Abstract
Background:
Insomnia is common among cancer survivors. Though behavioral treatments for insomnia are effective, access is limited. Stepped care delivery models may provide insomnia treatment that is more efficient and accessible to cancer survivors.
Methods:
Fifty-one survivors (mean age=55 years) with elevated Insomnia Severity Index (ISI) scores (≥12) first received STEP-1: a single, sleep education session. Those reporting elevated ISI scores one month later were offered STEP-2: a 3-session, group cognitive-behavioral treatment for insomnia previously demonstrated efficacious. Participants were “treatment responders” if their ISI score improved by ≥6 points and “remitted” if their post-treatment ISI score was <12. Mood was assessed with the Profile of Mood States-Short Form (POMS-SF).
Results:
Following STEP-1, ISI scores improved (17.1 to 11.2; p<.001) with 45% responding and 41% remitted. Insomnia remission after STEP-1 was associated with lower insomnia severity and shorter duration of sleep problems at baseline. Of the 30 survivors (59%) with unremitted insomnia after STEP-1, 14 (47%) participated in STEP-2. Following STEP-2, ISI scores improved (16.9 to 8.8; p<.001), with 79% responding and 71% remitted. STEP-2 participation was associated with interest in sleep treatment at baseline, but not demographic/health-related variables. Mood improved significantly following both STEP-1 and STEP-2 (POMS-SF; p<.001).
Conclusions:
A stepped care approach to treating cancer survivors’ insomnia has the potential to improve treatment accessibility. A sizable proportion of survivors can benefit from two different low-intensity approaches that could be delivered by non-sleep specialists. For those requiring more intensive care, assessing treatment interest can identify those likely to engage.
Keywords: insomnia, cancer survivor, cognitive-behavioral therapy for insomnia, stepped care
Precis:
Insomnia is common, but poorly managed among cancer survivors. A stepped care approach to its treatment was successful and the delivery of low-intensity behavioral insomnia interventions can result in improved access to care.
Introduction
There are over 15.5 million cancer survivors in the United States and this number is expected to increase due to improvements in early detection, better cancer therapies, and an aging population.1 As a result, there is greater emphasis on addressing their considerable survivorship concerns. Insomnia is one of the most commonly experienced survivorship difficulties, affecting up to 30% of patients years after treatment has ended.2, 3 More than simply a few bad nights of sleep, chronic sleep problems are associated with a wide range of significant health sequelae in the general and cancer population.4, 5
Based on compelling efficacy data, the American College of Physicians has recommended cognitive-behavioral therapy for insomnia (CBT-I) as the initial treatment for chronic insomnia disorder in adults.6 CBT-I addresses cognitive and behavioral factors that maintain insomnia using core treatment components of sleep restriction (shortening time spent in bed to consolidate sleep), stimulus control (restricting bedroom activities to create an association between the bed and sleep), sleep hygiene (development of good sleep habits), cognitive therapy (changing dysfunctional beliefs about sleep), and relaxation therapy.7 Though research has shown CBT-I significantly improves sleep in cancer survivors,8 it is not widely available in the community or at most cancer centers.9 Provider-level treatment barriers include lack of physician training about sleep, and a shortage of CBT-I specialists.10, 11 Patient-related barriers include limited understanding of the health consequences of insomnia, and lack of awareness of available behavioral treatments.12 In addition, CBT-I treatment can be burdensome, with 14–40% of participants estimated to withdraw before the conclusion of treatment, due to challenges such as the duration of standard treatment (approximately 6–8 sessions), and the challenges of making sleep-related behavioral changes.13
Given the prevalence of insomnia among cancer survivors and difficulties they encounter accessing evidence-based treatment, we conducted a trial of a stepped care insomnia program. Stepped care in psychological care delivery has been proposed to address treatment barriers such as the discrepancy between the limited supply of trained providers and the demand for treatment. In this model of care, an “entry level” treatment should be readily accessible, delivered at the lowest level of therapeutic intensity, inconvenience patients the least, be provided at the lowest cost, and require the least specialist time.14 Sleep hygiene recommendations meets all of these criteria, typically including general guidelines about individual behaviors (e.g., caffeine consumption, exercise) and environmental factors (e.g., bedroom noise level) that can affect sleep.15, 16 As this is the most commonly delivered treatment for insomnia,17 we chose to develop and deliver a ‘best practice’ version of sleep hygiene recommendations as the entry level treatment in our trial. For those whose insomnia does not resolve following this initial step, we offered a group-based CBT-I program that we have demonstrated to be efficacious among cancer survivors.18 We believe that these lower intensity approaches are more likely to be disseminated in hospitals without a behavioral sleep specialist on staff, and could also be easier for patients suffering from insomnia to engage in due to the lower commitment required.
Methods
Sample
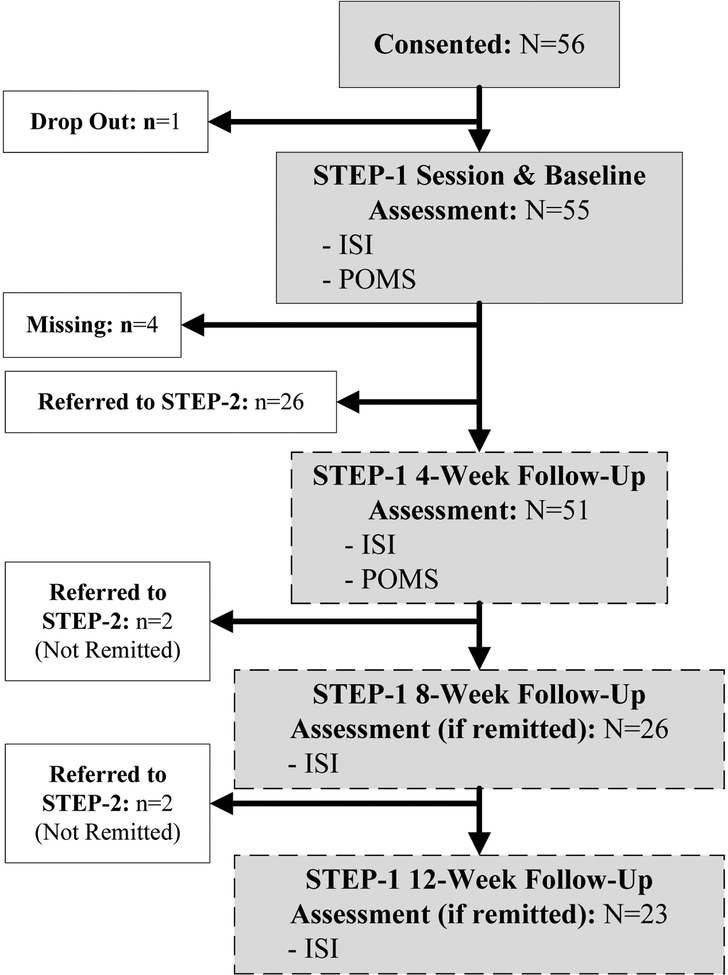
Participants were recruited by study staff at scheduled medical appointments in our cancer center, as well as oncologist referrals, clinic and newspaper advertisements, and mailed invitations to participants in a local cohort of cancer survivors. One hundred sixty-three adult cancer survivors (age ≥18 years) were screened for inclusion criteria: no active cancer therapy (excluding chemoprevention) in the past year, no cancer therapy or surgery planned in the next 6 months, an Insomnia Severity Index (ISI) score ≥12, and English fluency. Of those 163, 18 were ineligible based on ISI score, 40 because of another untreated sleep disorder, and 16 for other reasons (Supplemental Figure 1). Fifty-six participants enrolled on the trial; one did not complete any assessments or intervention sessions and was excluded, yielding a final sample of 55 (Table 1).
Table 1.
Demographic and Medical Characteristics of Study Participants (N=55)
| Participant Characteristics | M | SD | N | % | ||||
|---|---|---|---|---|---|---|---|---|
| Demographic Characteristics | ||||||||
| Age | 54.35 | 14.99 | ||||||
| Gender | ||||||||
| Female | 49 | 89.1 | ||||||
| Male | 6 | 10.9 | ||||||
| Race/Ethnicity* | ||||||||
| Caucasian | 50 | 90.9 | ||||||
| African American | 3 | 5.5 | ||||||
| Hispanic | 1 | 1.8 | ||||||
| Asian/Pacific Islander | 1 | 1.8 | ||||||
| Native American or Alaskan Native | 1 | 1.8 | ||||||
| Marital Status | ||||||||
| Married/Living as Married | 37 | 67.3 | ||||||
| Single | 9 | 16.4 | ||||||
| Divorced | 7 | 12.7 | ||||||
| Widowed | 2 | 3.6 | ||||||
| Education | ||||||||
| Received GED | 1 | 1.8 | ||||||
| Completed High School | 2 | 3.6 | ||||||
| Training after High School | 2 | 3.6 | ||||||
| Currently in College | 4 | 7.3 | ||||||
| Some College | 4 | 7.3 | ||||||
| College Graduate | 19 | 34.6 | ||||||
| Postgraduate Level | 23 | 41.8 | ||||||
| Employment Status* | ||||||||
| Working Full-Time | 26 | 47.3 | ||||||
| Working Part-Time | 8 | 14.5 | ||||||
| Student | 4 | 7.3 | ||||||
| Disabled and Unable to Work | 3 | 5.4 | ||||||
| Unemployed, Looking for Work | 1 | 1.8 | ||||||
| Unemployed, Not Looking for Work | 4 | 7.2 | ||||||
| Retired | 10 | 18.2 | ||||||
| Household Income | ||||||||
| Less than $25,000 | 7 | 13.7 | ||||||
| $25,000 to $49,999 | 9 | 17.6 | ||||||
| $50,000 to $74,999 | 7 | 13.7 | ||||||
| $75,000 to $99,999 | 13 | 25.5 | ||||||
| $100,000 or Greater | 14 | 27.5 | ||||||
| Missing | 1 | 1.8 | ||||||
| Medical Characteristics | ||||||||
| Years Since Cancer Diagnosis | 10.32 | 7.65 | ||||||
| Diagnosis | ||||||||
| Breast Cancer | 35 | 63.6 | ||||||
| Lymphoma | 9 | 16.4 | ||||||
| Leukemia | 5 | 9.1 | ||||||
| Other (Sarcoma, Head and Neck, Brain, Neuroblastoma, Thyroid, Wilms Tumor) |
6 | 10.9 | ||||||
Participants were able to choose more than one response..
Procedure
Study procedures were approved by the hospital IRB, conducted in accordance with the Declaration of Helsinki, and registered at clinicatrials.gov (). All participants provided written consent prior to participation.
Stepped Care Program
The Sleep Training Education Program (STEP) consists of two levels of intervention.
STEP-1 (Sleep Education):
The entry level in our stepped care approach was a single, hour-long sleep education session delivered by a clinical psychologist. The session content focused on: 1) providing psychoeducation about insomnia in cancer survivors; 2) introducing participants to sleep hygiene principles; 3) identifying two to three of the most relevant sleep hygiene strategies for each participant; and 4) developing a plan to consistently implement the recommended behavior changes over the next month.
STEP-2 (Group CBT-I):
The second level of intervention was a 3-session, group-based CBT-I program previously developed and tested for adult cancer survivors.18 Sessions were led by study investigators (ESZ and CJR) and supplemented by a workbook providing further information and examples tied to session material. Session 1 provided instruction on the etiology and maintenance of insomnia and proper completion of sleep diaries. Session 2 focused on stimulus control and sleep restriction, and provided participants with an individualized sleep schedule based on their sleep diary data. Session 3 instructed participants on sleep expansion, and sleep hygiene, and addressed cancer late-effects and maladaptive sleep cognitions, and long-term adherence.
Study Measures
Demographics and Medical History:
Demographic information, as well as medical information including cancer diagnosis and treatment, were collected by self-report and medical record review.
Insomnia Severity Index (ISI):19
The ISI is the most commonly used measure in insomnia research and has been validated in cancer populations.20 It has demonstrated adequate internal consistency and is sensitive to detect changes in perceived sleep difficulties with treatment.
Profile of Mood States–Short Form (POMS-SF):21
The POMS-SF is a 35-item measure, which assesses several dimensions of mood states and includes an overall Total Mood Disturbance (TMD) score.
Sleep Problem Information:
The participants were asked to estimate the duration of their sleep problems. In addition, they were asked to report the perceived burden of their sleep problems and their level of interest in seeking help for their sleep problems on a 1–10 scale (higher scores equating to more burden or greater interest).
Sleep Treatment Change:
Participants were asked to report any changes made in their use of sleep medications (over-the-counter or prescribed) during the study period.
Study Procedure
Participants were invited to start the intervention on the day of enrollment, or schedule for a later date. At study Baseline assessment prior to STEP-1, all participants completed the ISI and POMS-SF (Figure 1). Four weeks after the completion of STEP-1, participants completed these same measures and Sleep Treatment Change at the (STEP-1) 4-Week Follow-Up. If their ISI remained ≥12 at 4 weeks following STEP-1, they were referred to STEP-2. Participants with ISI <12 at this timepoint, were monitored with ISI assessments and referred to STEP-2 if their ISI score was ≥12 at either 8- or 12-weeks following STEP-1.
Figure 1.
STEP-1 Schema
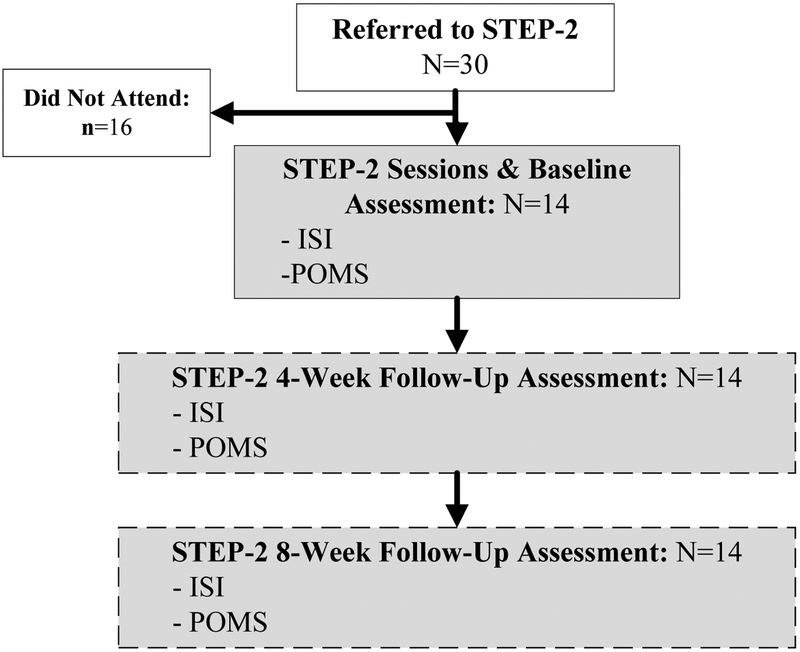
STEP-2 was offered to referred participants on 9 potential dates over a 15-month period. All participants referred to STEP-2 who did not attend were offered at least 3 group dates, included at least one weekday, weeknight, and weekend times, with at least 3 months prior notice for each group. At the initial STEP-2 session, participants completed the ISI and POMS-SF. The ISI, POMS-SF, and sleep treatment change were given at (STEP-2) 4-Week and 8-Week Follow-Up assessments (Figures 1 and 2). Median interval between STEP-1 completion and STEP-2 initiation was 1.5 months, with 78.6% of participants initiating STEP-2 within 3 months.
Figure 2.
STEP-2 Schema
Data Analysis
Descriptive statistics were used to describe demographic and medical characteristics. The primary analysis of change in ISI scores was conducted separately for each step of the intervention; baseline ISI scores were compared to ISI scores at each follow-up assessment using paired t-tests. Cohen’s d was calculated as a measure of effect size. Change in POMS-SF scores were analyzed similarly, with baseline POMS-SF scores being compared to POMS-SF scores at each follow-up assessment using paired t tests. For descriptive purposes, participants with ISI score decreases ≥1 point were considered “Improved.” Following published recommendation,22 those with ISI decreases ≥6 points were considered treatment “Responders.” Participants with post-intervention ISI scores < 12 were considered “Remitted.”23, 24 Differences between participants with remitted versus unremitted insomnia on demographic, medical, mood and sleep variables were evaluated with independent t tests. Additionally, among participants referred to STEP-2, differences between those who attended and those who did not were similarly assessed using independent t tests.
Four participants who attended the STEP-1 session, but did not complete any follow-up assessments, were not included in primary analyses. In a secondary analysis, we conservatively estimated their (STEP-1) 4-Week Follow-Up ISI and POMS-SF TMD scores using the last observation carried forward approach (which assumes they had no change on these measures after the intervention) and repeated the analyses on (STEP-1) 4-Week Follow-Up ISI and POMS-SF data.
Results
STEP-1 (Sleep Education)
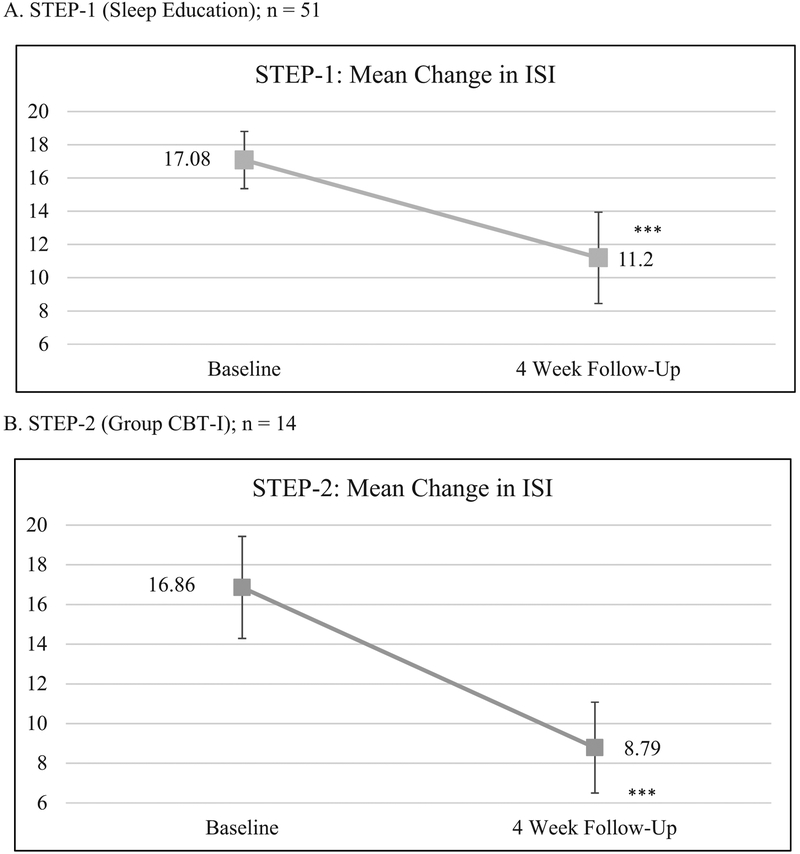
At STEP-1 4-Week Follow-Up, participants reported significant improvement in insomnia compared to baseline. Mean ISI scores decreased significantly from 17.1 to 11.2 (d=1.2; p<.001), and POMS-SF scores showed significant improvement in 5 of 7 mood subscales and the summary TMD scale (Table 2). At 4-week follow-up. 88.2% of the 51 participants reported improved sleep, 45.1% were treatment responders, and 51.0% had remitted (Table 3). Among the 26 participants whose insomnia remitted at 4-weeks, ISI scores continued to improve with further reductions in symptoms at 8- and 12-week follow-up. Compared to survivors with unremitted insomnia at this step, those with remitted insomnia had a shorter duration (4.1 years, SD=1.2, versus 4.8 years, SD=1.2, years; p<.05), and less perceived burden (6.2; SD=1.6 versus mean=7.2; SD=1.3; p<.05) from sleep problems, and less pain (mean=1.8; SD=1.1 versus mean=4.2; SD=2.5; p<.001). Survivors with remitted versus unremitted insomnia did not differ by demographic (age, gender, marital status, level of education, annual household income), psychological (psychological distress), medical (cancer diagnosis, cancer treatments, time since treatment), level of interest in help for sleep problems, and STEP-1 Baseline ISI score.
Table 2.
Change in ISI and POMS-SF Score at Follow-Up Time Points for STEP-1 (Sleep Education) and STEP-2 (Group CBT-I)
| Baseline | 4 Week Follow-Up | 8 Week Follow-Up | 12 Week Follow-Up | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | M (SD) | N | M (SD) | d | N | M (SD) | d | N | M (SD) | d | ||
| STEP-1 (Sleep Education) | ||||||||||||
| Insomnia Severity Index | 51 | 17.08 (3.44) | 51 | 11.20 (5.50)*** | 1.18 | 26a | 6.85 (3.87)*** | 1.62 | 23a | 6.09 (3.58)*** | 2.2 | |
| POMS-SF | ||||||||||||
| Total Mood Disturbance | 45 | 14.76 (17.74) | 45 | 8.00 (16.23)** | 0.50 | |||||||
| Anger Hostility | 47 | 2.87 (2.78) | 47 | 2.30 (2.58) | 0.18 | |||||||
| Confusion Bewilderment | 54 | 3.63 (3.29) | 47 | 3.15 (3.70)** | 0.41 | |||||||
| Depression Dejection | 47 | 2.19 (3.42) | 47 | 1.51 (1.82) | 0.26 | |||||||
| Fatigue Inertia | 55 | 9.62 (4.92) | 47 | 6.62 (4.70)*** | 0.66 | |||||||
| Tension Anxiety | 55 | 5.40 (4.64) | 47 | 4.45 (3.69)* | 0.31 | |||||||
| Vigor Activity | 54 | 9.15 (3.97) | 47 | 9.96 (4.36)* | 0.36 | |||||||
| STEP-2 (Group CBT-I) | ||||||||||||
| Insomnia Severity Index | 14 | 16.86 (5.14) | 14 | 8.79 (4.58)*** | 2.02 | 14 | 9.5 (3.35)*** | 1.53 | ||||
| POMS-SF | ||||||||||||
| Total Mood Disturbance | 14 | 15.79 (17.11) | 13 | 5.31 (10.16) | 0.49 | 14 | 7.71(10.59)* | 0.74 | ||||
| Anger Hostility | 14 | 2.50 (2.35) | 14 | 2.43 (1.95) | 0.03 | 14 | 2.36 (1.74) | 0.06 | ||||
| Confusion Bewilderment | 14 | 3.29 (4.08) | 13 | 1.77 (1.59) | 0.29 | 14 | 2.50 (3.39) | 0.26 | ||||
| Depression Dejection | 14 | 2.21 (2.99) | 14 | 1.50 (1.79) | 0.34 | 14 | 0.86 (0.77) | 0.50 | ||||
| Fatigue Inertia | 14 | 9.43 (5.71) | 14 | 6.00 (3.64)* | 0.65 | 14 | 6.86 (5.28)* | 0.74 | ||||
| Tension Anxiety | 14 | 5.14 (3.01) | 14 | 3.86 (3.09) | 0.53 | 14 | 3.14 (2.35)* | 0.71 | ||||
| Vigor Activity | 14 | 6.79 (4.64) | 14 | 8.71 (3.69)* | 0.76 | 14 | 8.00 (4.47) | 0.41 | ||||
p < .05,
p < .01,
p < .001
Paired t tests comparing follow up assessments with baseline assessments.
Only participants remitted at STEP-1 4-week follow-up completed the ISI at 8- and 12-weeks after STEP-1; non-remitted participants were referred to STEP-2.
Table 3.
Improved, Responded, and Remitted at 4-Week Follow-Up
| STEP-1 (Sleep Education) (n=51) |
STEP-2 (Group CBT-I) (n=14) |
|||||
|---|---|---|---|---|---|---|
| n (%) | n (%) | |||||
| Improved (ISI improved by ≥ 1 pt) | 45 (88.2) | 14 (100.0) | ||||
| Responded (ISI Improved by ≥ 6 pts) | 23 (45.1) | 12 (85.7) | ||||
| Remitted (ISI < 12) | 26 (51.0) | 12 (85.7) | ||||
On the sleep treatment change questions at 4-Week Follow-Up, one participant endorsed starting an over-the-counter medication for sleep, and three participants endorsed decreasing the amount of prescribed or over-the-counter medication they were using for sleep. Of note, to ensure our results were not overly influenced by the four STEP-1 participants who did not complete any follow-up assessments, we repeated our 4-Week Follow-Up analysis assuming they had no change in ISI or POMS-SF TMD scores following the intervention (last observation carried forward). Results including these additional 4 cases were highly similar, with mean ISI scores changed from 17.3 to 11.9 (d=1.1, p<.001) and POMS-SF TMD score changed from 15.6 to 9.4 (d=0.5, p<.005).
STEP-2 (Group CBT-I)
Based on continued insomnia symptoms following STEP-1, 30 survivors were referred to STEP-2 (25 survivors were referred based on an ISI score ≥12 at 4-weeks after STEP-1 and 5 based on ISI ≥12 at 8-weeks after STEP-1), which was offered at multiple times and dates (described in Study Procedure). A total of 14 survivors attended the STEP-2 intervention. At both 4-Week and 8-Week Follow-Up, the 14 participants who attended STEP-2 reported significant improvement in insomnia compared to Baseline with large effect sizes (d ≥1.5; p<.001). Overall mood scores (POMS-SF TMD) also improved, but difference from Baseline was significant only at 8-Week Follow-Up. POMS-SF subscale Fatigue and Vigor scores showed similar improvement at 4-Week Follow-Up and Fatigue scores also showed significant improvement at 8-Week Follow-Up. At 4 weeks after STEP-2, all 14 participants reported improved sleep. Of these 14 participants, 12 (85.7%) were treatment responders and 12 (85.7%) were remitted (Table 3). On the sleep treatment change questions at 4-Week & 8-Week Follow-Up, no participants endorsed starting a new sleep medication, but one participant reported decreasing use of an over-the-counter sleep medication at 8-Week Follow-Up.
Compared to survivors who did not attend STEP-2 (n=16), STEP-2 attendees reported a higher level of interest in seeking help for their sleep problems at their STEP-1 Baseline (mean=9.4; SD=0.9 versus mean=8.2; SD=1.3; p<.05). Attendees and non-attendees did not differ by demographic (age, gender, marital status, level of education, annual household income), psychological (psychological distress), medical (cancer diagnosis, cancer treatments, time since treatment, pain), burden of sleep problems, length of sleep problems, or STEP-2 Baseline ISI score.
Discussion
Stepped care models have been recommended as a way to improve access and deliver care efficiently, and have been successfully implemented in psychosocial care for cancer patients.25 Our findings demonstrate that stepped care is effective for insomnia in cancer survivors, and that a sizable proportion of cancer survivors suffering from insomnia experience meaningful symptom improvement from a low-intensity sleep hygiene education session.
In existing stepped care models for insomnia, levels of care are distinguished by how treatment is delivered. The first step is typically a form of self-directed therapy (e.g., Internet based CBT-I), up to the highest step of individual sessions with a sleep specialist.26–28 However, these levels of care are typically not differentiated by the intervention content or duration. This leaves an important barrier to treatment, as even the first treatment step still requires a significant patient commitment to the full course of CBT-I, which is approximately 6–8 sessions/modules. Ideally, the entry level to insomnia care should be the lowest dose proven to be associated with clinical improvement;26 our data demonstrate that more than half of cancer survivors with insomnia can benefit appreciably from one hour-long program that empowers patients by teaching them about sleep health, and provides concrete instruction on how to change their sleep behaviors. For those survivors whose insomnia does not resolve following this first step, a group-based CBT-I program that was designed to be a lower intensity intervention than standard CBT-I is also efficacious.18 Though we tested the STEP-1 and STEP-2 interventions as administered by PhD level clinicians, demonstrating their efficacy when delivered online or by paraprofessionals will be an important step to further increase their dissemination to survivors.
Our results have important implications for cancer centers and community oncology settings developing a sleep program. These findings provide information about which patients are most likely to benefit from a short course of care and which ones can be expected to engage in higher intensity levels of treatment. Existing literature has been mixed, without clear demographic, medical, or psychological characteristics consistently associated with CBT-I adherence.13 We found that cancer survivors who had experienced sleep problems for a shorter period of time, and perceived less burden from their sleep problems and less pain, were most likely to benefit from the single, sleep hygiene education session. This suggests it is crucial to identify patients with disturbed sleep as early as possible, before they have had to bear the negative effects of insomnia for too long.29 Routine screening for sleep disorders is already supported by clinical practice guidelines for survivors,30 and our data indicate that early identification and treatment may enhance efficacy of brief low-intensity interventions. In exploring adherence of participants referred for a second more intense level of treatment, we did not identify demographic or medical variables that were associated with greater likelihood of participation. Rather, participants reporting a greater level of interest in pursuing sleep treatment was predictive of enrollment in STEP-2. This is consistent with prior research demonstrating higher levels of baseline motivation to change sleep behaviors was associated with adhering to CBT-I recommendations in a sample of breast cancer survivors.31 Though consistent with these findings, the implications of this result are not entirely clear. The fact that survivors without strong motivation to improve their sleep are less likely to engage in more demanding treatments may not be a cause for great concern if it is viewed as reflecting their autonomy, values, and priorities. On the other hand, if low levels of motivation reflects a lack of self-efficacy and information about the health impact of insomnia and the benefits of treatment, the lack of motivation for change may itself be an important target for intervention. Qualitative and quantitative research methods will likely be needed to better understand factors effecting baseline motivation and its impact on adherence.
These findings demonstrating sleep hygiene education can be effective at improving insomnia symptoms may be surprising as sleep hygiene alone is often viewed as ineffective, and even used as the control condition in trials of CBT-I.16 However, it should be noted that in prior research, sleep hygiene is often delivered as a handout with limited instructions on how to actually enact the advised sleep behavior and/or environment changes, or guidance on reasonable expectations for a timeline for sleep improvements. Efficacy of the STEP-1 intervention here may reflect the delivery of the sleep hygiene information as part of a more comprehensive educational session about insomnia in cancer patients, and included structured information about how to make behavioral changes to improve sleep. Alternatively, the efficacy of STEP-1 in our participants may be because cancer patients are naïve to the basic principles of sleep hygiene, unlike patients seeking treatment in specialized sleep-medicine program. Replication of our findings and assessment of pre-treatment familiarity with sleep hygiene principles will be useful in evaluating these possible explanations.
This research is not without limitations. We acknowledge our sample is relatively homogenous (primarily White, higher socioeconomic level women) drawn from a single center. It is important to study behavioral sleep interventions in diverse populations. Next, we did not collect objective sleep data (e.g., actigraphy), though self-report on the ISI is a commonly used primary endpoint in insomnia intervention trials.8 Our study also lacked a control group, a limitation we plan to address in future trials. Finally, 17 of 56 participants (30.3%) did not complete some or all of the recommended intervention steps. This is consistent with attrition reported in previous CBT-I trials,13 but it is notable that 16 of 17 cases of attrition occurred when survivors chose not to attend STEP-2, while all survivors who began STEP-2 completed treatment. We did not directly assess why these participants who continued to experience insomnia after STEP-1, chose not to enroll in STEP-2. Consequently, we do not have data on the factors that influenced their decision, including the long-term course of their insomnia. Future research aimed at understanding these factors will be essential to helping survivors engage in available evidence-based treatments best suited to their needs.
It has been said of CBT-I that “doubts…do not reside in its efficacy, nor even in its effectiveness, but in its feasibility. Can [CBT-I] really become a first line treatment for insomnia in everyday practice?”26 as the American College of Physicians has recommended.6 Our efforts here seek to balance the desire for every patient with insomnia to receive the full course of the gold standard treatment, with the reality of survivorship care at most cancer centers which are appropriately focused primarily on delivering cancer treatment. The implementation of at least the first step in our program (a sleep education session) is reasonable to consider as a part of a commitment to quality survivorship care, even at less resourced sites. This represents a tremendous opportunity to successfully treat a common problem for cancer survivors that has significant health consequences when ignored.
Supplementary Material
Figure 3. Mean Change in ISI Scores.
A. STEP-1 (Sleep Education): n = 51 B. STEP-2 (Group CBT-I) (n=14) * p < .05, ** p < .01, *** p < .001 Paired t-tests comparing follow-up ISI scores with baseline ISI scores.
Funding
This trial was supported by NCI grant R03CA201459
Footnotes
Conflicts of Interest
Authors declare they have no conflicts of interest.
References
- 1.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66: 271–289. [DOI] [PubMed] [Google Scholar]
- 2.Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19: 895–908. [DOI] [PubMed] [Google Scholar]
- 3.Zhou ES, Recklitis CJ. Insomnia in adult survivors of childhood cancer: a report from project REACH. Support Care Cancer. 2014;22: 3061–3069. [DOI] [PubMed] [Google Scholar]
- 4.Roth T Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3: S7–10. [PMC free article] [PubMed] [Google Scholar]
- 5.Trudel-Fitzgerald C, Zhou ES, Poole EM, et al. Sleep and survival among women with breast cancer: 30 years of follow-up within the Nurses’ Health Study. Br J Cancer. 2017;116: 1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of Chronic Insomnia Disorder in Adults: A Clinical Practice Guideline From the American College of PhysiciansManagement of Chronic Insomnia Disorder in Adults. Ann Intern Med. 2016;165: 125–133. [DOI] [PubMed] [Google Scholar]
- 7.Perlis ML, Jungquist C, Smith MT, Posner D. Cognitive behavioral treatment of insomnia: a session-by-session guide. New York, NY: Springer Science and Business Media, 2008. [Google Scholar]
- 8.Johnson JA, Rash JA, Campbell TS, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016;27: 20–28. [DOI] [PubMed] [Google Scholar]
- 9.Zhou ES, Partridge AH, Syrjala KL, Michaud AL, Recklitis CJ. Evaluation and treatment of insomnia in adult cancer survivorship programs. J Cancer Surviv. 2017;11: 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mindell JA, Bartle A, Wahab NA, et al. Sleep education in medical school curriculum: a glimpse across countries. Sleep Med. 2011;12: 928–931. [DOI] [PubMed] [Google Scholar]
- 11.Thomas A, Grandner M, Nowakowski S, Nesom G, Corbitt C, Perlis ML. Where are the Behavioral Sleep Medicine Providers and Where are They Needed? A Geographic Assessment. Behav Sleep Med. 2016;14: 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stinson K, Tang NK, Harvey AG. Barriers to treatment seeking in primary insomnia in the United Kingdom: a cross-sectional perspective. Sleep. 2006;29: 1643–1646. [DOI] [PubMed] [Google Scholar]
- 13.Matthews EE, Arnedt JT, McCarthy MS, Cuddihy LJ, Aloia MS. Adherence to cognitive behavioral therapy for insomnia: a systematic review. Sleep Med Rev. 2013;17: 453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bower P, Gilbody S. Stepped care in psychological therapies: access, effectiveness and efficiency. Narrative literature review. Br J Psychiatry. 2005;186: 11–17. [DOI] [PubMed] [Google Scholar]
- 15.Hauri P Current concepts: the sleep disorders. Kalamazoo, MI: The Upjohn Company; 1977. [Google Scholar]
- 16.Stepanski EJ, Wyatt JK. Use of sleep hygiene in the treatment of insomnia. Sleep Med Rev. 2003;7: 215–225. [DOI] [PubMed] [Google Scholar]
- 17.Moss TG, Lachowski AM, Carney CE. What all treatment providers should know about sleep hygiene recommendations. The Behavior Therapist. 2013. [Google Scholar]
- 18.Zhou ES, Partridge AH, Recklitis CJ. A pilot trial of brief group cognitive-behavioral treatment for insomnia in an adult cancer survivorship program. Psychooncology. 2017;26: 843–848. [DOI] [PubMed] [Google Scholar]
- 19.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2: 297–307. [DOI] [PubMed] [Google Scholar]
- 20.Savard MH, Savard J, Simard S, Ivers H. Empirical validation of the Insomnia Severity Index in cancer patients. Psychooncology. 2005;14: 429–441. [DOI] [PubMed] [Google Scholar]
- 21.Curran SL, Andrykowski MA, Studts JL. Short Form of the Profile of Mood States (POMS-SF): Psychometric information. Psychol Assess. 1995;7: 80. [Google Scholar]
- 22.Yang M, Morin CM, Schaefer K, Wallenstein GV. Interpreting score differences in the Insomnia Severity Index: using health-related outcomes to define the minimally important difference. Curr Med Res Opin 2009;25: 2487–2494. [DOI] [PubMed] [Google Scholar]
- 23.Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34: 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gagnon C, Belanger L, Ivers H, Morin CM. Validation of the Insomnia Severity Index in primary care. J Am Board Fam Med. 2013;26: 701–710. [DOI] [PubMed] [Google Scholar]
- 25.Singer S, Danker H, Roick J, et al. Effects of stepped psychooncological care on referral to psychosocial services and emotional well-being in cancer patients: A cluster-randomized phase III trial. Psychooncology. 2017;26: 1675–1683. [DOI] [PubMed] [Google Scholar]
- 26.Espie CA. “Stepped care”: a health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatment. Sleep. 2009;32: 1549–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincent N, Walsh K. Stepped care for insomnia: an evaluation of implementation in routine practice. J Clin Sleep Med. 2013;9: 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mack LJ, Rybarczyk BD. Behavioral treatment of insomnia: a proposal for a stepped-care approach to promote public health. Nat Sci Sleep. 2011;3: 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou ES, Clark K, Recklitis CJ, Obenchain R, Loscalzo M. Preferences for Help With a Sleep Problem Before Starting Cancer Treatment. J Pain Symptom Manag. 2019;57: e5–e8. [DOI] [PubMed] [Google Scholar]
- 30.Denlinger CS, Ligibel JA, Are M, et al. Survivorship: sleep disorders, version 1.2014. J Natl Compr Canc Netw. 2014;12: 630–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews EE, Schmiege SJ, Cook PF, Berger AM, Aloia MS. Adherence to cognitive behavioral therapy for insomnia (CBTI) among women following primary breast cancer treatment: a pilot study. Behav Sleep Med. 2012;10: 217–229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.