Abstract
Cryptococcus neoformans is an opportunistic encapsulated pathogen that causes life-threatening meningoencephalitis in individuals with immunosuppression. We compared the interactions of C. neoformans planktonic and biofilm-derived cells with J774.16 macrophage-like cells. Planktonic cells are more phagocytized and killed by J774.16 cells than biofilm-derived fungal cells. Biofilm-derived cryptococci possess larger capsule size and release significantly more capsular polysaccharide than planktonic cells in culture. Biofilm-derived fungi exhibited upregulation of genes involved in capsular production. Capsular-specific monoclonal antibody 18B7 demonstrated differential binding to the surface of planktonic and biofilm-derived cryptococci providing a plausible strategy for fungal evasion of macrophages and persistence. Future studies are necessary to elucidate how C. neoformans biofilm-derived cells regulate their virulence factors when interacting with cells of the immune system.
Keywords: antibodies, capsule, C. neoformans, fungal biofilms, macrophages, phagocytosis
1. Introduction
Cryptococcus neoformans is an encapsulated opportunistic yeast-like fungus that affects immunocompromised individuals resulting in life-threatening meningoencephalitis. The fungus capsular polysaccharide is mainly composed of glucuronoxylomannan (GXM). Abundant amounts of GXM are released during cryptococcal infection (Goldman et al. , 1995), causing deleterious effects on the host immune response (Vecchiarelli, 2000). Additionally, active C. neoformans GXM shedding is required for adhesion to a solid support and subsequent biofilm formation (Martinez and Casadevall, 2005). Cryptococcal biofilms consist of a complex network of yeast cells enmeshed in a substantial amount of extracellular polysaccharide matrix (Martinez and Casadevall, 2005). C. neoformans adheres and forms biofilms on medical devices such as ventriculoatrial shunt catheters (Bach et al. , 1997, Walsh et al. , 1986), polytetrafluoroethylene peritoneal dialysis fistula (Braun et al. , 1994), and prosthetic cardiac valves (Banerjee et al. , 1997). Due to the increasing use of prosthetic devices in the treatment of cryptococcal meningoencephalitis, it is important to understand the role of C. neoformans biofilms on infection and interaction with cells of the immune system.
Macrophages play an important role in preventing fungal colonization and disease. These leukocytes can phagocytize C. neoformans yeast cells and this fungus can replicate intracellularly, release and accumulate capsular polysaccharide in the phagolysosome, and escape macrophages in the form of microcolonies via lytic or non-lytic exocytosis (Alvarez and Casadevall, 2006, Tucker and Casadevall, 2002). Since exocytosed microcolonies (Alvarez et al. , 2008) or biofilm-derived fungal cells (Martinez and Casadevall, 2005) can disseminate to multiple organs after reaching circulation, we compared the ability of biofilm-derived cells and their planktonic counterparts in preventing phagocytosis and killing by J774.16 macrophage-like cells. We assessed differences in C. neoformans capsule size, GXM release, and expression of capsular-related genes between these phenotypes. In addition, fluorescent microscopy was utilized to determine whether differences in phagocytosis and killing between planktonic and biofilm-derived cryptococci were associated to GXM-specific monoclonal antibody (mAb) binding to the fungus or modifications to the fungal cell surface. This study is important because it expands our current knowledge of C. neoformans-host cell interactions.
2. Material and methods
2.1. Cryptococcus neoformans
C. neoformans strain H99 (serotype A) was isolated and kindly provided by John Perfect at Duke University. C. neoformans strain B3501 (serotype D) was commercially acquired from the American Type Culture Collection. Yeasts were grown in Sabouraud dextrose broth (pH 5.6; Becton Dickinson) for 24 h at 30°C in an orbital shaker (Thermo Fisher) set at 150 rpm (to early stationary phase).
2.2. Biofilm formation
C. neoformans cells were then collected by centrifugation, washed twice with phosphate-buffered saline (PBS), counted using a hemacytometer, and suspended at 107 cells per mL in minimal medium (20 mg/mL thiamine, 30 mM glucose, 26 mM glycine, 20 mM MgSO4 · 7H2O, and 58.8 mM KH2PO4; pH 5.5; Sigma). For each strain, 100 μL of the suspension were added into 900 μL of fresh minimal medium in each individual well of polystyrene 6-well plates (Corning) and incubated at 37°C. Biofilms were formed over 48 h. Following the adhesion stage, the wells containing C. neoformans biofilms were gently washed three times with PBS to remove non-adhered cryptococcal cells using a multichannel pipette. Mature cryptococcal biofilms were scraped from the bottom of each well using mechanical force with a 200 μL pipette tip, a 1 mL suspension was transferred to a 2-mL microcentrifuge tube, and sonicated to detach the cells as described with a few modifications of the protocol (Merritt et al. , 2005). Briefly, the sonicator microtip was inserted into each microcentrifuge tube and the biofilm-derived cells were sonicated for 8 sec at 40% power. During the sonication process, each microcentrifuge tube was kept on ice to reduce the possibility of fungal death due to increase in temperature. To verify the impact of the sonication procedure on cell viability, we performed viable counts on separate cultures of H99 and B3501 planktonic or biofilm-derived cells before and after sonication. We only found 5% reduction in the sonicated cryptococcal cells in planktonic and biofilm-derived preparations (data not shown).
2.3. Measurement of biofilm metabolic activity by XTT reduction assay
A semiquantitative measurement of C. neoformans biofilm formation was obtained from the 2, 3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium-hydroxide (XTT; Sigma) reduction assay. For C. neoformans strains, 50 μL of XTT salt solution (1 mg/ml in PBS) and 4 μL of menadione solution (1 mM in acetone; Sigma) were added to each well at various time points (2, 4, 6, 8, 16, 24, and 48 h). Microtiter plates were incubated at 37°C for 5 h. Fungal mitochondrial dehydrogenase activity reduces XTT tetrazolium salt to XTT formazan, resulting in colorimetric change that correlates with cell viability (Meshulam et al. , 1995). The colorimetric change was measured using a microtiter plate reader (Bio-Tek) at 492 nm. In all the experiments, microtiter wells containing heat-killed C. neoformans and minimal medium alone were included as negative controls.
2.4. Confocal microscopy
C. neoformans biofilms were incubated for 45 min in 75 μL of PBS containing the fluorescent stain FUN-1 (10 μM; Molecular Probes). Then, wells were blocked with PBS (1% BSA). MAb 18B7 (2 μg/mL) was added, and the plate was incubated. Fluorescein isothiocyanate (FITC; Molecular Probes)-conjugated goat anti-mouse (GAM)-IgG1 at 1 μg/mL in PBS (1% BSA) was applied. Between steps, the wells were washed with 0.05% Tween 20 (Thermo Fisher) in Tris-buffered saline (TBS; Thermo Fisher). All incubations were done at 37°C for 1 h. FUN-1 (excitation wavelength, 470 nm; emission, 590 nm) is converted to orange-red cylindrical intravacuolar structures by metabolically active cells, while mAb 18B7, when bound by FITC-conjugated GAM IgG1 (excitation wavelength, 488 nm; emission, 530 nm), labels GXM and fluoresces green. Microscopic examinations of biofilms formed in glass-bottom plates were performed with confocal microscopy. Depth measurements across the width of the device were taken at regular intervals using an upright Leica TCS SP5 confocal laser scanning microscope (Leica). To determine the structure of the biofilms, a series of horizontal (x-y) optical sections with a thickness of 1.175 μm were taken throughout the full length of the biofilm. Confocal images of green (18B7-FITC) and red (FUN-1) fluorescence were recorded simultaneously using a multichannel mode. Z-stack images and measurements were corrected utilizing the Leica LASX software (Leica) in the deconvolution mode.
2.5. Planktonic cell growth
Cells of C. neoformans were grown in minimal medium for 48 h at 37°C in an orbital shaker incubator set at 150 rpm, collected by centrifugation, washed twice with PBS, and counted with a hemacytometer before each experiment. As previously described and determined using the XTT reduction assay (Martinez and Casadevall, 2007), we used a density of 5 x 106 C. neoformans planktonic cells for comparison to the biofilms.
2.6. Phagocytosis assay using J774.16 macrophage-like cells
J774.16 is a well-characterized murine macrophage-like cell line that is extensively used to study C. neoformans-macrophage interactions (Feldmesser et al. , 2000a, Tucker and Casadevall, 2002). J774.16 cells were incubated in 96-well microtiter plates (Corning). Monolayers of 105 J774.16 cells were washed thrice with PBS, feeding medium [59% Dulbecco’s modified eagle medium (Gibco), 10% NCTC-109 medium (Gibco), 10% fetal calf serum (Atlanta Biologicals), and 1% non-essential aminoacids (Gibco); pH 7.4], (Aslanyan et al. , 2017) supplemented with IFN-γ (100 U/mL; BD) and bacterial lipopolysaccharide (LPS; 1 μg/mL; Sigma) added, quickly followed by the addition of pre-incubated planktonic or biofilm-related cryptococci with mAb 18B7 [anti-cryptococcal GXM IgG1 generated and generously provided by Arturo Casadevall at Johns Hopkins Bloomberg School of Public Health; 2 μg/mL] for 1 h, in a macrophage (105 cells):yeast (106 cells) ratio of 1:10 per well. The plates were incubated for 2 h at 37 °C and 5% CO2 for phagocytosis. For microscopic determination of phagocytosis, the monolayer co-culture was washed thrice with PBS to remove non-adherent cells, giemsa stained (Thermo Fisher), fixed with cold methanol (Thermo Fisher), and viewed with light microscopy with use of an Axiovert 200 M inverted microscope (Carl Zeiss) at a magnification of 40X. Images were collected using an AxioCam MrC digital camera using the Zen 2011 digital imaging software (Carl Zeiss). The phagocytic index was determined to be the number of internalized yeast cells over the number of 100 macrophages per well. Internalized cells were differentiated from attached cells by the presence in a well-defined phagocytic vacuole. For flow cytometry, adhered cells with internalized cryptococci opsonized with mAb 18B7-conjugated to FITC were removed after treatment with pre-warmed cellstripper solution (Corning) for 5 min at 37 °C and 5% CO2. Samples were processed (10,000 events per sample) on a BD Accuri C6 flow cytometer and C. neoformans phagocytosis by macrophages was analyzed using the FCS express software. The percentage of infected macrophages in both the traditional method and flow cytometry was 75% for each condition.
2.7. Killing assay
The viability and non-viability of fungi can be differentiated during phagocytosis by acridine orange staining, while extracellular organisms are quenched with crystal violet (Gacser et al. , 2007). The phagocytosis of cryptococci by J774.16 cells was performed under similar conditions as described above in an 8-chamber polystyrene tissue culture glass slide (BD). After phagocytosis and 24 h incubation at 37°C, macrophages with intracellular cryptococci were washed with Hank’s Balanced Salt Solution (HBSS; pH 7.2; Thermo Fisher), and the slides were stained with 0.01% acridine orange (Sigma) for 45 sec by the method of Pruzanski and Saito (Pruzanski and Saito, 1988). The slides were gently washed with HBSS and stained for 45 sec with 0.05% crystal violet (Sigma) dissolved in 0.15 M NaCl (Sigma). Finally, the slides were rinsed 3 times with PBS, mounted on microscope coverslips, and sealed at the edge with nail polish. The percentage of C. neoformans planktonic and biofilm-related cell killing was determined by fluorescence microscope (Axiovert 200 M inverted microscope; Zeiss). Intracellular living and dead cryptococci fluoresce green and red, respectively. For each experiment 10 fields in each well were counted per well, and at least 100 macrophages with phagocytized fungal cells were analyzed in each well.
2.8. India ink staining and capsule measurements
An aliquot of 10 μL of planktonic or biofilm-derived yeast cells was mixed with India ink and visualized with light microscopy. The capsule size of 100 cells was measured in these images using ImageJ 1.39u software (NIH). Capsule size was defined as the difference between the diameter of the total cell (capsule included) and the cell body diameter, defined by the cell wall.
2.9. C. neoformans GXM release determinations
C. neoformans capsular GXM released in culture was measured by capture ELISA as described previously (Martinez et al. , 2004). Briefly, microtiter polystyrene plates (Coming) were coated with goat anti-mouse IgM (1 μg/mL; Southern Biotech) and blocked with 1% bovine serum albumin in PBS (1% BSA; Thermo Fisher). Next, the IgM GXM binding mAb 2D10 (2 μg/mL; a gift from Arturo Casadevall) was added as a capture antibody (Mukherjee et al. , 1994), and the plate was incubated for 1 h. The solution to be tested for GXM was collected from supernatant of each biofilm well after 48-h biofilm formation then added, serially diluted on the plate, and incubated for 1 h. The ELISA was completed by adding, in successive steps, mAb 18B7 (2 μg/mL) in PBS (1% BSA), 1 μg of alkaline phosphatase-labeled goat anti-mouse IgG1/mL (Southern Biotech), and 50 μL of p-nitrophenyl phosphate (5 mg/mL; Sigma) in substrate buffer. Between every step, the wells were washed with 0.05% Tween 20 in Tris-buffered saline. All incubations were performed at 37 or 4°C overnight.
2.10. RNA extraction and cDNA synthesis
For RNA extraction, C. neoformans planktonic or sonicated biofilm-derived cells were suspended at a density of 5 x 108 in 5 mL of PBS and homogenized with 0.5-mm-diameter zirconium-silica glass beads (Thermo Fisher) using a beater for 4 min to ensure complete lysis. Cell debris was removed by centrifugation at 10,000 rpm for 10 min at room temperature. RNA extraction was performed using the Ambion RNA purification kit (Thermo Fisher), following the manufacturer’s instructions. To remove any genomic DNA carryover, the samples were treated with DNase I (Qiagen) for 30 min at 37°C, followed by heat inactivation for 5 min at 65°C. Then, 1 μg of total RNA was used to synthesize cDNA with the Bio-Rad iScript reverse transcriptase kit (Bio-Rad), following the manufacturer’s instructions. The control reaction was set up using all components of the reaction mixture but without the reverse transcriptase enzyme (i.e., no reverse transcriptase).
2.11. Real-Time Polymerase Chain Reaction (RT-PCR)
The genes selected for quantification were cap 10 (Chang and Kwon-Chung, 1999), cap59 (Chang and Kwon-Chung, 1994), cas1 (Janbon et al. , 2001), cmt1 (Sommer et al. , 2003), grasp (Kmetzsch et al. , 2011), and man1 (Wills et al. , 2001), all involved in capsule synthesis. The primers used for RT-PCR analysis are described in Table 1. The efficiency of each primer was tested by using a 10-fold serial dilution of the cDNA mixture, and only primers with efficiencies between 95% and 105% were used for the analysis. The expression of genes was determined by quantitative PCR using iQ SYBR Green Supermix (Bio-Rad). Two different control reactions were included in the analysis, i.e., a no-template control and a no reverse transcriptase control. We used actin rRNA as the reference gene (forward, 5′-CAGCTCGTGTCGTGAGATGT-3′; reverse, 5′-CGTAAGGGCCATGATGACTT-3′). Relative expression was determined using the cycle threshold (ΔΔCT) method on a Mastercycler RealPlex2 system (Eppendorf). Reactions were set up using 300 nM primers and 5 μL of the cDNA template (diluted 1:10). The cycling conditions used were as follows: 55°C for 30 min and then 40 amplification cycles of 95°C for 15 s, 55°C for 30 s, and 72°C for 30 s. The samples were cooled to 55°C, and a melting curve for temperatures between 55°C and 95°C, with 0.5°C increments, was recorded. All reactions were carried out in triplicate. Target gene expression was measured using expression relative to that of the actin reference gene. Data analysis was carried out using Mastercycler ep realplex software (Eppendorf).
Table 1.
Primers used in the qRT-PCR studies.
| Gene | Forward | Reverse |
|---|---|---|
| cap10 | TCTTCTTCCGTCATTCGTTC | TTCTCCCGTACCTCTTCTTG |
| cap59 | AACCGAACGAAGAAACCTC | ACCCCAGCACCACACATACTC |
| cas1 | TATCTCTTCCTCGCCGACAG | TAGCCATTCAGTGATTTCGC |
| cmt1 | CTCGTACAGCCTATTCCAAC | GACATCACCTCTCCTGAAAC |
| grasp | GAGGGAGAGGGAATAGTAGTG | CTTTTGACGCTTCTTCTGAC |
| man1 | CTCGTACAGCCTATTCCAAC | GACATCACCTCTCCTGAAAC |
2.12. Fluorescent microscopy
For immunofluorescence studies, slides were coated with poly-l-lysine (0.1 mg/mL; Sigma), and 106 yeast cells were allowed to air dry on slides so that organisms adhered. For mAb binding experiments, mAb 18B7 (2 μg/mL) was added in PBS with 1% BSA. FITC-labeled goat anti-mouse (GAM)-IgG1 (Southern Biotech) was added at 2 μg/mL after application of unconjugated mAb. All incubations were performed at 37°C for 30 min, and slides were washed thrice with PBS between each application of reagents. Slides were washed again with PBS, 30 μL of mounting medium (0.1 M n-propyl gallate-50% glycerol in PBS; Sigma) was added, and coverslips were placed. The slides were viewed in an Olympus AX41 microscope (Olympus) with fluorescent filters attached. Fluorescent images were recorded with an Olympus DP70 camera and processed with Olympus DPC software. Finally, we determined the percentage of cells per field with Ab punctate binding pattern on their surface by dividing the number of cryptococci showing this phenotype over the total number of cells. For the fluorescent intensity studies, the cells were first incubated with calcofluor white (CW; 10 μg/mL; Sigma) dye in PBS for 10 min in the dark at room temperature and washed thrice in PBS. CW specifically stains chitin contained in the cell wall of several eukaryote microorganisms including C. neoformans (Alanio et al. , 2011). CW was used to compare cell wall differences in fungal planktonic and biofilm-derived cells. Then, mAb 18B7-FITC-conjugated GAM IgG1 was added to delineate the fungus polysaccharide capsule (size) and the sample was processed using a Leica TCS SP5 confocal laser scanning microscope as described above. Lastly, CW and FITC fluorescent intensities were assessed using ImageJ 1.39u software.
2.13. Statistical analysis
All data were subjected to statistical analysis using GraphPad Prism 7.0 (GraphPad Software). P values for individual comparisons were calculated using student’s t-test analysis. P values of <0.05 were considered significant.
3. Results
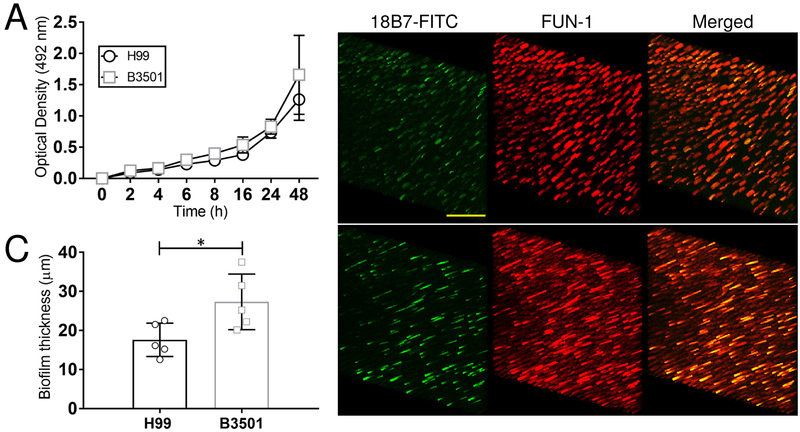
3.1. C. neoformans strains H99 and B3501 cells form robust biofilms.
We first validated previous observations and demonstrated that cells of C. neoformans strains H99 and B3501 form strong biofilms (Fig. 1). Using the XTT reduction assay, we showed that H99 and B3501 cryptococci had similar metabolic activity after 48 h (Fig. 1A). We used confocal microscopy to associate the XTT reduction assay findings with the visual properties on biofilm metabolism and architecture (Fig. 1B). Regions of red fluorescence (FUN-1) represent metabolically active cells, and the green fluorescence (mAb 18B7-FITC-conjugated GAM IgG1) indicates GXM. Similar to previous description, C. neoformans serotype A strain H99 cells displayed a uniform distribution and biofilm formation across the field with high metabolic activity on yeasts surrounded by extracellular GXM material (Fig. 1B; upper panel). In contrast, C. neoformans serotype D strain B3501 exhibited scattered biofilm aggregates with highly metabolically active fungal cells encased in substantial quantities of GXM (Fig. 1B; lower panel). In depth Z-stack reconstruction showed that on average C. neoformans B3501 biofilms (27.3 μm; range: 20.14-37.52 μm) were thicker compared to H99 biofilms (17.6 μm; range: 12.58-22.56 μm) (P<0.05) (Fig. 1C). These data confirmed that H99 and B3501 cryptococci form vigorous biofilms on abiotic surfaces.
Fig. 1. Biofilm formation by C. neoformans strains H99 (serotype A) and B3501 (serotype D).
(A) The kinetics of biofilm formation by C. neoformans strains H99 and B3501 was compared and determined using XTT reduction assay. Each time point denotes the average of four independent measurements per strain and error bars indicate standard deviations (SDs). (B) Confocal microscopic images of C. neoformans H99 and B3501 strain biofilms after 48 h. Representative images of mature fungal biofilms showed metabolically active (red; FUN-1-stained) cells embedded in the polysaccharide extracellular material (green; stained with mAb 18B7-FITC-conjugated GAM IgG1). The pictures were taken at a magnification of ×63. Scale bar, 20-μm. (C) The thickness of the cryptococcal biofilms was determined using Z-stack reconstruction. Bars are the averages of the results for five independent measurements (each symbol represents an individual measurement) per strain, and error bars denote SDs. Asterisk denotes P-value significance (P < 0.05) calculated by Student’s t-test. For A-C, each experiment was performed twice, and similar results were obtained.
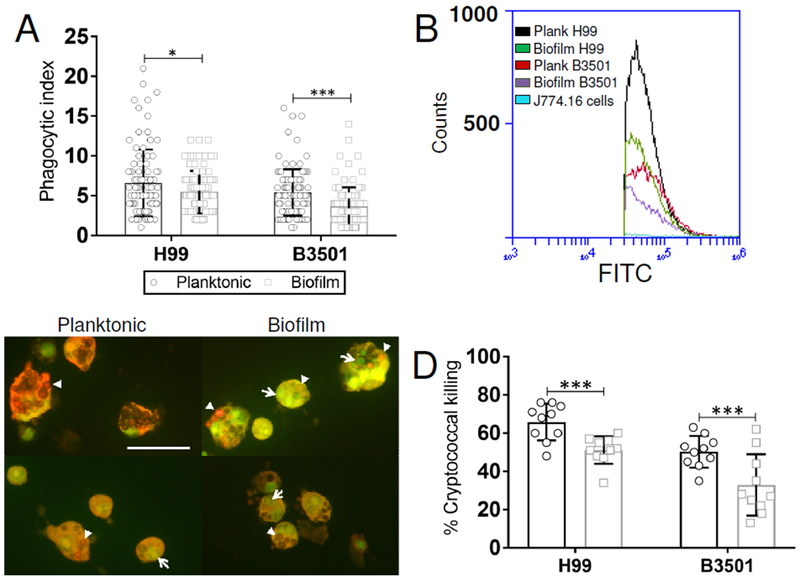
3.2. J774.16 cells evinced reduced phagocytosis and killing of biofilm-derived cryptococci.
We investigated the ability of J774.16 macrophage-like cells in phagocytizing planktonic and biofilm-derived C. neoformans strains H99 and B3501 cells. Using a traditional phagocytosis assay, we found that planktonic cryptococcal cells are more significantly phagocytized than biofilm-related cryptococci (H99, P<0.05; B3501, P<0.0001) (Fig. 2A). To validate our findings, we analyzed phagocytosis using flow cytometry (Fig. 2B). We confirmed that the planktonic phenotype of C. neoformans strains H99 (>16%) and B3501 (>18%) were more susceptible to phagocytosis by macrophages than their biofilm-related counterparts. Interestingly, C. neoformans strain H99 planktonic and biofilm-derived cells were more easily phagocytized than those of either planktonic or biofilm-derived B3501 cells (Fig. 2B). Then, we used fluorescent microscopy to examine the efficacy of J774.16 cells in killing phagocytized planktonic (upper and lower left panels) or biofilm-derived (upper and lower right panels) H99 and B3501 cryptococci (Fig. 2C). Macrophage-like cells were more efficient in killing phagocytized H99 (65.8%) and B3501 (50.3%) planktonic cells than biofilm-derived cells (H99, 51.1% and B3501, 32.9%; P<0.0001) (Fig. 2D). The results suggest that planktonic fungal cells are more susceptible to be engulfed and killed by phagocytic cells than biofilm-derived cells.
Fig. 2. Biofilm-derived C. neoformans cells are less phagocytized and killed than planktonic cells by J774.16 macrophage-like cells.
(A) The phagocytic indices (ratio of number of intracellular yeast cells to the number of macrophages counted) were determined after 2 h incubation of 105 J774.16 cells with mAb 18B7 (IgG1)-opsonized 106 planktonic and biofilm-related C. neoformans strains H99 (serotype A) and B3501 (serotype D). Bars represent the means of 100 cells (each symbol represents 1 macrophage) and error bars indicate standard deviations (SDs). Asterisks denote P-value significance (* P<0.05 and *** P<0.0001) calculated using student’s t-test analysis. (B) Phagocytosis of FITC-conjugated mAb 18B7-labeled planktonic and biofilm-derived C. neoformans strains H99 and B3501 by J774.16 cells was determined using flow cytometry. Representative plots (black, planktonic H99; green, biofilm H99; red, planktonic B3501; purple, biofilm B3501; and light blue, J774.16 cells alone) of internalized FITC-labeled fungi by macrophage-like cells are shown. Each plot was generated after 10,000 events were analyzed. (C) Killing of planktonic and biofilm-derived H99 and B3501 cryptococci by J774.16 cells was assessed using fluorescent microscopy. Dead yeast cells (arrowheads) show bright red or brown fluorescence whereas living fungi show green fluorescence (arrows). Scale bar, 20-μm. (D) Percentage of planktonic and biofilm-derived C. neoformans H99 and B3501 cells killed by macrophage-like cells. Bars represent the means of 10 fields (each symbol represents 1 field consisting of 100 macrophages) and error bars indicate SDs. Asterisks denote P-value significance (*** P<0.0001) calculated using student’s t-test analysis. The legend in A applies to this graph. For A-D, the experiments were performed twice, and similar results were obtained.
3.3. Biofilm-related cryptococci show larger capsule and abundant release of polysaccharide.
Reduced phagocytosis of biofilm-related cryptococci by J774.16 cells might also be associated to large capsule size and extensive capsular polysaccharide shedding. Therefore, we used India ink staining and light microscopy to investigate morphological changes in capsular size of H99 and B3501 cryptococcal planktonic or biofilm-related cells (Fig. 3). Images of cryptococcal biofilm-related cells display larger polysaccharide capsules than planktonic cells (Fig. 3A). Capsule measurements indicate that yeast cells within biofilms developed a significantly abundant capsule size compared to planktonic cells (H99, P<0.0001; B3501, P<0.05) (Fig. 3B). Given that C. neoformans releases large amounts of capsular material in cultures and tissues (Cherniak and Sundstrom, 1994, Goldman, Lee, 1995), we compared the ability of planktonic and biofilm-derived cells of C. neoformans H99 and B3501 strains to shed capsular polysaccharide in culture by capture ELISA (Fig. 3C). Biofilm-derived cryptococci released higher amounts of capsular polysaccharide in culture than planktonic cells (H99 and B3501, P<0.0001) (Fig. 3C). Taken together, our observations indicate that large capsule size and extensive release of polysaccharide by biofilm-derived cryptococci reduce phagocytosis by J774.16 macrophage-like cells.
Fig. 3. Biofilm-related C. neoformans cells display larger capsule and higher polysaccharide released than planktonic cells.
(A) Representative India ink images displaying the capsule size of cryptococcal planktonic and biofilm-related cells. Biofilm-derived fungal cells exhibited larger capsules than planktonic cells. The pictures were taken using a ×100-power field. Scale bar, 5-μm. (B) Capsule size measurements of planktonic and biofilm-derived C. neoformans cells were performed. The capsule size of 100 cells (each circle represents 1 macrophage) was measured. (C) GXM concentration in the supernatant of C. neoformans cultures of planktonic and biofilm-related cells. For B and C, bars represent the average and error bars indicate SDs. Asterisks denote P-value significance (* P<0.05 and *** P<0.0001) calculated using student’s t-test analysis. The legend in B applies to this graph. The experiments were performed twice, and similar results were obtained.
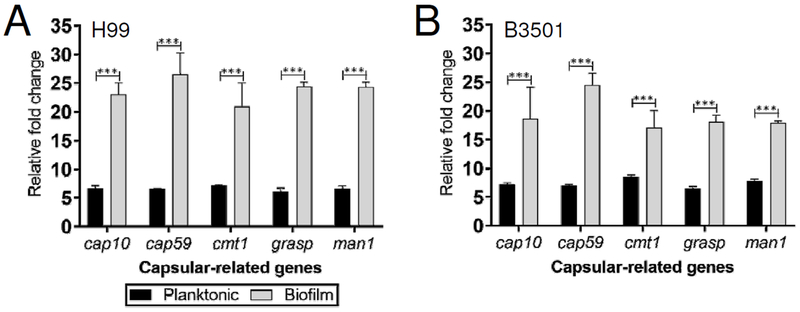
3.4. Biofilm-derived cryptococci show higher expression of genes involved in capsule formation than planktonic cells.
We used RT-PCR to evaluate the differential expression of capsular-related genes (cap10, cap59, cmt1, grasp, and man1) by planktonic and biofilm-related cells of C. neoformans strains H99 and B3501. C. neoformans strain H99 biofilm-derived cells displayed ~3-fold upregulation for each capsular-associated gene relative to their planktonic counterparts (P<0.0001) (Fig. 4A). Likewise, biofilm-derived cryptococci of strain B3501 evinced ~2-fold upregulation of cap10, cmt1, grasp, and man1 compared to planktonic cells (P<0.0001) (Fig. 4B). Additionally, cap59 showed ~3-fold increase in biofilm-related fungi compared to their planktonic counterparts (P<0.0001). Our findings provide evidence that capsular-related genes are upregulated in cryptococcal biofilms.
Fig. 4. Capsular-related genes are significantly expressed in biofilm-derived cryptococcal cells.
The relative fold changes of expression of capsular-related genes (cap10, cap59, cmt1 grasp, and man1) were compared in planktonic and biofilm-derived cells from C. neoformans strains (A) H99 and (B) B3501. The actin gene was used as a reference. Bars represent the means of 5 samples and error bars indicate SDs. Asterisks denote P-value significance (*** P<0.0001) calculated using student’s t-test analysis. The legend applies to A and B. The experiments were performed twice, and similar results were obtained.
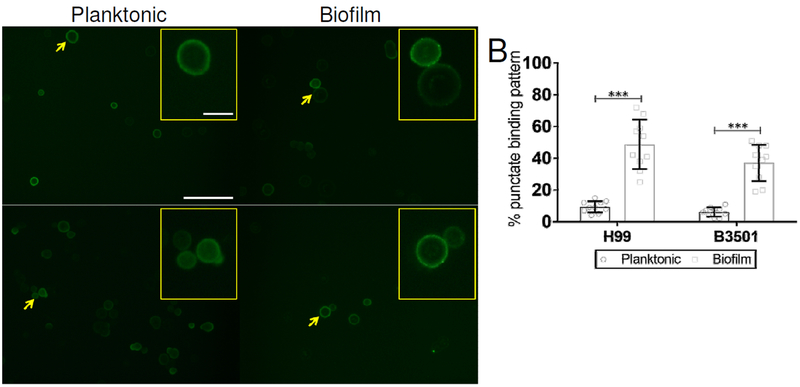
3.5. Biofilm-related cryptococci display a punctate fluorescence pattern after mAb 18B7 binding.
Analysis of mAb binding to cryptococcal cells has revealed that an annular and a punctate fluorescence pattern on the fungus capsular surface is associated with increased and reduced protection in murine models, respectively (Feldmesser, Kress, 2000a, Feldmesser et al. , 2000b). We examined whether mAb 18B7 binding to the surface of biofilm-derived cryptococci showcased a different fluorescent pattern compared to planktonic cells, thus, providing a plausible explanation for their impaired phagocytosis by J774.16 cells (Fig. 5). Fluorescent images of C. neoformans planktonic cells display an annular pattern whereas biofilm-derived fungi show a punctate pattern after GXM-binding of mAb 18B7 conjugated to FITC (Fig. 5A). We quantified the percentage of fungal cells exhibiting a mAb 18B7 punctate binding pattern in either planktonic or biofilm phenotypes (Fig. 5B). Biofilm-derived cells of strains H99 and B3501 demonstrated a punctate fluorescent pattern of 48.8% and 37.1%, respectively. While only 9.4% of H99 (P<0.0001) and 6.1% of B3501 (P<0.0001) planktonic cells presented similar mAb 18B7 punctate fluorescent binding pattern. Our results indicate that modification of the capsule and surface differential capsular-specific mAb binding to the surface of cryptococcal planktonic and biofilm-derived cells determine fungal phagocytosis by macrophages.
Fig. 5. C. neoformans biofilm-related cells display a punctate fluorescence pattern after the binding of GXM-binding mAb 18B7 conjugated to FITC.
(A) Immunofluorescent images of C. neoformans planktonic and biofilm-related cells after incubation with mAb 18B7-FITC-conjugated goat anti-mouse IgG1 stained to label the capsular polysaccharide. Each yellow arrow indicates magnified yeast cells in the inset. Scale bars, field: 20-μm and inset: 5-μm. (B) Percentage of cryptococci showing a mAb 18B7-FITC punctate binding pattern. Bars represent the means of multiple fields and error bars denote SDs. Asterisks denote P-value significance (*** P<0.0001) calculated using student’s t-test analysis. The experiments were performed twice, and similar results were obtained.
3.6. Biofilm-derived cryptococci showcase increased capsular size.
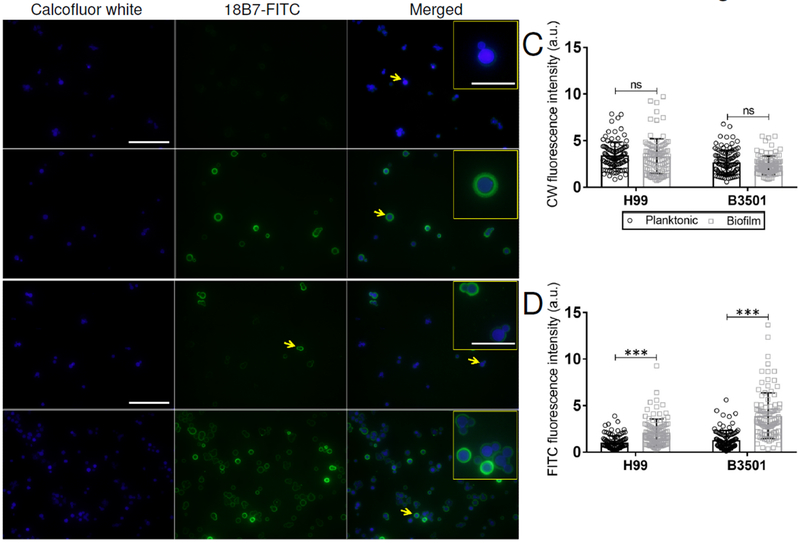
We examined whether planktonic or biofilm-derived fungi exhibited cell wall staining differences using the CW dye. Similarly, we used 18B7 conjugated to FITC to stain and delineate the fungus polysaccharide capsule. Fluorescent confocal microscopy was performed and the images taken were analyzed to determine the differences in fluorescence intensity between planktonic and biofilm-related yeast cells using imageJ 1.39 software. We did not observe any differences in CW (blue) fluorescence intensity between planktonic and biofilm-derived cells from either C. neoformans serotype A (Fig. 6A; left panels and 6C) or D (Fig. 6B; left panels and 6C) strains. Nevertheless, H99 (P<0.0001) and B3501 (P<0.0001) biofilm-derived cryptococci exhibited significant capsular (mAb 18B7-FITC; green) fluorescence intensity or size compared to the planktonic yeasts (Fig. 6A–B; center panels and 6D). Planktonic H99 (Fig. 6A; right panels and inset) and B3501 (Fig. 6B; right panels and inset) cells had smaller capsular halos than biofilm-derived fungal cells. These findings validate the differential capsular size between planktonic and biofilm-derived fungal cells and demonstrate that alterations to the capsular surface (e.g., chemistry, size, charge, etc.) of C. neoformans, not to the cell wall, of these phenotypes regulate phagocytosis of fungal cells by macrophages.
Fig. 6. Biofilm-derived cryptococcal cells exhibit increased capsular-associated fluorescent intensity.
Immunofluorescent images of C. neoformans (A) H99 and (B) B3501 planktonic and biofilm-related cells after incubation with calcofluor white dye (CW; blue) and mAb 18B7-FITC-conjugated goat anti-mouse IgG1 (green) stained to label the cell wall and capsular polysaccharide, respectively. Each yellow arrow indicates magnified yeast cells in the inset. Scale bars, field: 20-μm and inset: 5-μm. (C) CW and (D) FITC fluorescence intensities were determined using the NIH imageJ 1.39u software. Bars represent the means of 100 cells (each symbol represents an individual cell) and error bars denote SDs. Asterisks denote P-value significance (*** P<0.0001) calculated using student’s t-test analysis, ns denotes not statistically significant comparisons. The legend in C also applies to D. The experiments were performed twice, and similar results were obtained.
3.7. cas1 is only upregidated in C. neoformans strain H99 biofilm-derived cells.
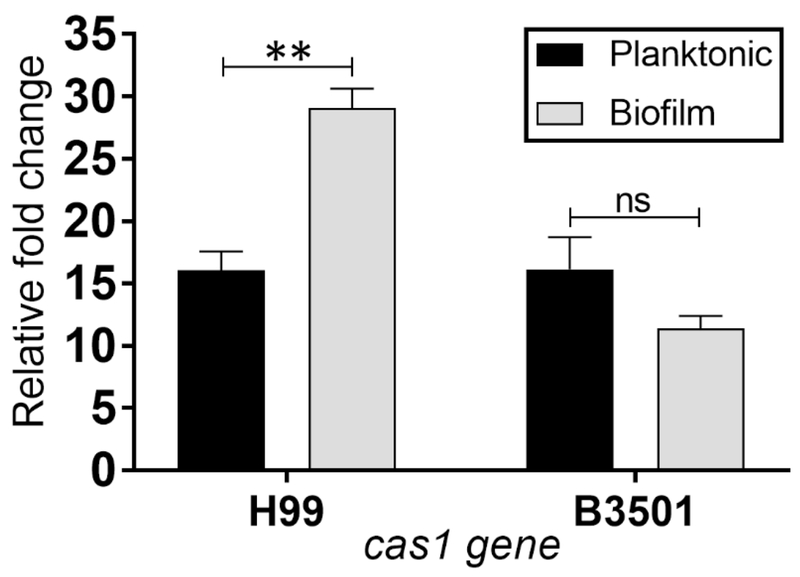
cas1 is a capsule synthesis gene that encodes for an enzyme necessary for the synthesis of O-acetyl residues on the GXM structure (Janbon, Himmelreich, 2001). Since we observed that biofilm-derived cells had an altered GXM-specific mAb binding to the surface of the fungus capsule, we investigated whether these modifications were associated to variations in O-acetylation of the capsular surface between biofilm-derived and planktonic cells. Hence, we examined cas1 expression in the two phenotypes of strains H99 and B3501 (Fig. 7). We found that cas1 was significantly expressed in H99 biofilm-related cells compared to their planktonic counterparts (P<0.001). In contrast, B3501 biofilm-derived cells displayed a downregulation trend in the expression of cas1 relative to planktonic cells. These findings suggest that cas1 expression in H99 biofilm-related cells might be important in phagocytosis inhibition by macrophages.
Fig. 7. C. neoformans H99 biofilm-derived cells show an increase in cas1 expression.
The relative fold changes of expression of the capsular-O-acetylation-associated gene cas1 were compared in planktonic and biofilm-derived cells from C. neoformans strains H99 and B3501. The actin gene was used as a reference. Bars represent the means of 3 samples and error bars indicate SDs. Asterisks denote P-value significance (** P<0.001) calculated using student’s t-test analysis, ns denotes not statistically significant comparisons. The experiments were performed twice, and similar results were obtained.
4. Discussion
We investigated J774.16 macrophage-like cells phagocytosis of C. neoformans planktonic and biofilm-derived cells. We observed reduced phagocytosis of cryptococcal cells related to biofilms by macrophages. Sugar composition analysis of the exopolymeric material of cryptococcal biofilms have shown the presence of other polysaccharides in addition to GXM when compared to planktonic cells (Martinez and Casadevall, 2007). C. neoformans is capable of altering its capsular polysaccharide highlighting the fungus’s ability to adapt to environmental stimuli or stress (Denham et al. , 2018, Patel et al. , 2013). In addition, GXM imparts negative charge to the cryptococcal cell surface making difficult cell-to-cell interactions and preventing phagocytic cells to adhere and engulf the yeast cells (Nosanchuk and Casadevall, 1997). Our results suggest that fungal biofilms may be a niche that promotes alterations in the composition and production of C. neoformans capsule, which can be advantageous to the microbe during pathogenesis.
Fungal cells with large capsule have anti-phagocytic activity. Our findings demonstrated that an increase in capsular size of biofilm-derived cryptococci interferes with antibody-mediated phagocytosis by J774.16 macrophage-like cells. Physical changes to the C. neoformans capsule result from responses to environmental factors (Denham, Verma, 2018), including iron levels (Vartivarian et al. , 1993), microbial competition (Abdulkareem et al. , 2015), and CO2 concentrations (Granger et al. , 1985). Although various signaling pathways that may contribute to capsular size regulation by C. neoformans have been explored, the architectural differences of the capsule in response to environmental changes are poorly elucidated. Aspects that contribute to increase capsule synthesis by C. neoformans are multifactorial and may include increase polysaccharide shedding, complex polysaccharide assembly, and production of physically modified or larger polysaccharide fibers.
C. neoformans extensively releases its polysaccharide capsule in tissues (Goldman, Lee, 1995) causing adverse effects on macrophage migration, proliferation, and phagocytosis (Vecchiarelli, 2000). Moreover, active capsular polysaccharide production is required for cryptococcal biofilm formation (Martinez and Casadevall, 2005). Upregulation of capsular-related genes by biofilm-derived cells during interactions with macrophages stimulates yeast cells to copiously produce large amounts of polysaccharide resulting in the survival of C. neoformans. This strategy allows C. neoformans biofilm-derived cells to colonize and inhabit inside of macrophages resulting in a persistent infection. For example, mAb-mediated phagocytosis of cryptococcal cells results in intracellular replication, host cell cytoplasmic polysaccharide accumulation, and phagosomal extrusion in a microcolony-like arrangement (Alvarez, Saylor, 2008). Our results are in agreement with Benaducci and colleagues which demonstrated that cap59, lac1, and ure1 expression is upregulated on cryptococcal-biofilm cells isolated from the model Galleria mellonella after infection (Benaducci et al. , 2016). In this regard, there is limited data describing the importance of C. neoformans non-capsular virulence factors such as lac1 and ure1 in biofilm formation. Expression of lac1 in C. neoformans encodes for laccase, a phenoloxidase in the cell wall that utilizes L-dopa to produce melanin, an important virulence factor contributing to the protection of the fungus against environmental stress (Rosas and Casadevall, 1997) and oxidative damage by host phagocytes (Zhu et al. , 2001). We have found that melanized C. neoformans biofilm-derived cells are less susceptible to antifungal drugs than melanized planktonic cells (Martinez and Casadevall, 2006). Nevertheless, there is no study investigating the role of melanin production on C. neoformans biofilms and their interactions with immune cells. Likewise, ure1, a gene that encodes for urease, a metalloenzyme that accelerates the hydrolysis of urea to ammonia and carbamate under physiological conditions resulting in an increased pH. Urease facilitates the invasion of C. neoformans to the central nervous system by enhancing yeast sequestration within the brain microcapillaries in the blood–brain barrier during blood stream dissemination (Olszewski et al. , 2004). It would be interesting to assess the involvement of urease in cryptococcoma formation, a biofilm-like structure and a hallmark of cryptococcal meningitis. Furthermore, examination of genes related to phospholipase (Chen et al. , 1997) and protease (Vu et al. , 2014, Xu et al. , 2014) production is necessary to elucidate their function in C. neoformans biofilm formation and pathogenesis.
Differences in levels of GXM binding by mAbs are related to the efficacies of those antibodies in protecting C. neoformans-infected animals (Feldmesser, Rivera, 2000b). For example, an annular GXM binding pattern is indicative of protection, whereas punctate binding is indicative of the opposite. We examined the binding of GXM-specific mAb on C. neoformans capsular polysaccharide to determine whether differences in the distribution of immunoglobulins on the surface of planktonic or biofilm-derived cryptococci might be important in preventing fungal phagocytosis by macrophages. Interestingly, biofilm-derived yeasts displayed a punctate or limited GXM-binding pattern with mAb 18B7, as opposed to the annular pattern exhibited by planktonic cells. Alterations to C. neoformans polysaccharide capsule surface can prevent the binding of immune molecules (e.g., Abs, complement, defensins, etc.) and interfere with their recognition by immune cells (Feldmesser, Rivera, 2000b). Thus, changes in the microbial phenotype should be taken in consideration for treatment of biofilm-related infections.
We also investigated differences in the expression of cas1, an enzyme required for GXM O-acetylation (Janbon, Himmelreich, 2001), between biofilm and planktonic cryptococci. Increasing or reducing GXM O-acetylation in the virulence of C. neoformans has provided conflicting results. For example, a cas1 mutant strain lacking O-acetylation in the capsular material was associated with increased C. neoformans virulence (Janbon, Himmelreich, 2001, Kozel et al. , 2003). In contrast, a spontaneous variant strain with poorly O-acetylated GXM have demonstrated attenuated virulence (Cleare et al. , 1999). We found that H99 biofilm-derived cells had increased cas1 expression compared to their planktonic counterparts. Since cas1 mutants display thinner capsules due to the chemical de-O-acetylation (Janbon, Himmelreich, 2001, Young and Kozel, 1993), increased cas1 expression in H99 biofilm-derived cryptococci may promote capsular enlargement and release by fungal cells resulting in uniform biofilms (Abdulkareem, Lee, 2015) which might be more resistant to attack and elimination by immune cells. Conversely, there was no difference in the expression of cas1 between B3501 planktonic and biofilm-derived cells. C. neoformans strain B3501 cells form biofilms structurally different to those of strain H99 cells (Abdulkareem, Lee, 2015). C. neoformans strain B3501 exhibits scattered biofilms (Abdulkareem, Lee, 2015) in a flower-like arrangement (Lopes et al. , 2017). Given that cas1 possibly plays an important role in the structure of cryptococcal biofilms, this glycosyltransferase might be a good candidate for future studies investigating the dramatic architectural differences between these strains. Likewise, the studies can be expanded by including multiple serotype A and D isolates to determine variations in cas1 expression and establish a correlation with fungal cell spatial distribution and biofilm structure.
In conclusion, we demonstrated that biofilm-related cryptococci are more difficult to phagocytize and kill by macrophages. Biofilm-derived cryptococci exhibited large capsule size, surface modifications, and release of high amounts of polysaccharide in culture suggesting that cells with this phenotype might be difficult to eradicate by immune cells. Our study suggest that differential gene expression between biofilm-derived and planktonic cells should be further investigated using a murine model of infection to gain a better understanding of disease progression, antimicrobial efficacy, and immune responses. Recent studies by Benaducci et al. using G. mellonella as infection model indicates that the biofilm phenotype may contribute to the fungus virulence (Benaducci, Sardi Jde, 2016). Therefore, future studies are warranted to understand how C. neoformans biofilm-derived and planktonic cells regulate their virulence factors when interacting with cells of the immune system.
HIGHLIGHTS.
The interactions of C. neoformans biofilm-derived cells with macrophages were evaluated.
Biofilm-derived cryptococci are more difficult to phagocytize and killed by macrophages.
Increased capsule size and polysaccharide released was evinced in fungal biofilm cells.
Upregulation of capsular-related genes was shown in biofilm-derived cells.
Altered capsular-specific mAb binding to the surface biofilm-derived cells was observed.
Acknowledgements
L.R.M. was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) under award number R01AI145559.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References
- Abdulkareem AF, Lee HH, Ahmadi M, Martinez LR. Fungal serotype-specific differences in bacterial-yeast interactions. Virulence. 2015;6:652–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanio A, Desnos-Ollivier M, Dromer F. Dynamics of Cryptococcus neoformans-macrophage interactions reveal that fungal background influences outcome during cryptococcal meningoencephalitis in humans. MBio. 2011;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez M, Casadevall A. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr Biol. 2006;16:2161–5. [DOI] [PubMed] [Google Scholar]
- Alvarez M, Saylor C, Casadevall A. Antibody action after phagocytosis promotes Cryptococcus neoformans and Cryptococcus gattii macrophage exocytosis with biofilm-like microcolony formation. Cell Microbiol. 2008;10:1622–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslanyan L, Ekhar VV, DeLeon-Rodriguez CM, Martinez LR. Capsular specific IgM enhances complement-mediated phagocytosis and killing of Cryptococcus neoformans by methamphetamine-treated J774.16 macrophage-like cells. International immunopharmacology. 2017;49:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach MC, Tally PW, Godofsky EW. Use of cerebrospinal fluid shunts in patients having acquired immunodeficiency syndrome with cryptococcal meningitis and uncontrollable intracranial hypertension. Neurosurgery. 1997;41:1280–2; discussion 2-3. [DOI] [PubMed] [Google Scholar]
- Banerjee U, Gupta K, Venugopal P. A case of prosthetic valve endocarditis caused by Cryptococcus neoformans var. neoformans. J Med Vet Mycol. 1997;35:139–41. [PubMed] [Google Scholar]
- Benaducci T, Sardi Jde C, Lourencetti NM, Scorzoni L, Gullo FP, Rossi SA, et al. Virulence of Cryptococcus sp. Biofilms In Vitro and In Vivo using Galleria mellonella as an Alternative Model. Front Microbiol. 2016;7:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun DK, Janssen DA, Marcus JR, Kauffman CA. Cryptococcal infection of a prosthetic dialysis fistula. Am J Kidney Dis. 1994;24:864–7. [DOI] [PubMed] [Google Scholar]
- Chang YC, Kwon-Chung KJ. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol Cell Biol. 1994;14:4912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Kwon-Chung KJ. Isolation, characterization, and localization of a capsule-associated gene, CAP10, of Cryptococcus neoformans. J Bacteriol. 1999;181:5636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SC, Wright LC, Santangelo RT, Muller M, Moran VR, Kuchel PW, et al. Identification of extracellular phospholipase B, lysophospholipase, and acyltransferase produced by Cryptococcus neoformans. Infect Immun. 1997;65:405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherniak R, Sundstrom JB. Polysaccharide antigens of the capsule of Cryptococcus neoformans. Infect Immun. 1994;62:1507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleare W, Cherniak R, Casadevall A. In vitro and in vivo stability of a Cryptococcus neoformans [corrected] glucuronoxylomannan epitope that elicits protective antibodies. Infect Immun. 1999;67:3096–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denham ST, Verma S, Reynolds RC, Worne CL, Daugherty JM, Lane TE, et al. Regulated Release of Cryptococcal Polysaccharide Drives Virulence and Suppresses Immune Cell Infiltration into the Central Nervous System. Infect Immun. 2018;86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmesser M, Kress Y, Novikoff P, Casadevall A. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect Immun. 2000a;68:4225–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmesser M, Rivera J, Kress Y, Kozel TR, Casadevall A. Antibody interactions with the capsule of Cryptococcus neoformans. Infect Immun. 2000b;68:3642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacser A, Trofa D, Schafer W, Nosanchuk JD. Targeted gene deletion in Candida parapsilosis demonstrates the role of secreted lipase in virulence. J Clin Invest. 2007;117:3049–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman DL, Lee SC, Casadevall A. Tissue localization of Cryptococcus neoformans glucuronoxylomannan in the presence and absence of specific antibody. Infect Immun. 1995;63:3448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DL, Perfect JR, Durack DT. Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide. J Clin Invest. 1985;76:508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janbon G, Himmelreich U, Moyrand F, Improvisi L, Dromer F. Cas1p is a membrane protein necessary for the O-acetylation of the Cryptococcus neoformans capsular polysaccharide. Mol Microbiol. 2001;42:453–67. [DOI] [PubMed] [Google Scholar]
- Kmetzsch L, Joffe LS, Staats CC, de Oliveira DL, Fonseca FL, Cordero RJ, et al. Role for Golgi reassembly and stacking protein (GRASP) in polysaccharide secretion and fungal virulence. Mol Microbiol. 2011;81:206–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel TR, Levitz SM, Dromer F, Gates MA, Thorkildson P, Janbon G. Antigenic and biological characteristics of mutant strains of Cryptococcus neoformans lacking capsular O acetylation or xylosyl side chains. Infect Immun. 2003;71:2868–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes W, Vainstein MH, De Sousa Araujo GR, Frases S, Staats CC, de Almeida RMC, et al. Geometrical Distribution of Cryptococcus neoformans Mediates Flower-Like Biofilm Development. Front Microbiol. 2017;8:2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LR, Casadevall A. Specific antibody can prevent fungal biofilm formation and this effect correlates with protective efficacy. Infect Immun. 2005;73:6350–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LR, Casadevall A. Susceptibility of Cryptococcus neoformans biofilms to antifungal agents in vitro. Antimicrob Agents Chemother. 2006;50:1021–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LR, Casadevall A. Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light. Appl Environ Microbiol. 2007;73:4592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LR, Moussai D, Casadevall A. Antibody to Cryptococcus neoformans glucuronoxylomannan inhibits the release of capsular antigen. Infect Immun. 2004;72:3674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt JH, Kadouri DE, O’Toole GA. Growing and analyzing static biofilms. Curr Protoc Microbiol. 2005;Chapter 1:Unit 1B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshulam T, Levitz SM, Christin L, Diamond RD. A simplified new assay for assessment of fungal cell damage with the tetrazolium dye, (2,3)-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)-tetrazolium-5-carboxanil ide (XTT). J Infect Dis. 1995;172:1153–6. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Lee S, Mukherjee J, Scharff MD, Casadevall A. Monoclonal antibodies to Cryptococcus neoformans capsular polysaccharide modify the course of intravenous infection in mice. Infect Immun. 1994;62:1079–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosanchuk JD, Casadevall A. Cellular charge of Cryptococcus neoformans: contributions from the capsular polysaccharide, melanin, and monoclonal antibody binding. Infect Immun. 1997;65:1836–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski MA, Noverr MC, Chen GH, Toews GB, Cox GM, Perfect JR, et al. Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. Am J Pathol. 2004;164:1761–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D, Desai GM, Frases S, Cordero RJ, DeLeon-Rodriguez CM, Eugenin EA, et al. Methamphetamine enhances Cryptococcus neoformans pulmonary infection and dissemination to the brain. MBio. 2013;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzanski W, Saito S. Comparative study of phagocytosis and intracellular bactericidal activity of human monocytes and polymorphonuclear cells. Application of fluorochrome and extracellular quenching technique. Inflammation. 1988;12:87–97. [DOI] [PubMed] [Google Scholar]
- Rosas AL, Casadevall A. Melanization affects susceptibility of Cryptococcus neoformans to heat and cold. FEMS Microbiol Lett. 1997;153:265–72. [DOI] [PubMed] [Google Scholar]
- Sommer U, Liu H, Doering TL. An alpha-1,3-mannosyltransferase of Cryptococcus neoformans. J Biol Chem. 2003;278:47724–30. [DOI] [PubMed] [Google Scholar]
- Tucker SC, Casadevall A. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc Natl Acad Sci U S A. 2002;99:3165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartivarian SE, Anaissie EJ, Cowart RE, Sprigg HA, Tingler MJ, Jacobson ES. Regulation of cryptococcal capsular polysaccharide by iron. J Infect Dis. 1993;167:186–90. [DOI] [PubMed] [Google Scholar]
- Vecchiarelli A Immunoregulation by capsular components of Cryptococcus neoformans. Med Mycol. 2000;38:407–17. [DOI] [PubMed] [Google Scholar]
- Vu K, Tham R, Uhrig JP, Thompson GR 3rd, Na Pombejra S, Jamklang M, et al. Invasion of the central nervous system by Cryptococcus neoformans requires a secreted fungal metalloprotease. MBio. 2014;5:e01101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh TJ, Schlegel R, Moody MM, Costerton JW, Salcman M. Ventriculoatrial shunt infection due to Cryptococcus neoformans: an ultrastructural and quantitative microbiological study. Neurosurgery. 1986;18:373–5. [DOI] [PubMed] [Google Scholar]
- Wills EA, Roberts IS, Del Poeta M, Rivera J, Casadevall A, Cox GM, et al. Identification and characterization of the Cryptococcus neoformans phosphomannose isomerase-encoding gene, MAN1, and its impact on pathogenicity. Mol Microbiol. 2001;40:610–20. [DOI] [PubMed] [Google Scholar]
- Xu CY, Zhu HM, Wu JH, Wen H, Liu CJ. Increased permeability of blood-brain barrier is mediated by serine protease during Cryptococcus meningitis. J Int Med Res. 2014;42:85–92. [DOI] [PubMed] [Google Scholar]
- Young BJ, Kozel TR. Effects of strain variation, serotype, and structural modification on kinetics for activation and binding of C3 to Cryptococcus neoformans. Infect Immun. 1993;61:2966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Gibbons J, Garcia-Rivera J, Casadevall A, Williamson PR. Laccase of Cryptococcus neoformans is a cell wall-associated virulence factor. Infect Immun. 2001;69:5589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]