOI] [PubMed] [Google Scholar]
- 15.Murad MH, Coburn JA, Coto-Yglesias F, et al. Glycemic control in non-critically ill hospitalized patients: a systematic review and meta-analysis. J Clin Endocrinol Metab 2012;97:49–58 [DOI] [PubMed] [Google Scholar]
- 16.Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care 2007;30:367–369 [DOI] [PubMed] [Google Scholar]
- 17.Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: Basal Plus trial. Diabetes Care 2013;36:2169–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg R, Schuman B, Hurwitz S, Metzger C, Bhandari S. Safety and efficacy of saxagliptin for glycemic control in non-critically ill hospitalized patients. BMJ Open Diabetes Res Care 2017;5:e000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umpierrez GE, Gianchandani R, Smiley D, et al. Safety and efficacy of sitagliptin therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes: a pilot, randomized, controlled study. Diabetes Care 2013;36:3430–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gianchandani RY, Pasquel FJ, Rubin DJ, et al. The efficacy and safety of co-administration of sitagliptin with metformin in patients with type 2 diabetes at hospital discharge. Endocr Pract 2018;24:556–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz S, DeFronzo RA. Is incretin-based therapy ready for the care of hospitalized patients with type 2 diabetes? The time has come for GLP-1 receptor agonists! Diabetes Care 2013;36:2107–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasquel FJ, Gianchandani R, Rubin DJ, et al. Efficacy of sitagliptin for the hospital management of general medicine and surgery patients with type 2 diabetes (Sita-Hospital): a multicentre, prospective, open-label, non-inferiority randomised trial. Lancet Diabetes Endocrinol 2017;5:125–133 [DOI] [PubMed] [Google Scholar]
- 23.Jespersen MJ, Knop FK, Christensen M. GLP-1 agonists for type 2 diabetes: pharmacokinetic and toxicological considerations. Expert Opin Drug Metab Toxicol 2013;9:17–29 [DOI] [PubMed] [Google Scholar]
- 24.Nyström T, Gutniak MK, Zhang Q, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab 2004;287:E1209–E1215 [DOI] [PubMed] [Google Scholar]
- 25.Sokos GG, Bolukoglu H, German J, et al. Effect of glucagon-like peptide-1 (GLP-1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am J Cardiol 2007;100:824–829 [DOI] [PubMed] [Google Scholar]
- 26.Nathanson D, Ullman B, Löfström U, et al. Effects of intravenous exenatide in type 2 diabetic patients with congestive heart failure: a double-blind, randomised controlled clinical trial of efficacy and safety. Diabetologia 2012;55:926–935 [DOI] [PubMed] [Google Scholar]
- 27.Finfer S, Chittock DR, Su SY, et al.; NICE-SUGAR Study Investigators . Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009;360:1283–1297 [DOI] [PubMed] [Google Scholar]
- 28.Griesdale DE, de Souza RJ, van Dam RM, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ 2009;180:821–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Preiser JC, Devos P, Ruiz-Santana S, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med 2009;35:1738–1748 [DOI] [PubMed] [Google Scholar]
- 30.Moghissi ES, Korytkowski MT, DiNardo M, et al.; American Association of Clinical Endocrinologists; American Diabetes Association . American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009;32:1119–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheen AJ. Pharmacokinetics of dipeptidylpeptidase-4 inhibitors. Diabetes Obes Metab 2010;12:648–658 [DOI] [PubMed] [Google Scholar]
- 32.Umpierrez GE, Gianchandani R, Smiley D, et al. Safety and efficacy of sitagliptin therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes: a pilot, randomized, controlled study. Diabetes Care 2013;36:3430–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abuannadi M, Kosiborod M, Riggs L, et al. Management of hyperglycemia with the administration of intravenous exenatide to patients in the cardiac intensive care unit. Endocr Pract 2013;19:81–90 [DOI] [PubMed] [Google Scholar]
- 34.Besch G, Perrotti A, Mauny F, et al. Clinical effectiveness of intravenous exenatide infusion in perioperative glycemic control after coronary artery bypass graft surgery: a phase II/III randomized trial. Anesthesiology 2017;127:775–787 [DOI] [PubMed] [Google Scholar]
- 35.Polderman JAW, van Steen SCJ, Thiel B, et al. Peri-operative management of patients with type-2 diabetes mellitus undergoing non-cardiac surgery using liraglutide, glucose-insulin-potassium infusion or intravenous insulin bolus regimens: a randomised controlled trial. Anaesthesia 2018;73:332–339 [DOI] [PubMed] [Google Scholar]
- 36.Fujishima Y, Maeda N, Inoue K, et al. Efficacy of liraglutide, a glucagon-like peptide-1 (GLP-1) analogue, on body weight, eating behavior, and glycemic control, in Japanese obese type 2 diabetes. Cardiovasc Diabetol 2012;11:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inoue K, Maeda N, Kashine S, et al. Short-term effects of liraglutide on visceral fat adiposity, appetite, and food preference: a pilot study of obese Japanese patients with type 2 diabetes. Cardiovasc Diabetol 2011;10:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.