Abstract
Circumstantial evidence supports the hypothesis that the sexually dimorphic vasopressin (AVP) innervation of the brain tempers sickness behavior in males. Here we test this hypothesis directly, by comparing sickness behavior in animals with or without ablations of BNST AVP cells, a major source of sexually dimorphic AVP in the brain. We treated male and female AVP-iCre+ and AVP-iCre− mice that had been injected with viral Cre-dependent caspase-3 executioner construct into the BNST with lipopolysaccharide (LPS) or sterile saline, followed by behavioral analysis. In all groups, LPS treatment reliably reduced motor behavior, increased anxiety-related behavior, and reduced sucrose preference and consumption. Male mice, whose BNST AVP cells had been ablated (AVP-iCre+), displayed only minor reductions in LPS-induced sickness behavior, whereas their female counterparts displayed, if anything, an increase in sickness behaviors. All saline-treated mice with BNST AVP cell ablations consumed more sucrose than did control mice, and males, but not females, with BNST AVP cell ablations showed reduced preference for novel conspecifics compared to control mice. These data confirm that BNST AVP cells control social behavior in a sexually dimorphic way, but do not play a critical role in altering sickness behavior.
Keywords: Arginine vasopressin, sickness behavior, social behavior, bed nucleus of the stria terminalis
1. Introduction
Most animals experience pathogen-induced sickness during their lifetime. While each pathogen brings its own set of inflammatory responses and other symptoms, sickness often causes general behavioral changes such as lethargy, reduced ingestive behavior, and social withdrawal (Kelley et al., 2003). These behavioral changes are generally thought to complement physiological responses, such as fever, in speeding up recovery (Hart, 1988). While such behavioral changes may be beneficial for survival, long-term or inappropriate inflammation may contribute to mental health conditions such as depression (Miller and Raison, 2016). Consequently, understanding the ways in which inflammation alters behavior may help treat such conditions.
The physiological basis of sickness behavior involves multiple pathways that relay information about peripheral inflammation, such as vagus nerve activity and humoral immune signaling (D’Mello and Swain, 2017). Ultimately, peripheral inflammation activates brain regions such as the paraventricular hypothalamus (PVH), medial amygdala (MeA), bed nucleus of the stria terminalis (BNST), and preoptic area (Goehler et al., 2000; Sagar et al., 1995), all of which have been associated with behavioral profiles altered during sickness (Konsman et al., 2008; Lacosta et al., 1999; Taylor et al., 2012).
Some of these areas, such as the posterior BNST and MeA, contain arginine vasopressin (AVP) cells that have been indirectly implicated in regulating fever and sickness behavior (Pittman et al., 1998a, 1998b; Sens et al., 2017). For example, in rats, fever increases BNST neuronal activity (Mathieson et al., 1989), and electrical stimulation of the BNST reduces fever (Naylor et al., 1988). This may be due to effects of AVP in the septum, a target of BNST/MeA AVP projections, as septal AVP administration also reduces fever. This effect is testosterone-dependent, and is found in males but not in females (Pittman et al., 1998b, 1988), mirroring the sex differences in BNST AVP expression, which is more pronounced in males than in females (De Vries and Panzica, 2006), which suggest that BNST AVP cells modulate the fever response.
The same cells may also regulate sickness behavior. For example, in male rats intracerebroventricular injections of AVP reduce sickness behavior, whereas AVP antagonism exacerbates sickness behavior (Dantzer et al., 1991). These effects are also testosterone-dependent; effects of inflammation and AVP administration are more pronounced in castrated animals, which cease to produce AVP in the BNST (Dantzer et al., 1991; De Vries and Panzica, 2006). However, whether BNST AVP cells modulate sickness behavior has not been directly tested. We do so here by selectively ablating AVP cells in the BNST of male and female AVP-iCre mice via injections of viral vector containing a Cre-dependent cell death construct (caspase-3/Tev) and testing effects of ablation on sickness behavior. We induced sickness behavior via intraperitoneal injections of lipopolysaccharide (LPS), a component of gram-negative bacterial cell walls, commonly used as a proxy for bacterial infections (Dantzer et al., 1998), followed by tests for behaviors altered in sickness. We predicted that BNST AVP cell ablation would intensify sickness behavior, more so in males than in females.
2. Material and Methods
2.1. Animals
All mice were maintained at 22°C on a 12:12 reverse light cycle with food and water available ad libitum, housed in individually ventilated cages (Animal Care Systems), and provided with corncob bedding, a nestlet square, and a housing tube. All animal procedures were approved by the Georgia State University Institutional Animal Care and Use Committee (IACUC) and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Founding AVP-iCre mice were obtained from Dr. Michihiro Mieda (Kanazawa University, Japan). These mice were generated using a bacterial artificial chromosome that expressed codon-improved Cre recombinase (Shimshek et al., 2002) under the transcriptional control of the AVP promoter (AVP-iCre mice). In these animals, iCre expression is found in the bed nucleus of the stria terminalis and the medial amygdala, as well as in hypothalamic areas (Mieda et al., 2015). Subjects were derived by crossing heterozygous iCre+ mutants to wildtype C57Bl/6J mice and genotyped (ear punch) by polymerase chain reaction (PCR) at 21-24 days of age (Transnetyx). Both iCre+ and iCre− littermates were used in behavioral experiments. All subjects were used in a prior experiment (Rigney et al., 2019) and all surgical procedures described below were conducted as part of that study. Stimulus animals for the three-chamber test were adult C57B6/J mice of both sexes, group housed in the same room conditions as the experimental animals.
In total, 45 animals with confirmed BNST AVP cell ablation (Rigney et al., 2019) were used for the behavioral testing described below: 11 Cre− males, 13 Cre – females, 13 Cre+ males, and 8 Cre+ females. Two female subjects (1 Cre+ and 1 Cre−) did not recover from initial LPS treatment and were euthanized and removed from all analyses. The remaining 43 animals were tested on all behavioral measures described below. Video recording error forced removal of 1 Cre+ female from the tail suspension test, and removal of 1 Cre+ and 1 Cre− female from the three-chamber social test analyses. Bottle failure and fluid leakage forced the removal of 6 females (5 Cre−, 1 Cre+) from the sucrose preference analysis.
2.2. Viral Vectors
BNST neurons with AVP promoter-driven Cre-expression were ablated using an adeno-associated virus (AAV-flex-taCasp3-TEVp) (University of North Carolina at Chapel Hill Vector Core) that encodes Cre-dependent pro-caspase-3. This enzyme activates an apoptotic signaling cascade, cleaving multiple structural and regulatory proteins critical for cell survival and maintenance (Unger et al., 2015; Yang et al., 2013) and thereby inducing far less inflammation than other lesion approaches (Morgan et al., 2014). High titer AAV of serotype 2/1 (3×1012 IU/mL) was purchased from the University of North Carolina at Chapel Hill Vector Core (Rigney et al., 2019).
2.3. Stereotaxic surgery
All surgeries were carried out using 1.5-3% isoflurane gas anesthesia in 100% oxygen; 3 mg/kg of carprofen was given before surgery to reduce pain. Mice were positioned in a stereotaxic frame (David Kopf Instruments) with ear and incisor bars holding bregma and lambda level. After a midline scalp incision, a hand operated drill was used to make holes in the skull, exposing the dura. For all subjects, 500 nl of AAV-flex-taCasp3-TEVp was delivered bilaterally to the BNST (coordinates: AP −0.01 mm; ML ±0.75 mm; DV 4.8 mm (Paxinos and Franklin, 2012) at a rate of 100 nl/min using a 5 μl Hamilton syringe with a 30-gauge beveled needle mounted on a stereotaxic injector. Following virus delivery, the syringe was left in place for 15 minutes and slowly withdrawn from the brain (Rigney et al., 2019).
2.4. Experimental Procedure
All behavior tests were done in the dark phase under red lighting, and animals were acclimated to the behavior testing suite for at least one hour before testing. At least one week after tests for social and communicative behavior described in (Rigney et al., 2019) and seven weeks after viral ablation, subjects were weighed and injected intraperitoneally with either 1 mg/kg LPS (from e. coli 0111:B4, Millipore-Sigma) or sterile saline one hour before dark phase (ZT11). While the LPS dose used is highly variable across previous studies, inflammation and sickness behavior after LPS administration have generally been reported to occur from 2 hours to over 24 hours post-injection (Biesmans et al., 2013; Dantzer et al., 2008), and the dose of LPS used in this study reliably induces sickness behavior (Lacosta et al., 1999). Therefore, the open field test (OFT) was conducted three hours following LPS or saline injections, and the elevated zero maze (EZM) test was conducted immediately following the OFT. Sucrose preference was then assessed in the home cage over a 20-hour period, starting 5 hours after LPS or saline injections). Immediately following this (25 hours post-injection), animals were tested in the three-chamber social interaction test and tail suspension test (TST). This entire sequence was repeated one week later, with animals that first received LPS now receiving saline and vice versa as indicated in Fig. 1. An interval of one week between treatments was chosen, because LPS causes sickness for only up to four days post-injection (Weiland et al., 2007). To make sure there were no residual effects of the initial LPS treatment results were compared across treatment order.
Figure 1.
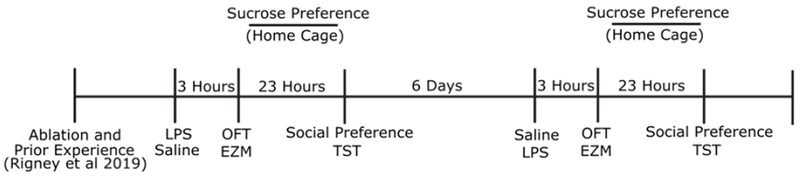
Experimental timeline.
2.5. Open-Field and Elevated Zero Maze
Three hours after injections, animals were placed in an 43cm x 43cm x 30cm open field chamber for 10 min and behavior was automatically tracked (Med Associates). Data was analyzed in two 5-min blocks, to separate behavior during the initial exploration period (first 5-min block, early exploration phase) from behavior after habituation to the chamber (second 5-min block, late exploration phase) (Gould et al., 2009; Walsh and Cummins, 1976). Distance traveled and time spent in the center area were analyzed as measures of locomotion and anxiety-like behavior, respectively.
Immediately after OFT, subjects were tested on an elevated zero maze (EZM). This apparatus consists of a 5.5 cm wide circular platform of internal diameter 35 cm raised 50 cm off the ground, with two equally spaced enclosed compartments covering half of the platform. Subject activity was tracked using automated software (AnyMaze) with the time and speed in each zone (closed, open), total distance traveled, and immobility time recorded.
2.6. Sucrose Preference
For at least 2 days before LPS/saline injections, subjects were acclimated to having two water bottles placed in their home cage. After OFT/EZM assessment, approximately 5 hours after LPS injections, subjects were returned to their home cage, and bottles were replaced with pre-weighed bottles, one containing sucrose solution (2.5% in tap water) and the other tap water. Animals had access to both sucrose solution and water for the next 20 hours, until the start of the next day’s testing. The bottles were then removed and reduction in liquid was measured. A control water bottle in a nearby empty cage showed a <1 mL loss over the same period and room conditions. Preference was calculated as the percentage of sucrose consumed compared to total consumption (Sucrose / (Sucrose + Water) *100%).
2.7. Social Preference
To measure social preference and social novelty-preference, animals were tested in a large plexiglass chamber (20.3 x 42 x 22 cm) divided into three equal compartments with openings between sections, 26 hours after LPS injections. Subjects were habituated to the apparatus for 5 min before testing. Subjects were temporarily removed while stimuli, contained within smaller cages (8cm diameter, 18cm height, 3-mm diameter bars, 7.4mm spacing) were placed in the center of each of the two outer chambers. First, to test for social preference, a novel toy object and a novel, same-sex stimulus animal were placed in opposite cages. Subjects and stimuli animals had limited ability to directly contact each other; they were able to pass extremities (e.g. paws, tail) through the smaller cage bars during investigation. The subjects were then returned to the apparatus and allowed 10 minutes to explore the apparatus. At the end of this test, the subjects were removed again, and the toy object replaced with a novel same-sex stimulus animal (from a different cage from the first stimulus) to test for recognition of social novelty. The subject was then placed into the center chamber again and given 10 minutes to explore the apparatus. The position of object and original animal was counterbalanced across trials but did not change between social preference and social novelty preference tests. Each trial was video-recorded, and the time spent in each chamber and in active investigation, defined by the subject’s snout within 2 cm of the stimulus cage were manually scored from video files (Noldus Observer) by an experimenter blind to the subjects’ genotype.
2.8. Tail Suspension
After the social preference/novelty tests, subjects underwent a 5-minute tail suspension test (TST) as a measure of stress-coping behavior (Castagne et al., 2011). Time spent hanging immobile (not struggling) was scored as was latency to first immobile period. Animals were suspended by their tails by a strip of tape (~15cm) attached to an overhang, during which they were recorded and later scored (Noldus Observer) by an experimenter blind to the genotype of the subjects.
2.9. Tissue Collection and FISH
After completion of all testing, animals were sacrificed via CO2 asphyxiation. Brains were rapidly removed and flash frozen in 2-methylbutane before storage at −80° C. Frozen tissue was sectioned coronally in 20μm sections, and processed for AVP mRNA fluorescent in situ hybridization (FISH) to confirm AVP cell deletion, as described in detail in Rigney et al. (2019). Ablation was specific to AVP cells in the BNST with no indication of non-targeted cell loss in the BNST. Nearby AVP hypothalamic cell populations were intact. Only data from subjects with confirmed BNST ablation (defined as over 90% AVP cell loss) were analyzed in this report.
2.10. Data Analysis
Statistical analysis was conducted using SPSS (IBM). OFT, EZM, Sucrose preference test, and TST, data was analyzed using a mixed-model three-way ANOVA. Treatment (LPS, Saline) was the within-subjects variable; both sex (M, F) and genotype (Cre+, Cre−) were the between-subject variables. Post-hoc t-tests were used to analyze genotype effects following significant ANOVA interactions. The social preference data was analyzed using a mixed-model three-way ANOVA with chamber (stimulus 1, stimulus 2) as a within-subjects variable, sex (M, F) as a between-subjects variable, and genotype (Cre+, Cre−) as a between-subjects variable. This analysis was performed for each treatment (LPS, Saline) separately, in order to better analyze the impact of genotype and sex on social investigation.
3. Results
3.1. Open Field Test and Elevated Zero Maze
LPS caused acute sickness behavior in all animals, with some specific measures affected by sex and genotype. As expected (Lacosta et al., 1999; Swiergiel and Dunn, 2007), LPS injections decreased the overall distance traveled in the OFT by all subjects, in both early exploration (FTreatment 1,39 = 396.29, p < 0.001, ηp2 = 0.91) and late exploration phases (FTreatment 1,39 = 309.54, p < 0.001, ηp2 = 0.89); no other differences were apparent (Fig 2A, B).
Figure 2.
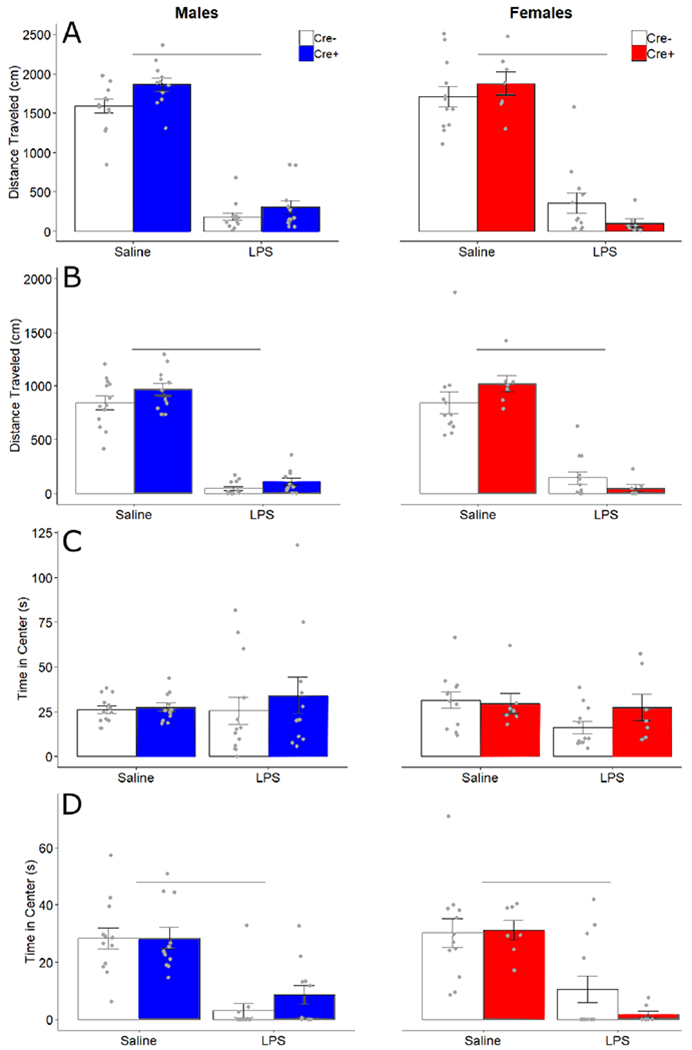
Open field behavior. Mean ± SEM of total distance (cm) traveled in the early (A) and late (B) exploration phases, and of time (seconds) spent in the center of the open field in early (C) and late (D) exploration phases for male and female Cre− (white bars) and Cre+ (filled bars) mice. Points indicate individual data. Horizontal bars indicate significant differences between treatments.
LPS treatment did not affect time spent in the center zone during the early exploration phase of the OFT (FTreatment 1,39 = 0.45, p= 0.51) (Fig 2C) but did decrease time spent in the anxiogenic center zone during the late exploration phase in the OFT test (FTreatment 1,39 = 74.847, p < 0.001, ηp2 = 0.66) (Fig 2D). There were no genotype or sex effects on any other measures.
In the elevated zero maze, LPS treatment decreased distance traveled (FTreatment 1,39 = 285.55, p < 0.001, ηp2 = 0.88) with an interaction of Treatment by Sex by Genotype (F1, 39 = 4.723, p = 0.036, ηp2 = 0.108). Post hoc comparisons for effects of genotype trended towards decreased distance in Cre+ females as compared to Cre− females after LPS treatment (t = 2.17, p = 0.052), but no obvious differences between Cre+ and Cre− males after LPS treatment (t = −1.11, p = 0.28). There were no apparent differences between genotypes for saline treated males (t = 0.27, p= 0.98) or females (t = −0.99, p = 0.35) (Fig 3A).
Figure 3.
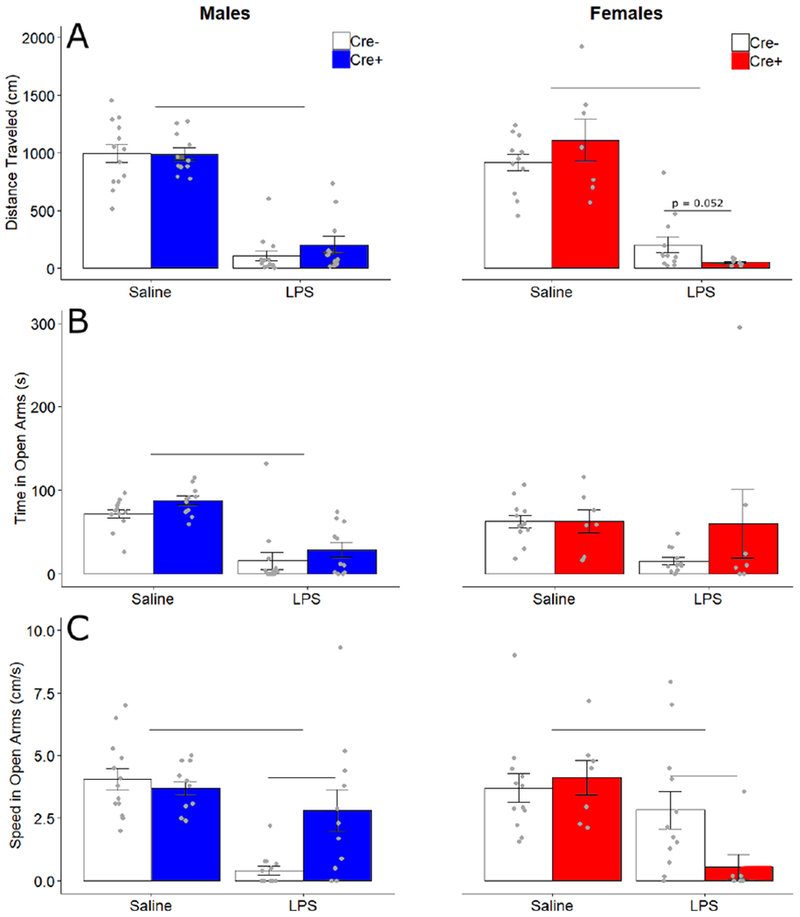
Elevated zero maze behavior. Mean ± SEM of (A) total distance traveled (cm), (B) time spent (seconds) in open arms of the maze, and (C) average speed (cm/seconds) in the open arms of the maze for male and female Cre− (white bars) and Cre+ (filled bars) mice. Points indicate individual data. Horizontal bars indicate treatment differences and genotype differences.
LPS treatment decreased time spent in the anxiogenic open arms (F1,39 = 30.37, p < 0.001, ηp2 = 0.44), with an interaction of Treatment by Sex (FTreatment x Sex 1,39 = 0.47, p= 0.037, ηp2 = 0.107). No other effects or interactions were detected (Fig 3B). LPS injections decreased subjects’ speed in the open arms (F1,39 = 31.50, p < 0.001, ηp2 = 0.48) in a manner dependent on sex and genotype (FTreatment x Genotype x Sex 1,39 = 11.668, p = 0.001, ηp2 = 0.23; FGenotype x Sex 1,39 = 5.343, p = 0.026, ηp2 = 0.12). Post-hoc comparisons for genotype revealed that in LPS-treated subjects, Cre+ males move faster than Cre− males (t=−2.8, p=.017), while Cre+ females move slower than Cre− females (t= 2.52, p = 0.022). No differences in genotype were detected in saline treated males (t = 0.69, p= 0.50) or females (t = −0.47, p = 0.64) (Fig 3C), suggesting that ablating AVP cells does not affect overall activity levels.
3.2. Sucrose Preference and Tail Suspension Tests
Technical problems with several water bottles caused leakage, thus data from six female subjects (5 Cre−, 1 Cre+) were removed from this analysis. LPS treatment decreased preference (percentage of sucrose consumed) for sucrose in all animals (F1,33 = 32.88, p < 0.001, ηp2 = 0.499). Overall, males had a higher preference for sucrose than females (F1,33 = 4.761, p = 0.036, ηp2 = 0.126). No other significant effects or interactions were detected for preference (Fig 4A). Total consumption was lowered by LPS (F1,33 = 38.959, p< 0.001). This difference was most likely driven by changes in sucrose consumption rather than water consumption as LPS decreased sucrose (F1,33 = 50.111, p < 0.001, ηp2 = 0.603) but not water consumption (Fig 4 B, C). There was also a Treatment x Genotype interaction (F1,33 = 4.64, p = 0.039, ηp2 = 0.123), and a trending effect of genotype (F1,33 = 3.796, p = 0.06, ηp2 = 0.1) for sucrose consumption. Follow up post-hoc tests for genotype effects show Cre+ animals (combined sexes) consumed more sucrose than Cre− animals after saline treatment (t = −2.27, p = 0.031), but not after LPS treatment (t = 0.20, p = 0.84). There were no other significant effects or interactions for sucrose consumption (Fig 4B). Body weights for all animals, taken immediately before injection, did not differ by treatment or genotypes, but, unsurprisingly, males were heavier than females (FSex 1,33 = 41.29, p < 0.001, ηp2= 0.51) (Table 1).
Figure 4.
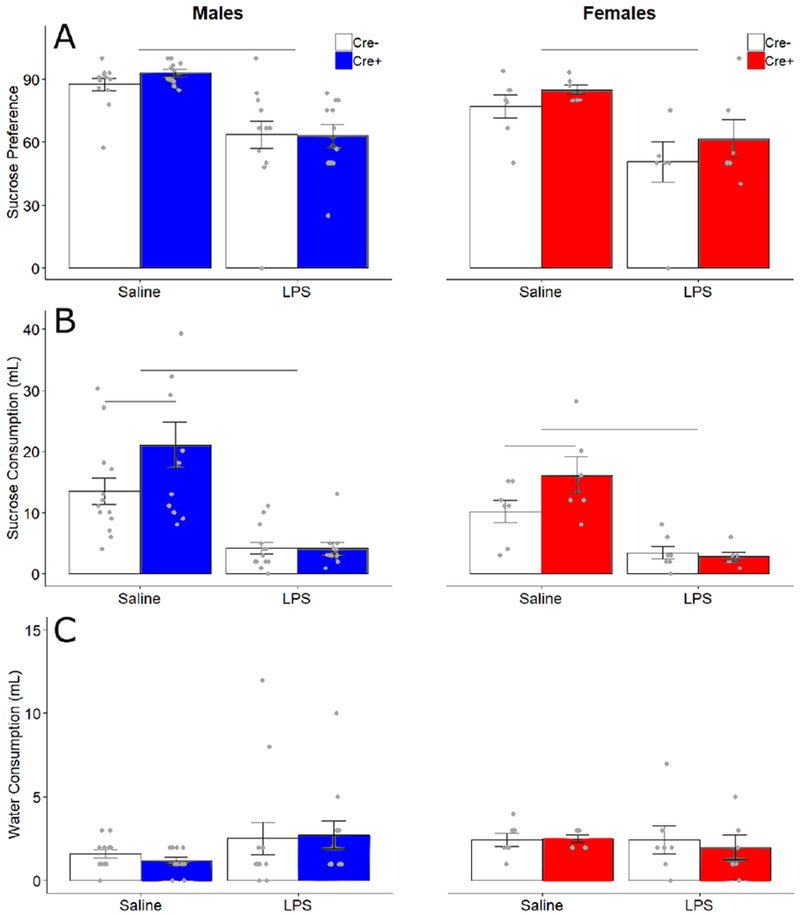
Sucrose and water consumption and preference. Mean ± SEM of (A) preference score (percentage of sucrose consumption compared to total consumption), (B) consumption (mL) of 2.5% sucrose solution, and (C) consumption (mL) of water for male and female Cre− (white bars) and Cre+ (filled bars) groups. Points indicate individual data. Horizontal bars indicate treatment differences and genotype differences
Table 1.
Body weight and tail suspension test data. Mean ± SEM for body weight at time of injection and TST measures.
| Measure | Male Cre− Sal | Male Cre+ Sal | Male Cre− LPS | Male Cre+ LPS | Female Cre− Saline | Female Cre+ Saline | Female Cre− LPS | Female Cre+ LPS |
|---|---|---|---|---|---|---|---|---|
| Body weight | 27.08 ± 1.706 | 26.82 ± 1.537 | 27.46 ± 2.367 | 27.00 ± 2.431 | 23.67 ± 2.103 | 23.14 ± 1.215 | 24.17 ± 1.850 | 23.29 ± 1.604 |
| Time immobile | 168.54 ± 31.16 | 155.72 ± 31.57 | 155.93 ± 42.72 | 178 ± 29.1 | 151.2 ± 42.18 | 156 ± 39.82 | 130.76 ± 44.9 | 134.55 ± 29.25 |
| Latency to first immobile period | 31.93 ± 18.55 | 37.75 ± 19.56 | 29.89 ± 20.95 | 26.06 ± 18.96 | 51.48 ± 37.96 | 51.67 ± 50.44 | 32.13 ± 24.18 | 41.62 ± 25.92 |
| Number of immobile periods | 12.62 ± 2.84 | 13.36 ± 3.35 | 13.31 ±2.84 | 14.64 ± 4.589 | 10.08 ± 2.54 | 12.33 ± 3.386 | 13 ± 5.170 | 13.33 ± 4.179 |
Time spent immobile, latency to first immobile period, and the number of immobile periods were measured in the tail suspension test (TST). Males spent more time immobile than females (FSex 1,38 = 4.893, p = 0.033, ηp2 = 0.114); there were no other effects detected. There were no effects on latency to first immobile period. LPS treatment caused an increase in the number of immobile periods (FTreatment 1,38 = 4.534, p=.040, ηp2=.107); there were no other effects on immobile periods. (Table 1).
3.3. Sociability tests
All subjects preferred investigating an animal over an object following both LPS and saline treatment (Saline F1,37 = 38.538, p < 0.001, ηp2 = 0.510; LPS F = 21.895, p < 0.001, ηp2 = 0.372). In saline-treated animals, there was a trend towards an interaction of Stimulus by Genotype by Sex (F1,37 = 3.59, p = 0.066). There were no other significant effects or interaction in saline conditions (Fig 5). LPS effects lasted at least one day, as LPS-treated animals showed significantly fewer zone crosses the day following injection than did saline-treated animals (12.34±7.97 vs. 24.32±7.29, mean ± SEM.; F1,37 = 64.47, p < 0.001, ηp2 = 0.64).
Figure 5.
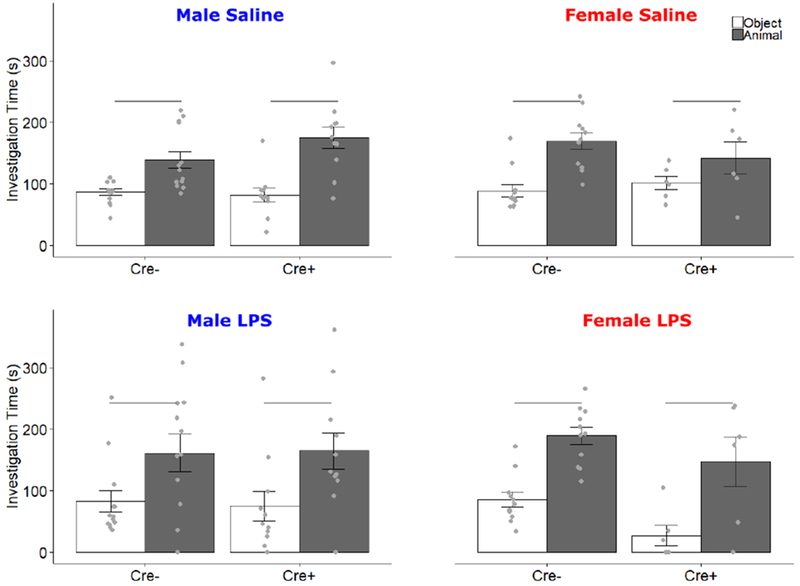
Social Preference Test. Data for Saline and LPS treatments are presented separately. Mean ± SEM of active investigation (s) of both object (white bars) and same-sex stimulus animal (filled bars) during the social preference test. Points indicate individual data. Horizontal bars indicate investigation differences.
To test subjects’ preference for social novelty, the object was replaced with a novel stimulus animal. For saline-treated subjects, there was a main effect of stimulus (F1,37 = 20.56, p < 0.001, ηp2 = 0.36), and an interaction of Stimulus by Genotype by Sex (F1,37 = 4.916, p = 0.033, ηp2 = 0.117). In males, there was a main effect of stimulus (F1,22 = 10.79, p = 0.003) and an interaction of Stimulus by Genotype (F1, 22 = 5.56, p = 0.028). To further elucidate this interaction, post-hoc t-tests were conducted for each genotype; Cre− males showed a preference for the novel animal (t = −4.9, p = 0.0004), while Cre+ males did not (t = −.55, p = 0.59). In females, there was a main effect of stimulus (F1,16 = 9.11, p = 0.008) but no interaction of Stimulus x Genotype. There were no other main effects or interactions in saline-treated animals. After LPS treatment, there was no preference for either the novel or original stimulus across subjects (Fig 6). Once again, LPS effects lasted at least one day, as LPS-treated animals showed significantly fewer zone crosses the day following injection than did saline-treated animals (9.12 ± 6.90 vs., 18.63 ± 5.14, mean ± SEM.; F1,37 = 56.41, p < 0.001, ηp2 = 0.60)
Figure 6.
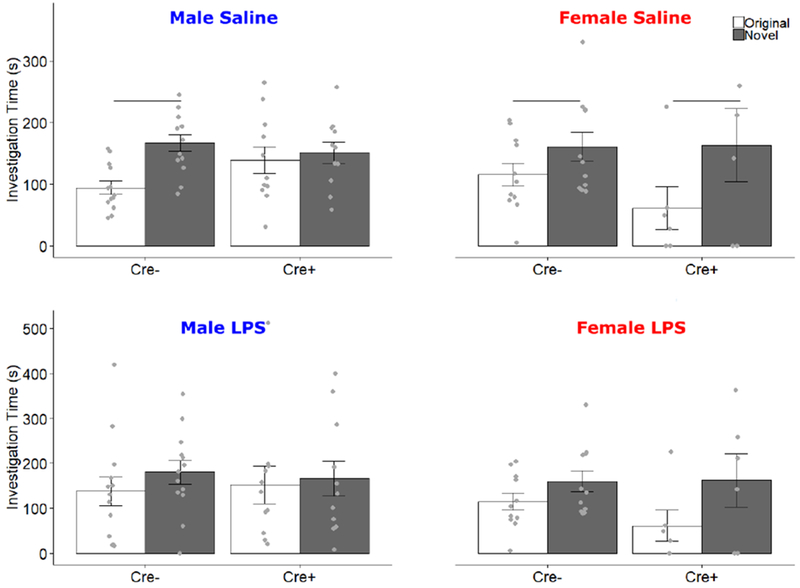
Social Novelty Preference Test. Data for Saline and LPS treatments are presented separately. Mean ± SEM of active investigation (s) of both original (white bars) and novel (filled bars) same-sex stimulus animals during the social novelty preference test. Points indicate individual data. Horizontal bars indicate investigation differences.
3.4. Testing order
We compared behavioral results between animals that received LPS first with those that received saline first. We did not find an effect in the majority of measures, except that in the first block of the OFT, saline-treated animals in the second trial (which received LPS in the first trial) traveled slightly less distance (F1,39 = 4.955, p=.032) than saline-treated animals in the first trial, irrespective of genotype. Additionally, all tail suspension measures, which were not affected by treatment or genotype, were affected by testing order (Time Immobile: F1,38 = 18.35, p < 0.001 ; Latency: F1,38 = 29.65, p < 0.001; Immobile Periods: F1,38 = 9.63, p = 0.004). This suggests that only the TST was significantly affected by repeated testing.
4. Discussion
Although previous studies have suggested that BNST AVP cells in rats may play a role in reducing sickness behavior (e.g., (Dantzer et al., 1991)), our results in mice do not align with this; if anything, males were affected opposite to our prediction. For example, ablating BNST AVP cells reduced sickness behavior in response to LPS in males. The same treatment significantly altered other behaviors in control conditions. Specifically, it reduced preference for social novelty in males and increased sucrose intake in both sexes. Therefore, these data support the idea that BNST AVP cells play a more prominent role in male than in female behavior, but they do not suggest a critical role in LPS-induced sickness behavior.
LPS reliably induced sickness behavior, seen especially as reductions in mobility and increases in anhedonia, but did so in all animals, irrespective of BNST AVP cell ablation. If BNST AVP cells reduce sickness behavior, one would expect that removal of these cells enhance behavioral effects of LPS. On the contrary, ablating BNST AVP cells mitigated the effects of LPS treatment on speed of locomotion in the elevated zero maze in males. However, ablating these cells in females produced effects in the predicted direction, enhancing the reduction in speed after LPS treatment. While this sex difference is still in line with the sex- and steroid-dependent function of these cells, if AVP significantly modulates sickness behavior in mice as it does in rats, it suggests that other AVP-expressing systems, such as the paraventricular nucleus of the hypothalamus (PVN) and suprachiasmatic nucleus (SCN), may be more significant players. PVN cells, including a large number of its AVP-expressing cells, respond to LPS with increased fos activation (Matsunaga et al., 2000). A central role of the PVN in sickness behavior is also suggested by studies showing correlated changes in behavior and neurochemical parameters in the PVN following LPS treatment (Lacosta et al., 1999; Stone et al., 2006). Likewise, the SCN is an important regulator of immune responding, as lesions of this structure exacerbate reactions to LPS (Guerrero-Vargas et al., 2014). Additionally, the SCN sends AVP projections to nuclei that regulate behaviors changed by LPS injection, such as the PVN, BNST, and medial preoptic area (Rood et al., 2013), and is therefore well positioned to play a role in sickness behavior.
Our results do not eliminate a possible role of sex- and steroid-dependent AVP in sickness, as there are sexually dimorphic AVP populations in the MeA as well as the BNST (De Vries and Panzica, 2006; Rood and De Vries, 2011), both of which have been implicated in fever suppression in rats (Federico et al., 1992a, 1992b; Pittman et al., 1998a). Therefore, one may have to ablate both populations to see a significant effect on sickness behavior as the remaining AVP cells in the MeA may compensate for the loss of BNST AVP and be sufficient to reduce sickness behavior in response to LPS. However, ablating BNST AVP cells strongly affected male social behavior, indicating that these lesions were extensive enough to impair AVP-dependent behavior.
Our data add strong evidence for a sexually dimorphic role of BNST AVP cells in the control of social behavior. As mentioned above, indirect evidence had already implicated these cells in social behavior. For example, injections of AVP antagonists (Bluthe et al., 1993, 1990; Caldwell et al., 2008; Veenema et al., 2012, 2013) or V1aR gene manipulations (Bielsky et al., 2005, 2004) affect social behavior, often in a sex-dependent manner. As we previously reported, BNST AVP cell deletion generates deficits in social communication in males only (Rigney et al., 2019). Here we find BNST cell ablation in males eliminates social recognition memory as measured by a bias for investigating novel individuals (social novelty preference), without changing social interest.
The effects of BNST AVP cell ablation on behavior found in the present study cannot be unequivocally linked to sexually-dimorphic AVP expression as we removed whole cells, which not only express AVP but also other neuroactive substances, such as galanin (Miller et al., 1993; Planas et al., 1995), which also contributes to social behavior (Wu et al., 2014). Consequently, behavioral effects may be caused by removal of AVP, co-transmitters, or both. However, since this manipulation eliminated male social recognition, a behavior that depends on AVP within BNST projection sites (Bielsky et al., 2005, 2004; Bluthe et al., 1993, 1990; Bychowski et al., 2013; Veenema et al., 2012), it is likely that our results are due primarily to removal of BNST AVP production.
Unexpectedly, BNST AVP cell ablation caused an increase in sucrose consumption in both males and females. While it is possible that this increase is due to increased fluid intake, this is not likely because water consumption was not altered by cell deletion. It is also possible that this increase was driven by increased hunger or overactivity. However, subjects whose BNST AVP cells were ablated did not differ from controls in body weight or activity levels during behavioral tests, suggesting no difference in caloric intake or energy expenditure. Perhaps these cells can modulate hedonic drive, such that their removal increased the desire for sucrose. Subnuclei of the BNST respond to sucrose with changes in dopamine and norepinephrine release (Park et al., 2012), so it is possible that AVP cells may contribute to sucrose reward. At any rate, the effects of BNST AVP cells on sucrose consumption suggest a wider role for these cells than just modulation of social behaviors.
4.1. Conclusions
Given the sex- and steroid-dependent nature of BNST AVP cells, we expected to find sex differences in the effects of their ablation on behavior, with males exhibiting greater impacts than females. AVP had been previously shown to reduce sickness behaviors, specifically in males, with the BNST indirectly implicated as a source for this effect. Our data, however, suggest that these cells are not critical for reducing LPS-induced sickness behavior, but they are critical for recognition of social novelty in males. This highlights the BNST, and specifically its AVP cells, as critical nodes for male social behavior.
Acknowledgments
Funding: This work was supported by the National Institutes of Health Grant R21 MH111104
Footnotes
Declarations of interest: none
References
- Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ, 2005. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: A gene replacement study. Neuron 47, 503–513. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ, 2004. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology 29, 483–493. [DOI] [PubMed] [Google Scholar]
- Biesmans S, Meert TF, Bouwknecht JA, Acton PD, Davoodi N, De Haes P, Kuijlaars J, Langlois X, Matthews LJR, Ver Donck L, Hellings N, Nuydens R, 2013. Systemic Immune Activation Leads to Neuroinflammation and Sickness Behavior in Mice. Mediators Inflamm. 2013, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthe RM, Gheusi G, Dantzer R, 1993. Gonadal steroids influence the involvement of arginine vasopressin in social recognition in mice. Psychoneuroendocrinology 18, 323–335. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Schoenen J, Dantzer R, 1990. Androgen-dependent vasopressinergic neurons are involved in social recognition in rats. Brain Res. 519, 150–157. [DOI] [PubMed] [Google Scholar]
- Bychowski ME, Mena JD, Auger CJ, 2013. Vasopressin infusion into the lateral septum of adult male rats rescues progesterone-induced impairment in social recognition. Neuroscience 246, 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, Lee HJ, Macbeth AH, Young WS, 2008. Vasopressin: Behavioral roles of an “original” neuropeptide. Prog. Neurobiol 84, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagne V, Moser P, Sylvain R, Porsolt RD, 2011. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr. Protoc. Neurosci 8, 1–14. [DOI] [PubMed] [Google Scholar]
- D’Mello C, Swain MG, 2017. Immune-to-Brain Communication Pathways in Inflammation-Associated Sickness and Depression. Curr. Top. Behav. Neurosci 31, 73–94. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Kelley KW, 1991. Androgen-dependent vasopressinergic neurotransmission attenuates interleukin-1-induced sickness behavior. Brain Res. 557, 115–120. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Laye S, Bret-Dibat J-L, Parnet P, Kelley KW, 1998. Cytokines and Sickness behavior. Ann. New York Acad. Sci 840, 586–590. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Connor JCO, Freund GG, Johnson RW, Kelley KW, 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci 9, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, Panzica GC, 2006. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: Different mechanisms, similar endpoints. Neuroscience 138, 947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federico P, Malkinson TJ, Cooper KE, Pittman QJ, Veale WL, 1992a. Vasopressin perfusion within the medial amygdaloid nucleus attenuates prostaglandin fever in the urethane-anaesthetized rat. Brain Res. 587, 319–326. [DOI] [PubMed] [Google Scholar]
- Federico P, Veale WL, Pittman QJ, 1992b. Vasopressin-induced antipyresis in the medial amygdaloid nucleus of consious rats. Am. J. Physiol 262, 901–908. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RPA, Hansen MK, Anderson K, Maier SF, Watkins LR, 2000. Vagal immune-to-brain communication: A visceral chemosensory pathway. Auton. Neurosci. Basic Clin 85, 49–59. [DOI] [PubMed] [Google Scholar]
- Gould TD, Dao DT, Kovacsics CE, 2009. The Open Field Test, in: Mood and Anxiety Related Phenotypes in Mice. pp. 1–20. [Google Scholar]
- Guerrero-Vargas NN, Salgado-Delgado R, Basualdo MDC, García J, Guzmán-Ruiz M, Carrero JC, Escobar C, Buijs RM, 2014. Reciprocal interaction between the suprachiasmatic nucleus and the immune system tunes down the inflammatory response to lipopolysaccharide. J. Neuroimmunol 273, 22–30. [DOI] [PubMed] [Google Scholar]
- Hart B, 1988. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev 12, 123–137. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Bluthé RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR, 2003. Cytokine-induced sickness behavior. Brain. Behav. Immun 17, 112–118. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Veeneman J, Combe C, Poole S, Luheshi GN, Dantzer R, 2008. Central nervous action of interleukin-1 mediates activation of limbic structures and behavioural depression in response to peripheral administration of bacterial lipopolysaccharide. Eur. J. Neurosci 28, 2499–2510. [DOI] [PubMed] [Google Scholar]
- Lacosta S, Merali Z, Anisman H, 1999. Behavioral and neurochemical consequences of lipopolysaccharide in mice: Anxiogenic-like effects. Brain Res. 818, 291–303. [DOI] [PubMed] [Google Scholar]
- Mathieson WB, Federico P, Veale WL, Pittman QJ, 1989. Single-unit activity in the bed nucleus of the stria terminalis during fever. Brain Res. 486, 49–55. [DOI] [PubMed] [Google Scholar]
- Matsunaga W, Miyata S, Takamata A, Bun H, Nakashima T, Kiyohara T, 2000. LPS-induced Fos expression in oxytocin and vasopressin neurons of the rat hypothalamus. Brain Res. 858, 9–18. [DOI] [PubMed] [Google Scholar]
- Mieda M, Ono D, Hasegawa E, Okamoto H, Honma K ichi, Honma S, Sakurai T, 2015. Cellular clocks in AVP neurons of the scn are critical for interneuronal coupling regulating circadian behavior rhythm. Neuron 85, 1103–1116. [DOI] [PubMed] [Google Scholar]
- Miller AH, Raison CL, 2016. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 16, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Kolb PE, Raskind MA, 1993. Extra-hypothalamic vasopressin neurons coexpress galanin messenger RNA as shown by double in situ hybridization histochemistry. J. Comp. Neurol 329, 378–384. [DOI] [PubMed] [Google Scholar]
- Morgan CW, Julien O, Unger EK, Shah NM, Wells JA, 2014. Turning ON Caspases with Genetics and Small Molecules. Methods Enzym. 544, 179–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor AM, Pittman QJ, Veale WL, 1988. Stimulation of vasopressin release in the ventral septum of the rat brain suppresses prostaglandin E1 fever. J. Physiol 399, 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Wheeler RA, Fontillas K, Keithley RB, Carelli RM, Wightman MR, 2012. Catecholamines in the Bed Nucleus of the Stria Terminalis Reciprocally Respond to Reward and Aversion. Biol. Psychiatry 71, 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin K, 2012. The mouse brain in stereotaxic coordinates, 4th ed Academic Press, San Diego, CA. [Google Scholar]
- Pittman QJ, Chen X, Mouihate A, Hirasawa M, Martin S, 1998a. Arginine vasopressin, fever and temperature regulation. Prog. Brain Res 119, 383–392. [DOI] [PubMed] [Google Scholar]
- Pittman QJ, Chen X, Mouihate A, Martin S, 1998b. Vasopressin-Induced Antipyresis: Sex- and Experience-Dependent Febriles Responses. Ann. New York Acad. Sci 53–61. [DOI] [PubMed] [Google Scholar]
- Pittman QJ, Malkinson TJ, Kasting NW, Veale WL, 1988. Enhanced fever following castration: possible involvement of brain arginine vasopressin. Am. J. Physiol 254, 513–517. [DOI] [PubMed] [Google Scholar]
- Planas B, Kolb PE, Raskind MA, Miller MA, 1995. Sex Difference in Coexpression by Galanin Neurons Accounts for Sexual Dimorphism of Vasopressin in the Bed Nucleus of the Stria Terminalis. Endocrinology 136, 727–733. [DOI] [PubMed] [Google Scholar]
- Rigney N, Whylings J, Mieda M, Vries G.J. De, Petrulis A, 2019. Sexually Dimorphic Vasopressin Cells Modulate Social Investigation and Communication in Sex-Specific Ways. eNeuro 6, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood BD, De Vries GJ, 2011. Vasopressin innervation of the mouse (Mus musculus) brain and spinal cord. J. Comp. Neurol 519, 2434–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood BD, Stott RT, You S, Smith CJW, Woodbury ME, De Vries GJ, 2013. Site of origin of and sex differences in the vasopressin innervation of the mouse (Mus musculus) brain. J. Comp. Neurol 521, 2321–2358. [DOI] [PubMed] [Google Scholar]
- Sagar SM, Price KJ, Kasting NW, Sharp FR, 1995. Anatomic patterns of FOS immunostaining in rat brain following systemic endotoxin administration. Brain Res. Bull 36, 381–392. [DOI] [PubMed] [Google Scholar]
- Sens J, Schneider E, Mauch J, Schaffstein A, Mohamed S, Fasoli K, Saurine J, Britzolaki A, Thelen C, Pitychoutis PM, 2017. Lipopolysaccharide administration induces sex-dependent behavioural and serotonergic neurochemical signatures in mice. Pharmacol. Biochem. Behav 153, 168–181. [DOI] [PubMed] [Google Scholar]
- Shimshek DR, Kim J, Hübner MR, Spergel DJ, Buchholz F, Casanova E, Stewart AF, Seeburg PH, Sprengel R, 2002. Codon-improved Cre recombinase (iCre) expression in the mouse. Genesis 32, 19–26. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lehmann ML, Lin Y, Quartermain D, 2006. Depressive Behavior in Mice Due to Immune Stimulation is Accompanied by Reduced Neural Activity in Brain Regions Involved in Positively Motivated Behavior. Biol. Psychiatry 60, 803–811. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH, Dunn AJ, 2007. Effects of Interleukin-1β and Lipopolysaccharide on Behavior of Mice in the Elevated Plus-Maze and Open Field Tests. Pharmacol. Biochem. Behav 86, 651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PV, Veenema AH, Paul MJ, Bredewold R, Isaacs S, De Vries GJ, 2012. Sexually dimorphic effects of a prenatal immune challenge on social play and vasopressin expression in juvenile rats. Biol. Sex Differ 3, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger EK, Burke KJJ, Yang CF, Bender KJ, Fuller PM, Shah NM, 2015. Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep. 10, 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema A, Bredewold R, De Vries G, 2012. Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Horm. Behav 61, 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, De Vries GJ, 2013. Sex-specific modulaion of juvenile social play by vasopressin. Psychoneuroendocrinology 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh R, Cummins R, 1976. The Open-Field Test: a critical review. Psychol. Bull 83, 482–504. [PubMed] [Google Scholar]
- Weiland TJ, Voudouris NJ, Kent S, 2007. CCK 2 receptor nullification attenuates lipopolysaccharide-induced sickness behavior. Am J Physiol Regul Inegr Comp Physiol 292, 112–123. [DOI] [PubMed] [Google Scholar]
- Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, Dulac CG, 2014. Galanin neurons in the medial preoptic area govern parental behavior. Nature 509, 325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Chiang M, Gray DC, Prabhakaran M, Juntti SA, Unger EK, Wells JA, Shah NM, 2013. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell 153, 896–909. [DOI] [PMC free article] [PubMed] [Google Scholar]


