Abstract
Autism Spectrum Disorders (ASD) are complex and multifactorial. Previous investigations have revealed associations between allergic disease and ASD, which are characterized by impaired communication skills. In this study we observed an association between allergic disease and communication skills development as assessed by the Ages and Stages Questionnaire (ASQ) communication score, as a proxy for ASD, among children who participated in the Vitamin D Antenatal Asthma Reduction Trial (VDAART). In particular, we observed significant associations between both a diagnosis of eczema at age 3 years (OR=1.87; confidence interval [CI]: 0.97–3.47; p=0.054) and a diagnosis of food allergy at age 6 years (OR=3.61; 95% CI: 1.18–9.85; p=0.015) with ASQ communication score. Plasma metabolomics analyses suggest that dysregulated tryptophan metabolism may contribute to the pathogenesis of these co-morbidities.
Keywords: Autism, ASD, Ages and Stages Questionnaire, Allergy, Atopy, VDAART
1. Introduction
Autism spectrum disorder (ASD) is a complex and heterogeneous developmental disorder characterized by difficulty with communication and interaction, spanning various social contexts[1]. The etiology of this condition is still being explored, however prior evidence points to a multifactorial etiopathogenesis resulting from complex interactions between genetic[2, 3] and environmental factors[4]. These environmental factors are hypothesized to underlie the increasing prevalence of ASD; which is estimated to be as high as one in every 59 people in the United States, and between 1% to 2% globally[5]. Interestingly, there is a growing body of evidence linking ASD to allergic disease[6–8]; the prevalence of which has also increased over the last few decades [9]. However, the mechanisms underlying this purported relationship are not fully elucidated[8].
The Ages and Stages Questionnaire (ASQ) is a widely used tool for assessment of a child’s early development. The ASQ surveys five developmental skill domains: communication, gross-motor, fine-motor, problem solving, and personal-social. The communication domain has been used as a proxy measure to identify children who are at a higher risk of a later ASD diagnosis [10–12]. We previously observed a significant relationship between a low ASQ communication score at age 3 with a subsequent diagnosis of ASD among children from the Vitamin D Antenatal Asthma Reduction Trial (VDAART)[11]. Our results suggested that dysregulated tryptophan metabolism may play an important role in delayed development of communication skills and therefore potentially in ASD diagnosis. Tryptophan metabolism has also been implicated in the pathophysiology of allergic disorders[13]. Therefore, in the current study, we investigated the relationship between the ASQ communication score and allergic disease in children from VDAART[14] with a specific focus on tryptophan metabolism.
2. Methods
2.1. Study Population
This study was nested within the VDAART; the details of which have been published previously[14]. In brief, pregnant, women with a history of allergic disease who were between 10 to 18 weeks of gestation were recruited and randomized 1:1 to a daily dose of vitamin D or a placebo and their offspring followed-up. This trial was approved by the IRBs of the participating institutions and all participants provided written consent at the time of enrollment.
2.2. Data Collection
VDAART included eczema and food allergy as secondary outcomes. Atopic eczema was defined as parental report of physician diagnosed eczema, a persistent rash and the presence of sensitization to at least one allergen or a total serum IgE above the geometric mean value[14]. Food allergy was defined by a parent-reported allergic reaction to a food by a given age with serum specific IgE to that food >= 0.35 kU/l. We used data based on the ages 3 and 6 follow-ups. Asthma and recurrent wheeze were defined by a self-report of a doctor’s diagnosis and wheezing as described previously [15]. The children in VDAART are now between 6 and 8.5 years old, and study follow up is ongoing.
2.3. ASQ
The ASQ 3rd Edition was administered to the primary caregivers of the VDAART children at their age one, two and three year visits. In this analysis we used data from the age 3 ASQ. The questionnaire included five developmental domains for assessment: gross motor skills, fine motor skills, problem solving ability, personal/social skills, and communication. For each domain, based on their score children are categorized as: (i) “On Schedule for developing normally” (above the mean); (ii) “Requires Monitoring” (1–2 standard deviations below the mean); (iii) “Needs further evaluation” (>2 standard deviations below the mean).
2.4. Tryptophan metabolism
A subset of children had metabolomic profiling available on plasma collected at age 3 years. Plasma sample profiling was performed using ultrahigh performance liquid chromatography-tandem mass spectroscopy (LC/MS) by Metabolon Inc, as described previously[16, 17]. Based on our previous findings [11] we focused on the 15 available plasma metabolites in the tryptophan metabolism sub-pathway (as defined by Metabolon Inc).
2.5. Statistical Analyses
ASQ communication score at age 3 was our outcome of interest, concurrent to the age three metabolomic profiling. We used a binary cut-off for communication score comparing those “On schedule for developing normally” to those either requiring “further evaluation” or “monitoring”, based on the recommendations of Hardy et al[10] and our previous work, in which we identified a significant relationship between this metric and future development of ASD [11]. We define the combination of the lower two categories as a ‘low communication score’. We used a chi squared test of independence and logistic regression models adjusting for race, gender and study site to investigate differences in baseline characteristics and in the frequency of allergic conditions (asthma, food allergy and eczema at ages 3 and 6) between children classified as “on schedule” as compared “low communication score”. VDAART was a randomized double-blinded trial which supplemented mothers with vitamin D or a placebo throughout pregnancy[14], therefore we additionally ran our analyses stratifying by intervention arm. Similarly, given the known sex disparity in ASD[1], we further ran a sex-stratified analysis.
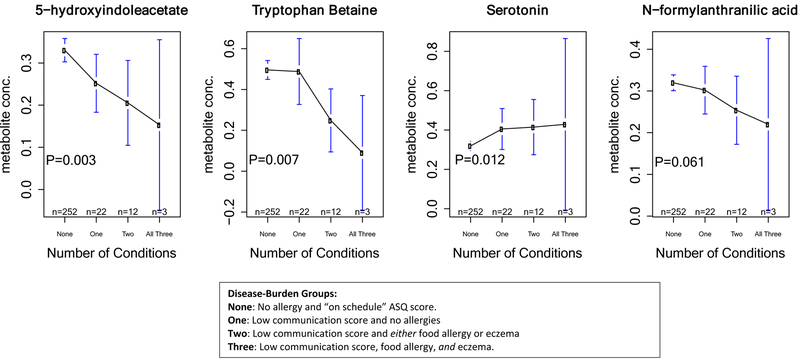
We then explored whether disease burden at age 3 or 6 years was associated with greater dysregulation in the tryptophan pathway, using analysis of variance (ANOVA) to look for differences in the levels of 15 tryptophan metabolites across 4 groups: ‘control’ (no relevant condition, n=252); ‘1 condition’ (low communication score, n=22); ‘2 conditions’ (low communication score and either food allergy or eczema, n=12) or ‘3 conditions’ (children with eczema, food allergy and a low communication score, n=3). Subjects with an “on schedule” ASQ communication score and food allergy or eczema were excluded (n=114).
3. Results
3.1. Study Population
Of 806 children in VDAART, 715 (88.7%) had completed ASQ questionnaires at age 3. Based on their communication score; 652 (91.2%) were classified as “On Schedule” (score >41.44), sixty-three (8.81%) had a low communication score: (42 (5.87%) “required monitoring” (score 30.99 – 41.44) and 21 (2.94%) “needed further evaluation” (scores <30.99))(Supplemental Figure 1).
Children in the low communication score group were more likely to be diagnosed with ASD by year 8 (11.1% vs 1.2%; p<0.001) (Supplemental Table 1). Children with low communication scores were also less likely to be from San Diego than Boston or St. Louis (17.5% in San Diego, 41.3% in St. Louis, 41.3% in Boston; p=0.012), and more likely to be male (68.3% vs 51.7% male; p = 0.017). There were no major differences by racial group or vitamin D treatment group.
3.2. ASQ-3 and Allergic Conditions
Children in the low communication score group were more likely to report food allergy (Table 1). This association was significant for children with food allergy at age 6 (17.6% in the low score group vs. 4.7% on-schedule group; p=0.007) and for those with food allergies at either age 3 or age 6 (13.5% vs 5.0%; p=0.029). After adjustment for site, gender, and race, we observed that children diagnosed with food allergy at either age 3 or 6 were almost three times more likely to be in the lowest ASQ-3 communication score categories (OR=2.77; 95% CI:1.03–6.64; p=0.030) (Table 1). This association appeared to be driven by the age 6 diagnoses (OR=3.62; 95% CI: 1.19–9.86; p=0.015). There were also more children with eczema at age 3 in the low communication score group (27.0% vs 16.0% p=0.040), and after adjustment for confounders, we found that children diagnosed with eczema at age 3 had almost double the risk of being in low communication score category, compared to children without eczema (OR=1.87; CI(95%): 0.97,3.47; p=0.054). However, this association was not evident based on age 6 eczema diagnosis. Children with a low communication score at age three were also more likely to report a diagnosis of asthma at both age 3 and age 6, but these associations did not reach significance.
Table 1:
ASQ Communication Score at Age 3 and the association with Food Allergy, Eczema and Asthma
| ASQ Communication Score Category at Age 3 | ||||||
|---|---|---|---|---|---|---|
| On Schedule | Low Communication Score | Chi Square P-Value | Odds Ratio | Confidence Interval | Logistic Regression P-Value | |
| n(%) | n(%) | |||||
| Food Allergy | ||||||
| Year 3 | ||||||
| No | 449(96.1) | 45(91.8) | ||||
| Yes | 18(3.9) | 4(8.2) | 0.294 | 1.94 | 0.53,5.71 | 0.264 |
| Year 6 | ||||||
| No | 342(95.3) | 28(82.4) | ||||
| Yes | 17(4.70) | 6(17.6) | 0.007 | 3.62 | 1.18,9.85 | 0.015 |
| Year 3 or 6 | ||||||
| No | 495(95.0) | 45(86.5) | ||||
| Yes | 26(5.0) | 7(13.5) | 0.029 | 2.77 | 1.03,6.63 | 0.030 |
| Eczema | ||||||
| Year 3 | ||||||
| No | 548(84.0) | 46(73.0) | ||||
| Yes | 104(16.0) | 17(27.0) | 0.040 | 1.87 | 0.97, 3.47 | 0.054 |
| Year 6 | ||||||
| No | 520(88.6) | 52(89.7) | ||||
| Yes | 67(11.4) | 6(10.3) | 0.978 | 0.807 | 0.29,1.88 | 0.645 |
| Year 3 or 6 | ||||||
| No | 459(78.2) | 41(70.7) | ||||
| Yes | 128(21.8) | 17(29.3) | 0.254 | 1.33 | 0.69,2.48 | 0.384 |
| Asthma | ||||||
| Year 3 | ||||||
| No | 478(73.4) | 39(61.9) | ||||
| Yes | 173(26.6) | 24(38.1) | 0.071 | 1.45 | 0.82,2.50 | 0.191 |
| Year 6 | ||||||
| No | 360(55.2) | 28(44.4) | ||||
| Yes | 292(44.8) | 35(55.6) | 0.132 | 1.37 | 0.80,2.34 | 0.249 |
| Year 3 or 6 | ||||||
| No | 358(55.0) | 28(44.4) | ||||
| Yes | 293(45.0) | 35(55.6) | 0.141 | 1.36 | 0.80,2.32 | 0.261 |
Data from 199 children missing for food allergy at age 3, 322 missing for food allergy at age 6, 142 missing for food allergy at age 3 or 6.
Data from 70 children missing for eczema at age 6, 70 missing for eczema at age 3 or 6.
Data from 1 child missing for asthma at age 3 and 1 missing for asthma at age 3 or 6.
3.3. Sensitivity Analyses
There was evidence that the ASQ-3 communication score - allergic disease relationship was stronger in those children whose mothers did not receive vitamin D supplementation during pregnancy (Supplemental Table 2). This was particularly evident for the association between ASQ communication score and food allergy at age 6; OR 10.15 (95% CI 2.09, 47.14) p=0.003 in those whose mothers received placebo during pregnancy, compared to OR 1.70 (95% CI 0.24, 7.43) p=0.525 in those whose mothers received vitamin D. Conversely, there was no evidence of a sex-specific effect, and an interaction analyses was non-significant (p>0.05) for all investigated variables (results not shown).
3.4. Tryptophan Metabolism
The subgroup of children with metabolomic profiling were representative of the parent VDAART population in terms of race, gender, and treatment group, however, there was a slightly higher percentage of children from St. Louis (44.7% vs 36.2%, p=0.002 Supplemental Table 3). 5-hydroxyindoleacetate, tryptophan betaine, serotonin, and N-formylanthranilic acid were all significantly different across disease-burden groups. 5-hydroxyindoleacetate, N-formylanthranilic acid and tryptophan betaine levels decreased with increasing disease burden; and were lowest in subjects with all three conditions. The opposite trend emerged for serotonin, with higher levels of this metabolite in children with all three conditions and decreasing progressively with lower disease burden. (Figure 1).
Figure 1:
Age 3 Year Metabolite Concentrations Across Disease-Burden Group
4. Discussion
The reported prevalence of both ASD and allergic disorders continue to rise globally[9]; and these diseases often co-occur, suggesting a common etiology[6, 7]. In this study, we investigated the relationship between poor early life communication development, as assessed by the ASQ-3 communication score, and allergic disease, with the hypothesis that the ASQ communication score is a good proxy for early detection of ASD. In order to explore potential biochemical modulators of this relationship, we leveraged plasma metabolomic data on a subset of these children with a focus on tryptophan metabolism.
In this analysis, we observed that children classified in the lowest two categories of communications skills development at age three were more likely to be diagnosed with food allergy or eczema, an association that persisted after adjustment for study site, race, and gender. This finding is in agreement with a growing body of literature reporting positive associations between allergy and ASD[6, 7].
There is a strong biological rationale for a link between allergic disorders and ASQ. Although the etiology of ASD is largely unknown, it is commonly agreed to be multifactorial [4], with compelling evidence to suggest that immune dysregulation may play a role. For example, subjects with ASD often display neuro-inflammation in brain tissue[18, 19]. Given the well-established relevance of immunologic perturbations to allergic conditions [17], it is possible that there are shared biological mechanisms linking these conditions[6, 7]. Recent literature suggests that an ASD diagnosis may render an individual more susceptible to allergic disease, potentially relating to the activation of mast cells that accompany atopy [20]. Studies have also reported higher levels of Th2 cytokines, elevated serum IgE, and eosinophils in the blood of subjects with autism lending greater plausibility to the hypothesized relationship between ASDs and allergic response [6, 21].
In previous work, we determined that tryptophan metabolism may be involved in communication skills development, which is in agreement with recent research into abnormal tryptophan levels and ASD[22]. Intriguingly, the tryptophan pathway has also been identified as relevant to the pathophysiology of allergic disease, with alterations in tryptophan metabolism reported in patients suffering from allergy and eczema[13, 23]. Therefore, we examined tryptophan metabolites as a possible common biochemical link between allergy and ASD risk, focusing on a burden of disease, classifying subjects according ASQ score and number of allergic conditions. Three metabolites in the tryptophan pathway significantly varied across subjects with different burdens of disease, and one was borderline significant. Levels of serotonin increased, while tryptophan betaine and 5-hydroxyindolacetate, and N-formylanthranilic acid decreased with increasing burdens.
Higher plasma serotonin in subjects with ASD as well as allergy has also been observed [24, 25]. In the plasma of children with ASD, the tryptophan biosynthesis pathway may be biased towards the production of serotonin over other downstream products [26]. Serotonin is involved in multiple aspects of neurological function that may influence the development of ASD such as regulation of mood, sleeping and appetite patterns, and social interaction [24]. Increased serotonin levels have also been linked to allergic disease, with reports that human mast cells may synthesize and release serotonin, thus illuminating a potential link to allergic response[25, 27]. This may explain why we also identified other metabolites downstream of serotonin catabolism, such as 5-hydroxyindolacetate, as significant.
Our study generated compelling results linking allergic disease to delayed communication skills development and provides evidence to support dysregulated tryptophan metabolism as a possible common etiological factor. However, there were a number of limitations. In this study, age 3 ASQ communication score served as a proxy for ASD, which we were underpowered to explore in this cohort, with only 15 cases. The use of the ASQ communication score as a proxy is based on evidence from the literature[10, 12], however we note that the evidence remains limited and we emphasize that ASDs are complex and multifactorial potentially affecting many other developmental domains. As such we caution that our results pertain to ASQ assessed communication skills and extrapolation to ASD is exploratory.
Even when considering ASQ low-communication score, we were limited by sample size particularly for the tryptophan analysis; there were only three subjects in our highest disease burden condition resulting in large confidence intervals. In contrast, within the confines of the existing VDAART dataset we were only able to assess IgE mediated food allergy. A proportion of food allergy is non IgE mediated and therefore our numbers for food allergy are likely to be an underestimate. Nevertheless, we believe that these results generate biologically meaningful hypotheses for the improved understanding of these complex conditions. Further studies in larger cohorts with more children in the lowest ASQ communication score categories, and which additionally consider other developmental domains are warranted.
It has been suggested that distress due to the irritation and discomfort experienced as a result of allergic disease may exacerbate the behavioral symptoms of ASD and communication behavior [28]. However, due to the cross-sectional nature of this study, we were not able to establish causality in terms of direction of effect between allergic disease and communication skills development. Rather, we hypothesize that these conditions all share a common underlying etiology, potentially involving epigenetic changes in utero. Maternal health during pregnancy, especially during critical windows of development, has been shown to influence the health and behavior of offspring[29]. Disruption to the mother’s immune systems, and in particular maternal allergic asthma, have been shown to increase the risk of ASDs and other developmental neurological disorders in their children[30]. Similarly, maternal history of immunological disorders has been shown to predict the risk of asthma, allergy and eczema[31, 32]. It is suggested these effects may be mediated through epigenetic changes passed down from mother to child as evidenced by transcriptomic dysregulation of common pathways, including those involved in immune function[29, 33]. Intriguingly, serotonin synthesis, which is particularly sensitive to perturbations to the immune system during development, has been proposed to be central to this link[33].
There were also significant differences in ASQ communication score across study sites. In our San Diego site, which had the highest socioeconomic status of the sites there were no subjects in the lowest category, which may reflect unmeasured biases related to parental reports of child development. We controlled for study site in our analysis to account for this discrepancy and determined that our results for food allergy and eczema retained significance after adjustment for study site and other potential confounders.
The significance of our results is two-fold. Firstly, this study adds to the small but growing body of literature on allergy and communication skills development in early life. Secondly, we propose a biological basis for the observed comorbidity of allergy and ASD using a metabolomic analysis of plasma. Allergic disease is one of the most common chronic health conditions globally [34]; and determining a common etiological pathway, which may relate to dysregulated tryptophan metabolism, with ASD could have important implications for both disorders. Interestingly, vitamin D has been suggested as protective for both neurological development, specifically ASD[35], and for allergic disease[36]. We observed that the relationship between ASD and allergic disease was strongest among VDAART children whose mothers were not supplemented with vitamin D during their pregnancy, suggesting that the common pathology underlying thee disorders could potentially be ameliorated with vitamin D supplementation.
These preliminary findings provide compelling hypothesis that are worthy of future research and more in-depth biochemical analyses in larger cohorts with well characterized neurological data and ASD diagnoses.
Supplementary Material
Communication domain of the Ages and Stages Questionnaire represents proxy for autism development
Children with poor communication skills development more likely to report food allergy and eczema
Autism; eczema and food allergy may share etiology involving dysregulated tryptophan metabolism
Funding:
This study is an ancillary study of the Vitamin D Antenatal Asthma Reduction Trial (VDAART). The trial was funded by the National Heart, Lung, and Blood Institute (U01 HL091528). RSK was supported by K01 HL146980 from the NHLBI. RSK, JLS, and SC were also supported through R01HL123915 and R01HL141826. HM was supported by 2L30 HL129467-02A1 from NHLBI. PK was supported by the US National Institutes of Health grant P01HL132825-01
Footnotes
Trial Registration: VDAART: ClinicalTrials.gov
References
- 1.Masi A, DeMayo MM, Glozier N, Guastella AJ. An Overview of Autism Spectrum Disorder, Heterogeneity and Treatment Options. Neurosci Bull. 2017;33(2):183–93. Epub 2017/02/19. doi: 10.1007/s12264-017-0100-y.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X, Zou H, Brown WT. Genes associated with autism spectrum disorder. Brain Res Bull. 2012;88(6):543–52. Epub 2012/06/13. doi: 10.1016/j.brainresbull.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Liu X, Takumi T. Genomic and genetic aspects of autism spectrum disorder. Biochem Biophys Res Commun. 2014;452(2):244–53. Epub 2014/09/01. doi: 10.1016/j.bbrc.2014.08.108.. [DOI] [PubMed] [Google Scholar]
- 4.Posar A, Visconti P. Autism in 2016: the need for answers. J Pediatr (Rio J). 2017;93(2):111–9. Epub 2016/11/13. doi: 10.1016/j.jped.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Data & Statistics on Autism Spectrum Disorder National Center on Birth Defects and Developmental Disabilities: Center for Disease Control and Prevention; [cited 2019 July 16]. [Google Scholar]
- 6.Xu G, Snetselaar LG, Jing J, Liu B, Strathearn L, Bao W. Association of Food Allergy and Other Allergic Conditions With Autism Spectrum Disorder in Children. JAMA Netw Open. 2018;1(2):e180279 Epub 2019/01/16. doi: 10.1001/jamanetworkopen.2018.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyall K, Van de Water J, Ashwood P, Hertz-Picciotto I. Asthma and Allergies in Children With Autism Spectrum Disorders: Results From the CHARGE Study. Autism Res. 2015;8(5):567–74. Epub 2015/02/28. doi: 10.1002/aur.1471.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyazaki C Allergies in Children with Autism Spectrum Disorder: a Systematic Review and Meta-analysis. Review Journal of Autism and Developmental Disorders. 2015;2(4):374–401. doi: 10.1007. [Google Scholar]
- 9.Thomsen SF. Epidemiology and natural history of atopic diseases. Eur Clin Respir J. 2015;2 Epub 2015/11/12. doi: 10.3402/ecrj.v2.24642.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardy S, Haisley L, Manning C, Fein D. Can Screening with the Ages and Stages Questionnaire Detect Autism? J Dev Behav Pediatr. 2015;36(7):536–43. Epub 2015/09/09. doi: 10.1097/DBP.0000000000000201.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly RS, Boulin A, Laranjo N, Lee-Sarwar K, Chu SH, Yadama AP, et al. Metabolomics and Communication Skills Development in Children; Evidence from the Ages and Stages Questionnaire. Metabolites. 2019;9(3). Epub 2019/03/08. doi: 10.3390/metabo9030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oien RA, Schjolberg S, Volkmar FR, Shic F, Cicchetti DV, Nordahl-Hansen A, et al. Clinical Features of Children With Autism Who Passed 18-Month Screening. Pediatrics. 2018;141(6). Epub 2018/05/23. doi: 10.1542/peds.2017-3596.. [DOI] [PubMed] [Google Scholar]
- 13.Gostner JM, Becker K, Kofler H, Strasser B, Fuchs D. Tryptophan Metabolism in Allergic Disorders. Int Arch Allergy Immunol. 2016;169(4):203–15. Epub 2016/05/11. doi: 10.1159/000445500.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Litonjua AA, Lange NE, Carey VJ, Brown S, Laranjo N, Harshfield BJ, et al. The Vitamin D Antenatal Asthma Reduction Trial (VDAART): rationale, design, and methods of a randomized, controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children. Contemp Clin Trials. 2014;38(1):37–50. Epub 2014/03/13. doi: 10.1016/j.cct.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O’Connor GT, et al. Effect of Prenatal Supplementation With Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years: The VDAART Randomized Clinical Trial. JAMA. 2016;315(4):362–70. Epub 2016/01/28. doi: 10.1001/jama.2015.18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blighe K, Chawes BL, Kelly RS, Mirzakhani H, McGeachie M, Litonjua AA, et al. Vitamin D prenatal programming of childhood metabolomics profiles at age 3 y. Am J Clin Nutr. 2017;106(4):1092–9. Epub 2017/08/25. doi: 10.3945/ajcn.117.158220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly RS, Sordillo JE, Lasky-Su J, Dahlin A, Perng W, Rifas-Shiman SL, et al. Plasma metabolite profiles in children with current asthma. Clin Exp Allergy. 2018;48(10):1297–304. Epub 2018/05/29. doi: 10.1111/cea.13183.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zerbo O, Leong A, Barcellos L, Bernal P, Fireman B, Croen LA. Immune mediated conditions in autism spectrum disorders. Brain Behav Immun. 2015;46:232–6. Epub 2015/02/15. doi: 10.1016/j.bbi.2015.02.001.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57(1):67–81. Epub 2004/11/17. doi: 10.1002/ana.20315.. [DOI] [PubMed] [Google Scholar]
- 20.Theoharides TC, Tsilioni I, Patel AB, Doyle R. Atopic diseases and inflammation of the brain in the pathogenesis of autism spectrum disorders. Transl Psychiatry. 2016;6(6):e844 Epub 2016/06/29. doi: 10.1038/tp.2016.77.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khakzad MR, Javanbakht M, Soltanifar A, Hojati M, Delgosha M, Meshkat M. The evaluation of food allergy on behavior in autistic children. Rep Biochem Mol Biol. 2012;1(1):37–42. Epub 2012/10/01.. [PMC free article] [PubMed] [Google Scholar]
- 22.Kaluzna-Czaplinska J, Jozwik-Pruska J, Chirumbolo S, Bjorklund G. Tryptophan status in autism spectrum disorder and the influence of supplementation on its level. Metab Brain Dis. 2017;32(5): 1585–93. Epub 2017/06/14. doi: 10.1007/s11011-017-0045-x.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito M, Ogawa K, Takeuchi K, Nakada A, Heishi M, Suto H, et al. Gene expression of enzymes for tryptophan degradation pathway is upregulated in the skin lesions of patients with atopic dermatitis or psoriasis. J Dermatol Sci. 2004;36(3):157–64. Epub 2004/11/16. doi: 10.1016/j.jdermsci.2004.08.012.. [DOI] [PubMed] [Google Scholar]
- 24.Muller CL, Anacker AMJ, Veenstra-VanderWeele J. The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience. 2016;321:24–41. Epub 2015/11/19. doi: 10.1016/j.neuroscience.2015.11.010.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciprandi G, De Amici M, Tosca M, Alesina R, Marseglia G, Fuchs D. Serotonin in allergic rhinitis: a possible role for behavioural symptoms. Iran J Allergy Asthma Immunol. 2011;10(3):183–8. Epub 2011/09/06. doi: 010.03/ijaai.183188.. [PubMed] [Google Scholar]
- 26.Chugani DC, Muzik O, Behen M, Rothermel R, Janisse JJ, Lee J, et al. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann Neurol. 1999;45(3):287–95. Epub 1999/03/11.. [DOI] [PubMed] [Google Scholar]
- 27.Machaffie RA, Menebroker LR, Mahler DJ, Barak AJ. Studies in allergy. II. Serum serotonin levels in nonallergic, pretreatment, and posttreatment allergic human beings and in normal and sensitized guinea pigs. J Allergy. 1960;31:106–10. [DOI] [PubMed] [Google Scholar]
- 28.Hughes HK, Mills Ko E, Rose D, Ashwood P. Immune Dysfunction and Autoimmunity as Pathological Mechanisms in Autism Spectrum Disorders. Front Cell Neurosci. 2018;12:405 Epub 2018/11/30. doi: 10.3389/fncel.2018.00405.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogel Ciernia A, LaSalle J. The landscape of DNA methylation amid a perfect storm of autism aetiologies. Nat Rev Neurosci. 2016;17(7):411–23. Epub 2016/05/07. doi: 10.1038/nrn.2016.41.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, et al. Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol. 2014;10(11):643–60. Epub 2014/10/15. doi: 10.1038/nrneurol.2014.187.. [DOI] [PubMed] [Google Scholar]
- 31.Bin L, Leung DY. Genetic and epigenetic studies of atopic dermatitis. Allergy Asthma Clin Immunol. 2016;12:52 Epub 2016/10/26. doi: 10.1186/sl3223-016-0158-5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohya MN Y, Akasawa A. Is maternal history of asthma, atopic dermatitis, food allergy, or allergic rhinitis the predictor of the same allergic disease of her child? Journal of Allergy and Clinical Immunology. 2005;115(2):S104. [Google Scholar]
- 33.Schwartzer JJ, Careaga M, Chang C, Onore CE, Ashwood P. Allergic fetal priming leads to developmental, behavioral and neurobiological changes in mice. Transi Psychiatry. 2015;5:e543 Epub 2015/04/08. doi: 10.1038/tp.2015.40.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997–2011. NCHS Data Brief. 2013;(121):1–8. [PubMed] [Google Scholar]
- 35.Cannell JJ. Vitamin D and autism, what’s new? Rev Endocr Metab Disord. 2017;18(2): 183–93. Epub 2017/02/22. doi: 10.1007/slll54-017-9409-0.. [DOI] [PubMed] [Google Scholar]
- 36.Mirzakhani HA-GA, Weiss ST, Litonjua AA. Vitamin D and the development of allergic disease: how important is it? Clin Exp Allergy. 2015. doi: 10.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.