Abstract
Purpose
After successful surgery for primary hyperparathyroidism, bone mineral density (BMD) does not improve equally in all patients. As no trial has so far aimed to influence normalization of BMD, it was the goal of this investigation to determine whether pharmacological treatment is effective in improving regain of BMD after successful parathyroidectomy in patients with preoperatively diagnosed osteoporosis or osteopenia and to evaluate when treatment may be indicated.
Methods
In this randomized, placebo-controlled, double-blind trial, 52 patients were treated with strontium ranelate 2 g daily + 1000 mg calcium + 800 IU vitamin D (strontium group; SG) or with 1000 mg calcium + 800 IU vitamin D alone (placebo group; PG) for 1 year. The main outcome measures were BMD (lumbar spine, femoral neck, radius) and bone turnover markers.
Results
The baseline characteristics were similar in both groups. Absolute BMD (1.007 ± 0.197 vs. 0.897 ± 0.137 g/cm2; p = 0.024) and both relative (9.94 vs. 3.94%; p < 0.001) and absolute (0.09 ± 0.06 vs. 0.03 ± 0.04 g/cm2; p < 0.001) changes in lumbar-spine BMD were significantly higher in the SG than in the PG. Compared to baseline, BMD significantly increased in both groups at the lumbar spine (p < 0.001 and p = 0.001, respectively) and femoral neck (both p < 0.001), whereas radius BMD only changed significantly in the SG. However, the proportion of patients with osteoporosis/osteopenia significantly declined only at the lumbar spine in the SG (from 69.0 to 37.9%; p = 0.034), whereas no decrease was found in the PG. No severe adverse events occurred.
Conclusions
Postoperative anti-osteoporotic treatment can positively influence regain of BMD mainly in the lumbar spine and should be considered. Without treatment, most patients and especially those with low preoperative markers of bone turnover remained osteoporotic/osteopenic 1 year after surgery.
Electronic supplementary material
The online version of this article (10.1007/s00423-019-01815-9) contains supplementary material, which is available to authorized users.
Keywords: Primary hyperparathyroidism, Parathyroidectomy, Bone mineral density, Osteoporosis, Biomarkers
Introduction
Primary hyperparathyroidism (pHPT) affects bone metabolism by advancing turnover through increase in both bone formation and resorption, resulting in a net decrease in bone mineral density (BMD) and strength [1, 2]. In the literature, the incidence of osteopenia or osteoporosis in patients with pHPT has been estimated at 39% to 59%, affecting BMD at all sites measured (lumbar spine, femur, and radius) [3–5]. The fracture risk, especially in vertebral spine, is thus increased up to fivefold [6–8] and is associated with low BMD [9]. Parathyroidectomy (PTX) is the only causal treatment for pHPT and is recommended even in patients without severe symptoms, yet reduced BMD [10].
Only three randomized trials have addressed the effect of PTX on BMD vs. observation alone. PTX resulted in a minor but significant increase in BMD of 0.5% to 4% after 1 year [11, 12] and a 3.3% increase only at the lumbar spine after 5 years [13] in patients with mild disease. Improvement of BMD is not found in all patients, as another prospective trial showed no improvement whatsoever following PTX in more than half of postmenopausal women [14]. Thus, the fracture risk is increased by up to 10 or more years following successful surgery [6, 15, 16]. No investigations have so far focused on patients with pHPT and preoperatively diagnosed osteoporosis or osteopenia, who generally are at an advanced risk of fractures, especially not for their postoperative change in BMD and bone turnover.
To our knowledge, no trial has as yet addressed the potentially positive influence of treatment with anti-osteoporotic medication on BMD and bone metabolism after PTX. Basically, these medications can have two physiological effects: they either reduce bone resorption or increase bone formation. While the main effect of bisphosphonate therapy is the reduction of bone resorption, it seems more reasonable to stimulate bone formation to advance the increase in BMD after PTX. Strontium ranelate (SR) is one of the few osteoporosis-specific medications that both stimulate osteoblasts and thereby bone formation and inhibit osteoclasts (reducing bone resorption) [17, 18], leading to a decrease in vertebral and non-vertebral fractures [19, 20]. A meta-analysis of four trials has substantiated the positive effect of 2 g SR daily on the fracture risk in primary osteoporosis [21], [22]. Improved bone microarchitecture [23] may at least partially account for this effect.
Although the use of SR has been restricted (for the potentially elevated risk of thromboembolism and cardiovascular events), it at least continues to serve as an example of an anti-osteoporotic medication for patients with low BMD after successful PTX. The rationale is to stimulate bone formation, re-improve bone strength, increase BMD to the largest extent and as quickly as possible, and thus potentially prevent fractures.
Another new target of pharmacological intervention to increase bone formation is the Wnt signaling pathway, measured by the biochemical parameters sclerostin (SOST) and Dickkopf-1 (DKK-1). Although there is evidence that this pathway is important for the effects of parathyroid hormone (PTH) on bone metabolism [24], the dynamics of SOST and DKK-1 after PTX for PHPT have not yet been investigated. This pathway is of special interest, as the novel therapeutic agent romosozumab, an antibody against SOST, has shown potent anti-osteoporotic effects [25].
Thus, the aim of this study was to focus on osteopenic and osteoporotic patients after PTX for pHPT in an attempt to investigate the effect of an anti-osteoporotic medication with an anabolic effect on the change of BMD and on classical biochemical markers of bone metabolism. Additionally, the parameters SOST and DKK-1 were analyzed as potential future targets of intervention for stimulating bone formation.
Materials and methods
Patients
All postmenopausal women and men with biochemically proven pHPT and osteopenia (T-score ≤ 1 and ≥ 2.5) or osteoporosis (T-score ≤ 2.5) according to WHO criteria [26] consulting the Department of Surgery, Medical University of Vienna, were asked to participate in this study prior to PTX. Following the recently published guidelines for the diagnosis and definitive management of pHPT, all patients fulfilled the criteria for surgical intervention [10, 27–29].
The exclusion criteria before surgery included anamnestic pulmonary embolism or deep venous thrombosis, blood coagulation disorder or coagulopathy, phenylketonuria, renal impairment (creatinine clearance < 30 mL/h), severe hepatic disorder, severe systemic disorder, thyroid dysfunction, and immobilization. Additionally, the intake of drugs with potential effects on BMD, such as glucocorticoids, lithium, estrogen replacement therapy, selective estrogen receptor modulators, bisphosphonates (oral: last 3 months, parenteral: past year), and denosumab (past year), was not allowed. The patients were excluded after surgery in the case of malignant disease (thyroid or parathyroid cancer, except for microcarcinoma of the thyroid gland), persistent or recurrent pHPT (postoperative hypercalcemia), four-gland hyperplasia, multiple endocrine neoplasia, hereditary pHPT, or familial hypocalcuric hypercalcemia (calcium/creatinine ratio < 0.01). In accordance with European Medicines Agency regulations, additional exclusion criteria (ischemic cardiac disease, peripheral arterial obstructive disease, cerebrovascular disease, and uncontrolled arterial hypertonia) were introduced in April 2013. Since then, electrocardiograms have been included in prestudy screening and were also performed after the 12-month study period.
The study procedures of this double-blind, placebo-controlled, randomized trial were approved by the Ethics Committee of the Medical University of Vienna (EKNr: 2142008), the Austrian Agency for Health and Food Safety (Österreichische Agentur für Gesundheit und Ernährungssicherheit), ClinicalTrials.gov (identifier: NCT01222026), and European Union Drug Regulating Authorities Clinical Trials. All study participants gave written informed consent in accordance with the Declaration of Helsinki.
Study design and treatment protocol
PTX was performed according to the current indications for surgery [10, 27]. Within 1 week after surgery, all enrolled patients received daily supplements of 1000 mg of calcium and 800 IU of vitamin D. Four weeks after surgery, the patients were randomly assigned to be given placebo (placebo group; PG) or SR 2 g daily (strontium group; SG) for 1 year (sequentially numbered containers). The primary outcome variable was BMD at the lumbar spine. The secondary outcome parameters were BMD at other sites and biochemical parameters.
BMD
The BMD measurements at the lumbar spine, left femoral neck, and non-dominant radius (one-third distal [1/3 radius], mid-distal [MID radius], and ultradistal [UD radius]) were performed using a dual-energy X-ray absorption (DXA) device (HOLOGIC 4500; Hologic Inc., Waltham, MA, USA; coefficient of variation: 2%). All measurements were conducted using the standard procedures recommended by the manufacturer. The BMD values are expressed as g/cm2 and T-scores.
Biochemical parameters
Overnight fasting venous blood samples were taken before surgery, 4 weeks, and 3, 6, and 12 months after surgery. Routine parameters (PTH, ionized calcium [Ca++], phosphate, 25-hydroxyvitamin D [25(OH)D], 1,25-dihydroxyvitamin D [1,25(OH)D]) were determined using standard methods. Additionally, bone turnover markers were studied: bone-specific alkaline phosphatase (BAP; Liaison Analyzer, DiaSorin Inc., Stillwater, MN, USA; detection limit: 0.1 μg/L; intra-assay coefficient of variation: 3.3–4.3%, inter-assay coefficient of variation: 6.1–8.1%), osteocalcin (OC; Cobas 8000 Analyzer, Roche Diagnostics, Rotkreuz, Switzerland; detection limit: 0.01 ng/mL; intra-assay coefficient of variation: 0.9–1.3%, inter-assay coefficient of variation: 1.2–2.3%), and CrossLaps (CTX; Cobas 8000 Roche Analyzer, Roche Diagnostics, Rotkreuz, Switzerland; detection limit: 0.5 ng/mL; intra-assay coefficient of variation: 1.2–4.7%, inter-assay coefficient of variation: 1.5–5.7%). Serum was frozen at − 70 °C until analysis for osteoprotegerin (OPG, BI-20403; colorimetric sandwich immunoassays, Biomedica, Vienna, Austria; detection limit: 0.07 pmol/L; intra-assay coefficient of variation: ≤ 3%, inter-assay coefficient of variation: ≤ 5%, according to the manufacturer’s data), receptor activator of nuclear factor-kappa B ligand (RANKL, BI-20452; colorimetric sandwich immunoassays, Biomedica, Vienna, Austria; detection limit: 0.02 pmol/L; intra-assay coefficient of variation: ≤ 9%; inter-assay coefficient of variation: ≤ 6%, according to the manufacturer’s data), SOST (BI-20492; colorimetric sandwich immunoassays, Biomedica, Vienna, Austria; detection limit: 2.6 pmol/L; intra-assay coefficient of variation: ≤ 5%; inter-assay coefficient of variation: ≤ 6%, according to the manufacturer’s data), and DKK-1 (BI-20412; colorimetric sandwich immunoassays, Biomedica, Vienna, Austria; detection limit: 0.38 pmol/L; intra-assay coefficient of variation: ≤ 8.0%, inter-assay coefficient of variation: ≤ 12.0%, according to the manufacturer’s data).
Statistical analysis
Normal distribution was assessed with the Shapiro-Wilk test and visual inspection of histograms. After descriptive analysis, parametric tests (t test for baseline characteristics and BMD) and non-parametric tests (Wilcoxon’s rank-sum test for biochemical parameters) were used for group comparison. In order to compare the two time points, longitudinal changes within each group were evaluated with the paired t test (BMD) or the Wilcoxon’s signed rank test (biochemical parameters). Fisher’s exact test was used to test for differences in binominal proportions. As the sample size was inadequate for the Chi-square test of homogeneity, Fisher’s exact test was also applied to test for differences in multinomial distributions. Spearman’s rank order correlation was run to assess the relationship between the increase in BMD and preoperative biochemical parameters.
Data are given as mean (± standard deviation [SD]) or median (25th quartile; 75th quartile). P values < 0.05 were considered significant.
Sample size
The sample size was calculated in cooperation with the Institute of Medical Statistics, Medical University of Vienna, to provide a statistical power of 80% accepting an alpha error of 5%. Background data were taken from the literature. The 1-year treatment effect of SR was estimated as 3% BMD change in the lumbar spine. The SD in the literature was ± 3.7% [19, 20]. Thirty patients were to be recruited in each group (including an estimated 20% loss of follow-up).
Results
Baseline parameters
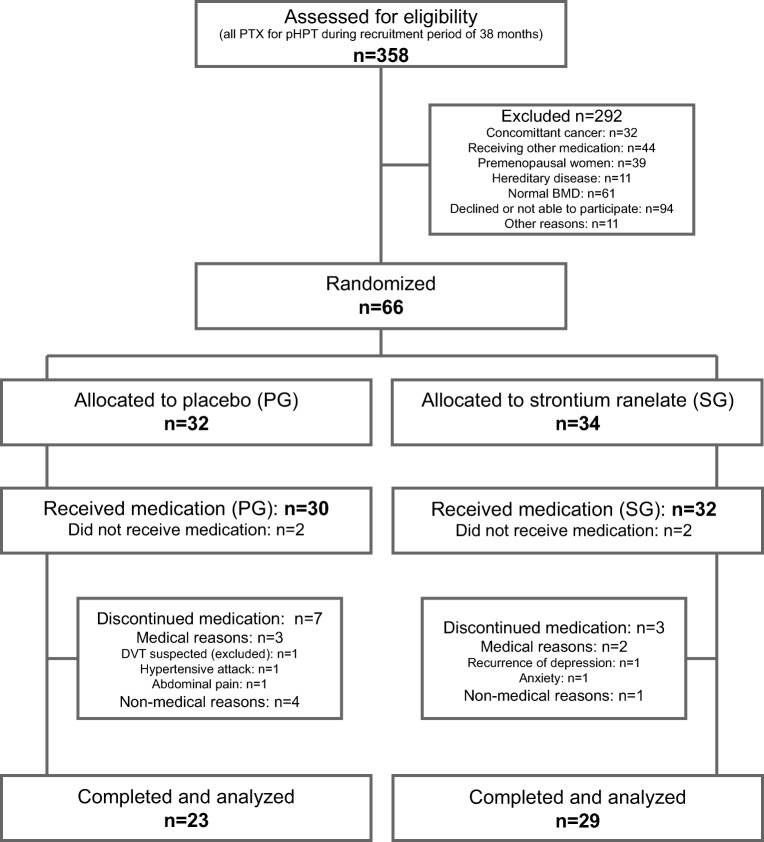
Of the 358 patients who underwent PTX due to biochemically proven pHPT at our institution during the study period, 291 were ineligible or not interested in participating (Fig. 1). Four of the 66 randomly assigned subjects (SG: 34; PG: 32) were excluded before they received medication because of hypercalcemia and intolerance of calcium supplementation. The study medication was finally administered to 62 patients. Patient withdrawals were due to medical reasons (SG: 2; PG: 3), protocol violations (PG: 2), and nonmedical reasons (SG: 1; PG: 2). Thus, 29 patients in the SG and 23 in the PG were included in the statistical analysis (per protocol).
Fig. 1.
Flow diagram of screening, randomization, and completion
Of these 52 patients, 8 had typical and 44 only minor or no symptoms of pHPT. Open minimally invasive PTX (OMIP) was performed in 20 patients, unilateral exploration for PTX in 8 patients, and bilateral exploration for PTX in 24 patients. In most cases (n = 50), histological analysis revealed an adenoma of the parathyroid gland; only two patients had hyperplasia.
The baseline characteristics were similar and did not differ significantly between the groups. There were no surgical complications (Table 1).
Table 1.
Baseline characteristics; mean (± SD) or median (25th percentile; 75th percentile)
| Placebo group (n = 23) | Strontium group (n = 29) | p | |
|---|---|---|---|
| Gender f/m | 16/7 | 16/13 | |
| Type of operation | |||
| OMIP | 10 | 11 | |
| Unilateral exploration | 2 | 6 | |
| Bilateral exploration | 11 | 12 | |
| Surgical complications | |||
| Bleeding | 0 | 0 | |
| Recurrent nerve palsy | 0 | 0 | |
| Age (years) | 63 (± 10) | 63 (± 12) | 0.949 |
| Height (cm) | 168 (± 8) | 170 (± 10) | 0.481 |
| Weight (kg) | 76 (± 15) | 80 (± 16) | 0.388 |
| BMI | 27.1 (± 4.8) | 27.8 (± 4.4) | 0.561 |
| Baseline BMD lumbar spine (g/cm2) | 0.864 (± 0.134) | 0.917 (± 0.185) | 0.260 |
| Baseline T-score lumbar spine | − 1.8 (± 1.2) | − 1.4 (± 1.5) | 0.300 |
| Baseline BMD femoral neck (g/cm2) | 0.680 (± 0.123) | 0.686 (± 0.114) | 0.867 |
| Baseline T-score femoral neck | − 1.6 (± 1.1) | − 1.7 (± 0.9) | 0.953 |
| Baseline BMD 1/3 radius (g/cm2) | 0.585 (0.085) | 0.605 (± 0.088) | 0.410 |
| Baseline T-score 1/3 radius | − 2.4 (± 1.2) | − 2.4 (± 1.0) | 0.821 |
| Baseline BMD MID radius (g/cm2) | 0.512 (± 0.077) | 0.520 (± 0.080) | 0.718 |
| Baseline T-score MID radius | − 2.3 (± 1.2) | − 2.4 (± 1.0) | 0.815 |
| Baseline BMD UD radius (g/cm2) | 0.396 (± 0.091) | 0.390 (± 0.077) | 0.805 |
| Baseline T-score UD radius | − 1.6 (± 1.1) | − 1.7 (± 0.9) | 0.601 |
| PTH (pg/mL) | 123.9 (90.8; 162.2) | 141.3 (97.4; 162.9) | 0.747 |
| Ca++ (mmol/L) | 1.39 (1.36; 1.43) | 1.41 (1.38; 1.50) | 0.264 |
| Phosphate (mmol/L) | 0.70 (0.57; 0.83) | 0.64 (0.57; 0.74) | 0.342 |
| 25(OH)D (nmol/L) | 38.9 (26.8; 47.4) | 39.8 (29.8; 52.1) | 0.719 |
| 1,25(OH)D (nmol/L) | 62.0 (49.0; 77.0) | 64.0 (54.0; 96.0) | 0.612 |
| BAP (ng/mL) | 20.4 (14.6; 28.3) | 15.9 (10.8; 27.7) | 0.339 |
| OC (ng/mL) | 41.8 (22.8; 59.6) | 33.2 (25.2; 43.3) | 0.315 |
| CTX (ng/mL) | 0.84 (0.42; 1.01) | 0.63 (0.52; 0.79) | 0.325 |
| P1NP (ng/mL) | 61.5 (47.0; 89.0) | 52.0 (43.0; 74.5) | 0.482 |
| 24 h CrCl (mL/min) | 107.7 (96.4; 132.8) | 121.6 (89.4; 161.5) | 0.768 |
| Patients with 24 h CrCl < 60 mL/min (n) | 0 | 2a | |
| OPG (pmol/L) | 4.46 (3.64; 5.64) | 4.65 (3.63; 5.47) | 0.869 |
| RANKL (pmol/L) | 0.87 (0.51; 1.42) | 0.74 (0.52; 0.99) | 1 |
| SOST (pmol/L) | 19.63 (15.55; 29.31) | 20.84 (14.85; 28.49) | 0.934 |
| DKK-1 (pmol/L) | 25.10 (18.99; 35.69) | 32.32 (23.71; 41.04) | 0.129 |
aBoth patients had a 24 h CrCl of > 60 mL/min 1 year after surgery
OMIP open minimally invasive parathyroidectomy, BMI body mass index, BMD bone mineral density, 1/3 radius one-third distal radius, MID radius Mid-distal radius, UD radius ultradistal radius, PTH parathyroid hormone, Ca++ ionized calcium, 25(OH)D 25-hydroxyvitamin D, 1,25(OH)D 1,25-dihydroxyvitamin D, BAP bone-specific alkaline phosphatase, OC osteocalcin, CTX CrossLaps, P1NP procollagen type 1 N-terminal propeptide, 24 h CrCl measured 24-h creatinine clearance, OPG osteoprotegerin, RANKL receptor activator of nuclear factor-kappa B ligand, SOST sclerostin, DKK-1 Dickkopf-1
Change in BMD
At the lumbar spine, absolute BMD after 1 year was higher in the SG than in the PG (1.007 ± 0.197 g/cm2 vs. 0.897 ± 0.137 g/cm2; p = 0.024). Additionally, the percentage change of BMD after 1 year was more than twofold higher in the SG than in the PG (9.94 vs. 3.94%; p < 0.001). Also, the absolute BMD change was significantly higher in the SG (0.09 ± 0.06 g/cm2 vs. 0.03 ± 0.04 g/cm2; p < 0.001). At all other sites, both the percentage and absolute changes in BMD were higher in the SG but did not reach the level of significance (Table 2).
Table 2.
Relative changes (Δ) in BMD after 1 year for both (female + male), female and male patients: % (± SD)
| Placebo group | Strontium group | p | |
|---|---|---|---|
| Δ BMD lumbar spine (%) | |||
| Both | 3.94 (± 4.49) | 9.94 (± 6.33) | < 0.001 |
| Female | 4.98 (±4.28) | 10.71 (± 6.18) | 0.005 |
| Male | 1.73 (± 4.42) | 8.99 (± 6.63) | 0.009 |
| Δ BMD femoral neck (%) | |||
| Both | 4.84 (± 4.55) | 5.87 (± 5.86) | 0.504 |
| Female | 5.62 (± 4.83) | 6.21 (± 6.28) | 0.772 |
| Male | 3.27 (± 3.75) | 5.42 (± 5.49) | 0.326 |
| Δ BMD 1/3 radius (%) | |||
| Both | 0.00 (± 3.36) | 0.42 (± 4.06) | 0.690 |
| Female | 0.75 (± 3.00) | 0.40 (± 4.80) | 0.810 |
| Male | − 1.61 (± 3.76) | 0.45 (± 3.11) | 0.241 |
| Δ BMD MID radius (%) | |||
| Both | 0.41 (± 2.86) | 1.64 (± 3.23) | 0.166 |
| Female | 0.97 (± 2.29) | 2.09 (± 3.71) | 0.316 |
| Male | − 0.78 (± 3.75) | 1.08 (± 2.57) | 0.271 |
| Δ BMD UD radius (%) | |||
| Both | 1.84 (± 5.76) | 3.02 (± 5.86) | 0.474 |
| Female | 2.90 (± 5.37) | 4.37 (± 6.14) | 0.482 |
| Male | − 0.43 (± 6.33) | 1.37 (± 5.26) | 0.535 |
SD standard deviation, BMD bone mineral density, 1/3 radius one-third distal radius, MID radius mid-distal radius, UD radius ultradistal radius
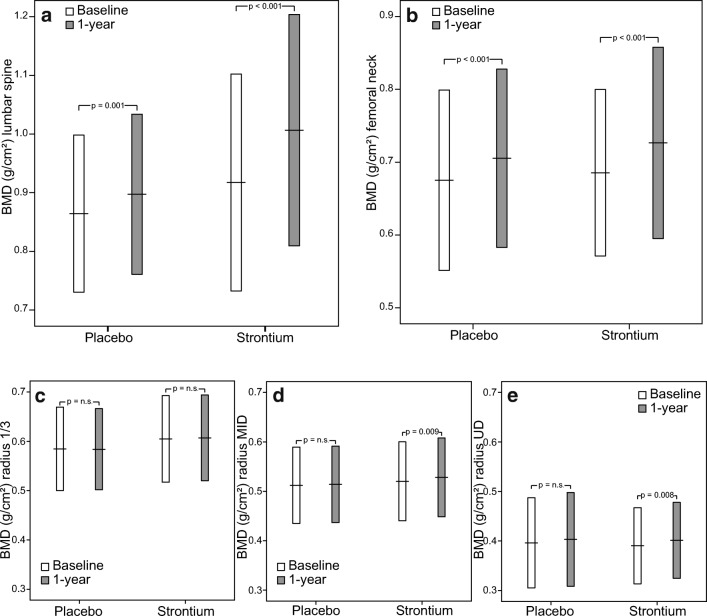
Comparing baseline and 1-year controls in both the SG and the PG, there was a significant increase in BMD at the lumbar spine (both p < 0.001) and the femoral neck (p < 0.001 and p = 0.001). Radius BMD (except 1/3 radius) changed significantly in the SG only (p = 0.008 and 0.009, respectively) (Fig. 2, Supplementary Data 1). Both the percentages and absolute changes did not differ significantly between female and male patients, neither in the SG nor in the PG.
Fig. 2.
Bone mineral density (BMD; mean ± 1SD) at baseline and after 1 year in lumbar spine (a), femoral neck (b), one-third distal radius (1/3 radius, c), mid-distal radius (MID radius, d), and ultradistal radius (UD radius, e)
When summing up osteoporosis and osteopenia, the percentage of lumbar-spine disorders before treatment was 68.9% in the SG and 77.3% in the PG. A statistically significant decline to 37.9% (p = 0.034) was seen in the SG, whereas no relevant change was found in the PG (72.7%; p = 1.000). Although statistically not significant anymore, there was a marked increase in patients with normal BMD values at the lumbar spine in the SG (from 31.0% at baseline to 62.1% after 1 year, p = 0.061), whereas this increase was not found in the PG (from 22.7% at baseline to 27.6% after 1 year, p = 0.734; Supplementary Data 2).
Initial osteoporosis and osteopenia at the femoral neck were identified in 89.3% and 68.2% of the patients in the SG and the PG, respectively. After the 1-year follow-up period, there was a trend towards a decrease in osteoporotic/osteopenic patients in the SG with a 17.9% reduction to 71.4% (p = 0.177). This reduction was not found in the PG (66.7% after 1 year; p = 1.000).
At the UD radius, the changes from 79.3 to 65.5% in the SG and from 81.8 to 73.9% in the PG did not prove to be statistically different (p = 0.379 and 0.722, respectively). At the MID radius and 1/3 radius, the percentages of osteoporosis and osteopenia remained nearly unchanged in both groups at more than 80% (p = 1.000 for both; p = 1.000 and p = 0.491, respectively).
Change in biochemical parameters
The PTH and Ca++ levels decreased and phosphate increased as indices of successful pHPT treatment. Comparing the groups after 1 year, no statistically significant differences were seen except for the Ca++ levels: the decrease in Ca++ levels was significantly higher in the SG compared to the PG (− 0.22 vs. − 0.12 mmol/L; p = 0.004) (Table 3).
Table 3.
Biochemical parameters: intra-group changes (baseline to 1 year) and comparison between groups after 1 year
| Placebo group | Strontium group | Between-group after 1 year | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 1 year | p | Baseline | 1 year | p | p | |
| PTH (pg/mL) | 123.9 (90.8; 162.2) | 36.2 (15.9; 51.1) | < 0.001 | 141.3 (97.4; 162.9) | 29.5 (± 23.5; 41.0) | < 0.001 | 0.362 |
| Ca++ (mmol/L) | 1.39 (1.36; 1.43) | 1.25 (1.21; 1.28) | < 0.001 | 1.41 (1.38; 1.50) | 1.22 (1.18; 1.24) | < 0.001 | 0.004 |
| Phosphate (mmol/L) | 0.70 (0.57; 0.83) | 1.03 (0.90; 1.17) | < 0.001 | 0.64 (0.57; 0.74) | 1.04 (0.95; 1.25) | < 0.001 | 0.265 |
| 25(OH)D (nmol/L) | 38.9 (26.8; 47.4) | 76.1 (61.6; 84.3) | < 0.001 | 39.8 (29.8; 52.1) | 67.9 (54.7; 83.4) | < 0.001 | 0.352 |
| 1,25(OH)D (nmol/L) | 62.0 (49.0; 77.0) | 46.0 (40.0; 65.0) | 0.017 | 57.0 (41.0; 73.0) | 57.0 (41.0; 73.0) | 0.011 | 0.525 |
| BAP (ng/mL) | 20.4 (14.6; 28.3) | 11.4 (8.1; 12.4) | 0.01 | 15.9 (10.8; 27.7) | 9.3 (8.1; 12.1) | 0.001 | 0.410 |
| OC (ng/mL) | 41.8 (22.8; 59.6) | 14.9 (12.7; 20.8) | < 0.001 | 33.2 (25.2; 43.3) | 14.3 (11.6; 17.8) | < 0.001 | 0.519 |
| CTX (ng/mL) | 0.84 (0.42; 1.01) | 0.19 (0.15; 0.28) | < 0.001 | 0.63 (0.52; 0.79) | 0.16 (0.14; 0.24) | < 0.001 | 0.366 |
| P1NP (ng/mL) | 61.5 (47.0; 89.0) | 24.0 (20.0; 31.0) | < 0.001 | 52.0 (43.0; 74.5) | 24.0 (22.0; 31.0) | < 0.001 | 0.890 |
| 24 h CrCl (mL/min) | 107.7 (96.4; 132.8) | 91.4 (78.9; 129.0) | 0.05 | 121.6 (89.4; 161.5) | 112.6 (90.6; 136.2) | 0.194 | 0.113 |
| OPG (pmol/L) | 4.46 (3.64; 5.64) | 4.97 (3.57; 7.48) | 0.087 | 4.65 (3.63; 5.47) | 5.05 (3.38; 6.27) | 0.976 | 0.525 |
| RANKL (pmol/L) | 0.87 (0.51; 1.42) | 0.58 (0.37; 1.35) | 0.465 | 0.74 (0.52; 0.99) | 0.65 (0.42; 0.94) | 0.068 | 1 |
| SOST (pmol/L) | 19.63 (15.55; 29.31) | 20.33 (16.16; 41.01) | 0.043 | 20.84 (14.85; 28.49) | 21.28 (18.42; 29.01) | 0.121 | 0.920 |
| DKK-1 (pmol/L) | 25.10 (18.99; 35.69) | 34.07 (20.06; 39.39) | 0.008 | 32.32 (23.71; 41.04) | 34.40 (29.39; 45.43) | 0.021 | 0.221 |
PTH parathyroid hormone, Ca++ ionized calcium, 25(OH)D 25-hydroxyvitamin D, 1,25(OH)D 1,25-dihydroxyvitamin D, BAP bone-specific alkaline phosphatase, OC osteocalcin, CTX CrossLaps, P1NP procollagen type 1 N-terminal propeptide, 24 h CrCl measured 24-h creatinine clearance, OPG osteoprotegerin, RANKL receptor activator of nuclear factor-kappa ligand, SOST sclerostin, DKK-1 Dickkopf-1
The markers of bone formation and markers of bone resorption decreased significantly in the SG and the PG (Table 3). Both parameters of the Wnt signaling pathway showed an increase: DKK-1 increased significantly in both groups and SOST showed a small increase in both groups that was only significant in the PG (p = 0.043 vs. p = 0.121 in the SG; Table 3).
Effects of preoperative PTH on biochemical markers of bone metabolism
At baseline, the PTH levels were positively correlated with Ca++ (r = 0.496; p < 0.001), OC (r = 0.482; p < 0.001), CTX (r = 0.420; p < 0.005), and procollagen type 1 N-terminal propeptide (P1NP; r = 0.332; p < 0.05), but not with BAP, OPG, RANKL, SOST, or DKK-1.
Correlation of BMD increase with preoperative parameters
In the PG, there was a strong and positive correlation between the preoperative levels of OC, P1NP, and CTX and the increase in lumbar-spine BMD (PG: r = 0.634, r = 0.750, r = 0.634, respectively). This correlation was weaker in the SG (SG: r = 0.413, r = 0.329, r = 0.329, respectively) (Table 4). At the femoral neck, a relevant correlation between BAP and increase in BMD (r = 0.510) was only identified in the PG.
Table 4.
Correlation between absolute change in lumbar spine BMD and preoperative levels of markers of bone metabolism; R: Spearman correlation index
| Placebo group | Strontium group | |||
|---|---|---|---|---|
| Spearman index (R) | p | Spearman index (R) | p | |
| Age | 0.085 | 0.706 | 0.139 | 0.473 |
| PTH | 0.292 | 0.187 | 0.232 | 0.226 |
| Ca++ | 0.292 | 0.187 | 0.256 | 0.180 |
| OC | 0.634 | 0.002 | 0.352 | 0.061 |
| P1NP | 0.750 | < 0.001 | 0.413 | 0.029 |
| CTX | 0.634 | 0.002 | 0.274 | 0.159 |
| BAP | 0.229 | 0.413 | 0.488 | 0.029 |
| OPG | − 0.116 | 0.627 | − 0.162 | 0.450 |
| RANKL | 0.600 | 0.400 | − 0.800 | 0.200 |
| SOST | 0.098 | 0.682 | 0.079 | 0.713 |
| DKK-1 | − 0.030 | 0.904 | − 0.206 | 0.335 |
BMD bone mineral density, PTH parathyroid hormone, Ca++ ionized calcium, OC osteocalcin, P1NP procollagen type 1 N-terminal propeptide, CTX CrossLaps, BAP bone-specific alkaline phosphatase, OPG osteoprotegerin, RANKL receptor activator of nuclear factor-kappa ligand, SOST sclerostin, DKK-1 Dickkopf-1
No significant correlation was seen between the preoperative levels of PTH, OPG, RANKL, SOST, or DKK-1 and gain in BMD at any site measured.
Adverse events
The intake of study medication did not induce any adverse events. In particular, no patient in either group developed cardiovascular disorders or thromboembolic complications during the study. No cases of vertebral or non-vertebral fractures occurred.
Discussion
This is the first prospective, randomized, double-blind trial to investigate the effect of anti-osteoporotic treatment on the postoperative course of BMD and bone metabolism after successful PTX, focusing only on patients with preoperatively advanced bone involvement (diagnosed osteoporosis or osteopenia). The rationale of this intervention was to enhance the speed and extent of bone density normalization in the early postoperative course. With intervention, the percentage of BMD increase at the lumbar spine was more than twofold higher in the treatment group than in the placebo group and the percentage of patients with osteopenia or osteoporosis declined from 69.0 to 37.9%. The additional effect of 6% lumbar-spine BMD change (PG: 3.94%; SG: 9.94%) shown in this study was equal to the 1-year baseline change after SR intake found in patients with primary osteoporosis but without pHPT (postmenopausal women and men). This treatment effect has been seen to reduce both vertebral and non-vertebral fractures [19, 20, 30]. As the fracture risk is prolonged up to 10 or more years following successful PTX in patients with pHPT [6, 15, 16], this effect seems preferable. The data presented here thus demonstrate that an anti-osteoporotic medication may additionally and positively influence bone remineralization, while producing similar effects as in patients without preceding PTX for pHPT. The effect on lumbar-spine BMD seems to be of even greater importance, as this site appears to be more strongly affected by pHPT [31, 32].
Although statistically not significant, a tendency towards a more pronounced gain in BMD at all sites was observed in females compared to males in either group (see Table 2). Especially for postmenopausal women who per se are at an elevated risk of fractures, any positive effect on bone stability seems important, since this trial showed that surgical cure of pHPT without additional treatment failed to reduce the proportion of patients with osteoporosis or osteopenia 1 year after PTX at any site in spite of adequate vitamin D and calcium supplementation. In a prospective and another retrospective trial, a further decrease in BMD was even documented in 15% to 31% [14, 33].
The information on biochemical markers presented here offers a broad insight into postoperative changes following PTX. Basically, the levels of Ca++ at diagnosis were only slightly elevated (1.39 and 1.41 mmol/L in the SG and PG, respectively; normal range 1.16–1.32 mmol/L) as consequence to earlier diagnoses in Western countries due to screening programs with more frequently asymptomatic patients [31]. Nevertheless, alterations in markers of bone metabolism were documented in all patients demonstrating relevant bone involvement, albeit with a high level of variability (thus suggesting individual differences in pHPT-induced bone affection). The serum levels of CTX, a bone resorption marker, as well as those of the bone formation markers OC and P1NP significantly declined in all subjects at follow-up compared to baseline (Table 3). Additionally, the two antagonists of the Wnt signaling pathway (“antagonists” of bone formation)—SOST and DKK-1—that have not yet been evaluated following PTX, slightly increased in both treatment groups over the 1-year follow-up. Both the increase in SOST and DKK-1 and the reduction of “classical” markers of bone metabolism suggest that PTX reduces bone resorption as well as bone formation. These findings are in line with our previous study, also showing reduced bone turnover after PTX surgery [34, 35]. Therefore, it seems unreasonable to postoperatively apply a mainly antiresorptive treatment such as bisphosphonates that have even shown to bear potentially negative effects in pHPT-related bone involvement [36]. Although SR was limited in its use in 2017 (due to an advanced risk of thromboembolism and cardiovascular events), it seems reasonable to consider other anti-osteoporotic medications stimulating bone formation to improve BMD after PTX. Alternatively, the Wnt signaling pathway may be an interesting starting point for further investigation, as both SOST and DKK-1 levels were seen to increase postoperatively in this trial. Romosozumab, an antibody against SOST, is a promising new therapeutic agent against osteoporosis, improving bone formation, and microarchitecture [25]. It was recently approved by the FDA and might be another potential pharmacological option. This assumption is in line with findings that expression of SOST in bone is enhanced after PTX for secondary (renal) HPT [37].
In addition to the effect of PTH in pHPT, low BMD may also be caused by such other factors as primary osteoporosis, especially in postmenopausal women. We therefore attempted to establish markers of bone metabolism that may potentially reflect bone involvement by pHPT. OC and CTX were strongly correlated (as strongly as Ca++) with preoperative PTH levels, and both markers, yet not PTH, showed a correlation with postoperative increase in BMD at the lumbar spine. These findings are in accordance with the study authored by Hansen et al., showing a correlation between increase in volumetric BMD and CTX (no data for OC) [38], but contradict the findings of Rolighed et al. who detected a positive association between preoperative PTH levels and postoperative BMD increase [39]. Preoperative biochemical markers may help to identify patients in whom a pronounced positive effect of successful PTX on postoperative BMD may be expected. They may also assist in differentiating such patients from those in whom primary osteoporosis and other factors may additionally strongly affect bone quality and who are less likely to improve after cure of pHPT, thus requiring immediate additional therapy.
Essentially, our investigation had several strengths: it was the first to investigate the effect of an anti-osteoporotic treatment immediately after successful PTX. Apart from its prospective, randomized, and double-blind design, the study population was very homogeneous, including only osteopenic and osteoporotic patients. Moreover, all patients were supplied with adequate standardized vitamin D and calcium doses, as shown to be important after PTX for pHPT [40].
A limitation of this trial is that SR was restricted in its use at the end of patient recruitment. Some of the measured effect of SR is likely caused by the overestimation of BMD in DXA scans of patients treated with SR (due to the higher atomic number of strontium compared to calcium [41]). However, even if 25% of the additional effect (approx. 6%) in the SG was caused by overestimation of BMD, the increase would still be 3.5% higher than in the PG (3.94% vs. SG: 7.47%; p = 0.01).
Conclusion
In conclusion, this randomized, double-blind, placebo-controlled trial indicated that the treatment of osteopenic or osteoporotic patients following PTX increases BMD mainly at the lumbar spine. The rationale of this trial was emphasized by the fact that without treatment, nearly all patients remained osteoporotic or at least osteopenic even with an adequate supply of vitamin D and calcium. In patients with high levels of OC and CTX, an increase in BMD is more likely than in those with low markers of bone metabolism. Thus, at least patients with osteoporosis/osteopenia with a lower probability of improvement after PTX should either be monitored closely for improvement in bone density or even receive bone-specific treatment immediately after PTX to reduce the risk of potential complications. As strontium ranelate was restricted in its use, other medications with positive effects on bone formation should be evaluated and the Wnt pathway may be another target for therapy.
Electronic supplementary material
(DOCX 19 kb).
Acknowledgments
The authors wish to thank Mrs. Daniela Truzla for her important logistic support and patience.
Abbreviations
- SG
Strontium group
- PG
Placebo group
- pHPT
Primary hyperparathyroidism
- BMD
Bone mineral density
- PTX
Parathyroidectomy
- PTH
Parathyroid hormone
- Ca++
Ionized calcium
- 25(OH)D
25-hydroxyvitamin D
- 1,25(OH)D
1,25-dihydroxyvitamin D
- BAP
Bone-specific alkaline phosphatase
- CTX
CrossLaps
- P1NP
Procollagen type 1 N-terminal propeptide
- 24 h CrCl
Measured 24-h creatinine clearance
- OPG
Osteoprotegerin
- RANKL
Receptor activator of nuclear factor-kappa B ligand
- DKK-1
Dickkopf-1
- 1/3 radius
One-third distal radius
- MID radius
Mid-distal radius
- UD radius
Ultradistal radius
- DXA
Dual-energy X-ray absorption
- OC
Osteocalcin
- SD
Standard deviation
- SOST
Sclerostin
- SR
Strontium ranelate
- OMIP
Open minimally invasive parathyroidectomy
Authors’ contributions
Study conception and design: MN, PP, KK, PR, BN. Acquisition of data: PR, AS, CS, BN, MN. Analysis and interpretation of data: MN, UF, PP, KK. Drafting manuscript: MN, UF, KK, PP. Critical revision of manuscript: MN, UF, KK, PP, BN, AS. Approving final version of manuscript: MN, UF, PR, AS, CS, BN, PP, KK.
Funding information
Open access funding provided by Medical University of Vienna. This work was supported by the Austrian National Bank (OENB) Jubilee Fund, Project No. 13245. Both verum and placebo medication was provided by Servier.
Compliance with ethical standards
The study procedures of this double-blind, placebo-controlled, randomized trial were approved by the Ethics Committee of the Medical University of Vienna (EKNr: 2142008), the Austrian Agency for Health and Food Safety (Österreichische Agentur für Gesundheit und Ernährungssicherheit), ClinicalTrials.gov (identifier: NCT01222026), and European Union Drug Regulating Authorities Clinical Trials. All study participants gave written informed consent in accordance with the Declaration of Helsinki.
Conflict of interest
PP has received research support and/or honoraria from Amgen GmbH, Biomedica GmbH, DePuySynthes, Fresenius Kabi Austria, Meda Pharma/Mylan GmbH, Shire Austria GmbH, TAmiRNA GmbH, and UCB Pharma. All authors have declared that no competing financial interests or other conflicts of interest exist.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siilin H, Rastad J, Ljunggren O, Lundgren E. Disturbances of calcium homeostasis consistent with mild primary hyperparathyroidism in premenopausal women and associated morbidity. J Clin Endocrinol Metab. 2008;93(1):47–53. doi: 10.1210/jc.2007-0600. [DOI] [PubMed] [Google Scholar]
- 2.Cusano NE, Rubin MR, Silva BC, Tay YD, Williams JM, Agarwal S, Omeragic B, Guo XE, Bilezikian JP. Skeletal microstructure and estimated bone strength improve following parathyroidectomy in primary hyperparathyroidism. J Clin Endocrinol Metab. 2018;103(1):196–205. doi: 10.1210/jc.2017-01932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker MD, Cong E, Lee JA, Kepley A, Zhang C, McMahon DJ, Silverberg SJ. Vitamin D in primary hyperparathyroidism: effects on clinical, biochemical, and Densitometric presentation. J Clin Endocrinol Metab. 2015;100(9):3443–3451. doi: 10.1210/jc.2015-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viccica G, Cetani F, Vignali E, Miccoli M, Marcocci C. Impact of vitamin D deficiency on the clinical and biochemical phenotype in women with sporadic primary hyperparathyroidism. Endocrine. 2017;55(1):256–265. doi: 10.1007/s12020-016-0931-8. [DOI] [PubMed] [Google Scholar]
- 5.Reid LJ, Muthukrishnan B, Patel D, Seckl JR, Gibb FW. Predictors of nephrolithiasis, osteoporosis and mortality in primary hyperparathyroidism. J Clin Endocrinol Metab. 2019;104:3692–3700. doi: 10.1210/jc.2018-02483. [DOI] [PubMed] [Google Scholar]
- 6.Vestergaard P, Mollerup CL, Frokjaer VG, Christiansen P, Blichert-Toft M, Mosekilde L. Cohort study of risk of fracture before and after surgery for primary hyperparathyroidism. Bmj. 2000;321(7261):598–602. doi: 10.1136/bmj.321.7261.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Geronimo S, Romagnoli E, Diacinti D, D'Erasmo E, Minisola S. The risk of fractures in postmenopausal women with primary hyperparathyroidism. Eur J Endocrinol. 2006;155(3):415–420. doi: 10.1530/eje.1.02225. [DOI] [PubMed] [Google Scholar]
- 8.Khosla S, Melton LJ, 3rd, Wermers RA, Crowson CS, O'Fallon W, Riggs B. Primary hyperparathyroidism and the risk of fracture: a population-based study. J Bone Miner Res. 1999;14(10):1700–1707. doi: 10.1359/jbmr.1999.14.10.1700. [DOI] [PubMed] [Google Scholar]
- 9.Eller-Vainicher C, Battista C, Guarnieri V, Muscarella S, Palmieri S, Salcuni AS, Guglielmi G, Corbetta S, Minisola S, Spada A, Hendy GN, Cole DE, Chiodini I, Scillitani A. Factors associated with vertebral fracture risk in patients with primary hyperparathyroidism. Eur J Endocrinol. 2014;171(3):399–406. doi: 10.1530/EJE-14-0343. [DOI] [PubMed] [Google Scholar]
- 10.Bilezikian JP, Brandi ML, Eastell R, Silverberg SJ, Udelsman R, Marcocci C, Potts JT., Jr Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the fourth international workshop. J Clin Endocrinol Metab. 2014;99(10):3561–3569. doi: 10.1210/jc.2014-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambrogini E, Cetani F, Cianferotti L, Vignali E, Banti C, Viccica G, Oppo A, Miccoli P, Berti P, Bilezikian JP, Pinchera A, Marcocci C. Surgery or surveillance for mild asymptomatic primary hyperparathyroidism: a prospective, randomized clinical trial. J Clin Endocrinol Metab. 2007;92(8):3114–3121. doi: 10.1210/jc.2007-0219. [DOI] [PubMed] [Google Scholar]
- 12.Rao DS, Phillips ER, Divine GW, Talpos GB. Randomized controlled clinical trial of surgery versus no surgery in patients with mild asymptomatic primary hyperparathyroidism. J Clin Endocrinol Metab. 2004;89(11):5415–5422. doi: 10.1210/jc.2004-0028. [DOI] [PubMed] [Google Scholar]
- 13.Lundstam K, Heck A, Godang K, Mollerup C, Baranowski M, Pernow Y, Aas T, Hessman O, Rosen T, Nordenstrom J, Jansson S, Hellstrom M, Bollerslev J, Group SS Effect of surgery versus observation: skeletal 5-year outcomes in a randomized trial of patients with primary HPT (the SIPH study) J Bone Miner Res. 2017;32(9):1907–1914. doi: 10.1002/jbmr.3177. [DOI] [PubMed] [Google Scholar]
- 14.Sitges-Serra A, Garcia L, Prieto R, Pena MJ, Nogues X, Sancho JJ. Effect of parathyroidectomy for primary hyperparathyroidism on bone mineral density in postmenopausal women. Br J Surg. 2010;97(7):1013–1019. doi: 10.1002/bjs.7044. [DOI] [PubMed] [Google Scholar]
- 15.Lundstam K, Heck A, Mollerup C, Godang K, Baranowski M, Pernow Y, Varhaug JE, Hessman O, Rosen T, Nordenstrom J, Jansson S, Hellstrom M, Bollerslev J, Group SS Effects of parathyroidectomy versus observation on the development of vertebral fractures in mild primary hyperparathyroidism. J Clin Endocrinol Metab. 2015;100(4):1359–1367. doi: 10.1210/jc.2014-3441. [DOI] [PubMed] [Google Scholar]
- 16.Ramos L, Piedra M, Munoz P, Vazquez LA, Garcia-Unzueta MT, Montalban C, Amado JA. Bone mineral density evolution and incidence of fractures in a cohort of patients with primary hyperparathyroidism treated with parathyroid surgery vs active surveillance during 6 years of follow-up. Endocrinol Diabetes Nutr. 2019;66(1):41–48. doi: 10.1016/j.endinu.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Marie PJ. Strontium ranelate: a physiological approach for optimizing bone formation and resorption. Bone. 2006;38(2 Suppl 1):S10–S14. doi: 10.1016/j.bone.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 18.Marie PJ. Strontium ranelate: new insights into its dual mode of action. Bone. 2007;40:S5–S8. doi: 10.1016/j.bone.2007.02.003. [DOI] [Google Scholar]
- 19.Meunier PJ, Roux C, Seeman E, Ortolani S, Badurski JE, Spector TD, Cannata J, Balogh A, Lemmel EM, Pors-Nielsen S, Rizzoli R, Genant HK, Reginster JY. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med. 2004;350(5):459–468. doi: 10.1056/NEJMoa022436. [DOI] [PubMed] [Google Scholar]
- 20.Reginster JY, Seeman E, De Vernejoul MC, Adami S, Compston J, Phenekos C, Devogelaer JP, Curiel MD, Sawicki A, Goemaere S, Sorensen OH, Felsenberg D, Meunier PJ. Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: treatment of peripheral osteoporosis (TROPOS) study. J Clin Endocrinol Metab. 2005;90(5):2816–2822. doi: 10.1210/jc.2004-1774. [DOI] [PubMed] [Google Scholar]
- 21.O'Donnell S, Cranney A, Wells GA, Adachi JD, Reginster JY. Strontium ranelate for preventing and treating postmenopausal osteoporosis. Cochrane Database Syst Rev. 2006;4:CD005326. doi: 10.1002/14651858.CD005326.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seeman E, Devogelaer JP, Lorenc RS, Spector T, Brixen K, Balogh A, Stucki G, Reginster JY. Strontium ranelate reduces the risk of vertebral fractures in patients with osteopenia. J Bone Miner Res. 2007;10:1359. doi: 10.1359/jbmr.071105. [DOI] [PubMed] [Google Scholar]
- 23.Ammann P. Strontium ranelate: a physiological approach for an improved bone quality. Bone. 2006;38(2 Suppl 1):15–18. doi: 10.1016/j.bone.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 24.Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O'Brien CA, Manolagas SC, Jilka RL. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146(11):4577–4583. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- 25.Chavassieux Pascale, Chapurlat Roland, Portero‐Muzy Nathalie, Roux Jean‐Paul, Garcia Pedro, Brown Jacques P, Libanati Cesar, Boyce Rogely W, Wang Andrea, Grauer Andreas. Bone‐Forming and Antiresorptive Effects of Romosozumab in Postmenopausal Women With Osteoporosis: Bone Histomorphometry and Microcomputed Tomography Analysis After 2 and 12 Months of Treatment. Journal of Bone and Mineral Research. 2019;34(9):1597–1608. doi: 10.1002/jbmr.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanis JA, Melton LJ, 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9(8):1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 27.Bilezikian JP. Primary hyperparathyroidism. Endocr Pract. 2012;18(5):781–790. doi: 10.4158/EP12166.RA. [DOI] [PubMed] [Google Scholar]
- 28.Udelsman R, Akerstrom G, Biagini C, Duh QY, Miccoli P, Niederle B, Tonelli F. The surgical management of asymptomatic primary hyperparathyroidism: proceedings of the fourth international workshop. J Clin Endocrinol Metab. 2014;99(10):3595–3606. doi: 10.1210/jc.2014-2000. [DOI] [PubMed] [Google Scholar]
- 29.Wilhelm SM, Wang TS, Ruan DT, Lee JA, Asa SL, Duh QY, Doherty GM, Herrera MF, Pasieka JL, Perrier ND, Silverberg SJ, Solorzano CC, Sturgeon C, Tublin ME, Udelsman R, Carty SE. The American Association of Endocrine Surgeons Guidelines for definitive Management of Primary Hyperparathyroidism. JAMA Surg. 2016;151(10):959–968. doi: 10.1001/jamasurg.2016.2310. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman JM, Audran M, Bianchi G, Braga V, Diaz-Curiel M, Francis RM, Goemaere S, Josse R, Palacios S, Ringe JD, Felsenberg D, Boonen S. Efficacy and safety of strontium ranelate in the treatment of osteoporosis in men. J Clin Endocrinol Metab. 2013;98(2):592–601. doi: 10.1210/jc.2012-3048. [DOI] [PubMed] [Google Scholar]
- 31.Liu JM, Cusano NE, Silva BC, Zhao L, He XY, Tao B, Sun LH, Zhao HY, Fan WW, Romano ME, Ning G, Bilezikian JP. Primary hyperparathyroidism: a tale of two cities revisited - New York and Shanghai. Bone Res. 2013;1(2):162–169. doi: 10.4248/BR201302005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eller-Vainicher C, Filopanti M, Palmieri S, Ulivieri FM, Morelli V, Zhukouskaya VV, Cairoli E, Pino R, Naccarato A, Verga U, Scillitani A, Beck-Peccoz P, Chiodini I. Bone quality, as measured by trabecular bone score, in patients with primary hyperparathyroidism. Eur J Endocrinol. 2013;169(2):155–162. doi: 10.1530/EJE-13-0305. [DOI] [PubMed] [Google Scholar]
- 33.Dy BM, Grant CS, Wermers RA, Kearns AE, Huebner M, Harmsen WS, Thompson GB, Farley DR, Richards ML. Changes in bone mineral density after surgical intervention for primary hyperparathyroidism. Surgery. 2012;152(6):1051–1058. doi: 10.1016/j.surg.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Kerschan-Schindl K. Bone turnover in hyperparathyroidism. Wien Med Wochenschr. 2013;163(17–18):391–396. doi: 10.1007/s10354-012-0125-9. [DOI] [PubMed] [Google Scholar]
- 35.Kerschan-Schindl K, Riss P, Krestan C, Rauner M, Bieglmayer C, Gleiss A, Fialka-Moser V, Niederle B, Pietschmann P. Bone metabolism in patients with primary hyperparathyroidism before and after surgery. Horm Metab Res. 2012;44(6):476–481. doi: 10.1055/s-0032-1308998. [DOI] [PubMed] [Google Scholar]
- 36.Yeh MW, Zhou H, Adams AL, Ituarte PH, Li N, Liu IL, Haigh PI. The relationship of parathyroidectomy and bisphosphonates with fracture risk in primary hyperparathyroidism: an observational study. Ann Intern Med. 2016;164(11):715–723. doi: 10.7326/M15-1232. [DOI] [PubMed] [Google Scholar]
- 37.Pires GO, Vieira IO, Hernandes FR, Teixeira AL, Oliveira IB, Dominguez WV, Dos Reis LM, Montenegro FM, Moyses RM, Carvalho AB, Jorgetti V. Effects of parathyroidectomy on the biology of bone tissue in patients with chronic kidney disease and secondary hyperparathyroidism. Bone. 2019;121:277–283. doi: 10.1016/j.bone.2019.01.029. [DOI] [PubMed] [Google Scholar]
- 38.Hansen S, Hauge EM, Rasmussen L, Jensen JE, Brixen K. Parathyroidectomy improves bone geometry and microarchitecture in female patients with primary hyperparathyroidism: a one-year prospective controlled study using high-resolution peripheral quantitative computed tomography. J Bone Miner Res. 2012;27(5):1150–1158. doi: 10.1002/jbmr.1540. [DOI] [PubMed] [Google Scholar]
- 39.Rolighed L, Vestergaard P, Heickendorff L, Sikjaer T, Rejnmark L, Mosekilde L, Christiansen P. BMD improvements after operation for primary hyperparathyroidism. Langenbeck's Arch Surg. 2013;398(1):113–120. doi: 10.1007/s00423-012-1026-5. [DOI] [PubMed] [Google Scholar]
- 40.Kaderli RM, Riss P, Dunkler D, Pietschmann P, Selberherr A, Scheuba C, Niederle B. The impact of vitamin D status on hungry bone syndrome after surgery for primary hyperparathyroidism. Eur J Endocrinol. 2018;178(1):1–9. doi: 10.1530/EJE-17-0416. [DOI] [PubMed] [Google Scholar]
- 41.Blake GM, Lewiecki EM, Kendler DL, Fogelman I. A review of strontium ranelate and its effect on DXA scans. J Clin Densitom. 2007;10(2):113–119. doi: 10.1016/j.jocd.2007.01.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 19 kb).