Abstract
Background
People with schizophrenia have a range of different symptoms, including positive symptoms (hallucinations and delusions), negative symptoms (such as social withdrawal and lack of affect), and cognitive impairment. The standard medication for people with schizophrenia is antipsychotics. However, these medications may not be effective for all symptoms of schizophrenia, as cognitive and negative symptoms are usually hard to treat. Additional therapies or medications are available for the management of these symptoms. Modafinil, a wakefulness‐promoting agent most frequently used in narcolepsy or shift work sleep disorder, is one intervention that is theorised to have an effect of these symptoms.
Objectives
The primary objective of this review was to assess the effects of modafinil for people with schizophrenia or related disorders.
Search methods
On 27 April 2015, 24 May 2017, and 31 October 2019, we searched the Cochrane Schizophrenia Group's register of trials, which is based on regular searches of CENTRAL, MEDLINE, Embase, AMED, BIOSIS, CINAHL, PsycINFO, PubMed, and registries of clinical trials. There are no language, time, document type, or publication status limitations for the inclusion of records in the register.
Selection criteria
We selected all randomised controlled trials comparing modafinil with placebo or other treatments for people with schizophrenia or schizophrenia‐spectrum disorders.
Data collection and analysis
We independently extracted data from the included studies. We analysed dichotomous data using risk ratios (RR) and 95% confidence intervals (CI). We analysed continuous data using mean difference (MD) with a 95% CI. We used a random‐effects model for the meta‐analysis. We used GRADE to complete a 'Summary of findings' table and assessed risk of bias for the included studies.
Main results
Eleven studies including a total of 422 participants contributed to data analyses. Most studies had a small population size (average 38 people per study) and were of short duration. We also detected a high risk of bias for selective outcome reporting in just under 50% of the trials. We therefore rated the overall methodological quality of the included studies as low. We considered seven main outcomes of interest: clinically important change in overall mental state, clinically important change in cognitive functioning, incidence of a clinically important adverse effect/event, clinically important change in global state, leaving the study early for any reason, clinically important change in quality of life, and hospital admission. All studies assessed the effects of adding modafinil to participants' usual antipsychotic treatment compared to adding placebo to usual antipsychotic treatment.
Six studies found that adding modafinil to antipsychotic treatment may have little or no effect on overall mental state of people with schizophrenia, specifically the risk of worsening psychosis (RR 0.91, 95% CI 0.28 to 2.98; participants = 209; studies = 6, low‐quality evidence). Regarding the effect of modafinil on cognitive function, the trials did not report clinically important change data, but one study reported endpoint scores on the MATRICS Consensus Cognitive Battery (MCCB): in this study we found no clear difference in scores between modafinil and placebo treatment groups (MD −3.10, 95% CI −10.9 to 4.7; participants = 48; studies = 1, very low‐quality evidence). Only one study (N = 35) reported adverse effect/event data. In this study one serious adverse event occurred in each group (RR 0.84, 95% CI 0.06 to 12.42; participants = 35; studies = 1, very low‐quality evidence).
One study measured change in global state using the Clinical Global Impression ‐ Improvement Scale. This study found that adding modafinil to antipsychotic treatment may have little or no effect on global state (RR 6.36, 95% CI 0.94 to 43.07, participants = 21; studies = 1, very low‐quality evidence). Nine studies found that modafinil has no effect on numbers of participants leaving the study early (RR 1.26, 95% CI 0.63 to 2.52 participants = 357; studies = 9, moderate‐quality evidence). None of the trials reported clinically important change in quality of life, but one study did report quality of life using endpoint scores on the Quality of Life Inventory, finding no clear difference between treatment groups (MD −0.2, 95% CI −1.18 to 0.78; participants = 20; studies = 1, very low‐quality evidence). Finally, one study reported data for number of participants needing hospitalisation: one participant in each group was hospitalised (RR 0.84, 95% CI 0.06 to 12.42; participants = 35; studies = 1, very low‐quality evidence).
Authors' conclusions
Due to methodological issues, low sample size, and short duration of the clinical trials as well as high risk of bias for outcome reporting, most of the evidence available for this review is of very low or low quality. For results where quality is low or very low, we are uncertain or very uncertain if the effect estimates are true effects, limiting our conclusions. Specifically, we found that modafinil is no better or worse than placebo at preventing worsening of psychosis; however, we are uncertain about this result. We have more confidence that participants receiving modafinil are no more likely to leave a trial early than participants receiving placebo. However, we are very uncertain about the remaining equivocal results between modafinil and placebo for outcomes such as improvement in global state or cognitive function, incidence of adverse events, and changes in quality of life. More high‐quality data are needed before firm conclusions regarding the effects of modafinil for people with schizophrenia or related disorders can be made.
Plain language summary
Modafinil for schizophrenia
Review question: Is adding modafinil to antipsychotic treatment effective and safe for people with schizophrenia?
Background
Schizophrenia is a complicated and chronic mental disorder that usually presents with a wide range of symptoms. The standard treatment (antipsychotics) is considered to be effective for the positive symptoms (such as delusions and hallucinations or bizarre thoughts). However, there are negative and cognitive symptoms (such as social withdrawal, lack of affect, problems with memory) that are not adequately treated with these medications. These symptoms tend to be chronic and can therefore have a long‐term impact on an individual's quality of life. Additional treatments are often added to antipsychotic treatment, of which one is modafinil, a wakefulness‐promoting medication usually used for sleep disorders.
Searching for evidence
We ran an electronic search of Cochrane Schizophrenia's register of trials in April 2015, May 2017, and October 2019 for trials that randomised (allocated participants to treatment groups using a random method) people with schizophrenia to receive add‐on modafinil (modafinil added to their standard care) or to receive add‐on placebo. We identified 67 records that referred to 25 studies.
Evidence found
Eleven studies met the review requirements and reported data that could be used in analyses. However, the trials included small numbers of participants and were of short duration; schizophrenia is a long‐term health problem that ideally requires studies of longer duration. Our analysis of the data showed there is no clear difference between add‐on modafinil and add‐on placebo for improving mental state or global state, changing cognitive functioning, causing participants to leave a study early, producing adverse effects, or affecting rates of hospitalisation. However, most of these results were based on very low‐ or low‐quality data, therefore it is uncertain if these statistical effect sizes found by our data analyses are true effects.
Conclusions
The results of this review indicate no clear difference in effectiveness and safety between add‐on modafinil and add‐on placebo, however these results are not conclusive as they are based low‐ or very low‐quality evidence. Based on the current evidence we were unable to provide an answer to our review question as to whether modafinil is better than placebo for improving the symptoms of schizophrenia, or if it is safe to use for people with schizophrenia. More high‐quality research is needed.
Summary of findings
Summary of findings for the main comparison. Modafinil compared to placebo for people with schizophrenia or related disorders.
| Modafinil compared to placebo for people with schizophrenia or related disorders | ||||||
| Patient or population: schizophrenia or related disorders Setting: inpatient and outpatient Intervention: modafinil (plus usual antipsychotic) Comparison: placebo (plus usual antipsychotic) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with modafinil | |||||
| Mental state: clinically important change in general mental state ‐ worsening psychosis Follow‐up: mean 8 weeks | Study population | RR 0.91 (0.28 to 2.98) | 209 (6 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 40 per 1000 | 36 per 1000 (11 to 119) | |||||
| Cognitive function: average endpoint score** assessed with: MATRICS Consensus Cognitive Battery Follow‐up: mean 2 weeks | The mean cognitive function: ranged from 29.7 to 32.8 points. | MD 3.1 points lower (10.9 lower to 4.7 higher) | ‐ | 48 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 3 5 | **Clinically important change data not reported by trials. Used data from short‐term trials. However, another trial reported this outcome after a single‐dose administration; the results also do not show a difference between the 2 groups. |
| Adverse effect/event(s) ‐ serious adverse events Follow‐up: mean 8 weeks | Study population | RR 0.84 (0.06 to 12.42) | 35 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 5 6 | ||
| 63 per 1000 | 53 per 1000 (4 to 776) | |||||
| Global state: clinically important change in global state assessed with: Clinical Global Impression‐Improvement scale Follow‐up: mean 8 weeks | Study population | RR 6.36 (0.94 to 43.07) | 21 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 5 6, 4 | ||
| 100 per 1000 | 636 per 1000 (94 to 1000) | |||||
| Leaving the study early ‐ for any reason Follow‐up: range 7 days to 9 weeks | Study population | RR 1.26 (0.63 to 2.53) | 357 (9 RCTs) | ⊕⊕⊕⊝ MODERATE 78 | ||
| 98 per 1000 | 123 per 1000 (62 to 247) | |||||
| Quality of life: average endpoint score** assessed with: Quality of Life Inventory Follow‐up: mean 8 weeks | The mean quality of life was 4 points. | MD 0.2 points lower (1.18 lower to 0.78 higher) | ‐ | 20 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 35 9 | **Clinically important change data not reported by trials. Lower scores indicate less quality of life. The range of possible values goes from 1 to 7. |
| Service use: hospital admission Follow‐up: mean 8 weeks | Study population | RR 0.84 (0.06 to 12.42) | 35 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 35 10 | ||
| 63 per 1000 | 53 per 1000 (4 to 776) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very certain that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately certain in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our certainty in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little certainty in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level due to indirectness. The follow‐up time frame (about eight weeks) in the trials was insufficient to properly assess this outcome. 2 Downgraded one level due to imprecision as the treatment effect was not consistent with benefit and harm. 3 Downgraded two levels due to indirectness. The trial did not have sufficient time to properly assess this outcome, continous score data not direct measure of clinically important change.
4 Downgraded one level due to indirectness: The trial did not have sufficient time to properly assess this outcome. 5 Downgraded one level due to imprecision. The treatment effect is not consistent with benefits and harms, the confidence interval is wide, and the sample size is low. 6 Downgraded one level due to risk of bias. The trial did not adequately report random sequence generation, allocation, and blinding. 7 No downgrade. Many studies had high risk of bias for selective reporting, however this did not apply to the outcome leaving the study early, which was adequately reported. Also, there was concern about one trial stopping early, but the study weight was 8.4%, and the outcome leaving the study early was adequately reported. 8 Downgraded one level due to imprecision. The treatment effect was not consistent with benefit and harms. 9 No downgrade. The trial did not adequately report random sequence generation, allocation, and blinding but had low risk for attrition and reporting bias, therefore we decided not to downgrade for risk of bias across domains. 10 Downgraded by on level due to risk of bias. The trial had unclear risk for random sequence generation, allocation, and blinding but high risk for attrition and reporting bias.
Background
Description of the condition
Schizophrenia is a severe psychotic disorder. It ranks among the top 25 illnesses responsible for the global burden of disease (Vos 2015), with a yearly incidence of 15 per 100,000 in men and 10 per 100,000 in women, and a point prevalence of 4.6 per 1000 (McGrath 2004). It is characterised by a long duration of symptoms, with onset typically beginning in early adulthood and a mean recovery rate of only 13.5% (Jääskeläinen 2012).
Like most psychiatric illnesses, it is diagnosed by the use of operationalised diagnostic criteria. Symptoms are categorised as positive (hallucinations or delusions; disorganised behaviour or speech) and negative (flat affect or poverty of speech) (APA 2013). However, impairments in cognition including attention, memory, and executive functions have also been proposed as core symptoms, despite not yet having been elevated to the level of diagnostic criteria (Kahn 2013). The neurobiology of schizophrenia is complex and is still poorly understood; classically a dysfunction of dopaminergic neurotransmission was implicated as the explanation of this condition (Howes 2009), but evidence has shown that other abnormalities in glutamate neurotransmission, abnormalities in synaptic pruning (Keshavan 1994), and oxidative stress are also involved in the disorder (Owen 2016).
Description of the intervention
Modafinil is a central nervous system wake‐promoting stimulant agent indicated for the treatment of excessive daytime sleepiness for people suffering from narcolepsy, obstructive sleep apnoea, and other sleep disorders (Bastoji 1988; Lyons 1991). Structurally, modafinil is a benzhydrylsulfinylacetamine compound and has a distant similarity to dextroamphetamine (Duteil 1979). It has a rapid rate of absorption, which is slowed if administered with food. It has a volume distribution of 0.9 L/kg and is approximately 90% metabolised by the liver (McClellan 1998; Moachon 1996). The most frequently used dosage range of modafinil is 200 to 400 mg per day (usually administered as a single dose), but pharmacokinetic studies in healthy normal participants, and randomised controlled trials for the treatment of narcolepsy found that up to 600 mg per day is well‐tolerated (Wong 1999).
Some studies have shown that modafinil increases neuronal activation in several regions of the cortex in some animals including mice, rats, and humans (Engber 1998; Ghahremani 2011; Gozzi 2012; Hunter 2006; Urbano 2007). Affected neurotransmitters throughout the brain include increased thalamic glutamate levels (Dawson 2010), noradrenaline in the prefrontal cortex (de Saint Hilaire 2001), serotonin in the dorsal raphe‐cortical system (Ferraro 2005), dopamine in the caudate nucleus (Andersen 2010), orexin in the perifornical area (Boutrel 2004), and a decreased level of GABAergic neurotransmission in the cortex, striatum, and posterior hypothalamus (Scammell 2000).
How the intervention might work
Given the proposed mechanisms of actions of modafinil, it is plausible that some of the deficits shown in people with schizophrenia might be impacted positively by the use of modafinil, as many of the neurotransmitters affected by this medication are altered in the current understanding of the biology of schizophrenia (Owen 2016). Also, clinical studies of modafinil have shown that modafinil improves mood and executive functions of healthy individuals (Battleday 2015; Randall 2003). Furthermore, modafinil has a positive impact on cognition in individuals with neuropsychiatric conditions, including schizophrenia (Turner 2004; Turner 2004a; Turner 2004b). Finally, modafinil might have a role in the treatment of the fatigue and insomnia associated with antipsychotic medication use (Makela 2003; Prasuna 2015). Modafinil therefore potentially has a role in the treatment of cognitive and negative symptoms of schizophrenia, and some of the side effects associated with antipsychotic use.
Why it is important to do this review
The lack of interventions that improve either negative symptoms or cognitive functions in people with schizophrenia is concerning. Considering the significant impact of negative symptoms on quality of life and functionality (Kirkpatrick 2006), and given that the largest randomised study of people with schizophrenia showed that an improvement in cognition was directly associated with an improved quality of life (Mohamed 2008), we considered it important to evaluate modafinil as an intervention that might have a positive impact on negative symptoms and cognition in the individuals with schizophrenia. Modafinil has shown potential for enhancing cognitive function in other populations, Turner 2004a; Turner 2004b, and has a theoretical impact on negative symptomatology (Pierre 2007).
Objectives
The primary objective of this review was to assess the effects of modafinil for people with schizophrenia or related disorders.
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant randomised controlled trials. We included trials that are described as 'double‐blind' ‐ in which randomisation is implied ‐ in a sensitivity analysis (see Sensitivity analysis). We excluded quasi‐randomised studies, such as those that allocate intervention by alternate days of the week. Where additional treatments were administered as well as modafinil, we only included data if the adjunct treatment was evenly distributed between groups and only the modafinil was randomised.
Types of participants
Adults, as defined in the trials, with schizophrenia or related disorders, including schizophreniform disorder, schizoaffective disorder, and delusional disorder, by any means of diagnosis. There is no clear evidence that the schizophrenia‐like psychoses are caused by fundamentally different disease processes or require different treatment approaches (Carpenter 1994).
We were interested in ensuring that information was as relevant as possible to the current care of people with schizophrenia, and so highlighted the current clinical state clearly (acute, early postacute, partial remission, remission), as well as the stage (prodromal, first episode, early illness, persistent), and whether the studies focused primarily on people with particular problems (e.g. negative symptoms, treatment‐resistant illnesses).
Types of interventions
1. Modafinil: any dose/administration
2. Placebo
3. Other treatments: interventions other than placebo used in the trials as comparators to modafinil
All interventions are in addition to standard care, where standard care is defined as the care patients would normally receive.
Types of outcome measures
We grouped outcomes into single dose, short term (chronic dose for up to 12 weeks), medium term (chronic dose for up to 26 weeks), and long term (chronic dose for over 26 weeks).
We endeavoured to report binary outcomes recording clear and clinically meaningful degrees of change (e.g. global impression of much improved, or more than 50% improvement on a rating scale, as defined in the trials) before any other outcomes. We thereafter listed other binary outcomes, and then continuous outcomes.
For outcomes such as 'clinically important change', 'any change', and 'relapse', we used the definition used in each of the trials.
We used data from valid scales; see Data extraction and management.
Primary outcomes
1. Mental state
1.1 Clinically important change in general mental state
2. Cognitive functioning
2.1 Clinically important change in overall cognitive functioning
3. Adverse effect/event(s); clinically important adverse effect
Secondary outcomes
1. Mental state
1.1 Average endpoint or change score general mental state scale 1.2 Clinically important change in specific (positive or negative) symptoms of schizophrenia 1.3 Average endpoint or change score on specific mental state scale
2. Cognitive functioning
2.1 Clinically important change in specific aspects of cognitive functioning (e.g. IQ, memory, learning, attention, fluency, control, executive functioning) 2.2 Average endpoint or change score on overall cognitive functioning scale 2.3 Average endpoint or change score on specific aspect of cognitive functioning scale
3. Adverse effect/event(s)
3.1 Number of participants with at least one treatment‐emergent adverse effect 3.2 Number of participants with at least one serious adverse effect 3.3 Clinically important specific adverse effects (e.g. cardiac effects, death, movement disorders, probating increase and associated effects, fatigue, sedation, insomnia, seizures, weight gain, effects on white blood cell count) 3.4 Average endpoint or change score on adverse effects scale 3.5 Death (natural or suicide)
4. Behaviour/emotional state
4.1 Clinically important change in overall behaviour 4.2 Clinically important change in specific aspects of behaviour/emotion (e.g. anxiety, aggression, mood) 4.3 Average endpoint or change score on behaviour scale
5. Global state
5.1 Relapse 5.2 Time to relapse 5.3 Clinically important change in global state 5.4 Any change in global state 5.5 Average endpoint or change score on global state scale
6. Functioning
6.1 Clinically important change in general functioning 6.2 Average endpoint or change score on general functioning scale 6.3 Clinically important change in specific aspects of functioning, such as social or life skills 6.4 Any change in specific aspects of functioning, such as social or life skills 6.5 Average endpoint or change score on specific aspects of functioning, such as social or life skills scale 6.6 Employment status (employed/unemployed)
7. Leaving the study early
7.1 For specific reason
8. Quality of life
8.1 Clinically important change in general quality of life 8.2 Average endpoint or change score on general quality of life scale
9. Service use
9.1 Hospital admission/readmission 9.2 Average number of days in hospital
10. Satisfaction with treatment
10.1 Recipient of care satisfied with treatment 10.2 Recipient of care average endpoint or change score on satisfaction scale 10.3 Carer satisfied with treatment 10.4 Carer average endpoint or change score on satisfaction scale
'Summary of findings' table
We used the GRADE approach to interpret findings (Schünemann 2011), and employed GRADEpro GDT to export data from our review and create a 'Summary of findings' table. 'Summary of findings' tables provide outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of the effect of the interventions examined, and the sum of available data on all outcomes we rate as important to patient care and decision making. We selected the following main outcomes for inclusion in the 'Summary of findings' table.
Mental state: clinically important change in general mental state
Cognitive functioning: clinically important change in overall cognitive functioning
Adverse effect/event(s): clinically important adverse effect
Global state: clinically important change in global state
Leaving the study early: for any reason
Quality of life: clinically important change in quality of life
Service use: hospital admission
If data were not available for these prespecified outcomes but were available for similar outcomes, we presented the closest outcome to the prespecified one in the table but took this into account when grading the finding.
Search methods for identification of studies
Electronic searches
Cochrane Schizophrenia Group's Study‐Based Register of Trials
On 27 April 2015, 24 May 2017, and 31 October 2019, the Information Specialist searched the register using the following search strategy:
*Modafinil* in Intervention of STUDY
In such a study‐based register, searching the major concept retrieves all the synonyms and relevant studies. This is because the studies have already been organised based on their interventions and linked to the relevant topics (Shokraneh 2017). This allows rapid and accurate searches that reduce waste in the next steps of systematic reviewing (Shokraneh 2019).
According to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2019), the Information Specialist compiles this register from systematic searches of major resources and their monthly updates (unless otherwise specified):
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library;
MEDLINE;
Embase;
Allied and Complementary Medicine (AMED);
BIOSIS;
Cumulative Index to Nursing and Allied Health Literature (CINAHL);
PsycINFO;
PubMed;
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov);
World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp);
ProQuest Dissertations and Theses Global and its quarterly update;
Chinese databases (Chinese Biomedical Literature Database, China Knowledge Resource Integrated Database, and Wanfang) and their annual updates.
The register also includes handsearches and conference proceedings (see Group's website). There are no limitations on language, date, document type, or publication status.
Searching other resources
1. Reference searching
We inspected references of all included studies for further relevant studies.
2. Personal contact
We contacted the first author of each included study for information regarding unpublished trials. We noted the outcome of this contact in the Characteristics of included studies or Characteristics of studies awaiting classification tables.
Data collection and analysis
Selection of studies
Two review authors (SAC and AOV) independently inspected citations from the searches and identified relevant abstracts; one review author (JO) independently re‐inspected a random 20% sample of these abstracts to ensure reliability of selection. Where disputes arose, we acquired the full report for more detailed scrutiny. Three review authors (SAC, AOV, and JO) then obtained and inspected full reports of the abstracts or reports meeting the review criteria. One review author (GEA) re‐inspected a random 20% of these full reports in order to ensure reliability of selection. Where it was not possible to resolve disagreement by discussion, we attempted to contact the authors of the study concerned for clarification.
Data extraction and management
1. Extraction
Review authors JO and either SAC or AOV extracted data from all included studies. In addition, to ensure reliability, one review author (GAQ) independently extracted data from a random sample of these studies comprising 10% of the total. We attempted to extract data presented only in graphs and figures whenever possible but included this information only if two review authors independently obtained the same result. If studies were multicentre, then we extracted data relevant to each where possible. Any disagreements were discussed and decisions documented. We attempted to contact authors through an open‐ended request in order to obtain missing information or for clarification where necessary. Two review authors (SCO and LEC) helped clarify issues regarding any remaining problems and final decisions were documented.
2. Management
2.1 Forms
We extracted data onto standard, pre‐designed, simple forms developed in Microsoft Word 2016 for non‐numerical data and a simple spreadsheet in Microsoft Excel 2016 for numerical data.
2.2 Scale‐derived data
We included continuous data from rating scales only if: a) the psychometric properties of the measuring instrument have been described in a peer‐reviewed journal (Marshall 2000); b) the measuring instrument has not been written or modified by one of the trialists for that particular trial; and c) the instrument was a global assessment of an area of functioning and not subscores which are not, in themselves, validated or shown to be reliable. However, there are exceptions: we included subscores from mental state scales measuring positive and negative symptoms of schizophrenia. Ideally, the measuring instrument should either be i.) a self‐report or ii.) completed by an independent rater or relative (not the therapist). We realise that this is not often reported clearly; we have noted if this was the case or not in Description of studies.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data: change data can remove a component of between‐person variability from the analysis; however, calculation of change needs two assessments (baseline and endpoint), which can be difficult to obtain in unstable and difficult‐to‐measure conditions such as schizophrenia. We decided to primarily use endpoint data, and only use change data if the former were not available. Where necessary, we combined endpoint and change data in the analysis, as we preferred to use mean differences (MDs) rather than standardised mean differences (SMDs) throughout (Higgins 2011a).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we applied the following standards to relevant continuous data before inclusion.
For endpoint data from studies including fewer than 200 participants:
a) when a scale starts from the finite number zero, we subtracted the lowest possible value from the mean, and divided this by the standard deviation (SD). A value lower than one strongly suggests that the data are skewed, and we excluded these data. A ratio higher than one but less than two suggests that the data are skewed: we entered these data and tested whether their inclusion or exclusion would change the results substantially. Finally, if the ratio was larger than two, we included these data because it is less likely that they are skewed (Altman 1996; Higgins 2011a);
b) if a scale starts from a positive value (such as the Positive and Negative Syndrome Scale (PANSS), which can have values from 30 to 210) (Kay 1986), we modified the calculation described above to take the scale starting point into account. In these cases skewed data are present if 2 SD > (S − S min), where S is the mean score and 'S min' is the minimum score.
Note: we entered all relevant data from studies of more than 200 participants in the analysis irrespective of the above rules because skewed data pose less of a problem in large studies. We also entered all relevant change data, as when continuous data are presented on a scale that includes the possibility of negative values (such as change data), it is difficult to tell whether or not data are skewed.
2.5 Common measurement
To facilitate comparison between trials, where relevant we converted variables that can be reported in different metrics, such as days in the hospital (mean days per year, per week, or per month), to a common metric (e.g. mean days per month).
2.6 Conversion of continuous to binary
Where possible, we attempted to convert outcome measures to dichotomous data. This can be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that if there is a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS), Overall 1962, or the PANSS (Kay 1986), this could be considered as a clinically significant response (Leucht 2005a; Leucht 2005b). If data based on these thresholds were not available, we used the primary cut‐off presented by the authors of the original study.
2.7 Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicates a favourable outcome for modafinil. Where adhering to this made it impossible to avoid outcome titles with clumsy double‐negatives (e.g. 'not un‐improved'), we reported data where the left of the line indicates an unfavourable outcome and noted this in the relevant graphs.
Assessment of risk of bias in included studies
Two review authors (SAC and JO) independently assessed risk of bias using the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions to evaluate trial quality (Higgins 2011b). This set of criteria is based on evidence of associations between potential overestimation of effect and the level of risk of bias of the article that could be due to aspects of sequence generation, allocation concealment, blinding, incomplete outcome data, and selective reporting, or the way in which these 'Risk of bias' domains are reported.
Where inadequate details of randomisation and other trial characteristics were provided, we attempted to contact the study authors to obtain the additional information. We reported non‐concurrence in quality assessment, but if disputes arose regarding the category to which a trial was to be allocated, we resolved this by discussion.
We noted the level of risk of bias in the text of the review and in Figure 1, Figure 2, and Table 1.
1.
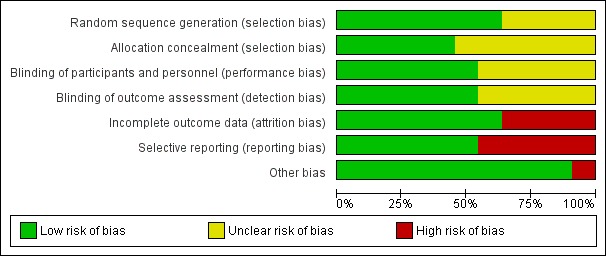
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
1. Binary data
For binary outcomes, we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI), as it has been shown that RR is more intuitive than odds ratios (Boissel 1999), and that clinicians tend to interpret odds ratios as RR (Deeks 2000). Although the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH), with their CIs, are intuitively attractive to clinicians, they are problematic to calculate and interpret in meta‐analyses (Hutton 2009). For binary data presented in the 'Summary of findings' table/s, where possible we calculated illustrative comparative risks.
2. Continuous data
For continuous outcomes, we estimated MD between groups. We preferred not to calculate effect size measures (SMD). However, if scales of very considerable similarity were used, we presumed there was a small difference in measurement, and calculated effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice), but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intraclass correlation in clustered studies, leading to a unit of analysis error whereby P values are spuriously low, CIs unduly narrow, and statistical significance overestimated (Divine 1992). This causes type I errors (Bland 1997; Gulliford 1999).
Where clustering has been incorporated into the analysis of primary studies, we presented these data as if from a non‐cluster‐randomised study, but adjusted for the clustering effect.
Where clustering was not accounted for in primary studies, we presented data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. We contacted the first authors of studies to obtain intraclass correlation coefficients (ICCs) for their clustered data and adjusted for this by using accepted methods (Gulliford 1999).
We sought statistical advice and have been advised that the binary data from cluster trials presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the ICC: thus design effect = 1 + (m − 1) * ICC (Donner 2002). If the ICC was not reported, we assumed it to be 0.1 (Ukoumunne 1999).
If cluster studies have been appropriately analysed and ICCs and relevant data documented in the report taken into account, synthesis with other studies was possible using the generic inverse‐variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. This occurs if an effect (e.g. pharmacological, physiological, or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, participants can differ significantly from their initial state at entry to the second phase, despite a wash‐out phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both carry‐over and unstable conditions are very likely in severe mental illness, we only used data from the first phase of cross‐over studies.
3. Studies with multiple treatment groups
Where a study involved more than two treatment arms, if relevant, we presented the additional treatment arms in comparisons. If data were binary, we simply added these and combined within the two‐by‐two table. If data were continuous, we combined data according to the formula for combining data in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Where additional treatment arms were not relevant, we did not reproduce these data.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). We chose that, for any particular outcome, should more than 50% of data be unaccounted for, we would not reproduce these data or use them within analyses. If, however, more than 50% of data in one arm of a study were lost, but the total loss was less than 50%, we would address this in the 'Summary of findings' table by downgrading the quality of the evidence. Finally, we would also downgrade quality in the 'Summary of findings' table if the loss was 25% to 50% in total.
2. Binary
In the case where attrition for a binary outcome was between 0% and 50% and where these data were not clearly described, we presented data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat analysis). Those participants leaving the study early were all assumed to have the same rates of negative outcome as those who completed, with the exception of the outcome of death and adverse effects. For these outcomes, the rate of those who stayed in the study ‐ in that particular arm of the trial ‐ was used for those who did not. We undertook a sensitivity analysis testing how prone the primary outcomes were to change when data only from participants who completed the study to that point were compared to the intention‐to‐treat analysis using the above assumptions.
3. Continuous
3.1 Attrition
We used data where attrition for a continuous outcome was between 0% and 50%, and data only from participants who completed the study to that point were reported.
3.2 Standard deviations
If SDs were not reported, we attempted to obtain the missing values from the authors. If this was not possible, where there were missing measures of variance for continuous data, but an exact standard error (SE) and CIs available for group means, and either P value or t value available for differences in mean, we could calculate SDs according to the rules described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). When only the SE is reported, SDs are calculated using the formula SD = SE * √(n). Detailed formulae for estimating SDs from P, t, or F values, CIs, ranges, or other statistics are presented in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). If these formulae did not apply, we calculated the SDs according to a validated imputation method that is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study's outcome and thus to lose information. Nevertheless, we examined the validity of the imputations in a sensitivity analysis that excluded imputed values.
3.3 Assumptions about participants who left the trials early or were lost to follow‐up
Various methods are available to account for participants who left the trials early or were lost to follow‐up. Some trials only present the results of study completers; others use the method of last observation carried forward (LOCF); whilst more recently, methods such as multiple imputation or mixed‐effects models for repeated measurements (MMRM) have become more of a standard. Whilst the latter methods seem to be somewhat better than LOCF (Leon 2006), we feel that the high percentage of participants leaving the studies early and differences between groups in their reasons for doing so is often the core problem in randomised schizophrenia trials. We therefore did not exclude studies based on the statistical approach used. However, by preference we used the more sophisticated approaches, that is we preferred to use MMRM or multiple imputation to LOCF, and only presented completer analyses if some kind of intention‐to‐treat data were not available at all. Moreover, we addressed this issue in the 'incomplete outcome data' domain of the 'Risk of bias' tool.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for participants who were clearly outliers or situations that we had not predicted would arise and discussed such situations or participant groups where found.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods that we had not predicted would arise and discussed any such methodological outliers.
3. Statistical heterogeneity
3.1 Visual inspection
We investigated the possibility of statistical heterogeneity by visually inspecting graphs.
3.2 Employing the I² statistic
We investigated heterogeneity between studies by considering the I² statistic alongside the Chi² P value. The I² statistic provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I² depends on the magnitude and direction of effects as well as the strength of evidence for heterogeneity (e.g. P value from Chi² test, or a confidence interval for I²). We interpreted an I² estimate greater than or equal to 50% and accompanied by a statistically significant Chi² statistic as evidence of substantial heterogeneity (Deeks 2011). When we found substantial levels of heterogeneity in the primary outcome, we explored reasons for the heterogeneity (see Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011). We are aware that funnel plots may be useful in investigating reporting biases, but that they are of limited power to detect small‐study effects. We did not use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar size. In such cases where funnel plots are possible, we will seek statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for preference of use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us, and the random‐effects model takes into account differences between studies, even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model, in that it puts added weight onto small studies, which are often the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. We chose to use a random‐effects model for all analyses.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses
1.1 Primary outcomes
We attempted to undertake subgroup analyses comparing the results for the following:
enrolment of acutely exacerbated or chronically ill patients;
treatment duration 12 weeks versus >12 weeks.
2. Investigation of heterogeneity
We reported if inconsistency was high. Firstly, we investigated whether data had been entered correctly. Secondly, if the data were correct, we inspected the graph visually and removed outlying studies successively to see if homogeneity was restored. We decided for this review that should this occur with data contributing to the summary finding of no more than 10% of the total weighting, we would present data. If not, we would not pool these data and would discuss any issues. We know of no supporting research for this 10% cut‐off, but are investigating use of prediction intervals as an alternative to this unsatisfactory state.
When unanticipated clinical or methodological heterogeneity was obvious, we simply stated hypotheses regarding this for future reviews or versions of this review. We did not anticipate undertaking analyses relating to this.
Sensitivity analysis
1. Implication of randomisation
We included trials in a sensitivity analysis if they were described in some way that implies randomisation. For primary outcomes, if the inclusion of these trials did not result in a substantive difference, the trials remained in the analyses. If their inclusion did result in statistically significant differences, we did not add the data from these lower‐quality studies to the results of the higher‐quality trials, but presented these data within a subcategory.
2. Assumptions for lost binary data
Where assumptions needed to be made regarding participants lost to follow‐up (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumption compared with completer data only. If there was a substantial difference, we reported the results and discussed them, but continued to employ our assumption.
Where assumptions needed to be made regarding missing SD data (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumption compared with completer data only. We undertook a sensitivity analysis to test how prone results were to change when completer data only were compared to the imputed data using the above assumption. If there was a substantial difference, we reported the results and discussed them, but continued to employ our assumption.
3. Risk of bias
We analysed the effects of excluding trials judged to be at high risk of bias across one or more of the 'Risk of bias' domains (implied as randomised with no further details available, allocation concealment, blinding, and outcome reporting) for the meta‐analysis of the primary outcome. If the exclusion of trials at high risk of bias did not alter the direction of effect or the precision of the effect estimates substantially, then we included relevant data from these trials.
4. Imputed values
We undertook a sensitivity analysis to assess the effects of including data from trials where we used imputed values for ICC in calculating the design effect in cluster‐randomised trials.
If we noted substantial differences in the direction or precision of effect estimates in any of the sensitivity analyses listed above, we did not pool data from the excluded trials with the other trials contributing to the outcome, but presented them separately.
5. Fixed‐effect and random‐effects
We synthesised data using a random‐effects model; however, we also synthesised data for the primary outcome using a fixed‐effect model in this line in order to evaluate whether this altered the significance of the results.
Results
Description of studies
For detailed descriptions of the studies, see Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The initial search in 2015 identified 61 records, and the updated search in 2017 yielded five further records. We found another reference during the process of writing the review (Lees 2017), as it was not indexed at the time of the search. The 67 records corresponded to 25 different studies; we excluded two of these records after reading the title and abstract, leaving 65 records for 23 studies that appeared to be relevant. We retrieved and inspected the 65 full‐text articles and excluded 12 studies with 32 references with reasons. There were no ongoing studies (Figure 3). We included 11 studies (33 references) in the review.
3.
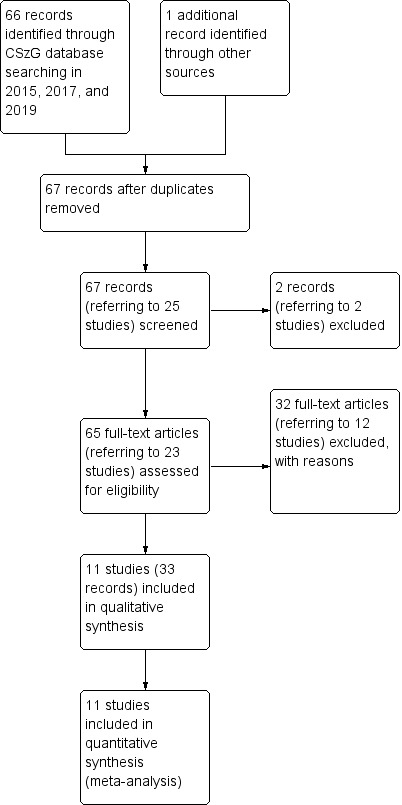
Study flow diagram.
Included studies
We identified 11 studies (33 references) for inclusion in the review.
1. Study design
Most studies used a parallel‐group design, but Lees 2017 and Spence 2005 were cross‐over studies. We used only the results of the first phase for the cross‐over studies, as specified in Unit of analysis issues.
2. Length of trials
All trials were short term as they lasted 12 weeks or less (average duration ˜7 weeks). Prasuna 2015 was the longest study, with a duration of 12 weeks, and Spence 2005 was the shortest, lasting one week.
3. Participants
The 11 included studies involved a total of 422 participants (range 19 to 72; average ˜38 people per study). Most studies included people with schizophrenia, delusional disorder, and schizoaffective psychoses. However, Michalopoulou 2015 also included healthy volunteers, but we only used the data from the participants with schizophrenia. Prasuna 2015 also included individuals with affective psychoses, but we decided to include this study as most of the participants were relevant to our review (see Differences between protocol and review). All of the studies used operational criteria for the diagnosis with the exception of Prasuna 2015, which included participants “taking antipsychotics”, but did not explain the method of the diagnosis.
Some studies examined specific groups of participants. Kumar 2010 studied participants with troublesome drowsiness or hyper‐salivation, and Pierre 2007, Shafti 2016, and Spence 2005 studied participants with prominent negative symptoms.
4. Intervention
All of the studies compared oral modafinil with placebo. Two studies used a single dose of modafinil (Pierre 2007; Spence 2005). The most commonly used dose was 200 mg (5 of 11 studies); only Freudenreich 2009 used higher doses of modafinil (mean dose of 250 mg/day of modafinil at the end of the study). Participants in all of the included studies were also receiving a treatment with another antipsychotic.
5. Settings
Three studies included inpatients, with Spence 2005 only admitting participants to the hospital for surveillance during the 24 hours after administration of the medication. Seven studies included outpatients. The setting in Prasuna 2015 was unclear.
6. Outcomes
A variety of scales were used to assess mental state, cognitive function, global effect, quality of life, and adverse events. The reporting of many of these was poor. We contacted the study authors to obtain more information (see Appendix 1); only the authors of Lees 2017 and Spence 2005 responded to our request and provided the necessary data.
6.1 Outcome scales
We have only presented details of scales that provided usable data for the analyses below. The reasons for not including data provided by other instruments can be found under 'outcomes' in the Characteristics of included studies.
Mental state
i. Brief Psychiatric Rating Scale (BPRS) (Overall 1962)
This is used to assess the severity of abnormal mental state. The original scale has 16 items, but a revised 18‐item scale is commonly used. Each item is defined on a seven‐point scale varying from 'not present' to 'extremely severe', scoring from zero to six or one to seven. Scores can range from zero to 126, with high scores indicating more severe symptoms.
ii. Positive and Negative Syndrome Scale (PANSS) (Kay 1987)
This schizophrenia scale has 30 items, each of which can be defined on a seven‐point scoring system varying from one ‐ absent to seven ‐ extreme. This scale can be divided into three subscales for measuring the severity of general psychopathology, positive symptoms (PANSS‐P), and negative symptoms (PANSS‐N). A high score indicates greater severity. iii. Scale for the Assessment of Positive Symptoms (SAPS) (Andreasen 1984)
This six‐point scale provides a global rating of positive symptoms such as delusions, hallucinations, and disordered thinking. Higher scores indicate more symptoms.
iv. Scale for the Assessment of Negative Symptoms (SANS) (Andreasen 1983)
This scale permits a global rating of the following negative symptoms: alogia (impoverished thinking), affective blunting, avolition‐apathy, anhedonia‐asociality, and attention impairment. Assessments are made on a six‐point scale from zero (not at all) to five (severe). Higher scores indicate more symptoms.
Cognitive function
i. MATRICS Consensus Cognitive Battery (MCCB) (Nuechterlein 2008)
The MCCB is a battery of tests developed by the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative. It measures multiple domains: speed of processing, attention/vigilance, working memory, verbal learning, reasoning and problem solving, and social cognition. It also calculates an overall composite score for all 10 tests. Higher scores indicate better cognition.
ii. The Cambridge Neuropsychological Test Automated Battery (CANTAB) (Robbins 1994)
The CANTAB is administered in a touch‐sensitive screen. The battery is composed of 22 tests that measure distinct areas of cognitive function such as visual memory, visual attention, learning and memory, working memory, planning, set‐shifting, sustained attention, and fluid intelligence. The scoring depends on the type of test that was performed. It measures multiple cognitive domains with different tests: Intra‐Extra Dimensional Set Shift (IED), One Touch Stockings of Cambridge (OTS), Motor Screening Task (MOT), Rapid Visual Information Processing (RVP), Reaction Time (RTI), Verbal Recognition Memory (VRM), Paired Associates Learning (PAL), and Spatial Working Memory (SWM). It does not have an overall score; scoring depends on the particular test.
iii. Controlled Word Association Test (COWAT) (Benton 1994)
This test evaluates verbal fluency. Participants are given one letter (C, F, L) and are asked to name the most number of words beginning with this letter in 1 minute. Higher scores indicate more fluidity.
iv. Degraded Stimulus‐Continuous Performance test (DSCP‐T) (Nuechterlein 1986)
This task evaluates attention and visual vigilance. Subjects are presented with a series of stimuli (numbers or letters) and are asked to discriminate the target stimuli from other distracting stimuli by pressing a button. Higher scores indicate more attention and visual vigilance.
v. Oculomotor Delayed Response Test (ODRT) (Goldman‐Rakic 1998)
In this task, subjects fixate on a central stimulus whilst a target appears unpredictably in the peripheral space, memorising the location of the target, and after a varied delay period, the fixation cue disappears, which cues the participant to make an eye movement to the location at which the target had appeared. The response measures accuracy, response time, hemifield errors, and perseverative errors. Higher ratings are associated with better overall working memory.
vi. Continuous Performance Test, Identical Pairs Version (CPT‐IP) (Cornblatt 1988)
This test is a measure of visual sustained attention. It involves monitoring responses to certain stimuli (numbers) as they are presented briefly one at a time. Higher scores indicate better attention.
vii. Rey Auditory Verbal Learning Test (RAVL) (Lezak 1995)
This test evaluates a wide diversity of functions: short‐term auditory‐verbal memory, rate of learning, learning strategies, retroactive and proactive interference, presence of confabulation of confusion in memory processes, retention of information, and differences between learning and retrieval. Different summary scores are derived from raw RAVL scores. Higher scores are related to better cognitive functioning.
Adverse event
i. Simpson Angus Scale (SAS) (Simpson 1970)
This scale contains 10 items: gait, arm dropping, shoulder shaking, elbow rigidity, wrist rigidity, leg pendulousness, head dropping, glabella tap, tremor, and salivation. Each item is rated between zero and four. A total score is obtained by adding the items and dividing by 10. Scores of up to 0.3 are considered within the normal range. Higher scores indicate greater severity.
Global state
i. Clinical Global Impression scale (CGI) (Guy 1976)
This scale is used to assess both the severity of illness and clinical improvement, by comparing the conditions of the person standardised against other people with the same diagnosis. A seven‐point scoring system is usually used, with low scores showing decreased severity or overall improvement, or both. CGI‐Severity (CGI‐S) is a component of the CGI that rates illness severity, and CGI‐Improvement (CGI‐I) rates improvement. High scores indicate a worse outcome.
Behaviour
i. Nurses' Observation Scale for Inpatient Evaluation (NOSIE) (Honigfeld 1965)
This is an 80‐item scale with items rated on a five‐point scale from zero (not present) to four (always present). Ratings are based on behaviour over the previous three days. The seven headings are social competence, social interest, personal neatness, co‐operation, irritability, manifest psychosis, and psychotic depression. The total score ranges from zero to 320, with high scores indicating a poor outcome.
Quality of life
i. Quality of Life Interview (QOLI) (Lehman 1988)
The QOLI is a scale administered by an interviewer that was developed for individuals with severe mental illness. It assesses the subjective experience and the objective characteristics of patients in eight life domains: living situation, daily activities and functioning, family relations, social relations, finances, work and school, legal and safety issues, and health. Higher scores indicate better quality of life; the range of possible values go from 1 to 7.
ii. Psychological General Well‐Being Index (PGWBI) (Dupuy 1984)
The PGWBI sets aside the evaluation of physical well‐being and focuses on a self‐reported evaluation of people well‐being in six dimensions: anxiety, depressed mood, positive well‐being, self‐control, general health, and vitality. It consists of 22 items with six point response scale, representing intensity and frequency.
Excluded studies
We excluded 12 studies with 32 references from this review (Characteristics of excluded studies).
Four trials used armodafinil as the intervention (Kane 2008; NCT00373672; NCT00487942; NCT00772005). Another four studies reported imaging outcomes or cumulative activity (Minzenberg 2007; Minzenberg 2010b; NCT00057707; NCT00423943), which were not the focus of this review. Minzenberg 2010a had no control group; randomisation was performed to the dose and not to the participants. Leblanc 2006 was a cross‐over clinical trial; the author provided data but the information was not usable. Scoriels 2011 and Turner 2004 were also cross‐over clinical trials, which did not report data for the first phase of the trial, making the data unusable for our review; we attempted to contact the study authors during the writing of this review but have not received a response.
Ongoing studies
We are not aware of any ongoing study.
Risk of bias in included studies
We performed a 'Risk of bias' assessment for each trial. Our judgements regarding the overall risk of bias in individual studies are illustrated in Figure 1 and Figure 2.
Allocation
All included studies were reported to be randomised. Freudenreich 2009, Pierre 2007, Sevy 2005, and Shafti 2016 did not report a specific method used for randomisation, therefore we classified these studies as having an unclear risk of bias for random sequence generation. Six studies did not report the method of concealment and were classified as at unclear risk of bias for allocation concealment.
Blinding
All studies were reported to be double‐blind. If the study was described as 'double‐blind' but no other information was provided, we assessed the trial as at unclear risk of bias. We judged five studies as at unclear risk of both performance bias and detection bias (Freudenreich 2009; Lohr 2013; Pierre 2007; Sevy 2005; Spence 2005). The remaining studies described the method of blinding and were classified as at low risk of bias.
Incomplete outcome data
We rated seven studies as at low risk of attrition bias, due either to adequate analysis of the data or no participants lost during the trial. We assessed four studies as at high risk of attrition bias. Freudenreich 2009 lost 33% of the participants in the control group, and Kumar 2010 did not address the type of analyses that were undertaken for participants who left early, and reasons for leaving early were imbalanced between groups. Lees 2017 did not report the groups to which the participants who left early belonged, and Prasuna 2015 did not report the type of analysis undertaken with regard to their missing data.
Selective reporting
Six studies were free from selective reporting. Five studies had a high risk of bias for selective reporting, with five of them reporting continuous data poorly or in methods not imputable in the meta‐analysis.
Other potential sources of bias
We identified no other potential sources of bias in 10 of the 11 included studies. We judged Kumar 2010 as at high risk of bias for this domain because it was stopped early and did not meet the target sample.
Effects of interventions
See: Table 1
1. COMPARISON 1: MODAFINIL (+ usual antipsychotic) versus PLACEBO (+ usual antipsychotic) ‐ all short‐term data
1.1 Mental state: 1a. Overall: clinically important change ‐ worsening psychosis
Six trials reported 'worsening psychosis', but no specific definition of worsening psychosis was given by any of the trials. Overall, 3.8% of participants in the trials experienced an event of worsening psychosis, but there was no clear difference between the modafinil and placebo groups for number of participants experiencing an episode of worsening psychosis (risk ratio (RR) 0.91, 95% confidence interval (CI) 0.28 to 2.98; participants = 209; studies = 6, Analysis 1.1; low‐quality evidence).
1.1. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 1 Mental state: 1a. Overall: clinically important change ‐ worsening psychosis.
1.2 Mental state: 1b. Overall: average total score (BPRS, endpoint, high = poor)
Two studies measured average endpoint scores on the BPRS (Analysis 1.2). There was no clear difference between modafinil and placebo groups (mean difference (MD) −0.66, 95% CI −5.65 to 4.32; participants = 40; studies = 2). This outcome had important levels of heterogeneity (I² = 56%). There was no clear difference between groups in either study; the heterogeneous results might be explained by the different population recruited in Pierre 2007, which had prominent negative symptoms.
1.2. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 2 Mental state: 1b. Overall: average total score (BPRS, endpoint, high = poor).
1.3 Mental state: 1c. Overall: average total score (PANSS, endpoint, high = poor)
One study measure average endpoint scores on the PANSS (Analysis 1.3). There was no clear difference between groups (MD 2.20, 95% CI −9.56 to 13.96; participants = 24; studies = 1).
1.3. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 3 Mental state: 1c. Overall: average total score (PANSS, endpoint, high = poor).
1.4 Mental state: 1d. Overall: average total score (PANSS, endpoint, high = poor, skewed data)
These continuous data (2 trials, N = 83) had such large SDs as to suggest that analysis would be inadvisable. We have therefore reported these data as 'other data' (Analysis 1.4).
1.4. Analysis.
Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 4 Mental state: 1d. Overall: average total score (PANSS, endpoint, high = poor, skewed data).
| Mental state: 1d. Overall: average total score (PANSS, endpoint, high = poor, skewed data) | ||||||
|---|---|---|---|---|---|---|
| Study | Mean (Modafinil group) | SD (Modafinil group) | N (Modafinil Group) | Mean (Placebo group) | SD (Placebo group) | N (Placebo group) |
| Arbabi 2012 | 56.47 | 13.47 | 22 | 66.52 | 10.7 | 20 |
| Michalopoulou 2015 | 55.2 | 11.9 | 19 | 53.3 | 13.3 | 22 |
1.5 Mental state: 2a. Specific: positive symptoms: i. average score (SAPS, endpoint, high = poor)
One study reported positive symptoms using SAPS (Analysis 1.5). There was no clear difference between groups (MD 1.45, 95% CI −1.38 to 4.28; participants = 50; studies = 1) .
1.5. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 5 Mental state: 2a. Specific: positive symptoms: i. average score ‐ short term (SAPS, endpoint, high = poor).
1.6 Mental state: 2b. Specific: positive symptoms: ii. average score (PANSS, endpoint, high = poor, skewed data)
Continuous data from three trials were skewed.We have therefore reported these data as 'other data' (Analysis 1.6).
1.6. Analysis.
Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 6 Mental state: 2b. Specific: positive symptoms: ii. average score ‐ short term (PANSS scale, endpoint, high = poor, skewed data).
| Mental state: 2b. Specific: positive symptoms: ii. average score ‐ short term (PANSS scale, endpoint, high = poor, skewed data) | ||||||
|---|---|---|---|---|---|---|
| Study | Mean (Modafinil group) | SD (Modafinil group) | N (Modafinil Group) | Mean (Placebo group) | SD (Placebo group) | N (Placebo group) |
| Arbabi 2012 | 11.86 | 5.77 | 22 | 14.34 | 4.54 | 20 |
| Lohr 2013 | 15.7 | 4.8 | 12 | 14.5 | 4.8 | 12 |
| Michalopoulou 2015 | 12.1 | 3.3 | 19 | 12.5 | 4.7 | 22 |
1.7 Mental state: 3a. Specific: negative symptoms ‐ clinically important change (improvement)
One study reported clinically important change (improvement) in negative symptoms (Analysis 1.7). The study defined improvement as a reduction of more than 20% in the severity of the SANS. There was a clear difference favouring modafinil between groups (RR 2.33, 95% CI 1.07 to 5.09; participants = 50; studies = 1).
1.7. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 7 Mental state: 3a. Specific: negative symptoms ‐ clinically important change (improvement) (> 20% reduction SANS).
1.8 Mental state: 3b. Specific: negative symptoms: i. average score (PANSS, endpoint, high = poor)
One study reported negative symptoms using average endpoint scores on the PANSS (Analysis 1.8). There was no clear difference between groups (MD −1.30, 95% CI −5.15 to 2.55; participants = 24; studies = 1).
1.8. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 8 Mental state: 3b. Specific: negative symptoms: i. average score ‐ short term (PANSS, endpoint, high = poor).
1.9 Mental state: 3b. Specific: negative symptoms: ii. average score (SANS scale, endpoint, high = poor)
One study reported negative symptoms using average endpoint scores on the SANS (Analysis 1.9). There was no clear difference between groups (MD −2.01, 95% CI −4.23 to 0.21; participants = 70; studies = 2).
1.9. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 9 Mental state: 3c. Specific: negative symptoms: ii. average score (SANS, endpoint, high = poor).
1.10 Mental state: 3c. Specific: negative symptoms: iii. average score (PANSS, endpoint, high = poor, skewed data)
These continuous data were skewed. We have therefore reported these data as 'other data' (Analysis 1.10).
1.10. Analysis.
Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 10 Mental state: 3d. Specific: negative symptoms: iii. average score ‐ short term (PANSS, endpoint, high = poor, skewed data).
| Mental state: 3d. Specific: negative symptoms: iii. average score ‐ short term (PANSS, endpoint, high = poor, skewed data) | ||||||
|---|---|---|---|---|---|---|
| Study | Mean (Modafinil group) | SD (Modafinil group) | N (Modafinil Group) | Mean (Placebo group) | SD (Placebo group) | N (Placebo group) |
| Arbabi 2012 | 15.13 | 4.3 | 22 | 18.73 | 4.11 | 22 |
1.11 Cognitive function: 1a. Overall: average score: i. single dose (MCCB composite score, endpoint, high = good)
One study reported overall cognitive functioning using MCCB composite scores (Analysis 1.11). There was no clear difference between groups (MD 7.93, 95% CI −0.57 to 16.43; participants = 40; studies = 1).
1.11. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 11 Cognitive function: 1a. Overall: average score: i. single dose (MCCB composite score, endpoint, high = good).
1.12 Cognitive function: 1b. Overall: average score: ii. short term (MCCB composite score, endpoint, high = good)
One study reported overall cognitive functioning using MCCB composite scores (Analysis 1.12). There was no clear difference between groups (MD −3.10, 95% CI −10.90 to 4.70; participants = 48; studies = 1).
1.12. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 12 Cognitive function: 1b. Overall: average score: ii. short term (MCCB composite score, endpoint, high = good).
1.13 Adverse event/effect(s): 1a. General: any adverse event
One study reported incidence of any adverse event. There was no clear difference between modafinil and placebo groups in the short term (RR 1.00, 95% CI 0.92 to 1.09; participants = 46; studies = 1, Analysis 1.13).
1.13. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 13 Adverse event/effect(s): 1a. General: any adverse event.
1.14 Adverse event/effect(s): 1b. General: any serious adverse events
A single study (N = 35) reported this outcome. There was no clear difference between modafinil and placebo groups (RR 0.84, 95% CI 0.06 to 12.42, Analysis 1.14, very low‐quality evidence).
1.14. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 14 Adverse event/effect(s): 1b. General: any serious adverse event.
1.15 Adverse event/effect(s): 2. Specific ‐ cardiovascular
One study reported cardiovascular effects (Analysis 1.15).
1.15. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 15 Adverse event/effect(s): 2. Specific ‐ cardiovascular.
1.15.1 Chest pain
There was no clear difference between modafinil and placebo groups for reports of chest pain (RR 1.00, 95% CI 0.07 to 15.08; participants = 48; studies = 1).
1.15.2 Hypertension
There was no clear difference between modafinil and placebo groups for reports of hypertension (RR 3.00, 95% CI 0.13 to 70.16; participants = 48; studies = 1).
1.15.3 Palpitations
There was no clear difference between modafinil and placebo groups for reports of palpitations (RR 0.33, 95% CI 0.01 to 7.80; participants = 48; studies = 1).
1.15.4 Tachycardia
There was no clear difference between modafinil and placebo groups for reports of tachycardia (RR 0.33, 95% CI 0.01 to 7.80; participants = 48; studies = 1).
1.16 Adverse event/effect(s): 3. Specific ‐ gastrointestinal
Four studies reported data for this outcome. There were no clear differences between modafinil and placebo groups for any of the gastrointestinal adverse event/effect(s) reported below (Analysis 1.16).
1.16. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 16 Adverse event/effect(s): 3. Specific ‐ gastrointestinal.
1.16.1 Abdominal pain
(RR 0.42, 95% CI 0.04 to 4.23; participants = 35; studies = 1).
1.16.2 Bitter taste
(RR 3.00, 95% CI 0.14 to 65.90; participants = 20; studies = 1).
1.16.3 Constipation
(RR 0.33, 95% CI 0.01 to 7.80; participants = 48; studies = 1).
1.16.4 Diarrhoea
(RR 2.00, 95% CI 0.19 to 20.61; participants = 48; studies = 1).
1.16.5 Dyspepsia
(RR 2.00, 95% CI 0.19 to 20.61; participants = 48; studies = 1).
1.16.6 Nausea
(RR 1.14, 95% CI 0.48 to 2.73; participants = 94; studies = 2).
1.17 Adverse event/effect(s): 4. Specific ‐ infectious
One study reported data for this outcome. Again, there were no clear differences between treatment groups for any of the infectious adverse event/effect(s) reported below (Analysis 1.17).
1.17. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 17 Adverse event/effect(s): 4. Specific ‐ infectious.
1.17.1 Flu syndrome
(RR 4.00, 95% CI 0.48 to 33.22; participants = 48; studies = 1).
1.17.2 Pharyngitis
(RR 3.00, 95% CI 0.13 to 70.16; participants = 48; studies = 1).
1.17.3 Rhinitis
(RR 0.75, 95% CI 0.19 to 3.00; participants = 48; studies = 1).
1.18 Adverse event/effect(s): 5. Specific ‐ movement disorder ‐ average scores (SAS, endpoint, high = poor, skewed data)
These continuous data were skewed and are presented as 'other data' (Analysis 1.18).
1.18. Analysis.
Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 18 Adverse event/effect(s): 5. Specific ‐ movement disorder ‐ average score (SAS, endpoint, high = poor, skewed data).
| Adverse event/effect(s): 5. Specific ‐ movement disorder ‐ average score (SAS, endpoint, high = poor, skewed data) | ||||||
|---|---|---|---|---|---|---|
| Study | Mean (Modafinil group) | SD (Modafinil group) | N (Modafinil Group) | Mean (Placebo group) | SD (Placebo group) | N (Placebo group) |
| Lohr 2013 | 4.1 | 3.46 | 12 | 5.3 | 4.5 | 12 |
| Sevy 2005 | 0.3 | 0.7 | 10 | 0.6 | 1.1 | 10 |
| Shafti 2016 | 5.71 | 3.39 | 25 | 5.98 | 4.01 | 25 |
1.19 Adverse event/effect(s): 6. Specific ‐ musculoskeletal
One study reported musculoskeletal effects and found no clear difference between modafinil and placebo groups (Analysis 1.19).
1.19. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 19 Adverse event/effect(s): 6. Specific ‐ musculoskeletal.
1.19.1 Back pain
(RR 2.00, 95% CI 0.19 to 20.61; participants = 48; studies = 1).
1.20 Adverse event/effect(s): 7. Specific ‐ neurological
Several trials reported this outcome and found no clear difference between modafinil and placebo groups for any of the neurological adverse event/effect(s) reported below (Analysis 1.20).
1.20. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 20 Adverse event/effect(s): 7. Specific ‐ neurological.
1.20.1 Dizziness
(RR 1.41, 95% CI 0.65 to 3.05; participants = 129; studies = 3).
1.20.2 Fatigue
(RR 5.95, 95% CI 0.33 to 107.25; participants = 35; studies = 1).
1.20.3 Headache
(RR 2.11, 95% CI 0.97 to 4.62; participants = 129; studies = 3).
1.20.4 Sedation
(RR 0.60, 95% CI 0.16 to 2.22; participants = 46; studies = 1).
1.20.5 Tinnitus
(RR 3.00, 95% CI 0.14 to 65.90; participants = 20; studies = 1).
1.20.6 Tremor
(RR 2.00, 95% CI 0.41 to 9.87; participants = 46; studies = 1).
1.21 Adverse event/effect(s): 8. Specific ‐ psychiatric
Three trials reported psychiatric adverse effects and found no clear difference between modafinil and placebo groups for any of the adverse effects reported below (Analysis 1.21).
1.21. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 21 Adverse event/effect(s): 8. Specific ‐ psychiatric.
1.21.1 Anxiety
(RR 2.23, 95% CI 0.54 to 9.26; participants = 82; studies = 2).
1.21.2 Depression
(RR 1.68, 95% CI 0.17 to 16.91; participants = 35; studies = 1).
1.21.3 Insomnia
(RR 1.16, 95% CI 0.49 to 2.74; participants = 114; studies = 3).
1.21.4 Irritability
(RR 0.20, 95% CI 0.01 to 3.70; participants = 20; studies = 1).
1.21.5 Nervousness
(RR 9.00, 95% CI 0.51 to 158.52; participants = 48; studies = 1).
1.22 Adverse event/effect(s): 9. Specific ‐ various other effects
Four studies reported the incidence of various adverse effects and found no clear differences between groups for any of the adverse event/effect(s) reported below (Analysis 1.22).
1.22. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 22 Adverse event/effect(s): 9. Specific ‐ various other effects.
1.22.1 Anorexia
(RR 3.00, 95% CI 0.13 to 70.16; participants = 48; studies = 1).
1.22.2 Oedema
(RR 3.00, 95% CI 0.14 to 65.90; participants = 20; studies = 1).
1.22.3 Sexual dysfunction
(RR 1.00, 95% CI 0.15 to 6.51; participants = 46; studies = 1).
1.22.4 Weight gain
(RR 0.50, 95% CI 0.10 to 2.47; participants = 46; studies = 1).
1.23 Adverse event/effect(s): 10. Specific ‐ sleep ‐ average hours of sleep at endpoint
There was no clear difference in average hours slept by endpoint of trial between groups (MD 0.80, 95% CI −0.03 to 1.63; participants = 70; studies = 1, Analysis 1.23).
1.23. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 23 Adverse event/effect(s): 10. Specific ‐ sleep ‐ average hours of sleep at endpoint.
1.24 Adverse event/effect(s): 11. Specific ‐ weight ‐ average weight in kilograms at endpoint
There was no clear difference in the average weight (kg) of participants at endpoint between groups (MD 4.63, 95% CI −9.75 to 19.01; participants = 20; studies = 1, Analysis 1.24).
1.24. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 24 Adverse event/effect(s): 11. Specific: weight ‐ average weight in kilograms at endpoint.
1.25 Adverse event/effect(s): 12a. Specific: fatigue: i. average scores (CGI‐I, endpoint, high = poor, skewed data)
These continuous data from a single trial had such large SDs as to suggest that analysis within Review Manager 5 would be inadvisable. We have therefore presented these data as 'other data' (Analysis 1.25).
1.25. Analysis.
Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 25 Adverse event/effect(s): 12a. Specific: fatigue: i. average scores (CGI‐I, endpoint, high = poor, skewed data).
| Adverse event/effect(s): 12a. Specific: fatigue: i. average scores (CGI‐I, endpoint, high = poor, skewed data) | ||||||
|---|---|---|---|---|---|---|
| Study | Mean (Modafinil group) | SD (Modafinil group) | N (Modafinil Group) | Mean (Placebo group) | SD (Placebo group) | N (Placebo group) |
| Sevy 2005 | 3.5 | 0.7 | 10 | 3.3 | 1.2 | 10 |
1.26 Adverse events: 12b. Specific: fatigue : ii. average scores (FSS, endpoint, high = poor, skewed data)
These continuous data were skewed and are presented as 'other data' (Analysis 1.26).
1.26. Analysis.
Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 26 Adverse event/effect(s): 12b. Specific: fatigue: ii. average scores (FSS, endpoint, high = poor, skewed data).
| Adverse event/effect(s): 12b. Specific: fatigue: ii. average scores (FSS, endpoint, high = poor, skewed data) | ||||||
|---|---|---|---|---|---|---|
| Study | Mean (Modafinil group) | SD (Modafinil group) | N (Modafinil Group) | Mean (Placebo group) | SD (Placebo group) | N (Placebo group) |
| Sevy 2005 | 32.3 | 7.2 | 10 | 34.9 | 14.2 | 10 |
1.27 Behaviour: average total score (NOSIE, endpoint, high = poor)
We identified one study relevant to this outcome (N = 50). There was no clear difference between modafinil and placebo groups (MD −1.72, 95% CI −7.25 to 3.81, Analysis 1.27).
1.27. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 27 Behaviour: average total score (NOSIE, endpoint, high = poor).
1.28 Global state: 1. Clinically important change in global state ‐ no improvement (CGI‐I = 4)
One study reported improvement in global state, in Pierre 2007 a participant was considered not clinically improved their CGI‐I score was 4 or less. There was no clear difference between groups for this outcome (RR 6.36, 95% CI 0.94 to 43.07; participants = 21; studies = 1, Analysis 1.28; very low‐quality evidence).
1.28. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 28 Global state: 1. Clinically important change in global state ‐ no improvement (CGI = 4).
1.29 Global state: 2. Relapse ‐ single dose
Spence 2005 reported relapse data. In this trial, a participant relapsed four days after administration of modafinil, but a causality for the use of modafinil could not be established. Analysis showed there was no clear difference in number of participants relapsing in the modafinil and placebo groups (RR 2.70, 95% CI 0.13 to 58.24; participants = 17; studies = 1, Analysis 1.29).
1.29. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 29 Global state: 2. Relapse ‐ single dose.
1.30 Global state: 3a. Average total score (CGI‐I scale, endpoint, high = poor)
Two studies reported endpoint CGI‐I scores. There was no clear difference between treatment groups. (MD ‐0.46, 95% CI ‐1.34 to 0.42; participants = 40; studies = 2, Analysis 1.30). Heterogeneity was high for this outcome (I² = 80%).
1.30. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 30 Global state: 3a. Average total score (CGI‐I scale, endpoint, high = poor).
1.31 Global state: 3b. Average total score (CGI‐S scale, endpoint, high = poor)
Two studies reported endpoint CGI‐S scores. There was no clear difference between treatment groups. (MD ‐0.24, 95% CI ‐0.63 to 0.15; participants = 70; studies = 2, Analysis 1.31).
1.31. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 31 Global state: 3b. Average total score (CGI‐S scale, endpoint, high = poor).
1.32 Leaving the study early: 1a. Any reason
Nine studies reported leaving the study early. There was no clear difference between groups (RR 1.26, 95% CI 0.63 to 2.52; participants = 357; studies = 9, Analysis 1.32,moderate‐quality evidence). Overall, 11.2% of participants did not complete the studies. Shafti 2016 was the only study in which no participants left the study early; this trial was completed in an inpatient setting.
1.32. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 32 Leaving the study early: 1a. Any reason.
1.33 Leaving the study early: 1b. Any reason ‐ single dose
There was no clear difference between groups for this outcome (RR 2.70, 95% CI 0.13 to 58.24; participants = 17; studies = 1, Analysis 1.33).
1.33. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 33 Leaving the study early: 1b. Any reason ‐ single dose.
1.34 Leaving the study early: 2. Due to adverse event
Two studies reported this outcome. There was no clear difference between modafinil and placebo groups (RR 1.08, 95% CI 0.05 to 22.20; participants = 109; studies = 2, Analysis 1.34). Heterogeneity was high for this outcome (I² = 64%), which might be explained by the different setting and type of patient: one study took place in a highly specialised clozapine clinic in the USA (Freudenreich 2009), whilst the other study was conducted in India and included people taking antipsychotics regardless of the diagnosis (Prasuna 2015). The five events occurring in the modafinil group were in Prasuna 2015: three participants discontinued the study due to headache and two participants due to insomnia.
1.34. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 34 Leaving the study early: 2. Due to adverse event.
1.35 Quality of life: 1a. General: i. average total score (QOLI, endpoint, high = good)
We found a single study (N = 20) relevant to this outcome. There was no clear difference between modafinil and placebo groups (MD −0.2, 95% CI −1.18 to 0.78, Analysis 1.35).
1.35. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 35 Quality of life: 1a. General: i. average total score (QOLI, endpoint, high = good).
1.36 Quality of life: 1b. General: ii. average total score (PGWBI, endpoint, high = good)
We identified one study relevant to this outcome (N = 50). There was no clear difference between modafinil and placebo groups (MD −0.68, 95% CI −5.52 to 4.16, Analysis 1.36).
1.36. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 36 Quality of life: 1b. General: ii. average total score (PGWBI, endpoint, high = good).
1.37 Service utilisation: hospital admission
Freudenreich 2009 (N = 35) reported one hospital admission for a participant in each group. The participant in the modafinil group was hospitalised in a psychiatric facility, whilst the participant in the placebo group had surgery due to appendicitis. There was no clear difference between groups (RR 0.84, 95% CI 0.06 to 12.42, Analysis 1.37).
1.37. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 37 Service use: hospital admission.
2. SENSITIVITY ANALYSIS
Performing a sensitivity analysis was only relevant for the primary outcome of mental state: clinically important change.
2.1 Mental state: clinically important change ‐ worsening psychosis ‐ trial with participants with affective psychoses
When we post hoc excluded the results from Prasuna 2015, the study that included participants who were on atypical antipsychotics for less than two weeks irrespective of the diagnosis, the direction of effect for worsening psychosis remained unchanged (RR 0.77, 95% CI 0.21 to 2.77; participants = 137; studies = 5, Analysis 1.38).
1.38. Analysis.

Comparison 1 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term, Outcome 38 SENSITIVITY ANALYSIS: Mental state: overall ‐ worsening psychosis ‐ TRIAL WITH PARTICIPANTS WITH AFFECTIVE PSYCHOSES.
3. Missing outcomes
As shown in this review, there has been a focus on the use of modafinil for people with schizophrenia for cognitive enhancement and to treat negative symptoms. However, economic outcomes, social functioning, and satisfaction with treatment have not been evaluated. Furthermore, binary data for improvement in mental state was only reported for negative symptoms outcomes but not for general improvement in mental state.
4. SUBGROUP ANALYSIS
Subgroup analysis was not possible as we only obtained data from chronically ill participants, and all of the trials lasted 12 weeks or less.
5. COMPARISON 2: MODAFINIL (+ usual antipsychotic) versus PLACEBO (+ usual antipsychotic) ‐ subscale data ‐ all short term
Four trials reported subscores from various cognitive functioning scales. As these are not global assessments of an area of functioning, the results are shown in Appendix 2.
Discussion
Summary of main results
1. General
The use of modafinil for schizophrenia has been of interest to researchers as an intervention to improve the negative and the cognitive symptoms of schizophrenia without worsening the positive symptoms. The first clinical trial on the use of modafinil for schizophrenia was published more than a decade ago (Turner 2004). This trial showed promising results for cognitive improvement in people with schizophrenia, but replication of these results has not been possible, as was shown in this review. We included 11 studies with approximately 37 outcomes; the included studies had rather small sample sizes and short follow‐up time. The overall findings of this review are that it is unclear if adding modafinil to usual antipsychotic treatment affects symptoms of schizophrenia when compared to adding placebo to usual antipsychotic treatment.
2. Overall mental state: worsening psychosis
We found that 3.8% of participants experienced an event of worsening psychosis in the trials, but there was no difference between groups in the incidence of this event. However, it is worth noting that we assessed the evidence as of low quality, limited by poor reporting by trials regarding methods of randomisation, allocation concealment, and blinding. Also, worsening psychosis was not further defined in any of the trials. Finally, it should be noted that the severity of a psychotic episode can vary, and episodes can have long‐lasting effects in the life of the person experiencing the episode as well as those around them. Based on the current evidence, we can draw no firm conclusions on the impact of modafinil for mental state.
3. Cognitive function: overall ‐ MATRICS Consensus Cognitive Battery composite score
Only two studies reported on cognitive function using the MCCB. Lees 2017 used a single dose of modafinil, and Michalopoulou 2015 used modafinil for 10 days plus cognitive training. We included Michalopoulou 2015 in the 'Summary of findings' table, but neither of the clinical trials showed an important difference between groups. Unfortunately, we were unable to use data from Scoriels 2011, Turner 2004, and Leblanc 2006, given that they are cross‐over trials that did not report data for the first phase. With these limitations in mind, being mindful that more data exist on the use of modafinil for cognition that was not included in our analysis, we are uncertain if modafinil has any effect on cognition for schizophrenia given the very low quality of the evidence.
4. Leaving the study early: any reason
The rate of attrition for randomised participants was 11.2%. This level of attrition is within acceptable ranges for clinical trials of schizophrenia (Xia 2009). The results of this review showed that modafinil did not seem to affect participants leaving the study early due to any reason. However, the results were limited, as the condition of schizophrenia is a chronic one, and the follow‐up time in the trials was insufficient (mean = 8 weeks). Also, whilst we assessed the quality of the evidence as moderate, translating this result to real‐world settings is problematic, especially given that participants abandon the trials for distinct reasons and motives, and these were not adequately reported in many of the included trials. Overall, the conclusions that can be drawn based on the current evidence are limited.
5. Adverse events: general ‐ any serious adverse events
Only one trial reported serious adverse events (Freudenreich 2009). Two serious adverse events occurred in this trial, one in each group. One participant had worsening psychosis in the placebo group, and one participant was psychiatrically admitted to the hospital in the modafinil group (no further details were provided regarding the reason for hospitalisation). Serious adverse events occurred in 5.7% of participants in this trial, raising concerns about the safety of modafinil in people with schizophrenia. However, the evidence is of poor quality due to the small sample size, imprecise results, lack of a direction of effect, and short follow‐up time. Furthermore, external validity is limited as the trial was done in a highly specialised setting. No firm conclusions can be drawn due to the very low quality of the evidence.
6. Global state: clinically important change in global state ‐ no improvement
There was no important difference between groups for clinically important change in global state. However, this result is frail and is limited in multiple ways. Firstly, the data come from a small trial with 21 participants and an insufficient follow‐up time, which lasted eight weeks (Pierre 2007). Furthermore, methods of randomisation, allocation, and blinding were not properly reported, which limits our ability to draw conclusions with the available data. Finally, clinicians seem to interpret CGI ratings on relative change rather than on absolute change of symptoms, suggesting that the baseline severity of the patient influences the assigned value by the clinicians (Leucht 2006). Overall, we assessed the quality of the evidence as very low, with no firm conclusions drawn given the limiting factors stated above.
7. Quality of life: general
Evidence on quality of life was lacking. Two studies reported data for this outcome on two different scales, none of which showed a difference between treatment groups. Results were limited by small sample size, insufficient follow‐up time, and imprecision. The quality of the evidence is very low, limiting our ability to draw any conclusions.
8. Service use: hospital admission
Of the seven trials conducted in the outpatient setting, only Freudenreich 2009 reported hospital admission. There was no difference between groups for this outcome. In the placebo group, there was one hospitalisation for appendicitis, and in the modafinil group, one participant was admitted to a psychiatric hospital. Also, the external validity of these results is limited, given that the Freudenreich 2009 trial was performed in a highly specialised clozapine clinic in Boston, Massachusetts, USA. Regardless, we assessed the quality of the evidence as very low due to the short follow‐up time and lack of statistical power. Based on the current evidence, we were unable to draw any firm conclusions.
Overall completeness and applicability of evidence
1. Completeness
Evidence on the use of modafinil is scarce, and the available evidence comes from only short‐term trials. We believe that if the outcome reporting had been better, the results of this review would be more comprehensive. We attempted to obtain these data, as shown in Appendix 1, but only a few authors responded to our request. This review is yet another reflection of the usefulness of initiatives such as OpenTrials, where a proposal to publicly publish the data of the trials is made. Also, we found three cross‐over trials that only reported the results after the cross‐over, making the data unusable for this review. Additionally, publication bias is suspected, given that we obtained a manuscript that was left unpublished as it was never accepted for publication (Leblanc 2006). Even though we were not able to use the data from this trial, the fact that the manuscript was left unpublished raises suspicion for the possibility of publication bias.
2. Applicability
Modafinil is a medication that is not commonly used in schizophrenia, thus most of the trials included in this review are considered exploratory; this is one explanation for why most of the trials were based on small sample sizes (˜38 people per study). The small sample size of the trials limits confidence in the findings of this systematic review, and with imprecise results and without trials of adequate power, the question of whether modafinil might work for schizophrenia will remain unanswered.
Quality of the evidence
Overall, the quality of the evidence was poor to very poor, with evidence from only one outcome rated as of moderate quality (Figure 1). Although all of the studies reported some form of randomisation, sequence generation and blinding were not described satisfactorily in most studies. Seven out of 11 trials had a clinical trial registration, but selective reporting was still an issue throughout the review, given that more than half of the trials reported outcomes poorly or in methods that were not imputable in a meta‐analysis. Six of the included trials had industry influence, but the data did not seem to favour the intervention more than non‐industry funded studies. We included 76 outcomes in this review, with most of them showing a small effect size and imprecision.
Potential biases in the review process
The data were first extracted by JO and imputed in Review Manager 5. Later, SAC and AOV extracted the data separately and verified that the data extraction was correct. We are aware that this order may not have been standard, but we ensured that the data imputed to Review Manager 5 was verified by the two review authors.
Agreements and disagreements with other studies or reviews
Rosenthal 2004 and Turner 2004 were the first experimental studies done on modafinil for people with schizophrenia. They initially showed promising results for the treatment of fatigue and cognitive impairment, but the sample sizes were rather small as they were exploratory studies. Although these two studies were not included in this review, we found more recent studies that reported that some cognitive functions (sensorimotor skills and social cognition) are improved with the use modafinil and that fatigue is not improved.
Scoriels 2013 is a systematic review of randomised trials without meta‐analysis, which concluded that modafinil might benefit working memory. Our results for working memory, coming from one study (Lees 2017), were that modafinil is no different than placebo for this outcome. The results differ because the two studies that provided data for the Scoriels 2013 systematic review were excluded in our review as they were cross‐over trials (Turner 2004; Scoriels 2011), and data for phase one of the trials were not available. We did not receive a response to our request for this information. As stated in our protocol, using endpoint data from cross‐over trials is problematic given the potential for a carry‐over effect.
Andrade 2015 is a systematic review with meta‐analysis with a similar scope to this review. However, it is distinct in multiple aspects: it combines the studies of modafinil and armodafinil; it uses a different method for data analysis; and evaluation for skewed data was not done. Regardless, the results of Andrade 2015 are similar for overall mental state, cognition, and adverse events. The result for negative symptoms in Andrade 2015 differed that in our review given the different methods: the authors describe a "statistically significant reduction in negative symptoms". However, they recognised that the effect size of this result was small, making clinical significance questionable.
Authors' conclusions
Implications for practice.
1. For people with schizophrenia
People with schizophrenia or related disorders usually live with cognitive or negative symptoms that are not alleviated by antipsychotics. They should be aware that the evidence we found is of very low quality and currently shows that adding modafinil to standard care is no better or worse than for placebo for most of the outcomes studied.
2. For clinicians
Given the results of this systematic review and the low quality of the evidence, clinicians who wish to use modafinil for patients with schizophrenia should understand that the current evidence shows no real effect and is too weak to support its use. We encourage clinicians who wish to use modafinil or who have experience with the use of modafinil in people with schizophrenia or related disorders to randomise patients to clinical trials.
3. For policymakers
Given the low quality of the evidence and lack of economic and satisfaction outcomes, the use of modafinil for schizophrenia is not supported by the current evidence.
Implications for research.
1. General
Trial registration and complying with reporting of outcomes should be done for all trials. Also, we encourage researchers to share the data from their clinical trials. The data made possible by the participants of the studies should be made available for good use.
2. Trials
A good argument needs to be provided for doing more clinical trials on the use of modafinil for schizophrenia. If a clinical trial is planned, we encourage the use of pragmatic outcomes and adequate reporting according to the CONSORT statement. Also, the need for a larger sample is required to improve the quality of the evidence, or the results might not be sufficiently powered to change the results of this systematic review.
History
Protocol first published: Issue 9, 2010 Review first published: Issue 12, 2019
| Date | Event | Description |
|---|---|---|
| 31 October 2019 | Amended | Search rerun and no new studies found. |
| 24 May 2017 | Amended | Search rerun and five references added as studies awaiting classification. |
| 9 June 2015 | Amended | Search was run and 61 references added as studies awaiting classification. |
Acknowledgements
The Cochrane Schizophrenia Group Editorial Base in Nottingham produces and maintains standard text for use in the Methods sections of their reviews. We have used this text as the basis of what appears here and adapted it as required. We would also like to thank Linda Scoriels, Jennifer Barnett, and Peter Jones for their contribution to the protocol.
Parts of this review were generated using RevMan HAL v 4.2. You can find more information about RevMan HAL here.
We thank Dr Richard Drake, Dr Tom Farrow, and Dr Marc‐Andre Roy for providing data from their clinical trials. Also, many thanks to Clive Adams and Farhad Shokraneh for the support given to JO during his month at the University of Nottingham. We also appreciate the work done by the original team who developed the protocol for this review.
Appendices
Appendix 1. Author contacts
| Study | Contact date | Solicited data | Response |
| Arbabi 2012 | 13‐Aug‐17 | Extrapyramidal symptoms rating scale (mean and SD). | Did not respond. |
| Freudenreich 2009 | 11‐Aug‐17 | ESS, FSS, PANSS, SANS, and COGBAT (mean and SD) ‐ the data are presented in a graph or as a slope. Mean and SD for weight and BMI. Method of randomisation. |
Did not respond. |
| Kumar 2010 | 8‐Aug‐17 | Participants leaving the study early for each group. NHRS, ESS, PANSS, IDEAS, and CGI‐I (mean scores and SD). Adverse events for each group. |
Did not respond. |
| Leblanc 2006 | 11‐Aug‐17 | Method of randomisation. How many participants were allocated to each group. To which group participants who left the study belonged and the reasons for leaving. Data from PANSS, CGI, SOFAS, and ESRS (mean and SD). |
Information received in the form of an unpublished draft, no information was usable for this review (20 August 2017). |
| Lees 2017 | 9‐Aug‐17 | Number randomised to the modafinil and placebo group in the first phase of the trial. MCCB (composite and domains) and CANTAB (mean and SD) for the first phase of the trial for each group. The number of participants with schizophrenia that left the study before the cross‐over and the group to which they were assigned. Scores (mean and SD) for the MCCB (composite and domains) and CANTAB for the first phase of the trial for each group. |
Information received and used in this review (15 August 2017). |
| Lohr 2013 | 9‐Aug‐17 | Data reported in a graph: SAS, PANSS (mean and SD). Data not reported in the results: AIMS, ESS, CGI‐S, BARS (mean and SD). Adverse events that occurred in both groups. |
Did not respond. |
| Michalopoulou 2015 | 4‐Aug‐17 | PANSS and UPSA‐B (mean score and SD) from visit 14; we used data from visit 16. | Did not respond. |
| Prasuna 2015 | 17‐Aug‐17 | BPRS, CGI‐S, EDD (mean and SD). List of adverse events. |
Did not respond. |
| Sevy 2005 | 12‐Aug‐17 | Method of randomisation and allocation. The list of adverse events (only the adverse events for the modafinil group are reported in the report). SANS total (mean and SD). |
Did not respond. |
| Spence 2005 | 16‐Aug‐17 | The group to which the participant who relapsed belonged. | Information received and used in this review (18 August 2017). |
| Turner 2004 | 14‐Aug‐17 | CANTAB (mean and SD) for the first phase of the trial. | Did not respond. |
AIMS = Abnormal Involuntary Movement Scale. BARS = Barnes Akathisia Rating Scale. BMI = Body Mass Index. BPRS = Brief Psychiatric Rating Scale. CANTAB = Cambridge Neuropsychological Test Automated Battery. CGI‐I = Clinical Global Impression‐Improvement. CGI‐S = Clinical Global Impression‐Severity. COGBAT = Cognitive Basic Assessment. ESRS = Extrapyramidal Symptom Rating Scale. ESS = Epworth Sleepiness Scale. EDD = Excessive Daytime Drowsiness Scale. FSS = Fatigue Severity Scale. IDEAS = Indian Disability Evaluation Assessment Scale. MCCB = MATRICS Consensus Cognitive Battery. NHRS = Nocturnal Hyper‐salivation Rating Scale. PANSS = Positive and Negative Syndrome Scale. SANS = Scale for the Assessment of Negative Symptoms. SAS = Simpson‐Angus Scale. SD = Standard deviation. SOFAS = Social and Occupational Functioning Assessment Scale. UPSA‐B = University of California San Diego (UCSD) Performance‐based Skills Assessment.
Appendix 2. Subscale results
2.1 Cognitive function: 1a. Specific: attention/vigilance: i. average score (DS‐CPT score, endpoint, high = good)
There was no clear difference between groups for this outcome (MD −0.01, 95% CI −0.07 to 0.05; participants = 20; studies = 1, Analysis 2.1).
2.1. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 1 Cognitive function: 1a. Specific: attention/vigilance: i. average score (DS‐CPT score, endpoint, high = good).
2.2 Cognitive function: 1b. Specific: attention/vigilance: ii. average score (MCCB score, endpoint, high = good)
There was no clear difference between groups for this outcome (MD −2.20, 95% CI −9.95 to 5.55; participants = 48; studies = 1, Analysis 2.2)
2.2. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 2 Cognitive function: 1b. Specific: attention/vigilance: ii. average score (MCCB score, endpoint, high = good).
2.3 Cognitive function: 1c. Specific: attention/vigilance: iii. average score ‐ single dose (MCCB score, endpoint, high = good)
There was no clear difference between groups for this outcome (MD 4.51, 95% CI −1.70 to 10.72; participants = 40; studies = 1, Analysis 2.3).
2.3. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 3 Cognitive function: 1c. Specific: attention/vigilance: iii. average score ‐ single dose (MCCB score, endpoint, high = good).
2.4 Cognitive function: 2a. Specific: flexibility of attention: i. average score ‐ single dose (CANTAB ‐ IED stages completed, endpoint, high = good)
There was no clear difference between groups for this outcome (MD 0.27, 95% CI −0.27 to 0.81; participants = 40; studies = 1, Analysis 2.4.
2.4. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 4 Cognitive function: 2a. Specific: flexibility of attention: i. average score ‐ single dose (CANTAB ‐ IED stages completed, endpoint, high = good).
2.5 Cognitive function: 2b. Specific: flexibility of attention: ii. average score ‐ single dose (CANTAB ‐ IED errors adjusted, endpoint, high = poor, skewed)
These continuous data were skewed and are presented as 'other data' (Analysis 2.5).
2.5. Analysis.
Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 5 Cognitive function: 2b. Specific: flexibility of attention: ii. average score ‐ single dose (CANTAB ‐ IED errors adjusted, endpoint, high = poor, skewed data).
| Cognitive function: 2b. Specific: flexibility of attention: ii. average score ‐ single dose (CANTAB ‐ IED errors adjusted, endpoint, high = poor, skewed data) | ||||||
|---|---|---|---|---|---|---|
| Study | Mean (Modafinil group) | SD (Modafinil group) | N (Modafinil Group) | Mean (Placebo group) | SD (Placebo group) | N (Placebo group) |
| Lees 2017 | 26.08 | 19.13 | 23 | 34.6 | 20.8 | 17 |
2.6 Cognitive function: 3. Specific: fluency ‐ average score (COWAT score, endpoint, high = good)
There was no clear difference between groups for this outcome (MD −3.50, 95% CI −14.33 to 7.33; participants = 20; studies = 1, Analysis 2.6).
2.6. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 6 Cognitive function: 3. Specific: fluency: average score (COWAT score, endpoint, high = good).
2.7 Cognitive function: 4. Specific: reaction time ‐ average score ‐ single dose (CANTAB ‐ RTI simple accuracy score, endpoint, high = good)
There was no clear difference between groups for this outcome (MD 0.18, 95% CI −0.71 to 1.07; participants = 40; studies = 1, Analysis 2.7).
2.7. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 7 Cognitive function: 4. Specific: reaction time: average score ‐ single dose (CANTAB ‐ RTI simple accuracy score, endpoint, high = good).
2.8 Cognitive function: 5a. Specific: reasoning and problem solving: i. average score ‐ short term (MCCB score, endpoint, high = good)
There was no clear difference between groups for this outcome (MD −0.20, 95% CI −6.14 to 5.74; participants = 48; studies = 1, Analysis 2.8).
2.8. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 8 Cognitive function: 5a. Specific: reasoning and problem solving: i. average score ‐ short term (MCCB score, endpoint, high = good).
2.9 Cognitive function: 5b. Specific: reasoning and problem solving: ii. average score ‐ single dose (MCCB score, endpoint, high = good)
There was no clear difference between groups for this outcome (MD 4.35, 95% CI −2.12 to 10.82; participants = 40; studies = 1, Analysis 2.9).
2.9. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 9 Cognitive function: 5b. Specific: reasoning and problem solving: i. average score ‐ single dose (MCCB score, endpoint, high = good).
2.10 Cognitive function: 6a. Specific: sensorimotor skills: i. average score ‐ single dose (CANTAB ‐ MOT mean error, endpoint, high = poor)
There was no clear difference between groups for this outcome (MD 0.12, 95% CI −1.22 to 1.46; participants = 40; studies = 1, Analysis 2.10).
2.10. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 10 Cognitive function: 6a. Specific: sensorimotor skills: i. average score ‐ single dose (CANTAB ‐ MOT mean error, endpoint, high = poor).
2.11 Cognitive function: 6b. Specific: sensorimotor skills: ii. average score ‐ single dose (CANTAB ‐ MOT mean latency, endpoint, high = poor)
There was a clear difference in scores favouring the modafinil group for this outcome (MD −113.44, 95% CI −220.11 to −6.77; participants = 40; studies = 1; Analysis 2.11).
2.11. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 11 Cognitive function: 6b. Specific: sensorimotor skills: ii. average score ‐ single dose (CANTAB ‐ MOT mean latency, endpoint, high = poor).
2.12 Cognitive function: 7a. Specific: short‐term auditory‐verbal memory: i. average score (RAVL ‐ delayed recall, endpoint, high = good)
There was no clear difference between groups for this outcome (MD −1.30, 95% CI −3.55 to 0.95; participants = 20; studies = 1, Analysis 2.12).
2.12. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 12 Cognitive function: 7a. Specific: short‐term auditory‐verbal memory: i. average score (RAVL test ‐ delayed recall, endpoint, high = good).
2.13 Cognitive function: 7b. Specific: short‐term auditory‐verbal memory: ii. average score (RAVL ‐ immediate recall, endpoint, high = good)
There was no clear difference between groups for this outcome (MD −7.20, 95% CI −14.69 to 0.29; participants = 20; studies = 1, Analysis 2.13).
2.13. Analysis.
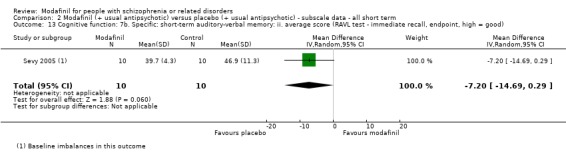
Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 13 Cognitive function: 7b. Specific: short‐term auditory‐verbal memory: ii. average score (RAVL test ‐ immediate recall, endpoint, high = good).
2.14 Cognitive function: 8a. Specific: short‐term memory: i. average score (DMST 4‐second delay score, endpoint, high = good, skewed data)
These continuous data were skewed and are presented as 'other data' (Analysis 2.14).
2.14. Analysis.
Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 14 Cognitive function: 8a. Specific: short‐term memory: i. average score (DMST 4‐second delay score, endpoint, high = good, skewed data).
| Cognitive function: 8a. Specific: short‐term memory: i. average score (DMST 4‐second delay score, endpoint, high = good, skewed data) | ||||||
|---|---|---|---|---|---|---|
| Study | Mean (Modafinil group) | SD (Modafinil group) | N (Modafinil Group) | Mean (Placebo group) | SD (Placebo group) | N (Placebo group) |
| Sevy 2005 | 12.1 | 6.2 | 10 | 15.10 | 3.0 | 10 |
2.15 Cognitive function: 8b. Specific: short‐term memory: ii. average score (DMST no‐delay score, endpoint, high = good)
There was no clear difference between groups for this outcome (MD −0.80, 95% CI −2.77 to 1.17; participants = 20; studies = 1, Analysis 2.15).
2.15. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 15 Cognitive function: 8b. Specific: short‐term memory: ii. average score (DMST no delay score, endpoint, high = good).
2.16 Cognitive function: 9a. Specific: social cognition: i. average score ‐ single dose (MCCB score, endpoint, high = good)
There was a clear difference in scores favouring the modafinil group for this outcome (MD 9.28, 95% CI 4.16 to 14.40; participants = 40; studies = 1, Analysis 2.16).
2.16. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 16 Cognitive function: 9a. Specific: social cognition: i average score ‐ single dose (MCCB score, endpoint, high = good).
2.17 Cognitive function: 9b. Specific: social cognition: ii. average score ‐ short term (MCCB score, endpoint, high = good)
There was no clear difference between groups for this outcome (MD 1.50, 95% CI −4.60 to 7.60; participants = 48; studies = 1, Analysis 2.17).
2.17. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 17 Cognitive function: 9b. Specific: social cognition: ii. average score ‐ short term (MCCB score, endpoint, high = good).
2.18 Cognitive function: 10a. Specific: spatial planning/working memory: i. average score ‐ single dose (CANTAB ‐ OTS choices to correct, endpoint, high = good)
There was no clear difference between groups for this outcome (MD −0.19, 95% CI −0.46 to 0.08; participants = 40; studies = 1, Analysis 2.18).
2.18. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 18 Cognitive function: 10a. Specific: spatial planning/working memory: i. average score ‐ single dose (CANTAB ‐ OTS choices to correct, endpoint, high = good).
2.19 Cognitive function: 10b. Specific: spatial planning/working memory: ii. average score ‐ single dose (CANTAB ‐ OTS mean latency to correct, endpoint, high = good)
These data were skewed and are presented as 'other data' (Analysis 2.19).
2.19. Analysis.
Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 19 Cognitive function: 10b. Specific: spatial planning/working memory: ii. average score ‐ single dose (CANTAB ‐ OTS mean latency to correct, endpoint, high = good, skewed data).
| Cognitive function: 10b. Specific: spatial planning/working memory: ii. average score ‐ single dose (CANTAB ‐ OTS mean latency to correct, endpoint, high = good, skewed data) | ||||||
|---|---|---|---|---|---|---|
| Study | Mean (Modafinil group) | SD (Modafinil group) | N (Modafinil Group) | Mean (Placebo group) | SD (Placebo group) | N (Placebo group) |
| Lees 2017 | 20092.56 | 10232.46 | 23 | 22087.40 | 4817.7 | 17 |
2.20 Cognitive function: 10c. Specific: spatial planning/working memory: iii. average score ‐ single dose (CANTAB ‐ OTS problems solved on the first choice, endpoint, high = good)
There was no clear difference between groups for this outcome (MD 0.96, 95% CI −0.72 to 2.64; participants = 40; studies = 1, Analysis 2.20).
2.20. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 20 Cognitive function: 10c. Specific: spatial planning/working memory: iii. average score ‐ single dose (CANTAB ‐ OTS problems solved on the first choice, endpoint, high = good).
2.21 Cognitive function: 11a. Specific: speed of processing: i. average score ‐ single dose (MCCB score, endpoint, high = good)
There was no clear difference between groups for this outcome (MD 4.19, 95% CI −5.46 to 13.84; participants = 40; studies = 1, Analysis 2.21).
2.21. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 21 Cognitive function: 11a. Specific: speed of processing: i. average score ‐ single dose (MCCB score, endpoint, high = good).
2.22 Cognitive function: 11b. Specific: speed of processing: ii. average score ‐ short term (MCCB score, endpoint, high = good)
There was no clear difference between groups for this outcome (MD −3.40, 95% CI −11.62 to 4.82; participants = 48; studies = 1, Analysis 2.22).
2.22. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 22 Cognitive function: 11b. Specific: speed of processing: ii. average score ‐ short term (MCCB score, endpoint, high = good).
2.23 Cognitive function: 12a. Specific: sustained attention: i. average score ‐ single dose (CANTAB ‐ RVP probability of a hit, endpoint, high = good)
There was a clear difference in scores favouring the modafinil group for this outcome (MD 0.13, 95% CI 0.03 to 0.23; participants = 40; studies = 1, Analysis 2.23).
2.23. Analysis.
Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 23 Cognitive function: 12a. Specific: sustained attention: i. average score ‐ single dose (CANTAB ‐ RVP probability of a hit, endpoint, high = good).
2.24 Cognitive function: 12b. Specific: sustained attention: ii. average score (CPT‐IP 2‐digit, endpoint, high = good, skewed data)
These continuous data were skewed and are presented as 'other data' (Analysis 2.24).
2.24. Analysis.
Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 24 Cognitive function: 12b. Specific: sustained attention: ii. average score (CPT‐IP 2 digit, endpoint, high = good, skewed data).
| Cognitive function: 12b. Specific: sustained attention: ii. average score (CPT‐IP 2 digit, endpoint, high = good, skewed data) | ||||||
|---|---|---|---|---|---|---|
| Study | Mean (Modafinil group) | SD (Modafinil group) | N (Modafinil Group) | Mean (Placebo group) | SD (Placebo group) | N (Placebo group) |
| Sevy 2005 | 2.5 | 1.3 | 10 | 2.6 | 1 | 10 |
2.25 Cognitive function: 12c. Specific: sustained attention: iii. average score (CPT‐IP 3‐digit, endpoint, high = good, skewed data)
These continuous data were skewed and are presented as 'other data' (Analysis 2.25).
2.25. Analysis.
Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 25 Cognitive function: 12c. Specific: sustained attention: iii. average score (CPT‐IP 3 digit, endpoint, high = good, skewed data).
| Cognitive function: 12c. Specific: sustained attention: iii. average score (CPT‐IP 3 digit, endpoint, high = good, skewed data) | ||||||
|---|---|---|---|---|---|---|
| Study | Mean (Modafinil group) | SD (Modafinil group) | N (Modafinil Group) | Mean (Placebo group) | SD (Placebo group) | N (Placebo group) |
| Sevy 2005 | 2.1 | 1.4 | 10 | 1.8 | 1.1 | 10 |
2.26 Cognitive function: 12d. Specific: sustained attention: iv. average score (CPT‐IP 4‐digit, endpoint, high = good, skewed data)
These continuous data were skewed and are presented as 'other data' (Analysis 2.26).
2.26. Analysis.
Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 26 Cognitive function: 12d. Specific: sustained attention: iv. average score (CPT‐IP 4 digit, endpoint, high = good, skewed data).
| Cognitive function: 12d. Specific: sustained attention: iv. average score (CPT‐IP 4 digit, endpoint, high = good, skewed data) | ||||||
|---|---|---|---|---|---|---|
| Study | Mean (Modafinil group) | SD (Modafinil group) | N (Modafinil Group) | Mean (Placebo group) | SD (Placebo group) | N (Placebo group) |
| Sevy 2005 | 1.2 | 1.0 | 10 | 1.2 | 0.9 | 10 |
2.27 Cognitive function: 13a. Specific: verbal learning: i. average score ‐ single dose (MCCB score, endpoint, high = good)
There was no clear difference between groups for this outcome (MD 1.62, 95% CI −5.65 to 8.89; participants = 40; studies = 1, Analysis 2.27).
2.27. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 27 Cognitive function: 13a. Specific: verbal learning: i. average score ‐ single dose (MCCB score, endpoint, high = good).
2.28 Cognitive function: 13b. Specific: verbal learning: ii. average score ‐ short term (MCCB score, endpoint, high = good)
There was no clear difference between groups for this outcome (MD −3.20, 95% CI −8.82 to 2.42; participants = 48; studies = 1, Analysis 2.28).
2.28. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 28 Cognitive function: 13b. Specific: verbal learning: ii. average score ‐ short term (MCCB score, endpoint, high = good).
2.29 Cognitive function: 14. Specific: verbal learning/memory: average score (CVLT, endpoint, high = good)
These continuous data were skewed and are reported as 'other data' (Analysis 2.29).
2.29. Analysis.
Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 29 Cognitive function: 14. Specific: verbal learning/memory ‐ average score (CVLT test, endpoint, high = good).
| Cognitive function: 14. Specific: verbal learning/memory ‐ average score (CVLT test, endpoint, high = good) | ||||||
|---|---|---|---|---|---|---|
| Study | Mean (Modafinil group) | SD (Modafinil group) | N (Modafinil Group) | Mean (Placebo group) | SD (Placebo group) | N (Placebo group) |
| Pierre 2007 | 43.2 | 9.5 | 10 | 39.7 | 21.1 | 10 |
2.30 Cognitive function: 15a. Specific: verbal memory: i. average score ‐ single dose (CANTAB ‐ VRM free recall correct immediate, endpoint, high = good)
There was no clear difference between groups for this outcome (MD 0.23, 95% CI −1.16 to 1.62; participants = 40; studies = 1, Analysis 2.30).
2.30. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 30 Cognitive function: 15a. Specific: verbal memory: i. average score ‐ single dose (CANTAB ‐ VRM free recall correct immediate, endpoint, high = good).
2.31 Cognitive function: 15b. Specific: verbal memory: ii. average score ‐ single dose (CANTAB ‐ VRM free recall novel words immediate, endpoint, high = good)
These continuous data were skewed and are presented as 'other data' (Analysis 2.31).
2.31. Analysis.
Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 31 Cognitive function: 15b. Specific: verbal memory: ii. average score ‐ single dose (CANTAB ‐ VRM free recall novel words immediate, endpoint, high = good, skewed data).
| Cognitive function: 15b. Specific: verbal memory: ii. average score ‐ single dose (CANTAB ‐ VRM free recall novel words immediate, endpoint, high = good, skewed data) | ||||||
|---|---|---|---|---|---|---|
| Study | Mean (Modafinil group) | SD (Modafinil group) | N (Modafinil Group) | Mean (Placebo group) | SD (Placebo group) | N (Placebo group) |
| Lees 2017 | 0.34 | 0.714 | 23 | 0.47 | 0.8 | 17 |
2.32 Cognitive function: 16. Specific: visual attention/task switching ‐ average score (TMT, endpoint, high = good)
These continuous data were skewed and are presented as 'other data' (Analysis 2.32).
2.32. Analysis.
Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 32 Cognitive function: 16. Specific: visual attention/task switching ‐ average score (TMT, endpoint, high = good, skewed data).
| Cognitive function: 16. Specific: visual attention/task switching ‐ average score (TMT, endpoint, high = good, skewed data) | ||||||
|---|---|---|---|---|---|---|
| Study | Mean (Modafinil group) | SD (Modafinil group) | N (Modafinil Group) | Mean (Placebo group) | SD (Placebo group) | N (Placebo group) |
| Pierre 2007 | 134.1 | 73.6 | 10 | 122.4 | 55.9 | 10 |
2.33 Cognitive function: 17a. Specific: visual learning: i. average score ‐ single dose (MCCB score, endpoint, high = good)
There was no clear difference between groups for this outcome (MD 7.16, 95% CI −0.54 to 14.86; participants = 40; studies = 1, Analysis 2.33).
2.33. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 33 Cognitive function: 17a. Specific: visual learning: i. average score ‐ single dose (MCCB score, endpoint, high = good).
2.34 Cognitive function: 17b. Specific: visual learning: ii. average score (MCCB score, endpoint, high = good)
There was no clear difference between groups for this outcome (MD −1.80, 95% CI −10.07 to 6.47; participants = 48; studies = 1, Analysis 2.34).
2.34. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 34 Cognitive function: 17b. Specific: visual learning: ii. average score (MCCB score, endpoint, high = good).
2.35 Cognitive function: 18a. Specific: visual memory/new learning: i. average score ‐ single dose (CANTAB ‐ PAL total errors adjusted, endpoint, high = poor)
These continuous data were skewed and are reported as 'other data' (Analysis 2.35).
2.35. Analysis.
Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 35 Cognitive function: 18a. Specific: visual memory/new learning: i. average score ‐ single dose (CANTAB ‐ PAL total errors adjusted, endpoint, high = poor, skewed data).
| Cognitive function: 18a. Specific: visual memory/new learning: i. average score ‐ single dose (CANTAB ‐ PAL total errors adjusted, endpoint, high = poor, skewed data) | ||||||
|---|---|---|---|---|---|---|
| Study | Mean (Modafinil group) | SD (Modafinil group) | N (Modafinil Group) | Mean (Placebo group) | SD (Placebo group) | N (Placebo group) |
| Lees 2017 | 11.17 | 10.43 | 23 | 28.29 | 29.96 | 17 |
2.36 Cognitive function: 18b. Specific: visual memory/new learning: ii. average score ‐ single dose (CANTAB ‐ PAL errors 6 shapes adjusted, endpoint, high = poor)
These continuous data were skewed and are presented as 'other data' (Analysis 2.36).
2.36. Analysis.
Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 36 Cognitive function: 18b. Specific: visual memory/new learning: ii. average score ‐ single dose (CANTAB ‐ PAL errors 6 shapes adjusted, endpoint, high = poor, skewed data).
| Cognitive function: 18b. Specific: visual memory/new learning: ii. average score ‐ single dose (CANTAB ‐ PAL errors 6 shapes adjusted, endpoint, high = poor, skewed data) | ||||||
|---|---|---|---|---|---|---|
| Study | Mean (Modafinil group) | SD (Modafinil group) | N (Modafinil Group) | Mean (Placebo group) | SD (Placebo group) | N (Placebo group) |
| Lees 2017 | 1.78 | 2.72 | 23 | 7.11 | 8.44 | 17 |
2.37 Cognitive function: 19a. Specific: working memory: i. average score ‐ single dose (MCCB score, endpoint, high = good)
There was no clear difference between groups for this outcome (MD 3.45, 95% CI −2.99 to 9.89; participants = 40; studies = 1, Analysis 2.37).
2.37. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 37 Cognitive function: 19a. Specific: working memory: i. average score ‐ single dose (MCCB score, endpoint, high = good).
2.38 Cognitive function: 19b. Specific: working memory: ii. average score (MCCB score, endpoint, high = good)
There was no clear difference between groups for this outcome (MD −3.80, 95% CI −11.18 to 3.58; participants = 48; studies = 1, Analysis 2.38).
2.38. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 38 Cognitive function: 19b. Specific: working memory: ii. average score (MCCB score, endpoint, high = good).
2.39 Cognitive function: 19c. Specific: working memory: iii. average score (ODRT 2‐second delay, endpoint, high = good, skewed data)
These continuous data were skewed and are presented as 'other data' (Analysis 2.39).
2.39. Analysis.
Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 39 Cognitive function: 19c. Specific: working memory: iii. average score (ODRT 2‐second delay, endpoint, high = good, skewed data).
| Cognitive function: 19c. Specific: working memory: iii. average score (ODRT 2‐second delay, endpoint, high = good, skewed data) | ||||||
|---|---|---|---|---|---|---|
| Study | Mean (Modafinil group) | SD (Modafinil group) | N (Modafinil Group) | Mean (Placebo group) | SD (Placebo group) | N (Placebo group) |
| Sevy 2005 | 36.9 | 19 | 10 | 40.9 | 10.4 | 10 |
2.40 Cognitive function: 19d. Specific: working memory: iv. average score (ODRT direct touch, endpoint, high = good, skewed data)
These continuous data were skewed and are presented as 'other data' (Analysis 2.40).
2.40. Analysis.
Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 40 Cognitive function: 19d. Specific: working memory: iv. average score (ODRT direct touch, endpoint, high = good, skewed data).
| Cognitive function: 19d. Specific: working memory: iv. average score (ODRT direct touch, endpoint, high = good, skewed data) | ||||||
|---|---|---|---|---|---|---|
| Study | Mean (Modafinil group) | SD (Modafinil group) | N (Modafinil Group) | Mean (Placebo group) | SD (Placebo group) | N (Placebo group) |
| Sevy 2005 | 8.2 | 4.5 | 10 | 7.6 | 2.5 | 10 |
2.41 Cognitive function: 20. Specific: working memory/strategy ‐ average score ‐ single dose (CANTAB ‐ SWM strategy, endpoint, high = good)
There was no clear difference between groups for this outcome (MD −2.39, 95% CI −5.17 to 0.39; participants = 40; studies = 1, Analysis 2.41).
2.41. Analysis.

Comparison 2 Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term, Outcome 41 Cognitive function: 20. Specific: working memory/strategy ‐ average score ‐ single dose (CANTAB ‐ SWM strategy, endpoint, high = good).
Footnotes
CANTAB ‐ OTS ‐ Cambridge Neuropsychological Test Automated Battery. CANTAB ‐ IED = Cambridge Neuropsychological Test Automated Battery ‐ Intra‐Extra Dimensional Set Shift. CANTAB ‐ RTI = Cambridge Neuropsychological Test Automated Battery ‐ Reaction Time. CANTAB ‐ RVP = Cambridge Neuropsychological Test Automated Battery ‐ Rapid Visual Information Processing. CANTAB ‐ PAL = Cambridge Neuropsychological Test Automated Battery ‐ Paired Associates Learning. CANTAB ‐ SWM = Cambridge Neuropsychological Test Automated Battery ‐ Spatial Working Memory. CANTAB ‐ MOT = Cambridge Neuropsychological Test Automated Battery ‐Motor Screening Task. COWAT = Controlled Word Association Test. CPT‐IP = Continuous performance test‐identical pair. CVLT = California verbal learning test DMST = Delayed match to sample task. DS‐CPT = Degraded Stimulus‐Continuous Performance test. MCCB = MATRICS Consensus Cognitive Battery. ODRT = Oculomotor Delayed Response Test. RAVL = Rey Auditory Verbal Learning Test. TMT = Trail Making Test
Appendix 3. Methods in published protocol
Criteria for considering studies for this review
Types of studies
All relevant randomised controlled trials will be included. Where trials are described as 'double‐blind' but are only implied as being randomised, they will be included in a sensitivity analysis. If there are no substantive differences within primary outcomes (Types of outcome measures) when these 'implied randomisation' studies are added, then we will include these in the final analysis. If there are substantive differences, these studies will not be included in the analysis and we will describe the results of the sensitivity analysis in the text. Randomised cross‐over studies will be eligible but only data up to the point of first cross‐over because of the likely carry‐over effects of all treatments (Elbourne 2002). We will exclude quasi randomised studies such as those allocating by using alternate days of the week.
Types of participants
Participants will be adults (18‐65 years) diagnosed with schizophrenia and other types of schizophrenia‐like psychoses (e.g. schizophreniform and schizoaffective disorders), irrespective of the diagnostic criteria used. There is no clear evidence that the schizophrenia‐like psychoses are caused by fundamentally different disease processes or require different treatment approaches (Carpenter 1994).
Types of interventions
1. Modafinil: any dose/administration 2. Placebo Both interventions are in addition to care as usual.
Types of outcome measures
We will group outcomes into single dose, short‐term (chronic dose for up to 12 weeks), medium‐term (chronic dose for up to 26 weeks) and long‐term (chronic dose for over 26 weeks).
Primary outcomes
1. Mental state (with particular reference to the positive and negative symptoms of schizophrenia) 1.1 No clinically important change in general mental state score 1.2 Average endpoint general mental state score 1.3 Average change in general mental state scores 1.4 No clinically important change in positive symptoms of schizophrenia 1.5 Average endpoint positive symptom score 1.6 Average change in positive symptom scores 1.7 No clinically important change in negative symptoms of schizophrenia 1.8 Average endpoint negative symptom score 1.9 Average change in negative symptom scores
2. Cognitive functioning 2.1 No clinically important change in overall cognitive functioning 2.2 No clinically important change in specific aspects of cognitive functioning (e.g., IQ, memory, learning, attention, fluency, control, executive functioning) 2.3 Average endpoint of overall cognitive functioning score 2.4 Average change of overall cognitive functioning scores 2.5 Average endpoint specific cognitive functioning score 2.6 Average change specific cognitive functioning scores
Secondary outcomes
1. Behaviour/Emotional state 1.1 No clinically important change in overall behaviour 1.2 No clinically important change in specific aspects of behaviour/emotion (e.g., anxiety, aggression, mood) 1.3 Average endpoint behaviour score 1.4 Average change in behaviour scores
2. Global state 2.1 Relapse 2.2 Time to relapse 2.3 No clinically important change in global state 2.4 Not any change in global state 2.5 Average endpoint global state score 2.6 Average change in global state scores
3. Social/General functioning 3.1 Average endpoint general functioning score 3.2 Average change in general functioning scores 3.3 No clinically important change in specific aspects of functioning, such as social or life skills 3.4 Not any change in specific aspects of functioning, such as social or life skills 3.5 Average endpoint specific aspects of functioning, such as social or life skills 3.6 Average change in specific aspects of functioning, such as social or life skills 3.7 Employment status (employed/unemployed) 4. Quality of life 4.1 No clinically important change in general quality of life 4.2 Average endpoint general quality of life score 4.3 Average change in general quality of life scores
5. Service Use 5.1 Mean days in hospital 5.2 Number of participants admitted to hospital/re‐hospitalised 6. Satisfaction with treatment 6.1 Recipient of care not satisfied with treatment 6.2 Recipient of care average satisfaction score 6.3 Recipient of care average change in satisfaction scores 6.4 Carer not satisfied with treatment 6.5 Carer average satisfaction score 6.6 Carer average change in satisfaction scores 7. Adverse effects 7.1 Number of participants with at least one treatment‐emergent adverse effect 7.2 Clinically important specific adverse effects (e.g. cardiac effects, death, movement disorders, probating increase and associated effects, fatigue, sedation, seizures, weight gain, effects on white blood cell count) 7.3 Average endpoint in specific adverse effects 7.4 Average change in specific adverse effects 7.5 Death (natural or suicide)
8. Leaving the study early (any reason, adverse events, inefficacy of treatment)
Search methods for identification of studies
Electronic searches
On April 27, 2015, the Trials Search Co‐ordinator (TSC) searched the Cochrane Schizophrenia Group's Study‐Based Register of Trials using the following search strategy:
*Modafinil* in Intervention of STUDY
In such study‐based register, searching the major concept retrieves all the relevant keywords and studies because all the studies have already been organised based on their interventions and linked to the relevant topics.
The Cochrane Schizophrenia Group's Register of Trials is compiled by systematic searches of major resources (including AMED, BIOSIS, CINAHL, EMBASE, MEDLINE, PsycINFO, PubMed, and registries of clinical trials) and their monthly updates, hand‐searches, grey literature, and conference proceedings (see Group Module). There are no language, date, document type, or publication status limitations for inclusion of records into the register.
Searching other resources
Studies will also be identified by cross‐referencing of reviews and included studies. We will also contact laboratories that are in the process of studying these effects. Studies will also be identified from conferences and congresses.
Data collection and analysis
Selection of studies
Two authors (LS and JHB) will independently read citations identified from the search and include studies according to the criteria for review. Irrelevant articles will be discarded by a review of the title and its abstract. In the presence of any suggestion that the article could possibly be relevant, it will be retrieved for further assessment. Any disagreements will be resolved by discussion. If it is impossible to resolve disagreements these studies will be added to those awaiting assessment and the authors of the papers will be contacted for clarification. Non‐concurrence in trial selection will be reported.
Data extraction and management
1. Extraction
Two independent reviewers (LS and JHB) will extract data from the selected trials using the double entry method. In the event of a difference between the reviewers, they will seek to resolve the difference by further scrutiny of the original trial reports, and may involve a third reviewer and/or contact the authors for further information.
2. Management
Data will be extracted onto standard, simple forms.
3. Scale‐derived data
We will include continuous data from rating scales only if: (a) the psychometric properties of the measuring instrument have been described in a peer‐reviewed journal (Marshall 2000); (b) the measuring instrument was not written or modified by one of the trialist; (c) the measuring instrument is either (i) a self‐report or (ii) completed by an independent rater or relative.
Assessment of risk of bias in included studies
Two review authors (LS and JHB) will independently assess risk of bias in accordance with the Cochrane Collaboration's tools for assessing quality and risk of bias (Higgins 2008). This tool encourages consideration of how the sequence was generated, how allocation was concealed, the integrity of blinding, the completeness of outcome data, selective reporting and other biases. The risk of bias in each domain, and overall, are assessed and categorised into: A. Low risk of bias: plausible bias unlikely to seriously alter the results (categorised as 'Yes' in Risk of Bias table) B. High risk of bias: plausible bias that seriously weakens confidence in the results (categorised as 'No' in Risk of Bias table) C. Unclear risk of bias: plausible bias that raises some doubt about the results (categorised as 'Unclear' in Risk of Bias table) Trials with high risk of bias (defined as at least 3 out of 5 domains categorised as 'No') will not be included in the meta‐analysis. If the raters disagree, the final rating will be made by consensus with the involvement of another member of the review group. Where inadequate details of randomisation and other characteristics of trials are provided, authors of the studies will be contacted in order to obtain further information. Non‐concurrence in quality assessment will be reported.
Measures of treatment effect
1. Binary outcomes
Where binary outcomes (proportions) are used, we will calculate fixed‐effects model relative risks (RR) (Furukawa 2002), and 95% confidence intervals for each outcome. We will perform a sensitivity analysis for heterogeneity, and if this is significant, we will use a random effects model. The relative risk will be chosen over the odds ratio because the latter tends to overstate effect size when event rates are high (Higgins 2008). 2. Continuous data
2.1 Summary statistic
For continuous outcomes we will estimate a Weighted Mean Difference (WMD) between groups. WMDs are based on the fixed‐effects model, unless there is significant heterogeneity, in which case we will use the random‐effects model. We will calculate Standardised Mean Differences (SMD) for continuous outcomes measured with different scales. 2.2 Endpoint versus change data
Since there is no principal statistical reason why endpoint and change data should measure different effects (Higgins 2008), we will use scale endpoint data which is easier to interpret from clinical point of view. If endpoint data are not available, we will use change data. 2.3 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we aim to apply the following standards to all data before inclusion: (a) standard deviations (SD) and means (M) are reported in the paper or obtainable from the authors; (b) when a scale starts from the finite number zero, the SD, when multiplied by two, is less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution (Altman 1996)); (c) if a scale starts from a positive value the calculation described above will be modified to take the scale starting point into account. In these cases skew is present if 2SD>(S‐S min), where S is the mean score and S min is the minimum score. Endpoint scores on scales often have a finite start and end point and these rules can be applied. When continuous data are presented on a scale which includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not. These skewed data will be excluded and then included to see if they make a substantive difference in the results. If it does, data will be excluded. If it does not, data will be included and results discussed. 2.4 Data synthesis
When standard errors instead of standard deviations are presented, the former will be converted to the standard deviations. If standard deviations are not reported and cannot be calculated from available data, authors will be asked to supply the data. In the absence of data from authors, we will calculate the SD using the p‐values and the sample size of the group(s) present in individual studies (Chapter 8.5.2.5 "P‐values" from the Cochrane Handbook version 4.2.6).
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby p‐values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes Type I errors (Bland 1997; Gulliford 1999). Where clustering has not been accounted for in primary studies, we will present the data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intra class correlation coefficients of their clustered data and to adjust for this by using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will also present these data as if from a non‐cluster randomised study, but adjusted for the clustering effect. The binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the intra‐class correlation coefficient (ICC) [Design effect = 1+(m‐1)*ICC] (Donner 2002). If the ICC has not been reported it will be assumed to be 0.1 (Ukoumunne 1999).
2. Studies with multiple treatment groups
Where a study involved more than two treatment groups, if relevant, the additional treatment groups will be presented in additional relevant comparisons. Groups will either be combined in two categories of treatment‐no treatment, where appropriate, to create a single pair‐wise comparison. Alternatively, if groups differ among them, groups will be split so they can be compared to each group. However data will not be double‐counted. Where the additional treatment groups were not relevant, these data will not be reproduced.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow up data must lose credibility (Xia 2009). Should more than 40% of data be unaccounted for we will not reproduce these data or use them within analyses, with the exception of the analysis of numbers leaving the study early. 2. Binary
In the case where attrition for a binary outcome is between 0 and 40% and outcomes of these people are described, we will include these data as reported. Where these data are not clearly described, data will be presented on a 'once‐randomised‐always‐analyse' basis, assuming an intention to treat analysis. Those lost to follow up will be all assumed to have a negative outcome. For example, for the outcome of employment, those who were lost to follow up will be considered all unemployed. A final sensitivity analysis will be undertaken testing how prone the primary outcomes are to change when 'completer' data only are compared to the intention to treat analysis using the negative assumption. 3. Continuous
In the case where attrition for a continuous outcome is between 0 and 40% and completer‐only data are reported, we will reproduce these. 4. Intention‐to‐treat (ITT)
Intention‐to‐treat (ITT) will be used when available. We anticipate that in some studies, in order to do an ITT analysis, the method of last observation carried forward (LOCF) would be employed within the study report. As with all methods of imputation to deal with missing data, LOCF introduces uncertainty about the reliability of the results. Therefore, where LOCF data have been used in the analysis, it will be indicated in the review.
Assessment of heterogeneity
Overall, if possible, results will be calculated based on the fixed‐effects model. We plan to quantify the impact of statistical heterogeneity in the meta‐analysis using I2, a measure of the degree of inconsistency in the studies' results. This measure describes the percentage of total variation across studies due to heterogeneity rather than chance. The values of I2 lie between 0% and 100%, and a simplified categorisation of heterogeneity that we plan to use is of low (I2 value of 25%); moderate (I2 value of 50%); and high (I2 value of 75%) (Higgins 2003). In addition, a Chi2 test will be included to assess whether observed differences in results are compatible with chance alone. A low p‐value provides evidence of heterogeneity of intervention effects.
Assessment of reporting biases
A funnel plot will be used to assess whether the review is subject to publication bias where 10 or more studies are available. If asymmetry is detected we will also assess other possible causes such as selection bias, reporting bias, true heterogeneity and artefact. The possible existence of small study effects will be examined by Egger's regression method (Egger 1997) as well as by visual inspection of the graph. Where available we will compare the study protocols to published reports to assess for outcome reporting bias. Otherwise we will compare the 'Methods' section of the study to the 'Results' section of the report. If we suspect outcome reporting bias we will contact authors for data.
Data synthesis
We will synthesise data performing meta‐analysis using the fixed‐effects model. If heterogeneity is present among the included studies, we will use the random‐effects model. Where available, the analyses will be based on intention‐to‐treat data from the individual studies. The data from included trials will be combined in a meta‐analysis if they are sufficiently homogeneous, both clinically and statistically.
Subgroup analysis and investigation of heterogeneity
If necessary, we will perform subgroup analysis in order to investigate heterogeneity in the case scenario I2 was superior to 50%. If the number of studies allows, analyses will be performed taking factors that may contribute to the existence of clinical heterogeneity into account. These might include diagnosis, age, sex or different doses of modafinil administered.
Sensitivity analysis
We will perform sensitivity analyses to determine the impact of study quality on outcome, including and excluding studies with missing data. We will also investigate the effect of including studies with implied randomisation and high attrition rates by sensitivity analyses. In addition, different doses of modafinil will be compared with regard to the primary outcomes of mental state and cognitive function using sensitivity analyses.
Appendix 4. Checklist to aid consistency and reproducibility of GRADE assessments
| Trial limitations | SoF outcome 1 (Mental state ‐ clinically important change ‐ worsening psychosis) | SoF outcome 1 (Cognitive function: 1. Overall ‐ average score (MCCB high = good)) | SoF outcome 3 (Adverse events ‐ serious adverse effect) | SoF outcome 4 (Global state) | SoF outcome 5 (Leaving the study early) | SoF outcome 6 (Quality of life ‐ any change as defined by each study) | SoF outcome 7 (Service utilisation: hospital admission) | |
| Risk of bias a | Was random sequence generation used (i.e. no potential for selection bias)? | Unclear | Yes | Unclear | Unclear | Yes | Yes | Yes |
| Was allocation concealment used (i.e. no potential for selection bias)? | Unclear | Yes | Unclear | Unclear | Yes | Unclear | Yes | |
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or was the outcome not likely to be influenced by lack of blinding? | Unclear | Yes | Unclear | Unclear | Yes | Yes | Yes | |
| Was there blinding of outcome assessment (i.e. no potential for detection bias) or was the outcome measurement not likely to be influenced by lack of blinding? | Unclear | Yes | Unclear | Unclear | Yes | Yes | Yes | |
| Was an objective outcome used? | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | |
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?e | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | |
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | |
| No other biases reported (i.e. no potential of other bias)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Did the trials end up as scheduled (i.e. not stopped early)? | Yes | Yes | Yes | Yes | No (*) | Yes | Yes | |
| Inconsistencyb | Point estimates did not vary widely? | No (*) | N/A | N/A | N/A | Yes | N/A | N/A |
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least 1 of the included studies point estimate; some: confidence intervals overlap but not all overlap at least 1 point estimate; no: at least 1 outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? |
Substantial | N/A | N/A | N/A | Substantial | N/A | N/A | |
| Was the direction of effect consistent? | No (*) | N/A | N/A | N/A | Yes | N/A | N/A | |
| What was the magnitude of statistical heterogeneity (as measured by I²) ‐ low (I² < 40%), moderate (I² 40% to 60%), high (I² > 60%)? | Low | N/A | N/A | N/A | Low | N/A | N/A | |
| Was the test for heterogeneity statistically significant (P < 0.1)? | Not statistically significant | N/A | N/A | N/A | Not statistically significant | N/A | N/A | |
| Indirectness | Were the populations in the included studies applicable to the decision context? | Applicable | Applicable | Applicable | N/A | Applicable | N/A | Applicable |
| Were the interventions in the included studies applicable to the decision context? | Applicable | Applicable | Applicable | N/A | Applicable | Applicable | Applicable | |
| Was the included outcome not a surrogate outcome? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Was the outcome time frame sufficient? | Insufficient (*) | Insufficient (*) | Insufficient (*) | Insufficient (*) | Sufficient | Insufficient (*) | Insufficient (*) | |
| Were the conclusions based on direct comparisons? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Imprecisionc | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | No (*) | No (*) | No (*) | No (*) | Yes | No (*) | No (*) |
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100 to 300 participants, low: < 100 participants)?e | Intermediate | Low (*) | Low (*) | Low (*) | Low (*) | Low (*) | Low (*) | |
| What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5 to 10 studies, small: < 5 studies)?e | Moderate | Small (*) | Small (*) | Moderate | Small (*) | Small (*) | Small (*) | |
| Was the outcome a common event (e.g. occurs more than 1/100)? | Yes | N/A | N/A | Yes | Yes | N/A | Yes | |
| Publication biasd | Was a comprehensive search conducted? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was grey literature searched? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| There was no industry influence on studies included in the review? | No (*) | Yes | Yes | Yes | Yes | Yes | Yes | |
| There was no evidence of funnel plot asymmetry? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| There was no discrepancy in findings between published and unpublished trials? | N/A | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
MCCB = MATRICS Consensus Cognitive Battery. N/A = not applicable. SOF = 'Summary of findings' table.
aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual trials. bQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I² statistic.
(*): key item for potential downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of findings' table. cWhen judging the width of the confidence interval, it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful. dQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry, and discrepancies between published and unpublished trials. eDepends on the context of the systematic review area.
Data and analyses
Comparison 1. Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ all short term.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mental state: 1a. Overall: clinically important change ‐ worsening psychosis | 6 | 209 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.28, 2.98] |
| 2 Mental state: 1b. Overall: average total score (BPRS, endpoint, high = poor) | 2 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐5.65, 4.32] |
| 3 Mental state: 1c. Overall: average total score (PANSS, endpoint, high = poor) | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 2.20 [‐9.56, 13.96] |
| 4 Mental state: 1d. Overall: average total score (PANSS, endpoint, high = poor, skewed data) | Other data | No numeric data | ||
| 5 Mental state: 2a. Specific: positive symptoms: i. average score ‐ short term (SAPS, endpoint, high = poor) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 1.45 [‐1.38, 4.28] |
| 6 Mental state: 2b. Specific: positive symptoms: ii. average score ‐ short term (PANSS scale, endpoint, high = poor, skewed data) | Other data | No numeric data | ||
| 7 Mental state: 3a. Specific: negative symptoms ‐ clinically important change (improvement) (> 20% reduction SANS) | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [1.07, 5.09] |
| 8 Mental state: 3b. Specific: negative symptoms: i. average score ‐ short term (PANSS, endpoint, high = poor) | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐5.15, 2.55] |
| 9 Mental state: 3c. Specific: negative symptoms: ii. average score (SANS, endpoint, high = poor) | 2 | 70 | Mean Difference (IV, Random, 95% CI) | ‐2.01 [‐4.23, 0.21] |
| 10 Mental state: 3d. Specific: negative symptoms: iii. average score ‐ short term (PANSS, endpoint, high = poor, skewed data) | Other data | No numeric data | ||
| 11 Cognitive function: 1a. Overall: average score: i. single dose (MCCB composite score, endpoint, high = good) | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 7.93 [‐0.57, 16.43] |
| 12 Cognitive function: 1b. Overall: average score: ii. short term (MCCB composite score, endpoint, high = good) | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐10.90, 4.70] |
| 13 Adverse event/effect(s): 1a. General: any adverse event | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.92, 1.09] |
| 14 Adverse event/effect(s): 1b. General: any serious adverse event | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.06, 12.42] |
| 15 Adverse event/effect(s): 2. Specific ‐ cardiovascular | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 Chest pain | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 15.08] |
| 15.2 Hypertension | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 70.16] |
| 15.3 Palpitations | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.80] |
| 15.4 Tachycardia | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.80] |
| 16 Adverse event/effect(s): 3. Specific ‐ gastrointestinal | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 Abdominal pain | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.04, 4.23] |
| 16.2 Bitter taste | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.14, 65.90] |
| 16.3 Constipation | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.80] |
| 16.4 Diarrhoea | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.19, 20.61] |
| 16.5 Dyspepsia | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.19, 20.61] |
| 16.6 Nausea | 2 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.48, 2.73] |
| 17 Adverse event/effect(s): 4. Specific ‐ infectious | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.1 Flu syndrome | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 4.0 [0.48, 33.22] |
| 17.2 Pharyngitis | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 70.16] |
| 17.3 Rhinitis | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.19, 3.00] |
| 18 Adverse event/effect(s): 5. Specific ‐ movement disorder ‐ average score (SAS, endpoint, high = poor, skewed data) | Other data | No numeric data | ||
| 19 Adverse event/effect(s): 6. Specific ‐ musculoskeletal | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19.1 Back pain | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.19, 20.61] |
| 20 Adverse event/effect(s): 7. Specific ‐ neurological | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 20.1 Dizziness | 3 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.65, 3.05] |
| 20.2 Fatigue | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 5.95 [0.33, 107.25] |
| 20.3 Headache | 3 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [0.97, 4.62] |
| 20.4 Sedation | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.6 [0.16, 2.22] |
| 20.5 Tinnitus | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.14, 65.90] |
| 20.6 Tremor | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.41, 9.87] |
| 21 Adverse event/effect(s): 8. Specific ‐ psychiatric | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 21.1 Anxiety | 2 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 2.23 [0.54, 9.26] |
| 21.2 Depression | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.17, 16.91] |
| 21.3 Insomnia | 3 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.49, 2.74] |
| 21.4 Irritability | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.01, 3.70] |
| 21.5 Nervousness | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [0.51, 158.52] |
| 22 Adverse event/effect(s): 9. Specific ‐ various other effects | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 22.1 Anorexia | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 70.16] |
| 22.2 Oedema | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.14, 65.90] |
| 22.3 Sexual dysfunction | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.15, 6.51] |
| 22.4 Weight gain | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.10, 2.47] |
| 23 Adverse event/effect(s): 10. Specific ‐ sleep ‐ average hours of sleep at endpoint | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.03, 1.63] |
| 24 Adverse event/effect(s): 11. Specific: weight ‐ average weight in kilograms at endpoint | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 4.63 [‐9.75, 19.01] |
| 25 Adverse event/effect(s): 12a. Specific: fatigue: i. average scores (CGI‐I, endpoint, high = poor, skewed data) | Other data | No numeric data | ||
| 26 Adverse event/effect(s): 12b. Specific: fatigue: ii. average scores (FSS, endpoint, high = poor, skewed data) | Other data | No numeric data | ||
| 27 Behaviour: average total score (NOSIE, endpoint, high = poor) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐1.72 [‐7.25, 3.81] |
| 28 Global state: 1. Clinically important change in global state ‐ no improvement (CGI = 4) | 1 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 6.36 [0.94, 43.07] |
| 29 Global state: 2. Relapse ‐ single dose | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 2.7 [0.13, 58.24] |
| 30 Global state: 3a. Average total score (CGI‐I scale, endpoint, high = poor) | 2 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐1.34, 0.42] |
| 31 Global state: 3b. Average total score (CGI‐S scale, endpoint, high = poor) | 2 | 70 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.63, 0.15] |
| 32 Leaving the study early: 1a. Any reason | 9 | 357 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.63, 2.52] |
| 33 Leaving the study early: 1b. Any reason ‐ single dose | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 2.7 [0.13, 58.24] |
| 34 Leaving the study early: 2. Due to adverse event | 2 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.05, 22.20] |
| 35 Quality of life: 1a. General: i. average total score (QOLI, endpoint, high = good) | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.18, 0.78] |
| 36 Quality of life: 1b. General: ii. average total score (PGWBI, endpoint, high = good) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐5.52, 4.16] |
| 37 Service use: hospital admission | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.06, 12.42] |
| 38 SENSITIVITY ANALYSIS: Mental state: overall ‐ worsening psychosis ‐ TRIAL WITH PARTICIPANTS WITH AFFECTIVE PSYCHOSES | 5 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.21, 2.77] |
Comparison 2. Modafinil (+ usual antipsychotic) versus placebo (+ usual antipsychotic) ‐ subscale data ‐ all short term.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arbabi 2012.
| Methods |
Allocation: randomised.
Blindness: double‐blind.
Duration: 8 weeks. Settings: inpatients. Design: parallel. Country: Iran. Study dates: January 2008 to January 2011. |
|
| Participants |
Diagnosis: schizophrenia (DSM‐IV‐TR). N = 46. Age: ˜34 years. Sex: 31 M ,11 F. History: minimum score of 60 on the PANSS, length of illness 86 to 96 months. |
|
| Interventions |
1. Modafinil: modafinil 200 mg (100 mg mid‐morning and evening) + risperidone 6 mg/day. 2. Placebo: placebo + risperidone 6 mg/day. Participants were allowed to take biperiden for the management of extrapyramidal symptoms. |
|
| Outcomes |
Usable data:
Unable to use:
|
|
| Notes | Iranian Clinical Trials Registry (IRCT138903131556N16). Funding: Tehran University of Medical Sciences. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized to receive modafinil or placebo in a 1:1 ratio using a computer‐generated code and the randomization was stratified by site." |
| Allocation concealment (selection bias) | Low risk | "The assignments were kept in sealed opaque envelopes until data analysis." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Throughout the study, the person who administered the medications, the rater and the patients were blind to assignments." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Throughout the study, the person who administered the medications, the rater and the patients were blind to assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was done. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | We found no other bias. |
Freudenreich 2009.
| Methods |
Allocation: randomised.
Blindness: double‐blind.
Duration: 8 weeks. Settings: outpatients. Design: parallel. Country: USA. Study dates: September 2003 to September 2007. |
|
| Participants |
Diagnosis: schizophrenia or schizoaffective disorder (DSM‐IV). N = 37. Age: ˜45 years. Sex: 27 M, 8 F. History: treated with clozapine, length of illness 18 to 20 years. |
|
| Interventions |
1. Modafinil: modafinil (flexible dose 50 mg to 300 mg) + clozapine. 2. Placebo: placebo + clozapine. |
|
| Outcomes |
Usable data:
Unable to use:
|
|
| Notes | Trial registry: NCT00573417. Funding: Cephalon Inc (modafinil manufacturer). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned." Comment: probably done. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double blind" Comment: Probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double blind" Comment: Probably done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6/18 (33%) in the placebo group did not complete the study. |
| Selective reporting (reporting bias) | High risk | Continuous data were presented as a slope, so we were unable to impute the information in the meta‐analysis. |
| Other bias | Low risk | We found no other bias. |
Kumar 2010.
| Methods |
Allocation: randomised.
Blindness: double‐blind.
Duration: 9 weeks. Settings: outpatients. Design: parallel. Country: India. Study dates: October 2007 to October 2008. |
|
| Participants |
Diagnosis: schizophrenia or schizoaffective disorder (DSM‐IV). N = 34. Age: not reported. Sex: not reported. History: troublesome drowsiness or hyper‐salivation. |
|
| Interventions |
1. Modafinil: modafinil (100 mg to 200 mg) + clozapine. 2. Placebo: placebo + clozapine. |
|
| Outcomes |
Usable data:
Unable to use:
|
|
| Notes | Trial registry: CTRI/2007/091/000020. Funding: Fluid Research Fund of CMC Vellore; modafinil and placebo supplied by Sun Pharmaceuticals. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Offsite computer‐generated, variable‐block‐size randomization." |
| Allocation concealment (selection bias) | Low risk | "Pharmacist‐dispensed, pre‐packed, serially numbered, containers ensured allocation concealment." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Participant, investigator, observer and data‐entry‐blinded trial." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Participant, investigator, observer and data‐entry‐blinded trial." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Imbalance in numbers or reasons for missing data." |
| Selective reporting (reporting bias) | High risk | Outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis. |
| Other bias | High risk | Early stopping of trial without meeting the target population. |
Lees 2017.
| Methods |
Allocation: randomised.
Blindness: double‐blind.
Duration: 4 weeks. Settings: outpatient. Design: cross‐over. Country: UK. Study dates: unclear. |
|
| Participants |
Diagnosis: schizophrenia (DSM‐IV) and healthy volunteers N = 46 (participants with schizophrenia) Age: ˜25 years Sex: 30 M, 10 F (participants that completed the study). History: clinically stable in a non‐acute phase. |
|
| Interventions |
1.Modafinil: single‐dose modafinil (200 mg) + second‐generation antipsychotic. 2. Placebo: placebo + second‐generation antipsychotic. |
|
| Outcomes |
Usable data:
Unable to use:
|
|
| Notes | Data not presented for phase A of the study, but this information was provided by the author. Trial registry: ISRCTN66900787. Funding: EU Innovative Medicines Initiative to the NEWMEDS programme. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Carried out via an online system at the King's Clinical Trials Unit, was by minimisation." |
| Allocation concealment (selection bias) | Low risk | "Carried out via an online system at the King's Clinical Trials Unit, was by minimisation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Capsules were supplied in coded bottles containing identical capsules of modafinil and placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Capsules were supplied in coded bottles containing identical capsules of modafinil and placebo" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Six schizophrenia participants withdrew following randomisation" Comment: Unable to obtain the information for this. |
| Selective reporting (reporting bias) | Low risk | Outcomes of interest in the review were reported in a manner not imputable in a meta‐analysis, however data provided by author. |
| Other bias | Low risk | We found no other bias. |
Lohr 2013.
| Methods |
Allocation: randomised.
Blindness: double‐blind.
Duration: 8 weeks. Settings: outpatients. Design: parallel. Country: USA. Study dates: unclear. |
|
| Participants |
Diagnosis: schizophrenia or schizoaffective disorder (DSM‐IV). N = 24. Age: ˜48 years. Sex: all male. Length of illness:
|
|
| Interventions |
1. Modafinil: modafinil (50 to 200 mg) + stable dose of a second‐generation antipsychotic. 2: Placebo: placebo + stable dose of a second‐generation antipsychotic. |
|
| Outcomes |
Usable data:
Unable to use:
|
|
| Notes | Trial registry: NCT00546403. Funding: Cephalon Inc (modafinil manufacturer). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was stratified by excessive daytime sleepiness (EDS) symptoms." |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “Double‐blind” Comment: Probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | “Double‐blind” Comment: Probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were analysed, only 1 participant left the study early. |
| Selective reporting (reporting bias) | High risk | Outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis. |
| Other bias | Low risk | We found no other bias. |
Michalopoulou 2015.
| Methods |
Allocation: randomised.
Blindness: double‐blind.
Duration: 2 weeks. Settings: outpatients. Design: parallel. Country: UK Study dates: December 2010 to September 2012. |
|
| Participants |
Diagnosis: schizophrenia (DSM‐IV). N = 49. Age: ˜36.3 years. Sex: 35 M, F 13. Length of illness: ˜12 years. History: chronic, clinically stable. |
|
| Interventions |
1. Modafinil: modafinil 200 mg + antipsychotic treatment + cognitive training (10 days). 2. Placebo: placebo + antipsychotic treatment + cognitive training (10 days). |
|
| Outcomes |
Usable data:
Unable to use:
|
|
| Notes | Trial registry: ISRCTN60687844. Funding: Innovative Medicines Initiative Joint Undertaking. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Stratified randomisation was performed for two preselected factors (gender and smoking status) with known effects on cognition." |
| Allocation concealment (selection bias) | Low risk | "To ensure concealment of the randomisation assignment, medication was provided in coded bottles containing identical capsules of active drug or placebo." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "To ensure concealment of the randomisation assignment, medication was provided in coded bottles containing identical capsules of active drug or placebo." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All raters were blind to treatment assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis using mixed‐effect models. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | We found no other bias. |
Pierre 2007.
| Methods |
Allocation: randomised.
Blindness: double‐blind.
Duration: 8 weeks. Settings: outpatients. Design: parallel. Country: USA. Study dates: March 2002 to March 2006. |
|
| Participants |
Diagnosis: schizophrenia or schizoaffective disorder (DSM‐IV). N = 21. Age: ˜49 years. Sex: 19 M, 1 F (only participants who were analysed). History: prominent negative symptoms as defined by a scale for the assessment of negative symptoms total score > 20. |
|
| Interventions |
1. Modafinil: modafinil (flexible dose 100 mg to 200 mg) + antipsychotic medication + 60% were taking an antidepressant. 2. Placebo: placebo + antipsychotic medication + 60% were taking an antidepressant. |
|
| Outcomes |
Usable data:
Unable to use:
|
|
| Notes | Funding: Stanley Research Foundation and West Coast College of Biological Psychiatry/Janssen. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned" Comment: Probably done. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “Double blind assessments were performed” Comment: Probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | “Double blind assessments were performed” Comment: Probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Last observation carried forward used for analysis. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in the protocol were addressed in the results. |
| Other bias | Low risk | We found no other bias. |
Prasuna 2015.
| Methods |
Allocation: randomised.
Blindness: double‐blind.
Duration: 12 weeks. Settings: unclear. Design: parallel. Country: India. Study dates: Unclear. |
|
| Participants |
Diagnosis: individuals who were on atypical antipsychotics for less than 2 weeks, irrespective of diagnosis (> 60% of patients with a schizophrenia spectrum disorder). N = 72 (36 with schizophrenia, 2 with delusional disorder, 3 with acute psychosis, 7 with psychotic depression, 15 mania with psychosis). Age: unclear. Sex: 31 M, 32 F (only participants who finished the study). History: unclear. |
|
| Interventions |
1. Modafinil: modafinil (dose 200 mg) + atypical antipsychotic medication. 2. Placebo: placebo + atypical antipsychotic medication. |
|
| Outcomes |
Usable data
Unable to use:
|
|
| Notes | Funding: Sun Pharmaceuticals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was done centrally, by an outside agency." |
| Allocation concealment (selection bias) | Low risk | "Randomization was done centrally, by an outside agency." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Similar looking placebo capsules." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Similar looking placebo capsules." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12.5% (9/72) of participants left the study early, no information on the type of analysis done. |
| Selective reporting (reporting bias) | High risk | Data presented in a method not imputable in a meta‐analysis. |
| Other bias | Low risk | We found no other bias. |
Sevy 2005.
| Methods |
Allocation: randomised.
Blindness: double‐blind.
Duration: 8 weeks. Settings: outpatients. Design: parallel. Country: USA. Study dates: 18 May 2001 to 11 September 2003. |
|
| Participants |
Diagnosis: schizophrenia or schizoaffective disorder (DSM‐IV). N = 24. Age: ˜38 years. Sex: 10 M, 14 F. History: moderately ill (CGI‐S ≥ 4). |
|
| Interventions |
1. Modafinil: modafinil (dose 100 mg to 200 mg) + atypical antipsychotic medication. 2: Placebo: placebo + atypical antipsychotic medication. |
|
| Outcomes |
Usable data:
Unable to use:
|
|
| Notes | Funding: Cephalon Inc (modafinil manufacturer). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned” Comment: Probably done. |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “Double blind” Comment: Probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | “Double blind” Comment: Probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Intention‐to‐treat analyses that included all randomised subjects.” |
| Selective reporting (reporting bias) | High risk | Adverse events only reported for the modafinil group. |
| Other bias | Low risk | We found no other bias. |
Shafti 2016.
| Methods |
Allocation: randomised.
Blindness: double‐blind.
Duration: 8 weeks. Settings: inpatients. Design: parallel. Country: Iran. Study dates: unclear. |
|
| Participants |
Diagnosis: schizophrenia (DSM‐V). N = 50. Age: ˜40 years. Sex: male. Length of illness: ˜12 years. History: chronic, high negative symptoms score. |
|
| Interventions |
1. Modafinil: modafinil 200 mg + haloperidol (5 to 10 mg). 2. Placebo: placebo + haloperidol (5 to 10 mg). |
|
| Outcomes |
Usable data:
Unable to use:
Not used in this review:
|
|
| Notes | No clinical trial registry. Funding: unclear. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomly allocated.” |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The placebo and modafinil tablets had the same shape and color to make it difficult for the patients and the physician to differentiate them from each other." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The placebo and modafinil tablets had the same shape and color to make it difficult for the patients and the physician to differentiate them from each other." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "While patients were free to withdraw from the study at any stage without prejudice, there was no dropout in the course of the evaluation in any of the groups." |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in the methods section are adequately reported in the results. |
| Other bias | Low risk | We found no other bias. |
Spence 2005.
| Methods |
Allocation: randomised.
Blindness: double‐blind.
Duration: 1 week. Settings: inpatient (admitted for 24 hours observation) Design: cross‐over. Country: UK. Study dates: unclear. |
|
| Participants |
Diagnosis: schizophrenia (DSM‐IV). N = 19. Age: ˜37 years. Sex: all males. Length of illness: ˜14 years. History: prominent negative symptomatology. |
|
| Interventions |
1. Modafinil: single‐dose modafinil 100 mg + "most patients were receiving oral antipsychotics". 2: Placebo: placebo + "most patients were receiving oral antipsychotics". |
|
| Outcomes |
Usable data:
Not used in this review:
|
|
| Notes | Trial registry: ISRCTN54567688. Funding: "Investigator‐led award". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation, performed by a pharmacist (not a member of the research team), was achieved by drawing labelled counters; this ensured that approximately equivalent numbers of patients received modafinil and placebo on day 1, and vice versa on day 2." |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “Double blind” Comment: Probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | “Double blind” Comment: Probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data would not have impacted the data extracted for this meta‐analysis. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in the protocol were reported in the report. |
| Other bias | Low risk | None detected. |
General F = females. M = males. N = number. SD = standard deviation.
Cognitive scales COGBAT = Cognitive Basic Assessment. CANTAB = Cambridge Neuropsychological Test Automated Battery. COWAT = Controlled word association test. CPT‐IP = Continuous performance test‐identical pair. CVLT = California verbal learning test DMST = Delayed match to sample task. MATRICS = Measurement and Treatment Research to Improve Cognition in Schizophrenia. MCCB = MATRICS Consensus Cognitive Battery. ODRT = Oculomotor delayed response test. TMT = Trail Making Test RAVL = Rey auditory verbal learning test.
Quality of life scales PGWBI = Psychological General Well‐Being Index. QOLI = Quality of Life Interview.
Mental state scales BPRS = Brief Psychiatric Rating Scale. CGI = Clinical Global Impression CGI‐I = Clinical Global Impression ‐ Improvement Scale. CGI‐S = Clinical Global Impression ‐ Severity Scale. NOSIE = Nurses' Observation Scale for Inpatient Evaluation. PANSS = Positive and Negative Syndrome Scale. SANS = Scale for the Assessment of Negative Symptoms. SAPS = Scale for the Assessment of Positive Symptoms.
Other DSM = Diagnostic and Statistical Manual of Mental Disorders. DSM‐IV‐TR = Diagnostic and Statistical Manual, Fourth edition, Text Revision. FMRI = Functional magnetic resonance imaging. UPSA‐B = University of California San Diego (UCSD) Performance‐based Skills Assessment‐Brief. Side effect scales AIMS = Abnormal Involuntary Movement Scale. BARS = Barnes Akathisia Rating Scale. DAI = Drug Attitude Inventory. DSM = Diagnostic and Statistical Manual of Mental Disorders. ESS = Epworth Sleepiness Scale. ESRS = Extrapyramidal Symptom Rating Scale. FSS = Fatigue Severity Scale. NHRS = Nocturnal Hyper‐salivation Rating Scale. PANSS = Positive and Negative Syndrome Scale. SAS = Simpson‐Angus Scale. SOFAS = Social and Occupational Functioning Assessment Scale. UKU = Udvalg for Kliniske Undersøgelser.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Kane 2008 |
Allocation: randomised. Participants: people with schizophrenia. Interventions: armodafinil versus placebo, not modafinil. |
| Leblanc 2006 |
Allocation: randomised.
Participants: people with schizophrenia spectrum disorder.
Interventions: modafinil + second‐generation antipsychotic vs placebo + second‐generation antipsychotic. Reason for exclusion: unable to use data ‐ we contacted the authors as the data should have been useful but was reported in a method not imputable in a meta‐analysis. We received no response. |
| Minzenberg 2007 |
Allocation: randomised cross‐over trial. Participants: people with clinically stable chronic schizophrenia. Interventions: modafinil vs placebo. Outcomes: imaging outcomes (FMRI), not protocol outcome. |
| Minzenberg 2010a |
Allocation: intra‐participant dose randomisation. Participants: people with schizophrenia. Interventions: modafinil vs different doses of modafinil. Outcomes: performance on measures of working memory, episodic memory, and cognitive control, not protocol outcomes. |
| Minzenberg 2010b |
Allocation: randomised. Participants: people with schizophrenia. Interventions: modafinil vs placebo. Outcome: imaging results (FMRI), not protocol outcome. |
| NCT00057707 |
Allocation: randomised. Participants: people with schizophrenia and healthy volunteers. Interventions: modafinil vs placebo. Outcome: connectivity of brain regions underlying working memory, not protocol outcome. |
| NCT00373672 |
Allocation: randomised. Participants: people with schizophrenia. Interventions: armodafinil versus placebo, not modafinil. |
| NCT00423943 | Allocation: randomised. Participants: people with schizophrenia. Interventions: modafinil vs placebo. Outcomes: EEG and prepotency task, not protocol outcomes. |
| NCT00487942 |
Allocation: randomised. Participants: people with schizophrenia. Interventions: armodafinil versus placebo, not modafinil. |
| NCT00772005 |
Allocation: randomised. Participants: people with schizophrenia. Interventions: armodafinil versus placebo, not modafinil. |
| Scoriels 2011 |
Allocation: randomised.
Participants: people with first‐episode psychosis (Melbourne criteria). Interventions: modafinil + second‐generation antipsychotic vs placebo + second‐generation antipsychotic. Reason for exclusion: no usable data. Cross‐over trial, data were not presented for phase A of the study, see protocol. |
| Turner 2004 |
Allocation: randomised.
Participants: people with schizophrenia (DSM‐IV). Interventions: single‐dose modafinil 200 mg + antipsychotics vs single‐dose placebo (lactose) + antipsychotic. Reason for exclusion: no usable data. Cross‐over trial, data were not presented for phase A of the study, see protocol. |
DSM = Diagnostic and Statistical Manual of Mental Disorders. EEG = electroencephalogram FMRI = Functional magnetic resonance imaging
Differences between protocol and review
We have made a number of minor changes to the published protocol.
Title: We changed the title to more accurately reflect the types of participants included in the review.
Objectives: We clarified that review does include people with schizophrenia or related disorders as described in 'Types of participants' in the published protocol.
Outcomes: We changed the order of the primary outcomes, as we considered mental state to be of more importance for people with schizophrenia.
We have changed wording of outcomes to reflect latest terminology in Cochrane Schizophrenia Group (CSzG) methods template. For example no clinically important change is now clinically important change.
We have updated sections of our methods to reflect updates to CSzG methods template. These are not major changes to the methods, the changes are refinements and clarifications to previous statements. For published protocol methods please see Appendix 3
The background was updated with up‐to‐date references.
We included Prasuna 2015 a trial in which some of the participants had an affective psychosis, we made a sensitivity analysis regarding this outcome and the results were not changed.
We decided to present outcomes for the sub‐scores of scales measuring cognitive outcomes because one of the potential indications for modafinil was improvement of cognitive function, thus, we considered these outcomes relevant.
We decided to establish an appendix' Checklist to aid consistency and reproducibility of GRADE assessments' (Meader 2014) in order to aid with the standardisation of the 'Summary of findings' tables (Appendix 4).
Contributions of authors
Javier Ortiz‐Orendain: writing background, selection of studies, data extraction, 'Summary of findings' table, completion of report.
Sergio A Covarrubias‐Castillo: writing background, selection of studies, data extraction, 'Summary of findings' table, completion of report.
Alan Omar Vazquez‐Alvarez: writing background, selection of studies, data extraction, completion of report.
Santiago Castiello de Obeso: methodological support, completion of report.
Gustavo E Arias‐Quiñones: writing of background, completion of report.
Maya Seegers: writing of background, completion of report.
Luis Enrique Colunga‐Lozano: methodological support, 'Summary of findings' table, completion of report.
Sources of support
Internal sources
No sources of support supplied
External sources
Pinsent Darwin Fund of the University of Cambridge, UK.
NIHR CLAHRC for Cambridgeshire & Peterborough, UK.
Cambridge Cognition Ltd., UK.
Declarations of interest
Javier Ortiz‐Orendain: None
Sergio A Covarrubias‐Castillo: None
Alan Omar Vazquez‐Alvarez: None
Santiago Castiello de Obeso: None
Gustavo E Arias‐Quiñones: None
Maya Seegers: None
Luis Enrique Colunga‐Lozano: None
New
References
References to studies included in this review
Arbabi 2012 {published data only}
- Arbabi M, Bagheri M, Rezaei F, Ahmadi‐Abhari S‐A, Tabrizi M, Khalighi‐Sigaroudi F, et al. A placebo‐controlled study of the modafinil added to risperidone in chronic schizophrenia. Psychopharmacology 2012;220(3):591‐8. [CSzG: 23895; IRCT138903131556N16] [DOI] [PubMed] [Google Scholar]
Freudenreich 2009 {published data only}
- Freudenreich O, Henderson DC, Macklin EA, Evins AE, Fan X, Cather C, et al. Modafinil for clozapine‐treated schizophrenia patients. a double‐blind, placebo‐controlled trial. 12th International Congress on Schizophrenia Research; 2009 Mar 28‐Apr 1; California (CA). San Diego (CA): Oxford University Press, 2009:358. [CSzG: 20180]
- Freudenreich O, Henderson DC, Macklin EA, Evins AE, Fan XD, Cather C, et al. Modafinil for clozapine‐treated schizophrenia patients: a double‐blind, placebo‐controlled pilot trial. Journal of Clinical Psychiatry 2009;70(12):1674‐80. [CSzG: 19643; MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson DC, Freudenreich O, Borba CPC, Wang X, Copeland PM, Macklin E, et al. Effects of modafinil on weight, glucose and lipid metabolism in clozapine‐treated patients with schizophrenia. Schizophrenia Research 2011;130(1‐3):53‐6. [CSzG: 23071] [DOI] [PubMed] [Google Scholar]
- NCT00573417. A placebo‐controlled trial of modafinil (Provigil) added to clozapine in patients with schizophrenia. http://www.clinicaltrials.gov 2007. [CSzG: 15613]
Kumar 2010 {published data only}
- Kumar S, Tharyan P, Thomas N, Adams CE. Modafinil for clozapine induced adverse effects in people with schizophrenia and schizoaffective disorder in remission: a randomized, placebo‐controlled trial stopped early for harms. 8th Winter Symposium on Evidence Informed Healthcare; 2010 Jan 11‐14; Vellore, India. Vellore, India, 2010. [CSzG: 19145]
Lees 2017 {published and unpublished data}
- ISRCTN66900787. Neuroimaging effects of a single dose of modafinil on brain activation in patients with schizophrenia: a randomised controlled trial. http://isrctn.org/ISRCTN66900787 2013. [CSzG: 28544]
- Lees J, Michalopoulou PG, Lewis SW, Preston S, Bamford C, Collier T, et al. Modafinil and cognitive enhancement in schizophrenia and healthy volunteers: the effects of test battery in a randomised controlled trial. Psychological Medicine 2017;47(13):2358‐68. [CSzG: 38245; DOI: 10.1017/S0033291717000885] [DOI] [PubMed] [Google Scholar]
Lohr 2013 {published data only}
- Liu L, Lohr JB, Caligiuri MP, Perin T, May T, DelaPena‐Murphy J, et al. Modafinil as an adjunctive treatment strengthened circadian activity rhythms in schizophrenia. Sleep 2013;36:A309‐10. [CSzG: 28532] [Google Scholar]
- Lohr JB, Liu L, Caligiuri MP, Kash TP, May TA, Murphy JD, et al. Modafinil improves antipsychotic‐induced parkinsonism but not excessive daytime sleepiness, psychiatric symptoms or cognition in schizophrenia and schizoaffective disorder: a randomized, double‐blind, placebo‐controlled study. Schizophrenia Research 2013;150(1):289‐96. [CSzG: 28612] [DOI] [PubMed] [Google Scholar]
- NCT00546403. Modafinil augmentation therapy for excessive daytime sleepiness and negative symptoms in patients with schizophrenia. http://www.clinicaltrials.gov 2007. [CSzG: 15638]
Michalopoulou 2015 {published data only}
- ISRCTN60687844. A pilot study of cognitive enhancer and cognitive training combination: testing a therapeutic paradigm for cognitive impairment in schizophrenia. http://www.controlled‐trials.com 2010. [CSzG: 21917]
- Michalopoulou P, Lewis S, Drake R, Reichenberg A, Emsley R, Kalpakidou A, et al. The effects of modafinil on cognitive training: a proof‐of‐concept trial in patients with schizophrenia. Schizophrenia Research 2014;153(Suppl 1):S216‐7. [CSzG: 28841] [Google Scholar]
- Michalopoulou PG, Lewis SW, Drake R, Reichenberg A, Emsley R, Kalpakidou AK, et al. The effects of a combined intervention for cognition in schizophrenia on COGSTATE schizophrenia battery. European Psychiatry 2015; Vol. 30:255. [CSzG: 32196; MEDLINE: ]
- Michalopoulou PG, Lewis SW, Drake RJ, Reichenberg A, Emsley R, Kalpakidou AK, et al. Modafinil combined with cognitive training: pharmacological augmentation of cognitive training in schizophrenia. European Neuropsychopharmacology 2015; Vol. 28:1178‐89. [CSzG: 29897; MEDLINE: ] [DOI] [PubMed]
- Michalopoulou PG, Lewis SW, Drake RJ, Reichenberg A, Emsley R, Kalpakidou AK, et al. The effects of a single dose of modafinil on MATRICS Consensus Cognitive Battery performance in chronic schizophrenia. Schizophrenia Bulletin 2015;41:S324. [CSzG: 29551] [Google Scholar]
- Reichenberg A. The effects of modafinil and cognitive training on cognitive performance. Neuropsychopharmacology 2012;38(S1):S36. [CSz: 25055] [Google Scholar]
Pierre 2007 {published data only}
- Peloian JH, Pierre JM. Modafinil: a candidate for pharmacotherapy of negative symptoms in schizophrenia. Progress in Neurotherapeutics and Neuropsychopharmacology 2008;3(1):259‐74. [CSzG: 16053] [Google Scholar]
- Pierre J. Modafinil for schizophrenia. Stanley Foundation Research Programs 2009. [CSzG: 17281]
- Pierre J, Peloian JH, Wirshing DA, Wirshing WC, Marder SR. A double‐blind placebo controlled trial of modafinil for negative symptoms in schizophrenia. Schizophrenia Bulletin 2005;31:501. [CSzG: 11634] [DOI] [PubMed] [Google Scholar]
- Pierre JM, Peloian JH, Wirshing DA, Wirshing WC, Marder SR. A double‐blind placebo controlled trial of modafinil for negative symptoms in schizophrenia. Neuropsychopharmacology 2005;30(Suppl 1):S207. [CSzG: 14785] [DOI] [PubMed] [Google Scholar]
- Pierre JM, Peloian JH, Wirshing DA, Wirshing WC, Marder SR. A randomized, double‐blind, placebo‐controlled trial of modafinil for negative symptoms in schizophrenia. Journal of Clinical Psychiatry 2007;68(5):705‐10. [CSzG: 14567; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Prasuna 2015 {published data only}
- Prasuna PL, Sudhakar TP. Impact of modafinil add‐on with atypical anti‐psychotics on excessive daytime drowsiness. Indian Journal of Psychological Medicine 2015; Vol. 37, issue 4:388‐92. [CSzG: 33412; MEDLINE: ] [DOI] [PMC free article] [PubMed]
- Prasuna PL, Vijay Sagar KJ, Sudhakar TP, Rao GP. A placebo controlled trial on add‐on modafinil on the anti‐psychotic treatment emergent hyperglycemia and hyperlipidemia. Indian Journal of Psychological Medicine 2014;36(2):158‐63. [CSzG: 29051] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhakar TP, Rao PG, Prasuna PL, Sagar KJV. Study of effects of modafinil add‐on therapy on excessive day time drowsiness and weight gain in patients on atypical antipsychotics. Indian Journal of Psychological Medicine 2008;30(1):24‐31. [CSzG: 27938] [Google Scholar]
Sevy 2005 {published data only}
- Sevy S, Rosenthal MH, Alvir J, Meyer S, Visweswaraiah H, Gunduz‐Bruce H, et al. Double‐blind, placebo‐controlled study of modafinil for fatigue and cognition in schizophrenia patients treated with psychotropic medications. Journal of Clinical Psychiatry 2005;66(7):839‐43. [CSzG: 12195; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shafti 2016 {published data only}
Spence 2005 {published data only}
- Farrow TFD, Hunter MD, Haque R, Spence SA. Modafinil and unconstrained motor activity in schizophrenia: double‐blind crossover placebo‐controlled trial. British Journal of Psychiatry 2006;189:461‐2. [CSzG: 13355; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Green R, Hunter M, Spence S. Modafinil modulates prefrontal function in schizophrenia. Schizophrenia Research 2004;67(1):144. [CSzG: 11297] [Google Scholar]
- Hunter M, Ganesan VS, Spence SA. Effects of modafinil on prefrontal function and voluntary behaviour in chronic schizophrenia. Schizophrenia Bulletin 2005;31:511‐2. [CSzG: 11606] [Google Scholar]
- Hunter MD, Ganesan V, Wilkinson ID, Spence SA. Impact of modafinil on prefrontal executive function in schizophrenia. American Journal of Psychiatry 2006;163(12):2184‐6. [CSzG: 13946; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- ISRCTN54567688. Acute effects of modafinil on cognitive function and activation of prefrontal cortex in people with schizophrenia. http://www.isrctn.com/ISRCTN54567688 2003. [CSzG: 14543]
- Spence SA, Green RD, Wilkinson ID, Hunter MD. Modafinil modulates anterior cingulate function in chronic schizophrenia. British Journal of Psychiatry 2005;187(1):55‐61. [CSzG: 12423; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Kane 2008 {published data only}
- Kane JM, D'Souza DC, Patkar AA, Youakim J, Tiller J, Yang R, et al. Efficacy and tolerability of adjunctive armodafinil in patients with schizophrenia. International Congress on Schizophrenia Research; 2009 Mar 28 ‐ Apr 1; San Diego, California, USA. 2009.
- Kane JM, D'Souza DC, Patkar AA, Youakim JM, Tiller JM, Yang R, et al. Armodafinil as adjunctive therapy in adults with cognitive deficits associated with schizophrenia: A 4‐week, double‐blind, placebo‐controlled study. Journal of Clinical Psychiatry 2010;71(11):1475‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kane JM, D'Souza DC, Patkar AA, Yuakim J, Tiller J, Yang R, et al. Effects of adjunctive armodafinil in schizophrenia. American College of Neuropsychopharmacology Annual Meeting; 2008 Oct 1‐11; Scottsdale, Arizona. 2008.
- Tiller J, D'Souza D, Keefe RSE, Kane JM, Patkar AA, Youakim JM. Effects of adjunctive armodafinil on patients with schizophrenia. Neurology 2009;11(3):336. [Google Scholar]
Leblanc 2006 {published data only (unpublished sought but not used)}
- Leblanc J, Letourneau K, Demers MF, Bouchard RH, Roy MA. The impact of modafinil as an adjunct to a second generation antipsychotic on cognitive functioning in patients with first psychotic episode. Schizophrenia Research 2006;86(Suppl 1):S131. [Google Scholar]
- Letourneau K, Demers MF, Simard M, Emond C, Roy MA. A 10‐week, double‐blind, placebo controlled, cross‐over trial of adjunctive modafinil for neurocognitive impairments in schizophrenia. 12th International Congress on Schizophrenia Research; 2009 Mar 28‐Apr 1; San Diego, CA. San Diego (CA): Oxford Univ Press, 2009:283.
- NCT00314639. The impact of modafinil as an adjunctive to a second generation antipsychotic on cognitive functioning in schizophrenia and schizophrenia spectrum psychosis. http://www.clinicaltrials.gov 2006.
Minzenberg 2007 {published data only}
- Minzenberg MJ, Ursu S, Carter CS. Modafinil effects on cognitive control in schizophrenia: a double‐blind, placebo‐controlled single‐dose pharmaco‐FMRI study. Schizophrenia Bulletin 2007;33(2):379‐80. [Google Scholar]
Minzenberg 2010a {published data only}
- Minzenberg M, Carter CS. Modafinil effects on cognition in schizophrenia: a dose‐ranging study. Clinical and Translational Science 2010;3(2):S48. [Google Scholar]
- Minzenberg M, Prado JN, Yoon JH, Ragland JD, Carter CS. Dose‐response effects of single‐dose modafinil on cognition in schizophrenia. Biological Psychiatry 2010;67(9):87‐8. [Google Scholar]
- NCT00711464. A dose‐response study of modafinil effects on cognition in healthy adults and in schizophrenia patients. http://www.clinicaltrials.gov 2008.
Minzenberg 2010b {published data only}
- Minzenberg M, Yoon J, Prado JN, Soosman S, Carter C. Subcortical/cortical effects of single‐dose modafinil during cognitive control performance in schizophrenia. Neuropsychopharmacology 2010;35:S282. [Google Scholar]
- Minzenberg M, Yoon J, Nunez del Prado J, Soosman S, Carter C. Subcortical/cortical effects of single‐dose modafinil during cognitive control performance in schizophrenia. 49th Annual meeting of the American College of Neuropsychopharmacology; 2010 Dec 5‐9; Miami, Florida 2010.
- Minzenberg M, Yoon J, Soosman S, Prado JN, Watrous A, Carter C. Modafinil effects on locus coeruleus and ventral tegmental area during cognitive control performance in schizophrenia. Biological Psychiatry 2011;9(Suppl 1):282. [Google Scholar]
- Minzenberg M, Yoon JH, Nunez Del Prado J, Soosman S, Watrous AJ, Carter CS. Subcortical/cortical effects of modafinil during cognitive control in schizophrenia. Schizophrenia Bulletin 2011;37:290. [Google Scholar]
NCT00057707 {published data only}
- Apud JA, Rasetti R, Li C, Chen Q, Cheng X, Hochheiser J, et al. Modafinil modulates activation and functional coupling of the prefrontal cortex during working memory in patients with schizophrenia. Neuropsychopharmacology 2012;38(S1):S433. [Google Scholar]
- NCT00057707. Randomized, double‐blinded, placebo controlled study of the effects of modafinil on cognitive function in patients with schizophrenia and normal controls based on COMT genotype. http://www.clinicaltrials.gov 2006.
NCT00373672 {published data only}
- Bobo WV, Woodward ND, Sim MY, Jayathilake K, Meltzer HY. The effect of adjunctive armodafinil on cognitive performance and psychopathology in antipsychotic‐treated patients with schizophrenia/schizoaffective disorder: a randomized, double‐blind, placebo‐controlled trial. Schizophrenia Research 2011;130(1‐3):106‐13. [DOI] [PubMed] [Google Scholar]
- NCT00373672. Effects of modafinil on the tolerability and efficacy for cognition of atypical antipsychotic drugs in patients with schizophrenia or schizoaffective disorder. http://www.clinicaltrials.gov 2006.
NCT00423943 {published data only}
- Minzenberg MJ, Yoon JH, Cheng Y, Carter CS. Modafinil effects on middle‐frequency oscillatory power during rule selection in schizophrenia. Neuropsychopharmacology 2014;39(13):3018‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Yoon JH, Cheng Y, Carter CS. Sustained modafinil treatment effects on control‐related gamma oscillatory power in schizophrenia. Neuropsychopharmacology 2016; Vol. 41, issue 5:1231‐40. [MEDLINE: ] [DOI] [PMC free article] [PubMed]
- NCT00423943. Modafinil for treatment of cognitive dysfunction in schizophrenia. http://www.clinicaltrials.gov 2007.
NCT00487942 {published data only}
- NCT00487942. A 4‐week, double‐blind, placebo‐controlled, parallel‐group, fixed‐dosage study to evaluate the efficacy and safety of armodafinil as adjunctive therapy in adults with cognitive deficits associated with schizophrenia. http://www.clinicaltrials.gov 2007. [DOI] [PubMed]
NCT00772005 {published data only}
- Kane JM, Yang R, Youakim JM. Adjunctive armodafinil for negative symptoms in adults with schizophrenia: a double‐blind, placebo‐controlled study. Schizophrenia Research 2012;135(1‐3):116‐22. [DOI] [PubMed] [Google Scholar]
- Kane JM, Youakim JM, Yang R, Tiller J. Adjunctive armodafinil for negative symptoms in patients with schizophrenia: a double‐blind, placebo‐controlled study. Schizophrenia Bulletin 2011;37:307‐8. [DOI] [PubMed] [Google Scholar]
- NCT00772005. Study to evaluate the efficacy and safety of armodafinil as adjunctive therapy in adults with schizophrenia. http://www.clinicaltrials.gov 2008.
Scoriels 2011 {published data only}
- Sahakian B, Robbins T. Cognitive enhancing drugs in preclinical models of schizophrenia and improvements in cognition by modafinil in patients with chronic and first episode schizophrenia. Schizophrenia Bulletin 2011;37:319. [Google Scholar]
- Scoriels L, Barnett JH, Murray GK, Cherukuru S, Fielding M, Cheng F, et al. Effects of modafinil on emotional processing in first episode psychosis. Biological Psychiatry 2011;69(5):457‐64. [DOI] [PubMed] [Google Scholar]
- Scoriels L, Barnett JH, Soma PK, Sahakian BJ, Jones PB. Effects of modafinil on cognitive functions in first episode psychosis. Psychopharmacology 2012;220(2):249‐58. [DOI] [PubMed] [Google Scholar]
Turner 2004 {published data only}
- Sahakian BJ. Study of the cognitive effects of modafinil in schizophrenia. National Research Register 2002; Vol. 4.
- Turner DC, Clark L, Pomarol‐Clotet E, McKenna P, Robbins TW, Sahakian BJ. Modafinil improves cognition and attentional set shifting in patients with chronic schizophrenia. Neuropyschopharmacology 2004;29(7):1363‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Turner DC, Sahakian BJ. Analysis of the cognitive enhancing effects of modafinil in schizophrenia. In: Cummings JL editor(s). Progress in Neurotherapeutics and Neuropsychopharmacology. New York, NY: Cambridge University Press, 2006:133‐47, xi. [Google Scholar]
Additional references
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andersen 2010
- Andersen ML, Kessler E, Murnane KS, McClung JC, Tufik S, Howell LL. Dopamine transporter‐related effects of modafinil in rhesus monkeys. Psychopharmacology 2010;210(3):439‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andrade 2015
- Andrade C, Kisely S, Monteiro I, Rao S. Antipsychotic augmentation with modafinil or armodafinil for negative symptoms of schizophrenia: systematic review and meta‐analysis of randomized controlled trials. Journal of Psychiatric Research 2015;31(60):14‐21. [DOI: 10.1016/j.jpsychires.2014.09.013] [DOI] [PubMed] [Google Scholar]
Andreasen 1983
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS). Iowa City, Iowa: University of Iowa, 1983. [Google Scholar]
Andreasen 1984
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, Iowa: University of Iowa, 1984. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM‐5. Washington, DC: American Psychiatric Publishing, 2013. [DOI: 10.1176/appi.books.9780890425596] [DOI] [Google Scholar]
Bastoji 1988
- Bastoji H, Jouvet M. Successful treatment of idiopathic hypersomnia and narcolepsy with modafinil. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 1988;12(5):695‐700. [DOI] [PubMed] [Google Scholar]
Battleday 2015
- Battleday RM, Brem AK. Modafinil for cognitive neuroenhancement in healthy non‐sleep‐deprived subjects: a systematic review. European Neuropsychopharmacology 2015;25(11):1865‐81. [DOI: 10.1016/j.euroneuro.2015.07.028] [DOI] [PubMed] [Google Scholar]
Benton 1994
- Benton AL, Hamsher KD. Multilingual Aphasia Examination.. Multilingual Aphasia Examination. 3rd Edition. Iowa City: AJA Associates, 1989. [Google Scholar]
Bland 1997
- Bland JM, Kerry SM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [Aperçu sur la problématique des indices d'efficacité thérapeutique, 3: comparaison des indices et utilisation. Groupe d'Etude des Indices D'efficacite]. Therapie 1999;54(4):405‐11. [PubMed] [Google Scholar]
Boutrel 2004
- Boutrel B, Koob GF. What keeps us awake: the neuropharmacology of stimulants and wakefulness promoting medications. Sleep 2004;27(6):1181‐94. [DOI] [PubMed] [Google Scholar]
Carpenter 1994
- Carpenter WT Jr, Buchanan RW. Schizophrenia. New England Journal of Medicine 1994;330:681‐90. [DOI] [PubMed] [Google Scholar]
Cornblatt 1988
- Cornblatt BA, Lenzenweger MF, Erlenmeyer‐Kimling L. The Continuous Performance Test, Identical Pairs Version: II. Contrasting attentional profiles in schizophrenic and depressed patients. Psychiatry Research 1988;29:65‐85. [DOI] [PubMed] [Google Scholar]
Dawson 2010
- Dawson N, Thompson RJ, McVie A, Thomson DM, Morris BJ, Pratt JA. Modafinil reverses phencyclidine‐induced deficits in cognitive flexibility, cerebral metabolism, and functional brain connectivity. Schizophrenia Bulletin 2010;38(3):457‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Saint Hilaire 2001
- Saint Hilaire Z, Orosco M, Rouch C, Blanc G, Nicolaidis S. Variations in extracellular monoamines in the prefrontal cortex and medial hypothalamus after modafinil administration: a microdialysis study in rats. Neuroreport 2001;12(16):3533‐7. [DOI] [PubMed] [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. 8th International Cochrane Colloquium; 2000 Oct 25‐28; Cape Town. Cape Town: The Cochrane Collaboration, 2000.
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Divine 1992
- Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7:623‐9. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. [DOI] [PubMed] [Google Scholar]
Dupuy 1984
- Dupuy HJ. The Psychological General Well‐Being Index (PGWBI). In: Wenger NK, Mattson ME, Furburg CD, Elinson J editor(s). Assessment of Quality of Life in Clinical Trials of Cardiovascular Therapies. New York: Le Jacq Publishing Inc, 1984:170‐83. [Google Scholar]
Duteil 1979
- Duteil J, Rambert FA, Pessonnier J, Gombert R, Assous E. A possible α‐adrenergic mechanism for drug (CRL 40028)‐induced hyperactivity. European Journal of Pharmacology 1979;59(1‐2):121‐3. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder CSO. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;13:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne D, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Engber 1998
- Engber TM, Koury EJ, Dennis SA, Miller MS, Contreras PC, Bhat RV. Differential patterns of regional c‐Fos induction in the rat brain by amphetamine and the novel wakefulness‐promoting agent modafinil. Neuroscience Letters 1998;241(2):95‐8. [DOI] [PubMed] [Google Scholar]
Ferraro 2005
- Ferraro L, Fuxe K, Agnati L, Tanganelli S, Tomasini MC, Antonelli T. Modafinil enhances the increase of extracellular serotonin levels induced by the antidepressant drugs fluoxetine and imipramine: a dual probe microdialysis study in awake rat. Synapse 2005;55(4):230‐41. [DOI] [PubMed] [Google Scholar]
Furukawa 2002
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(7):7‐10. [DOI] [PubMed] [Google Scholar]
Ghahremani 2011
- Ghahremani DG, Tabibnia G, Monterosso J, Hellemann G, Poldrack RA, London ED. Effect of modafinil on learning and task‐related brain activity in methamphetamine‐dependent and healthy individuals. Neuropsychopharmacology 2011;36(5):950‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldman‐Rakic 1998
- Goldman‐Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational knowledge. Handbook of Physiology: The Nervous System V. Vol. 5, Bethesda: American Physiological Society, 1987:373‐417. [Google Scholar]
Gozzi 2012
- Gozzi A, Colavito V, Etet PF, Montanari D, Fiorini S, Tambalo S, et al. Modulation of fronto‐cortical activity by modafinil: a functional imaging and fos study in the rat. Neuropsychopharmacology 2013;37(3):822‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gulliford 1999
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy U. Early Clinical Drug Evaluation (ECDEU) Assessment Manual for Psychopharmacology. National Institute of Mental Health, 1976. [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated September 2008). The Cochrane Collaboration, 2008. Available from handbook.cochrane.org.
Higgins 2011a
- Higgins JPT, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Honigfeld 1965
- Honigfeld G, Gillis RD, Klett CJ. Nurses' observation scale for inpatient evaluation: a new scale for measuring improvement in chronic schizophrenia. Journal of Clinical Psychology 1965;21:65‐71. [DOI] [PubMed] [Google Scholar]
Howes 2009
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III ‐ the final common pathway. Schizophrenia Bulletin 2009;35(3):549‐62. [DOI: 10.1093/schbul/sbp006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hunter 2006
- Hunter MD, Ganesan V, Wilkinson ID, Spence SA. Impact of modafinil on prefrontal executive function in schizophrenia. American Journal of Psychiatry 2006;163:2184‐6. [DOI] [PubMed] [Google Scholar]
Hutton 2009
- Hutton JL. Number needed to treat and number needed to harm are not the best way to report and assess the results of randomised clinical trials. British Journal of Haematology 2009;146(1):27‐30. [DOI] [PubMed] [Google Scholar]
Jääskeläinen 2012
- Jääskeläinen E, Juola P, Hirvonen N, McGrath J, Saha S, Isohanni M, et al. A systematic review and meta‐analysis of recovery in schizophrenia. Schizophrenia Bulletin 2012;39(6):1296‐306. [DOI: 10.1093/schbul/sbs130] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kahn 2013
- Kahn RS, Keefe RSE. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry 2013;70(10):1107‐12. [DOI: 10.1001/jamapsychiatry.2013.155] [DOI] [PubMed] [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Vol. Positive and Negative Syndrome Scale (PANSS) Manual, North Tonawanda, NY: Multi‐Health Systems, 1986. [Google Scholar]
Kay 1987
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin 1987;13(2):261‐76. [DOI] [PubMed] [Google Scholar]
Keshavan 1994
- Keshavan MS, Anderson S, Pettergrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. Journal of Psychiatric Research 1994;28(3):236‐65. [DOI: 10.1016/0022-3956(94)90009-4] [DOI] [PubMed] [Google Scholar]
Kirkpatrick 2006
- Kirkpatrick B, Fenton WS, Carpenter WT, Marder SR. The NIMH‐MATRICS consensus statement on negative symptoms. Schizophrenia Bulletin 2006;32(2):214‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M, et al. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. [DOI: 10.1002/9781119536604.ch4] [DOI]
Lehman 1988
- Lehman AF. A quality of life interview for the chronically mentally ill. Evaluation and Program Planning 1988;11(1):51‐62. [Google Scholar]
Leon 2006
- Leon AC, Mallinckrodt CH, Chuang‐Stein C, Archibald DG, Archer GE, Chartier K. Attrition in randomized controlled clinical trials: methodological issues in psychopharmacology. Biological Psychiatry 2006;59(11):1001‐5. [DOI] [PubMed] [Google Scholar]
Leucht 2005a
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophrenia Research 2005;79(2‐3):231‐8. [DOI] [PubMed] [Google Scholar]
Leucht 2005b
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry 2005;187:366‐71. [DOI] [PubMed] [Google Scholar]
Leucht 2006
- Leucht S, Kane JM, Etschel E, Kissling W, Hamann J, Engel RR. Linking the PANSS, BPRS, and CGI: clinical implications. Neuropsychopharmacology 2006;31(10):2318–25. [DOI: 10.1038/sj.npp.1301147] [DOI] [PubMed] [Google Scholar]
Lezak 1995
- Lezak MD. Neuropsychological Assessment. 3rd Edition. New York: Oxford University Press, 1995. [Google Scholar]
Lyons 1991
- Lyons T, French J. Modafinil ‐ the unique properties of a new stimulant. Aviation, Space, and Environmental Medicine 1991;62(5):432‐5. [PubMed] [Google Scholar]
Makela 2003
- Makela EH, Miller K. Three case reports of modafinil use in treating sedation induced by antipsychotic medications. Journal of Clinical Psychiatry 2003;64(4):485‐6. [DOI] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. [DOI] [PubMed] [Google Scholar]
McClellan 1998
- McClellan KJ, Spencer CM. Modafinil: a review of its pharmacology and clinical efficacy in the management of narcolepsy. CNS Drugs 1988;9(4):311‐24. [DOI] [PubMed] [Google Scholar]
McGrath 2004
- McGrath J, Saha S, Welham J, Saadi O, MacCauley C, Chant D. A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Medicine 2004;2(1):13. [DOI: 10.1186/1741-7015-2-13] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3(82):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Microsoft Excel 2016 [Computer program]
- Microsfot corporation. Microsoft Excel. Version 2016. Redmond, WA: Microsfot corporation, 2016.
Microsoft Word 2016 [Computer program]
- Microsfot corporation. Microsoft Word. Version 2016. Redmond, WA: Microsfot corporation, 2016.
Moachon 1996
- Moachon G, Kanmacher I, Clenet M, Matinier D. Pharmacokinetic profile of modafinil. Drugs of Today 1996;32:327‐37. [Google Scholar]
Mohamed 2008
- Mohamed S, Rosenheck R, Swartz M, Stroup S, Lieberman JA, Keefe RS. Relationship of cognition and psychopathology to functional impairment in schizophrenia. American Journal of Psychiatry 2008;165(8):978‐87. [DOI] [PubMed] [Google Scholar]
Nuechterlein 1986
- Nuechterlein KH, Edell WS, Norris M, Dawson ME. Attentional vulnerability indicators, thought disorder, and negative symptoms. Schizophrenia Bulletin 1986;12(3):408. [DOI] [PubMed] [Google Scholar]
Nuechterlein 2008
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch D, Cohen J, et al. The MATRICS Consensus Cognitive Battery, Part 1: Test selection, reliability, and validity. American Journal of Psychiatry 2008;165(2):203‐13. [DOI] [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Owen 2016
- Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet 2016;388(10039):86‐97. [DOI: 10.1016/S0140-6736(15)01121-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Randall 2003
- Randall DC, Shneerson JM, Plaha KK, File SE. Modafinil affects mood, but not cognitive function, in healthy young volunteers. Human Psychopharmacology 2003;18:163‐73. [DOI] [PubMed] [Google Scholar]
Robbins 1994
- Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia 1994;5:266‐81. [DOI] [PubMed] [Google Scholar]
Rosenthal 2004
- Rosenthal MH, Bryant SL. Benefits of adjunct modafinil in an open‐label, pilot study in patients with schizophrenia. Clinical Neuropharmacology 2004;27(1):38‐43. [PUBMED: 15090936] [DOI] [PubMed] [Google Scholar]
Scammell 2000
- Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagisawa M, Miller MS, et al. Hypothalamic arousal regions are activated during modafinil‐induced wakefulness. Journal of Neuroscience 2000;20(22):8620‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Scoriels 2013
- Scoriels L, Jones PB, Sahakian BJ. Modafinil effects on cognition and emotion in schizophrenia and its neurochemical modulation in the brain. Neuropharmacology 2013;64:168‐84. [DOI: 10.1016/j.neuropharm.2012.07.011] [DOI] [PubMed] [Google Scholar]
Shokraneh 2017
- Shokraneh F, Adams CE. Study‐based registers of randomized controlled trials: starting a systematic review with data extraction or meta‐analysis. BioImpacts 2017;7(4):209‐17. [DOI: 10.15171/bi.2017.25] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shokraneh 2019
- Shokraneh F, Adams CE. Study‐based registers reduce waste in systematic reviewing: discussion and case report. Systematic Reviews 2019;8:129. [DOI: 10.1186/s13643-019-1035-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simpson 1970
- Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica 1970;Suppl 212:11‐9. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Turner 2004a
- Turner DC, Clark L, Dowson J, Robbins TW, Sahakian BJ. Modafinil improves cognition and response inhibition in adult attention‐deficit/hyperactivity disorder. Biological Psychiatry 2004;55(10):1031‐40. [DOI] [PubMed] [Google Scholar]
Turner 2004b
- Turner DC, Clark L, Pomarol‐Clotet E, McKenna P, Robbins TW, Sahakian BJ. Modafinil improves cognition and attentional set shifting in patients with chronic schizophrenia. Neuropsychopharmacology 2004;29:1363‐73. [DOI] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1‐75. [PubMed] [Google Scholar]
Urbano 2007
- Urbano FJ, Leznik E, Llinás RR. Modafinil enhances thalamocortical activity by increasing neuronal electrotonic coupling. Proceedings of the National Academy of Sciences 2007;104(30):12554‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vos 2015
- Vos T, Barber RM, Bell B, Bertozzi‐Villa A, Biryukov S, Bolliger I, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386(9995):743‐800. [DOI: 10.1016/S0140-6736(15)60692-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 1999
- Wong YN, Simcoe D, Hartman LN, Laughton WB, King SP, McCormick GC, et al. A double‐blind, placebo‐controlled, ascending‐dose evaluation of the pharmacokinetics and tolerability of modafinil tablets in healthy male volunteers. Journal of Clinical Pharmacology 1999;39(1):340. [DOI] [PubMed] [Google Scholar]
Xia 2009
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7. [Google Scholar]


