Abstract
C-C chemokine receptor type 5, also known as CCR5 or CD195, is best known as a viral co-receptor that facilitates entry of HIV into cells. Evidence that CCR5 knockout mice display fewer dopamine neurons, lower striatal dopamine levels, and reduced locomotor activation compared to wild types also suggest a link between CCR5 receptors and cocaine dependence. Here, we tested the hypothesis using male Sprague-Dawley rats that cocaine-induced locomotor activation and conditioned place preference (CPP) are inhibited by a FDA-approved CCR5 antagonist (maraviroc), and that CCR5 gene expression in mesolimbic substrates is enhanced by repeated cocaine exposure. Pretreatment with maraviroc (1, 2.5, 5 mg/kg, IP) reduced hyperlocomotion induced by acute cocaine (10 mg/kg) without affecting spontaneous locomotor activity. For CPP experiments, rats conditioned with cocaine (10 mg/kg × 4 days, IP) were injected with maraviroc (1, 2.5, 5 mg/kg, IP) before each injection of cocaine. Maraviroc dose-dependently inhibited development of cocaine CPP, with a dose of 5 mg/kg producing a significant reduction. In rats treated repeatedly with cocaine (10 mg/kg × 4 days, IP), CCR5 gene expression was upregulated in the nucleus accumbens and ventral tegmental area but mRNA levels of CCR5 ligands (i.e., CCL3, CCL4 and CCL5) were not affected. Our results suggest that mesolimbic CCR5 receptors are dysregulated by cocaine exposure and, similar to CXCR4 and CCR2 receptors, influence behavioral effects related to the abuse liability of cocaine.
Keywords: chemokine, maraviroc, CCR5, RANTES, cocaine, addiction
1. Introduction
A critical role for dopamine, glutamate and traditional neurotransmitter systems in the acute and chronic effects of cocaine has been established, but strategies targeting these systems have not yet resulted in an approved medication for cocaine use disorder. This therapeutic void suggests that important biological substrates underlying the abuse liability of cocaine remain to be discovered. One such system is the neuroimmune system, which is dysregulated during cocaine exposure (Batchell et al., 2017; Cotto et al., 2018). The key cellular components of the immune system are glial cells, and drugs that inhibit glial activation, such as propentofylline, ibudilast, and minocycline, reduce preclinical effects of cocaine related to addiction but also produce adverse effects, including immunosuppression, that may limit clinical utility (Zhang et al., 2017; Poland et al., 2016). Thus, an important next step is to identify and characterize specific endogenous elements of the immune system, such as cytokines and chemokines, that might more selectively modulate behavioral effects of cocaine.
Chemokines themselves are small proteins secreted by immune cells that are well recognized for neuroinflammatory and chemotactic functions but may also contribute to cocaine dependence (Cui et al., 2014). Plasma levels of several chemokines (e.g. CXCL12, CCL2, CX3CL1) are increased in mice following acute cocaine exposure (Araos et al., 2015), and reduced in cocaine abusers during abstinence. Two chemokine receptor systems, CXCR4 and CCR2, are known to influence behavioral and neurochemical effects of cocaine. CXCL12, an endogenous ligand of CXCR4, enhances cocaine-induced hyperlocomotion following ICV administration (Trecki and Unterwald, 2009). CXCL12 injected into the substantia nigra increases extracellular dopamine levels in the dorsal striatum through CXCR4 receptor activation (Skrzydelski et al. 2007; Guyon et al., 2014). Behavioral studies reveal that systemic administration of a CXCR4 receptor antagonist, AMD3100, inhibits both development and expression of cocaine conditioned place preference (CPP) and cocaine-induced locomotor activation in rats (Kim et al., 2017). Similarly, AMD3100 reduces CPP and locomotor activation produced by the ‘bath salt’ synthetic cathinone MDPV (3,4-methlylenedioxypyrovalerone), a psychostimulant with a mechanism of action similar to cocaine (Oliver et al., 2018). At the cellular level, CXCL12 mRNA levels in the mesolimbic circuit are increased in rats exposed to chronic cocaine or MDPV (Kim et al., 2017; Oliver et al., 2018). Another chemokine receptor, CCR2, and its cognate ligand, CCL2, are implicated in cocaine’s effects as CCL2 administration enhances striatal dopamine levels and locomotor activity, and CCR2 genetic knockdown enhances cocaine locomotor sensitization (Guyon et al., 2009; Trocello et al., 2011).
Evidence suggests that the CCR5 system may also contribute to the behavioral and neurochemical effects of cocaine. For example, CCR5 knockout mice have lower numbers of dopamine neurons, reduced dopamine levels in the striatum, and reduced locomotor activity compared to wild type mice (Choi et al., 2013). CCL5 (RANTES), an endogenous agonist of CCR5, is co-localized with tyrosine hydroxylase (TH) positive cells in the VTA, suggesting that dopamine neurons in the mesolimbic pathway produce and express CCL5 (Lanfranco et al., 2017). CCL3, another endogenous CCR5 ligand, is constitutively expressed by astrocytes and dopamine neurons in the substantia nigra and striatum (Kalkonde et al., 2007), and CCR5 and its ligands modulate neurotransmitter release and ion channel gating (Meucci et al., 1998). CCR5 gene expression is also enhanced by cocaine exposure in lymphocytes and by methamphetamine or dopamine exposure in a THP1 human macrophage cell line (Basova et al., 2018).
CCR5 is also one of the few chemokine receptors expressed in the brain with a FDA-approved antagonist (i.e., maraviroc). Maraviroc is a CNS entry inhibitor that prevents the HIV protein gp120 from associating with CCR5 (Dorr et al., 2005). Maraviroc also inhibits the binding of CCL3, CCL4 and CCL3 to CCR5 with IC50 values of 3.3, 7.2 and 5.2 nM, respectively, and suppresses downstream CCR5 signaling without promoting internalization of CCR5 (Walker et al., 2005). Moreover, maraviroc shows negligible affinity for other receptors, including CCR2, which shares sequence homology with CCR5 and interacts with other CCR5 antagonists (Paterlini et al., 2002).
The present study tested the hypothesis that cocaine dysregulates the CCR5 receptor system in the mesolimbic circuit and produces rewarding and locomotor-stimulant effects that are reduced by maraviroc. Locomotor activity was quantified because hyperlocomotion resulting from increased dopamine transmission is a hallmark behavioral feature of cocaine and an early indicator of abuse liability. Rewarding effects of cocaine were quantified by CPP, which is a learned behavior displayed by vertebrates and invertebrates that occurs when a subject prefers a specific environment that was previously paired with a rewarding event (Huston et al., 2013).
2. Materials and Methods
2.1. Animals and Chemicals
Male Sprague-Dawley rats (250–275 g) from Taconic Biosciences (Hudson, NY) were used. All animal use procedures were conducted in accordance with the National Research Council and the National Academies Press publication for the Care and Use of Laboratory Animals (adopted for use by the National Institutes of Health) and approved by the Temple University Institutional Animal Care and Use Committee. Rats were housed in a controlled environment (21–23 °C) on a 12-h light/dark cycle and provided food and water ad libitum. Cocaine hydrochloride was purchased from Sigma-Aldrich (St Louis, MO, USA) and dissolved in physiological saline. Maraviroc was purchased from Cayman Chemical Company (Ann Arbor, MI, USA) and dissolved in a vehicle of 10% DMSO/saline. All drugs were injected intraperitoneally (IP) in a volume of 1 ml/kg. Rats were assigned randomly to experimental groups with appropriate sample sizes, and trained experimenters were blinded to group and outcome assignments.
2.2. Locomotor activity
Locomotor activity was measured as described using a Digiscan DMicro system (Accuscan Inc.) consisting of transparent plastic chambers (45 cm × 20 cm × 20cm) set inside metal frames equipped with 16 infrared light emitters and detectors (Hicks et al., 2018). Following a 60-min habituation in activity chambers, rats were injected with maraviroc (1, 2.5 or 5 mg/kg) or saline. Thirty min later, all rats were injected with cocaine (10 mg/kg) and activity was measured for 90 min. Separate experiments assessed potential effects of maraviroc on spontaneous locomotor activity. Following the 60-min habituation interval, rats were injected with maraviroc (5 mg/kg) or saline, and locomotor activity was measured for 120 min.
2.3. Conditioned place preference (CPP)
CPP experiments were conducted as described using CPP chambers (45 cm × 20 cm × 20 cm) consisting of 2 compartments separated by a removable door (Gregg et al., 2015; Hicks et al., 2018). A 30-min pre-test was conducted on day 1 to determine initial compartment preference. The compartment in which a rat spent less time was designated as the cocaine-paired side. The day after the pre-test, a 4-day conditioning paradigm with morning and afternoon sessions was initiated. In the morning, rats pretreated with saline or maraviroc (5 mg/kg) were injected 15 min later with cocaine (10 mg/kg) and confined to the cocaine-paired compartment for 30 min. In the afternoon session, rats were injected with saline and placed in the opposite compartment for 30 min. On day 6, a post-test was conducted in which rats were placed into the chamber and given free access to roam both compartments for 30 min. Two lower doses of maraviroc (1, 2.5 mg/kg) were tested to obtain dose-effect data.
2.4. Gene expression
Gene expression experiments were conducted as described (Kim et al., 2017). Rats were injected with cocaine (10 mg/kg) or saline once daily for 4 days and were returned to home cages after each injection. Compared to CPP studies, the cocaine exposure paradigm was identical, but the context was different (no conditioning for gene expression study). Thirty min following the last injection, rats were euthanized and brains were flash frozen. The VTA and nucleus accumbens were dissected from frozen slices using 1 and 2 mm round punches respectively. RNA was isolated using the Quick-RNA Miniprep kit (Zymo Research, Irvine, CA, USA), and cDNA was synthesized using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Quantitative real-time PCR was carried out with TaqMan Fast Advanced Master Mix and the TaqMan Gene Expression Assays for CCR5 (Rn023132969_s1), CCL5 (Rn00579590_m1), CCL4 (Rn00671924_m1), CCL3 (Rn01464736_g1) and the internal control gene 45S rRNA (Rn03928990_g1)/18S rRNA (Hs99999901_s1) using the StepOnePlus Real-Time PCR System (Applied Biosystems). Relative gene expression was measured according to the 2-ΔΔCT method.
2.5. Statistical analysis
Two-way ANOVA was used to analyze locomotor data (maraviroc treatment × time) and CPP data (maraviroc pretreatment × cocaine treatment). In cases of significant ANOVA, a Bonferroni post-hoc test was used to identify differences between individual groups. Gene expression data were analyzed by a Student’s t-test. Statistical significance was set at p < 0.05 in all cases.
3. Results
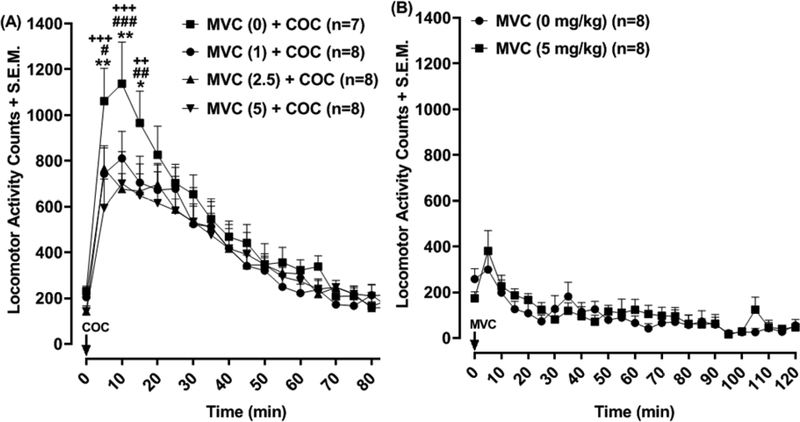
3.1. CCR5 antagonist reduced cocaine locomotor activation (Fig. 1)
Fig. 1. Effects of maraviroc on cocaine locomotor activation.
Rats were treated with MVC (1, 2.5, 5 mg/kg) 30 min before exposure to cocaine (10 mg/kg). Data are presented as locomotor activity counts/5 min. *p < 0.05 or **p < 0.01 compared to MVC (1) + COC, #p < 0.05, ##p < 0.01 or ###p < 0.001 compared to MVC (2.5) + COC, and ++p < 0.01 or +++p < 0.001 compared to MVC (5) + COC. Box) Effects of MVC (5 mg/kg) by itself: Data are presented as locomotor counts from 0–60 min following injection with MVC or VEH.
For time-course data, two-way ANOVA revealed a significant effect of time [F (18, 486) = 65.32, p < 0.0001], and a significant interaction (treatment × time) [F (54, 486) = 1.490, p < 0.05], but not a significant effect of treatment [F (3, 27) = 0.7046, p > 0.05] (Fig. 1). Within the first 15 min following cocaine exposure (10 mg/kg), hyperlocomotion in rats treated with maraviroc (1, 2.5, 5 mg/kg) was significantly reduced compared to maraviroc-naïve rats (MVC (0) + COC): [5 min: 1 mg/kg (p < 0.01), 2.5 mg/kg (p < 0.05), and 5 mg/kg (p < 0.001); 10 min: 1 mg/kg (p < 0.01), 2.5 mg/kg (p < 0.001), and 5 mg/kg (p < 0.001); and 15 min: 1 mg/kg (p < 0.05), 2.5 mg/kg (p < 0.01) and 5 mg/kg (p < 0.01). In separate experiments assessing effects of maraviroc (5 mg/kg) on spontaneous locomotor activity (Fig. 1, box), two-way ANOVA indicated an effect of time [F (24, 336) = 7.867, p < 0.0001]. However, there was not an effect of maraviroc treatment [F (1, 336) = 0.29, p > 0.05] or a significant interaction [F (24, 336) = 0.8182, p > 0.05].
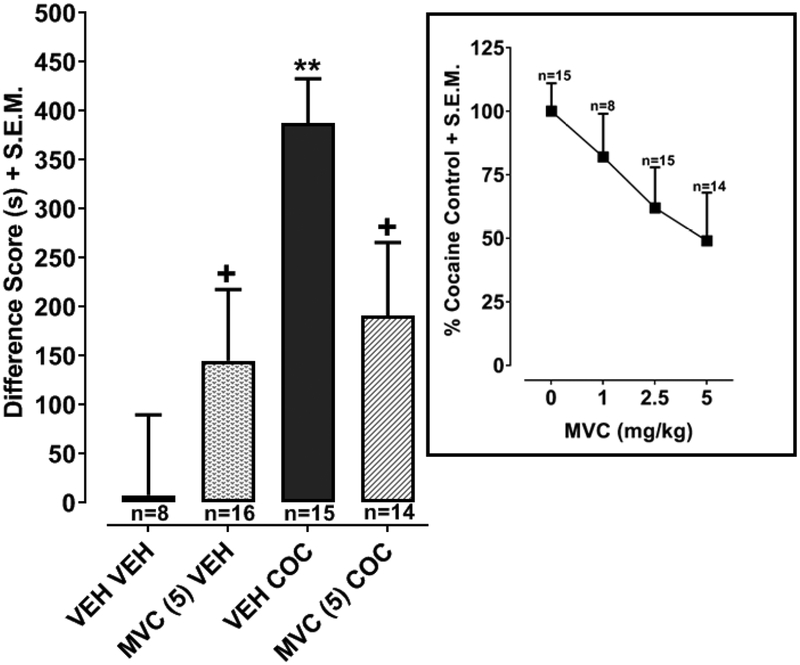
3.2. CCR5 antagonist reduced cocaine CPP (Fig. 2)
Fig. 2. Effects of maraviroc on development of cocaine CPP.
Data are presented as a difference score (difference in time spent on cocaine-paired side between post-test and pre-test). **p < 0.01 compared to VEH and +p < 0.05 compared to VEH COC. Box) Dose-effects of maraviroc (1, 2.5, 5 mg/kg) are presented as % CPP in rats treated cocaine by itself.
Two-way ANOVA revealed effects of treatment [F(1, 49) = 8.80, p < 0.01] and a significant interaction [F(1, 49) = 5.41, p < 0.05] but not a significant effect of pretreatment [F(1, 49) = 0.17, p > 0.05] (Fig. 2). Rats treated with 10 mg/kg cocaine (VEH + COC) displayed greater CPP than drug-naïve rats (VEH + VEH; p < 0.01). In rats treated with 10 mg/kg cocaine, pretreatment with maraviroc (5 mg/kg) (MVC + COC) reduced CPP by approximately 50% compared to pretreatment with vehicle (VEH + COC) (p < 0.01). In rats naïve to cocaine treatment, CPP following pretreatment with maraviroc (5 mg/kg) (MVC + VEH) was not significantly different from vehicle pretreatment (VEH + VEH) (p > 0.05). Two lower doses of maraviroc (1 and 2.5 mg/kg) were tested against cocaine, and dose-effect data were graphed as % CPP produced by cocaine alone (Fig. 2, box) [F(3, 51) = 2.027, p > 0.05].
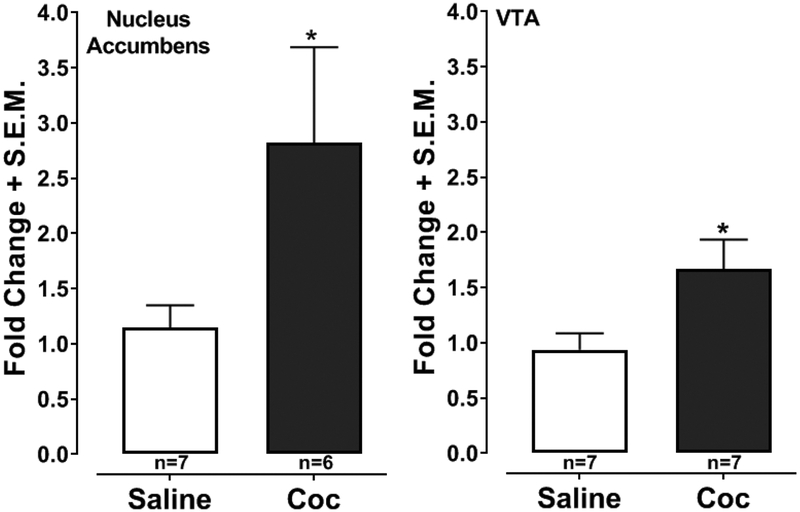
3.3. Cocaine enhanced CCR5 mRNA levels in the nucleus accumbens and VTA (Fig. 3)
Fig. 3. Effects of repeated cocaine exposure on CCR5 mRNA levels in the nucleus accumbens and VTA.
Gene expression (CCR5, CCL3, CCL4, CCL5) in the nucleus accumbens of rats injected with cocaine (10 mg/kg) or saline for 4 days was quantified by rt-PCR. Data are presented as the fold- change in mRNA expression compared with saline-injected controls. *p < 0.05, Student’s t-test.
Repeated cocaine exposure significantly increased CCR5 mRNA levels in the nucleus accumbens relative to vehicle-injected controls (2.45-fold increase, p < 0.05, Student’s t-test) but did not significantly affect mRNA levels of CCL3, CCL4 and CCL5 (p > 0.05, Student’s t-test) (Fig. 3). Similar effects were detected in the VTA, where repeated cocaine treatment again produced a significant enhancement of CCR5 gene expression (1.78-fold increase relative to vehicle-treated controls, Student’s t-test) but did not affect levels of CCL3, CCL4 and CCL5) (p > 0.05) (data not shown).
4. Discussion
Our results suggest a role for the CCR5 system in cocaine reward. Daily pretreatment with a CCR5 antagonist (maraviroc) during cocaine conditioning reduced the development of cocaine CPP, and cocaine-exposed rats treated with maraviroc displayed a brief, but significant, reduction in locomotor activity. At the cellular level, repeated cocaine exposure increased CCR5 gene expression in substrates of the mesolimbic pathway but did not affect primary ligands of CCR5 (i.e., CCL3, CCL4 and CCL5). The mechanism underlying maraviroc efficacy is unclear, but, based on pharmacodynamic profile, CCR5 receptor antagonism is the most parsimonious explanation. This interpretation infers that the rewarding and locomotor-stimulant effects of cocaine require active CCR5 receptors and is consistent with activation of two other chemokine systems, CXCR4 and CCR2, also facilitating psychostimulant-induced behaviors (Oliver et al., 2018; Kim et al., 2017; Trecki and Unterwald, 2009; Trocello et al., 2011).
CCR5 receptors are expressed in cortical and striatal regions that contribute to cocaine dependence (Avdoshina et al., 2011), but the CCR5 system, relative to CXCR4 and CCR2 systems, is much less defined in terms of cocaine’s in vivo action. Evidence that CCR5 knockout mice, compared to wild types, display lower numbers of dopaminergic neurons in the substantia nigra and reduced levels of dopamine in the striatum suggest that CCR5 receptors modulate dopamine systems that contribute to cocaine reward (Choi et al., 2013). Accordingly, in our study, CCR5 antagonism by maraviroc during cocaine administration may have lowered the normal elevation in accumbal dopamine levels produced by a cocaine reuptake block, thus reducing place preference and locomotor activation resulting from increased mesolimbic dopamine transmission. Genetic deletion of CCR5 also increases the expression of monoamine oxidase (MAO), an enzyme that catabolizes dopamine, in the midbrain of mice (Choi et al., 2013). Thus, it is possible that CCR5 antagonism with maraviroc, similar to CCR5 deletion, produced enhancement of MAO that contributed to the reduction in cocaine place preference and locomotor activation observed here.
Mechanisms underlying the CCR5-cocaine interaction, including a potential role for dopamine, are unclear. Given that CCR5 mRNA levels in the VTA and nucleus accumbens were enhanced following repeated cocaine exposure in our gene expression experiments, one possibility is that the enhanced dopamine transmission that is known to occur during cocaine exposure promotes downstream recruitment and enhancement of the mesolimbic CCR5 system, including increased CCR5 mRNA synthesis, which contributes to the rewarding and locomotor-stimulant effects of cocaine. Activation of dopamine D1- and D2-like receptors does enhance calcium mobilization caused by activation of Gαq-coupled receptors such as CCR5 (Gaskill et al., 2014; Arai and Charo, 1996). Dopamine-CCR5 crosstalk has been demonstrated with regard to HIV, where dopamine receptor activation increases HIV entry into primary human macrophages through a mechanism that requires CCR5 receptor activation (Gaskill et al., 2014).
A limitation of the present study is that protein levels, which do not always correlate with changes in gene expression, were not quantified. Consequently, the lack of effect of cocaine on mRNA levels of CCR5 ligands (e.g. CCL3, CCL4, CCL5) does not preclude the possibility that cocaine increases protein levels or the cellular release of these, or other ligands, that activate CCR5 (e.g. CCL3L1 and CCL8) (Corbisier et al., 2015). Future studies will also examine CCR5 immunoreactivity during cocaine exposure. CCR5 immunoreactivity is primarily co-localized with the neuronal marker NeuN, with CCR5 always being localized to the cytoplasm of neuronal perikaryon and processes (Avdoshina et al., 2011), suggesting that CCR5 receptors are associated with neuronal cell bodies in the VTA and nucleus accumbens. Because the present study only used a single pharmacological approach, antagonism with maraviroc, future work will employ protein knockdown of CCR5 to better demonstrate a role for CCR5 receptors in the in vivo action of cocaine. It will also be important to test effects maraviroc on cocaine intake in self-administration assays that better model aspects (e.g. reinforcement, motivation, and relapse) of cocaine use disorder in humans.
In summary, we showed that CCR5 receptors, similar to CCR2 and CXCR4 receptors, contribute to psychostimulant reward and locomotor activation. The FDA-approved CCR5 antagonist maraviroc reduced cocaine place preference and hyperlocomotion, and CCR5 gene expression in the mesolimbic pathway was enhanced by chronic cocaine exposure. The present evidence suggests that interactions between chemokine CCR5 and brain reward systems exist and contribute to the rewarding effects of cocaine.
Research Highlights.
Repeated cocaine exposure increases mesolimbic CCR5 gene expression.
Chemokine CCR5 antagonist maraviroc reduces cocaine reward.
CCR5 antagonist maraviroc reduces cocaine hyperlocomotion.
CCR5 and cocaine interactions are identified in the brain.
Chemokine and neuroimmune systems may influence the abuse liability of cocaine.
Acknowledgements
This work was supported by National Institute on Drug Abuse grants R01DA039139, R01DA045499, P30DA013429 and T32DA007237.
Footnotes
Conflict of Interest: All authors declare that they have no conflicts of interest.
References
- Araos P, Pedraz M, Serrano A, Lucena M, Barrios V, García-Marchena N, Campos Cloute R, Ruiz JJ, Romero P, Suárez J, 2015. Plasma Profile of Pro-Inflammatory Cytokines and Chemokines in Cocaine Users Under Outpatient Treatment: Influence of Cocaine Symptom Severity and Psychiatric Co-Morbidity. Addict. Biol 20, 756–772. [DOI] [PubMed] [Google Scholar]
- Arai H, Charo IF, 1996. Differential Regulation of G-Protein-Mediated Signaling by Chemokine Receptors. J. Biol. Chem 271, 21814–9. [DOI] [PubMed] [Google Scholar]
- Avdoshina V, Becker J, Campbell LA, Parsadanian M, Mhyre T, Tessarollo L, Mocchetti I, 2011. Neurotrophins Modulate the Expression of Chemokine Receptors in the Brain. J. Neurovirol 17, 58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Jones JD, Heinzerling KG, Beardsley PM, Comer SD, 2017. Glial and neuroinflammatory targets for treating substance use disorders. Drug Alcohol Depend. 180, 156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basova L, Najera JA, Bortell N, Wang D, Moya R, Lindsey A, Semenova S, Ellis RJ, Marcondes MCG, 2018. Dopamine and Its Receptors Play a Role in the Modulation of CCR5 Expression in Innate Immune Cells Following Exposure to Methamphetamine: [DOI] [PMC free article] [PubMed]
- Choi DY, Lee MK, Hong JT, 2013. Lack of CCR5 Modifies Glial Phenotypes and Population of the Nigral Dopaminergic Neurons, but Not MPTP-induced Dopaminergic Neurodegeneration. Neurobiol. Dis 49, 159–68. [DOI] [PubMed] [Google Scholar]
- Cotto B, Li H, Tuma RF, Ward SJ, Langford D, 2018. Cocaine-mediated activation of microglia and microglial MeCP2 and BDNF production. Neurobiol. Dis 117, 28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Shurtleff D, Harris RA, 2014. Chapter One-Neuroimmune Mechanisms of Alcohol and Drug Addiction. Int. Rev. Neurobiol 118, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M, Mori J, Rickett G, Smith-Burchnell C, Napier C, Webster R, Armour D, Price D, Stammen B, Wood A, Perros M, 2005. Antimicrob. Agents Chemother. 49, 4721–4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Yano HH, Kalpana GV, Javitch J, Berman JW, 2014. Dopamine Receptor Activation Increases HIV Entry Into Primary Human Macrophages. PLoS One. 9, e108232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RA, Baumann MH, Partilla JS, Bonano JS, Vouga A, Tallarida CS, Velvadapu V, Smith GR, Peet MM, Reitz AB, 2015. Stereochemistry of mephedrone neuropharmacology: enantiomer-specific behavioural and neurochemical effects in rats. Br. J. Pharmacol 172, 883–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon A, Skrzydelski D, De Giry I, Rovère C, Conductier G, Trocello JM, Daugé V, Kitabgi P, Rostène W, Nahon JL, Mélik Parsadaniantz S, 2009. Long Term Exposure to the Chemokine CCL2 Activates the Nigrostriatal Dopamine System: A Novel Mechanism for the Control of Dopamine Release. Neuroscience. 162, 1072–80. [DOI] [PubMed] [Google Scholar]
- Guyon A, 2014. CXCL12 chemokine and its receptors as major players in the interactions between immune and nervous systems. Front. Cell. Neurosci 8, 65–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks C, Huang P, Ramos L, Nayak SU, Caro Y, Reitz AB, Smith GR, Lee DY, Rawls SM, Liu-Chen LY, 2018. Dopamine D1-Like Receptor Agonist and D2-Like Receptor Antagonist (−)-Stepholidine Reduces Reinstatement of Drug-Seeking Behavior for 3,4-Methylenedioxypyrovalerone (MDPV) in Rats. ACS Chem. Neurosci 9, 1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MC, Cherng CG, Tsai YP, Chiang CY, Chuang JY, Kao SF, Yu L, 2009. Chronic Treatment With Monoamine Oxidase-B Inhibitors Decreases Cocaine Reward in Mice. Psychopharmacology. 205, 141–9. [DOI] [PubMed] [Google Scholar]
- Huston JP, Silva MA, Topic B, Müller CP, 2013. What’s conditioned in conditioned place preference? Trends Pharmacol. Sci 34, 162–166. [DOI] [PubMed] [Google Scholar]
- Kalkonde YV, Morgan WW, Sigala J, Maffi SK, Condello C, Kuziel W, Ahuja SS, Ahuja SK, 2007. Chemokines in the MPTP Model of Parkinson’s Disease: Absence of CCL2 and its Receptor CCR2 Does Not Protect Against Striatal Neurodegeneration. Brain Res. 1128, 1–11. [DOI] [PubMed] [Google Scholar]
- Kim J, Connelly KL, Unterwald EM, Rawls SM, 2017. Chemokines and Cocaine: CXCR4 Receptor Antagonist AMD3100 Attenuates Cocaine Place Preference and Locomotor Stimulation in Rats. Brain Behav. Immun 62, 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfranco MF, Mocchetti I, Burns MP, Villapol S, 2018. Glial- and Neuronal-Specific Expression of CCL5 mRNA in the Rat Brain. Front. Neuroanat 11, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ, 1998. Chemokines Regulate Hippocampal Neuronal Signaling and gp120 Neurotoxicity. Proc. Natl. Acad. Sci. U. S. A 95, 14500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver CF, Simmons SJ, Nayak SU, Smith GR, Reitz AB, Rawls SM, 2018. Chemokines and ‘Bath Salts’: CXCR4 Receptor Antagonist Reduces Rewarding and Locomotor-Stimulant Effects of the Designer Cathinone MDPV in Rats. Drug Alcohol Depend. 186, 75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterlini MG, 2002. Structure modeling of the chemokine receptor CCR5: implications for ligand binding and selectivity. Biophys. J 83, 3012–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland RS, Hahn Y, Knapp PE, Beardsley PM, Bowers MS, 2016. Ibudilast attenuates expression of behavioral sensitization to cocaine in male and female rats. Neuropharmacology. 109, 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzydelski D, Guyon A, Dauge V, Rovere C, Apartis E, Kitabgi P, Nahon JL, Rostene W, Parsadaniantz SM, 2007. The chemokine stromal cell-derived factor-1/CXCL12 activates the nigrostriatal dopamine system. J. Neurochem 102, 1175–1183. [DOI] [PubMed] [Google Scholar]
- Trecki J, Unterwald EM, 2009. Modulation of cocaine-induced activity by intracerebral administration of CXCL12. Neuroscience 161, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trocello J, Rostene W, Melik-Parsadaniant S, Godefroy D, Roze E, Kitabgi P, Kuziel WA, Chalon S, Caboche J, Apartis E, 2011. Implication of CCR2 Chemokine Receptor in Cocaine-Induced Sensitization. J. Mol. Neurosci 44, 147–51. [DOI] [PubMed] [Google Scholar]
- Walker DK, Abel S, Comby P, Muirhead GJ, Nedderman AN, Smith DA, 2005. Species differences in the disposition of the CCR5 antagonist, UK-427,857, a new potential treatment for HIV. Drug Metab. Disposit 33, 587–595. [DOI] [PubMed] [Google Scholar]