Abstract
Importance:
Although the overall rate of spontaneous resolution in congenital nasolacrimal duct obstruction (CNLDO) and efficacy of probing have been documented in the literature, the optimal timing of intervention has not been established.
Objective:
To report new findings regarding spontaneous resolution in a large cohort of children with CNLDO
Design, Setting, and Participants:
The medical records of 1998 consecutive infants diagnosed with CNLDO from January 1, 1995, through December 31, 2004, while residing in Olmsted County, Minnesota were retrospectively reviewed. Data were analyzed between January 1, 2015, and January 2017.
Main Outcome(s) and Measure(s):
Rate of spontaneous resolution over time and by sex.
Results:
The cohort, diagnosed at a median age of 1.2 months (interquartile range, 0.4–3.6), was 48% girls and 89% white. Among the 1998 cases, 1669 (83.5%) spontaneously resolved, 289 (14.5%) underwent treatment, and the remaining 40 (2.0%) were lost to follow-up. Of the 1958 followed infants, 47.3% spontaneously resolved by 3 months of age, 66.4% by 6, 75.7% by 9, and 78.4% by 12 months. The rate of resolution was 35% faster (95% CI=23%−47%; p<0.001) at <1 month vs 3 months, 43% faster (95% CI=27%−43%; p<0.001) at 3 months vs. 6 months, 39% faster (95% CI=16%−64%; p<0.001) at 6 months vs. 9 months, and insignificantly different at 9 vs. 12 months (HR=0.99; 95% CI=0.80–1.22; p=0.78). Males resolved 24% faster than females (95% CI=13%−37%; p<0.001), and unilateral obstructions resolved 16% faster than bilateral (95% CI=5%−29%; p=0.005) ones. Children probed at 15months or older had decreased odds of resolution after probing (OR=0.11; 95% CI=0.01–0.89; p=0.04) compared to children probed at 12–14 months of age.
Conclusions & Relevance:
Based on this large cohort of children with CNLDO, probing between 9 and 15 months of age may be reasonable given that the rate of spontaneous resolution plateaued after 9 months and initial probing success declined after 15 months. This time frame supports both an earlier and narrower range of ages for intervention compared to the current practice of probing after one year of age.
Introduction
Congenital nasolacrimal duct obstruction (CNLDO) occurs in 1 in 9 newborns1 and is characterized by persistent tearing and intermittent mucopurulent discharge from one or both eyes. Standard early management includes hydrostatic nasolacrimal massage and topical antibiotics. While the obstruction will spontaneously resolve in most infants, it does not in up to 25% of affected children.2–8 Mechanical probing of the nasolacrimal duct has been accepted as a first-line treatment for persistent CNLDO; however, a consensus on the optimal timing for this intervention has not been established. To capitalize on the condition’s high frequency of spontaneous resolution,3–7 some authors propose waiting until the child is 12 or 13 months of age to probe. Others contend that delaying probing can increase the risk of inflammation and fibrosis which may decrease the success rates of subsequent probings.8–10 The purpose of this study is to describe the natural course of spontaneous resolution in a cohort of 1998 infants diagnosed with congenital nasolacrimal duct obstruction and to suggest a reasonable time frame for surgical intervention.
Methods
The medical records of 1998 consecutive patients younger than five years of age diagnosed with congenital nasolacrimal duct obstruction, while residing in Olmsted County, Minnesota, from January 1, 1995 through December 31, 2004, were retrospectively reviewed. The inclusion criteria and demographic data of the 1998 patients have been previously reported.1 Institutional Review Board (IRB) approval was obtained from Mayo Clinic and Olmsted Medical Group. The population of Olmsted County is relatively isolated from other urban areas and virtually all medical care is provided to its residents by Mayo Clinic, Olmsted Medical Group, and their affiliated hospitals. All patient-physician encounters in the county, including summary information on demographics, clinical examinations, diagnoses, and surgical interventions, are collected through the Rochester Epidemiology Project (REP), a computerized medical record linkage system utilized in this study.11
Data regarding sex, laterality, natural history and management were recorded for each patient. The date upon which the symptoms of dacryostenosis resolved was established by parental history. If the date was not documented, resolution was calculated to occur between the last documentation of CNLDO and the subsequent infant evaluation negative for the condition. For example, if a child, when examined at 2 months of age, was found to have dacryostenosis that was absent at the examination at 4 months of age, the calculated date of resolution was the midpoint between the 2 exams, or at 3 months of age. Most infants had well-child examinations at 2 days, 2 weeks, 1 month, 2 months, 4 months, 6 months, 9 months, and 12 months of age. Any additional sick child visits provided increased surveillance over relatively short intervals of time. Multiple examinations over the years, in both primary and subspecialty care, were reviewed to confirm full resolution of symptoms.
Categorical and continuous variables were descriptively summarized using frequencies and percentages and medians and ranges, respectively. The rates of spontaneous resolution, beginning at 12 months of age vs. 9 months, were compared by first left-truncating follow-up for all patients still being followed at 9 and 12 months of age (i.e. beginning follow-up at 9 and 12 months, respectively). A marginal Cox regression model clustered on each patient was then used to estimate the relative risk of spontaneous resolution beginning at 12 months compared to 9 months.12 Similar methods were used to compare 3 months and 0 months, 6 months and 3 months, and 9 months and 6 months. Kaplan-Meier curves were used to summarize overall unresolved over time for the entire cohort, and to compare time to resolution by sex and laterality. Distribution of variables across the need for surgical treatment were compared using chi-square/Fisher exact tests (where appropriate) for categorical variables and 2-sample t-tests for continuous variables. Associations between successful probing intervention and probing age were examined using the following multivariable logistic regression. A successful probing was defined as the absence of epiphora postoperatively, whether the condition was unilateral or bilateral preoperatively. Other variables included in the model were diagnosis age (categorized as <1 month, 1–2 months, 3–5 months, and 6+ months), sex, and presence of bilateral CNLDO. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). All tests were 2-sided.
Results
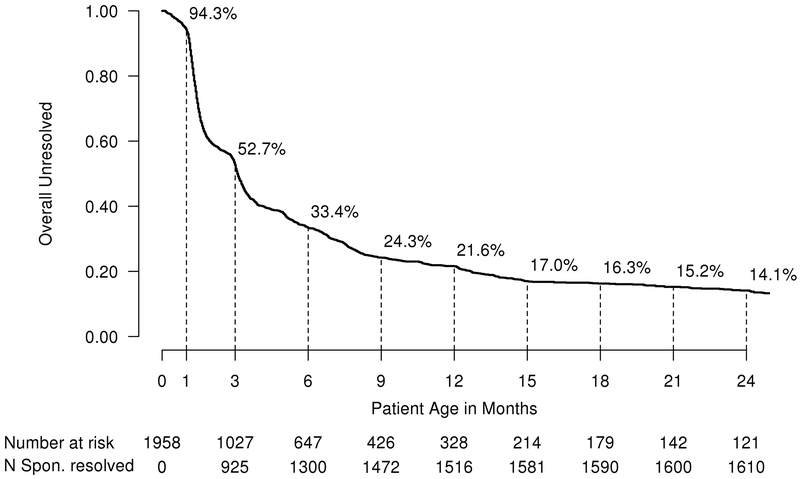
Among the 1998 infants diagnosed over the 10-year period, 1669 (83.5%) spontaneously resolved, 289 (14.5%) required surgical management, and 40 (2.0%) were lost to follow-up. The principal cohort of this study is the 1958 followed infants, which includes the 1669 that spontaneously resolved and the 289 that required surgical intervention. The 1669 who spontaneously resolved were diagnosed at a median age of 1.0 month compared to 5.0 months for the 289 who required treatment (p<0.001). Figure 1 illustrates graphically the number and percent of the 1958 observed infants who had not yet spontaneously resolved by age in months. The median age at resolution was 2.4 months (interquartile range, 1.3 to 5.3; range, 0 to 87) for the 1669 who spontaneously resolved, while the 289 surgically managed patients underwent their first procedure at median age of 14.0 months (interquartile range, 9.5 to 21.1; range 1 to 248).
Figure 1.
Overall Percent Without Spontaneous Resolution Over Time Using Kaplan-Meier Methods in 1958 infants.
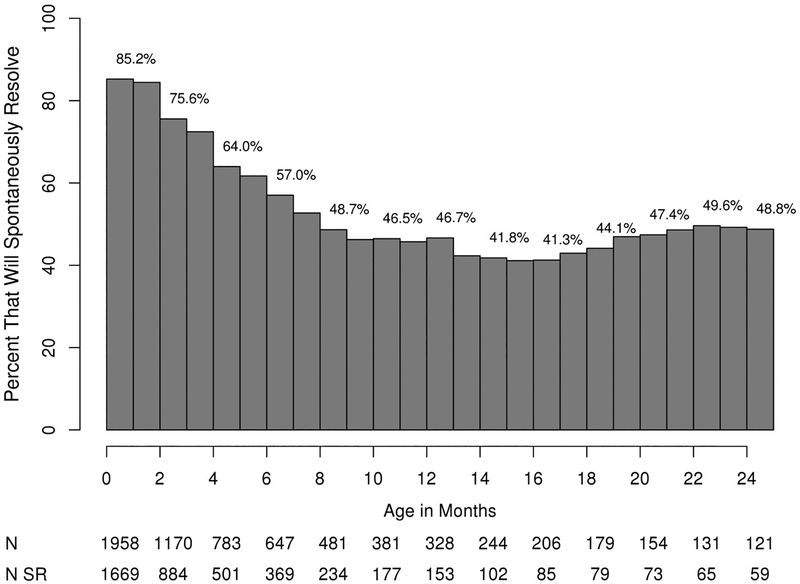
The rate of spontaneous resolution was highest in the first few months of life, declining until 9 months of age when the rate flattened thereafter as shown in Figure 2. The rate of resolution was 35% faster (95% CI=23%−47%; p <0.001) at <1month vs. 3 months, 43% faster (95% CI=27%−43%; p<0.001) at 3 months vs. 6 months, 39% faster (95% CI=16%−64%; p<0.001) at 6 months vs. 9 months, and insignificantly different at 9 vs. 12 months (HR=0.99; 95% CI=0.80–1.22; p=0.78). Males resolved faster than females [males: median=2.9 months, 95% CI=2.4–3.0; females: median=3.4 months, 95% CI=3.2–3.7; p<0.001] (see eFigure1 in the Supplement), and unilateral obstructions resolved faster than bilateral [unilateral: median=3.1, 95% CI=3.0–3.2; bilateral: median=3.3, 95% CI=3.1–3.6; p=0.002] ones (see eFigure2 in the Supplement).
Figure 2.
Percent Of Children That Eventually Spontaneously Resolved At Any Time After Specified Age. N=Total Number; N SR=Number that spontaneously resolved
Two hundred seventy-two (94.1%) of the 289 surgically managed patients eventually underwent a probing, of which 242 (89%) resolved without additional treatment. After adjusting for the effects of age at diagnosis, sex, and laterality, children probed at 15 months of age or older had decreased odds of resolution after probing (OR=0.11; 95% CI=0.01–0.89; p=0.04) relative to children probed at 12–14 months (Table). When patients aged <9 months and 9–11 months were compared to patients probed aged 12–14 months, there was no difference in their success rates (P=0.09 and P=0.45, respectively, Table).
Table.
Associations Between Probing Age and Congenital Nasolacrimal Duct Obstruction Resolution Using Multivariable Logistic Regression
| Characteristic | Total N=272 | CNLDO Resolution Without Additional Treatment N=242 | Odds Ratio (95% CI) | p-value |
|---|---|---|---|---|
| Probing Age | ||||
| Probing <9 months age | 76 | 68 (89.5%) | 0.16 (0.02, 1.35) | 0.09 |
| Probing 9–11 months age | 55 | 52 (94.5%) | 0.41 (0.04, 4.10) | 0.45 |
| Probing 12–14 months age | 46 | 45 (97.8%) | 1.00 (ref) | – |
| Probing 15+ months age | 95 | 77 (81.1%) | 0.11 (0.01, 0.89) * | 0.04 |
| Age at Diagnosis | ||||
| <1 month | 51 | 47 (92.2%) | 1.00 (ref) | – |
| 1–2 months | 43 | 41 (95.3%) | 1.91 (0.33, 11.24) | 0.47 |
| 3–5 months | 42 | 38 (90.5%) | 0.85 (0.19, 3.73) | 0.83 |
| 6+ | 136 | 116 (85.3%) | 0.59 (0.18, 1.96) | 0.39 |
| Sex | ||||
| Female | 151 | 132 (87.4%) | 1.00 (ref) | – |
| Male | 121 | 110 (90.9%) | 1.38 (0.61, 3.11) | 0.43 |
| Bilateral | ||||
| No | 181 | 164 (90.6%) | 1.00 (ref) | – |
| Yes | 91 | 78 (85.7%) | 0.80 (0.36, 1.81) | 0.60 |
An Odds Ratio significantly <1.0 can be interpreted as a decreased odds of successful CNLDO resolution without further intervention relative to the referent group.
Discussion
In this population-based cohort of 1958 followed infants, complete resolution of congenital nasolacrimal duct obstruction with non-surgical management was achieved in 78.4% by the first year of life. The rate of resolution was highest in the first months of life, decreasing until 9 months of age, after which the rate changed minimally. The obstruction resolved faster in males than females and in unilateral disease compared to bilateral disease. Patients probed at 15 months of age or older had lower odds of resolution without additional treatment compared to those probed at 12–14 months of age, with no difference in the success rates between patients aged <9 months and 9–11 months of age.
Spontaneous resolution has been reported to occur in over 90% of infants managed conservatively by several clinical studies.2–4,7, 13 The majority of these studies, however, were of selected populations with less than 200 patients. Their small sample sizes and associated biases may have provided imprecise and unrepresentative estimates. While the current study corroborates the tendency of CNLDO to resolve without surgical treatment as suggested in the literature, it does so with a relatively lower one-year resolution rate. In this cohort diagnosed over a 10-year period, 78.4% of the observed infants spontaneously resolved by 12 months, and among the 1669 that never required surgical treatment, 90.8% had resolved by the first year of life.
The rate of resolution decreases with increasing age, as observed by other investigators,3,6 and confirmed in this cohort. Beginning at soon after birth, the spontaneous resolution rate declines by approximately 30% every 3 months until 9 months of age at which time the rate plateaus. Although the rate of resolution is no better at 9 months of age compared to 12 months, there are fewer patients at one year of age who have the potential to resolve. In this study, nearly nine of ten patients who spontaneously resolved did so by 9 months of age.
The condition resolved faster in males than in females which has not been demonstrated previously. Several reports have suggested that the bony nasolacrimal canal and fossa is significantly smaller in females,14,15 by an average of 0.35 millimeters, compared to males.15 It is possible that the slower resolution of CNLDO in girls is due, at least in part, to the smaller diameter of the nasolacrimal canal leading to more chronic and complex obstructions.16, 17 Resolution of CNLDO also occurred faster in unilateral disease than bilateral disease, consistent with prior reports.16, 18,19
Several studies have described an age-dependent decrease in success rates of initial probings from greater than 90% when done before 12 months of age to 50–70% of those done after.13,16,18, 20 One group noted a similar decline when comparing the success of probings before 6 months to those between 6 and 12 months of age.20 The Pediatric Eye Disease Investigator Group (PEDIG), however, reporting on a prospective cohort of 718 children, did not find a relationship between age at surgery and surgical success up to 36 months of age.19 The findings of this cohort confirm a negative correlation between increasing age after 12 months and a successful initial surgical intervention, which was 90.2%, 83.1%, 71.4%, 64.7% at ages 6–12, 12–18, 18–24, and greater than 24 months of age, respectively. The poorer results in older children have been attributed to the development of more complicated obstructions from chronic infections and scarring.21 Moreover, as the mean age of CNLDO diagnosis was 5.0 months in the group who received surgical treatment compared to 1.0 months in those who spontaneously resolved, the decreasing success of probings in older children is likely due to natural selection. Presumably, later presenting children may have more complicated obstructions that reduce successful outcomes.
The optimal timing of the first probing remains controversial. A number of studies advocate delaying surgical procedures until after the first year of life, citing CNLDO’s high rate of spontaneous resolution.3–7 Others, however, favor early probing, citing the decline in favorable outcomes with increasing age as well as the previous reports of morbidities associated with persistent CNLDO.8–10 Conflicting conclusions based upon underpowered studies, case series, and expert opinions promote the ongoing controversy. Given that the overall resolution rate changes minimally after 9 months of age, it may be reasonable to consider intervening earlier than the current practice of probing at 12 months of age or older. Probing beyond 12 months, moreover, as demonstrated by this study and others, appears to be less successful than when performed before one year of age. Based on the findings of this study, an initial probing between 9 months and 15 months of age appears most advantageous.
While probing under general anesthesia is recommended for better procedural control and completion, recent studies on the association of childhood anesthesia and the development of cognitive impairment have questioned whether some procedures, including probings under anesthesia, is worth the risk. These studies, however, suggest that multiple, not single, exposures to anesthetics and cumulative exposure greater than 120 minutes were associated with increased learning disabilities.22 Neurotoxicity risk stratified by age under 18 months is nonexistent in the literature; that is, there is no calculated difference in anesthesia risk for a 9-month old compared to a one year old. Findings from the landmark Pediatric Anesthesia Neurodevelopment Assessment (PANDA) study also demonstrated that one brief anesthetic, of a duration less than 80 minutes, was not associated with cognitive or behavioral abnormalities in exposed children when compared to their unexposed sibling.23 A probing typically involves less than 20 minutes of anesthetic exposure, and 80 to 90% of children require only one procedure.19 An argument for earlier probing is that by delaying procedures, older children could develop more complicated obstructions with higher probing failure rates, and ultimately require additional surgical procedures and increased exposure to general anesthesia.
There are a number of limitations to the findings in this study. Its retrospective design is limited by non-standardized and incomplete documentation. Accurately determining the age at resolution, for example, was problematic. Although most infants experienced 8 to 9 well-child exams by 18 months of age, both parental observation and the calculated date of resolution could have been calculated imprecisely if they were not. However, underestimations of the age at resolution would be expected to be balanced by overestimations with no systemic bias and no effect on the observations regarding sex, laterality, and the total number of resolutions. Although the REP system is uniquely designed to capture all of a patient’s medical visits in Olmsted County, some residents may have sought care outside of the county, leading to an overestimation of spontaneous resolution rate in this population. Finally, our ability to generalize these findings to other populations is limited by the demographics of Olmsted County, a relatively homogeneous semi-urban Caucasian population.
Given that the rate of spontaneous resolution appears to plateau after 9 months of age and a successful probing outcome declines beyond 15 months of age, surgical intervention between these time intervals appears to be a reasonable management strategy for infants with nasolacrimal duct obstruction. This time frame establishes both an earlier and narrower range of ages for intervention compared to the current general practice of probing after one year of age. Further prospective investigation is needed to definitively determine the most appropriate age for surgical intervention in children with CNLDO.
Supplementary Material
KEY POINTS:
Question: What are the trends for spontaneous resolution in congenital nasolacrimal duct obstruction, and when is an appropriate time to intervene?
Findings: In this large population-based study 0f 1998 infants with congenital nasolacrimal duct obstruction, the rate of spontaneous resolution plateaued after 9 months of age, and the success rate of the initial probing declined after 15 months of age.
Meaning: These findings suggest that surgical intervention may be appropriate during a new time frame, between 9 and 15 months, capitalizing on the condition’s changing rate of resolution as well as the declining success rate of the initial probing.
Acknowledgements:
Funding: Design and conduct of the study was made possible in part by an unrestricted grand from Research to Prevent Blindness, Inc. Data collection, management, and analysis was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest:
No conflicting relationship exists for any author.
REFERENCES
- 1.Sathiamoorthi S, Frank RD, Mohney BG. Incidence and Clinical Characteristics of Congenital Nasolacrimal Duct Obstruction. Under consideration for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson LB, Calhoun JH, Menduke H. Medical management of congenital nasolacrimal duct obstruction. Ophthalmology 1985;92(9):1187–1190. [DOI] [PubMed] [Google Scholar]
- 3.MacEwen CJ, Young JDH. Epiphora during the first year of life. Eye 1991;5:596–600. [DOI] [PubMed] [Google Scholar]
- 4.Nucci P, Capoferri C, Alfarano R, Brancato R. Conservative management of congenital nasolacrimal duct obstruction. J Pediatr Ophthalmol Strabismus 1989;26(1):39–43. [DOI] [PubMed] [Google Scholar]
- 5.Price HW. Dacryostenosis. J Pediatr 1947;30:302–5. [DOI] [PubMed] [Google Scholar]
- 6.Paul TO, Shepherd R. Congenital nasolacrimal duct obstruction: natural history and the timing of optimal intervention. J Pediatr Strabismus 1994;31:362–7. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RA, Robb RM. The natural course of congenital obstruction of the nasolacrimal duct. J Pediatr Ophthalmol Strabismus 1978;15:246–50. [DOI] [PubMed] [Google Scholar]
- 8.Robb RM. Success rate of nasolacrimal duct probing at time intervals after 1 year of age. Ophthalmology 1998;105:1308–10. [DOI] [PubMed] [Google Scholar]
- 9.Ffookes OO. Dacryocystitis in infancy. Br J Ophthalmol 1962;46: 422–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weil BA: Dacryocystitis, in Viers ER(ed): The Lacrimal System, Proceedings of the First International Symposium. St. Louis, CV Mosby Co, 1971, p 118. [Google Scholar]
- 11.Melton LJ. History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. [DOI] [PubMed] [Google Scholar]
- 12.Therneau T, Grambsch P. Modeling Survival Data: Extending the Cox Model. Springer-Verlag, 2000. [Google Scholar]
- 13.Katowitz JA, Welsh MG. Timing of initial probing and irrigation in congenital nasolacrimal duct obstruction. Ophthalmology 1987; 94:698–705. [DOI] [PubMed] [Google Scholar]
- 14.Groessl SA, Sires BS, Lemke BN. An anatomical basis for primary acquired nasolacrimal duct obstruction. Arch Ophthalmol. 1997;115(1):71–74. [DOI] [PubMed] [Google Scholar]
- 15.Janssen AG, Mansour K, Bos JJ, Castelijns JA. Diameter of the bony lacrimal canal: normal values and values related to nasolacrimal duct obstruction: assessment with CT. AJNR Am J Neuroradiol 2001;22(5):845–850. [PMC free article] [PubMed] [Google Scholar]
- 16.Mannor GE, Rose GE, Frimpong-Ansah K, Ezra E. Factors affecting the success of nasolacrimal duct probing for congenital nasolacrimal duct obstruction. Am J Ophthalmol 1999;127:616–617. [DOI] [PubMed] [Google Scholar]
- 17.Honavar S, Vasudha EP, Rao GN. Outcome of probing for congenital nasolacrimal duct obstruction in older Children. Am J Ophthalmol 2000;130:42–48. [DOI] [PubMed] [Google Scholar]
- 18.Kashkouli MB, Beigi B, Parvaresh MM, Kassaee A, Tabatabaee Z. Late and very late initial probing for congenital nasolacrimal duct obstruction: What is the cause of failure? Br J Ophthalmol 2003;87:1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pediatric Eye Disease Investigator Group. Primary treatment of nasolacrimal duct obstruction with probing in children younger than 4 years. Ophthalmology 2008;115:577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciftci F, Akman A, Sonmez M, et al. Systematic, combined treatment approach to nasolacrimal duct obstruction in different age groups. Eur J Ophthalmol 2000;10:324. [DOI] [PubMed] [Google Scholar]
- 21.Kushner BJ. The management of nasolacrimal duct obstruction in children between 18 months and 4 years old. AAPOS 1998; 2(1):57–60. [DOI] [PubMed] [Google Scholar]
- 22.Flick RP, Katusic SK, Colligan RC, et al. Cognitive and Behavioral Outcomes After Early Exposure to Anesthesia and Surgery. Pediatrics 2011;128(5):e1053–e1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun LS, Li G, Miller TLK, et al. Association Between a Single General Anesthesia Exposure Before Age 36 Months and Neurocognitive Outcomes in Later Childhood. JAMA 2016;315(21):2312–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.