Abstract
OBJECTIVE
This study evaluated the association of time in range (TIR) of 70–180 mg/dL (3.9–10 mmol/L) with the development or progression of retinopathy and development of microalbuminuria using the Diabetes Control and Complications Trial (DCCT) data set in order to validate the use of TIR as an outcome measure for clinical trials.
RESEARCH DESIGN AND METHODS
In the DCCT, blood glucose concentrations were measured at a central laboratory from seven fingerstick samples (seven-point testing: pre- and 90-min postmeals and at bedtime) collected during 1 day every 3 months. Retinopathy progression was assessed every 6 months and urinary microalbuminuria development every 12 months. Proportional hazards models were used to assess the association of TIR and other glycemic metrics, computed from the seven-point fingerstick data, with the rate of development of microvascular complications.
RESULTS
Mean TIR of seven-point profiles for the 1,440 participants was 41 ± 16%. The hazard rate of development of retinopathy progression was increased by 64% (95% CI 51–78), and development of the microalbuminuria outcome was increased by 40% (95% CI 25–56), for each 10 percentage points lower TIR (P < 0.001 for each). Results were similar for mean glucose and hyperglycemia metrics.
CONCLUSIONS
Based on these results, a compelling case can be made that TIR is strongly associated with the risk of microvascular complications and should be an acceptable end point for clinical trials. Although hemoglobin A1c remains a valuable outcome metric in clinical trials, TIR and other glycemic metrics—especially when measured with continuous glucose monitoring—add value as outcome measures in many studies.
Introduction
Hemoglobin A1c (A1C) became the gold standard for assessing glycemic management after the landmark Diabetes Control and Complications Trial (DCCT) demonstrated the strong association between A1C levels and the risk of chronic diabetic vascular complications, and laboratory methods were developed so that A1C levels could be readily measured with a high degree of precision. Although its important role in diabetes management as a clinical trials outcome and as a predictor of long-term diabetic complications cannot be overstated, A1C does have certain limitations.
A1C is a measure of hyperglycemia, but it provides no indication of hypoglycemia, glycemic variability, or daily patterns of glycemia. Notably, considerable interindividual variability exists in the relationship between A1C and mean glucose concentration so that for an individual patient, A1C may or may not be a good indicator of glycemia (1). This is not necessarily important when comparing groups in a clinical trial or computing population averages, but it can be important in the management of an individual patient. There are certain well-known causes of A1C–mean glucose discordance, such as hemoglobinopathy, hemolytic anemia, and chronic renal failure (2); but even when no known condition affecting red blood cells is present, there is a wide range of possible mean glucose concentrations for a given A1C level (1). This is likely due, in great part, to interindividual variation in red blood cell life span (3). The frequent discordance between mean glucose and A1C has been known since at least 1990 (4), but awareness has increased in recent years as continuous glucose monitoring (CGM) has become more prevalent (5).
In the last year, several organizations published consensus statements on the role of CGM and specific metrics to use for assessing overall glycemic management, hyperglycemia, hypoglycemia, and glycemic variability (6,7), and a conference was held with representatives of all organizations to reach an agreement on the consensus statements (8). Over time and in these consensus statements, time in range (TIR) of 70–180 mg/dL (3.9–10 mmol/L) has been popularized as an important metric to be derived from CGM data to classify glycemic management. A survey showed that patients also recognized TIR as an important outcome (8,9).
Despite the rapidly increasing use of CGM, particularly in type 1 diabetes (5), and the recognition by clinicians of its value, the U.S. Food and Drug Administration has not accepted CGM metrics as outcomes for making efficacy claims in clinical trials conducted for the approval of a new drug or device (8). The Food and Drug Administration has indicated the need to demonstrate the clinical relevance of CGM outcomes, similar to the DCCT’s demonstration of the association of A1C levels with vascular complications (10). For a hypoglycemia outcome such as time below 54 mg/dL, evidence already exists to provide clinical validation of its importance in that hypoglycemia is associated with a number of occurrences that are clinically relevant. They include an increased risk of subsequent severe clinical hypoglycemia events; defective glucose counterregulation/impaired hypoglycemia awareness, which has been associated with an increased risk of severe clinical hypoglycemic events; cognitive function impairment; an increase in cardiac arrhythmias (mortality); an increase in car accidents; an adverse effect on quality of life, including sleep; and reduced work productivity (11–16).
However, for commonly reported CGM metrics, such as TIR and time in hyperglycemia, the case of clinical relevance cannot be as easily made. The metric TIR typically refers to the percentage of time that glucose concentrations are between 70 and 180 mg/dL (3.9 and 10 mmol/L) and as such is primarily determined by the amount of hyperglycemia. It is correlated with A1C, easily understood by individuals with diabetes, and readily computed from CGM profiles. Regulators have not accepted the argument that because TIR is largely a measure of hyperglycemia and is correlated with A1C, it therefore must be associated with risk for vascular complications. Therefore, merely showing an association with A1C has been considered insufficient evidence to have TIR accepted as a clinically meaningful outcome.
To conduct a prospective, longitudinal study to assess the association of TIR with the development of complications would take many years and be costly. Fortunately, the DCCT collected data that are valuable for assessing this issue. During the DCCT, participants were asked to collect a fingerstick blood sample seven times a day (pre- and 90 min postmeals and at bedtime) once every 3 months for blood glucose measurement by a central laboratory (referred to as “seven-point testing”). These data, although sparse compared with the 288 daily data points obtained with CGM, which was not available during the DCCT, nevertheless provided the opportunity to evaluate the association of TIR and hyperglycemia metrics with the major DCCT outcomes of retinopathy and microalbuminuria. We used Cox proportional hazards modeling to assess the longitudinal association between TIR as a time-dependent variable and the rates of retinopathy progression and microalbuminuria development.
Research Design and Methods
Public DCCT data sets were obtained from the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) Central Database Repository and with the assistance of the Biostatistics Center at George Washington University. An Institutional Review Board waiver was received to conduct the analyses. The DCCT methods and cohort characteristics have been well described in many publications and are not repeated here (10,17).
The analyses used data collected during the DCCT (1983–1993). Glycemic data used in the analyses included A1C and seven-point glucose concentration blood samples, measured every 3 months at a central laboratory. Outcome data included reading center retinopathy gradings from fundus photographs obtained every 6 months and urinary microalbuminuria assessment from the albumin excretion rate (AER) obtained every 12 months. As reported by the DCCT, retinopathy progression was defined as a sustained progression of three or more steps from baseline at two consecutive visits (for analysis, timed from the first of the two visits), and microalbuminuria was defined as an AER ≥30 mg/24 h at two consecutive visits (for analysis, timed from the first of the two visits).
Of the 1,441 DCCT participants, 1 participant had no retinopathy or microalbuminuria assessments and was not included in the analyses. For the retinopathy assessment, the DCCT definitions of primary prevention (no retinopathy at baseline, n = 726) and secondary intervention cohorts (retinopathy present at baseline, n = 714) were used. For the microalbuminuria assessment, the analysis cohort included 1,283 with an AER <30 mg/24 h at baseline.
The degree of completeness of the seven-point testing data has been reported (18). Among the 1,440 participants included in this analysis, blood glucose testing data were available for 32,528 (88%) of the possible 37,057 quarterly data collections, with the seven-point testing profile complete for 24,892 (77%) of those. To address the missing glucose data, the multiple imputation method applied by Lachin et al. (18) in prior analyses of the DCCT data set was used.
TIR 70–180 mg/dL was computed each quarter by calculating the percentage of the seven-point profile samples that were 70–180 mg/dL. In addition, the following glucose metrics were similarly computed: mean glucose, percentage time >180 mg/dL, percentage time >250 mg/dL, area under the curve 180 mg/dL, and high blood glucose index (19).
To assess the association of each glycemic metric with the retinopathy and microalbuminuria outcomes, two separate discrete Cox proportional hazards regression models were constructed to assess the effects of each glucose metric on the retinopathy outcome and the microalbuminuria outcome. One model was unadjusted, and the other model was stratified by the Early Treatment Diabetic Retinopathy Study (ETDRS) level of retinopathy at baseline and adjusted for duration of diabetes separately for the primary and secondary cohorts. The average of each glucose metric up to each event time was used as a time-dependent covariate in the model unless otherwise specified. The treatment group difference for each glucose metric was assessed using linear regression models adjusting for the glucose metric at baseline.
All reported CIs are at the 95% level. Analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC).
Results
Criteria for the retinopathy outcome were met by 271 of 1,440 participants (19%), and criteria for the microalbuminuria outcome were met by 116 of 1,283 participants (9%).
TIR
Mean TIR for the 1,440 participants was 41 ± 16%: 52 ± 10% in the intensive treatment group versus 31 ± 12% in the conventional treatment group (P < 0.001). The full distribution of TIR values according to treatment group can be seen in Supplementary Fig. 1. Among the glucose values out of the range of 70–180 mg/dL, a median of 84% were >180 mg/dL and 16% were <70 mg/dL. The correlation of mean TIR with mean A1C was −0.79.
For each outcome, TIR was substantially lower in those developing the microvascular complication compared with those not developing the outcome (Supplementary Table 1). Mean TIR was 32 ± 15% in the 271 participants developing the retinopathy outcome compared with 44 ± 15% in the 1,169 participants not developing the retinopathy outcome. Similarly for microalbuminuria, mean TIR was 32 ± 14% in the 116 participants developing the outcome versus 42 ± 16% in the 1,167 participants not developing the outcome.
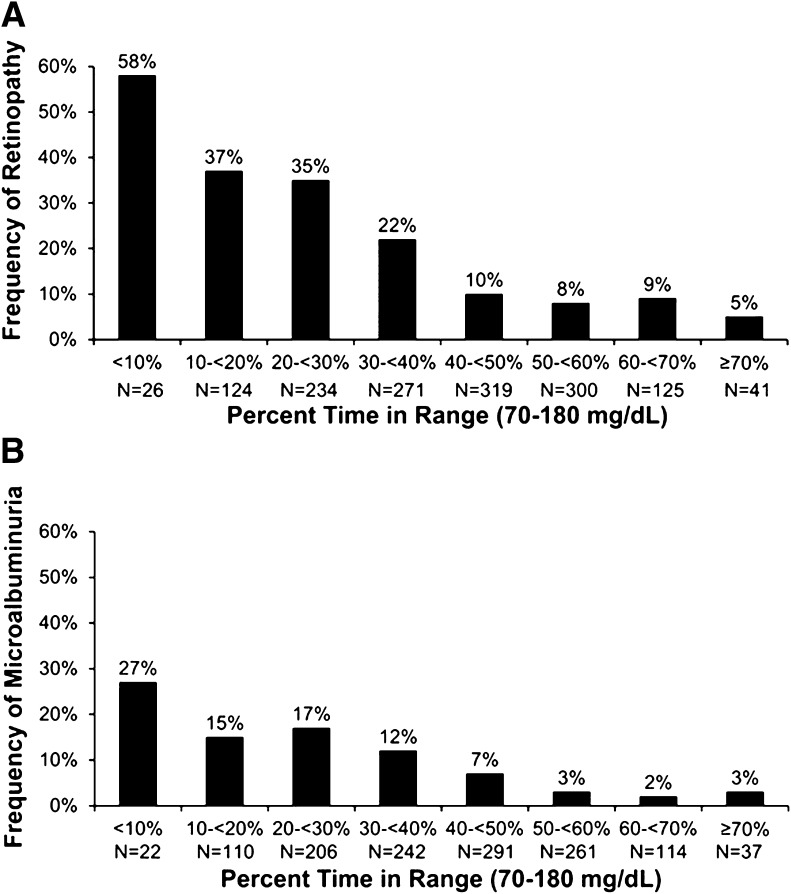
In concert with these findings, hazard ratios for the development of retinopathy or microalbuminuria were increased substantially with lower time in range (Fig. 1 and Table 1). The adjusted hazard rate of development of the retinopathy outcome was increased by 64% (95% CI 51–78), and development of the microalbuminuria outcome was increased by 40% (95% CI 25–56), for each 10 percentage points lower TIR (Table 2) (P < 0.001 for each). Results were similar in analyses conducted separately on the primary and secondary cohorts based on baseline retinopathy level (Tables 1 and 2).
Figure 1.
Frequency of development of microvascular complication according to level of TIR (70–180 mg/dL) computed from quarterly seven-point blood glucose testing. A: Retinopathy. B: Microalbuminuria.
Table 1.
Hazard ratios for development of retinopathy and microalbuminuria outcomes according to TIR
| Retinopathy outcome |
Microalbuminuria outcome |
|||||||
|---|---|---|---|---|---|---|---|---|
| TIR | N | N (%) with outcome | Unadjusted HR (95% CI)* | Adjusted HR (95% CI)† | N | N (%) with outcome | Unadjusted HR (95% CI)* | Adjusted HR (95% CI)† |
| Overall | ||||||||
| ≥50% | 466 | 36 (8) | 1.00 | 1.00 | 412 | 10 (2) | 1.00 | 1.00 |
| 40 to <50% | 319 | 32 (10) | 1.54 (0.94–2.55) | 1.61 (0.97–2.65) | 291 | 20 (7) | 2.44 (1.05–5.67) | 2.40 (1.03–5.58) |
| 30 to <40% | 271 | 59 (22) | 3.38 (2.22–5.15) | 3.37 (2.20–5.15) | 242 | 29 (12) | 4.74 (2.16–10.40) | 4.39 (2.01–9.58) |
| <30% | 384 | 144 (38) | 6.23 (4.25–9.13) | 6.93 (4.69–10.24) | 338 | 57 (17) | 6.68 (3.35–13.29) | 6.98 (3.49–13.96) |
| Primary cohort | ||||||||
| ≥50% | 228 | 8 (4) | 1.00 | 1.00 | 222 | 3 (1) | 1.00 | 1.00 |
| 40 to <50% | 159 | 10 (6) | 2.43 (0.91–6.53) | 2.43 (0.90–6.54) | 153 | 4 (3) | 1.25 (0.19–8.41) | 1.24 (0.18–8.36) |
| 30 to <40% | 131 | 25 (19) | 7.08 (3.09–16.23) | 6.51 (2.82–15.02) | 123 | 6 (5) | 3.68 (1.00–13.47) | 3.61 (0.98–13.21) |
| <30% | 208 | 64 (31) | 11.29 (5.13–24.85) | 11.16 (5.05–24.64) | 200 | 23 (12) | 7.35 (2.22–24.26) | 7.24 (2.19–23.94) |
| Secondary cohort | ||||||||
| ≥50% | 238 | 28 (12) | 1.00 | 1.00 | 190 | 7 (4) | 1.00 | 1.00 |
| 40 to <50% | 160 | 22 (14) | 1.30 (0.73–2.31) | 1.38 (0.77–2.45) | 138 | 16 (12) | 2.91 (1.05–8.02) | 2.92 (1.05–8.08) |
| 30 to <40% | 140 | 34 (24) | 2.39 (1.43–3.97) | 2.50 (1.50–4.17) | 119 | 23 (19) | 5.11 (1.91–13.65) | 4.76 (1.79–12.68) |
| <30% | 176 | 80 (45) | 4.95 (3.16–7.77) | 5.72 (3.60–9.09) | 138 | 34 (25) | 6.93 (2.90–16.59) | 6.78 (2.83–16.25) |
Strata based on TIR averaged over the entire DCCT study period. HR, hazard ratio.
*From discrete Cox proportional hazards regression models. P value, computed using TIR as a time-dependent continuous variable, is <0.001 for each cohort. †From discrete Cox proportional hazards regression models stratified by the ETDRS level of retinopathy at baseline and adjusted for the pre-DCCT glycemic exposure represented by the preexisting duration of diabetes separately for the primary and secondary cohorts. P value, computed using TIR as a time-dependent continuous variable, is <0.001 for each cohort. An additional model which included age and sex as covariates produced similar results.
Table 2.
Hazard ratios for development of retinopathy and microalbuminuria outcomes according to glycemic metrics
| Retinopathy outcome |
Microalbuminuria outcome |
||||
|---|---|---|---|---|---|
| HR per change of | Unadjusted HR (95% CI)* | Adjusted HR (95% CI)† | Unadjusted HR (95% CI)* | Adjusted HR (95% CI)† | |
| Overall | |||||
| TIR | 10% | 1.58 (1.46–1.71) | 1.64 (1.51–1.78) | 1.37 (1.23–1.53) | 1.40 (1.25–1.56) |
| Mean glucose | 15 mg/dL | 1.20 (1.17–1.23) | 1.22 (1.19–1.26) | 1.15 (1.10–1.20) | 1.16 (1.11–1.21) |
| Time >180 mg/dL | 10% | 1.48 (1.39–1.57) | 1.52 (1.43–1.62) | 1.31 (1.20–1.43) | 1.33 (1.21–1.45) |
| Time >250 mg/dL | 10% | 1.43 (1.36–1.51) | 1.48 (1.40–1.57) | 1.30 (1.21–1.41) | 1.33 (1.22–1.44) |
| AUC 180 mg/dL | 20 | 1.34 (1.28–1.40) | 1.39 (1.32–1.45) | 1.27 (1.18–1.36) | 1.29 (1.20–1.39) |
| HBGI | 5 | 1.36 (1.30–1.42) | 1.40 (1.34–1.47) | 1.27 (1.19–1.37) | 1.30 (1.21–1.40) |
| A1C | 0.5% | 1.32 (1.27–1.37) | 1.37 (1.31–1.42) | 1.22 (1.15–1.28) | 1.24 (1.17–1.31) |
| Primary cohort | |||||
| TIR | 10% | 1.71 (1.50–1.94) | 1.73 (1.52–1.97) | 1.61 (1.31–1.97) | 1.61 (1.31–1.98) |
| Mean glucose | 15 mg/dL | 1.24 (1.18–1.30) | 1.24 (1.19–1.30) | 1.22 (1.14–1.31) | 1.22 (1.14–1.31) |
| Time >180 mg/dL | 10% | 1.56 (1.41–1.73) | 1.58 (1.42–1.75) | 1.46 (1.24–1.72) | 1.46 (1.24–1.72) |
| Time >250 mg/dL | 10% | 1.50 (1.38–1.64) | 1.53 (1.40–1.67) | 1.43 (1.24–1.64) | 1.43 (1.24–1.65) |
| AUC 180 mg/dL | 20 | 1.43 (1.32–1.54) | 1.43 (1.33–1.55) | 1.40 (1.25–1.57) | 1.40 (1.25–1.56) |
| HBGI | 5 | 1.44 (1.33–1.55) | 1.45 (1.34–1.57) | 1.41 (1.26–1.59) | 1.41 (1.25–1.58) |
| A1C | 0.5% | 1.38 (1.30–1.46) | 1.41 (1.32–1.50) | 1.25 (1.13–1.37) | 1.25 (1.14–1.37) |
| Secondary cohort | |||||
| TIR | 10% | 1.53 (1.39–1.69) | 1.59 (1.43–1.76) | 1.32 (1.15–1.51) | 1.31 (1.14–1.49) |
| Mean glucose | 15 mg/dL | 1.18 (1.15–1.23) | 1.21 (1.16–1.25) | 1.13 (1.08–1.20) | 1.13 (1.07–1.19) |
| Time >180 mg/dL | 10% | 1.45 (1.34–1.56) | 1.49 (1.37–1.62) | 1.28 (1.15–1.42) | 1.27 (1.14–1.41) |
| Time >250 mg/dL | 10% | 1.41 (1.32–1.51) | 1.46 (1.35–1.57) | 1.29 (1.16–1.42) | 1.28 (1.15–1.41) |
| AUC 180 mg/dL | 20 | 1.31 (1.24–1.39) | 1.36 (1.28–1.44) | 1.23 (1.12–1.35) | 1.23 (1.11–1.35) |
| HBGI | 5 | 1.33 (1.26–1.41) | 1.37 (1.29–1.46) | 1.24 (1.13–1.36) | 1.23 (1.12–1.36) |
| A1C | 0.5% | 1.30 (1.24–1.37) | 1.34 (1.27–1.41) | 1.24 (1.15–1.33) | 1.23 (1.14–1.32) |
AUC, area under the curve; HBGI, high blood glucose index; HR, hazard ratio.
*From discrete Cox proportional hazards regression models using a time-dependent version of each glucose metric. P value <0.001 for each model.
†From discrete Cox proportional hazards regression models using a time-dependent version of each glucose metric stratified by the ETDRS level of retinopathy at baseline and adjusted for the pre-DCCT glycemic exposure represented by the preexisting duration of diabetes separately for the primary and secondary cohorts. P value <0.001 for each model. An additional model which included age and sex as covariates produced similar results.
Other Glycemic Metrics
Mean glucose concentration and hyperglycemia metrics computed from seven-point testing correlated with TIR from −0.91 to −0.97 and with A1C by 0.81 to 0.84. Higher mean glucose concentrations and higher degrees of hyperglycemia were present in those with versus without each outcome (P < 0.001 for each metric for each outcome) (Supplementary Table 1) and associated with increased hazard ratios for the development of the outcomes (P < 0.001 for each metric for each outcome) (Table 2 and Supplementary Table 2). Similar to TIR, there was a substantial difference between treatment groups in each metric (P < 0.001 for each hyperglycemic metric) (Table 3).
Table 3.
Summary of glucose metrics according to treatment group
| Treatment group |
||
|---|---|---|
| Intensive (n = 711) | Conventional (n = 729) | |
| % TIR | 52 ± 10 | 31 ± 12 |
| Mean glucose (mg/dL) | 160 ± 30 | 233 ± 47 |
| % Time >180 mg/dL | 34 ± 12 | 64 ± 15 |
| % Time >250 mg/dL | 13 (8, 20) | 41 (30, 53) |
| AUC 180 mg/dL | 23 (15, 35) | 71 (49, 97) |
| HBGI | 8 (6, 11) | 21 (16, 27) |
| A1C (%) | 7.3 ± 1.0 | 9.1 ± 1.3 |
| % Time <70 mg/dL | 13 (9, 17) | 5 (2, 8) |
| % Time <54 mg/dL | 5 (3, 7) | 2 (1, 3) |
| Coefficient of variation (%) | 53 ± 7 | 46 ± 7 |
Values shown are mean ± SD or median (quartiles) as appropriate for the distribution. For treatment group comparison of each metric, P value <0.001 from a linear regression model adjusted for the baseline value. Due to skewed distributions, van der Waerden scores were used for % time >250 mg/dL, AUC 180 mg/dL, HBGI, % time <70 mg/dL, and % time <54 mg/dL. AUC, area under the curve; HBGI, high blood glucose index.
Conclusions
Using the DCCT data set, we have demonstrated that TIR of 70–180 mg/dL, computed from quarterly seven-point blood glucose testing, has a strong association with the risk of development or progression of retinopathy and development of microalbuminuria. TIR was substantially higher in the DCCT intensive treatment group (52%) compared with the conventional therapy group (31%). The findings, which parallel the DCCT A1C results, were similar for mean glucose and hyperglycemia metrics computed from the seven-point testing. Prior studies analyzing the DCCT data have demonstrated an association between mean glucose computed from the seven-point testing and microvascular complications (20–23), but we are not aware of a prior analysis of TIR. The difference in mean TIR between those developing and not developing a microvascular outcome was 10–12%, representing about 2.5 h/day and an A1C difference of 1.0–1.4%.
With advances in CGM technology, including accuracy and ease of use, CGM has supplanted seven-point testing as the optimal method to obtain information on glycemia throughout the day. It is reasonable to assume that the association of seven-point testing–measured TIR with complications also would apply to CGM-measured TIR, particularly because two studies have demonstrated similar TIR results comparing CGM and blood glucose measurements. In a study of 161 subjects conducted by the Diabetes Research in Children Network (DirecNet), mean TIR was 49% measured with CGM and 50% measured with eight-point testing (an overnight measurement added to the seven-point profile) (24). In another study combining data from six inpatient studies, mean TIR was 60% with both CGM and with paired blood glucose measurements made with a YSI analyzer or by a central laboratory (25). Because the amount of glucose data available to compute TIR and other metrics from CGM is so substantially greater than the amount from periodic seven-point testing, it is possible that the association of CGM-measured TIR with the risk of microvascular complications could be of even greater magnitude than that found using quarterly seven-point testing. Studies have shown that 10–14 days of CGM data provide for an estimate of TIR and other metrics that correlates well with 3 months of CGM data (26).
There are certain limitations inherent in using seven-point testing data compared with CGM data. With seven-point testing, there is limited ability to assess glycemic variability and interday variation within and between individuals. In addition, the seven-point data are solely from daytime, and thus the calculation of TIR and the other metrics does not include the overnight period. This could produce an underestimate of TIR; however, it is unlikely to have an effect on the association of TIR with complications. Although a similar association between TIR and microvascular complications might be expected with type 2 diabetes, it is important to recognize that the DCCT cohort consists solely of individuals with type 1 diabetes. During the DCCT, there were too few cardiovascular events for a meaningful assessment of the association of TIR with such events.
A1C remains a valuable metric as an outcome in clinical trials. It can be measured with precision and does not rely on participant use of a device. However, there are studies where CGM-measured TIR or other glycemia metrics would have advantages as an outcome metric. Based on these results, a compelling case can be made that TIR is strongly associated with the risk of microvascular complications and should be an acceptable end point for clinical trials.
Supplementary Material
Article Information
Acknowledgments. James S. Hirsch, Needham, MA, provided editing assistance.
Funding. The DCCT and its follow-up, the Epidemiology of Diabetes Interventions and Complications (EDIC) study, were conducted by the DCCT/EDIC Research Group and supported by National Institutes of Health grants and contracts and by the General Clinical Research Center Program, National Center for Research Resources. The data from the DCCT/EDIC study were supplied by the NIDDK Central Repositories. This manuscript was not prepared under the auspices of the DCCT/EDIC study and does not represent analyses or conclusions of the DCCT/EDIC study group, the NIDDK Central Repositories, or the National Institutes of Health. Funding to support the conduct of the analyses reported herein was provided by the Jaeb Center for Health Research Foundation.
Duality of Interest. Jaeb Center for Health Research has received research funding or study supplies from Abbott, Ascenia, Bigfoot, Dexcom, Roche, and Tandem and consulting fees from Insulet and Eli Lilly. Health Partners Institute contracts for R.M.B’s services, which include research support, consulting, or scientific advisory board participation for Abbott Diabetes Care, Becton Dickinson, Dexcom, Eli Lilly, Glooko, Hygieia, Johnson & Johnson, Medtronic, Merck, Novo Nordisk, Roche, and Sanofi. The diaTribe Foundation receives donations from a number of manufacturers and academic institutions in the diabetes field. Several academic institutions, government bodies, and manufacturers in the diabetes field subscribe to Close Concerns’ fee-based newsletter, Closer Look. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. R.W.B. wrote the manuscript. R.M.B. contributed to the manuscript and reviewed and edited the manuscript. T.D.R., C.K., and Z.L. performed statistical analyses and reviewed and edited the manuscript. A.S.B. and K.L.C. reviewed and edited the manuscript. R.W.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-1444/-/DC1.
See accompanying article, p. 345.
References
- 1.Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care 2017;40:994–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welsh KJ, Kirkman MS, Sacks DB. Role of glycated proteins in the diagnosis and management of diabetes: research gaps and future directions. Diabetes Care 2016;39:1299–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malka R, Nathan DM, Higgins JM. Mechanistic modeling of hemoglobin glycation and red blood cell kinetics enables personalized diabetes monitoring. Sci Transl Med 2016;8:359ra130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yudkin JS, Forrest RD, Jackson CA, Ryle AJ, Davie S, Gould BJ. Unexplained variability of glycated haemoglobin in non-diabetic subjects not related to glycaemia. Diabetologia 1990;33:208–215 [DOI] [PubMed] [Google Scholar]
- 5.Foster NC, Miller K, DiMeglio L, et al. . Marked increases in CGM use has not prevented increases in HbA1c levels in participants in the T1D Exchange (T1DX) Clinic Network. Diabetes 2018;67(Suppl. 1):1689-P [Google Scholar]
- 6.Agiostratidou G, Anhalt H, Ball D, et al. . Standardizing clinically meaningful outcome measures beyond HbA1c for type 1 diabetes: a consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care 2017;40:1622–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danne T, Nimri R, Battelino T, et al. . International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beyond A1C Writing Group Need for regulatory change to incorporate beyond A1C glycemic metrics. Diabetes Care 2018;41:e92–e94 [DOI] [PubMed] [Google Scholar]
- 9.Runge AS, Kennedy L, Brown AS, et al. . Does time-in-range matter? Perspectives from people with diabetes on the success of current therapies and the drivers of improved outcomes. Clin Diabetes 2018;36:112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 11.Brod M, Christensen T, Thomsen TL, Bushnell DM. The impact of non-severe hypoglycemic events on work productivity and diabetes management. Value Health 2011;14:665–671 [DOI] [PubMed] [Google Scholar]
- 12.Fiallo-Scharer R, Cheng J, Beck RW, et al.; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group . Factors predictive of severe hypoglycemia in type 1 diabetes: analysis from the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized control trial dataset. Diabetes Care 2011;34:586–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brod M, Pohlman B, Wolden M, Christensen T. Non-severe nocturnal hypoglycemic events: experience and impacts on patient functioning and well-being. Qual Life Res 2013;22:997–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seaquist ER, Anderson J, Childs B, et al. . Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013;36:1384–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Hypoglycaemia Study Group Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2017;40:155–157 [DOI] [PubMed] [Google Scholar]
- 16.Novodvorsky P, Bernjak A, Chow E, et al. . Diurnal differences in risk of cardiac arrhythmias during spontaneous hypoglycemia in young people with type 1 diabetes. Diabetes Care 2017;40:655–662 [DOI] [PubMed] [Google Scholar]
- 17.The DCCT Research Group The Diabetes Control and Complications Trial (DCCT): design and methodologic considerations for the feasibility phase. Diabetes 1986;35:530–545 [PubMed] [Google Scholar]
- 18.Lachin JM, Bebu I, Bergenstal RM, et al.; DCCT/EDIC Research Group . Association of glycemic variability in type 1 diabetes with progression of microvascular outcomes in the Diabetes Control and Complications Trial. Diabetes Care 2017;40:777–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovatchev BP. Metrics for glycaemic control – from HbA1c to continuous glucose monitoring. Nat Rev Endocrinol 2017;13:425–436 [DOI] [PubMed] [Google Scholar]
- 20.Kilpatrick ES, Rigby AS, Atkin SL. The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care 2006;29:1486–1490 [DOI] [PubMed] [Google Scholar]
- 21.Kilpatrick ES, Rigby AS, Atkin SL. Effect of glucose variability on the long-term risk of microvascular complications in type 1 diabetes. Diabetes Care 2009;32:1901–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathan DM, McGee P, Steffes MW, Lachin JM; DCCT/EDIC Research Group . Relationship of glycated albumin to blood glucose and HbA1c values and to retinopathy, nephropathy, and cardiovascular outcomes in the DCCT/EDIC study. Diabetes 2014;63:282–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Service FJ, O’Brien PC. The relation of glycaemia to the risk of development and progression of retinopathy in the Diabetic Control and Complications Trial. Diabetologia 2001;44:1215–1220 [DOI] [PubMed] [Google Scholar]
- 24.Fiallo-Scharer R; Diabetes Research in Children Network Study Group . Eight-point glucose testing versus the continuous glucose monitoring system in evaluation of glycemic control in type 1 diabetes. J Clin Endocrinol Metab 2005;90:3387–3391 [DOI] [PubMed] [Google Scholar]
- 25.Beck RW, Calhoun P, Kollman C. Use of continuous glucose monitoring as an outcome measure in clinical trials. Diabetes Technol Ther 2012;14:877–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xing D, Kollman C, Beck RW, et al.; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group . Optimal sampling intervals to assess long-term glycemic control using continuous glucose monitoring. Diabetes Technol Ther 2011;13:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.