Abstract
Several experimental and clinical studies have transformed the traditional antimalarial role of chloroquine (CHQ) and related structural analogues to potent therapeutic agents for a host of nonmalarial indications. The expanding clinical applicability for these drugs includes rheumatological and cardiovascular disorders (CVD), chronic kidney disease (CKD), oncology, and a variety of nonmalarial infections. These clinical advancements are primarily related to pleiotropic pharmacological actions of these drugs, including immunomodulation, anti-inflammatory properties, and capabilities of inducing autophagy and apoptosis at a cellular level. Historically, many clinical benefits in nonmalarial indications were first recognized through serendipitous observations; however, with numerous ongoing systematic clinical studies, the clinical horizons of these drugs have a promising future.
Keywords: antimalarials, cardiovascular disease, chloroquine, chronic kidney disease, hydroxychloroquine, rheumatology
Introduction
Chloroquine (CHQ) and closely related structural analogues were originally developed for the treatment of malaria. However, as the clinical use for these drugs began to increase, many additional drug properties were discovered mainly through serendipitous observations. These clinical observations, followed by some well-designed studies, have substantially broadened the horizons for CHQ and analogues over the last few decades. CHQ and analogues have been proven beneficial for many rheumatological, cardiovascular diseases (CVD), and dermatological conditions, and there is a growing body of evidence to support their therapeutic potential in oncology, HIV infection, and chronic kidney disease (CKD). The goal of this review is to enunciate the rationale and the expanding clinical role of these drugs in these areas considering significant promise in recent times.
Drug development
CHQ and close structural analogues were developed around World War II to combat malarial infection in the US Army soldiers who were deployed in the South Pacific part of the world.1,2 For many years prior, the drug quinine, which is derived from cinchona tree bark, held the status of the first natural and effective antimalarial compound. In 1891, Paul Ehrlich and colleagues found that a synthetic dye, methylene blue, was selectively taken up by the malarial parasites.2 A few years later, the methyl group was replaced with a basic side chain to synthesize pamaquine that retained its antimalarial property. Further modifications led to the formation of compounds quinacrine, sontoquine, primaquine, and resochin, where the basic side chain was attached to several different heterocyclic ring systems. Resochin and its further modification, CHQ, were used widely for the malarial prophylaxis during the Second World War.2 During these times several investigators made serendipitous observations of the beneficial effects of CHQ on various cutaneous disorders as well as arthritis. However, the toxicological properties of this compound were concerning and limited its wide therapeutic applicability in its early phase.3 A decade later, the addition of a β-hydroxy chain to the CHQ molecule led to the development of hydroxychloroquine (HCQ), which reduced the toxicity of CHQ to a third of the original molecule. Since then, HCQ has been increasingly adopted for most of the non-malarial and chronic indications. At the same time, CHQ continues to be utilized for the prophylaxis and management of Plasmodium falciparum and Plasmodium vivax malaria in many parts of the world, including China, Korea, Mexico, Paraguay, Turkey, and so on. However, increasing concern over drug resistance has increasingly limited their application in favor of newer agents.4,5
Pharmacokinetics and safety
CHQ and HCQ are water soluble, commonly administered orally in clinical practice, and have a near-complete absorption from the gastrointestinal tract with about 75% bioavailability.6 The plasma concentrations of these agents are affected by their strong affinity for many blood constituents, including thrombocytes, granulocytes, and plasma proteins, including albumin and α-acid glycoprotein.2,7 The peak plasma concentration of the parent drug molecule is reached in about 4–12 h after an individual dose and stable plasma levels are usually achieved after 4–6 weeks of regular daily dosing, though there are significant interindividual variations.7,8 In the cases of chronic use, additional metabolites such as desethylhydroxychloroquine, desethylchloroquine, and bidesethylhydroxychloroquine are noted to accumulate, which influence the plasma levels. Measuring the blood concentration of these drugs is not routinely performed for efficacy or safety, but can be pursued in select patients for assessing adherence, especially when standard dosing regimens do not produce the desired clinical results.9 The elimination half-life of these compounds is long, ranging between 40 and 50 days, due to an extensive tissue uptake and volume of distribution. At therapeutic doses, a major fraction of these analogues, as well as their metabolites, bind avidly to several tissues in the body, which slows down the overall excretion process.10 The final availability at the desired target effector molecule depends on the complex interplay of absorption, distribution, metabolism, and excretion.11 Renal elimination is the principal route of excretion, and being a weak base, its excretion can be further potentiated by the acidification of the urine. Only small quantities are excreted through the biliary (bile) and secretory (sweat and saliva) system. As HCQ has comparable clinical efficacy and a better safety profile than CHQ, in modern medicine, for most non-malarial indications, it is a more commonly used formulation.12 Thus, in this article, we discuss the role and use of HCQ and CHQ interchangeably.
Over the last six decades of clinical use, CHQ and HCQ compounds have shown excellent safety profile with good long-term tolerance in not only the general population but in certain special populations as well, including among pregnant individuals and those with renal failure. Clinically, gastrointestinal intolerance, retinopathy, cutaneous hyperpigmentation and other skin reactions, myopathy, and hematological complications are the most relevant adverse effects.9 Among these, the risk of retinopathy with a specific type of irreversible pigmentary change is the most noticeable adverse effect and a major limiting factor for chronic use of HCQ. The incidence of retinopathy rises with the cumulative dose and duration of the therapy. A recent analysis of a large clinical database showed that at doses of 4–5 mg/kg/day of the actual body weight, the risk of retinopathy with 10 years of therapy is less than 2% and increases to over 20% if the exposure lengthens to 20 years.13 Development of the optical coherence tomography in recent times has improved the ability to detect subtle early changes of retinal pathology such as thinning of the foveal photoreceptor outer segment, thickening of the retinal pigment epithelium, and loss of the macular ganglion cell–inner plexiform layer, and so on. However, with the advent of such sensitive screening techniques it is yet to be seen whether there are distinct improvements in the clinical outcomes.14,15 The risk of retinopathy is minimal when detected early; however, the prognosis declines sharply once the reduction in the central foveal thickness and classic bull’s-eye lesion are evident.13 The American Academy of Ophthalmology recently revised its guidelines and now recommends limiting the chronic HCQ therapy to less than 5 mg/kg/day of the actual body weight.16,17 The guidelines recommend a baseline fundus exam to rule out preexisting maculopathy and annual screening with automated visual fields and retinal exams for patients on treatment for more than 5 years, even on acceptable doses and without concomitant risk factors should be performed.
Cutaneous manifestations, especially the hyperpigmentation related to HCQ, appears to be due to local bruising following deposition of iron in the soft tissue; however, the exact reason underlying increased skin propensity to bruising and/or inadequate resorption of pigment with HCQ is unclear. Unfortunately, similar to retinopathy, cutaneous hyperpigmentation may persist permanently.18 By contrast, the acute generalized exanthematous pustulosis related to HCQ usually resolves within 2 weeks after cessation of the medication.19 HCQ-related skeletal myopathy, an uncommon adverse effect improves with discontinuation of treatment; however, the improvement often takes weeks, likely because of the prolonged elimination half-life.20 Cardiomyopathy is another rare and disturbing adverse effect related to HCQ’s effects on the lysosomal function.21,22 It is largely of concern in conditions that require high doses and/or long-term therapy, and its occurrence with chronic low-dose therapy is rare.23,24 Analyses of the longitudinal cohorts in systemic lupus erythematosus (SLE) have shown that the incidence of the new-onset cardiac arrhythmias is significantly reduced among those exposed to the long-term low-dose HCQ therapy.25,26 Finally, cases of hemolytic anemia among those with genetic G6PD deficiency has been reported. While the utility of routinely checking G6PD status is uncertain and not recommended, a prior blood test may avoid this concern in the susceptible populations. Additionally, rare cases of hematological adverse effects such as agranulocytosis, anemia, aplastic anemia, leukopenia, and thrombocytopenia have been reported.11
Mechanisms of action
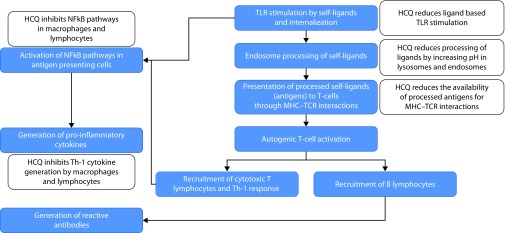
The antimalarial actions for the CHQ and the related analogues were considered to be rooted in their lysosomotropic properties. Being weak bases, these compounds accumulate heavily, nearly 1000-fold, in the acidic environment of the lysosomes of the malarial parasites. Here, they prevent the conversion of the toxic heme moiety into nontoxic crystalline hemozoin, allowing it to lyse the parasite as well as the housing red blood cell. Recognition of the multiple non-malarial benefits for these compounds has led the investigators to examine the putative mechanisms in these non-malarial disorders. Two broad mechanistic pathways have become apparent in these investigations. First, these compounds appear to have potent immunomodulatory effects as they affect multiple sequential steps in the process of immune recognition, response, and downstream generation of inflammation (Figure 1). These actions facilitate their application in the variety of chronic diseases with altered immune recognition and/or response, such as rheumatologic disease, CVD, CKD, dermatological diseases, and infectious disease, and so on. Similarly, over the last 2–3 decades, it has become clear that these compounds have prominent effects on autophagy and apoptosis processes, which have led their application in the field of oncology. The detailed mechanistic rationale for the application of these compounds in prominent disease states is further discussed under the individual clinical applications, in the sections below.
Figure 1.
Simplified schematic representation of the autoimmunity, and the multistep effects of chloroquine and the related compounds in reducing autoimmunity and inflammation.
TLR, Toll-like receptors; MHC, major histocompatibility complex; TCR, T-lymphocyte cell receptors; Th-1, T helper cell type 1; NFkB, nuclear factor kappa B.
HCQ in rheumatology
The rheumatologic applications of the CHQ and the related compounds were first realized when soldiers posted to the Pacific Islands at the time of World War II were provided malaria prophylaxis and they reported parallel benefits in many musculoskeletal and dermatological manifestations.3 Subsequent studies indicated a promising role for these compounds in several rheumatic diseases such as SLE, discoid lupus, rheumatoid arthritis (RA), Sjogren’s syndrome, psoriasis, among others.3 Modification of CHQ into HCQ and a resultant improvement in drug tolerance elevated the role of these compounds to everyday staple therapy for these disorders.3 Even in the current era of the highly targeted biological therapies, it is increasingly realized that the comparative long-term safety and efficacy provides HCQ a unique and essential role in the management of many rheumatologic disorders – as an adjunct therapy in severe forms of the disease or as a sole therapy in milder cases.27–29
Mechanisms of rheumatologic benefits
The serendipitous discovery of the clinical benefits in rheumatologic disorders led to many in vitro and in vivo investigations on the potential mechanistic role of HCQ in autoimmunity and inflammation. It is now recognized that HCQ has multistep and pleiotropic effects on innate and adaptive immunity such that it preferentially targets the process of autoimmunity without significantly interfering with the process of adaptive immunity necessary for fighting off the pathogens.30,31 Figure 1 illustrates a simplified mechanistic role from an immunological and inflammation perspective that applies to many non-malarial indications.
In many autoimmune and rheumatologic disorders, toll-like receptors (TLR) that recognize and interact with many endogenous ligands are considered important mediators for the pathophysiology. HCQ blocks the activation of this family of receptors, especially TLR 9, necessary for the recognition of autoantigens, which reduces the activation of the downstream immune pathways.32–35 Once intracellular, these weak basic compounds preferentially concentrate in the acidic environment of lysosomes and phagolysosomes with concentrations reaching up to 1000 times higher in these organelles. The acidic environment of these organelles is vital in digesting and clearing the peptides. The processed antigenic peptides are eventually presented to T-cells through major histocompatibility complex and T-cell receptor (MHC–TCR) interactions.30 However, the neutralizing lysosomotropic actions of HCQ reduces further processing of antigenic peptides, thus affecting the downstream immune cascade of T cell activation, and the generation and proliferation of the targeted T and B cells as well as autoantibodies.30 A lower natural affinity of the self-antigen MHC molecule (compared with the foreign antigen–MHC molecule) to the TCR further allows HCQ’s effects to be targeted toward inhibiting the autoimmunity rather than generating an immunosuppressed state.30 Pro-inflammatory cytokines generated either through nascent immune cells or through the targeted cytotoxic T cells are important mediators and propagators of the tissue injury in the autoimmune diseases.36 In vitro investigations have shown that HCQ at concentrations routinely achieved with chronic low-dose therapy potently inhibits the nuclear factor of kappa B (NFκB) pathways in the macrophages and T helper type 1 (Th-1) lymphocytes,37,38 thus reducing the generation of the pro-inflammatory cytokines such as interleukin (IL)-1, IL-6, tumor necrosis factor (TNF), interferon (IFN), and so on by about 60–80% at 24 hours.12,39 These effects have also been verified in the longitudinal lupus cohorts where long-term use of HCQ is associated with reduced levels of pro-inflammatory cytokines.40,41 Finally, apart from these direct immunological benefits, use of HCQ has also been associated with reduced musculoskeletal and metabolic complications in these disorders; either due to reduction in the propensity for adverse effects associated with alternate immunosuppressants, that is, steroids or by its direct actions as highlighted below in the section of CVD.42
Clinical data
HCQ is approved by the US Food and Drug Administration for the treatment of SLE and RA.11 Low-dose chronic HCQ therapy has been shown to positively affect the musculoskeletal syndrome of arthralgia/arthritis, myalgia, serositis, cutaneous disease, and hematological manifestations.43 One of the pivotal randomized, placebo-controlled studies in the early 1990s showed that an elective, withdrawal of HCQ among stable SLE patients more than doubled the risk of lupus flare (odds ratio [OR]: 2.5, 95% confidence interval [CI], 95% CI: 1.08–5.58) over the initial 6 months period, an effect which is maintained even in the long-term follow up.44,45 Since then, a number of studies further confirmed that the use of HCQ substantially reduces disease activity and the overall flare-up rates in SLE.41 Cohort studies have shown that HCQ use in lupus is associated with reduced incidence of new-onset organ damage, including grave complications such as nephritis and cerebritis.45–47 Furthermore, HCQ use in patients already diagnosed with lupus nephritis has been shown to slow the progression CKD and reduced the incidence of end-stage renal disease (ESRD).46,48 Other investigators have shown the risk for thrombotic events, CV events, and CV mortality are reduced with HCQ.49,50 Analysis of a large longitudinal lupus cohort (LUMINA study) also showed that chronic HCQ use was associated with a nearly 70% reduction in vascular events51 and a substantial reduction in CV deaths.49,50,52 Finally, cohort analyses from different parts of the world have consistently shown that the use of HCQ in SLE is associated with a substantial reduction in long-term mortality (about 38–84% reduced odds for death).52–55
Similar to SLE, HCQ has shown multiple benefits in the long-term management of RA. HCQ is used as a sole agent in patients with milder forms of disease or as an adjunct to disease-modifying agents, including biological in those with severe systemic manifestations.56 Cohort analyses in the RA have shown a significant reduction in the onset and progression of erosive arthritis.31 Furthermore, recent studies have shown improved long-term CV and renal outcomes and mortality.57
Apart from SLE and RA, HCQ has been tried in multiple other rheumatologic diseases, prominent among which are Sjogren’s syndrome and psoriasis, and so on;58 however, the benefits of these compounds are not well established. A recent metanalysis in Sjogren’s syndrome showed substantial heterogeneity in efficacy data that warrant high-quality randomized studies for further testing.59 In addition, due to propensity for a worsened skin condition, HCQ experience in psoriasis is largely limited to those populations with a possible coexistent lupus-like syndrome.60 In a nutshell, the currently available data indicate that HCQ has a central place in the management SLE and RA28; however, they argue for more focused and possibly randomized studies in other rheumatological disorders.
The chronic dosing of HCQ in rheumatologic disorders is usually recommended in a low-dose format traditionally, limited to less than 6.5 mg/kg/day61 to ensure safety while maximizing the duration of administration. Few early reports suggested that there is a good correlation between the blood levels of HCQ measured (>750–1000 ng/ml) and an individual’s disease responsive state.61 This led some investigators to suggest the need for a dose–response titration in patients with suboptimal response.62 It is now well recognized that these blood levels are primarily reflective of the adherence; however, titrating the dose for efficacy- or safety-based levels is unreliable given the large volume of distribution and multiple active metabolites.8 Furthermore, there is an increasing realization that the long-term toxicities of these compounds, especially severe adverse events such as retinal toxicity, are related to the cumulative dose exposure. As mentioned in the section above, routine monitoring of the drug levels and titration of dosing for these compounds is not recommended8,63, but the American Academy of Ophthalmology has limited the chronic low-dose regimens to less than 5 mg/kg/day calculated for the actual weight.16,17
HCQ in CVD
Although isolated reports suggested CV benefits several decades ago,64,65 meaningful benefits for these compounds gained recognition only over the last couple of decades, especially with the demonstration of reduced incidence of vascular events, vascular deaths and all-cause mortality in rheumatologic cohorts.49–52 There are mounting data to support that atherosclerosis is primarily an inflammatory disease66 and that the systemic inflammation evident in rheumatologic diseases worsens atherosclerosis and contributes to a higher CVD burden in these populations.67
Mechanisms underlying CVD benefits
Similar to rheumatological indications, the CV benefits seem related to multistep pleiotropic effects of these compounds on the immune system, with no clear consensus on a dominant mechanism. As CHQ and HCQ are both potent inhibitors of the TLR-9 pathway, they have the potential to block the macrophage transformation into a foam cell. The lysosomotropic action of HCQ interferes with the downstream activation of the Th-1 response and pro-inflammatory cytokine generation important in propagation of atherosclerosis.32–35 HCQ’s effects on the NFκB further reduce the release of pro-inflammatory cytokines such as IL-1, IL-6, TNFα, and IFNγ with resultant reduction in the tissue damage.12,39
In recent times, research work from our lab and other investigators have further demonstrated direct anti-atherosclerosis and vasculoprotective actions for HCQ in a variety of animal models, including the models of metabolic syndrome, diabetes, hyperlipidemia, and CKD.68 Razani and colleagues showed that these anti-atherosclerosis effects occur via a p53-dependent beneficial effect on cellular stress response pathways.69 Moreover, in vitro studies have shown that CHQ increases endothelial nitric oxide synthase resulting in improved nitric oxide release, which is beneficial to endothelial health and inhibits atherosclerosis.70 During in vivo testing of these benefits, low-dose HCQ was observed to reduce the progression of vascular stiffness in the presence of atherosclerosis likely related to the reduction in oxidative stress.70,71 Clinical studies have shown HCQ leads to improvement in endothelial function and vascular stiffness, as judged by improvement in flow-mediated dilation and reduction in aortic pulse wave velocity,72,73 and lower incidence of new-onset hypertension.74 Finally, these compounds have shown a significant effect on parameters for metabolic syndrome, including insulin sensitivity and lipid homeostasis with reductions in fasting glucose, LDL, and triglycerides levels with some reports showing an increase in HDL levels.42,74–76
Clinical data
From a clinical perspective, the beneficial role of HCQ in CVD has largely been derived from the analyses of longitudinal SLE and RA cohorts. Investigators have found that the long-term use of HCQ is not only associated with a significant reduction in the all-cause mortality, but also reduces the CV events and CV mortality.49–52 Recently, two separate RCTs found that the adjunct therapy with HCQ for the management of hyperlipidemia (with statins) and diabetes control (with routine antidiabetic management) led to more effective reductions in LDL and hemoglobin A1c, respectively, than either of them alone.77,78 Although these data imply HCQ affects atherosclerosis and metabolic syndrome, to date, evidence for direct benefits on major adverse CV events defined as the composite of total death; myocardial infarction; stroke and hospitalization because of heart failure; and revascularization, including percutaneous coronary intervention, and coronary artery bypass graft is not available. Two ongoing randomized studies have aimed to accomplish these tasks. The first among these, is a phase IIb randomized controlled trial (NCT03636152; also known as MaCK Study), plans to examine effects on atherosclerosis, vascular stiffness, and inflammation in patients diagnosed with CKD.79 The second study is a phase III, randomized placebo controlled trial (NCT02648464; also known as the OXI trial) that aims to evaluate the effects of HCQ on these composite major adverse CV events in patients hospitalized for acute coronary events.80 The results of these studies should provide fascinating insights into the future role of HCQ as a therapy for CVD in general as well as certain high CV risk special populations.
HCQ in nephrology
Limited investigators have examined the effect of the antimalarials on the renal parameters. Anecdotally, these analogues have been used for a variety of proliferative glomerulonephritis; though, such use was largely driven by the scientific evidence derived from its clinical benefits in lupus nephritis. A number of investigations in recent times have led to increasing interest in HCQ for the primary renal disorders.
Mechanisms underlying renal benefits
The mechanistic rationale for the use of CHQ analogues in renal diseases are primarily driven by immunological properties discussed hereinbefore in the rheumatologic disorders and CVD. The glomerular mesangial cells are derived from the monocyte/macrophage lineage and play a prominent role in the pathogenesis and progression of the autoimmune damage in primary glomerular diseases.81 Due to its inhibitory effects on a series of steps critical to the process of autoimmunity, that is, self-peptide recognition, its antigenic presentation and the resultant short-term and long-term downstream responses with the generation of cytotoxic cytokines and Th-1 type cellular immune response, respectively, HCQ has potentials to interrupt the pathophysiology of these disorders (Figure 1).82 A number of investigators in recent times have focused on the potentials of HCQ to affect the pathophysiological basis of the IgA nephropathy. Activation of the TLR-9 pathways by the common antigens has been shown to affect the severity of IgA nephropathy and HCQ is a potent inhibitor of this pathway.83 Exploiting these mechanistic rationales, Liu and colleagues were recently able to show a significant reduction in the severity of proteinuria in IgA nephropathy.84 Additionally, the mechanistic parallels between the progressive CVD and progressive CKD strongly argue for the role of these analogues in progressive CKD. In a few targeted investigations examining the effects of HCQ on renal parameters, investigators have shown the beneficial effects of HCQ on endothelial functions with resultant reduction in hypertension and renal hypertrophy, recognized markers of long-term renal outcomes.70,71 Although promising in nature, these hypotheses require further investigations.
Clinical data
A number of cohort studies have collectively shown that HCQ therapy in patients with autoimmune disorders such as SLE and RA is associated with reduction in the emergence of lupus nephritis among those with SLE without renal involvement, and reduced incidence of adverse renal outcomes including the development of ESRD in patients with SLE and RA.45–48,57 Whereas the MacK study aims to understand whether there are direct benefits of HCQ in renal progression,68 Liu and colleagues have shown that adjunct use of HCQ significantly reduces the proteinuria in IgA nephropathy, but the final word on their efficacy, especially in terms of hard endpoints, remains uncertain.82,84,85
CHQ in oncology
There is growing research to support CHQ’s important adjuvant role for the treatment of neoplasms. Similar to rheumatological disorders, the original observations about five decades ago were serendipitous wherein investigators noticed a significant decline in the incidence of Burkitt’s lymphoma among North African countries where CHQ was widely distributed for control of malaria.86 These observations were critical in promoting further research. Since then, CHQ and HCQ have been tested in many tumors including gliomas, breast cancer, metastatic cancer, multiple myeloma, lymphomas, head and neck cancers, and gastrointestinal cancers.
Mechanisms underlying oncological benefits
While immunological and anti-inflammatory properties have been the key factors underlying the benefits in rheumatological, CVD, and nephrological conditions, the most widely accepted view on the antineoplastic effects of CHQ is autophagy inhibition, which facilitates radiosensitization of tumors. Recent research suggests the antineoplastic effects of CHQ are likely independent from its autophagy-inhibiting activities as the autophagy-related pathways were found to inhibit cholesterol biosynthesis and thereby induce cell death.87 There is evidence that CHQ can profoundly influence cell metabolism through multitude of pathways such as inhibition of glyconeogenesis, mitochondrial metabolism, and amino acid metabolism.88 While CHQ has been found to induce p53-dependent apoptosis of neoplastic cells,87,89–91 the exact mechanism underlying activation of p53, which is the key node controlling cell survival and cell death, remains elusive.87 The chemosensitization behavior of CHQ is mainly related to the normalization of the tumor vasculature through reduction of vessel density, improvement of cell alignment, the formation of tight junctions, and promotion of quiescent phenotype of endothelial cells, which leads to the more effective delivery of chemotherapy drug.92 Although CHQ showed encouraging results in a study involving recurrent glioblastoma multiforme, the therapeutic role through potentiation of standard chemotherapy drugs was further confirmed in a double-blinded phase III clinical trial (n=30 newly diagnosed cases).93–95 Besides the direct suppression of cancer cells, these agents can potentially modulate the tumor microenvironment through their effects on the tumor vasculature, cancer-associated fibroblasts, and immune system.87 The CHQ property against T cell multiplication evoked in response to foreign antigens and major histocompatibility complex antigens in conjunction with reduced T cell cytokine production is leveraged for inhibition of graft-versus-host disease in patients who receive bone marrow transplantation.96
The dosing requirements of both CHQ and HCQ for their oncology application are substantially different compared with their other non-oncology use. Due to these, the concerns for their GI, hemodynamic, and cardiac side effects substantially increase, especially as these analogues have high bioavailability and long elimination half-life. In recent times, targeted nanoparticle-based delivery methods to enhance the delivery of the drug to the desired tissues have been proposed to mitigate the potential for toxicity.
CHQ in dermatology and infections
The roles of CHQ, HCQ, and their analogues has been examined in many dermatological disorders; however, the outcomes have not been consistently positive. While FDA has granted approval for the treatment of discoid lupus97 and small clinical studies have shown positive benefits for porphyria cutanea tarda,98,99 in a large randomized clinical trial of dermatomyositis, HCQ treatment for 24 weeks did not show significant improvements in mouth and eye dryness compared with that in the placebo group.58 The immunological properties of CHQ analogues have also been tested in many bacterial, viral, and parasitic infections such as Q fever, chikungunya fever, giardiasis, amoebiasis, Ebola virus, and HIV-1 infections.2 These investigations are at an early stage and require further testing in well-designed studies and large well-defined cohorts before clear conclusions can be drawn.
Conclusion
A series of developments over the last several decades has significantly transformed the role of CHQ and the related analogues in clinical medicine. While a rising prevalence of resistant malarial strains has substantially affected the therapeutic role of these agents in malaria, a series of serendipitous observations and some well-conducted experimental and clinical studies have significantly expanded the horizons in many chronic metabolic diseases and malignancies. From a mechanistic perspective, there are two broad reasons for an increasing number of non-malarial applications. The lysosomotropic, immunomodulatory, and anti-inflammatory potentials of chronic low-dose therapy appear to play a major role in rheumatologic diseases, CVD, CKD, dermatological and infectious diseases, and so on. At the same time, high doses of these analogues with prominent effects on autophagy and apoptosis processes are leveraged for their application in oncology. With the recent clarifications of the risk of retinal toxicity and the related dosing recommendations by the American Academy of Ophthalmology, it appears that these analogues are ready for a new chapter in their life.
Acknowledgements
Ashutosh Shukla reports the following VA Merit Grant supports from the Department of Veterans Affairs (I01CX001661: Management of cardiovascular disease in advanced CKD and I01HX002639: A system-wide strategy for KDE to improve the health and health services outcomes among Veterans). Aparna Wagle Shukla reports grants from the NIH and has received grant support from Benign Essential Blepharospasm Research foundation, Dystonia coalition, Dystonia Medical Research foundation, National Organization for Rare Disorders and NIH (KL2 and K23 NS092957-01A1).
Footnotes
Contributions: Ashutosh Shukla was involved in the execution, review and critique of the manuscript. Aparna Wagle Shukla was involved in the organization, review and critique of the manuscript. Both authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at http://www.drugsincontext.com/wp-content/uploads/2019/10/dic.2019-9-1-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2019 Shukla AM, Wagle Shukla A. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Article URL: https://www.drugsincontext.com/expanding-horizons-for-clinical-applications-of-chloroquine,-hydroxychloroquine,-and-related-structural-analogues/
Provenance: invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editor-in-Chief gordon.mallarkey@bioexcelpublishing.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
Peer review comments to author: 20 September 2019
References
- 1.Wallace DJ. The history of antimalarials. Lupus. 1996;5(Suppl 1):S2–S3. [PubMed] [Google Scholar]
- 2.Al-Bari MA. Chloroquine analogues in drug discovery: new directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J Antimicrob Chemother. 2015;70(6):1608–1621. doi: 10.1093/jac/dkv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallace DJ. The use of chloroquine and hydroxychloroquine for non-infectious conditions other than rheumatoid arthritis or lupus: a critical review. Lupus. 1996;5(1 Suppl):S59–S64. doi: 10.1177/096120339600500113. [DOI] [PubMed] [Google Scholar]
- 4.Price RN, von Seidlein L, Valecha N, Nosten F, Baird JK, White NJ. Global extent of chloroquine-resistant Plasmodium vivax: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(10):982–991. doi: 10.1016/S1473-3099(14)70855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC. CfDCaP. Malaria information and prophylaxis, by country. 2018. www.cdc.gov/malaria/travelers/country_table/a.html .
- 6.McLachlan AJ, Tett SE, Cutler DJ, Day RO. Bioavailability of hydroxychloroquine tablets in patients with rheumatoid arthritis. Br J Rheumatol. 1994;33(3):235–239. doi: 10.1093/rheumatology/33.3.235. [DOI] [PubMed] [Google Scholar]
- 7.Miller DR, Khalil SK, Nygard GA. Steady-state pharmacokinetics of hydroxychloroquine in rheumatoid arthritis patients. DICP. 1991;25(12):1302–1305. doi: 10.1177/106002809102501202. [DOI] [PubMed] [Google Scholar]
- 8.Miller DR, Fiechtner JJ, Carpenter JR, Brown RR, Stroshane RM, Stecher VJ. Plasma hydroxychloroquine concentrations and efficacy in rheumatoid arthritis. Arthritis Rheum. 1987;30(5):567–571. doi: 10.1002/art.1780300512. [DOI] [PubMed] [Google Scholar]
- 9.Shippey EA, Wagler VD, Collamer AN. Hydroxychloroquine: an old drug with new relevance. Cleve Clin J Med. 2018;85(6):459–467. doi: 10.3949/ccjm.85a.17034. [DOI] [PubMed] [Google Scholar]
- 10.Muller F, Konig J, Glaeser H, et al. Molecular mechanism of renal tubular secretion of the antimalarial drug chloroquine. Antimicrob Agents Chemother. 2011;55(7):3091–3098. doi: 10.1128/AAC.01835-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanofi-Aventis. Package insert, hydroxychloroquine sulfate, USP. Oct, 2006. [Accessed August 29, 2019]. [Google Scholar]
- 12.van den Borne BE, Dijkmans BA, de Rooij HH, le Cessie S, Verweij CL. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells. J Rheumatol. 1997;24(1):55–60. [PubMed] [Google Scholar]
- 13.Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132(12):1453–1460. doi: 10.1001/jamaophthalmol.2014.3459. [DOI] [PubMed] [Google Scholar]
- 14.Uslu H, Gurler B, Yildirim A, et al. Effect of hydroxychloroquine on the retinal layers: a quantitative evaluation with spectral-domain optical coherence tomography. J Ophthalmol. 2016;2016 doi: 10.1155/2016/8643174. 8643174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trenkic Bozinovic MS, Stankovic Babic G, Petrovic M, Karadzic J, Sarenac Vulovic T, Trenkic M. Role of optical coherence tomography in the early detection of macular thinning in rheumatoid arthritis patients with chloroquine retinopathy. J Res Med Sci. 2019;24:55. doi: 10.4103/jrms.JRMS_704_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmol. 2011;118(2):415–422. doi: 10.1016/j.ophtha.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF American Academy of Ophthalmology. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision) Ophthalmol. 2016;123(6):1386–1394. doi: 10.1016/j.ophtha.2016.01.058. [DOI] [PubMed] [Google Scholar]
- 18.Jallouli M, Frances C, Piette JC, et al. Hydroxychloroquine-induced pigmentation in patients with systemic lupus erythematosus: a case-control study. JAMA Dermatol. 2013;149(8):935–940. doi: 10.1001/jamadermatol.2013.709. [DOI] [PubMed] [Google Scholar]
- 19.Pearson KC, Morrell DS, Runge SR, Jolly P. Prolonged pustular eruption from hydroxychloroquine: an unusual case of acute generalized exanthematous pustulosis. Cutis. 2016;97(3):212–216. [PubMed] [Google Scholar]
- 20.Avina-Zubieta JA, Johnson ES, Suarez-Almazor ME, Russell AS. Incidence of myopathy in patients treated with antimalarials. A report of three cases and a review of the literature. Br J Rheumatol. 1995;34(2):166–170. doi: 10.1093/rheumatology/34.2.166. [DOI] [PubMed] [Google Scholar]
- 21.Cotroneo J, Sleik KM, Rene Rodriguez E, Klein AL. Hydroxychloroquine-induced restrictive cardiomyopathy. Eur J Echocardiogr. 2007;8(4):247–251. doi: 10.1016/j.euje.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Yogasundaram H, Putko BN, Tien J, et al. Hydroxychloroquine-induced cardiomyopathy: case report, pathophysiology, diagnosis, and treatment. Can J Cardiol. 2014;30(12):1706–1715. doi: 10.1016/j.cjca.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Costedoat-Chalumeau N, Hulot JS, Amoura Z, et al. Heart conduction disorders related to antimalarials toxicity: an analysis of electrocardiograms in 85 patients treated with hydroxychloroquine for connective tissue diseases. Rheumatology (Oxford) 2007;46(5):808–810. doi: 10.1093/rheumatology/kel402. [DOI] [PubMed] [Google Scholar]
- 24.Soong TR, Barouch LA, Champion HC, Wigley FM, Halushka MK. New clinical and ultrastructural findings in hydroxychloroquine-induced cardiomyopathy–a report of 2 cases. Hum Pathol. 2007;38(12):1858–1863. doi: 10.1016/j.humpath.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Teixeira RA, Borba EF, Pedrosa A, et al. Evidence for cardiac safety and antiarrhythmic potential of chloroquine in systemic lupus erythematosus. Europace. 2014;16(6):887–892. doi: 10.1093/europace/eut290. [DOI] [PubMed] [Google Scholar]
- 26.Kojuri J, Nazarinia MA, Ghahartars M, Mahmoody Y, Rezaian G, Liaghat L. Qt dispersion in patients with systemic lupus erythematosus: the impact of disease activity. BMC Cardiovasc Dis. 2012;12:11. doi: 10.1186/1471-2261-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 update of the eular recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78(6):736–745. doi: 10.1136/annrheumdis-2019-215089. [DOI] [PubMed] [Google Scholar]
- 28.Michaud M, Catros F, Ancellin S, Gaches F. Treatment of systemic lupus erythematosus: don’t forget hydroxychloroquine. Ann Rheum Dis. 2019 doi: 10.1136/annrheumdis-2019-215799. [DOI] [PubMed] [Google Scholar]
- 29.Abbasi M, Mousavi MJ, Jamalzehi S, et al. Strategies toward rheumatoid arthritis therapy; the old and the new. J Cell Physiol. 2019;234(7):10018–10031. doi: 10.1002/jcp.27860. [DOI] [PubMed] [Google Scholar]
- 30.Fox RI. Mechanism of action of hydroxychloroquine as an antirheumatic drug. Semin Arthritis Rheum. 1993;23(2 Suppl 1):82–91. doi: 10.1016/s0049-0172(10)80012-5. [DOI] [PubMed] [Google Scholar]
- 31.Rainsford KD, Parke AL, Clifford-Rashotte M, Kean WF. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23(5):231–269. doi: 10.1007/s10787-015-0239-y. [DOI] [PubMed] [Google Scholar]
- 32.Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21(12):1876–1890. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]
- 33.Baidya SG, Zeng QT. Helper T cells and atherosclerosis: the cytokine web. Postgrad Med J. 2005;81(962):746–752. doi: 10.1136/pgmj.2004.029827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michelsen KS, Wong MH, Shah PK, et al. Lack of toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101(29):10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu JQ, Wang DF, Yan XG, et al. A toll-like receptor 9-mediated pathway stimulates perilipin 3 (TIP7) expression and induces lipid accumulation in macrophages. Am J Physiol Endocrinol Metab. 2010;299(4):E593–E600. doi: 10.1152/ajpendo.00159.2010. [DOI] [PubMed] [Google Scholar]
- 36.Moudgil KD, Choubey D. Cytokines in autoimmunity: role in induction, regulation, and treatment. J Interferon Cytokine Res. 2011;31(10):695–703. doi: 10.1089/jir.2011.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jang CH, Choi JH, Byun MS, Jue DM. Chloroquine inhibits production of TNF-alpha, IL-1beta and IL-6 from lipopolysaccharide-stimulated human monocytes/macrophages by different modes. Rheumatology (Oxford) 2006;45(6):703–710. doi: 10.1093/rheumatology/kei282. [DOI] [PubMed] [Google Scholar]
- 38.Karres I, Kremer JP, Dietl I, Steckholzer U, Jochum M, Ertel W. Chloroquine inhibits proinflammatory cytokine release into human whole blood. Am J Physiol Regul Integr Comp Physiol. 1998;274(4):R1058–R1064. doi: 10.1152/ajpregu.1998.274.4.R1058. [DOI] [PubMed] [Google Scholar]
- 39.Picot S, Peyron F, Donadille A, Vuillez JP, Barbe G, Ambroise-Thomas P. Chloroquine-induced inhibition of the production of TNF, but not of IL-6, is affected by disruption of iron metabolism. Immunology. 1993;80(1):127–133. [PMC free article] [PubMed] [Google Scholar]
- 40.Picot S, Peyron F, Vuillez JP, Polack B, Ambroise-Thomas P. Chloroquine inhibits tumor necrosis factor production by human macrophages in vitro. J Infect Dis. 1991;164(4):830. doi: 10.1093/infdis/164.4.830. [DOI] [PubMed] [Google Scholar]
- 41.Willis R, Seif AM, McGwin G, Jr, et al. Effect of hydroxychloroquine treatment on pro-inflammatory cytokines and disease activity in SLE patients: data from LUMINA (LXXV), a multiethnic US cohort. Lupus. 2012;21(8):830–835. doi: 10.1177/0961203312437270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen YM, Lin CH, Lan TH, et al. Hydroxychloroquine reduces risk of incident diabetes mellitus in lupus patients in a dose-dependent manner: a population-based cohort study. Rheumatology (Oxford) 2015;54(7):1244–1249. doi: 10.1093/rheumatology/keu451. [DOI] [PubMed] [Google Scholar]
- 43.Fessler BJ, Alarcon GS, McGwin G, Jr, et al. Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis Rheum. 2005;52(5):1473–1480. doi: 10.1002/art.21039. [DOI] [PubMed] [Google Scholar]
- 44.Canadian Hydroxychloroquine Study Group. A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. N Engl J Med. 1991;324(3):150–154. doi: 10.1056/NEJM199101173240303. [DOI] [PubMed] [Google Scholar]
- 45.Tsakonas E, Joseph L, Esdaile JM, et al. A long-term study of hydroxychloroquine withdrawal on exacerbations in systemic lupus erythematosus. The Canadian Hydroxychloroquine study group. Lupus. 1998;7(2):80–85. doi: 10.1191/096120398678919778. [DOI] [PubMed] [Google Scholar]
- 46.Pons-Estel GJ, Alarcon GS, McGwin G, Jr, et al. Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: LXV, data from a multiethnic US cohort. Arthritis Rheum. 2009;61(6):830–839. doi: 10.1002/art.24538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pons-Estel BA, Sanchez-Guerrero J, Romero-Diaz J, et al. Validation of the Spanish, Portuguese and French versions of the Lupus Damage Index questionnaire: data from North and South America, Spain and Portugal. Lupus. 2009;18(12):1033–1052. doi: 10.1177/0961203309105590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kasitanon N, Fine DM, Haas M, Magder LS, Petri M. Hydroxychloroquine use predicts complete renal remission within 12 months among patients treated with mycophenolate mofetil therapy for membranous lupus nephritis. Lupus. 2006;15(6):366–370. doi: 10.1191/0961203306lu2313oa. [DOI] [PubMed] [Google Scholar]
- 49.Liu D, Li X, Zhang Y, et al. Chloroquine and hydroxychloroquine are associated with reduced cardiovascular risk: a systematic review and meta-analysis. Drug Des Devel Ther. 2018;12:1685–1695. doi: 10.2147/DDDT.S166893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Floris A, Piga M, Mangoni AA, Bortoluzzi A, Erre GL, Cauli A. Protective effects of hydroxychloroquine against accelerated atherosclerosis in systemic lupus erythematosus. Mediators Inflamm. 2018;2018 doi: 10.1155/2018/3424136. 3424136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Becker-Merok A, Nossent J. Prevalence, predictors and outcome of vascular damage in systemic lupus erythematosus. Lupus. 2009;18(6):508–515. doi: 10.1177/0961203308099233. [DOI] [PubMed] [Google Scholar]
- 52.Ruiz-Irastorza G, Egurbide MV, Pijoan JI, et al. Effect of antimalarials on thrombosis and survival in patients with systemic lupus erythematosus. Lupus. 2006;15(9):577–583. doi: 10.1177/0961203306071872. [DOI] [PubMed] [Google Scholar]
- 53.Alarcon GS, McGwin G, Bertoli AM, et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA l) Ann Rheum Dis. 2007;66(9):1168–1172. doi: 10.1136/ard.2006.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shinjo SK, Bonfa E, Wojdyla D, et al. Antimalarial treatment may have a time-dependent effect on lupus survival: data from a multinational Latin American inception cohort. Arthritis Rheum. 2010;62(3):855–862. doi: 10.1002/art.27300. [DOI] [PubMed] [Google Scholar]
- 55.Hernandez-Cruz B, Tapia N, Villa-Romero AR, Reyes E, Cardiel MH. Risk factors associated with mortality in systemic lupus erythematosus. A case-control study in a tertiary care center in Mexico City. Clin Exp Rheumatol. 2001;19(4):395–401. [PubMed] [Google Scholar]
- 56.Suarez-Almazor ME, Belseck E, Shea B, Homik J, Wells G, Tugwell P. Antimalarials for treating rheumatoid arthritis. Cochrane Database Syst Rev. 2000;(4):CD000959. doi: 10.1002/14651858.CD000959. [DOI] [PubMed] [Google Scholar]
- 57.Wu CL, Chang CC, Kor CT, et al. Hydroxychloroquine use and risk of CKD in patients with rheumatoid arthritis. Clin J Am Soc Nephrol. 2018;13(5):702–709. doi: 10.2215/CJN.11781017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gottenberg JE, Ravaud P, Puechal X, et al. Effects of hydroxychloroquine on symptomatic improvement in primary Sjogren syndrome: the JOQUER randomized clinical trial. JAMA. 2014;312(3):249–258. doi: 10.1001/jama.2014.7682. [DOI] [PubMed] [Google Scholar]
- 59.Wang SQ, Zhang LW, Wei P, Hua H. Is hydroxychloroquine effective in treating primary Sjogren’s syndrome: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2017;18(1):186. doi: 10.1186/s12891-017-1543-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luzar MJ. Hydroxychloroquine in psoriatic arthropathy: exacerbations of psoriatic skin lesions. J Rheumatol. 1982;9(3):462–464. [PubMed] [Google Scholar]
- 61.Frances C, Cosnes A, Duhaut P, et al. Low blood concentration of hydroxychloroquine in patients with refractory cutaneous lupus erythematosus: a French multicenter prospective study. Arch Dermatol. 2012;148(4):479–484. doi: 10.1001/archdermatol.2011.2558. [DOI] [PubMed] [Google Scholar]
- 62.Modi JV, Patel KR, Patel ZM, Patel HR, Dhanani SS, Shah BH. Dose response relationship of hydroxychloroquine sulphate in the treatment of rheumatoid arthritis: a randomised control study. Int J Pharm Sci Res. 2017;8(2):856–858. doi: 10.13040/ijpsr.0975-8232.8(2).856-58. [DOI] [Google Scholar]
- 63.Durcan L, Clarke WA, Magder LS, Petri M. Hydroxychloroquine blood levels in systemic lupus erythematosus: clarifying dosing controversies and improving adherence. J Rheumatol. 2015;42(11):2092–2097. doi: 10.3899/jrheum.150379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burrell ZL, Jr, Martinez AC. Chloroquine and hydroxychloroquine in the treatment of cardiac arrhythmias. N Engl J Med. 1958;258(16):798–800. doi: 10.1056/NEJM195804172581608. [DOI] [PubMed] [Google Scholar]
- 65.Sandler G, Ilahi MA, Lawson CW. Clinical evaluation of hydroxychloroquine, a desludging agent, in angina pectoris. Angiology. 1963;14(6):319–324. doi: 10.1177/000331976301400607. [DOI] [PubMed] [Google Scholar]
- 66.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 67.Shoenfeld Y, Gerli R, Doria A, et al. Accelerated atherosclerosis in autoimmune rheumatic diseases. Circulation. 2005;112(21):3337–3347. doi: 10.1161/CIRCULATIONAHA.104.507996. [DOI] [PubMed] [Google Scholar]
- 68.Shukla AM, Bose C, Karaduta OK, et al. Impact of hydroxychloroquine on atherosclerosis and vascular stiffness in the presence of chronic kidney disease. PLoS One. 2015;10(9):e0139226. doi: 10.1371/journal.pone.0139226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Razani B, Feng C, Semenkovich CF. P53 is required for chloroquine-induced atheroprotection but not insulin sensitization. J Lipid Res. 2010;51(7):1738–1746. doi: 10.1194/jlr.M003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Virdis A, Tani C, Duranti E, et al. Early treatment with hydroxychloroquine prevents the development of endothelial dysfunction in a murine model of systemic lupus erythematosus. Arthritis Res Ther. 2015;17(1):277. doi: 10.1186/s13075-015-0790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gomez-Guzman M, Jimenez R, Romero M, et al. Chronic hydroxychloroquine improves endothelial dysfunction and protects kidney in a mouse model of systemic lupus erythematosus. Hypertension. 2014;64(2):330–337. doi: 10.1161/HYPERTENSIONAHA.114.03587. [DOI] [PubMed] [Google Scholar]
- 72.Bengtsson C, Andersson SE, Edvinsson L, Edvinsson ML, Sturfelt G, Nived O. Effect of medication on microvascular vasodilatation in patients with systemic lupus erythematosus. Basic Clin Pharmacol Toxicol. 2010;107(6):919–924. doi: 10.1111/j.1742-7843.2010.00604.x. [DOI] [PubMed] [Google Scholar]
- 73.Selzer F, Sutton-Tyrrell K, Fitzgerald S, Tracy R, Kuller L, Manzi S. Vascular stiffness in women with systemic lupus erythematosus. Hypertension. 2001;37(4):1075–1082. doi: 10.1161/01.hyp.37.4.1075. [DOI] [PubMed] [Google Scholar]
- 74.Tanay A, Leibovitz E, Frayman A, Zimlichman R, Gavish D. Vascular elasticity of systemic lupus erythematosus patients is associated with steroids and hydroxychloroquine treatment. Ann N Y Acad Sci. 2007;1108(1):24–34. doi: 10.1196/annals.1422.003. [DOI] [PubMed] [Google Scholar]
- 75.Penn SK, Kao AH, Schott LL, et al. Hydroxychloroquine and glycemia in women with rheumatoid arthritis and systemic lupus erythematosus. J Rheumatol. 2010;37(6):1136–1142. doi: 10.3899/jrheum.090994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rempenault C, Combe B, Barnetche T, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2018;77(1):98–103. doi: 10.1136/annrheumdis-2017-211836. [DOI] [PubMed] [Google Scholar]
- 77.Pareek A, Chandurkar N, Thulaseedharan NK, et al. Efficacy and safety of fixed dose combination of atorvastatin and hydroxychloroquine: a randomized, double-blind comparison with atorvastatin alone among Indian patients with dyslipidemia. Curr Med Res Opin. 2015;31(11):2105–2117. doi: 10.1185/03007995.2015.1087989. [DOI] [PubMed] [Google Scholar]
- 78.Pareek A, Chandurkar N, Thomas N, et al. Efficacy and safety of hydroxychloroquine in the treatment of type 2 diabetes mellitus: a double blind, randomized comparison with pioglitazone. Curr Med Res Opin. 2014;30(7):1257–1266. doi: 10.1185/03007995.2014.909393. [DOI] [PubMed] [Google Scholar]
- 79.NIH USNLoM. HCQ for the management of CVD in CKD. 2018. [Accessed August 30, 2019]. https://clinicaltrials.gov/ct2/show/NCT03636152 .
- 80.Hartman O, Kovanen PT, Lehtonen J, Eklund KK, Sinisalo J. Hydroxychloroquine for the prevention of recurrent cardiovascular events in myocardial infarction patients: rationale and design of the oxi trial. Eur Heart J Cardiovasc Pharmacother. 2017;3(2):92–97. doi: 10.1093/ehjcvp/pvw035. [DOI] [PubMed] [Google Scholar]
- 81.Masuya M, Drake CJ, Fleming PA, et al. Hematopoietic origin of glomerular mesangial cells. Blood. 2003;101(6):2215–2218. doi: 10.1182/blood-2002-04-1076. [DOI] [PubMed] [Google Scholar]
- 82.Gao R, Wu W, Wen Y, Li X. Hydroxychloroquine alleviates persistent proteinuria in IgA nephropathy. Int Urol Nephrol. 2017;49(7):1233–1241. doi: 10.1007/s11255-017-1574-2. [DOI] [PubMed] [Google Scholar]
- 83.Suzuki H, Suzuki Y, Narita I, et al. Toll-like receptor 9 affects severity of IgA nephropathy. J Am Soc Nephrol. 2008;19(12):2384–2395. doi: 10.1681/ASN.2007121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu LJ, Yang YZ, Shi SF, et al. Effects of hydroxychloroquine on proteinuria in IgA nephropathy: a randomized controlled trial. Am J Kidney Dis. 2019;74(1):15–22. doi: 10.1053/j.ajkd.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 85.Yang YZ, Liu LJ, Shi SF, et al. Effects of hydroxychloroquine on proteinuria in immunoglobulin A nephropathy. Am J Nephrol. 2018;47(3):145–152. doi: 10.1159/000487330. [DOI] [PubMed] [Google Scholar]
- 86.Geser A, Brubaker G, Draper CC. Effect of a malaria suppression program on the incidence of African Burkitt’s lymphoma. Am J Epidemiol. 1989;129(4):740–752. doi: 10.1093/oxfordjournals.aje.a115189. [DOI] [PubMed] [Google Scholar]
- 87.Weyerhauser P, Kantelhardt SR, Kim EL. Re-purposing chloroquine for glioblastoma: potential merits and confounding variables. Front Oncol. 2018;8:335. doi: 10.3389/fonc.2018.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cotton DW, Sutorius AH. Inhibiting effect of some antimalarial substances on glucose-6-phosphate dehydrogenase. Nature. 1971;233(5316):197. doi: 10.1038/233197a0. [DOI] [PubMed] [Google Scholar]
- 89.Choi MM, Kim EA, Choi SY, Kim TU, Cho SW, Yang SJ. Inhibitory properties of nerve-specific human glutamate dehydrogenase isozyme by chloroquine. J Biochem Mol Biol. 2007;40(6):1077–1082. doi: 10.5483/bmbrep.2007.40.6.1077. [DOI] [PubMed] [Google Scholar]
- 90.Lee SW, Kim HK, Lee NH, et al. The synergistic effect of combination temozolomide and chloroquine treatment is dependent on autophagy formation and p53 status in glioma cells. Cancer Lett. 2015;360(2):195–204. doi: 10.1016/j.canlet.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 91.Zaidi AU, McDonough JS, Klocke BJ, et al. Chloroquine-induced neuronal cell death is p53 and Bcl-2 family-dependent but caspase-independent. J Neuropathol Exp Neurol. 2001;60(10):937–945. doi: 10.1093/jnen/60.10.937. [DOI] [PubMed] [Google Scholar]
- 92.Maes H, Kuchnio A, Peric A, et al. Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell. 2014;26(2):190–206. doi: 10.1016/j.ccr.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 93.Verbaanderd C, Maes H, Schaaf MB, et al. Repurposing drugs in oncology (ReDo)-chloroquine and hydroxychloroquine as anti-cancer agents. Ecancermedicalscience. 2017;11:781. doi: 10.3332/ecancer.2017.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sotelo J, Briceno E, Lopez-Gonzalez MA. Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2006;144(5):337–343. doi: 10.7326/0003-4819-144-5-200603070-00008. [DOI] [PubMed] [Google Scholar]
- 95.Bilger A, Bittner MI, Grosu AL, et al. FET-PET-based reirradiation and chloroquine in patients with recurrent glioblastoma: first tolerability and feasibility results. Strahlenther Onkol. 2014;190(10):957–961. doi: 10.1007/s00066-014-0693-2. [DOI] [PubMed] [Google Scholar]
- 96.Li GD. Nucleus may be the key site of chloroquine antimalarial action and resistance development. Med Hypotheses. 2006;67(2):323–326. doi: 10.1016/j.mehy.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 97.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2010;69(1):20–28. doi: 10.1136/ard.2008.101766. [DOI] [PubMed] [Google Scholar]
- 98.Singal AK, Kormos-Hallberg C, Lee C, et al. Low-dose hydroxychloroquine is as effective as phlebotomy in treatment of patients with porphyria cutanea tarda. Clin Gastroenterol Hepatol. 2012;10(12):1402–1409. doi: 10.1016/j.cgh.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gonzalez-Estrada A, Gomez-Morales LB, Gonzalez-Estrada A, Garcia-Morillo JS. Sporadic porphyria cutanea tarda: treatment with chloroquine decreases hyperglycemia and reduces development of metabolic syndrome. Eur J Intern Med. 2014;25(6):e76–e77. doi: 10.1016/j.ejim.2014.02.009. [DOI] [PubMed] [Google Scholar]