Abstract
OBJECTIVE
In people with type 2 diabetes, sodium–glucose cotransporter 2 inhibitors (SGLT2i) reduce cardiovascular risk and progression of diabetic kidney disease. Our aim was to determine whether sotagliflozin (SOTA), a dual SGLT1i and SGLT2i, had favorable effects on clinical biomarkers suggestive of kidney protection in adults with type 1 diabetes.
RESEARCH DESIGN AND METHODS
In this 52-week pooled analysis, 1,575 adults enrolled in the inTandem1 and inTandem2 trials were randomized to SOTA 200 mg, 400 mg, or placebo in addition to optimized insulin therapy. Changes in cardiorenal biomarkers were assessed.
RESULTS
At 52 weeks, in response to SOTA 200 and 400 mg, the placebo-corrected least squares mean change from baseline in estimated glomerular filtration rate was −2.0 mL/min/1.73 m2 (P = 0.010) and −0.5 mL/min/1.73 m2 (P = 0.52), respectively. Systolic blood pressure difference was −2.9 and −3.6 mmHg (P < 0.0001 for both); diastolic blood pressure changed by −1.4 (P = 0.0033) and −1.6 mmHg (P = 0.0008). In participants with baseline urinary albumin-to-creatinine ratio (UACR) ≥30 mg/g, UACR decreased by 23.7% (P = 0.054) and 18.3% (P = 0.18) for SOTA 200 and SOTA 400 mg, respectively, versus placebo. Increases in serum albumin and hematocrit and reductions in uric acid were observed throughout 52 weeks with both SOTA doses.
CONCLUSIONS
SOTA was associated with short- and long-term renal hemodynamic changes, which were similar to those seen with SGLT2i in type 2 diabetes. Further investigation around cardiorenal effects of SOTA in people with type 1 diabetes is justified.
Introduction
Diabetic kidney disease occurs in ∼20–40% of people with type 1 diabetes despite management of traditional renal risk factors (1). Sodium–glucose cotransporter 2 inhibitors (SGLT2i) act by blocking tubular glucose reuptake, leading to glucosuria and thereby lowering HbA1c and body weight. In addition to glucosuric effects, SGLT2i are natriuretic, leading to contraction of plasma volume, systolic blood pressure (SBP) lowering, and increases in hematocrit and serum albumin (2,3). Natriuresis also attenuates glomerular hyperfiltration by lowering intraglomerular pressure via activation of tubuloglomerular feedback, an effect that has been shown in mechanistic studies in young adults with type 1 diabetes (4,5). In the setting of type 2 diabetes, SGLT2i induce a drop in estimated glomerular filtration rate (eGFR) that stabilizes over time and also decrease albuminuria (6) and tubular injury (7,8). In addition, in cardiovascular (CV) safety trials in people with type 2 diabetes, SGLT2i improve albuminuria progression and hard renal outcomes (9–11), independent of glucose lowering (10,12). Sodium–glucose cotransporter (SGLT)2 inhibition–related natriuresis has also been linked with improved CV outcomes, as reflected by the association between increased hematocrit—as a marker of hemoconcentration—in the BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) and the reduction in CV death (13). From a metabolic perspective, consistent with type 2 diabetes data, SGLT2i reduce HbA1c and body weight, generally without increasing the risk of significant hypoglycemia, in people with type 1 diabetes (14–19).
Sotagliflozin (SOTA) is a dual inhibitor of SGLT1 and SGLT2. In addition to renal SGLT2 inhibition and its effect on urinary glucose excretion (UGE), SOTA reduces postprandial hyperglycemia by blunting glucose absorption via local SGLT1 inhibition in the gut (20). The efficacy and safety of SOTA in adults with type 1 diabetes have been studied in three phase 3 clinical studies: inTandem1, inTandem2, and inTandem3 (clinical trial reg. nos. NCT02384941, NCT02421510, NCT02531035, ClinicalTrials.gov) (21–23). In these trials, placebo-corrected HbA1c change from baseline ranged from −0.35% to −0.46% (P < 0.001) at week 24, with −2.0 to −3.5 kg (P < 0.001) reduction in body weight and no increased risk of hypoglycemia (21–23). These effects were maintained at week 52 in the inTandem1 and inTandem2 trials.
Despite what is known about glycemia-related parameters, the effects of dual SGLT1 and SGLT2 inhibition with SOTA on renal function, albuminuria, blood pressure, and hematocrit (as a marker for plasma volume) in people with type 1 diabetes have not yet been examined. An in-depth understanding of how SOTA impacts clinical parameters associated with CV and renal protection in people with type 1 diabetes is crucial to determine the rationale for long-term clinical outcome trials in this population. Accordingly, in the current analysis, our aim was to determine whether dual SGLT1 and SGLT2 inhibition with SOTA over a 52-week treatment period led to changes in eGFR, albuminuria, and blood pressure suggestive of renal protection in people with type 1 diabetes.
Research Design and Methods
Study Design and Population
The inTandem1 and inTandem2 trials are two multicenter, randomized, double-blind, placebo-controlled, parallel-group 52-week phase 3 studies of adults age 18 years and older with type 1 diabetes (21,22). The inTandem1 study was conducted between March 2015 and February 2017 at 75 study sites in the U.S. and Canada, whereas the inTandem2 study was conducted between May 2015 and June 2017 at 96 study sites in European countries and Israel. In brief, eligible participants (n = 1,575) with HbA1c at screening of 7.0–11.0% (53–97 mmol/mol) and eGFR >45 mL/min/1.73 m2 went through a 6-week insulin optimization period before randomization to SOTA (200 mg or 400 mg) or placebo. Following randomization, participants entered a 24-week, double-blind core treatment period, a 28-week double-blind long-term extension period, a 1-week laboratory follow-up period, and a final 30-day follow-up period. The primary outcome of both studies was change in HbA1c from baseline to week 24. The studies were conducted in accordance with international standards of good clinical practice and with approval by local institutional review boards (21,22).
End Points
In this pooled analysis, measures of kidney function included changes in eGFR and urine albumin-to-creatinine ratio (UACR) from baseline up to week 52. We also analyzed cardiorenal risk factors including body weight, SBP, and diastolic blood pressure (DBP), as well as other biochemical markers, such as hematocrit, serum albumin, and uric acid. The effect of SOTA was assessed in the overall population and subpopulation with follow-up records.
Urinary albumin and creatinine were obtained at baseline; at weeks 12, 24, and 52; and during the follow-up period from spot urine sample and were used to derive albumin-to-creatinine ratio. Serum creatinine was obtained from clinical chemistry samples and used to calculate eGFR using the MDRD study equation as per the study protocol. In a sensitivity analysis, eGFR according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula was calculated at baseline and over time. Vital signs, physical examination, and serum chemistry including uric acid and albumin were measured at each study time point (at screening, baseline, and weeks 4, 8, 12, 16, 20, 24, 32, 40, and 52 and during the follow-up period); hematocrit was measured at screening, baseline, and weeks 12, 24, 52 and during the follow-up period.
Statistical Analyses
Our analyses comprised the pooled population of inTandem1 and inTandem2 with all randomized participants who had taken at least one dose of study drug (modified intent-to-treat population). Data from the inTandem3 study were not included in this analysis due to differences in the trial design (24 weeks and no insulin optimization) and because inTandem3 did not include the SOTA 200 mg dose.
All analyses, except UACR and DBP subgroups, were prespecified. Prespecified analyses were also conducted with a subset of participants who had an “off drug” laboratory record during the laboratory follow-up period. “Off drug” is defined as 7 days after the last dose, with a window of 5–28 days. Post hoc analyses were conducted for change in UACR with baseline albumin status and change in DBP based on subgroups of DBP at baseline.
Analysis of the continuous efficacy end points postbaseline used a mixed-effect model repeat measure (MMRM) under the missing-at-random framework based on the restricted maximum likelihood method for estimation. All postbaseline observations collected during the treatment period were used in the MMRM analysis. The analysis model included fixed categorical effects of treatment, randomization strata of insulin delivery method (multiple daily injection, continuous subcutaneous insulin infusion), randomization strata of week −2 HbA1c (≤8.5% and >8.5% [≤69 mmol/mol and >69 mmol/mol]), study, time (study week), a treatment-by-time interaction, and baseline value (of the corresponding analysis variable)-by-time interaction as a covariate. Continuous end points assessed at off drug (a single postbaseline time point) were analyzed using ANCOVA models and used the observed-case data set. For UACR, geometric mean was used instead of arithmetic mean to control for the right-skewed data distribution. UACR was log10 transformed and was back transformed to obtain the geometric means. All statistical tests comparing treatment effects were two sided with a 5% significance level.
Data Availability
Qualified researchers may request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and data set specifications. Patient-level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com/.
Results
Baseline Characteristics
The baseline characteristics of the study population are shown in Table 1. In brief, participants were mostly of non-Hispanic white ethnicity and ∼50 years old with average diabetes duration of 20 years and normal renal function.
Table 1.
Baseline characteristics (after insulin therapy optimization) of participants randomized in the pooled analysis group
| Pooled analysis group (inTandem1 and inTandem2) |
|||
|---|---|---|---|
| Placebo | SOTA 200 mg | SOTA 400 mg | |
| Randomized, n | 526 | 524 | 525 |
| Age, years, mean (SD) | 42.5 (13.3) | 44.4 (13.7) | 44.0 (13.4) |
| Female, % | 48.5 | 49.4 | 51.8 |
| White race, % | 93.9 | 94.1 | 94.5 |
| Diabetes duration, years, mean (SD) | 21.2 (12.0) | 21.6 (12.5) | 21.5 (12.3) |
| CSII, %/MDI, % | 43.0/57.0 | 42.7/57.3 | 42.7/57.3 |
| BMI, kg/m2, mean (SD) | 28.5 (5.3) | 28.9 (5.6) | 28.7 (5.2) |
| Weight, kg, mean (SD) | 84.3 (17.6) | 84.5 (18.1) | 84.2 (18.1) |
| HbA1c, %, mean (SD) | 7.7 (0.8) | 7.7 (0.8) | 7.6 (0.8) |
| HbA1c, mmol/mol, mean (SD) | 60.3 (8.8) | 60.4 (8.4) | 60.0 (8.5) |
| Total daily insulin, IU/kg, mean (SD) | 0.75 (0.3) | 0.73 (0.3) | 0.73 (0.30) |
| SBP, mmHg, mean (SD) | 122.0 (14.6) | 121.5 (15.0) | 121.3 (14.3) |
| eGFR (MDRD), mL/min/1.73 m2 | |||
| Mean (SD) | 90.2 (18.5) | 89.3 (19.6) | 89.1 (18.3) |
| <60, n (%) | 24 (4.6) | 22 (4.2) | 25 (4.8) |
| ≥60, n (%) | 502 (95.4) | 502 (95.8) | 500 (95.2) |
| eGFR (CKD-EPI), mL/min/1.73 m2 | |||
| Mean (SD) | 98.2 (18.1) | 96.5 (18.3) | 97.0 (17.7) |
| <60, n (%) | 17 (3.2) | 16 (3.1) | 12 (2.3) |
| ≥60, n (%) | 509 (96.8) | 508 (96.9) | 513 (97.7) |
| UACR, mg/g | |||
| Geometric mean (CI) | 8.9 (8.0, 9.8) | 9.6 (8.7, 10.7) | 8.7 (7.9, 9.7) |
| Median (Q1:Q3) | 6.6 (4.3:13.0) | 7.0 (4.4:14.1) | 6.3 (4.2:12.3) |
| <30, n (%) | 451 (87.7) | 439 (85.6) | 450 (88.4) |
| Median (Q1:Q3) | 5.7 (4.1:9.2) | 6.1 (4.2:9.2) | 5.6 (4.0:9.4) |
| ≥30, n (%) | 63 (12.3) | 74 (14.4) | 59 (11.6) |
| Median (Q1:Q3) | 56.2 (35.7:197.2) | 61.3 (39.9:155.1) | 83.3 (48.0:305.0) |
CSII, continuous subcutaneous insulin infusion; MDI, multiple daily injections of insulin; Q, quartile.
Effect of SOTA on eGFR
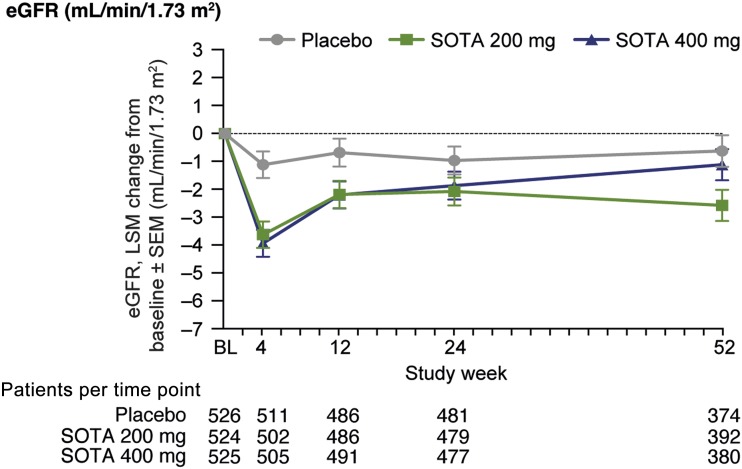
From a baseline eGFR of 89.3 mL/min/1.73 m2, the least squares (LS) mean changes were −2.5 and −2.8 mL/min/1.73 m2 (SE 0.6, P < 0.0001) in the 200 mg and 400 mg dose groups, respectively, versus placebo at week 4. From week 4 to 52, although lower than placebo, eGFR tended to return toward baseline. At 52 weeks, eGFR change from baseline was −2.0 mL/min/1.73 m2 (SE 0.8, P = 0.010) and −0.5 mL/min/1.73 m2 (SE 0.8, P = 0.52) for SOTA 200 mg and 400 mg, respectively, versus placebo (Fig. 1). Similar results were obtained when eGFR was calculated by CKD-EPI (Supplementary Fig. 1).
Figure 1.
eGFR change over time in overall population. LS mean (LSM) change from baseline (BL) vs. placebo at week 52 for SOTA 200 mg, −1.96 mL/min/1.73 m2 (95% CI −3.45, −0.47), P = 0.010, and SOTA 400 mg, −0.49 mL/min/1.73 m2 (−1.99, 1.00), P = 0.52.
In the subset of participants (n = 370) with off drug follow-up laboratory records, defined as 7 days after last dose, eGFR had returned to baseline compared with placebo: LS mean change from baseline to off drug records was 3.0 mL/min/1.73 m2 (SE 1.4, P = 0.031) for SOTA 200 mg and 2.7 mL/min/1.73 m2 (SE 1.3, P = 0.045) for SOTA 400 mg (Supplementary Fig. 2A). Placebo-corrected LS mean change from last on-treatment to off drug records also rose significantly with both SOTA 200 mg and 400 mg (Supplementary Fig. 3A).
Effect of SOTA on Albuminuria
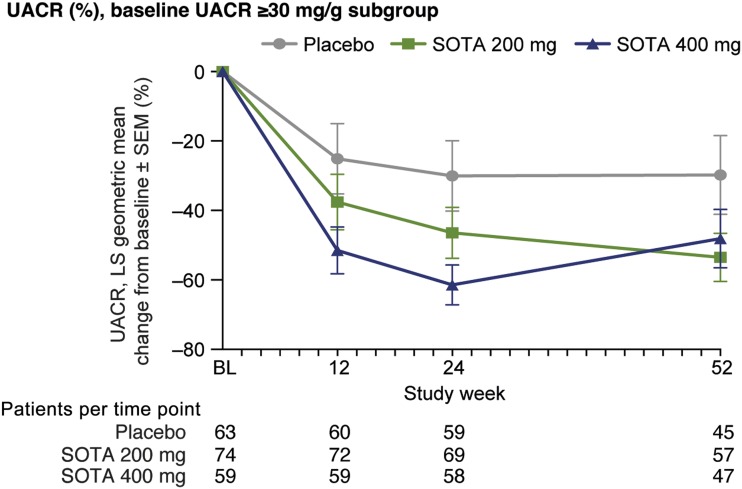
The majority of participants (87.2%) were normoalbuminuric at baseline (UACR <30 mg/g). In the post hoc analysis of a subgroup of participants (n = 196) with increased albuminuria (UACR ≥30 mg/g), LS mean UACR decreased by 16.4% (SE 12.0, P = 0.16) with the 200 mg dose and by 31.4% in the 400 mg dose group (SE 11.3, P = 0.0032) from baseline to week 24. At 52 weeks, UACR decreased by 23.7% (SE 12.9, P = 0.054) and 18.3% (SE 13.8, P = 0.18) for SOTA 200 mg and SOTA 400 mg versus placebo, respectively (Fig. 2).
Figure 2.
UACR change over time in subgroup of participants with baseline (BL) albuminuria (UACR ≥30 mg/g). Percentage change from baseline vs. placebo based on geometric mean estimated from MMRM model. At week 52, SOTA 200 mg, −23.7% (95% CI −48.9, 1.5), P = 0.054, and SOTA 400 mg, −18.3% (−45.3, 8.7), P = 0.18.
Effects of SOTA on Blood Pressure and Body Weight
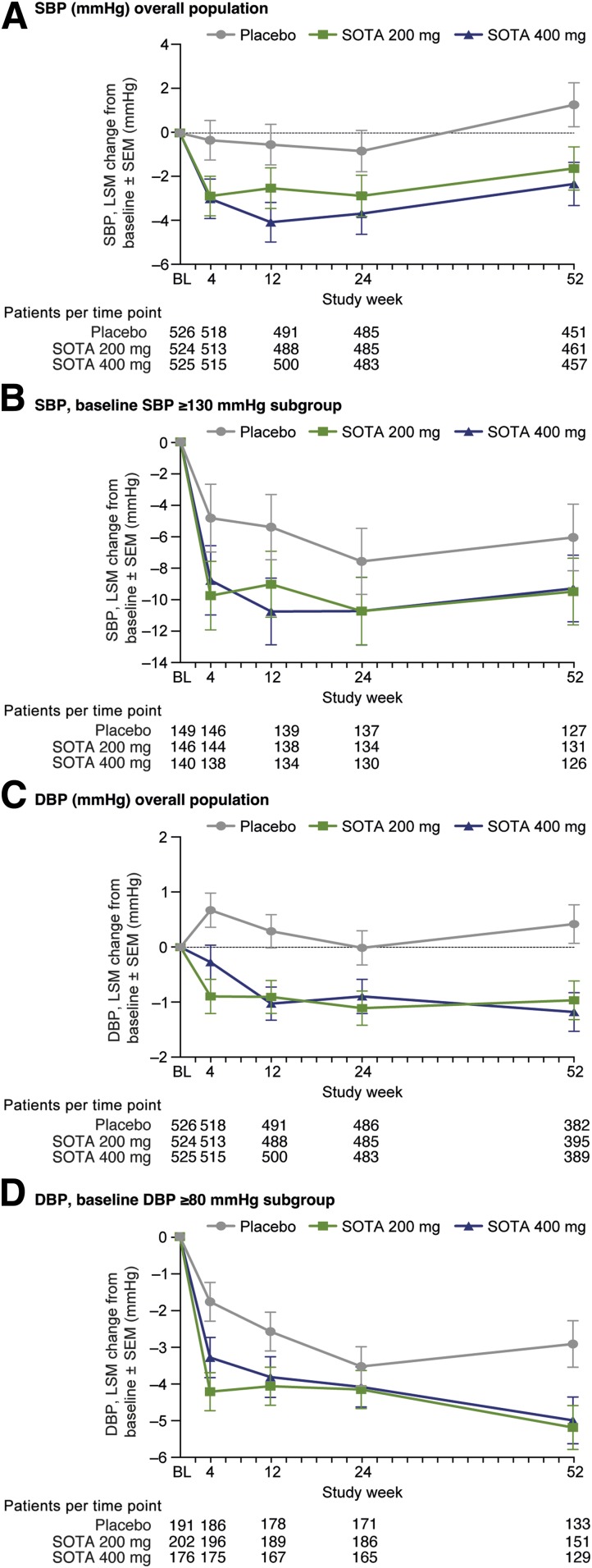
In the overall cohort, the placebo-corrected SBP LS mean change from baseline was −2.0 mmHg (SE 0.6, P = 0.0017) and −2.9 mmHg (SE 0.7, P < 0.0001) at week 12 and week 52, respectively, with SOTA 200 mg, and −3.5 mmHg (SE 0.6, P < 0.0001) and −3.6 mmHg (SE 0.7, P < 0.0001) with SOTA 400 mg (Fig. 3A).
Figure 3.
Blood pressure change over time. A: Changes in SBP in overall population. LS mean (LSM) change from baseline (BL) vs. placebo at week 52 for SOTA 200 mg, −2.9 mmHg (95% CI −4.3, −1.6), P < 0.0001, and SOTA 400 mg, −3.6 mmHg (−5.0 to −2.3), P < 0.0001. B: Changes in SBP in subgroup of patients with baseline SBP ≥130 mmHg. LS mean change from baseline vs. placebo at week 52 for SOTA 200 mg, −3.4 mmHg (−6.2, −0.7), P = 0.016, and SOTA 400 mg, −3.2 mmHg (−6.0, −0.4), P = 0.024. C: Changes in DBP in overall population. LS mean change from baseline vs. placebo at week 52 for SOTA 200 mg, −1.4 mmHg (−2.3, −0.5), P = 0.0033, and SOTA 400 mg, −1.6 mmHg (−2.5, −0.7), P = 0.0008. D: Changes in DBP in subgroup of patients with baseline DBP ≥80 mmHg. LS mean change from baseline vs. placebo at week 52 for SOTA 200 mg, −2.3 mmHg (−3.9, −0.6), P = 0.0064, and SOTA 400 mg, −2.1 mmHg (−3.8, −0.4), P = 0.016.
In the subgroup of participants who had SBP measurements after the washout period, mean SBP values for SOTA 200 mg and 400 mg arms tended to stay below mean values in the placebo group. Placebo-corrected LS mean changes from baseline or last on-treatment measurement to off drug measurement were not significant in either the 200 mg or 400 mg dose group (Supplementary Figs. 2B and 3B).
In a prespecified subgroup analysis, participants with SBP <130 mmHg at baseline, the mean placebo-corrected SBP change was −1.4 mmHg (SE 0.7, P = 0.047) with SOTA 200 mg and −2.8mmHg (SE 0.7, P < 0.0001) with SOTA 400 mg at 12 weeks. At 52 weeks, placebo-corrected changes were −2.7 mmHg (SE 0.8, P = 0.0005) and −3.7 mmHg (SE 0.8, P < 0.0001) with SOTA 200 mg and 400 mg, respectively.
In participants with SBP ≥130 mmHg at baseline, the mean placebo-corrected SBP change was −3.6 mmHg (SE 1.4, P = 0.010) with SOTA 200 mg and −5.4 mmHg (SE 1.4, P = 0.0002) with SOTA 400 mg at 12 weeks. At 52 weeks, placebo-corrected changes were −3.4 mmHg (SE 1.4, P = 0.016) and −3.2 mmHg (SE 1.4, P = 0.024) with SOTA 200 mg and 400 mg, respectively (Fig. 3B).
At 12 weeks, compared with placebo, the mean DBP change was −1.2 mmHg (SE 0.4, P = 0.0031) and −1.3 mmHg (SE 0.4, P = 0.0011) with SOTA 200 mg and SOTA 400 mg, respectively. At 52 weeks, placebo-corrected DBP changes were −1.4 mmHg (SE 0.5, P = 0.0033) and −1.6 mmHg (SE 0.5, P = 0.0008) with SOTA 200 mg and 400 mg, respectively (Fig. 3C).
In the subgroup of participants with available DBP measurements after a washout period, mean DBP values tended to increase back to values at baseline (Supplementary Fig. 2C). Placebo-corrected LS mean changes from baseline or last on-treatment measurement to off drug measurement were not significant in either the 200 mg or 400 mg dose groups (Supplementary Figs. 2C and 3C).
In the post hoc analysis with the subgroup of participants with baseline DBP ≥80 mmHg, mean placebo-corrected DBP at 52 weeks was −2.3 mmHg (SE 0.8, P = 0.0064) for SOTA 200 mg and −2.1 mmHg (SE 0.9, P = 0.016) for SOTA 400 mg (Fig. 3D).
Over 52 weeks, body weight was significantly decreased from baseline for both SOTA groups compared with placebo (P < 0.001). At week 24, the placebo-corrected LS mean differences from baseline were −2.2 kg for SOTA 200 mg and −3.0 kg for SOTA 400 mg (SE 0.2, P < 0.001, for both). This treatment difference persisted at week 52, with −2.7 kg and −3.6 kg (SE 0.2, P < 0.001, for both) for SOTA 200 mg and 400 mg versus placebo, respectively.
Effects of SOTA on Markers of Hemoconcentration and Plasma Uric Acid
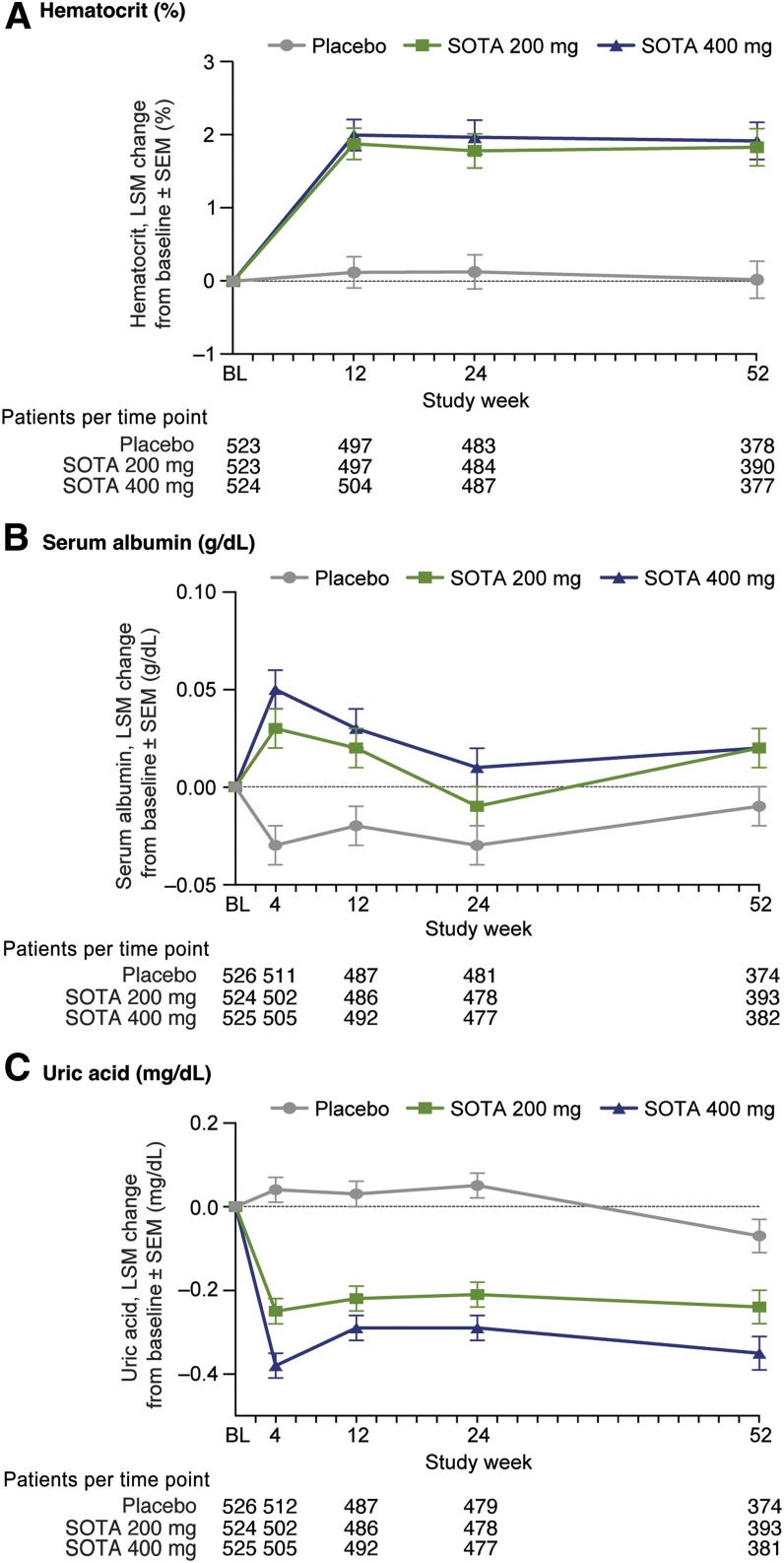
The mean hematocrit values were generally within normal ranges. A small increase of ∼4% relative to baseline hematocrit was observed by week 12 in the SOTA groups compared with the placebo group and appeared to be stable throughout the study. Mean serum hematocrit increased from 41.9% at baseline to 43.8% at week 12 for SOTA 200 mg and 42.0–44.0% for SOTA 400 mg. Relative to placebo, the LS mean difference was 1.8% and 1.9% for SOTA 200 mg and 400 mg, respectively (SE 0.2, P < 0.0001, for both). These changes persisted throughout the 52-week trial at both SOTA doses (P < 0.0001) (Fig. 4A and Supplementary Table 1). For participants with off drug hematocrit values, the LS mean change after washout versus placebo was significant in both the 200 mg and 400 mg dose groups (Supplementary Figs. 2D and 3D).
Figure 4.
A: Changes in hematocrit in overall population. B: Changes in serum albumin in overall population. C: Changes in uric acid in overall population. BL, baseline; LSM, LS mean.
Mean baseline serum albumin concentrations were similar, at ∼4.3 g/dL, for all groups. In response to SOTA, LS mean serum albumin increased 0.06 g/dL and 0.07 g/dL with SOTA 200 mg and 400 mg, respectively, at week 4 (SE 0.01, P < 0.0001, for both). At week 52, placebo-corrected LS mean change was 0.03 g/dL (SE 0.02, P = 0.036) for SOTA 200 mg and 0.03 g/dL (SE 0.02, P = 0.053) for SOTA 400 mg (Fig. 4B). For participants with off drug serum albumin values, the placebo-corrected LS mean serum albumin changes after washout were significant in both dose groups (Supplementary Figs. 2E and 3E).
SOTA also significantly reduced uric acid throughout 52 weeks (all P < 0.001). The placebo-corrected LS mean change in serum uric acid was −0.29 mg/dL and −0.42 mg/dL (SE 0.04, P < 0.0001, for both) at 4 weeks and −0.17 mg/dL (SE 0.05, P = 0.0003) and −0.28 mg/dL (SE 0.05, P < 0.0001) at 52 weeks for SOTA 200 mg and 400 mg, respectively (Fig. 4C and Supplementary Table 2). For participants with off drug serum uric acid values, the placebo-corrected LS mean uric acid changes after washout were not significant in both dose groups (Supplementary Figs. 2F and 3F).
Conclusions
In this pooled analysis of the inTandem1 and inTandem2 trials, the dual SGLT1i and SGLT2i SOTA showed beneficial effects on clinical parameters of cardiorenal health in adults with type 1 diabetes. The observed changes in factors that can be assessed in clinical practice—eGFR, UACR, blood pressure, hematocrit, serum albumin, and uric acid—mirror for the most part the effects observed in response to SGLT2i in trials with participants with type 2 diabetes. These findings are clinically important, as SGLT2i have been shown to reduce the risk of diabetic kidney disease progression, including hard renal end points, in various populations (9–11,24). It should be noted that despite these salutary effects on clinical outcomes, the mechanisms responsible for these benefits remain largely unknown. Yet, available analyses from published CV outcome trials strongly implicate glucose-independent mechanisms in cardiorenal benefits with SGLT2i (13).
In contrast with what is known in type 2 diabetes, in people with type 1 diabetes, existing clinical trial data (16,21–23,25,26) have reported improvements in glycemic control and body weight reduction. Because it is likely that there is substantial overlap between type 1 and type 2 diabetes in terms of factors leading to end-organ damage, it is crucial to understand whether inhibition of SGLTs has similar cardiorenal effects in people with type 1 diabetes in order to assess the potential for primary and secondary end-organ protection with these therapies.
As opposed to the more widely studied SGLT2i, SOTA is a dual SGLT1i and SGLT2i. Although SOTA concentrations are too low to inhibit SGLT1 in the kidney, it partially inhibits intestinal SGLT1, leading to blunted and delayed gastrointestinal glucose uptake, resulting in reduced postprandial glucose excursions. Other clinically important gut-based actions of SOTA include sustained increments in secretion of intestinal hormones (27–29), which may improve insulin sensitivity and mitochondrial bioenergetics (30,31), leading to additional nephroprotection (32–37). Reducing glucose concentrations by blunted glucose uptake or improved insulin sensitivity also lowers the tubular glucose load and consequently UGE, which may explain the difference in UGE with SOTA versus selective SGLT2i. While the clinical importance of different UGE rates, and, consequently, different levels of natriuresis with various SGLT inhibitors is not yet known, it is important to note that changes in renal function, BP, and markers of blood volume (hematocrit and albumin, discussed below), due to SGLT inhibition–related natriuresis, were observed with SOTA.
In several studies in people with type 2 diabetes, SGLT2 inhibition is associated with an initial “dip” in eGFR that stabilizes over time and is reversible after cessation of therapy. The most likely mechanism responsible for this initial change in eGFR is a hemodynamically mediated afferent vasoconstriction through tubuloglomerular feedback. It should be noted that the observed eGFR decrease in this cohort is smaller than in young adults with type 1 diabetes and hyperfiltration (5). This anticipated eGFR dip also occurred with SOTA early in the course of treatment and persisted at 52 weeks in the 200 mg dose SOTA group. Consistent with observations from studies involving patients with type 2 diabetes, after a washout period, eGFR increased significantly compared with the last value on treatment in the subgroup with values at both time points. In terms of preservation of kidney function, after cessation of therapy, eGFR was significantly higher in SOTA versus placebo-treated patients, suggesting that even after a very short duration of therapy, SOTA may prevent kidney function loss—which is an intriguing possibility in the post–Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy (CREDENCE) era that merits further study.
In people with type 2 diabetes, SGLT2 inhibition lowers UACR in the microalbuminuric range by 25–40% and by 30–50% in the setting of baseline macroalbuminuria, independent of treatment-induced changes in classical renal risk factors. Until now, little was known about the impact of dual SGLT1i and SGLT2i or selective SGLT2i on UACR in people with type 1 diabetes. In the current analysis, in addition to the eGFR dip, SOTA 400 mg was associated with a significant reduction in UACR early in the course of treatment at 24 weeks, which tended to persist over time—although changes at 52 weeks were no longer statistically significant. While the magnitude of the effect at 24 weeks with SOTA 400 mg was similar to that expected in people with type 2 diabetes and microalbuminuria, dedicated trials are required to fully understand the antialbuminuric impact of dual SGLT1i and SGLT2i in patients with type 1 diabetes.
SGLT2 inhibition induces modest but consistent SBP- and DBP-lowering effects in people with type 2 diabetes. In people with type 1 diabetes, in both mechanistic studies and in large glycemic control trials, effects of SGLT2i have generally demonstrated similar SBP and DBP lowering (5). With this background, in the current analysis with a dual SGLT1i and SGLT2i, mean SBP and DBP values declined acutely over 4 weeks. These changes persisted over time, and neither SBP nor DBP increased after the brief washout period. Furthermore, in participants above and below current blood pressure targets 130/80 mmHg, the impact of SOTA was comparable and resulted in clinically relevant blood pressure lowering.
In previous trials involving participants with type 2 diabetes, hematocrit increased by 3–7% relative to baseline values and remained elevated over the course of long-term clinical trials (38,39), likely due to hemoconcentration (13) or, alternatively, secondary to increased erythropoietin production (40). The clinical relevance of changes in hematocrit was demonstrated in EMPA-REG OUTCOME, in which the increase in hematocrit was most strongly associated with CV benefits in participants with type 2 diabetes (13). Accordingly, in the current analysis, the rise in hematocrit and serum albumin in people with type 1 diabetes in response to SOTA, as well as the rapid increase toward baseline in these parameters after the 7-day washout, may be clinically important, since this may lead to clinical benefits similar to those observed in SGLT2i trials in people with type 2 diabetes.
Finally, SGLT2 inhibition is associated with biochemical alterations linked with cardiorenal protection, including reductions in plasma uric acid concentrations. Serum uric acid is associated with kidney disease progression and with increased CV risk (39,41–43). SGLT2 inhibition lowers plasma uric acid by 10–15% in people with type 2 diabetes but has been scarcely investigated in people with type 1 diabetes. Based on the current analysis, dual SGLT1 and SGLT2 inhibition reduces uric acid in type 1 diabetes to an extent similar to that previously observed in people with type 2 diabetes in response to selective SGLT2i, and these changes persisted after a brief 7-day washout period, without a significant rise during the period between last value on therapy until the end of the washout.
There are several limitations worth mentioning. The cohort of participants included in this analysis was not enriched for risk factors associated with kidney disease such as albuminuria or impaired kidney function. Accordingly, only a small proportion of participants had micro- or macroalbuminuria at baseline. Furthermore, in accordance with the original, the primary study design focused on glycemic control, and UACR measurements were only taken on a single occasion using spot collections at each time point. Perhaps due to the limited number of participants with albuminuria and the single urine sample collected for each time point, we observed a directional decrease in UACR in the placebo group, which may represent regression to the mean. Despite these changes in the placebo group, placebo-corrected UACR declines were significant at 24 weeks in the SOTA 400 mg group, highlighting the need for dedicated future studies in people with type 1 diabetes and albuminuria at baseline to elucidate the effect of SGLT inhibitors on surrogate and hard renal outcomes. As a caveat, however, the consequence of having single UACR measures at each time point would be a bias toward the null. Therefore, the favorable effect of SOTA on albuminuria in this relatively small subset may reflect a lower range estimate of the effect—further emphasizing the need for dedicated studies in people with type 1 diabetes and albuminuria. We also recognize that some of the analyses were post hoc and exploratory and should therefore be considered hypothesis generating. Finally, eGFR was used to assess changes in kidney function. Estimating equations are recognized to have limited precision and accuracy in people with type 1 diabetes with preserved kidney function, and future studies should ideally use direct measures of glomerular filtration rate.
In people with type 1 diabetes, SOTA lowered blood pressure and induced mild hemoconcentration, and it was associated with an acute change in eGFR and a reduction in albuminuria. While it is difficult to know whether these changes are similar or attenuated versus patients with type 2 diabetes in the absence of head-to-head trials, our data suggest that dual SGLT1 and SGLT2 inhibition has the potential to confer cardiorenal protection in ways analogous to selective SGLT2 inhibition in people with type 1 and type 2 diabetes. Dedicated trials exploring renal and CV protective pathways are warranted in people with type 1 diabetes with preexisting cardiac or renal disease.
Supplementary Material
Article Information
Funding. D.H.v.R. is supported by fellowships from the Dutch Diabetes Foundation as well as the European Union (Marie Curie). D.C. is supported by a Department of Medicine, University of Toronto, Merit Award and receives support from the Canada Institutes of Health Research, Diabetes Canada, and the Heart and Stroke Richard Lewar Centre of Excellence. P.B. receives salary and research support from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (K23 DK116720-01), in addition to research support from the Thrasher Research Fund, JDRF, NIDDK/DiaComp, International Society of Pediatric and Adolescent Diabetes, Colorado Clinical and Translational Sciences Institute, Children’s Hospital Colorado Research Institute, and Center for Women’s Health Research at University of Colorado.
Duality of Interest. Lexicon Pharmaceuticals, Inc. designed and funded the studies, including the operational execution and medical monitoring of the studies. The analysis for this manuscript was funded by Sanofi. Sanofi and Lexicon Pharmaceuticals, Inc. have entered a license agreement effective November 2015 and are collaborating on the development and commercialization of SOTA. D.H.v.R. has received consulting fees or speaking honoraria or both from Sanofi, Boehringer Ingelheim–Eli Lilly, and Merck and has received operating funds from Boehringer Ingelheim–Eli Lilly, AstraZeneca, Merck, and Sanofi. F.P. reports having received research grants from AstraZeneca, Novo Nordisk, and Novartis and lecture fees from Novartis, Eli Lilly, Merck Sharp & Dohme (MSD), AstraZeneca, Sanofi, Novo Nordisk, and Boehringer Ingelheim and having served as a consultant for AstraZeneca, Bayer, Amgen, Novo Nordisk, and MSD. P.B. has received consulting fees and/or research support from Bayer, Bristol-Myers Squibb, and Horizon Pharma and speaking honorarium from Boehringer Ingelheim–Eli Lilly. P.B. is on the scientific advisory board for XORTX Therapeutics Inc. H.J.L.H. has received consulting fees and/or research support from Astellas, AstraZeneca, Abbvie, Boehringer Ingelheim, Gilead, Fresenius, Janssen, Merck, MundiPharma, and Mitsubishi Tanabe (honoraria paid to his employer). D.R.P. is an employee of Lexicon Pharmaceuticals and receives compensation in the form of salary and stock options. R.d.C.C., P.S.W., and M.L. are employees of Sanofi and receive compensation in the form of salary and/or stock options. D.C. has received consulting fees or speaking honoraria or both from Janssen, Boehringer Ingelheim–Eli Lilly, AstraZeneca, Merck, and Sanofi and has received operating funds from Janssen, Boehringer Ingelheim–Eli Lilly, AstraZeneca, and Merck. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. D.H.v.R., P.B., F.P., D.R.P., R.d.C.C., P.S.W., M.L., H.J.L.H., and D.C. contributed to the interpretation of data, provided critical edits, and reviewed and approved the final version. D.H.v.R., P.S.W., and D.C. drafted the report. D.H.v.R. and D.C. contributed to the study design. M.L. contributed to the acquisition of data. No writing assistance was received by the authors. M.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-0937/-/DC1.
References
- 1.American Diabetes Association 11. Microvascular complications and foot care: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019;42(Suppl. 1):S124–S138 [DOI] [PubMed] [Google Scholar]
- 2.Cherney D, Lund SS, Perkins BA, et al. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia 2016;59:1860–1870 [DOI] [PubMed] [Google Scholar]
- 3.Cherney DZI, Cooper ME, Tikkanen I, et al. Pooled analysis of phase III trials indicate contrasting influences of renal function on blood pressure, body weight, and HbA1c reductions with empagliflozin. Kidney Int 2018;93:231–244 [DOI] [PubMed] [Google Scholar]
- 4.Thomson SC, Blantz RC. Glomerulotubular balance, tubuloglomerular feedback, and salt homeostasis. J Am Soc Nephrol 2008;19:2272–2275 [DOI] [PubMed] [Google Scholar]
- 5.Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014;129:587–597 [DOI] [PubMed] [Google Scholar]
- 6.Cherney DZI, Zinman B, Inzucchi SE, et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2017;5:610–621 [DOI] [PubMed] [Google Scholar]
- 7.Dekkers CCJ, Petrykiv S, Laverman GD, Cherney DZ, Gansevoort RT, Heerspink HJL. Effects of the SGLT-2 inhibitor dapagliflozin on glomerular and tubular injury markers. Diabetes Obes Metab 2018;20:1988–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fioretto P, Del Prato S, Buse JB, et al.; DERIVE Study Investigators . Efficacy and safety of dapagliflozin in patients with type 2 diabetes and moderate renal impairment (chronic kidney disease stage 3A): the DERIVE Study. Diabetes Obes Metab 2018;20:2532–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 10.Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 11.Wiviott SD, Raz I, Bonaca MP, et al.; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357 [DOI] [PubMed] [Google Scholar]
- 12.Wanner C, Inzucchi SE, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–334 [DOI] [PubMed] [Google Scholar]
- 13.Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care 2018;41:356–363 [DOI] [PubMed] [Google Scholar]
- 14.Skrtić M, Yang GK, Perkins BA, et al. Characterisation of glomerular haemodynamic responses to SGLT2 inhibition in patients with type 1 diabetes and renal hyperfiltration. Diabetologia 2014;57:2599–2602 [DOI] [PubMed] [Google Scholar]
- 15.Škrtić M, Cherney DZ. Sodium-glucose cotransporter-2 inhibition and the potential for renal protection in diabetic nephropathy. Curr Opin Nephrol Hypertens 2015;24:96–103 [DOI] [PubMed] [Google Scholar]
- 16.Rosenstock J, Marquard J, Laffel LM, et al. Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care 2018;41:2560–2569 [DOI] [PubMed] [Google Scholar]
- 17.Rajasekeran H, Lytvyn Y, Bozovic A, et al. Urinary adenosine excretion in type 1 diabetes. Am J Physiol Renal Physiol 2017;313:F184–F191 [DOI] [PubMed] [Google Scholar]
- 18.Perkins BA, Cherney DZ, Partridge H, et al. Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of-concept trial. Diabetes Care 2014;37:1480–1483 [DOI] [PubMed] [Google Scholar]
- 19.Perkins BA, Cherney DZ, Soleymanlou N, et al. Diurnal glycemic patterns during an 8-week open-label proof-of-concept trial of empagliflozin in type 1 diabetes. PLoS One 2015;10:e0141085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lapuerta P, Zambrowicz B, Strumph P, Sands A. Development of sotagliflozin, a dual sodium-dependent glucose transporter 1/2 inhibitor. Diab Vasc Dis Res 2015;12:101–110 [DOI] [PubMed] [Google Scholar]
- 21.Buse JB, Garg SK, Rosenstock J, et al. Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the North American inTandem1 study. Diabetes Care 2018;41:1970–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danne T, Cariou B, Banks P, et al. HbA1c and hypoglycemia reductions at 24 and 52 weeks with sotagliflozin in combination with insulin in adults with type 1 diabetes: the European inTandem2 study. Diabetes Care 2018;41:1981–1990 [DOI] [PubMed] [Google Scholar]
- 23.Garg SK, Henry RR, Banks P, et al. Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med 2017;377:2337–2348 [DOI] [PubMed] [Google Scholar]
- 24.Perkovic V, Jardine MJ, Neal B, et al.; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306 [DOI] [PubMed] [Google Scholar]
- 25.Dandona P, Mathieu C, Phillip M, et al.; DEPICT-1 Investigators . Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2017;5:864–876 [DOI] [PubMed] [Google Scholar]
- 26.Mathieu C, Dandona P, Gillard P, et al.; DEPICT-2 Investigators . Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT-2 study): 24-week results from a randomized controlled trial. Diabetes Care 2018;41:1938–1946 [DOI] [PubMed] [Google Scholar]
- 27.Dobbins RL, Greenway FL, Chen L, et al. Selective sodium-dependent glucose transporter 1 inhibitors block glucose absorption and impair glucose-dependent insulinotropic peptide release. Am J Physiol Gastrointest Liver Physiol 2015;308:G946–G954 [DOI] [PubMed] [Google Scholar]
- 28.Powell DR, Smith M, Greer J, et al. LX4211 increases serum glucagon-like peptide 1 and peptide YY levels by reducing sodium/glucose cotransporter 1 (SGLT1)-mediated absorption of intestinal glucose. J Pharmacol Exp Ther 2013;345:250–259 [DOI] [PubMed] [Google Scholar]
- 29.Zambrowicz B, Freiman J, Brown PM, et al. LX4211, a dual SGLT1/SGLT2 inhibitor, improved glycemic control in patients with type 2 diabetes in a randomized, placebo-controlled trial. Clin Pharmacol Ther 2012;92:158–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Góralska J, Śliwa A, Gruca A, et al. Glucagon-like peptide-1 receptor agonist stimulates mitochondrial bioenergetics in human adipocytes. Acta Biochim Pol 2017;64:423–429 [DOI] [PubMed] [Google Scholar]
- 31.Parlevliet ET, de Leeuw van Weenen JE, Romijn JA, Pijl H. GLP-1 treatment reduces endogenous insulin resistance via activation of central GLP-1 receptors in mice fed a high-fat diet. Am J Physiol Endocrinol Metab 2010;299:E318–E324 [DOI] [PubMed] [Google Scholar]
- 32.Alkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, Harder DR. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke 1996;27:971–979 [DOI] [PubMed] [Google Scholar]
- 33.Miller RG, Mahajan HD, Costacou T, Sekikawa A, Anderson SJ, Orchard TJ. A contemporary estimate of total mortality and cardiovascular disease risk in young adults with type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 2016;39:2296–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harding JL, Shaw JE, Peeters A, Davidson S, Magliano DJ. Age-specific trends from 2000–2011 in all-cause and cause-specific mortality in type 1 and type 2 diabetes: a cohort study of more than one million people. Diabetes Care 2016;39:1018–1026 [DOI] [PubMed] [Google Scholar]
- 35.Lind M, Svensson AM, Kosiborod M, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med 2014;371:1972–1982 [DOI] [PubMed] [Google Scholar]
- 36.Groop PH, Thomas MC, Moran JL, et al.; FinnDiane Study Group . The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 2009;58:1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia 2010;53:2312–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 2016;134:752–772 [DOI] [PubMed] [Google Scholar]
- 39.Lytvyn Y, Har R, Locke A, et al. Renal and vascular effects of uric acid lowering in normouricemic patients with uncomplicated type 1 diabetes. Diabetes 2017;66:1939–1949 [DOI] [PubMed] [Google Scholar]
- 40.van Raalte DH, Cherney DZI. Sodium glucose cotransporter 2 inhibition and renal ischemia: implications for future clinical trials. Kidney Int 2018;94:459–462 [DOI] [PubMed] [Google Scholar]
- 41.Lytvyn Y, Škrtić M, Yang GK, et al. Plasma uric acid effects on glomerular haemodynamic profile of patients with uncomplicated type 1 diabetes mellitus. Diabet Med 2016;33:1102–1111 [DOI] [PubMed] [Google Scholar]
- 42.Lytvyn Y, Perkins BA, Cherney DZ. Uric acid as a biomarker and a therapeutic target in diabetes. Can J Diabetes 2015;39:239–246 [DOI] [PubMed] [Google Scholar]
- 43.Maahs DM, Caramori L, Cherney DZ, et al.; PERL Consortium . Uric acid lowering to prevent kidney function loss in diabetes: the Preventing Early Renal Function Loss (PERL) allopurinol study. Curr Diab Rep 2013;13:550–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and data set specifications. Patient-level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com/.