Abstract
Background.
Hearing impairment is common at an older age and has considerable social, health and economic implications. With an increase in the ageing population, there is a need to identify modifiable risk factors for hearing impairment. A shared aetiology with cardiovascular disease (CVD) has been advanced as CVD risk factors (e.g. obesity, type 2 diabetes) are associated with a greater risk of hearing impairment. Moreover, low-grade inflammation is implicated in the aetiology of CVD. Accordingly, our aim was to investigate the association between several markers of inflammation - C-reactive protein, fibrinogen and white blood cell count - and hearing impairment.
Methods.
Participants of the English Longitudinal Study of Ageing aged 50 to 93 were included. Inflammatory marker data from both wave 4 (baseline, 2008/09) and wave 6 (2012/13) were averaged to measure systemic inflammation. Hearing acuity was measured with a simple handheld tone-producing device at follow-up (2014/15).
Results.
Among 4879 participants with a median age of 63 years at baseline, 1878 (38.4%) people presented hearing impairment at follow-up. All three biomarkers were positively and linearly associated with hearing impairment independent of age and sex. After further adjustment for covariates, including cardiovascular risk factors (smoking, physical activity, obesity, diabetes, hypertension, cholesterol), memory and depression, only the association with white blood cell count remained significant: odds ratio per log-unit increase; 95% confidence interval =1.46; 1.11, 1.93.
Conclusions.
While white blood cell count was positively associated with hearing impairment in older adults, no relationships were found for two other markers of low-grade inflammation.
Keywords: Inflammation, Hearing impairment, Leukocyte count, C-reactive protein, Fibrinogen, Ageing
Introduction
Hearing impairment is common in older age, with more than half of the European population suffering from substantial hearing loss by age 80 years1. It is an important cause of disability2, with considerable social, economic and health implications3. Hearing loss represents a burden for individuals and health care systems, given that its management is costly, and it impacts on the economy owing to a loss in productivity and unemployment4. Moreover, hearing impairment is associated with dementia5, depression6, lower cognitive function7, quality of life8, and physical functioning9; themselves risk factors for premature mortality10. As age-related hearing loss is currently incurable, prevention is key, and there is a need to identify risk factors that may be subject to intervention.
Known risk factors for age-related hearing impairment include genetic predispositions11 and environmental exposures particularly noise, infections, and ototoxic drugs12, with emerging correlates being lower levels of insulin-like growth factor I13 and early life exposures as proxied by adult physical stature14. A vascular cause has also been hypothesized, linked to changes in the microcirculation of the cochlea and ischemic intracochlear injury15. Observational studies have found associations between cardiovascular disease (CVD) risk factors such as obesity16, diabetes17, smoking18, subclinical atherosclerosis19 and hearing loss. Inflammation is a hallmark of ageing20 and is associated with atherosclerosis21 and cardiovascular outcomes22 as well as a wide range of age-related diseases including altered physical functioning23 and dementia24. Chronic low-grade inflammation is termed “inflammageing”. Animal studies suggest a link between immune function, vascular pathology and hearing loss, by mechanisms involving disrupted vascular integrity in the cochlear stria vascularis and disturbed ion homeostasis of the endolymph25. The relationship between circulating inflammatory markers and hearing has, however, been little studied in ageing humans and results are inconsistent26–29. Three studies26,27,29 report cross-sectional associations between elevated markers of inflammation, namely white blood cell counts (WBCC), neutrophil count, interleukin-6 (IL-6) and C-reactive protein (CRP), and hearing impairment. A longitudinal study28 found no association between CRP, IL-6 and tumour necrosis factor-α (TNF-α) levels from a single time point and incident hearing impairment. However, a 10-year high CRP profile (long-term chronic inflammation) offered some predictive capacity for subsequent hearing impairment, although only in participants younger than 60 years at baseline28. This constituted the basis for a 3-year double blind randomized controlled trial of the effect of aspirin on age-related hearing loss15.
The aim of the present study was to examine the association between several markers of inflammation - CRP, fibrinogen and WBCC - and objectively measured hearing impairment up to 6 years later in a cohort of older adults.
Methods
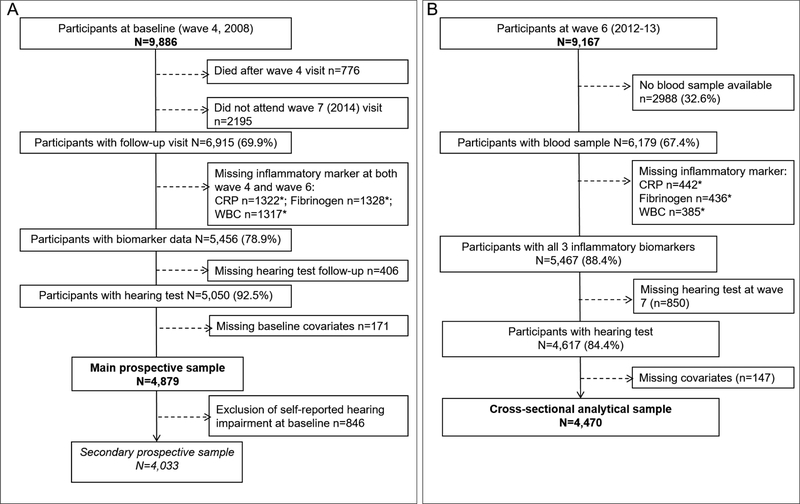
The English Longitudinal Study of Ageing (ELSA) is an on-going, prospective study of a nationally representative sample of men and women living in England aged ≥50 years30 at recruitment in 2002/2003 (wave 1). Data are collected every two years via face-to-face interviews, and nurse visits every 4 years for the assessment of clinical and biomarker data. At most waves, a “refreshment” sample of new younger participants is included to remain representative of the population aged 50 and older. Ethical approval was granted from NHS Research Ethics Committees31, and all participants provided verbal informed consent. For our main prospective analysis, wave 4 (2008/2009) was considered as baseline. We also conducted a cross-sectional analysis at wave 6. The main analytical sample consists of participants aged 50 to 93 years old with complete data on biomarkers at wave 4 or wave 6, covariates at wave 4 and hearing test at wave 7. In a secondary analysis, we excluded participants who self-reported hearing at baseline. The selection of the prospective and cross-sectional analytical samples is presented in Figure 1.
Figure 1.
Flow of subjects into the two analytical samples, prospective (panel A) and cross-sectional (panel B), the English Longitudinal Study of Ageing, 2008
* numbers non-mutually exclusive
The % given in parenthesis is the proportion of participants included at each selection step
Inflammatory biomarkers
At each nurse visit, a blood sample was drawn (available for 65% of the participants at wave 4 and 67% at wave 6). High-sensitivity serum CRP (mg/L) was analysed using the N Latex CRP mono Immunoassay on the Behring Nephelometer II Analyser (Dade Behring, Milton Keynes, UK). We excluded CRP values >20 (n=134 at wave 4 and n=112 at wave 6) because they are likely to reflect acute infection. Fibrinogen (g/L) was analysed on the Organon Teknika MDA 180 coagulation analyser (Organon Teknika, Durham, USA). WBCC was measured on a haematology-automated analyser (Abbott Diagnostics Cell-Dyn 4000 at wave 4 and Sysmex XE at wave 6). All analyses were conducted at the Royal Victoria Infirmary (Newcastle-upon-Tyne, UK) and details on internal quality can be found in the Health Survey for England technical report32 as the methodology is the same as in ELSA. The number of participants with available data for each biomarker and each wave is described in Supplemental Table 1.
Hearing acuity
The only available information on hearing acuity at baseline (wave 4) and at wave 6 was self-reported during the interview. Participants rated their hearing, including using hearing aid if wearing one, on a 5-point scale from ‘poor’ to ‘excellent’. From these responses, we identified participants with self-reported hearing impairment as rating ‘poor’ or ‘fair’. This self-reported measure has previously been shown to be accurate when compared with objectively measured hearing33.
At follow-up (wave 7, 2014/2015), participants took part in an objective hearing test unless they refused, reported use of a cochlear implant, had an ear infection or were unable to do the test (in total, 12% of the sample). The Siemens HearCheck device™34 was used, which has shown good sensitivity (ranging from 78% to 92%) and acceptable to good specificity (ranging from 62 to 95%) to detect hearing loss (at any intensity level) compared to the gold standard measure of hearing performance, pure tone audiometry34,35. HearCheck is a simple handheld appliance which produces three pure tones of mid-frequency (1 kHz), followed by three high-frequency (3 kHz) tones. Participants who wore hearing aids removed them for the test. The interviewer held the device against the participant’s ear (left, then right). Participants had to indicate when they heard a tone when a sound was made at three intensities (55, 35 and 20 dB HL at 1kHz and then 75, 55 and 35 dB HL at 3kHz). The main outcome, hearing impairment (including mild), was defined as hearing fewer than 6 tones in the best hearing ear, i.e. a hearing ‘threshold’ of 20dB HL at 1kHz and 35 dB HL at 3k Hz.
Covariates
Smoking status (current, former, never), alcohol drinking (≥5 days a week vs <5 days a week) and leisure-time physical activity (5 levels from low to high) were self-reported at baseline. Cognitive function (memory) was assessed using a word-list learning test (immediate and delayed), producing a continuous score ranging 0–20 (higher score represents a better memory). Depressive symptoms were ascertained using the eight-item Center for Epidemiologic Studies Depressive CES-D scale, with ‘caseness’ defined as a score ≥436. Educational attainment was classified as low (compulsory schooling), medium (up to high school) and high (university degree or higher). Height and weight were measured directly during the nurse visit, and body mass index (BMI) was defined as the weight (kg) divided by height squared (m2). Cholesterol was assayed using the DAX Oxidase assay. High-density lipoprotein (HDL)-cholesterol analysis was carried out on an Olympus 640analyser using the direct method. Total glycated haemoglobin (HbA1c) assay was measured using the Tosoh G7 analyser. Diabetes was defined as HbA1c ≥ 6.5% and/or self-reported doctor-diagnosed diabetes. Systolic and diastolic blood pressure (SBP and DBP) were measured with an Omron HEM-907 BP monitor three times in the sitting position after 5-min rest and leaving 1-min between each reading. An average of the second and third BP recordings was used. Hypertension was defined as presenting any of the following: SBP≥140, DBP≥90 mm Hg, or self-reported doctor-diagnosed hypertension.
Statistical analysis
For each of the three markers of inflammation (CRP, fibrinogen, WBCC), we averaged the two measurements of wave 4 and wave 6 to gain precision and better reflect chronic inflammation. Averaging two measurements may limit fluctuations due to measurement error or biologic variability37. If only one measurement was available (33% of the sample), we used the one that was available and only excluded participants who were missing both measurements. We also used this method for covariates BMI and HDL-cholesterol to limit missing values.
Baseline characteristics were compared between cases and non-cases of hearing impairment by means of t-test or chi-square. The distributions of CRP and WBCC were skewed, so these variables were natural log-transformed. Logistic regression models were fit to estimate the relationship (odds ratios [OR] and 95% confidence intervals CI) between inflammatory markers and hearing impairment. We conducted four sets of analysis. In the main longitudinal analysis (Longitudinal 1), the independent variable was long-term biomarker exposure (average wave 4 and wave 6) and the outcome was subsequent objective hearing loss at wave 7, adjusting for baseline (wave 4) self-reporter hearing. In a secondary longitudinal analysis (Longitudinal 2), we excluded participants with self-reported hearing impairment (i.e. rating their hearing as “fair” or “poor”) at baseline. We also conducted two cross-sectional analyses: using only wave 6 inflammatory biomarkers in relation to objective hearing impairment (Cross-sectional 1) and cross-sectional association between wave 6 inflammation markers and self-reported hearing impairment (Cross-sectional 2). In all four designs, various levels of adjustment were used. In models 1, we adjusted the estimates for baseline age, sex and self-reported hearing (for longitudinal 1 and 2 only). Models 2 further included smoking status (current, former, never), BMI (kg/m2), physical activity (5 levels), education (3 levels), memory (continuous score) and depression. Models 3 further included clinical cardiovascular risk factors that may be mediators: HDL-cholesterol, hypertension and diabetes. In cross-sectional analysis 1, we also included self-reported hearing at wave 6 in Model 4. Because of the strong association between smoking and both inflammatory markers (exposure)38 and hearing impairment (outcome)18,39, residual confounding may still occur when adjusting for smoking status and stratification can help better disentangle the association. Interactions with smoking were modelled by the cross-product terms. Potential nonlinear relations were examined using restricted cubic spline transformations. All analyses were performed using Stata 14 (StataCorp, TX, USA).
Results
Among 4879 participants with a median age of 63 years (interquartile range 58–70), 1878 (38.4%) had a hearing impairment at follow-up (2014). Compared to those without hearing impairment, those with the condition were older, more likely to be men, have received basic education, live alone, be sedentary, have hypertension and diabetes, have higher BMI, lower HDL-cholesterol, and lower memory score (Table 1). They were also more likely to have higher levels of CRP, fibrinogen and WBCC. Compared to the participants included in this analysis (Supplemental Table 2), the ones who were not included were older, had poorer reported hearing, lower memory score, education and physical activity level, were more likely to smoke and to live alone. The average of two measurements was available for two-thirds of the sample for all three biomarkers, and the remaining third had either a measurement at wave 4 or wave 6 only (Supplemental Table 1).
Table 1.
Baseline (2008) characteristics according to hearing impairment in 2014: the English Longitudinal Study of Ageing
| Overall | No hearing impairment | Hearing impairment | P-valuea | |
|---|---|---|---|---|
| N=4879 | N=3001 | N=1878 | ||
| Age (years) | 64.1 (8.02) | 61.6 (6.65) | 68.2 (8.34) | <0.001 |
| Female n(%) | 2670 (54.7%) | 1731 (57.7%) | 939 (50.0%) | <0.001 |
| Education | <0.001 | |||
| Degree | 929 (19.0%) | 682 (22.7%) | 247 (13.2%) | |
| Intermediate | 2861 (58.6%) | 1790 (59.6%) | 1071 (57.0%) | |
| No qualifications | 1089 (22.3%) | 529 (17.6%) | 560 (29.8%) | |
| Living with partner | 3564 (73.0%) | 2290 (76.3%) | 1274 (67.8%) | <0.001 |
| Depressive symptoms (CES-D≥4) | 566 (11.6%) | 325 (10.8%) | 241 (12.8%) | 0.038 |
| Memory score (0–20) | 11.1 (3.23) | 11.7 (3.06) | 10.2 (3.26) | <0.001 |
| Drinks alcohol daily | 1029 (23.2%) | 653 (23.7%) | 376 (22.4%) | 0.32 |
| Smoking status | 0.054 | |||
| Never smoker | 1983 (40.6%) | 1259 (42.0%) | 724 (38.6%) | |
| Ex-smoker | 2307 (47.3%) | 1382 (46.1%) | 925 (49.3%) | |
| Current smoker | 589 (12.1%) | 360 (12.0%) | 229 (12.2%) | |
| Physical activity | <0.001 | |||
| Sedentary | 518 (10.6%) | 223 (7.43%) | 295 (15.7%) | |
| Moderately inactive | 639 (13.1%) | 370 (12.3%) | 269 (14.3%) | |
| Moderately active | 1504 (30.8%) | 926 (30.9%) | 578 (30.8%) | |
| Active | 1060 (21.7%) | 675 (22.5%) | 385 (20.5%) | |
| Very active | 1158 (23.7%) | 807 (26.9%) | 351 (18.7%) | |
| Self-reported hearing | <0.001 | |||
| Excellent | 994 (20.4%) | 798 (26.6%) | 196 (10.4%) | |
| Very good | 1406 (28.8%) | 1010 (33.7%) | 396 (21.1%) | |
| Good | 1633 (33.5%) | 943 (31.4%) | 690 (36.7%) | |
| Fair | 700 (14.3%) | 230 (7.66%) | 470 (25.0%) | |
| Poor | 146 (2.99%) | 20 (0.67%) | 126 (6.71%) | |
| High blood pressure | 2394 (49.1%) | 1325 (44.2%) | 1069 (56.9%) | <0.001 |
| Diabetes | 513 (10.5%) | 272 (9.06%) | 241 (12.8%) | <0.001 |
| BMI (kg/m2) | 28.2 (5.02) | 28.0 (5.00) | 28.5 (5.03) | <0.001 |
| HDL-cholesterol (mmol/L) | 1.62 (0.43) | 1.64 (0.45) | 1.57 (0.41) | <0.001 |
| CRP (mg/L)b | 1.64 (1.60; 1.68) | 1.53 (1.48; 1.58) | 1.83 (1.76; 1.91) | <0.001c |
| Fibrinogen (g/L) | 3.15 (0.5) | 3.11 (0.49) | 3.21 (0.51) | <0.001 |
| WBCC (109 cell/L)b | 6.11 (6.06; 6.15) | 5.98 (5.93; 6.04) | 6.31 (6.23; 6.38) | <0.001c |
Values are mean (SD) or n (%) as appropriate
p-value of the chi-square test (categorical variables) or t-test (continuous variables) of the difference between the cases and non-cases of hearing impairment at the end of follow-up
geometric mean and 95% confidence interval
t-test performed on natural log-transformed values
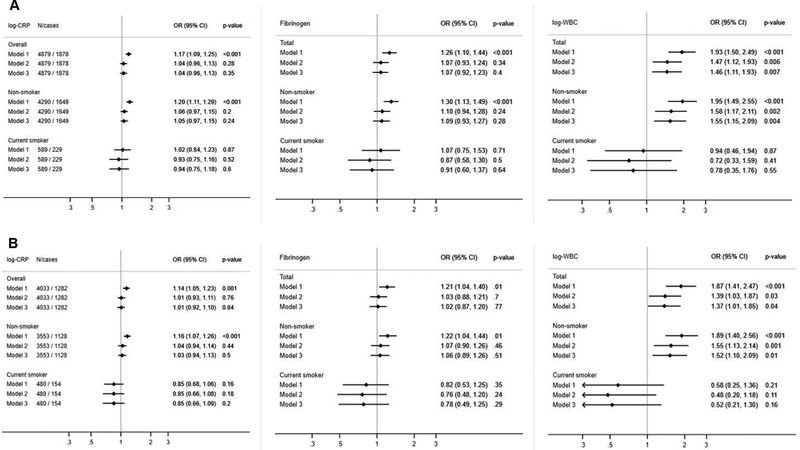
In models 1 (age and sex), all three biomarkers were positively and linearly associated with odds of hearing impairment: CRP OR 1 log-unit increase = 1.17; 95% CI: 1.09, 1.25; fibrinogen OR 1g/L increase = 1.26; 1.10, 1.44; WBCC, OR 1 log-unit increase = 1.93; 1.50, 2.49 (Figure 2A). The relation was linear for all three biomarkers, as the cubic splines did not improve the model fit. In Model 2 (adding smoking, BMI, physical activity, memory, depressive symptoms, and education), the association with CRP (OR: 1.04; 0.96, 1.13) and fibrinogen (1.07; 0.93, 1.24) became non-significant, whereas the association with WBCC remained (OR Model 2: 1.47; 1.12, 1.93). Further addition of CVD risk factors in the model did not attenuate this association (OR Model 3: 1.46; 1.11, 1.93). BMI and smoking were positively associated with hearing impairment, and largely explained the association of fibrinogen and CRP with hearing impairment as the estimates were attenuated and became non-significant after inclusion of these two factors in the models. Memory displayed a strong inverse association with hearing loss.
Figure 2.
Prospective associations between three inflammatory biomarkers (2008–2012) and odds of hearing impairment (2014), in the main analytical sample (panel A) and after exclusion of self-reported hearing impairment at baseline (panel B) the English Longitudinal Study of Ageing
Estimates are multivariate odds ratios (OR) and 95% confidence interval (95% CI)
Model 1 includes baseline age, sex and self-reported hearing at baseline
Model 2: Model 1 + smoking, physical activity, BMI, memory score, education, depression
Model 3: Model 2 + cardiovascular risk factors: diabetes, hypertension, HDL-cholesterol
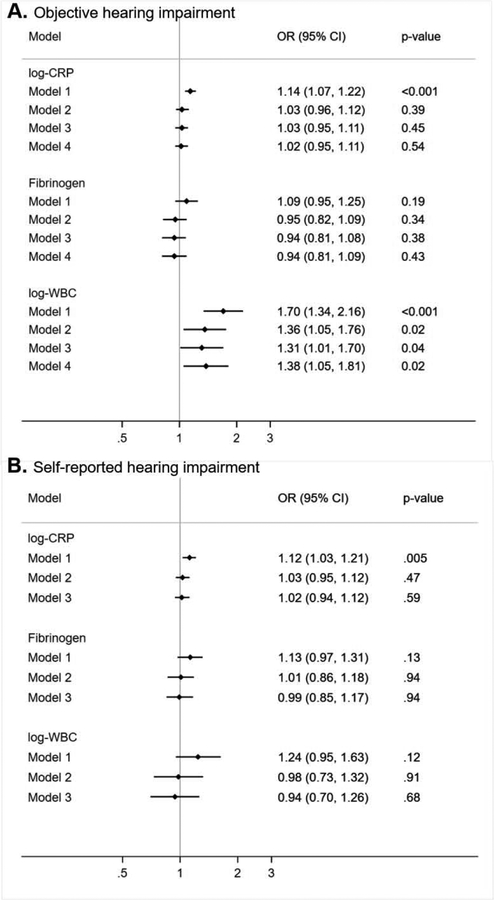
There was a suggested interaction between smoking status and WBCC (p-interaction =0.07) and with CRP (p=0.12). The associations with hearing impairment were positive in the non-smoker group (OR Model 1: 1.20; 1.11, 1.29 for CRP, 1.30; 1.13, 1.49 for fibrinogen and 1.95; 1.49, 2.55 for WBCC), whereas there was essentially no association in the smoker group (Figure 2A). The main findings were replicated when we excluded participants with self-reported hearing impairment at baseline (longitudinal analysis 2, Figure 2B). Results from cross-sectional analysis 1 of wave 6 biomarkers with objective hearing impairment are also very similar (Figure 3 A), even when adjusting for self-reported hearing (Model 4), whereas associations with self-reported hearing impairment (cross-sectional analysis 2) were all non-significant (Figure 3 B).
Figure 3.
Cross-sectional associations between inflammatory biomarkers and odds of hearing impairment (panel A) objectively measured (1587 cases) and (panel B) self-reported (875 cases), the English Longitudinal Study of Ageing, n=4470
Estimates are multivariate odds ratios (OR) and 95% confidence interval (95% CI)
Model 1 includes age and sex
Model 2: Model 1 + smoking, physical activity, BMI, memory score, education, depression
Model 3: Model 2 + cardiovascular risk factors: diabetes, hypertension, HDL-cholesterol
Model 4: Model 3 + self-reported hearing
Discussion
In a cohort study of English older adults, we found that three inflammatory markers investigated – CRP, fibrinogen and WBCC - were associated with age-related hearing loss in age and sex-adjusted models. The associations with CRP and fibrinogen were largely explained by BMI and smoking, whereas the association with WBCC was independent of these factors.
Only a few studies have examined the relationship between inflammation and hearing loss in older adults. Verschuur and colleagues found cross-sectional associations between raised WBCC and worse hearing in two UK samples, a national study of hearing26 and the Hertfordshire Ageing Study (HAS)27, where WBCC was the biomarker most strongly associated with hearing impairment compared to CRP and IL-6. Despite methodological differences due to the use of a simple hearing test device in our study as opposed to pure tone audiometry in the other studies, our study corroborates the cross-sectional association and extends those findings showing a prospective association between raised WBCC, measured on two occasions, and higher odds of hearing impairment 6 years later. Moreover, this association was apparent only in non-smokers, so the observed estimates are independent of smoking, which is a strong correlate of WBCC. A possible explanation is that, although generating an overall pro-inflammatory state, smoking has ambivalent effects on macrophage and can promote anti-inflammatory macrophage polarisation40. Therefore, smoking could selectively inhibit the effects of inflammation (high circulating levels of white blood cells) on hearing impairment, explaining the absence of a relationship between inflammation and hearing loss in smokers.
CRP showed a strong cross-sectional association with hearing impairment in participants aged 40–69 years of the National Health and Nutrition Examination Survey (NHANES) in the US29, as well as with hearing threshold in the HAS but not with maximum audiogram slope27. The only longitudinal analysis conducted in 1073 participants from the Epidemiology of Hearing Loss Study (EHLS)28 found no association when CRP or cytokines were measured once at baseline. However, it was found that long-term (10 years) high levels of CRP were associated with the incidence of hearing impairment independent of obesity, smoking and alcohol use but only in people aged < 60y. In our study smoking and BMI appeared to explain most of the association between medium-term high CRP and hearing loss. Comparability between the two studies is limited by the difference in assessment (pure tone air- and bone-conduction audiometry at 7 frequencies in EHLS, a simple handheld device in ELSA) and by the longer time of follow-up in EHLS (20 years).
Reducing fibrinogen levels in the circulation has been shown to be a promising therapy for idiopathic sudden sensorineural hearing loss, as fibrinogen increases blood viscosity and can reduce the blood flow into the cochlea41. However, the relationship between fibrinogen and age-related hearing loss has not been previously investigated. The association observed in our study was largely explained by lifestyle and CVD risk factors.
The identified factors involved in the pathology of age-related hearing loss include damage and loss of cochlear hair cells, damage of neural elements (loss of auditory nerve function, loss of spiral ganglion neurons in the cochlea) and biochemical damage to the inner ear (degeneration of the stria vascularis)12,42. However, not all older people are affected by hearing loss and multiple factors can contribute to the deterioration of hearing, including vascular damage27. An unhealthy lifestyle, combined with low-grade chronic inflammation that accompanies ageing, can influence hearing via an effect on cardiovascular disease reducing the blood supply in the cochlea or via direct inflammatory damage on the cochlea and on the spiral ganglion cells27. Our study supports both these mechanisms, given that inflammation and several CVD risk factors such as smoking, physical inactivity and obesity were predictive of an increased risk of hearing impairment. Inflammageing-related diseases such as obesity, hypertension and type 2 diabetes have been linked to poorer hearing in previous studies43. Moreover, our results and a large body of studies, including animal models, make a direct inflammatory role plausible in the aetiology of age-related hearing loss. First, inner ear disorders have been observed in autoimmune diseases such as lupus erythematosus or polyarthritis. Moreover, the recommended treatment of sudden sensorineural hearing loss are anti-inflammatory corticosteroids, and their effectiveness has been shown44. The network of tight junctions between the stria vascularis cells in the cochlear lateral wall prevents blood flow into the intrastrial region, known as the “fluid-blood” barrier. However, it is now established that this barrier is permeable to immune response signalling and that stria vascularis cells release pro-inflammatory cytokines through the tight junction barrier43, and that there can be inflammation in the cochlea. Mouse models suggest an important part of the cochlear response to age-related chronic hair cell degeneration is an immune response mediated by macrophages45. The only marker of inflammation associated with hearing loss in our study was white blood cells, and this may be related to the important role of macrophages, a type of white blood cell. The other two markers studied here, CRP and fibrinogen, are downstream in the inflammation response and have been found not to be causally related to cardiovascular disease outcomes46,47. Therefore we hypothesise that circulating cytokines such as IL-6 or TNF-α, that govern the inflammatory cascades upstream47 may be better predictors of hearing impairment.
The main strengths of this study are the well-characterised large nationally representative sample, followed up for 6 years and the inflammatory biomarkers measured at two-time points to increase accuracy and reflect chronic inflammation over 4 years. It is the first study to assess three inflammatory biomarkers prospectively in relation to hearing in such a large sample. However, the main limitation of our study is the assessment of hearing function. First, hearing at baseline was self-reported, which is known to differ from audiometric hearing loss as people can deny their hearing loss48; therefore participants with existing hearing problems at baseline are likely to have been included in the analysis. Moreover, compared to pure tone audiometry, the hearing test used has shown acceptable sensitivity and sensibility to detect mild to severe hearing loss34,35 but does not cover the same frequency range (the hearing test provides only two frequencies, 1kHz and 3 kHz at three intensities), which limits the comparison with existing studies and the interpretation of the findings to a certain extent. Pure tone audiometry is considered the gold standard to assess hearing sensitivity, measuring both air (at frequencies from 0.125kHz to 8 kHz) and bone (from 0.25 to 4 kHz) conduction hearing thresholds. The shape of the resulting audiogram informs on the disease such as conductive hearing loss (external and middle ear damage), or sensorineural hearing loss, typically at high frequency for age-related or noise-induced hearing loss (related to hair cell damage in the cochlea). However, even pure tone audiometry has limitations: it requires active cooperation from the patient, which can be problematic in elderly participants; moreover, it does not provide information about central auditory processing, nor the auditory processing of real-world signals such as conversations or music49. Despite the limitation inherent to the HearCheck method, this hearing test has been used to assess hearing loss in the Health Survey for England50, to provide the first up-to-date estimates of hearing impairment in the UK. In addition, the fact that the baseline and follow-up assessment of hearing performance are different is a methodological caveat as it does not allow us to assess incidence (new cases) of hearing loss. Another crucial information that was not available in our study is noise exposure. Finally, we had to exclude an important part of the sample who had missing data, and excluded participants were older and less healthy; therefore selection bias may have incurred, possibly leading to collider bias and to a more homogeneous population, thus distorting the association. However, despite the limitations presented, we observed enough variability in all our measurements, and believe that the generalisability of the associations should not be compromised. Moreover, we observed a strong association between WBCC and objective hearing impairment using different modelling and design approaches, which gives confidence in the robustness of our results.
In a cohort of English older adults, chronic inflammation measured by elevated WBCC, but not CRP or fibrinogen, was associated with hearing impairment. If results are replicated, monitoring and reducing the levels of inflammation alongside other cardiovascular risk factors has the potential to reduce the burden of age-related hearing loss.
Identifying modifying risk factors for hearing impairment is necessary, given that this condition is a major cause of disability and is incurable. Chronic elevated inflammation is a hallmark of ageing but the association of inflammation with hearing loss has been very little studied
In a population of over 4000 English older adults, inflammation measured by C-reactive protein, fibrinogen and white blood cell count were associated with higher risk of hearing impairment
The associations with CRP and fibrinogen were largely explained by body mass index and smoking but elevated white blood cell count remained associated with higher risk of hearing impairment independently of cardiovascular risk factors.
Systemic inflammation measured by white blood cell count appears to offer some predictive capacity for hearing impairment in older adults.
Funding:
The English Longitudinal Study of Ageing is supported by the National Institute on Aging (grant numbers: 2RO1AG7644 and 2RO1AG017644-01A1) and a consortium of the UK government departments coordinated by the Economic and Social Research Council. The funding bodies had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data sharing: ELSA data are open access at https://www.ukdataservice.ac.uk/ and Stata codes for the analyses are available from authors upon request
Conflicts of interest: The authors declare no competing financial interests
References
- 1.Roth TN, Hanebuth D, Probst R. Prevalence of age-related hearing loss in Europe: a review. Eur Arch Otorhinolaryngol 2011;268(8):1101–07. doi: 10.1007/s00405-011-1597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salomon JA, Haagsma JA, Davis A, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health 2015;3(11):e712–e23. doi: S2214-109X(15)00069-8 [pii];10.1016/S2214-109X(15)00069-8 [doi] [DOI] [PubMed] [Google Scholar]
- 3.Mohr PE, Feldman JJ, Dunbar JL, et al. The societal costs of severe to profound hearing loss in the United States. Int J Technol Assess Health Care 2000;16(4):1120–35. [DOI] [PubMed] [Google Scholar]
- 4.England NHS. Action Plan on Hearing Loss. London, 2015. [Google Scholar]
- 5.Lin FR, Metter EJ, O’Brien RJ, et al. Hearing loss and incident dementia. Arch Neurol 2011;68(2):214–20. doi: 68/2/214 [pii];10.1001/archneurol.2010.362 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amieva H, Ouvrard C, Meillon C, et al. Death, Depression, Disability and Dementia Associated with Self-Reported Hearing Problems: a 25-Year Study. J Gerontol A Biol Sci Med Sci 2018. doi: 10.1093/gerona/glx250 [DOI] [PubMed] [Google Scholar]
- 7.Yuan J, Sun Y, Sang S, et al. The risk of cognitive impairment associated with hearing function in older adults: a pooled analysis of data from eleven studies. Sci Rep 2018;8(1):2137. doi: 10.1038/s41598-018-20496-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nordvik O, Laugen Heggdal PO, Brannstrom J, et al. Generic quality of life in persons with hearing loss: a systematic literature review. BMC Ear Nose Throat Disord 2018;18:1. doi: 10.1186/s12901-018-0051-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liljas AE, Wannamethee SG, Whincup PH, et al. Hearing impairment and incident disability and all-cause mortality in older British community-dwelling men. Age Ageing 2016;45(5):662–67. doi: afw080 [pii];10.1093/ageing/afw080 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celis-Morales CA, Welsh P, Lyall DM, et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ 2018;361:k1651. doi: 10.1136/bmj.k1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann TJ, Keats BJ, Yoshikawa N, et al. A Large Genome-Wide Association Study of Age-Related Hearing Impairment Using Electronic Health Records. PLoS Genet 2016;12(10):e1006371. doi: 10.1371/journal.pgen.1006371 [published Online First: 2016/10/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidd III AR, Bao J. Recent advances in the study of age-related hearing loss: a mini-review. Gerontology 2012;58(6):490–6. doi: 10.1159/000338588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lassale C, Batty GD, Steptoe A, et al. Insulin-like Growth Factor 1 in relation to future hearing impairment: findings from the English Longitudinal Study of Ageing. Sci Rep 2017;7(1):4212. doi: 10.1038/s41598-017-04526-7 [published Online First: 2017/06/25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batty GD, Zaninotto P, Steptoe A, et al. Early Life Origins of Hearing Impairment in Older People. Epidemiology 2017;28(4):e34–e35. doi: 10.1097/EDE.0000000000000682 [published Online First: 2017/05/11] [DOI] [PubMed] [Google Scholar]
- 15.Lowthian JA, Britt CJ, Rance G, et al. Slowing the progression of age-related hearing loss: Rationale and study design of the ASPIRIN in HEARING, retinal vessels imaging and neurocognition in older generations (ASPREE-HEARING) trial. Contemp Clin Trials 2016;46:60–66. doi: 10.1016/j.cct.2015.11.014 [published Online First: 2015/11/28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engdahl B, Aarhus L, Lie A, et al. Cardiovascular risk factors and hearing loss: The HUNT study. Int J Audiol 2015;54(12):958–66. doi: 10.3109/14992027.2015.1090631 [doi] [DOI] [PubMed] [Google Scholar]
- 17.Horikawa C, Kodama S, Tanaka S, et al. Diabetes and risk of hearing impairment in adults: a meta-analysis. J Clin Endocrinol Metab 2013;98(1):51–58. doi: jc.2012-2119 [pii];10.1210/jc.2012-2119 [doi] [DOI] [PubMed] [Google Scholar]
- 18.Cruickshanks KJ, Nondahl DM, Dalton DS, et al. Smoking, central adiposity, and poor glycemic control increase risk of hearing impairment. J Am Geriatr Soc 2015;63(5):918–24. doi: 10.1111/jgs.13401 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer ME, Schubert CR, Nondahl DM, et al. Subclinical atherosclerosis and increased risk of hearing impairment. Atherosclerosis 2015;238(2):344–49. doi: S0021-9150(14)01653-0 [pii];10.1016/j.atherosclerosis.2014.12.031 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Otin C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell 2013;153(6):1194–217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Libby P. Inflammation in atherosclerosis. Nature 2002;420(6917):868–74. doi: 10.1038/nature01323 [DOI] [PubMed] [Google Scholar]
- 22.Emerging Risk Factors C, Kaptoge S, Di Angelantonio E, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010;375(9709):132–40. doi: 10.1016/S0140-6736(09)61717-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lassale C, Batty GD, Steptoe A, et al. Association of 10-year C-reactive protein trajectories with markers of healthy aging: findings from the English Longitudinal Study of Ageing. J Gerontol A Biol Sci Med Sci 2018. doi: 10.1093/gerona/gly028 [published Online First: 2018/02/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darweesh SKL, Wolters FJ, Ikram MA, et al. Inflammatory markers and the risk of dementia and Alzheimer’s disease: A meta-analysis. Alzheimers Dement 2018;14(11):1450–59. doi: 10.1016/j.jalz.2018.02.014 [published Online First: 2018/04/02] [DOI] [PubMed] [Google Scholar]
- 25.Shi X. Pathophysiology of the cochlear intrastrial fluid-blood barrier (review). Hear Res 2016;338:52–63. doi: 10.1016/j.heares.2016.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verschuur C, Agyemang-Prempeh A, Newman TA. Inflammation is associated with a worsening of presbycusis: evidence from the MRC national study of hearing. Int J Audiol 2014;53(7):469–75. doi: 10.3109/14992027.2014.891057 [published Online First: 2014/04/01] [DOI] [PubMed] [Google Scholar]
- 27.Verschuur CA, Dowell A, Syddall HE, et al. Markers of inflammatory status are associated with hearing threshold in older people: findings from the Hertfordshire Ageing Study. Age Ageing 2012;41(1):92–7. doi: 10.1093/ageing/afr140 [published Online First: 2011/11/17] [DOI] [PubMed] [Google Scholar]
- 28.Nash SD, Cruickshanks KJ, Zhan W, et al. Long-term assessment of systemic inflammation and the cumulative incidence of age-related hearing impairment in the epidemiology of hearing loss study. J Gerontol A Biol Sci Med Sci 2014;69(2):207–14. doi: glt075 [pii];10.1093/gerona/glt075 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bainbridge KE, Cheng YJ, Cowie CC. Potential mediators of diabetes-related hearing impairment in the U.S. population: National Health and Nutrition Examination Survey 1999–2004. Diabetes Care 2010;33(4):811–6. doi: 10.2337/dc09-1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steptoe A, Breeze E, Banks J, et al. Cohort profile: the English longitudinal study of ageing. Int J Epidemiol 2013;42(6):1640–48. doi: dys168 [pii];10.1093/ije/dys168 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NatCen Social Research. English Longitudinal Study of Ageing (ELSA): Wave One to Wave Seven - User Guide to the datasets 2016 [updated 2017. Available from: http://doc.ukdataservice.ac.uk/doc/5050/mrdoc/pdf/5050_elsa_user_guide_waves_1-7.pdf.
- 32.Craig RD C; Pickering K. Quality control of blood, saliva and urine analytes In: Spronston KMJ, ed. Health Survey for England 2004, Methodology and Documentation, 2004. [Google Scholar]
- 33.Ferrite S, Santana VS, Marshall SW. Validity of self-reported hearing loss in adults: performance of three single questions. Revista de saude publica 2011;45(5):824–30. [published Online First: 2011/08/03] [DOI] [PubMed] [Google Scholar]
- 34.Parving A, Sorup Sorensen M, Christensen B, et al. Evaluation of a hearing screener. Audiological Medicine 2008;6(2):115–19. [Google Scholar]
- 35.Fellizar-Lopez KR, Abes GT, Reyes-Quintos MR, et al. Accuracy of Siemens HearCheck Navigator as a screening tool for hearing loss. Philipp J Otolaryngol Head Neck Surg 2011;26(1):10–15. [Google Scholar]
- 36.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 37.Clarke R, Shipley M, Lewington S, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol 1999;150(4):341–53. [DOI] [PubMed] [Google Scholar]
- 38.Bakhru A, Erlinger TP. Smoking cessation and cardiovascular disease risk factors: results from the Third National Health and Nutrition Examination Survey. PLoS Med 2005;2(6):e160. doi: 10.1371/journal.pmed.0020160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang J, Ryou N, Jun HJ, et al. Effect of Cigarette Smoking and Passive Smoking on Hearing Impairment: Data from a Population-Based Study. PLoS One 2016;11(1):e0146608. doi: 10.1371/journal.pone.0146608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang DC, Chen CH. Cigarette Smoking-Mediated Macrophage Reprogramming: Mechanistic Insights and Therapeutic Implications. J Nat Sci 2018;4(11) [published Online First: 2019/02/26] [PMC free article] [PubMed] [Google Scholar]
- 41.Canis M, Heigl F, Suckfuell M. Fibrinogen/LDL apheresis is a promising rescue therapy for sudden sensorineural hearing loss. Clin Res Cardiol Suppl 2012;7:36–40. doi: 10.1007/s11789-012-0044-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gates GA, Mills JH. Presbycusis. Lancet 2005;366(9491):1111–20. doi: S0140-6736(05)67423-5 [pii];10.1016/S0140-6736(05)67423-5 [doi] [DOI] [PubMed] [Google Scholar]
- 43.Watson N, Ding B, Zhu X, et al. Chronic inflammation - inflammaging - in the ageing cochlea: A novel target for future presbycusis therapy. Ageing Res Rev 2017;40:142–48. doi: 10.1016/j.arr.2017.10.002 [published Online First: 2017/10/12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trune DR, Kempton JB, Gross ND. Mineralocorticoid receptor mediates glucocorticoid treatment effects in the autoimmune mouse ear. Hear Res 2006;212(1–2):22–32. doi: 10.1016/j.heares.2005.10.006 [published Online First: 2005/11/26] [DOI] [PubMed] [Google Scholar]
- 45.Kalinec GM, Lomberk G, Urrutia RA, et al. Resolution of Cochlear Inflammation: Novel Target for Preventing or Ameliorating Drug-, Noise- and Age-related Hearing Loss. Front Cell Neurosci 2017;11:192. doi: 10.3389/fncel.2017.00192 [published Online First: 2017/07/25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prins BP, Abbasi A, Wong A, et al. Investigating the Causal Relationship of C-Reactive Protein with 32 Complex Somatic and Psychiatric Outcomes: A Large-Scale Cross-Consortium Mendelian Randomization Study. PLoS Med 2016;13(6):e1001976. doi: 10.1371/journal.pmed.1001976 [published Online First: 2016/06/22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaptoge S, Seshasai SR, Gao P, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J 2014;35(9):578–89. doi: 10.1093/eurheartj/eht367 [published Online First: 2013/09/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi JS, Betz J, Deal J, et al. A Comparison of Self-Report and Audiometric Measures of Hearing and Their Associations With Functional Outcomes in Older Adults. J Aging Health 2016;28(5):890–910. doi: 10.1177/0898264315614006 [published Online First: 2015/11/11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Musiek FE, Shinn J, Chermak GD, et al. Perspectives on the Pure-Tone Audiogram. J Am Acad Audiol 2017;28(7):655–71. doi: 10.3766/jaaa.16061 [published Online First: 2017/07/20] [DOI] [PubMed] [Google Scholar]
- 50.Scholes S, Biddulph J, Davis A, et al. Socioeconomic differences in hearing among middle-aged and older adults: cross-sectional analyses using the Health Survey for England. BMJ Open 2018;8(2):e019615. doi: 10.1136/bmjopen-2017-019615 [published Online First: 2018/02/03] [DOI] [PMC free article] [PubMed] [Google Scholar]