Abstract
Although it is well established that type 2 diabetes (T2D) is generally due to the progressive loss of β-cell insulin secretion against a background of insulin resistance, the actual correlation of reduced β-cell mass to its defective function continues to be debated. There is evidence that a compensatory increase in β-cell mass, and the consequent insulin secretion, can effectively cope with states of insulin resistance, until hyperglycemia supervenes. Recent data strongly indicate that the mechanisms by which islets are able to compensate in response to insulin resistance in peripheral tissues is secondary to hyperplasia, as well as the activation of multiple cellular machineries with diverse functions. Importantly, islet cells exhibit plasticity in altering their endocrine commitment; for example, by switching from secretion of glucagon to secretion of insulin and back (transdifferentiation) or from an active secretory state to a nonsecretory quiescent state (dedifferentiation) and back. Lineage tracing (a method used to track each cell though its differentiation process) has demonstrated these potentials in murine models. A limitation to drawing conclusions from human islet research is that most studies are derived from human autopsy and/or organ donor samples, which lack in vivo functional and metabolic profiling. In this review, we specifically focus on evidence of islet plasticity in humans—from the normal state, progressing to insulin resistance to overt T2D—to explain the seemingly contradictory results from different cross-sectional studies in the literature. We hope the discussion on this intriguing scenario will provide a forum for the scientific community to better understand the disease and in the long term pave the way for personalized therapies.
α- and β-Cells in Humans: The Current Contradictory Scenario
Although the mechanisms responsible for type 2 diabetes (T2D) are still not completely understood, it is now well established that hyperglycemia is generally due to a progressive loss of β-cell insulin secretion against a background of insulin resistance. Investigating how β-cells and α-cells change in terms of number and/or secretory function is a rational approach to understanding the natural history of this complex and multifaceted disease (1).
In Tables 1 and 2, we summarize the reports on the quantification of human β-cells and α-cells. It is interesting to note that the results are often contradictory. Although some authors describe 52% β-cells per islet in control subjects (2), others found the same percentage in samples from individuals with diabetes (3,4). A similar contradiction is evident regarding the quantification of α-cells: some studies describe an increase in α-cells in individuals with diabetes (3,5), whereas others do not (4,6,7). These data make it challenging for readers to interpret results at a time when even β-cells have been classified into subpopulations (8–10).
Table 1.
The present scenario: β-cell/area and quantification data on human pancreata
| β-Cell quantification study | Unit | Control subjects | Change within control subjects (%) | Diabetes | Reduction diabetes vs. control subjects (%) |
|---|---|---|---|---|---|
| Rahier et al. (1) | Mass per pancreas | 0.888 ± 0.304 g | 0.573 ± 0.259 g | 36 | |
| Butler et al. (2) | % per islet | 52.0 ± 4.1% (lean) | 38.0 ± 3.9% (lean) | 26 | |
| Butler et al. (2) | % per islet | 45.4 ± 2.7% (obese) | 37.0 ± 2.3% (obese) | 17.7 | |
| Inaishi et al. (7) | % per total pancreas area | 1.48 ± 1.08% | 0.80 ± 0.54% | 46 | |
| Yoon et al. (5) | % per islet | 59.0 ± 10.3% | 38.3 ± 12.4% | 35.5 | |
| Marselli et al. (4) | % per islet | 72.1 ± 8.7% | 54.9 ± 6.3% | 24 | |
| Cinti et al. (3) | % per islet | 77.2 ± 1.8% | 53.1 ± 3.7% | 31 | |
| Yoneda et al. (12) | % per total pancreas area | NGT 1.60 ± 0.45% IGT 0.99 ± 0.51% | 38 | NewOns 0.93 ± 0.23% Longst 0.53 ± 0.1% | 43 |
| Mezza et al. (11) | % per total pancreas area | InsSens 0.58 ± 0.17% InsRes 1.10 ± 0.23% | 47 |
Data are means ± SE. InsRes, insulin resistant; InsSens, insulin sensitive; Longst, long-standing; NewOns, new onset. Rahier et al. (1) used the traditional method of measurement of β-cell mass. The other studies describe percentages of islet or total pancreas area occupied by β-cells as a surrogate for the total mass of endocrine cells.
Table 2.
The present scenario: α-cell/area and quantification data on human pancreata
| α-Cell quantification study | Unit | Individuals without diabetes | Increase (%) | Individuals with diabetes | Increase (%) | α-cell/β-cell increase (%) |
|---|---|---|---|---|---|---|
| Henquin and Rahier (6) | Mass | 0.347 ± 0.183 g | 0.366 ± 0.186 g | NS | 30 | |
| Inaishi et al. (7) | % per total pancreas area | 0.49 ± 0.44% | 0.35 ± 0.31% | NS | 11 | |
| Yoon et al. (5) | % per islet | 16.6 ± 2.8% | 26.1 ± 6.1% | 9.5 (1.6-fold change) | 52 | |
| Marselli et al. (4) | % per islet | 20.2 ± 5.3% | 23.3 ± 5.4% | NS | 15 | |
| Cinti et al. (3) | % per islet | 22.75 ± 1.6% | 37.36 ± 1.5% | 14.61 (1.6-fold change) | 30 | |
| Mezza et al. (11) | % per total pancreas area | InsSens 0.04 ± 0.01%InsRes 0.23 ± 0.06% | 0.19 (5.7-fold change) | 14 |
Data are means ± SE. InsRes, insulin resistant; InsSens, insulin sensitive. Henquin and Rahier (6) used the traditional method of measurement of α-cell mass. The other studies describe percentages of islet or total pancreas area occupied by α-cells as a surrogate for the total mass of endocrine cells.
In a previous study (11), we examined islet morphology in a subset of patients without diabetes, subclassified according to their insulin sensitivity (i.e., insulin resistant compared with non–insulin resistant) (Table 2). We observed that the ratio of α-cell to β-cell area was higher in insulin-resistant subjects without diabetes, due to a relatively greater increase in α-cell area (by approximately five times) compared with the increase in β-cell area. Interestingly, the α-cell area was inversely correlated with insulin sensitivity, meaning that the greater the insulin resistance, the greater the α-cell mass (11). Whatever the underlying mechanism, it is evident that not all subjects without diabetes have the same α-cell and β-cell masses, as each is strongly influenced by individual insulin resistance (and the secretory consequences).
In this context, it is important for the reader to interpret the basis for considering 52% β-cells per islet as “normal” in some studies (2,5), while 53% is labeled as “reduced” in others (3,4). When one carefully examines the control groups used in these studies, it might be inferred that the lower percentage of β-cell mass (2,5) is due to the selection of more insulin-sensitive subjects, in whom β-cell mass is sufficient to guarantee euglycemia; while the greater percentage of β-cell mass described in other studies (3,4) is due to the presence of relatively more insulin-resistant subjects, in whom the β-cell mass increases to compensate for the increased insulin demand.
Similarly, the reports describing α-cell mass also vary significantly. For example, although some studies report an increase in α-cells in patients with diabetes, others have observed a comparable α-cell mass between groups, attributing the increase in the α-cell/β-cell ratio to the reduced β-cell mass, rather than to an absolute increase in α-cells (Table 2).
Applying the same logic as above, one needs to consider the difference between the two groups of patients without diabetes (i.e., insulin sensitive vs. insulin resistant) as contributing to the contradictory results observed in T2D groups compared with “control groups.” Thus, the studies that describe an increase in α-cell mass may have compared T2D subjects with a homogeneous control group, which included relatively more insulin-sensitive control subjects (3,5), whereas the other studies, which reported comparable α-cell mass between groups, may have included both insulin-sensitive and insulin-resistant subjects and/or mostly insulin-resistant subjects as control subjects (1,4,6,7). Of course, other hypotheses cannot be excluded.
These studies provide examples of how crucial information may be missed if one does not adequately characterize control groups before assessing islet cell mass and, indirectly, this can potentially explain the lack of an increase in α-cell mass in some pancreas samples from patients with diabetes. Thus, the current contradictions might be only apparent.
Insulin Resistance and Islet Plasticity: The Emerging Scenario
Although the contradictory results in the studies discussed above are potentially due to lack of “uniform control subjects” across all studies, another variable that requires careful consideration in such studies is prediabetes.
Yoneda et al. (12) were among the first to “subclassify” control subjects (Table 1). In this study, pancreatic surgical samples from 42 patients were analyzed, and 32 of these patients were subjected to an oral glucose tolerance test. Subjects were then classified into the following groups: normal glucose tolerance (NGT), impaired glucose tolerance (IGT), or T2D. The authors reported a progressive decrease in the percentage of β-cell area across the groups with the largest decrease in patients with long-standing T2D. Considering that the greatest variation in β-cell mass was between NGT and IGT subjects, the major reduction in the percentage β-cell area was observed in the prediabetic phase, suggesting that significant changes in islet cell function and/or morphology occur during this period.
T2D is characterized by insulin resistance, which typically begins several years prior to hyperglycemia, and is detectable over the entire course of the disease. On the other hand, β-cell secretory function has a compensatory phase during which insulin secretion rises to compensate for insulin resistance, thereby maintaining euglycemia, and then falls off, leading to the increase in plasma glucose levels (13–15). Insulin resistance therefore seems to be the driver of the early compensative phase. Insulin resistance, however, is not limited to skeletal muscle, liver, adipose tissue, and other insulin-sensitive tissues as traditionally believed; indeed, it has been described in insulin-secreting β-cells (16,17) or α-cells (18), where it could modulate insulin secretion (19). It is therefore possible that insulin resistance in the same islet cells contributes, in part, to the failure of cell growth and secretory function during the euglycemic compensatory phase (20). However, it should be noted that insulin resistance is not invariably associated with subsequent hyperglycemia, since the triad insulin resistance plus increased insulin plus increased β-cell mass without hyperglycemia has been observed in several conditions, including obesity (21,22), pregnancy (23,24), and polycystic ovary syndrome (25). Thus, β-cells are able to compensate for insulin demand that occurs in different conditions. When the compensation mechanisms are lost, hyperglycemia appears.
Mechanisms of Compensation
Several studies (26–28) provide indirect evidence that islet cell mass is dynamic and capable of adapting to physiological or pathological conditions to maintain normoglycemia. In pregnant women, for example, there is a 40% increase in the relative volume of β-cells (percentage of β-cells/pancreas). Another model of pathophysiological β-cell compensation is obesity, which can lead to a 30-fold increase in β-cell mass in mice (29), but only an ∼30% increase in humans (1,30).
This capacity of the islet to modify its architecture and function (i.e., islet plasticity) has been described in several metabolic conditions. Our group has observed similar changes in pancreatic samples from individuals without diabetes (11,31). In these studies, we classified individuals on the basis of insulin sensitivity (measured with the gold standard euglycemic-hyperinsulinemic clamp) and observed that chronic insulin resistance, in the absence of hyperglycemia, directly impacts islets. This was evident from the 50% increase in the percentage of β-cell area in insulin-resistant subjects compared with insulin-sensitive control subjects. Insulin resistance did not directly modify β-cell size, suggesting that the increase in β-cell area is unlikely to be due to cell hypertrophy (11).
Although direct evidence in the context of human islet morphology is still limited, findings in murine and in vitro models, reviewed by Migliorini et al. (32), reveal that endocrine and exocrine cell types within the pancreas preserve a level of cellular plasticity. In particular, rodent models of insulin resistance (reviewed by Mezza and Kulkarni [33]) show increased β-cell mass and proliferation largely in an attempt to compensate for increasing insulin demand. Further, analyses of α-IRKO mice have demonstrated the significance of insulin signaling in the regulation of α-cell function and mass, where the mutant mice exhibited hyperglucagonemia, glucose intolerance, and an age-dependent progressive increase in β-cell area, whereas α-cell area was unchanged, leading to a relative decrease in cell area (18).
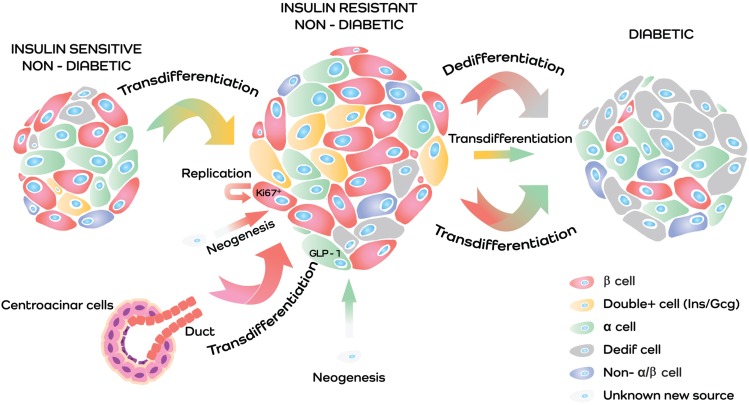
The precise origin of potential new β-cells in insulin resistance continues to be a topic of active investigation. Replication, neogenesis, and transdifferentiation (i.e., transdifferentiation of α-cells into β-cells and/or acinar/ductal transdifferentiation into insulin-producing cells) are all potential sources and warrant further investigation (Fig. 1) (11,31,34,35).
Figure 1.
Schematic representation of the hypothetical scenario of islet plasticity. Islet plasticity is the capacity of the islet to modify its morphology and function according to different metabolic conditions. A potential explanation of the present scenario is as follows: when insulin resistance increases insulin demand, islet plasticity guarantees a twofold increase in β-cells, whose origins are still debated but some hypotheses are transdifferentiation from centroacinar and duct cells (duct red cells and centroacinar violet cells), replication (red cell Ki67+), and neogenesis from an unknown source (white to red cell); a twofold increase in the α-cells transdifferentiated into insulin-producing cells (yellow double-positive cells); and a fivefold increase in the α-cells via neogenesis, with a consequent increase of a potential GLP-1 source (white to green cell). As with any compensatory mechanism, in a chronic condition, it is bound to fail. The exhausted β-cells undergo dedifferentiation (Dedif) (a resting state, red to gray cells), the double-positive cell switches back into the original α-cell (yellow cells to green cells), and the overstressed β-cells transdifferentiate into α-cells (red cell to green cell).
In contrast to data in rodents (36,37), studies in humans, to date, have found no evidence of replication or apoptosis of β-cells in insulin resistance–induced islet remodeling, whereas increased proportions of small islets, increased numbers of insulin+ cells within the ducts, and increased numbers in singlet insulin+ cells have all been observed in both pregnant and insulin-resistant individuals (11,27). Nevertheless, it is worth noting that human β-cells are receptive to proliferation in vitro as reported by multiple groups (34,35,38–42), despite data describing a lower rate of proliferation obtained through more intense stimulations.
Findings in mice have revealed plasticity and a potential for intraislet (e.g., α-cell) and extraislet (acinar and ductal) cell transdifferentiation in order to produce insulin (reviewed by Aguayo-Mazzucato and Bonner-Weir [43]). In humans, pancreatic ductal cells have been proposed as a possible source of these new β-cells. For example, a threefold increase in cells coexpressing insulin and the duct marker cytokeratin 19 has been observed in individuals with insulin resistance or IGT (11,12). Since the majority of human studies are cross-sectional, the origin of the insulin+ duct cell in humans continues to be a mystery. In this context, it is worth noting that pancreatic duct cells have been reported to serve as a pool for progenitors of both islet and acinar tissues after birth and during adulthood (44–46). Compelling as this evidence on human pancreatic sections and isolated human duct cells is, we should consider the ability of a pool of duct cells to transdifferentiate into insulin-producing cells, as a possible compensatory mechanism in insulin resistance–induced remodeling of islets.
Another potential origin of “new” β-cells is the pool of α-cells themselves. Transdifferentiation of α-cells into β-cells has been observed in several studies and different experimental settings (47–49). Single gene manipulations have been reported to suffice to induce α-cell conversion into β-cells, using an ectopic Pax4 overexpression model by Collombat et al. (48). In humans, an increase in α-cells has been reported in insulin-resistant subjects (11), where a greater increase in α-cell area compared with the increase in β-cell area explained the increase in the ratio. The mechanisms underlying this apparent increase in α-cell mass in humans remains unclear. However, various studies analyzing disruption in glucagon signaling induced in animal models (50–52) have found that α-cell hyperplasia, similar to that in insulin resistance, is due to an increase in α-cell proliferation and hormone production in states of glucagon resistance. Further, a hepatic–α-islet cell axis, where glucagon regulates serum amino acid availability, seems to induce elevated plasma amino acid levels and compensatory glucagon hypersecretion involving the expansion of pancreatic α-cell mass (53,54). In addition, the contribution of hyperinsulinemia to α-cell hyperplasia is supported by observations that IRS2 KO mice exhibit reduced α-cell mass and glucagon secretion (55), whereas in vitro insulin treatment of α-TC1 cells leads to increased α-cell proliferation by triggering mammalian target of rapamycin (56). In the context of increased α-cell mass observed in experimental conditions of glucagon and insulin resistance, an increase in the percentage of insulin/glucagon double-positive cells reported in individuals with insulin resistance and IGT links α-cell and β-cell function in vivo (11,12,31). The evidence of bihormonal cells has been interpreted as confirmation of α-cell transdifferentiation into β-cells; however, the exact significance and fate of these cells in the context of prediabetes in humans are important areas for further investigation. Of note, this increase in bihormonal cells was more apparent in larger islets compared with smaller ones, suggesting a continuing attempt to respond to greater insulin demand despite the already increased islet size. In vivo β-cell dysfunction has been correlated with islet dimensions and with the percentage of α-cell to β-cell transdifferentiation, suggesting that the β-cells themselves emit signals to induce their own regeneration (31). Thus, dysfunctional β-cells could drive the increase of β-cell mass, but also promote the process of producing new β-cells, thereby expanding the cell mass when secretory function decreases. However, few of these studies include comprehensive molecular and functional analyses to attest to the efficacy of reprogrammed β-cell populations. The recent identification of urocortin3−, insulin-expressing β-cells (1–2% of all β-cells) that are transcriptionally and functionally immature, suggests a naturally occurring α-cell to β-cell conversion process as part of islet homeostasis (57). Lineage-tracing studies have revealed that these cells represent an intermediate stage in both the transdifferentiation of α-cells to mature β-cells and in the inverse transition (57). The transcriptional profile of double hormone-positive cells could provide insights into the mechanisms underlying α-cell to β-cell conversion and unravel regulatory factors that allow this transition. Indeed, such rare double hormone-positive cells were captured by single-cell RNA-sequencing studies, but this observation was not further commented on or excluded as potential evidence of double hormone-positive cells (58,59). Recent reports have identified activation of the GABA signaling pathway as a means to induce transdifferentiation of pancreatic α-cells into β-cells. Whether the role of GABA in the transdifferentiation process is context dependent and/or requires very precise experimental conditions must be determined by further studies (60,61).
An additional contribution to compensation by α-cells is the intraislet production of glucagon-like peptide 1 (GLP-1) (62), which could potentially impact islet function and remodeling. As already known, GLP-1 enhances glucose-stimulated insulin secretion (63) and inhibits glucagon release (64). Interestingly, it has been suggested that the presence of GLP-1 in the islet has other effects, including the differentiation of progenitor cells into β-cells in the pancreatic duct epithelium (65,66), and the direct stimulation of proliferation and inhibition of apoptosis of β-cells (67–69). Therefore, the production of incretin hormones in the pancreatic islets might be an adaptive mechanism to improve β-cell function and survival under stress (70). Further, it has been recently suggested that GLP-1 stimulates β-cell autophagy and that this plays a role in the inhibition of apoptosis in human islets (71). Several groups have found that islet cells produce GLP-1 under various stress conditions, including obesity (72,73). Among other pathways, recent reports suggest that in vitro and in vivo factors or signals can promote the transdifferentiation of α-cells into β-cells (11,48,74,75), and that this transdifferentiation is driven by intraislet GLP-1 secretion. Pancreas extracts from glucagon receptor knockout mice (52) exhibit an increase in GLP-1 levels associated with an up to 10-fold increase in circulating levels of GLP-1 amide, the active form of the incretin hormone, whereas no changes were observed in GLP-1 levels in intestinal extracts, suggesting that the pancreas is one of the sources of circulating GLP-1. These reports have refueled research on the in vivo function of α-cells in both prediabetes and diabetes that is aimed at investigating the potential role of these cells in islet homeostasis.
Neogenesis from exocrine cells (including duct and centroacinar cells) and transdifferentiation (including α-cells to β-cells) promoted by insulin resistance and potentially driven by intraislet GLP-1 secretion might be one of the important mechanisms underlying the compensatory response to increased insulin demand.
Collectively, these results and other models (recently reviewed by Aguayo-Mazzucato and Bonner-Weir [43]) have demonstrated an extraordinary plasticity of islet and exocrine cells triggered by insulin resistance in order to maintain euglycemia in the prediabetic state.
Failure of Compensation
Despite the attempts at compensation discussed above, the natural history of diabetes is typically characterized by a progressive steep decline in β-cell function preceding the onset of overt disease (76). Until a few years ago, β-cell demise was considered the principal mechanism underlying this decline (2); however, data from rodents and correlative human studies have introduced a new alternative: β-cell dedifferentiation. The process of dedifferentiation was first described in vitro wherein culture conditions prompt mature β-cells to attain a mesenchymal cell phenotype while retaining the potential to redifferentiate into β-cells (77,78). Subsequently, lineage-tracing experiments in mice have demonstrated that stress switches off the β-cell gene leader FOXO1 and that the stunned β-cell, instead of dying, undergoes dedifferentiation by returning to a progenitor-like state.
In human pancreatic samples, a dedifferentiated β-cell has been defined as one that no longer contains pancreatic hormones, although still retaining endocrine features (i.e., synaptophysin and/or chromogranin A immunoreactivity) and the expression of progenitor markers such as ALDH1a3. The fact that dedifferentiated β-cells account for 30% of β-cells in patients with diabetes and ∼10% in “control subjects” suggests that dedifferentiation plays a role in insufficient insulin secretion in patients with diabetes (3,4,79,80). These recent data have been interpreted in different ways and raised several questions that need to be addressed: for example, are the dedifferentiated cells just degranulated or have they lost their identity? Should we consider these cells as attempting to regenerate (81)?
Importantly, findings in murine models (82,83) reveal that dedifferentiated β-cells have the ability to revert into insulin-producing cells under different experimental conditions, suggesting that even dedifferentiation contributes to the continuous process of islet remodeling from normal/prediabetes stages to overt diabetes. On the other hand, if the chronic metabolic inflexibility persists, the failure of dedifferentiation coupled with transdifferentiation of β-cells into other hormone-producing cells (e.g., α-cells) likely nullifies the extraordinary attempts at compensation (84).
Taken together, these data provide an alternative perspective on the changes in islet cell identity over the natural history of T2D. Although the increase in α-cells observed in insulin resistance acts as a potential source of β-cells to compensate for increased insulin demand (and the transition phase is characterized by the presence of insulin/glucagon double-positive cells), the persistence of metabolic inflexibility finally leads the β-cells to revert to the original α-cells, likely causing the hyperglucagonemia observed in T2D (Fig. 1) (3).
The Need to Link Function and Morphology: The Final Scenario
Most of the studies discussed in previous sections have evaluated autopsy pancreata or organs from donors (1,2,85,86). Despite the advantage of using the entire organ, the autopsied pancreatic specimens likely exhibit postmortem degeneration as a consequence of variable cold ischemia times (i.e., the time between organ removal and freezing of tissues, usually >24 h). This factor in turn could lead to tissue autolysis due to poor stability of cellular components and, therefore, limit the morphological quality of the samples (87–89), although replication and inflammation are probably not affected (90).
Beyond the variable morphology, the lack of detailed medical history and metabolic profiling precludes accurate classification of donors as control subjects and subclassification of patients with diabetes. Indeed, studies in which individuals with prediabetes were subclassified on the basis of glucose tolerance (NGT vs. IGT) or insulin sensitivity, and where pancreatic samples were collected after surgery for morphological studies, indicate that duct and α-cell transdifferentiation occur in insulin-sensitive and normal glucose-tolerant individuals. This suggests that islet remodeling represents a continuous process during the transition from normal to prediabetes to overt diabetes (11). Furthermore, among donors with diabetes, differences in disease duration and therapeutic approach could also affect islet plasticity (i.e., one can hypothesize that GLP-1 mimetics amplify the effects displayed by intraislet GLP-1). However, the latter has never been investigated in human cadavers.
These data support the hypothesis that a combination of insulin resistance and increased β-cell demand determine rapid changes in islet morphology and function, suggesting that the accurate subclassification of control subjects is the only way to trace a complete roadmap of islet plasticity.
The proposed outlook represents a plausible hypothesis that needs a point-to-point link between ex vivo islet morphology and in vivo functional markers of islets cells. To date, a continuing challenge in investigating human islet biology is the lack of accurate in vivo metabolic and hormonal profiling coupled with human tissue samples of appropriate quality for analyses. Moreover, since islet morphology and cellular composition may vary throughout the pancreas (91), only samples collected from the same pancreatic region should be compared. Furthermore, an accurate metabolic profiling needs sensitive, specific, and proven in vivo tools. The oral glucose tolerance test, hyperinsulinemic, euglycemic, and hyperglycemic clamp procedures and mixed meal tests performed using standard procedures, as previously reported (92–95), are promising solutions to understanding the metabolic features of patients whose pancreatic samples will be analyzed ex vivo (11).
Conclusions
The present scenario based on human in vivo and ex vivo data from the nondiabetic condition through to diabetes onset suggests that the seeming contradictions of quantification reports can be explained by the lack of an accurate in vivo metabolic characterization of subjects, who are not just patients with diabetes or without diabetes but who could also be insulin resistant or insulin sensitive and maybe more. There are currently extraordinary islet plasticity reports from different studies and in different metabolic conditions (3,11,12). The evolving scenario demonstrates the remarkable attempts of the human body to compensate for pathological conditions and slow the progression of disease by promoting higher rates of neogenesis and/or plasticity to redifferentiate insulin-producing cells. Investigating the modifications occurring in different metabolic conditions provides an excellent opportunity to identify possible targets to prevent, or possibly deviate, the natural history of T2D. One suitable approach to gain insights into signaling pathways in human islet cells is the time-consuming, yet unique, method of collecting islet samples in vivo from patients without diabetes and patients with diabetes who have undergone accurate metabolic and hormonal profiling.
All available data require further investigation: our interpretation (Fig. 1) of the present scenario is that, moving from an early defect in β-cell function, islet remodeling increases the number of insulin-producing cells potentially derived from duct cells, acinar cells, and/or proliferation of neogenesis from an unrevealed source. The α-cells, also involved in the increased cell count of islet remodeling, could become β-cells, but may also produce GLP-1. Intraislet GLP-1 could both potentiate β-cell survival and functions and also stimulate the transdifferentiation of duct cells and α-cells into new β-cells. When this attempt at compensation fails, the exhausted β-cell undergoes dedifferentiation. In a chronic metabolic inflexibility condition (e.g., long-standing diabetes), even dedifferentiation fails, nullifying all the extraordinary attempts at compensation (Fig. 1).
The emerging insights on islet plasticity evident in diverse metabolic conditions that manifest over the course of the development of overt diabetes will provide an excellent opportunity to design personalized therapies to potentiate compensatory processes, and/or prevent the mechanisms that trigger failure, to arrest the progression toward overt T2D.
Article Information
Acknowledgments. The authors thank Serena Rotunno (Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Roma, Italia), who provided editorial assistance in the preparation of this manuscript.
Funding. This study was supported by grants to T.M. from the European Foundation for the Study of Diabetes (EFSD)/Novo Nordisk, EFSD/Lilly, and EFSD/AstraZeneca; to F.C. from the EFSD/AstraZeneca; and to A.G. from the Università Cattolica del Sacro Cuore (Fondi Ateneo Linea D.3.2 Sindrome Metabolica and Linea D.1 2018) and from the Italian Ministry of Education, University and Research (PRIN 2015373Z39_006).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. T.M. and F.C. contributed equally to the writing of the manuscript. C.M.A.C. and A.P. contributed to the discussion and reviewed/edited the manuscript. R.N.K. and A.G. reviewed/edited the manuscript.
References
- 1.Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab 2008;10(Suppl. 4):32–42 [DOI] [PubMed] [Google Scholar]
- 2.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 3.Cinti F, Bouchi R, Kim-Muller JY, et al. Evidence of β-cell dedifferentiation in human type 2 diabetes. J Clin Endocrinol Metab 2016;101:1044–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marselli L, Suleiman M, Masini M, et al. Are we overestimating the loss of beta cells in type 2 diabetes? Diabetologia 2014;57:362–365 [DOI] [PubMed] [Google Scholar]
- 5.Yoon KH, Ko SH, Cho JH, et al. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab 2003;88:2300–2308 [DOI] [PubMed] [Google Scholar]
- 6.Henquin JC, Rahier J. Pancreatic alpha cell mass in European subjects with type 2 diabetes. Diabetologia 2011;54:1720–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inaishi J, Saisho Y, Sato S, et al. Effects of obesity and diabetes on α- and β-cell mass in surgically resected human pancreas. J Clin Endocrinol Metab 2016;101:2874–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benninger RKP, Hodson DJ. New understanding of β-cell heterogeneity and in situ islet function. Diabetes 2018;67:537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasteska D, Hodson DJ. The role of beta cell heterogeneity in islet function and insulin release. J Mol Endocrinol 2018;61:R43–R60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutierrez GD, Gromada J, Sussel L. Heterogeneity of the pancreatic beta cell. Front Genet 2017;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mezza T, Muscogiuri G, Sorice GP, et al. Insulin resistance alters islet morphology in nondiabetic humans. Diabetes 2014;63:994–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoneda S, Uno S, Iwahashi H, et al. Predominance of β-cell neogenesis rather than replication in humans with an impaired glucose tolerance and newly diagnosed diabetes. J Clin Endocrinol Metab 2013;98:2053–2061 [DOI] [PubMed] [Google Scholar]
- 13.Bailey T. Options for combination therapy in type 2 diabetes: comparison of the ADA/EASD position statement and AACE/ACE algorithm. Am J Med 2013;126(Suppl. 1):S10–S20 [DOI] [PubMed] [Google Scholar]
- 14.Ramlo-Halsted BA, Edelman SV. The natural history of type 2 diabetes. Implications for clinical practice. Prim Care 1999;26:771–789 [DOI] [PubMed] [Google Scholar]
- 15.Nathan DM. Clinical practice. Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med 2002;347:1342–1349 [DOI] [PubMed] [Google Scholar]
- 16.Kulkarni RN, Brüning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 1999;96:329–339 [DOI] [PubMed] [Google Scholar]
- 17.Accili D. Insulin action research and the future of diabetes treatment: the 2017 Banting Medal for Scientific Achievement Lecture. Diabetes 2018;67:1701–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawamori D, Kurpad AJ, Hu J, et al. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab 2009;9:350–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halperin F, Lopez X, Manning R, Kahn CR, Kulkarni RN, Goldfine AB. Insulin augmentation of glucose-stimulated insulin secretion is impaired in insulin-resistant humans. Diabetes 2012;61:301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okada T, Liew CW, Hu J, et al. Insulin receptors in beta-cells are critical for islet compensatory growth response to insulin resistance. Proc Natl Acad Sci U S A 2007;104:8977–8982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bagdade JD, Bierman EL, Porte D Jr. The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J Clin Invest 1967;46:1549–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeFronzo RA. Lilly lecture 1987. The triumvirate: β-Cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988;37:667–687 [DOI] [PubMed] [Google Scholar]
- 23.Kühl C. Insulin secretion and insulin resistance in pregnancy and GDM. Implications for diagnosis and management. Diabetes 1991;40(Suppl. 2):18–24 [DOI] [PubMed] [Google Scholar]
- 24.Kautzky-Willer A, Prager R, Waldhausl W, et al. Pronounced insulin resistance and inadequate β-cell secretion characterize lean gestational diabetes during and after pregnancy. Diabetes Care 1997;20:1717–1723 [DOI] [PubMed] [Google Scholar]
- 25.Dunaif A, Graf M, Mandeli J, Laumas V, Dobrjansky A. Characterization of groups of hyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. J Clin Endocrinol Metab 1987;65:499–507 [DOI] [PubMed] [Google Scholar]
- 26.Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC. β-Cell mass and turnover in humans: effects of obesity and aging. Diabetes Care 2013;36:111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butler AE, Cao-Minh L, Galasso R, et al. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia 2010;53:2167–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patti ME, McMahon G, Mun EC, et al. Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia 2005;48:2236–2240 [DOI] [PubMed] [Google Scholar]
- 29.Brüning JC, Winnay J, Bonner-Weir S, Taylor SI, Accili D, Kahn CR. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell 1997;88:561–572 [DOI] [PubMed] [Google Scholar]
- 30.Klöppel G, Löhr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res 1985;4:110–125 [DOI] [PubMed] [Google Scholar]
- 31.Mezza T, Sorice GP, Conte C, et al. β-Cell glucose sensitivity is linked to insulin/glucagon bihormonal cells in nondiabetic humans. J Clin Endocrinol Metab 2016;101:470–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Migliorini A, Bader E, Lickert H. Islet cell plasticity and regeneration. Mol Metab 2014;3:268–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mezza T, Kulkarni RN. The regulation of pre- and post-maturational plasticity of mammalian islet cell mass. Diabetologia 2014;57:1291–1303 [DOI] [PubMed] [Google Scholar]
- 34.Dirice E, Walpita D, Vetere A, et al. Inhibition of DYRK1A stimulates human β-cell proliferation. Diabetes 2016;65:1660–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kondegowda NG, Fenutria R, Pollack IR, et al. Osteoprotegerin and denosumab stimulate human beta cell proliferation through inhibition of the receptor activator of NF-κB ligand pathway. Cell Metab 2015;22:77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429:41–46 [DOI] [PubMed] [Google Scholar]
- 37.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 2007;12:817–826 [DOI] [PubMed] [Google Scholar]
- 38.Shirakawa J, Fernandez M, Takatani T, et al. Insulin signaling regulates the FoxM1/PLK1/CENP-a pathway to promote adaptive pancreatic β cell proliferation. Cell Metab 2017;25:868–882.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhawan S, Dirice E, Kulkarni RN, Bhushan A. Inhibition of TGF-β signaling promotes human pancreatic β-cell replication. Diabetes 2016;65:1208–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang P, Alvarez-Perez JC, Felsenfeld DP, et al. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat Med 2015;21:383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Ouaamari A, O-Sullivan I, Shirakawa J, et al. Forkhead box protein O1 (FoxO1) regulates hepatic serine protease inhibitor B1 (serpinB1) expression in a non-cell-autonomous fashion. J Biol Chem 2019;294:1059–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H, Bender A, Wang P, et al. Insights into beta cell regeneration for diabetes via integration of molecular landscapes in human insulinomas. Nat Commun 2017;8:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aguayo-Mazzucato C, Bonner-Weir S. Pancreatic β cell regeneration as a possible therapy for diabetes. Cell Metab 2018;27:57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonner-Weir S, Toschi E, Inada A, et al. The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatr Diabetes 2004;5(Suppl. 2):16–22 [DOI] [PubMed] [Google Scholar]
- 45.Bonner-Weir S, Aguayo-Mazzucato C, Weir GC. Dynamic development of the pancreas from birth to adulthood. Ups J Med Sci 2016;121:155–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inada A, Nienaber C, Katsuta H, et al. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci U S A 2008;105:19915–19919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thorel F, Népote V, Avril I, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 2010;464:1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collombat P, Xu X, Ravassard P, et al. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell 2009;138:449–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Courtney M, Gjernes E, Druelle N, et al. The inactivation of Arx in pancreatic α-cells triggers their neogenesis and conversion into functional β-like cells. PLoS Genet 2013;9:e1003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liang Y, Osborne MC, Monia BP, et al. Reduction in glucagon receptor expression by an antisense oligonucleotide ameliorates diabetic syndrome in db/db mice. Diabetes 2004;53:410–417 [DOI] [PubMed] [Google Scholar]
- 51.Chen M, Gavrilova O, Zhao WQ, et al. Increased glucose tolerance and reduced adiposity in the absence of fasting hypoglycemia in mice with liver-specific Gs alpha deficiency. J Clin Invest 2005;115:3217–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gelling RW, Du XQ, Dichmann DS, et al. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci U S A 2003;100:1438–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim J, Okamoto H, Huang Z, et al. Amino acid transporter Slc38a5 controls glucagon receptor inhibition-induced pancreatic alpha cell hyperplasia in mice. Cell Metab 2017;25:1348–1361.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dean ED, Li M, Prasad N, et al. Interrupted glucagon signaling reveals hepatic α cell axis and role for L-glutamine in α cell proliferation. Cell Metab 2017;25:1362–1373.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cantley J, Choudhury AI, Asare-Anane H, et al. Pancreatic deletion of insulin receptor substrate 2 reduces beta and alpha cell mass and impairs glucose homeostasis in mice. Diabetologia 2007;50:1248–1256 [DOI] [PubMed] [Google Scholar]
- 56.Liu Z, Kim W, Chen Z, et al. Insulin and glucagon regulate pancreatic α-cell proliferation. PLoS One 2011;6:e16096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van der Meulen T, Mawla AM, DiGruccio MR, et al. Virgin beta cells persist throughout life at a neogenic niche within pancreatic islets. Cell Metab 2017;25:911–926.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Segerstolpe Å, Palasantza A, Eliasson P, et al. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab 2016;24:593–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang YJ, Schug J, Won KJ, et al. Single-cell transcriptomics of the human endocrine pancreas. Diabetes 2016;65:3028–3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ackermann AM, Moss NG, Kaestner KH. GABA and artesunate do not induce pancreatic α-to-β cell transdifferentiation in vivo. Cell Metab 2018;28:787–792.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Meulen T, Lee S, Noordeloos E, et al. Artemether does not turn α cells into β cells. Cell Metab 2018;27:218–225.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marchetti P, Lupi R, Bugliani M, et al. A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets. Diabetologia 2012;55:3262–3272 [DOI] [PubMed] [Google Scholar]
- 63.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157 [DOI] [PubMed] [Google Scholar]
- 64.Seino Y, Yabe D. Glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1: incretin actions beyond the pancreas. J Diabetes Investig 2013;4:108–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y, Cao X, Li LX, Brubaker PL, Edlund H, Drucker DJ. β-Cell Pdx1 expression is essential for the glucoregulatory, proliferative, and cytoprotective actions of glucagon-like peptide-1. Diabetes 2005;54:482–491 [DOI] [PubMed] [Google Scholar]
- 66.Zhu X, Zhou A, Dey A, et al. Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects. Proc Natl Acad Sci U S A 2002;99:10293–10298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farilla L, Bulotta A, Hirshberg B, et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology 2003;144:5149–5158 [DOI] [PubMed] [Google Scholar]
- 68.Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ. Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J Biol Chem 2003;278:471–478 [DOI] [PubMed] [Google Scholar]
- 69.Buteau J, El-Assaad W, Rhodes CJ, Rosenberg L, Joly E, Prentki M. Glucagon-like peptide-1 prevents beta cell glucolipotoxicity. Diabetologia 2004;47:806–815 [DOI] [PubMed] [Google Scholar]
- 70.Linnemann AK, Davis DB. Glucagon-like peptide-1 and cholecystokinin production and signaling in the pancreatic islet as an adaptive response to obesity. J Diabetes Investig 2016;7(Suppl. 1):44–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zummo FP, Cullen KS, Honkanen-Scott M, Shaw JAM, Lovat PE, Arden C. Glucagon-like peptide 1 protects pancreatic β-cells from death by increasing autophagic flux and restoring lysosomal function. Diabetes 2017;66:1272–1285 [DOI] [PubMed] [Google Scholar]
- 72.Kilimnik G, Kim A, Steiner DF, Friedman TC, Hara M. Intraislet production of GLP-1 by activation of prohormone convertase 1/3 in pancreatic α-cells in mouse models of ß-cell regeneration. Islets 2010;2:149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Linnemann AK, Neuman JC, Battiola TJ, Wisinski JA, Kimple ME, Davis DB. Glucagon-like peptide-1 regulates cholecystokinin production in β-cells to protect from apoptosis. Mol Endocrinol 2015;29:978–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ben-Othman N, Vieira A, Courtney M, et al. Long-term GABA administration induces alpha cell-mediated beta-like cell neogenesis. Cell 2017;168:73–85.e11 [DOI] [PubMed] [Google Scholar]
- 75.Li J, Casteels T, Frogne T, et al. Artemisinins target GABAA receptor signaling and impair α cell identity. Cell 2017;168:86–100.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) [published correction appears in Lancet 1999;354:602]. Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 77.Weinberg N, Ouziel-Yahalom L, Knoller S, Efrat S, Dor Y. Lineage tracing evidence for in vitro dedifferentiation but rare proliferation of mouse pancreatic β-cells. Diabetes 2007;56:1299–1304 [DOI] [PubMed] [Google Scholar]
- 78.Lechner A, Nolan AL, Blacken RA, Habener JF. Redifferentiation of insulin-secreting cells after in vitro expansion of adult human pancreatic islet tissue. Biochem Biophys Res Commun 2005;327:581–588 [DOI] [PubMed] [Google Scholar]
- 79.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 2012;150:1223–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun J, Ni Q, Xie J, et al. β cell dedifferentiation in patients with T2D with adequate glucose control and nondiabetic chronic pancreatitis. J Clin Endocrinol Metab 2019;104:83–94 [DOI] [PubMed] [Google Scholar]
- 81.Butler AE, Dhawan S, Hoang J, et al. β-Cell deficit in obese type 2 diabetes, a minor role of β-cell dedifferentiation and degranulation. J Clin Endocrinol Metab 2016;101:523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ishida E, Kim-Muller JY, Accili D. Pair feeding, but not insulin, phloridzin, or rosiglitazone treatment, curtails markers of β-cell dedifferentiation in db/db mice. Diabetes 2017;66:2092–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Z, York NW, Nichols CG, Remedi MS. Pancreatic β cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab 2014;19:872–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Accili D, Talchai SC, Kim-Muller JY, et al. When β-cells fail: lessons from dedifferentiation. Diabetes Obes Metab 2016;18(Suppl. 1):117–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Opie EL. The relation of diabetes mellitus to lesions of the pancreas. Hyaline degeneration of the islands of Langerhans. J Exp Med 1901;5:527–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Richardson SJ, Morgan NG, Foulis AK. Pancreatic pathology in type 1 diabetes mellitus. Endocr Pathol 2014;25:80–92 [DOI] [PubMed] [Google Scholar]
- 87.Campbell-Thompson M. Organ donor specimens: what can they tell us about type 1 diabetes? Pediatr Diabetes 2015;16:320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marchetti P, Suleiman M, Marselli L. Organ donor pancreases for the study of human islet cell histology and pathophysiology: a precious and valuable resource. Diabetologia 2018;61:770–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sullivan BA, Hollister-Lock J, Bonner-Weir S, Weir GC. Reduced Ki67 staining in the postmortem state calls into question past conclusions about the lack of turnover of adult human β-cells. Diabetes 2015;64:1698–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kusmartseva I, Beery M, Philips T, et al. Hospital time prior to death and pancreas histopathology: implications for future studies. Diabetologia 2018;61:954–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang X, Misawa R, Zielinski MC, et al. Regional differences in islet distribution in the human pancreas--preferential beta-cell loss in the head region in patients with type 2 diabetes. PLoS One 2013;8:e67454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mari A. Mathematical modeling in glucose metabolism and insulin secretion. Curr Opin Clin Nutr Metab Care 2002;5:495–501 [DOI] [PubMed] [Google Scholar]
- 93.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 94.Meier JJ, Gallwitz B, Siepmann N, et al. Gastric inhibitory polypeptide (GIP) dose-dependently stimulates glucagon secretion in healthy human subjects at euglycaemia. Diabetologia 2003;46:798–801 [DOI] [PubMed] [Google Scholar]
- 95.Deacon CF, Nauck MA, Meier J, Hücking K, Holst JJ. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J Clin Endocrinol Metab 2000;85:3575–3581 [DOI] [PubMed] [Google Scholar]