Abstract
OBJECTIVE
To evaluate effects of the dual sodium–glucose cotransporter (SGLT) 1 and SGLT2 inhibitor sotagliflozin in combination with insulin on glucose time in range (TIR) and glucose excursions, postprandial glucose (PPG), and other glycemic metrics in adults with type 1 diabetes using masked continuous glucose monitoring (CGM).
RESEARCH DESIGN AND METHODS
Data sets from the inTandem1 (clinical trial reg. no. NCT02384941) and inTandem2 (clinical trial reg. no. NCT02421510) double-blind randomized trials evaluating sotagliflozin versus placebo in adults with type 1 diabetes treated with optimized insulin were pooled for analyses of masked CGM data from a subset of participants in each trial. The pooled cohort included patients randomized to receive placebo (n = 93), sotagliflozin 200 mg (n = 89), or sotagliflozin 400 mg (n = 96). The primary outcome was change from baseline to week 24 in glucose TIR (3.9–10.0 mmol/L [70–180 mg/dL]). Secondary end points included time below and above the target range and 2-h PPG level assessed after a standardized mixed meal.
RESULTS
Mean percentage of glucose TIR/percentage time spent at <3.9 mmol/L (<70 mg/dL) during week 24 was 51.6%/5.9%, 57.8%/5.5%, and 64.2%/5.5% with placebo, sotagliflozin 200 mg, and sotagliflozin 400 mg, respectively, which corresponded to a placebo-adjusted change from a baseline of +5.4%/−0.3% (P = 0.026; +1.3/−0.1 h/day) for sotagliflozin 200 mg and +11.7%/−0.1% (P < 0.001; +2.8/−0.02 h/day) for sotagliflozin 400 mg. Placebo-adjusted PPG reductions were 1.9 ± 0.7 mmol/L (35 ± 13 mg/dL; P = 0.004) and 2.8 ± 0.7 mmol/L (50 ± 13 mg/dL; P < 0.001) with sotagliflozin 200 and 400 mg, respectively.
CONCLUSIONS
Combined with optimized insulin in type 1 diabetes, sotagliflozin significantly increased glucose TIR without increasing time spent at <3.9 mmol/L and reduced PPG, thereby improving glycemic control.
Introduction
Managing type 1 diabetes is a challenge for even skilled, experienced patients and clinicians. Although insulin therapy is lifesaving for patients with type 1 diabetes, and intensive glycemic control reduces the complications of diabetes (1), it exposes patients to hypoglycemia and weight gain. Moreover, many patients have difficulty controlling glucose fluctuations, which can occur on a minute-by-minute basis. Because HbA1c does not reflect short-term variations in blood glucose, daily exposure to hypoglycemia and hyperglycemia, or the impact of blood glucose variations on patients’ quality of life, the international Type 1 Diabetes Outcomes Program recently recommended an additional set of type 1 diabetes outcomes measures beyond HbA1c level. These include glucose time in range (TIR), hypoglycemia, hyperglycemia, and the incidence of diabetic ketoacidosis (DKA) (2). Further refining glycemic outcomes beyond HbA1c, an international consensus group recently recommended 15 key metrics for continuous glucose monitoring (CGM) analyses and reporting, with particular emphasis on percentage of TIR at 3.9–10.0 mmol/L (70–180 mg/dL) and a means of alerting patients when glucose exceeds or is below this range (3).
The challenges of managing type 1 diabetes have prompted interest in insulin adjuncts, with the goal of improving glycemic control without increasing hypoglycemia or weight gain (4,5). To date, only pramlintide has been approved as an adjunct to insulin for the treatment of type 1 diabetes, but this agent requires prandial injections and is associated with an increased risk of severe hypoglycemia, nausea, and vomiting (4,6). Studies of incretin mimetics (i.e., dipeptidyl peptidase 4 inhibitors and glucagon-like peptide 1 receptor agonists) and metformin have shown few if any benefits (7–10), but sodium–glucose cotransporter (SGLT) inhibitors have shown promise in combination with insulin for type 1 diabetes management (11–16).
Sotagliflozin (LX4211) is a novel dual inhibitor of SGLT1 and SGLT2 that decreases renal glucose reabsorption through systemic SGLT2 inhibition. In addition, SGLT1 inhibition reduces glucose absorption in the proximal gastrointestinal tract, causing a blunting and delay of postprandial glucose (PPG) (17–19). The resulting lower peak PPG should reduce time above the goal blood glucose range and increase the time within that range (i.e., TIR), resulting in less glycemic variability. The inTandem phase 3 program consists of three completed international, randomized, double-blind, placebo-controlled trials of sotagliflozin combined with insulin for the treatment of type 1 diabetes (11–13). In each, sotagliflozin significantly reduced HbA1c, fasting plasma glucose, body weight, and blood pressure (in patients with SBP ≥130 mmHg) and increased the proportion of patients achieving HbA1c <7.0% and those achieving composite outcomes consisting of HbA1c <7.0% without severe hypoglycemia or DKA or HbA1c reductions ≥0.5% without severe hypoglycemia or DKA.
The inTandem1 and inTandem2 trials had identical trial designs in which insulin therapy was optimized for all patients starting 6 weeks before the initiation of oral therapy to identify incremental effects of sotagliflozin that could not be achieved by merely increasing insulin doses (12,13). These trials included a randomly selected subgroup of patients who underwent masked CGM to assess the effect of sotagliflozin on TIR and glycemic variability as well as the assessment of 2-h PPG to evaluate postmeal glucose excursions. Here, we describe the results of pooled analyses of data from the CGM substudies from inTandem1 and inTandem2.
Research Design and Methods
Design Overview
Prespecified pooled analyses were conducted using 24-week CGM substudy data from two phase 3, 52-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group trials of oral sotagliflozin 200 or 400 mg once daily in combination with optimized insulin in adults with type 1 diabetes who had inadequate glycemic control on insulin alone. The trials were conducted in the U.S. and Canada (inTandem1 [clinical trial reg. no. NCT02384941, ClinicalTrials.gov]) and Europe and Israel (inTandem2 [clinical trial reg. no. NCT02421510, ClinicalTrials.gov]); trial design details have been previously reported (12,13).
Participants in the CGM substudy of each trial underwent masked CGM with a Dexcom G4 monitor (Dexcom, Inc., San Diego, CA) during specified 1-week intervals throughout the first 24 weeks: week −1 to baseline, week 3–4, week 11–12, and week 23–24. Because it was possible that the CGM substudies of each trial would not meet their individual enrollment targets (n = 70/arm), the inTandem1 and inTandem2 protocols were modified before data were unmasked to include prespecified pooled analyses of CGM data. At baseline and week 24, CGM substudy participants had 2-h plasma PPG assessments after ingesting a standardized mixed liquid nutrition drink with bolus insulin matched to the carbohydrate content of the meal (Supplementary Data) (20,21).
The institutional review boards for each study center or the local ethics committees approved the protocol, consent form, and associated documents. All patients provided written informed consent. An independent statistician performed statistical analyses.
Study Population
The inTandem program included men and nonpregnant women ≥18 years of age who had type 1 diabetes treated with insulin delivered via multiple daily injections (MDI) or continuous subcutaneous insulin infusion (CSII) whose HbA1c was between 7.0% and 11.0% at screening. Full details have been published previously (12,13).
Interventions
After a 6-week insulin optimization phase, patients were randomly assigned in a 1:1:1 ratio to placebo, sotagliflozin 200 mg, or sotagliflozin 400 mg, given as two tablets administered orally once daily. Insulin optimization continued throughout the trial for all patients. Regardless of the HbA1c level achieved at the end of the optimization period, all patients were randomly assigned to treatment. Study personnel adjusted basal and bolus insulin doses to maintain fasting or preprandial blood glucose between 4.4 and 7.2 mmol/L (80 and 130 mg/dL) and 1- to 2-h PPG <10 mmol/L (<180 mg/dL) based on self-monitored blood glucose (SMBG) patterns (12,13).
End Points
The primary efficacy end point was the change from baseline to week 24 (week 23–24 period) in percentage of TIR of 3.9–10.0 mmol/L (70–180 mg/dL). Secondary end points included percentage time and area under the curve (AUC) outside the target range. These correspond to hyperglycemia (>10.0 mmol/L [>180 mg/dL]), hypoglycemia (<3.9 mmol/L [<70 mg/dL]), and the change from baseline to week 24 in AUC values above or below different thresholds (>10.0 or >13.9 mmol/L [>180 or >250 mg/dL]; <3.0 or <3.9 mmol/L [<55 or <70 mg/dL]) by time of day (full 24-h day, diurnal period [0600 to 2359 h], nocturnal period [0000 to 0559 h]) and the change from baseline in 2-h PPG (measured as plasma glucose) after a standardized mixed meal at week 24. To provide context on the risk of hypoglycemia, CGM TIR is expressed similarly to blood pressure, as percentage of TIR (3.9–10.0 mmol/L [70–180 mg/dL])/percentage time <3.9 mmol/L (<70 mg/dL). Glycemic variability was assessed with CGM data including the SD of glucose, mean daily glucose, mean amplitude of glycemic excursions (MAGE), and coefficient of variation (CV) as previously defined (22,23). Hypoglycemia by CGM was defined as at least 10 min of continuous CGM readings below the thresholds of 3.9 mmol/L (70 mg/dL) or 3.0 mmol/L (55 mg/dL).
Statistical Methods
Analyses of CGM data were based on the modified intent-to-treat subpopulation participating in the CGM substudy of each trial, which included all randomized CGM patients who had taken at least one dose of study drug. Three to 7 days worth of valid CGM recordings were required for a visit to be used for analysis; a visit consisted of a weekly session of recordings. For any day within a visit to be eligible, ≥80% of the data points had to have been nonmissing. Gaps in the CGM recording for those days included in a visit were imputed using a linear interpolation approach (24) of the planned 7 days. Because mealtime bolus insulin administration directly influences PPG, and to perform analyses under ideal design conditions to best evaluate any effects related to study treatment, the PPG analyses were based on the per-protocol population, which included all modified intent-to-treat patients who completed 24 weeks of treatment without significant protocol deviations, took bolus insulin and the study drug at the designated time at the standardized mixed meal, and completed all required mixed meal test procedures.
Continuous, longitudinal end point data were analyzed using the mixed-effects model for repeated measures method based on the restricted maximum likelihood method for estimation with several prespecified fixed effects, including first-order interactions with time, and the corresponding end point as the dependent variable in the model. ANCOVA was used for analyses of measures taken at a single time point postbaseline. For binary end points, the Cochran-Mantel-Haenszel test, stratified by the randomization stratification factors, was used. The treatment group comparisons were performed at week 24. Missing observations at week 24 were imputed as nonresponse in the Cochran-Mantel-Haenszel analysis.
Results
The pooled cohort included 278 adults (136 from inTandem1 and 142 from inTandem2) randomly assigned to receive placebo (n = 93), sotagliflozin 200 mg (n = 89), or sotagliflozin 400 mg (n = 96). Baseline characteristics were similar between groups (Table 1). In the total pooled analysis, 143 (51.4%) patients used MDI and 135 (48.6%) used CSII (Table 1).
Table 1.
Baseline characteristics
| Characteristic | Placebo (n = 93) | Sotagliflozin 200 mg (n = 89) | Sotagliflozin 400 mg (n = 96) | Total (N = 278) |
|---|---|---|---|---|
| inTandem1 participants, n (%) | 45 (48) | 44 (49) | 47 (49) | 136 (49) |
| inTandem2 participants, n (%) | 48 (52) | 45 (51) | 49 (51) | 142 (51) |
| Age (years) | 43.5 (14.2) | 44.2 (13.2) | 45.1 (12.1) | 44.3 (13.2) |
| Female sex, n (%) | 52 (55.9) | 47 (52.8) | 48 (50.0) | 147 (52.9) |
| Race or ethnic group, n (%)* | ||||
| White | 89 (95.7) | 86 (96.6) | 92 (95.8) | 267 (96.0) |
| Black | 2 (2.2) | 0 | 2 (2.1) | 4 (1.4) |
| Asian | 1 (1.1) | 0 | 0 | 1 (0.4) |
| Other | 1 (1.1) | 3 (3.4) | 2 (2.1) | 6 (2.2) |
| Hispanic/Latino ethnicity | 5 (5.4) | 4 (4.5) | 7 (7.3) | 16 (5.8) |
| HbA1c (%) | 7.6 (0.7) | 7.6 (0.6) | 7.6 (0.7) | 7.6 (0.7) |
| HbA1c (mmol/mol) | 59.5 (7.2) | 59.4 (7.0) | 59.1 (8.1) | 59.3 (7.4) |
| Diabetes duration (years) | 24.6 (12.8) | 22.2 (12.3) | 23.4 (12.0) | 23.4 (12.4) |
| Weight (kg) | 85.5 (17.9) | 86.4 (16.6) | 86.1 (16.9) | 86.0 (17.1) |
| BMI (kg/m2) | 29.4 (5.5) | 29.5 (4.8) | 29.7 (4.9) | 29.5 (5.0) |
| BMI ≥30 kg/m2, n (%) | 41 (44.1) | 41 (46.1) | 46 (47.9) | 128 (46.0) |
| Total daily insulin dose (IU/kg) | 0.8 (0.3) | 0.7 (0.2) | 0.8 (0.3) | 0.7 (0.3) |
| Daily insulin dose (IU/day) | ||||
| Total | 65.8 (34.6) | 60.3 (24.7) | 65.7 (33.0) | 64.0 (31.2) |
| Basal | 34.6 (15.9) | 31.3 (14.0) | 33.8 (15.0) | 33.3 (15.0) |
| Bolus and corrections | 31.2 (22.0) | 28.9 (15.3) | 31.9 (21.9) | 30.7 (20.0) |
| Insulin therapy, n (%) | ||||
| MDI | 44 (47.3) | 43 (48.3) | 48 (50.0) | 135 (48.6) |
| CSII | 49 (52.7) | 46 (51.7) | 48 (50.0) | 143 (51.4) |
Data are mean (SD) unless otherwise indicated.
*Determined according to patient self-report.
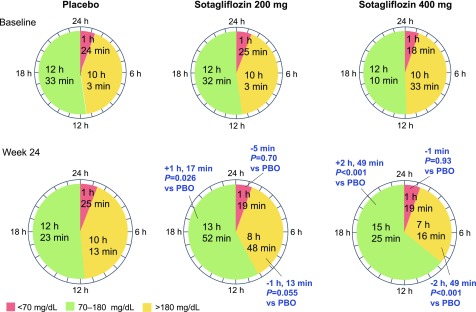
Of the pooled cohort, sufficiently complete CGM data for analyses were available for 61 of 93 patients (66%) receiving placebo; 63 of 89 patients (71%) receiving sotagliflozin 200 mg; and 72 of 96 patients (75%) receiving sotagliflozin 400 mg. The mean percentage of TIR/percentage time spent <3.9 mmol/L (<70 mg/dL) during week 24 was 51.6%/5.9%, 57.8%/5.5%, and 64.2%/5.5% with placebo, sotagliflozin 200 mg, and sotagliflozin 400 mg, respectively. Placebo-adjusted differences from baseline to week 24 in percentage of TIR were 5.4% ± 2.4% (95% CI 0.6–10.1; P = 0.026) and 11.7% ± 2.3% (95% CI 7.1–16.3; P < 0.001) with sotagliflozin 200 and 400 mg, respectively (Table 2), which was estimated to be an additional TIR of 1.3 h/day with sotagliflozin 200 mg and 2.8 h/day with sotagliflozin 400 mg (Fig. 1). Placebo-subtracted changes from baseline to week 24 in the percentage time spent >10.0 mmol/L (>180 mg/dL) were −5.0% ± 2.6% (95% CI −10.2 to 0.1; P = 0.055) and −11.8% ± 2.5% (95% CI −16.7 to −6.8; P < 0.001) in the sotagliflozin 200 and 400 mg groups, translating to −1.2 h/day and −2.8 h/day, respectively. Patients receiving sotagliflozin 200 and 400 mg spent less time at >13.9 mmol/L (>250 mg/dL) relative to placebo; differences were −3.7% ± 1.8% (95% CI −7.3 to −0.1; P = 0.045) and −7.7% ± 1.8% (95% CI −11.2 to −4.2; P < 0.001), or 0.9 and 1.9 fewer hours, respectively. No significant differences were observed in the percentage of time spent at <3.9 mmol/L (<70 mg/dL) or <3.0 mmol/L (<55 mg/dL) for either dose level of sotagliflozin compared with placebo (Table 2). Average week 24 ambulatory glucose profiles for each study group are shown in Supplementary Fig. 1.
Table 2.
Changes in CGM values and 2-h PPG at week 24*
| Placebo (n = 93) | Sotagliflozin 200 mg (n = 89) | Sotagliflozin 400 mg (n = 96) | |
|---|---|---|---|
| Time in target range, 3.9–10.0 mmol/L (70–180 mg/dL) | |||
| Percentage time 3.9–10.0 mmol/L (70–180 mg/dL)† | |||
| Patients, n | 61 | 63 | 72 |
| Mean baseline ± SD | 52.3 ± 13.8 | 52.2 ± 15.3 | 50.7 ± 14.8 |
| Mean week 24 ± SD | 51.6 ± 14.7 | 57.8 ± 15.9 | 64.2 ± 14.0 |
| Change from baseline, LSM ± SE (95% CI; P value) | −1.3 ± 1.8 (−4.8 to 2.3; 0.49) | 4.1 ± 1.8 (0.5–7.6; 0.024) | 10.5 ± 1.7 (7.1–13.8; <0.001) |
| Difference from placebo ± SE (95% CI; P value) | 5.4 ± 2.4 (0.6–10.1; 0.026) | 11.7 ± 2.3 (7.1–16.3; <0.001) | |
| Difference from placebo in hours per day corresponding to percentage time per day ± SE† | 1.3 ± 0.6 | 2.8 ± 0.6 | |
| Time in hyperglycemic range | |||
| Percentage time >10.0 mmol/L (>180 mg/dL)† | |||
| Patients, n | 61 | 63 | 72 |
| Mean baseline ± SD | 41.9 ± 15.4 | 41.9 ± 16.7 | 44.0 ± 17.1 |
| Mean week 24 ± SD | 42.6 ± 16.9 | 36.7 ± 16.8 | 30.3 ± 14.5 |
| Change from baseline, LSM ± SE (95% CI; P value) | 1.2 ± 2.0 (−2.7 to 5.1; 0.54) | −3.8 ± 2.0 (−7.7 to 0.1; 0.055) | −10.5 ± 1.9 (−14.2 to −6.8; <0.001) |
| Difference from placebo ± SE (95% CI; P value) | −5.0 ± 2.6 (−10.2 to 0.1; 0.055) | −11.8 ± 2.5 (−16.7 to −6.8; <0.001) | |
| Difference from placebo in hours per day corresponding to percentage time per day ± SE‡ | −1.2 ± 0.6 | −2.8 ± 0.6 | |
| Percentage time >13.9 mmol/L (>250 mg/dL)† | |||
| Patients, n | 61 | 63 | 72 |
| Mean baseline ± SD | 17.9 ± 12.4 | 18.6 ± 13.0 | 17.6 ± 12.2 |
| Mean week 24 ± SD | 17.4 ± 12.6 | 13.3 ± 12.7 | 8.6 ± 8.2 |
| Change from baseline, LSM ± SE (95% CI; P value) | 0.2 ± 1.4 (−2.5 to 2.9; 0.90) | −3.5 ± 1.4 (−6.2 to −0.8; 0.012) | −7.5 ± 1.3 (−10.0 to −5.0; <0.001) |
| Difference from placebo ± SE (95% CI; P value) | −3.7 ± 1.8 (−7.3 to −0.1; 0.045) | −7.7 ± 1.8 (−11.2 to −4.2; <0.001) | |
| Difference from placebo in hours per day corresponding to percentage time per day ± SE‡ | −0.9 ± 0.4 | −1.9 ± 0.4 | |
| Time in hypoglycemic ranges | |||
| Percentage time <3.9 mmol/L (<70 mg/dL)† | |||
| Patients, n | 61 | 63 | 72 |
| Mean baseline ± SD | 5.8 ± 5.3 | 5.9 ± 5.6 | 5.4 ± 6.1 |
| Mean week 24 ± SD | 5.9 ± 5.2 | 5.5 ± 5.6 | 5.5 ± 5.2 |
| Change from baseline, LSM ± SE (95% CI; P value) | 0.1 ± 0.7 (−1.2 to 1.4; 0.84) | −0.2 ± 0.7 (−1.5 to 1.1; 0.76) | 0.1 ± 0.6 (−1.2 to 1.3; 0.92) |
| Difference from placebo ± SE (95% CI; P value) | −0.3 ± 0.9 (−2.0 to 1.4; 0.70) | −0.1 ± 0.8 (−1.7 to 1.6; 0.93) | |
| Difference from placebo in hours per day corresponding to percentage time per day ± SE‡ | −0.1 ± 0.2 | −0.02 ± 0.2 | |
| Percentage time <3.9 mmol/L (<70 mg/dL) during nocturnal period (0000–0559 h) | |||
| Patients, n | 71 | 68 | 75 |
| Mean baseline ± SD | 8.2 ± 11.0 | 7.4 ± 9.8 | 7.6 ± 10.5 |
| Change from baseline, LSM ± SE (95% CI; P value) | −1.4 ± 1.2 (−3.8 to 1.0; 0.25) | −0.6 ± 1.3 (−3.0 to 1.9; 0.66) | 1.0 ± 1.2 (−1.4 to 3.3; 0.43) |
| Difference from placebo ± SE (95% CI; P value) | 0.9 ± 1.6 (−2.3 to 4.1; 0.59) | 2.4 ± 1.6 (−0.7 to 5.5; 0.13) | |
| Difference from placebo in hours per day corresponding to percentage time per day ± SE‡ | 0.2 ± 0.4 | 0.6 ± 0.4 | |
| Percentage time <3.9 mmol/L (<70 mg/dL) during diurnal period (0600–2359 h) | |||
| Patients, n | 61 | 63 | 71 |
| Mean baseline ± SD | 5.3 ± 5.1 | 5.6 ± 5.4 | 4.6 ± 5.1 |
| Change from baseline, LSM ± SE (95% CI; P value) | 0.6 ± 0.6 (−0.6 to 1.8; 0.35) | −0.07 ± 0.6 (−1.3 to 1.1; 0.91) | 0.2 ± 0.6 (−1.0 to 1.3; 0.79) |
| Difference from placebo ± SE (95% CI; P value) | −0.6 ± 0.8 (−2.2 to 0.9; 0.42) | −0.4 ± 0.8 (−1.9 to 1.1; 0.59) | |
| Difference from placebo in hours per day corresponding to percentage time per day ± SE‡ | −0.1 ± 0.2 | −0.1 ± 0.2 | |
| Percentage time <3.0 mmol/L (<55 mg/dL)† | |||
| Patients, n | 61 | 63 | 72 |
| Mean baseline ± SD | 2.4 ± 3.0 | 2.4 ± 3.7 | 2.3 ± 4.0 |
| Mean week 24 ± SD | 2.4 ± 3.2 | 2.1 ± 3.1 | 1.8 ± 2.6 |
| Change from baseline, LSM ± SE (95% CI; P value) | −0.1 ± 0.4 (−0.8 to 0.7; 0.88) | −0.2 ± 0.4 (−1.0 to 0.5; 0.57) | −0.5 ± 0.4 (−1.2 to 0.2; 0.16) |
| Difference from placebo ± SE (95% CI; P value) | −0.2 ± 0.5 (−1.1 to 0.8; 0.75) | −0.4 ± 0.5 (−1.4 to 0.5; 0.36) | |
| Difference from placebo in hours per day corresponding to percentage time per day ± SE‡ | −0.04 ± 0.1 | −0.1 ± 0.1 | |
| Percentage time <3.0 mmol/L (<55 mg/dL) during nocturnal period (00:00–05:59 h) | |||
| Patients, n | 71 | 68 | 75 |
| Mean baseline ± SD | 4.3 ± 7.3 | 3.3 ± 6.8 | 4.0 ± 7.3 |
| Change from baseline, LSM ± SE (95% CI; P value) | −0.8 ± 0.8 (−2.4 to 0.8; 0.35) | −0.6 ± 0.8 (−2.3 to 1.0; 0.44) | −0.2 ± 0.8 (−1.8 to 1.3; 0.78) |
| Difference from placebo ± SE (95% CI; P value) | 0.1 ± 1.1 (−2.0 to 2.2; 0.91) | 0.5 ± 1.0 (−1.5 to 2.6; 0.61) | |
| Difference from placebo in hours per day corresponding to percentage time per day ± SE‡ | 0.02 ± 0.3 | 0.1 ± 0.3 | |
| Percentage time <3.0 mmol/L (<55 mg/dL) during diurnal period (0600–2359 h) | |||
| Patients, n | 61 | 63 | 71 |
| Mean baseline ± SD | 1.9 ± 2.6 | 2.1 ± 3.5 | 1.7 ± 3.0 |
| Change from baseline, LSM ± SE (95% CI; P value) | 0.3 ± 0.3 (−0.3 to 0.9; 0.38) | −0.1 ± 0.3 (−0.8 to 0.5; 0.71) | −0.3 ± 0.3 (−0.9 to 0.3; 0.37) |
| Difference from placebo ± SE (95% CI; P value) | −0.4 ± 0.4 (−1.2 to 0.4; 0.33) | −0.6 ± 0.4 (−1.4 to 0.2; 0.17) | |
| Difference from placebo in hours per day corresponding to percentage time per day ± SE‡ | −0.1 ± 0.1 | −0.1 ± 0.1 | |
| Glycemic instability† | |||
| AUC >10.0 mmol/L (>180 mg/dL), mmol/L (mg/dL) × minutes/1,000 | |||
| Patients, n | 61 | 63 | 72 |
| Mean baseline ± SD | 2.4 ± 1.5 (43.6 ± 27.8) | 2.5 ± 1.7 (45.1 ± 30.2) | 2.4 ± 1.5 (44.0 ± 27.7) |
| Change from baseline, LSM ± SE (95% CI; P value) | 0.1 ± 0.2 (1.3 ± 3.2) (−0.3 to 0.4 [−4.9 to 7.5]; 0.68) | −0.4 ± 0.2 (−7.1 ± 3.2) (−0.7 to −0.1 [−13.3 to −0.9]; 0.024) | −0.9 ± 0.2 (−16.4 ± 3.0) (−1.2 to −0.6 [−22.2 to −10.6]; <0.001) |
| Difference from placebo ± SE (95% CI; P value) | −0.5 ± 0.2 (−8.4 ± 4.2) (−0.9 to −0.01 [−16.7 to −0.2]; 0.045) | −1.0 ± 0.2 (−17.7 ± 4.1) (−1.4 to −0.5 [−25.7 to −9.7]; <0.001) | |
| AUC >13.9 mmol/L (>250 mg/dL), mmol/L (mg/dL) × minutes/1,000 | |||
| Patients, n | 61 | 63 | 72 |
| Mean baseline ± SD | 0.8 ± 0.8 (14.7 ± 14.5) | 0.9 ± 0.9 (16.0 ± 16.3) | 0.8 ± 0.8 (14.0 ± 14.5) |
| Change from baseline, LSM ± SE (95% CI; P value) | 0.03 ± 0.1 (0.6 ± 1.7) (−0.1 to 0.2 [−2.7 to 3.8]; 0.73) | −0.2 ± 0.1 (−3.0 ± 1.6) (−0.3 to 0.01 [−6.3 to 0.2]; 0.07) | −0.4 ± 0.1 (−6.3 ± 1.5) (−0.5 to −0.2 [−9.4 to −3.3]; <0.001) |
| Difference from placebo ± SE (95% CI; P value) | −0.2 ± 0.1 (−3.6 ± 2.2) (−0.4 to 0.04 [−7.9 to 0.7]; 0.10) | −0.4 ± 0.1 (−6.9 ± 2.1) (−0.6 to −0.1 [−11.1 to −2.7]; 0.001) | |
| AUC <3.9 (<70 mg/dL), mmol/L (mg/dL) × minutes/1,000 | |||
| Patients, n | 61 | 63 | 72 |
| Mean baseline ± SD | 0.1 ± 0.1 (1.2 ± 1.4) | 0.1 ± 0.1 (1.2 ± 1.6) | 0.1 ± 0.1 (1.1 ± 1.7) |
| Change from baseline, LSM ± SE (95% CI; P value) | −0.002 ± 0.01 (−0.04 ± 0.2) (−0.02 to 0.02 [−0.4 to 0.3]; 0.81) | −0.006 ± 0.01 (−0.1 ± 0.2) (−0.02 to 0.01 [−0.4 to 0.2]; 0.57) | −0.01 ± 0.01 (−0.2 ± 0.2) (−0.03 to 0.01 [−0.4 to 0.2]; 0.35) |
| Difference from placebo ± SE (95% CI; P value) | −0.006 ± 0.01 (−0.1 ± 0.2) (−0.03 to 0.02 [−0.5 to 0.4]; 0.81) | −0.006 ± 0.01 (−0.1 ± 0.2) (−0.03 to 0.02 [−0.5 to 0.3]; 0.61) | |
| AUC <3.0 mmol/L (<55 mg/dL), mmol/L (mg/dL) × minutes/1,000 | |||
| Patients, n | 61 | 63 | 72 |
| Mean baseline ± SD | 0.02 ± 0.03 (0.3 ± 0.5) | 0.02 ± 0.04 (0.3 ± 0.7) | 0.02 ± 0.04 (0.3 ± 0.7) |
| Change from baseline, LSM ± SE (95% CI; P value) | −0.006 ± 0.006 (−0.1 ± 0.1) (−0.01 to 0.006 [−0.2 to 0.1]; 0.33) | −0.006 ± 0.006 (−0.1 ± 0.1) (−0.01 to 0.006 [−0.2 to 0.1]; 0.26) | −0.006 ± 0.006 (−0.1 ± 0.1) (−0.01 to 0.001 [−0.2 to 0.02]; 0.10) |
| Difference from placebo ± SE (95% CI; P value) | −0.0006 ± 0.006 (−0.01 ± 0.1) (−0.01 to 0.01 [−0.2 to 0.1]; 0.91) | −0.002 ± 0.006 (−0.03 ± 0.1) (−0.01 to 0.006 [−0.2 to 0.1]; 0.66) | |
| PPG | |||
| 2-h PPG, mmol/L (mg/dL)§ | |||
| Patients, n (per-protocol population) | 57 | 59 | 62 |
| Mean baseline ± SD | 12.9 ± 5.1 (232 ± 93) | 12.3 ± 5.4 (221 ± 96) | 11.4 ± 5.1 (205 ± 91) |
| Change from baseline, LSM ± SE (95% CI; P value) | −0.2 ± 0.6 (−3.6 ± 10.4) (−1.3 to 0.9 [−24.2 to 16.9]; 0.73) | −2.1 ± 0.6 (−38.3 ± 10.5) (−3.3 to −1.0 [−59.0 to −17.6]; <0.001) | −3.0 ± 0.6 (−53.3 ± 10.1) (−4.1 to −1.9 [−73.2 to −33.4]; <0.001) |
| Difference from placebo ± SE (95% CI; P value) | −1.9 ± 0.7 (−34.6 ± 13.2) (−3.4 to −0.5 [−60.6 to −8.6]; 0.009) | −2.8 ± 0.7 (−49.7 ± 13.1) (−4.2 to −1.3 [−75.5 to −23.9]; <0.001) | |
| Glycemic variability | |||
| CV, %† | |||
| No. patients | 61 | 63 | 72 |
| Mean baseline ± SD | 37.7 ± 5.9 | 37.9 ± 7.3 | 35.8 ± 7.4 |
| Change from baseline, LSM ± SE (95% CI; P value) | −1.0 ± 0.9 (−2.7 to 0.8; 0.27) | −2.5 ± 0.9 (−4.2 to −0.7; 0.005) | −2.0 ± 0.8 (−3.7 to −0.4; 0.016) |
| Difference from placebo ± SE (95% CI; P value) | −1.5 ± 1.2 (−3.8 to 0.8; 0.20) | −1.0 ± 1.1 (−3.3 to 1.2; 0.36) | |
| SD, mmol/L (mg/dL)† | |||
| Patients, n | 61 | 63 | 72 |
| Mean baseline ± SD | 3.6 ± 0.8 (65 ± 15) | 3.7 ± 0.8 (66 ± 14) | 3.4 ± 0.8 (62 ± 14) |
| Change from baseline, LSM ± SE (95% CI; P value) | −0.1 ± 0.1 (−1.5 ± 1.7) (−0.3 to 0.1 [−4.8 to 1.9]; 0.40) | −0.3 ± 0.1 (−6.0 ± 1.7) (−0.5 to −0.2 [−9.4 to −2.7]; <0.001) | −0.5 ± 0.1 (−8.2 ± 1.6) (−0.6 to −0.3 [−11.4 to −5.1]; <0.001) |
| Difference from placebo ± SE (95% CI; P value) | −0.3 ± 0.1 (−4.6 ± 2.3) (−0.5 to −0.01 [−9.0 to −0.2]; 0.042) | −0.4 ± 0.1 (−6.8 ± 2.2) (−0.6 to −0.1 [−11.1 to −2.5]; 0.002) | |
| Mean daily glucose, mmol/L (mg/dL) | |||
| Patients, n | 61 | 63 | 72 |
| Mean baseline ± SD | 9.7 ± 1.7 (175 ± 31) | 9.8 ± 1.8 (176 ± 33) | 9.9 ± 1.8 (178 ± 32) |
| Change from baseline, LSM ± SE (95% CI; P value) | 0.1 ± 0.2 (2.0 ± 3.6) (−0.3 to 0.5 [−5.0 to 9.1]; 0.57) | −0.3 ± 0.2 (−5.9 ± 3.6) (−0.7 to 0.1 [−12.9 to 1.1]; 0.10) | −0.9 ± 0.2 (−16.9 ± 3.4) (−1.3 to −0.6 [−23.5 to −10.3]; <0.001) |
| Difference from placebo ± SE (95% CI; P value) | −0.4 ± 0.3 (−7.9 ± 4.7) (−1.0 to 0.1 [−17.2 to 1.3]; 0.09) | −1.1 ± 0.3 (−18.9 ± 4.6) (−1.6 to −0.6 [−27.9 to −9.9]; <0.001) | |
| MAGE, mmol/L (mg/dL) | |||
| Patients, n | 61 | 63 | 72 |
| Mean baseline ± SD | 9.2 ± 2.0 (166 ± 35) | 9.1 ± 1.9 (163 ± 34) | 8.8 ± 2.0 (158 ± 36) |
| Change from baseline, LSM ± SE (95% CI; P value) | −0.2 ± 0.2 (−3.0 ± 4.2) (−0.6 to 0.3 [−11.3 to 5.4]; 0.48) | −0.9 ± 0.2 (−15.7 ± 4.2) (−1.3 to −0.4 [−24.0 to −7.4]; <0.001) | −1.4 ± 0.2 (−25.1 ± 3.9) (−1.8 to −1.0 [−32.8 to −17.3]; <0.001) |
| Difference from placebo ± SE (95% CI; P value) | −0.7 ± 0.3 (−12.7 ± 5.5) (−1.3 to −0.1 [−23.6 to −1.8]; 0.022) | −1.2 ± 0.3 (−22.1 ± 5.4) (−1.9 to −0.7 [−32.7 to −11.5]; <0.001) | |
| CGM hypoglycemic events | |||
| Hypoglycemic events per patient per day, <3.9 mmol/L (<70 mg/dL)‖ | |||
| Patients, n | 61 | 63 | 72 |
| Mean baseline ± SD | 1.1 ± 0.8 | 1.1 ± 0.7 | 0.9 ± 0.7 |
| Change from baseline, LSM ± SE (95% CI; P value) | 0.03 ± 0.1 (−0.2 to 0.2; 0.73) | −0.2 ± 0.1 (−0.4 to 0.03; 0.11) | −0.002 ± 0.1 (−0.2 to 0.2; 0.98) |
| Difference from placebo ± SE (95% CI; P value) | −0.2 ± 0.1 (−0.5 to 0.1; 0.15) | −0.04 ± 0.1 (−0.3 to 0.2; 0.78) | |
| Hypoglycemic events per patient per day, <3.0 mmol/L (<55 mg/dL)‖ | |||
| Patients, n | 61 | 63 | 72 |
| Mean baseline ± SD | 0.5 ± 0.5 | 0.4 ± 0.5 | 0.4 ± 0.4 |
| Change from baseline, LSM ± SE (95% CI; P value) | 0.1 ± 0.1 (−0.03 to 0.2; 0.14) | 0.003 ± 0.1 (−0.1 to 0.1; 0.96) | −0.04 ± 0.1 (−0.2 to 0.1; 0.44) |
| Difference from placebo ± SE (95% CI; P value) | −0.1 ± 0.1 (−0.3 to 0.1; 0.28) | −0.1 ± 0.1 (−0.3 to 0.02; 0.09) |
LSM, least squares mean.
*Conducted in a subgroup of patients who underwent blinded CGM with a Dexcom G4 monitor (Dexcom Inc.) during specified 1-week intervals throughout the first 24 weeks.
†Included in 2017 international consensus on use of CGM (3). The consensus group identified hypoglycemia cutoffs as follows: level 1, <3.9–3.0 mmol/L (<70–54 mg/dL); level 2, <3.0 mmol/L (<54 mg/dL).
‡Assuming 100% daily CGM data were available for analysis, 1.0% of daily CGM time = 0.24 h.
§To assess the change in PPG under standardized conditions, the per-protocol population was selected; 2-h plasma PPG values were obtained after a standardized mixed meal.
‖A CGM hypoglycemic event was defined by CGM sensor values of at least 10 continuous minutes below the thresholds of 3.9 mmol/L (70 mg/dL) or 3.0 mmol/L (55 mg/dL).
Figure 1.
Time spent in glycemic ranges of <3.9, 3.9–10.0, and >10.0 mmol/L (<70, 70–180, and >180 mg/dL) among patients monitored with blinded CGM. Time values were calculated by multiplying values for the percentage of time spent in specified ranges (Table 2) by 0.24 (assumes 100% data capture) to determine the number of hours, and the resulting right-of-decimal values by 60 to determine the number of minutes (e.g., the baseline percentage of TIR of 3.9–10.0 mmol/L in the placebo group was 52.3% × 0.24 = 12.552 h; 0.552 × 60 = 33 min). PBO, placebo.
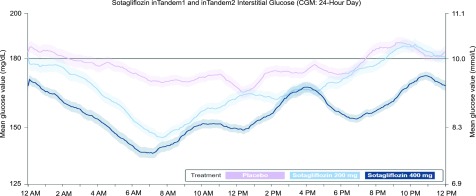
The AUC >10.0 mmol/L (>180 mg/dL) decreased significantly in both sotagliflozin groups by 0.5 ± 0.2 mmol/L (8.4 ± 4.2 mg/dL) × minutes/1,000 (95% CI −0.9 to −0.01; P = 0.045) with 200 mg and 1.0 ± 0.2 mmol/L (17.7 ± 4.1 mg/dL) × minutes/1,000 (95% CI −1.4 to −0.5; P < 0.001) with 400 mg relative to placebo (Table 2), a finding supported by CGM tracings of mean 24-h glucose excursions (Fig. 2). Severe hyperglycemia as measured by AUC >13.9 mmol/L (>250 mg/dL) decreased by 0.4 ± 0.1 mmol/L (6.9 ± 2.1 mg/dL) × minutes/1,000 (95% CI −0.6 to −0.2; P = 0.001) with sotagliflozin 400 mg but was not significantly different with sotagliflozin 200 mg (Table 2). Differences from placebo for hypoglycemic AUC values were not statistically significant (Table 2): <3.9 mmol/L (<70 mg/dL), −0.006 ± 0.01 mmol/L (−0.1 ± 0.2 mg/dL) for both sotagliflozin 200 and 400 mg; <3.0 mmol/L (<55 mg/dL), −0.0006 ± 0.006 mmol/L (−0.01 ± 0.1 mg/dL) for sotagliflozin 200 mg and −0.002 ± 0.006 mmol/L (−0.03 ± 0.1 mg/dL) for sotagliflozin 400 mg. The decline in mean glucose value from 12:00 to 6:00 a.m. in patients treated with sotagliflozin shown in Fig. 2 was not associated with a significant increase in nocturnal hypoglycemia.
Figure 2.
Average day represented by a 24-h CGM tracing consisting of interstitial glucose readings collected every 5 min from the week prior to the week 24 visit. The figure shows data collected from midnight (0000 h). The actual start time for 24-h readings may vary for each patient. Solid lines represent mean values from each treatment group (light purple = placebo [n = 93]; light blue = sotagliflozin 200 mg [n = 89]; dark blue = sotagliflozin 400 mg [n = 96]); shaded areas represent ± 1 SEM. Top of the target CGM range = 10.0 mmol/L (180 mg/dL). The decline in mean glucose value from 12:00 to 6:00 a.m. in patients treated with sotagliflozin was not associated with a significant increase in nocturnal hypoglycemia. Ambulatory glucose profiles (AGPs) for CGM data collected from each treatment group in the week prior to week 24 appear in the Supplementary Data. Patient and aggregate visualizations of CGM data, as well as AGPs, were generated by Cenduit, LLC (Durham, NC) and any reproductions must acknowledge Cenduit.
After a standardized mixed meal (Supplementary Data), the 2-h plasma PPG concentration decreased by 1.9 ± 0.7 mmol/L (35 ± 13 mg/dL) (95% CI −3.4 to −0.5; P = 0.009) with sotagliflozin 200 mg and by 2.8 ± 0.7 mmol/L (50 ± 13 mg/dL) (95% CI −4.2 to −1.3; P < 0.001) with sotagliflozin 400 mg relative to placebo.
The placebo-adjusted mean daily glucose concentration decreased by 0.4 ± 0.3 mmol/L (7.9 ± 4.7 mg/dL) (95% CI −1.0 to 0.1; P = 0.09) with sotagliflozin 200 mg and by 1.1 ± 0.3 mmol/L (18.9 ± 4.6 mg/dL) (95% CI −1.6 to −0.6; P < 0.001) with the sotagliflozin 400 mg dose. The MAGE also was reduced by 0.7 ± 0.3 mmol/L (12.7 ± 5.5 mg/dL) (95% CI −1.3 to −0.1; P = 0.022) and 1.2 ± 0.3 mmol/L (22.1 ± 5.4 mg/dL) (95% CI −1.9 to −0.7; P < 0.001) with sotagliflozin 200 and 400 mg, respectively, relative to placebo. The SD for glucose decreased in both treatment groups by 0.3 ± 0.1 mmol/L (4.6 ± 2.3 mg/dL) (95% CI −0.5 to −0.01; P = 0.042) and 0.4 ± 0.1 mmol/L (6.8 ± 2.2 mg/dL) (95% CI −0.6 to −0.1; P = 0.002) with sotagliflozin 200 and 400 mg, respectively, relative to placebo. The CV did not differ between the sotagliflozin and placebo groups.
The Supplementary Data include week 24 CGM data for individual but representative patients from each treatment group whose baseline HbA1c values were close to 6.5%, 7.0%, and 8.0%. At the baseline HbA1c threshold of 6.5% (Supplementary Fig. 2), sotagliflozin 200 and 400 mg were associated with dose-dependent decreases in the percentage of time at >10.0 mmol/L (180 mg/dL). At the higher HbA1c thresholds of 7.0% (Supplementary Fig. 3) and 8.0% (Supplementary Fig. 4), patients exhibited dose-dependent increases in the percentage of TIR, decreases in the percentage of time at >10.0 mmol/L, and decreases in the percentage of time at <3.9 mmol/L (70 mg/dL).
Compared with placebo, hypoglycemia by CGM (in number of events per patient per day) did not differ with sotagliflozin 200 or 400 mg at either hypoglycemic threshold (<3.0 mmol/L [55 mg/dL] and <3.9 mmol/L [<70 mg/dL]) (Table 2) (all P > 0.05). Similarly, no significant differences were observed in the percentage of time spent below hypoglycemic thresholds during nocturnal periods or for diurnal hypoglycemia (Table 2).
Conclusions
In pooled analyses of masked CGM data from two phase 3 trials involving adults with type 1 diabetes treated with optimized insulin therapy, dual inhibition of SGLT1 and SGLT2 with sotagliflozin significantly increased the percentage of the TIR 3.9–10.0 mmol/L (70–180 mg/dL), reduced the percentage of time spent at >10.0 and >13.9 mmol/L (180 and 250 mg/dL), and reduced PPG and glycemic variability. These findings were predicted as a result of the blunting and delay of glucose absorption due to SGLT1 inhibition, resulting in lower peak PPG (and less time spent above the goal glucose range) and more time spent in the goal glucose range, with a net result of less glycemic variability. These outcomes demonstrate that sotagliflozin-produced efficacy beyond HbA1c was achieved without an increase in percentage time below target range or increased hypoglycemia risk. With the exception of artificial pancreas studies, this has not been observed with insulin therapy alone (25).
High-dose (400 mg) sotagliflozin was associated with significant improvements in all CGM metrics recently recommended by an international CGM consensus group (3) except for CV, which was not statistically different between treatment groups (Table 2). Changes with high-dose sotagliflozin were consistently larger than those observed with low-dose sotagliflozin (200 mg). These observations are consistent with dose-dependent decreases in weight and blood pressure reported from inTandem1 and inTandem2 (12,13). In the main studies, the higher dose of sotagliflozin was also associated with more DKA and mechanism of action–related adverse events including diarrhea (SGLT1 inhibition) and genital mycotic infection (SGLT2 inhibition) (12,13). The evaluation of week 24 versus baseline CGM data for individual patients at various baseline HbA1c thresholds further demonstrated dose-related increases in TIR and decreases in time spent with glucose at <3.9 or >10.0 mmol/L, suggesting that sotagliflozin may provide a higher quality of HbA1c at the HbA1c thresholds studied, in a dose-dependent manner (26).
An international CGM consensus group identified <3.0 and >13.9 mmol/L (<54 and >250 mg/dL) as action thresholds for patients to avoid serious health consequences from hypoglycemia or hyperglycemia (the hyperglycemia action threshold was established in patients not treated with SGLT inhibitors) (3). The time spent below the <3.0 mmol/L threshold was not increased, and the time spent at >13.9 mmol/L significantly decreased by nearly an hour per day with sotagliflozin 200 mg and nearly 2 h with the 400 mg dose. Furthermore, a CGM AUC >13.9 mmol/L decreased significantly with high-dose sotagliflozin treatment. Time spent at >10.0 mmol/L (>180 mg/dL) decreased significantly with both dose levels by more than an hour with the lower dose and by nearly 3 h with the higher dose.
HbA1c is typically measured at 3-month intervals, and SMBG profiles are often insufficient to allow treatment intensification without an increased risk for hypoglycemia. The present analyses of CGM data during combination therapy with sotagliflozin and insulin show how sotagliflozin has the potential to simultaneously improve glycemia and reduce the risk for hypoglycemia, thereby increasing the TIR. CGM profiles of patients treated with sotagliflozin and placebo with similar baseline HbA1c values show a distinct improvement in the amplitude of glycemic excursions. Erratic swings of glucose levels out of the target range have been associated with patient-related outcomes such as perceived poor health and functioning or increased anxiety or absenteeism (27–29). In a study comparing basal-bolus to premixed insulin therapy, improved glycemic variability was associated with improved patient-reported outcomes (30). Likewise, the improvements in glycemic variability shown in this study may be related to significant improvements in patient-reported outcomes reported in the pivotal studies of sotagliflozin (12,13).
Improvements in TIR have been reported with other SGLT2 inhibitors (14,31). These studies were performed in patients with higher baseline mean HbA1c and glucose values than in the current study; therefore, improvements in TIR may have been amplified. In a small CGM substudy involving 89 patients with type 1 diabetes, 18 months of canagliflozin treatment increased the time spent with glucose values between 3.9 and 10.0 mmol/L (70 and 180 mg/dL), decreased the time spent above and below this range, and modestly improved glycemic variability indices (31). After 24 weeks in the DEPICT-1 Trial, dapagliflozin significantly reduced CGM mean glucose and MAGE and increased the percentage of time spent within the target range of 3.9–10.0 mmol/L (14). The effect of canagliflozin and dapagliflozin on PPG in the type 1 diabetes population has not been reported, although this was a prespecified end point of the studies (14,31).
Across the inTandem program, sotagliflozin treatment was associated with significant decreases in bolus insulin doses of 7–12% at 24 weeks (11–13). Nevertheless, sotagliflozin significantly reduced PPG by up to 2.8 mmol/L (50 mg/dL). A comparison of canagliflozin and dapagliflozin suggested that SGLT1 inhibition in the proximal intestine may confer greater PPG lowering (32). This finding is consistent with preclinical and clinical evidence showing that SGLT1 inhibition delays and reduces postprandial hyperglycemia and also increases the release of glucagon-like peptide 1 and polypeptide tyrosine (18,19,33).
Measures to reduce postprandial hyperglycemia usually increase the risk for hypoglycemia (34,35). Sotagliflozin-associated PPG reductions occurred without an increase in hypoglycemia as defined by CGM hypoglycemic events per day, percentage of time per day, or CGM AUC below the threshold of 3.0 mmol/L (<55 mg/dL) or 3.9 mmol/L (<70 mg/dL). A decline in mean glucose value from 12:00 to 6:00 a.m. was observed in this substudy but was not associated with an increased risk of nocturnal hypoglycemia. A decrease in mean glucose level between 12:00 and 6:00 a.m., but no increase in nocturnal hypoglycemia at week 24, was also observed with dapagliflozin (36). In the full inTandem1 and inTandem2 study populations, the incidence of documented hypoglycemia (by SMBG) and severe hypoglycemia was numerically lower with sotagliflozin 400 mg than with 200 mg (12,13).
A key limitation of this study was the masking of CGM data from investigators and patients, which may have resulted in an underrepresentation of the efficacy of sotagliflozin in patients with type 1 diabetes who use CGM. Also, the study required only limited use of masked CGM at 1-week intervals. The substudy population also predominantly comprised non-Hispanic whites, and the applicability of these results to other ethnic and racial groups is unclear.
In summary, when used in combination with optimized insulin in patients with type 1 diabetes, sotagliflozin significantly improved multiple measures of glycemic control beyond HbA1c. Compared with placebo, sotagliflozin-treated patients spent 1.3–2.8 h more time per day within the range of 3.9–10.0 mmol/L (70–180 mg/dL), with corresponding decreases in time spent at glucose levels of >10.0 mmol/L (>180 mg/dL). Decreases in PPG were accompanied by lower mean daily glucose and reductions in SD and the amplitude of glycemic excursions, while there was no increase in hypoglycemia. These data support the use of sotagliflozin in combination with insulin for the treatment of type 1 diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors thank the inTandem1 and inTandem2 trial investigators, staff, and patients for their participation. The authors also thank the following employees of Lexicon Pharmaceuticals, Inc., for reviewing the manuscript: Diane Gesty-Palmer, David Powell, Lisa Sherman, and Kristi Boehm. In addition, the authors thank Amanda Justice, who provided medical writing and editorial support, which was funded by Lexicon Pharmaceuticals, Inc. Finally, the authors thank Covance Inc. (Princeton, NJ) for providing the operational execution and medical monitoring of this study and Cenduit, LLC (Durham, NC), for visualization of CGM data.
Funding and Duality of Interest. This study was supported and conducted by Lexicon Pharmaceuticals, Inc. Lexicon Pharmaceuticals, Inc., and Sanofi entered into a license agreement effective in November 2015 and are collaborating on the development and commercialization of sotagliflozin. J.B.B. is supported by a grant from the National Institutes of Health (UL1-TR-002489). T.D. has acted as consultant, advisory board member, and steering committee member or speaker for Abbott, Medtronic, Roche, Lexicon Pharmaceuticals, Inc., Menarini, Boehringer Ingelheim, AstraZeneca, Novo Nordisk, Sanofi, Dexcom, and Eli Lilly and has received research grants from Abbott, AstraZeneca, Novo Nordisk, Medtronic, and Sanofi. B.C. has received research funding from Amgen, Pfizer, Sanofi, and Regeneron Pharmaceuticals, Inc. and has served on scientific advisory boards and received honoraria or consulting fees from Abbott, Akcea, Amgen, AstraZeneca, Genfit, Pierre Fabre, Eli Lilly and Company, Merck Sharp & Dohme, Novo Nordisk, Regeneron, Sanofi, and Servier. J.B.B. has received contracted consulting fees, paid to his institution, and travel support from Adocia, AstraZeneca, Dexcom, Elcelyx Therapeutics, Eli Lilly, Intarcia Therapeutics, Lexicon Pharmaceuticals, Inc., Metavention, NovaTarg, Novo Nordisk, Sanofi, Senseonics, and vTv Therapeutics; grant support from AstraZeneca, Boehringer Ingelheim, Johnson & Johnson, Lexicon Pharmaceuticals, Inc., Novo Nordisk, Sanofi, Theracos, and vTv Therapeutics; holds stock options in Mellitus Health, PhaseBio, and Stability Health; is a consultant to Neurimmune AG; and has served on the board of the AstraZeneca HealthCare Foundation. S.K.G. reports receiving grant support and travel support from Sanofi, Lexicon Pharmaceuticals, Inc., Novo Nordisk, MannKind, Roche Diagnostics, Zealand, Senseonics Inc., and Medtronic; research grants and advisory board fees from AstraZeneca; and grant support paid to his institution from Eli Lilly, Dexcom, and Johnson & Johnson. J.R. has served on scientific advisory boards and received honoraria or consulting fees from Eli Lilly, Sanofi, Novo Nordisk, Janssen, AstraZeneca, Boehringer Ingelheim, and Intarcia and has also received grants/research support from Merck, Pfizer, Sanofi, Novo Nordisk, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Genentech, Janssen, Lexicon Pharmaceuticals, Inc., Boehringer Ingelheim, and Intarcia. P.B. is employed by Lexicon Pharmaceuticals, Inc. J.A.K. serves as medical director of McNair Interests, a private equity group with investments in type 1 diabetes and other chronic illnesses, and is also an advisor for Sanofi and Lexicon. D.K.M. has received consulting fees and fees for serving on a clinical trial executive committee from Applied Therapeutics, Boehringer Ingelheim, Sanofi US, Novo Nordisk, and AstraZeneca; consulting fees from Lilly USA and Metavant Sciences, Ltd.; advisory board fees and fees for serving on a clinical trial executive committee from Merck Sharp & Dohme; fees for serving on a data monitoring committee from Janssen Research and Development and GlaxoSmithKline; fees for chairing the steering committees for Lexicon Pharmaceuticals, Inc.; and fees for serving on a clinical trial executive or steering committee from Eisai and Esperion. A.L.P. has participated on advisory boards for Abbott Diabetes Care, Becton Dickinson, Bigfoot, Eli Lilly and Company, Livongo, MannKind, Medscape, Merck, Novo Nordisk, Omada Health, Sanofi, and Zafgen; is chair of the type 1 diabetes steering committee at Lexicon Pharmaceuticals, Inc.; has participated in a speaker’s bureau for Novo Nordisk; and has received research funding from AstraZeneca, Dexcom, and MannKind. S.S. is employed by and holds stock in Lexicon Pharmaceuticals, Inc. P.S. owns stock in and was employed by Lexicon Pharmaceuticals, Inc. at the time the study was conducted and the manuscript was written and is now employed by Metavant Sciences, Ltd. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. T.D., J.A.K., D.K.M., and A.L.P. conceived and conducted the study, including acquisition, analysis, and interpretation of the data; participated in the drafting and critical revision of the manuscript; had full access to the data in the study; and had final responsibility for the decision to publish. B.C., J.B.B., S.K.G., and J.R. conducted the study, including acquisition, analysis, and interpretation of data; participated in the drafting and critical revision of the manuscript; had full access to the data in the study; and had final responsibility for the decision to publish. P.B. participated in the drafting and critical revision of the manuscript; conceived the study, including analysis and interpretation of data; contributed to the statistical design, analysis, and interpretation of data; oversaw the statistical analyses conducted by the independent statistician; reviewed the data quality prior to database lock; was involved in approving the protocol and its amendments; had full access to the data in the study; and had final responsibility for the decision to publish. S.S. conceived and conducted the study, including acquisition, analysis, and interpretation of the data; participated in the drafting and critical revision of the manuscript; reviewed the data quality prior to database lock; and had full access to the data in the study and final responsibility for the decision to publish. P.S. participated in the drafting and critical revision of the manuscript; conceived and conducted the study, including acquisition, analysis, and interpretation of data; reviewed the data quality prior to database lock; was involved in approving the protocol and its amendments; had full access to the data in the study; and had final responsibility for the decision to publish. T.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study (the inTandem1 and inTandem2 pooled CGM results) were presented in abstract form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Footnotes
Clinical trial reg. nos. NCT02384941 and NCT02421510, clinicaltrials.gov
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-2149/-/DC1.
J.A.K. is currently affiliated with McNair Interests and McNair Medical Institute, Houston, TX.
P.S. is currently affiliated with Metavant Sciences, Ltd., Durham, NC.
References
- 1.Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.Agiostratidou G, Anhalt H, Ball D, et al. Standardizing clinically meaningful outcome measures beyond HbA1c for type 1 diabetes: a consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care 2017;40:1622–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bode BW, Garg SK. The emerging role of adjunctive noninsulin antihyperglycemic therapy in the management of type 1 diabetes. Endocr Pract 2016;22:220–230 [DOI] [PubMed] [Google Scholar]
- 5.Lyons SK, Hermann JM, Miller KM, et al. Use of adjuvant pharmacotherapy in type 1 diabetes: international comparison of 49,996 individuals in the Prospective Diabetes Follow-up and T1D Exchange Registries. Diabetes Care 2017;40:e139–e140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ratner RE, Dickey R, Fineman M, et al. Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in type 1 diabetes mellitus: a 1-year, randomized controlled trial. Diabet Med 2004;21:1204–1212 [DOI] [PubMed] [Google Scholar]
- 7.Garg SK, Moser EG, Bode BW, et al. Effect of sitagliptin on post-prandial glucagon and GLP-1 levels in patients with type 1 diabetes: investigator-initiated, double-blind, randomized, placebo-controlled trial. Endocr Pract 2013;19:19–28 [DOI] [PubMed] [Google Scholar]
- 8.Ellis SL, Moser EG, Snell-Bergeon JK, Rodionova AS, Hazenfield RM, Garg SK. Effect of sitagliptin on glucose control in adult patients with type 1 diabetes: a pilot, double-blind, randomized, crossover trial. Diabet Med 2011;28:1176–1181 [DOI] [PubMed] [Google Scholar]
- 9.Petrie JR, Chaturvedi N, Ford I, et al.; REMOVAL Study Group . Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2017;5:597–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathieu C, Zinman B, Hemmingsson JU, et al.; ADJUNCT ONE Investigators . Efficacy and safety of liraglutide added to insulin treatment in type 1 diabetes: the ADJUNCT ONE Treat-To-Target Randomized Trial. Diabetes Care 2016;39:1702–1710 [DOI] [PubMed] [Google Scholar]
- 11.Garg SK, Henry RR, Banks P, et al. Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med 2017;377:2337–2348 [DOI] [PubMed] [Google Scholar]
- 12.Buse JB, Garg SK, Rosenstock J, et al. Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the North American inTandem1 study. Diabetes Care 2018;41:1970–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danne T, Cariou B, Banks P, et al. HbA1c and hypoglycemia reductions at 24 and 52 weeks with sotagliflozin in combination with insulin in adults with type 1 diabetes: the European inTandem2 study. Diabetes Care 2018;41:1981–1990 [DOI] [PubMed] [Google Scholar]
- 14.Dandona P, Mathieu C, Phillip M, et al.; DEPICT-1 Investigators . Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2017;5:864–876 [DOI] [PubMed] [Google Scholar]
- 15.Henry RR, Thakkar P, Tong C, Polidori D, Alba M. Efficacy and safety of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care 2015;38:2258–2265 [DOI] [PubMed] [Google Scholar]
- 16.Mathieu C, Dandona P, Gillard P, et al.; DEPICT-2 Investigators . Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT-2 Study): 24-week results from a randomized controlled trial. Diabetes Care 2018;41:1938–1946 [DOI] [PubMed] [Google Scholar]
- 17.Lapuerta P, Zambrowicz B, Strumph P, Sands A. Development of sotagliflozin, a dual sodium-dependent glucose transporter 1/2 inhibitor. Diab Vasc Dis Res 2015;12:101–110 [DOI] [PubMed] [Google Scholar]
- 18.Dobbins RL, Greenway FL, Chen L, et al. Selective sodium-dependent glucose transporter 1 inhibitors block glucose absorption and impair glucose-dependent insulinotropic peptide release. Am J Physiol Gastrointest Liver Physiol 2015;308:G946–G954 [DOI] [PubMed] [Google Scholar]
- 19.Zambrowicz B, Ogbaa I, Frazier K, et al. Effects of LX4211, a dual sodium-dependent glucose cotransporters 1 and 2 inhibitor, on postprandial glucose, insulin, glucagon-like peptide 1, and peptide tyrosine tyrosine in a dose-timing study in healthy subjects. Clin Ther 2013;35:1162–1173.e8 [DOI] [PubMed] [Google Scholar]
- 20.Lane JD, Barkauskas CE, Surwit RS, Feinglos MN. Caffeine impairs glucose metabolism in type 2 diabetes. Diabetes Care 2004;27:2047–2048 [DOI] [PubMed] [Google Scholar]
- 21.Greenbaum CJ, Mandrup-Poulsen T, McGee PF, et al.; Type 1 Diabetes Trial Net Research Group; European C-Peptide Trial Study Group . Mixed-meal tolerance test versus glucagon stimulation test for the assessment of beta-cell function in therapeutic trials in type 1 diabetes. Diabetes Care 2008;31:1966–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovatchev B, Cobelli C. Glucose variability: timing, risk analysis, and relationship to hypoglycemia in diabetes. Diabetes Care 2016;39:502–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovatchev BP. Metrics for glycaemic control - from HbA1c to continuous glucose monitoring. Nat Rev Endocrinol 2017;13:425–436 [DOI] [PubMed] [Google Scholar]
- 24.Fonda SJ, Lewis DG, Vigersky RA. Minding the gaps in continuous glucose monitoring: a method to repair gaps to achieve more accurate glucometrics. J Diabetes Sci Technol 2013;7:88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol 2017;5:501–512 [DOI] [PubMed] [Google Scholar]
- 26.Close KL, Wood R. Patient priorities for diabetes drugs: what does success look like? A download on 3,000+ patient views collected August 2016. Presented at FDA Workshop: Outcomes Measures for New Diabetes Therapies Beyond A1C, Silver Spring, MD, 2016. Available from https://diatribe.org/patient-priorities-diabetes-drugs-what-does-success-look. Accessed 3 January 2019.
- 27.Grigsby AB, Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. Prevalence of anxiety in adults with diabetes: a systematic review. J Psychosom Res 2002;53:1053–1060 [DOI] [PubMed] [Google Scholar]
- 28.Tunceli K, Bradley CJ, Lafata JE, et al. Glycemic control and absenteeism among individuals with diabetes. Diabetes Care 2007;30:1283–1285 [DOI] [PubMed] [Google Scholar]
- 29.Hart HE, Redekop WK, Bilo HJ, Berg M, Jong BM. Change in perceived health and functioning over time in patients with type I diabetes mellitus. Qual Life Res 2005;14:1–10 [DOI] [PubMed] [Google Scholar]
- 30.Testa MA, Gill J, Su M, Turner RR, Blonde L, Simonson DC. Comparative effectiveness of basal-bolus versus premix analog insulin on glycemic variability and patient-centered outcomes during insulin intensification in type 1 and type 2 diabetes: a randomized, controlled, crossover trial. J Clin Endocrinol Metab 2012;97:3504–3514 [DOI] [PubMed] [Google Scholar]
- 31.Rodbard HW, Peters AL, Slee A, Cao A, Traina SB, Alba M. The effect of canagliflozin, a sodium glucose cotransporter 2 inhibitor, on glycemic end points assessed by continuous glucose monitoring and patient-reported outcomes among people with type 1 diabetes. Diabetes Care 2017;40:171–180 [DOI] [PubMed] [Google Scholar]
- 32.Sha S, Polidori D, Farrell K, et al. Pharmacodynamic differences between canagliflozin and dapagliflozin: results of a randomized, double-blind, crossover study. Diabetes Obes Metab 2015;17:188–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polidori D, Sha S, Mudaliar S, et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study. Diabetes Care 2013;36:2154–2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cryer PE. Glycemic goals in diabetes: trade-off between glycemic control and iatrogenic hypoglycemia. Diabetes 2014;63:2188–2195 [DOI] [PubMed] [Google Scholar]
- 35.The Diabetes Control and Complications Trial Research Group Hypoglycemia in the diabetes control and complications trial. Diabetes 1997;46:271–286 [PubMed] [Google Scholar]
- 36.Mathieu C, Dandona P, Phillip M, et al. Glucose variables in T1D studies with dapagliflozin: pooled analysis of continuous glucose monitoring data from DEPICT-1 and 2. Late-breaking poster presented at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018. DOI: 10.2337/db18-125-LB. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.