Abstract
Objectives
Observational cohort studies in early RA are a key source of evidence, despite inconsistencies in methodological approaches. This narrative review assesses the spectrum of methodologies used in addressing centre-level effect and case-mix adjustment in early RA observational cohort studies.
Methods
An electronic search was undertaken to identify observational prospective cohorts of >100 patients recruited from two or more centres, within 2 years of an RA or early inflammatory arthritis diagnosis. References and author publication lists of all studies from eligible cohorts were assessed for additional cohorts.
Results
Thirty-four unique cohorts were identified from 204 studies. Seven percent of studies considered centre in their analyses, most commonly as a fixed effect in regression modelling. Reporting of case-mix variables in analyses varied widely. The number of variables considered in case-mix adjustment was higher following publication of the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement in 2007.
Conclusion
Centre effect is unreported or inadequately accounted for in the majority of RA observational cohorts, potentially leading to spurious inferences and obstructing comparisons between studies. Inadequate case-mix adjustment precludes meaningful comparisons between centres. Appropriate methodology to account for centre and case-mix adjustment should be considered at the outset of analyses.
Keywords: rheumatoid arthritis, early inflammatory arthritis, observational cohorts, centre effect, case-mix, methodology, narrative review
Rheumatology key messages
Centre effect is poorly reported and accounted for in early RA observational cohorts.
Case-mix adjustment varies widely in early RA observational cohorts.
Inadequate consideration of centre effect and case-mix can lead to spurious findings and impairs comparability.
Introduction
RA is an incurable inflammatory disease of the musculoskeletal system. Recent decades have witnessed an exponential growth in the publication of randomized controlled trials studying early aggressive interventions in RA. This evidence base has been the cornerstone of guidelines across the globe [1–3]. Observational research is crucial to understanding how trial evidence translates into real world practice.
While there is intense methodological scrutiny of clinical trials, there has been less historic attention given to the methodology of observational studies. This was addressed with the publication of the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement in October 2007, which established a checklist for reporting case–control, cohort and cross-sectional studies, with particular focus on the reporting of methods and results [4].
A key component of care is the environment within which it is delivered, what can be termed ‘centre effect’. Centre effect refers to how clinical outcomes can vary depending on the venue in which a patient receives treatment. Centre effect reflects both the way treatment is delivered in a particular unit and the case-mix of patients. The manner in which analysts of multicentre observational studies manage centre-level variation is crucial to ensure comparability, minimize bias and enhance causal inference [5].
Drivers of centre effects, related to the centre itself, include clinician treatment preferences, unit staffing and clinic capacity, and access to other health professionals. In addition, funding availability for higher cost drugs may vary by region. Case-mix between centres is also salient as there is geographic variation in patient-level factors, including sociodemographic characteristics and comorbidity.
Accounting for centre when comparing across patients
Clustering within centres should be accounted for during the design of a study (e.g. power calculations). Where the centre effect is treated as a nuisance factor this is typically done by incorporating a design effect. The design effect is a measure of variation of population distributions between centres, which allows power to be recalculated with centre clustering taken into account. This was initially developed for use in cluster randomized trials [6], but can be adapted to observational studies [7].
Once a study is established and data collected, the impact of centre should be considered. In its simplest form this could entail a description of differences in populations between centres. Failing to account for centre effect and/or case-mix in analyses will produce incorrect P-values and CIs. Clustering of data and potential effect modification within units can lead to an overestimation of the power of a study. It is true that including analysis of centre effect usually widens CIs, and makes P-values less likely to meet the <0.05 threshold, which is often adhered to without further considerations. Table 1a and b details common methods for reducing bias in estimates, and correcting CIs to account for centre clustering.
Table 1.
Methods to reduce bias in estimates and correct CIs to account for centre clustering
| Method | Notes |
|---|---|
| (a) Reducing bias in centre level point estimates | |
| Multilevel modelling | Allows within and between centre associations to be estimated within the same model (i.e. participant- and hospital-level factors) [8] |
| Generalized estimating equations | Extension of generalized linear models to incorporate within cluster correlation [9] |
| Bayesian hierarchical models | Calculates probability of observed data by simultaneously considering patient and centre level parameters [10] |
| Case-mix adjustment | This can account for when centres serve distinct populations with varying demographics [11] |
| (b) Correcting CIs to account for centre clustering | |
| Fixed effects | Assumes centre population is fixed and estimates effect variation within centres; will therefore not include any centre where only one exposure is present |
| Random effects | Assumes centre are a random sample from local population. Accounts for random variation in populations between centres. Does not require all exposures to be present in each centre |
| Cluster robust standard errors | Post-estimation calculation of standard errors accounting for clustering. Requires assumption that number of clusters goes to infinity [12] |
| Unconditional methods | Adjustment of CIs for effects of centre clustering; e.g. logistical regression with bootstrap resampling stratified by centre |
Statistical explanation footnote. To help understand this table, consider the example that you want to determine the odds of remission in patients with initial combination therapy vs monotherapy in a multicentre observational study. Imagine a hypothetical scenario where the odds ratio for remission is 1.2 (95% CI 1.1–1.3) for combination therapy. This would suggest 20% better odds of remission with the combination strategy. If there is a strong link between certain centres and remission this may bias the point estimate of the odds ratio, and (a) contains potential statistical methods to account for this bias. In addition, if there is significant variability across centres, standard models will under estimate the true width of the CIs. The statistical techniques in (b) are tools to improve the precision of the model.
Case-mix adjustment when comparing between centres
Hospitals within a region or country serve distinct populations with considerable differences in case-mix. When comparing between centres, comprehensive case-mix adjustment is crucial to ensure that estimated differences are not confounded by differences in case-mix, allowing for unbiased performance comparisons. There is a large body of work focussing on standardized case-mix approaches, for example the UK National Health Service has a standardized case-mix adjustment methodology for comparing outcomes nationally [13–15], and in other centralized healthcare systems [16]. Typically, case-mix approaches adjust for baseline sociodemographic factors such as age, gender, ethnicity and socio-economic position (SEP). SEP is defined as the socially derived economic factors that influence the position an individual or group hold within stratified society [17]. It is a method of defining an individual’s place in society. Many authors opt to measure SEP with a composite measure. An example is the Index of Multiple Deprivation, which utilizes income, employment, education, health, crime, barriers to housing and services, and living environment to rank the most to least deprived areas across England [18]. Alternatively, SEP can be considered using single variables including income, employment type and education. There is currently no consensus on appropriate case-mix adjustment in RA cohort studies, and the extent to which case-mix is included in analyses has not been investigated.
A narrative review was conducted to assess the spectrum of methodologies used in addressing centre-level effect and case-mix adjustment in multicentre early RA observational cohort studies. This review also addresses the impact of the STROBE statement on the reporting of centre-level effect and case-mix adjustment.
Methods
Eligibility criteria
Although this is a narrative review, we sought to identify relevant literature systematically using a systematic search of the literature. Specifically, the search focussed on prospective observational cohorts of adult patients with a diagnosis of RA or early inflammatory arthritis within 2 years of recruitment and exemplar studies using these cohorts. The 2-year disease duration was pragmatic. Early inflammatory arthritis typically accounts for the first 6 months from diagnosis, but a longer duration gave a higher probability of catching all eligible cohorts, accounting for the time taken to recruit newly diagnosed patients.
The rationale for limiting the search to prospective cohort studies of early RA was pragmatic, recognizing the magnitude of publications on cohort studies in the field. Cohort studies with 100 or more individuals and recruiting from two or more centres were included. Experimental, cross-sectional, pharmaco-economic and validation studies, and conference abstracts were excluded.
Search strategy
Three search strategies were employed for this review: an electronic search of databases; a review of reference lists of all articles identified as eligible for inclusion from the electronic search; and a review of the publications for all authors listed on eligible articles from the electronic search.
Electronic search
MEDLINE and Embase were searched from 1946 and 1974, respectively, to October 2017. Titles and abstracts were reviewed, identifying eligible studies.
Additional searches
The reference lists of all eligible studies from the electronic search were searched for further suitable manuscripts. In addition, publication histories for all authors on eligible studies were reviewed.
Data extraction
Detail on cohort characteristics, baseline case-mix data collection and adjustment, and consideration of centre-level effect in analyses were extracted and tabulated by a single author. A data extraction spreadsheet with the following column headings was used: First author; publication year; cohort name; data collection period; cohort type; country/countries conducted in; number of centres; sample size; disease duration on study entry; baseline sociodemographic variables included; outcome measure; and nature of centre effect inclusion in analyses.
Results
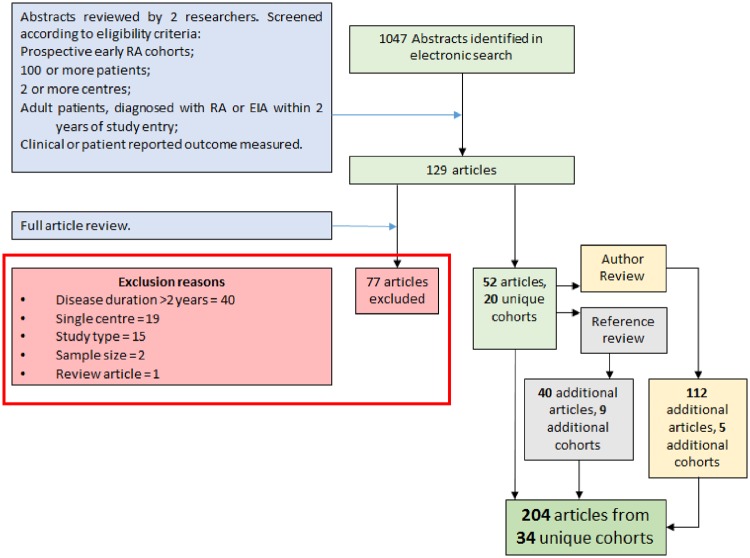
A total of 1047 studies were identified from the electronic search (see Fig. 1); 129 were selected for full review, of which 52 studies identified 20 unique cohorts that met eligibility criteria. Reference list review identified nine additional cohorts from 40 studies. The author review highlighted five further cohorts from 112 reviewed studies. This gave a total of 204 exemplar studies concerning 34 unique observational cohorts, listed in Table 2. Full reference details of all included studies can be found in supplementary Table S1, available at Rheumatology online.
Fig. 1.
Flow diagram of manuscript inclusion and exclusion
From an initial 1047 papers produced by a systematic literature search, 204 articles from 34 unique cohorts were identified for inclusion.
Table 2.
Early RA observational cohorts with case-mix details
| Cohort name or study first author | Study location | Data collection period | Centres (n) | Sample size | Symptom duration | Comorbidity | SEP | Smoking | Employment | Education |
|---|---|---|---|---|---|---|---|---|---|---|
| BARFOT | Sweden | 1992–2006 | 6 | 2800 | 6 weeks to 12 months | ✓ | × | ✓ | ✓ | × |
| CAPEA | Germany | 2010–13 | 118 | 1301 | <6 months | ✓ | × | ✓ | × | ✓ |
| CATCH | Canada | 2007 to present | 21 | 2524 | 6 weeks to 12 months | ✓ | × | ✓ | ✓ | ✓ |
| CLEAR | USA | 2001–05 | 5 | 300 | <24 months | × | × | ✓ | ✓ | ✓ |
| CONAART | Argentina | 2008–12 | 13 | 1045 | <24 months | × | ✓ | ✓ | ✓ | ✓ |
| Cornec 2012 | Brittany, France | 1995–97 | 7 | 270 | <12 months | × | × | × | × | × |
| DREAM | Netherlands | 2006–12 | 6 | 589 | <12 months | × | × | × | × | × |
| ERAN | UK and Ireland | 2002–12 | 23 | 1236 | <12 months | ✓ | ✓ | ✓ | ✓ | × |
| ERAS | England | 1986–2001 | 9 | 1465 | <24 months | ✓ | ✓ | ✓ | ✓ | ✓ |
| ESPOIR | France | 2002–05 | 14 | 814 | <6 months | ✓ | × | ✓ | ✓ | ✓ |
| ‘French Cohort’ | France | 2003–04 | 5 | 191 | <12 months | × | × | × | × | × |
| GLADAR | Latin America | 2004–05 | 46 | 1093 | <12 months | × | ✓ | × | × | ✓ |
| GREAT | South Africa | 2005–08 | 2 | 171 | <12 months | × | × | ✓ | ✓ | ✓ |
| Gremese 2013 | Italy | 2007–09 | 3 | 1795 | <12 months | ✓ | × | × | ✓ | × |
| Jamal 2011 | Canada | 2003–06 | 15 | 204 | <3 months | ✓ | × | × | × | ✓ |
| NHIRD | Taiwan | 1996–2011 | ND | 51 476 | ND | ✓ | × | × | × | × |
| Nijmegen Inception Cohort | Netherlands | 1985–2009 | 2 | 1157 | <12 months | ✓ | × | ✓ | × | × |
| NOAR | England | 1990–2011 | 77 | 3666 | <24 months | ✓ | ✓ | ✓ | ✓ | × |
| NOR-DMARD | Norway | 2000 to 2012 | 5 | 4126 | <5 years | ✓ | × | ✓ | × | ✓ |
| NOR-VEAC | Norway | 2004–10 | 6 | 1118 | <4 months | × | × | ✓ | × | ✓ |
| Olmstead County | USA | 1955–2007 | ND | 813 | ND | ✓ | × | ✓ | × | × |
| ORAR | Norway | 1994–97 | 2 | 894 | ND | × | × | × | × | ✓ |
| Pease 1999 | England | 1989 to ND | 2 | 422 | <24 months | × | × | × | × | × |
| RAMQ | Canada | 2002–11 | ND | 11 365 | ND | ✓ | × | × | ✓ | ✓ |
| Western Consortium | USA and Mexico | 1993–2000 | 26 | 263 | <14 months | × | × | × | × | × |
| SCQM database | Switzerland | 1997–2011 | ND | 592 | <12 months | ✓ | × | × | × | × |
| TIRA | Sweden | 1996–98, 2006–08 | 10 | 636 | 6 weeks to 12 months | × | × | ✓ | ✓ | ✓ |
| United Healthcare Register | USA | 2000–13 | ND | 195 433 | ND | ✓ | × | ✓ | × | × |
| Van der Heijde 1992 | Holland | ND | 2 | 147 | <12 months | × | × | × | × | ✓ |
| VERA | France | 1998–2002 | 5 | 310 | 4 weeks to 6 months | × | × | ✓ | ✓ | ✓ |
| Wagner 2007 | Austria, Hungary, and Slovenia | ND | 7 | 172 | Mean of 17 months | ✓ | ✓ | × | ✓ | ✓ |
| Westhoff 2007 and 2008 | Germany | 2000–01 | 54 | 1023 | <12 months | ✓ | × | ✓ | × | ✓ |
| YEAR | England | 1997–2009 | 14 | 1415 | <24 months | × | × | × | × | × |
| Ziegalasch 2017 | Sweden | ND | 3 | 176 | <12 months | × | × | × | × | × |
Thirty-four unique cohorts from the literature search. Most cohorts are from more economically developed regions, with a broad range of centres and sample sizes. Case-mix consideration varied widely. The NOR-DMARD cohort collected data on patients with a symptom duration of up to 5 years. It has been presented in this review as there are a number of studies that have presented sub-analyses on patients with <2 years symptom duration. SEP: any time a scoring system or index was utilized; ND: not documented; BARFOT: Better Anti-Rheumatic PharmacOTherapy; CAPEA: Course And Prognosis of Early Arthritis; CATCH: Canadian Early Arthritis Cohort; CLEAR: Consortium for the Longitudinal Evaluation of African-Americans with Early RA; CONAART: Consorcio Argentino de Artritis Temprana; DREAM: Dutch Rheumatoid Arthritis Monitoring; ERAN: Early RA Network; ERAS: Early RA Study; ESPOIR: Étude et Suivi des Polyarthrites Indifférenciées Récentes; GLADAR: Grupo Latino Americano de Estudio de Artritis Reumatoide; GREAT: Gauteng Region Early Arthritis Trial; NHIRD: National Health Insurance Research Database; NOAR: Norfolk Arthritis Register; NOR-DMARD: Norwegian DMARD registry; NOR-VEAC: Norwegian Very Early Arthritis Cohort; ORAR: Oslo RA register; RAMQ: Régie de l’assurance maladie du Québec; SCQM: Swiss Clinical Quality Management; TIRA: Swedish Early Intervention in RA; VERA: Very Early RA; YEAR: Yorkshire Early Arthritis Register.
The number of centres in each cohort ranged from 2 to 118, the number of participants from 147 to 195 433, with an average of 159 per centre. The majority of the 34 cohorts were conducted in Europe (24/34, 71%), with two (6%) from less economically developed regions. The period of data collection was between 1955 and 2017. Disease duration at study entry ranged from diagnosis to 24 months.
Centre-effect
Of the 204 included papers, 15 (7%) considered the effect of centre in their analyses, utilizing a range of methodologies as described in Table 3. Seven of the 15 papers included centre as a fixed effect in regression models. Four studies accounted for centre as a random effect in their modelling. Propensity modelling including centre as a covariate was undertaken in four studies. Lee et al. [21] used a Cox proportional hazards model, stratified by centre. Two studies described clinical differences of populations between centres, but did not include it as an effect in their analyses. There were no examples of consistent reporting of centre effect in multiple publications from the same cohort.
Table 3.
Included studies that considered the effect of centre in their analyses
| 1st Author and year | Cohort name | Centres (n) | Statistical modelling | Centre modelling | Centre effect |
|---|---|---|---|---|---|
| Albrecht 2015 [19] | CAPEA | 118 | Multivariate logistic regression modelling | Centre included as a fixed effect in regression model | Significant variation in prescribing glucocorticoids at baseline between practice types |
| Harris 2013 [20] | CATCH | 8 | Mixed linear and logistic regression modelling | Centre included as a random effect in regression models | Centre variation in DAS-28 change, remission and therapy choices |
| Lee 2013 [21] | CATCH | 18 | Cox proportional hazards modelling | Stratified by centre | Variation in fibromyalgia diagnoses across centres. Impact on risk estimates not reported |
| Dixey 2004 [22] | ERAS | 9 | Descriptive | Nil | NA |
| Young 2000 [23] | ERAS | 9 | Descriptive | Nil | NA |
| Nikiphorou 2017 [24] | ERAS/ERAN | ERAS 9, ERAN 23 | Mixed effects modelling | Centre included as a random effect in mixed effects models | Not reported |
| Escalas 2012 [25] | ESPOIR | 14 | Mixed effects modelling | Centre included as a random effect in mixed effects models | Adherence to European treatment recommendations associated with a lower risk of radiographic progression, maintained after adjustment for centre |
| Gaujoux-Viala 2017 [26] | ESPOIR | 14 | Multivariate logistic regression modelling | Centre included as a fixed effect in regression model | Optimal MTX treatment associated with higher rates of remission, and maintaining normal function. This was preserved after centre adjustment (unadjusted data not reported) |
| Krams 2016 [27] | ESPOIR | 14 | Multivariate logistic regression modelling | Centre included as a random effect in regression model | Age of onset and steroid dose associated with remission, after centre adjustment. (unadjusted data not reported) |
| Lukas 2011 [28] | ESPOIR | 14 | Multivariate logistic regression and propensity modelling | Centre included in logistic regression model to calculate propensity score | Centre a significant factor in propensity score for predicting treatment choice |
| Lie 2011 [29] | NOR-DMARD | 5 | Multivariate logistic regression and propensity modelling | Centre included in logistic regression model to calculate propensity score | Centre variation in numbers offered combination DMARDs after MTX monotherapy failure |
| Lie 2012 [30] | NOR-DMARD | 5 | Multivariate logistic regression and propensity modelling | Centre included in logistic regression model to calculate propensity score | Wide variation in SSZ prescribing between centres |
| Mueller 2017 [31] | SCQM | ND | Mixed effects modelling | ND | No centre effect observed (data not reported) |
| Jamal 2011 [32] | 15 | Multivariate logistic regression modelling | Centre included as a fixed effect in regression model. Generalized estimating equations performed to investigate for cluster sampling | No intra-centre clustering of results observed (data not reported) | |
| Van der Heijde 1992 [33] | 2 | Multivariate regression modelling | Centre included as fixed effect in regression model | No significant effect on outcomes (data not reported) |
Out of 204 included studies, 15 (7%) accounted for centre effect. Seven included centre as a fixed effect. Six did not report the magnitude of effect, and two described centre level differences. ND: not documented; NA: not applicable; CAPEA: Course And Prognosis of Early Arthritis; CATCH: Canadian Early Arthritis Cohort; ERAN: Early RA Network; ERAS: Early RA Study; ESPOIR: Étude et Suivi des Polyarthrites Indifférenciées Récentes; NOR-DMARD: Norwegian DMARD registry; SCQM: Swiss Clinical Quality Management.
The impact of centre on analyses varied. A paper from the Course And Prognosis of Early Arthritis (CAPEA) cohort by Albrecht et al. [19] reported that rheumatology practice type was associated with glucocorticoid prescription in multivariate logistic regression analysis. Lie et al. [30] showed significant centre-level variation in prescribing of sulfasalazine. Escalas et al. [25] included centre as a random effect in a mixed effects model assessing the association of EULAR treatment guideline adherence with radiographic disease progression. Centre inclusion increased the strength of association but widened the 95% CI. Six of the studies considered centre in their analyses but did not include data to allow an assessment of the magnitude of centre effect.
Case-mix adjustment
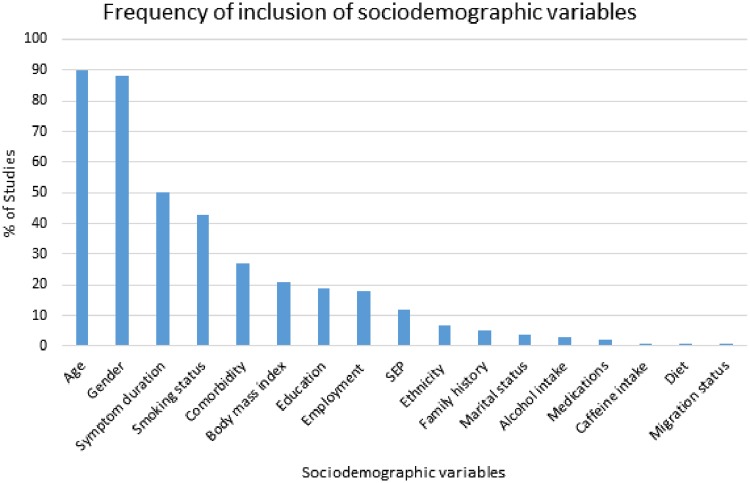
Reporting of sociodemographic variables in analyses varied widely between cohorts. Most studies made only a limited attempt to adjust for case-mix. Although age and gender were widely included in analysis models, comorbidity was accounted for in less than one-third of studies. Of the 204 included studies, 160 (78%) included age, gender and one or more additional sociodemographic variable. Fifty-nine (29%) included a measure of employment, salary or education status, while 16 (8%) utilized a composite SEP measure including Index of Multiple Deprivation, Carstair’s Index, and Graffar’s method. The Early RA Study (ERAS) conducted in England across nine centres had a high degree of case-mix adjustment with comorbidity, SEP with Carstair’s Index, smoking, employment and education were all considered, as well as age, gender, symptom duration, family history and BMI. In contrast, the Western Consortium of Rheumatalogists’ cohort only considered age, gender, family history and symptom duration. Fig. 2 displays the relative frequencies of sociodemographic variable consideration across all included studies. Of the 34 included cohorts, 28 (82%) collected time-dependent sociodemographic variables (such as employment status) only at baseline, with no further collection at follow-ups.
Fig. 2.
The frequency of inclusion of sociodemographic variables in collected studies
Age and gender were the most frequently considered sociodemographic variables in case-mix adjustment. SEP: any time a scoring system or index was utilized.
Impact of the STROBE statement
In the 42 studies predating the STROBE statement, 3 (7%) considered centre in their analyses, compared with 13 (8%) of the 162 published after STROBE. The mean number of case-mix covariates considered in pre-STROBE studies was 3, while in studies post-STROBE it was 4.2. An independent t-test showed that there was a significant difference in the number of case-mix covariates considered pre- and post-STROBE (see Table 4).
Table 4.
Centre adjustment and case-mix adjustment reporting pre and post STROBE
| Pre- STROBE | Post- STROBE | P-value | |
|---|---|---|---|
| Total studies | 42 | 162 | |
| Studies considering centre (%) | 3 (7%) | 13 (8%) | |
| Mean case-mix covariates (s.d.) | 3 (1.1) | 4.2 (1.7) | <0.0001 |
STROBE: STrengthening the Reporting of OBservational studies in Epidemiology.
Discussion
Only a minority of studies considered centre in their analyses. Centre had a significant effect in a number of studies where it was included, underlining the importance of its inclusion. Of those studies that did include centre, there was a high level of under reporting, with many authors not presenting unadjusted data. This makes interpreting the magnitude of centre-level effect impossible. Additionally, none of the cohorts had a uniform approach to centre effect across multiple publications. It is possible that centre has been handled in a way that will best support the findings, rather than the most statistically appropriate approach.
A range of methods were utilized to account for centre, with inclusion as a fixed covariate in a multivariate logistic regression model being the most common. The impact of centre will usually vary across individuals, so it is more appropriate to include centre as a random effect in a mixed effects model, rather than including it as a fixed covariate [34]. The pragmatist might suggest that by recommending inclusion of centre effect in analyses, we may have missed many important clinical correlations. The devil’s advocate would suggest that we may have published many false-positive results.
There was a wide spectrum of case-mix adjustment in the included studies. Inadequate adjustment for case-mix, particularly with data from multiple geographical areas, will likely lead to biased conclusions being drawn from results, and precludes meaningful comparisons between centres [16]. Age, gender and symptom duration were the most common factors adjusted for. Despite this, half of the studies did not include all of these factors in analyses, reflecting a lack of consensus on what constitutes adequate case-mix adjustment in early RA cohorts.
There were also variations in the degree of case-mix adjustment performed between studies from the same cohort. This may be due to a lack of access to full datasets for some studies. One consideration regarding degree of case-mix adjustment is the loss of power from adjusting for variables with incomplete data. This can be managed with imputation of missing results, but it is likely that a number of authors opted to retain power by excluding certain variables from case-mix adjustment, given that 62 of the 204 (30%) included studies had a sample size of fewer than 500 patients.
This review is a comprehensive summary of early RA cohort centre effect methodology. The initial literature search with an extended manual search led to the identification and review of over 200 articles from 34 early RA cohorts. The extended manual search of reference lists and authors identified over 150 of the included papers. The majority (85%) of the additional papers were from cohorts already identified during the initial literature search, giving confidence that we have captured all eligible early RA cohorts. By limiting the search to early RA, we were able to include cohorts with a higher homogeneity of study design, enabling clearer comparisons to be made between methodology choices.
As with any review, we are reliant upon what is published. It is possible that some studies did include centre in their analyses, but due to word counts this was not included in the published methods. Due to the volume of studies, it was not feasible to contact each individual author for clarification. Another limitation is that many of the cohorts have been published on by multiple academic groups. Papers often referred to original publications for detailed description of methods. These were reviewed, but it is likely that there has been a subtle evolution in methodology in subsequent studies that is not captured. Distinct academic groups may describe the methodology of a cohort in differing manners, making comparisons more challenging.
Nearly a quarter of the included studies were in print before the publication of the STROBE statement, which set out to standardize reporting of observational studies so that their strengths, weakness and generalizability could be more easily assessed [4]. Those studies that predated STROBE considered on average fewer covariates for case-mix adjustment. The quantity of variables considered in a given study should not be taken as an indicator of robust case-mix adjustment. However, the greater number of covariates in post-STROBE studies suggests that case-mix adjustment has been assigned greater importance.
We recommend that at the conception of new cohorts, a standardized approach to centre effect, including case-mix, is adopted to enhance comparability of results. This should include identifying the sociodemographic variables for case-mix adjustment. This may not always be possible if, for example, the introduction of a case-mix variable leads to an unacceptable reduction in power. Authors should, however, justify and mitigate the effects of not including all available case-mix variables in their analyses.
Multicentre observational cohort studies should consider if there is a centre effect impacting on their results. This is usually best served by including centre as a random effect in outcome analyses. Full reporting of unadjusted and adjusted results then allows readers to assess the magnitude of any centre effect.
Conclusion
This narrative review highlights an inconsistent approach to centre effect in early RA cohort studies, and the varying degrees to which case-mix is considered. This supports the recommendation that centre effect should be routinely accounted for in analyses, usually as a random effect rather than as a fixed effect. It is possible that authors did consider centre in their analyses, but that this was not included in the published manuscript as centre either had no effect, or had such an effect that the reported findings were no longer significant. Further research is needed to understand the most efficient approach to account for centre effect that have the least impact on power.
Funding: M.Y.’s salary is funded by the ARUK transforming MSK health grant, and by the BSR.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary Material
References
- 1. Smolen JS, Landewé R, Bijlsma J. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. [DOI] [PubMed] [Google Scholar]
- 2.Rheumatoid Arthritis in over 16s. Quality standard 33: NICE. https://www.nice.org.uk/guidance/qs33 (1 October 2018, date last accessed).
- 3. Singh JA, Saag KG, Bridges SL Jr. et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol (Hoboken) 2016;68:1–26. [DOI] [PubMed] [Google Scholar]
- 4. von Elm E, Altman DG, Egger M. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7. [DOI] [PubMed] [Google Scholar]
- 5. Localio AR, Berlin JA, Ten Have TR, Kimmel SE.. Adjustments for center in multicenter studies: an overview. Ann Intern Med 2001;135:112–23. [DOI] [PubMed] [Google Scholar]
- 6. Kerry SM, Bland JM.. Sample size in cluster randomisation. BMJ 1998;316:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vierron E, Giraudeau B.. Design effect in multicenter studies: gain or loss of power? BMC Med Res Methodol 2009;9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sera F, Ferrari P.. A multilevel model to estimate the within- and the between-center components of the exposure/disease association in the EPIC study. PLoS One 2015;10:e0117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liang K-Y, Zeger SL.. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22. [Google Scholar]
- 10. Dominici F,, Griswold M. Bayesian Hierarchical Models. 2005. http://www.biostat.jhsph.edu/bstcourse/bio607/lectures/Module2-2prpg.pdf (1 October 2018, date last accessed).
- 11. Mehta RH, Liang L, Karve AM. et al. Association of patient case-mix adjustment, hospital process performance rankings, and eligibility for financial incentives. JAMA 2008;300:1897–903. [DOI] [PubMed] [Google Scholar]
- 12. Cameron A, Miller DL.. A practitioner’s guide to cluster-robust inference. J Hum Resour 2015;50:317–72. [Google Scholar]
- 13.Digital N. PROMs Methodologies. 2018. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/patient-reported-outcome-measures-proms/proms-methodologies. (1 October 2018, date last accessed).
- 14. Guo P, Dzingina M, Firth AM. et al. Development and validation of a casemix classification to predict costs of specialist palliative care provision across inpatient hospice, hospital and community settings in the UK: a study protocol. BMJ Open 2018;8:e020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salisbury C, Wallace M, Montgomery AA.. Patients' experience and satisfaction in primary care: secondary analysis using multilevel modelling. BMJ 2010;341:c5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mesterton J, Lindgren P, Ekenberg Abreu A. et al. Case mix adjustment of health outcomes, resource use and process indicators in childbirth care: a register-based study. BMC Pregnancy Childbirth 2016;16:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Galobardes B, Lynch J, Smith GD. Measuring socioeconomic position in health research. British Medical Bulletin 2007;81–82:21–37. [DOI] [PubMed] [Google Scholar]
- 18. Statistics N. English indices of deprivation 2015, 2015. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2015. [Google Scholar]
- 19. Albrecht K, Callhoff J, Schneider M, Zink A.. High variability in glucocorticoid starting doses in patients with rheumatoid arthritis: observational data from an early arthritis cohort. Rheumatol Int 2015;35:1377–84. [DOI] [PubMed] [Google Scholar]
- 20. Harris JA, Bykerk VP, Hitchon CA. et al. Determining best practices in early rheumatoid arthritis by comparing differences in treatment at sites in the canadian early arthritis cohort. J Rheumatol 2013;40:1823–30. [DOI] [PubMed] [Google Scholar]
- 21. Lee YC, Lu B, Boire G. et al. Incidence and predictors of secondary fibromyalgia in an early arthritis cohort. Ann Rheum Dis 2013;72:949–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dixey J, Solymossy C, Young A.. Is it possible to predict radiological damage in early rheumatoid arthritis (RA)? A report on the occurrence, progression, and prognostic factors of radiological erosions over the first 3 years in 866 patients from the Early RA Study (ERAS). J Rheumatol Suppl 2004;69:48–54. [PubMed] [Google Scholar]
- 23. Young A, Dixey J, Cox N. et al. How does functional disability in early rheumatoid arthritis (RA) affect patients and their lives? Results of 5 years of follow-up in 732 patients from the Early RA Study (ERAS). Rheumatology (Oxford) 2000;39:603–11. [DOI] [PubMed] [Google Scholar]
- 24. Nikiphorou E, Norton S, Carpenter L. et al. Secular changes in clinical features at presentation of rheumatoid arthritis: increase in comorbidity but improved inflammatory states. Arthritis Care Res 2017;69:21–7. [DOI] [PubMed]
- 25. Escalas C, Dalichampt M, Combe B. et al. Effect of adherence to European treatment recommendations on early arthritis outcome: data from the ESPOIR cohort. Ann Rheum Dis 2012;71:1803–8. [DOI] [PubMed] [Google Scholar]
- 26. Gaujoux-Viala C, Rincheval N, Dougados M, Combe B, Fautrel B.. Optimal methotrexate dose is associated with better clinical outcomes than non-optimal dose in daily practice: results from the ESPOIR early arthritis cohort. Ann Rheum Dis 2017;76:2054–60. [DOI] [PubMed] [Google Scholar]
- 27. Krams T, Ruyssen-Witrand A, Nigon D. et al. Effect of age at rheumatoid arthritis onset on clinical, radiographic, and functional outcomes: the ESPOIR cohort. Joint Bone Spine 2016;83:511–5. [DOI] [PubMed] [Google Scholar]
- 28. Lukas C, Combe B, Ravaud P. et al. Favorable effect of very early disease-modifying antirheumatic drug treatment on radiographic progression in early inflammatory arthritis: data from the Étude et Suivi des polyarthrites indifférenciées récentes (study and followup of early undifferentiated polyarthritis). Arthritis Rheum 2011;63:1804–11. [DOI] [PubMed] [Google Scholar]
- 29. Lie E, van der Heijde D, Uhlig T. et al. Treatment strategies in patients with rheumatoid arthritis for whom methotrexate monotherapy has failed: data from the NOR-DMARD register. Ann Rheum Dis 2011;70:2103–10. [DOI] [PubMed] [Google Scholar]
- 30. Lie E, Uhlig T, van der Heijde D. et al. Effectiveness of sulfasalazine and methotrexate in 1102 DMARD-naive patients with early RA. Rheumatology (Oxford) 2012;51:670–8. [DOI] [PubMed] [Google Scholar]
- 31. Mueller RB, Reshiti N, Kaegi T. et al. Does addition of glucocorticoids to the initial therapy influence the later course of the disease in patients with early RA? Results from the Swiss prospective observational registry (SCQM). Clin Rheumatol 2017;36:59–66. [DOI] [PubMed] [Google Scholar]
- 32. Jamal S, Alibhai SM, Badley EM, Bombardier C.. Time to treatment for new patients with rheumatoid arthritis in a major metropolitan city. J Rheumatol 2011;38:1282–8. [DOI] [PubMed] [Google Scholar]
- 33. van der Heijde DM, van Riel PL, van Leeuwen MA. et al. Prognostic factors for radiographic damage and physical disability in early rheumatoid arthritis. A prospective follow-up study of 147 patients. Br J Rheumatol 1992;31:519–25. [DOI] [PubMed] [Google Scholar]
- 34. Kahan BC. Accounting for centre-effects in multicentre trials with a binary outcome - when, why, and how? BMC Med Res Methodol 2014;14:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.