Abstract
BACKGROUND:
The incidence of nasopharyngeal carcinoma (NPC) has been historically low in the United States. Although etiological factors differ by histological subtype, Epstein-Barr virus is accepted as the primary risk factor for nonkeratinizing NPC. In light of the changing epidemiology of viral-associated cancers, it is important to evaluate the temporal incidence of NPC in the United States.
METHODS:
Incidence and survival data from 1973 through 2015 were obtained from the Surveillance, Epidemiology, and End Results program. Stratified analyses were conducted to assess temporal trends in NPC by histological subtype, sex, and race. The data were analyzed using SAS and Joinpoint Regression Software to determine age-adjusted incidence rates, determine trends in the annual percent change, and calculate 5-year relative survival estimates and Kaplan-Meier curves.
RESULTS:
Although overall NPC incidence is decreasing in the United States, the nonkeratinizing differentiated subtype is starkly increasing, with an annual percent change of approximately 4% among white males (95% CI, 2.5%–5.2%), white females (95% CI, 1.9%–6.2%), and black males (95% CI, 2.0%, 5.7%); 2.7% among black females (95% CI, 0.8%, 4.6%); and 1.8% among women in the “other” race category (95% CI, 0.4%–3.3%). Racial disparities were noted, with 32% of nonkeratinizing NPC cases among blacks occurring before the age of 40 years. In addition, black males displayed consistently worse survival across all histological subtypes, whereas individuals in the “other” race category, particularly females, experienced the highest 5-year relative survival estimates.
CONCLUSIONS:
The current results indicate that the Epstein-Barr virus-related, differentiated NPC subtype is increasing across all sexes and races in the United States, with distinct incidence and survival disparities among blacks
Keywords: disparity; Epstein-Barr virus; incidence; nasopharyngeal carcinoma; Surveillance, Epidemiology, and End Results (SEER); survival
INTRODUCTION
Nasopharyngeal carcinoma (NPC) incidence varies greatly throughout the world. Although historically rare in the United States, NPC is endemic to southeast China, Southeast Asia, the Middle East, and North Africa.1 Arising from the surface epithelium of the posterior nasopharynx,2 NPC is currently classified by the World Health Organization (WHO) into 3 major histological subtypes: keratinizing squamous cell carcinoma (WHO type 1), nonkeratinizing carcinoma (WHO type 2), which can further be divided into differentiated and undifferentiated, and basaloid squamous cell carcinoma (WHO type 3).3 These pathological classifications have been repeatedly correlated with clinicopathologic features, treatment response, and prognosis,4,5 Basaloid squamous cell carcinoma is infrequent and thus is rarely reported in the literature.6 Nonkeratinizing NPC predominates in endemic regions, whereas the keratinizing subtype is most frequently observed in nonendemic regions, such as the United States.7 The unique geographic and ethnic distribution of NPC incidence throughout the world suggests a multifactorial etiology that involves viral, genetic, and environmental components.
NPC incidence has been reported to rise monotonically with age in nonendemic countries; whereas, in high-risk populations, such as southern China, incidence peaks between ages 45 and 54 years among both sexes, suggesting that early exposure to carcinogens and Epstein-Barr virus (EBV) may result in younger onset NPC.8 EBV, a herpesvirus most commonly known for causing mononucleosis, is generally accepted as the primary etiologic factor in nonkeratinizing NPC. Increased latent EBV infection has been reported in dysplastic nasopharyngeal epithelium and NPC, suggesting that the infection occurs in the early stages of carcinogenesis.9–12 Because of the ubiquitous nature of infection and the disproportionate global case distribution of NPC, several studies have assessed interactions between EBV and other lifestyle risk factors in high-risk populations. Dietary factors, namely, early life exposure to Cantonese salted dried fish and other preserved foods containing volatile N-nitrosamines, have been implicated in NPC carcinogenesis both through the activation of latent EBV13 and as independent carcinogens.14
In nonendemic regions like the United States, cigarette smoking and heavy alcohol consumption have been associated with keratinizing squamous cell carcinoma.15 Compared with never-smokers, heavy smokers reportedly have a 2-fold to 4-fold increased risk of NPC.15–22 Several studies have suggested that the effect conferred by smoking and alcohol primarily pertains to those diagnosed when older than 50 years, suggesting a differing etiology among younger patients.15 The increased prevalence of high-risk human papillomavirus (HPV) in oropharyngeal cancer etiology, paired with the similarities exhibited between the lymphoid and epithelium tissue of the oropharynx and nasopharynx, has led to investigations into the role of HPV in nasopharyngeal carcinogenesis. Although the results remain inconsistent, largely because of small sample sizes, there is evidence to suggest the role of HPV, and even HPV/EBV coinfection, in NPC.4,23–26
It is important to evaluate the incidence of NPC in the United States over time in light of the changing epidemiology of HPV-associated oral cancers. Thus, in these analyses, we assess NPC incidence and mortality trends in the United States from 1973 through 2015 using data from the Surveillance, Epidemiology, and End Results (SEER) program. In doing so, we aim to identify potential variation across race and sex and draw on the literature to make inferences on changing etiology over time.
MATERIALS AND METHODS
Data Selection and Criteria
Cancer incidence and survival data were obtained from the SEER program. For this analysis, data from the SEER 9 registries were used, which include data from the following registries: Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah registries. Data from 1973 through 2015 were available for all sites, with the exception of Seattle-Puget Sound and Atlanta, which joined in 1974 and 1975, respectively. Corresponding population estimates were obtained from SEER*Stat 8.3.5 data,27 which are based on the US Census Bureau’s Population Estimates Program.28 In addition to diagnoses, case information included age, sex, race, date of diagnosis, SEER historical staging, histology, cause of death classification, and survival time in months. Because of the small sample size of American Indians/Alaska Natives, this race group was combined with Asians/Pacific Islanders to create the “other” race grouping.
Tumor site and histology were identified according to the International Classification of Diseases for Oncology third edition (ICD-O-3).6,29 Histologic subtypes for NPC (site codes C11.0–C11.9) included: keratinizing squamous cell carcinoma (ICD-O histology codes 8070 and 8071), differentiated nonkeratinizing carcinoma (ICD-O histology codes 8072 and 8073), undifferentiated nonkeratinizing carcinoma (ICD-O histology codes 8020, 8021, and 8082), and “carcinoma not otherwise specified” (ICD-O histology code 8010).5 In accordance with WHO guidelines, differentiated and undifferentiated nonkeratinizing carcinomas were analyzed both separately and jointly to comprise the single nonkeratinizing group. Overall NPC was defined as the culmination of all the aforementioned histological groupings. Basaloid squamous cell carcinomas (ICD-O histology code 8083) were excluded from these analyses because of the small sample size (N = 30). Additional exclusion criteria included unknown race, nonmalignant carcinoma, and prior diagnosis of malignancy. Patient characteristics were assessed by histological subgrouping, and further evaluation of age at diagnosis (in 5 year groupings) was conducted by visually displaying case frequencies by race for overall, keratinizing, and nonkeratinizing NPC.
Sex-stratified exploratory analyses assessing incidence trends in extranodal natural killer (NK)/T-cell lymphoma, nasal type (ICD-O histology code 9719), were conducted for all effective years, from 2001 through 2015, to further evaluate the potential role of EBV in US malignancies.
Incidence Trends
Age-adjusted incidence rates (standardized to the US population) and annual percent change (APC) were assessed using a log-linear model stratified by race, sex, and histological classification in Joinpoint Regression Program version 4.5.0.1.30 Joinpoint uses a Monte Carlo permutation method to assess the number of joinpoints along with the slope in the trends as well as their corresponding significance.31 In circumstances for which no cases were reported within a given year, a half-case was added to the age strata with the largest population to enable computation on the log-linear scale.31,32 SAS 9.4 software27 was used to shape the data and generate descriptive statistics.
Survival Analysis
Individual-level data from the SEER*Stat case listing session was used to create Kaplan-Meier curves in SAS 9.4 software. Graphs were sex-specific, stratified by histologic type, and strata were compared using log-rank tests. Patients whose survival data were obtained from death certificates or autopsy only, as well as those with unknown survival duration, were excluded. In addition, patients with unknown age or those with a previous invasive cancer diagnosis were excluded from survival analyses. Five-year relative survival, calculated as the ratio of the observed survival for patients with NPC to the expected survival rate based on age, race, and calendar year, were computed using the Kaplan-Meier method in SEER*Stat. Expected survival life tables were constructed using the US Decennial Life Tables from the National Center for Health Statistics and from the US Annual Life Tables from the National Center for Health Statistics for the years 2001 through 2014.27
RESULTS
Between 1973 and 2015, 4447 cases of NPC were reported among males, and 1838 were reported among females in the United States. Regardless of histological subtype, males accounted for approximately 70% of the overall cancer burden. In general, the peak incidence occurred at age 55 years for both sexes, with the undifferentiated nonkeratinizing carcinoma subgroup representing the youngest mean age at diagnosis (49.34 and 49.15 years for males and females, respectively). Expository analyses revealed a bimodal distribution among blacks, with 25% of all patients being diagnosed at age <40 years compared with 12% and 21% of their white and other race counterparts, respectively. Although this trend was not apparent for patients with keratinizing NPC, 32% of nonkeratinizing NPCs among blacks, 21% among whites, and 22% among the other race group occurred at age <40 years (see Supporting Fig. 2). The keratinizing squamous cell carcinoma subtype made up the majority of NPC cases, largely because of its predominance among white patients, who accounted for 49% of the overall sample size. Nonkeratinizing NPC was diagnosed more frequently among blacks and predominated among the other race group. Among those with nonkeratinizing NPC, the undifferentiated cell type was more frequently reported across all races. SEER summary stage was available for cases diagnosed before 2004, and most cases were diagnosed as regional disease (Table 1).
TABLE 1.
Nasopharyngeal Carcinoma Distribution, 1973 to 2015, by Sex and Histology
| Variable | No. of Patients (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall NPC | Keratinizing Squamous Cell Carcinoma | Nonkeratinizing Carcinoma | Carcinoma NOS | |||||||
| Differentiated | Undifferentiated | |||||||||
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | |
| Total | 4447 (70.76) | 1838 (29.24) | 1979 (70.30) | 836 (29.70) | 710 (73.35) | 258 (26.65) | 996 (70.49) | 417 (29.51) | 762 (69.97) | 327 (30.03) |
| Age: Mean ± SD, y | 54.55 ± 15.00 | 55.36 ± 16.47 | 57.35 ± 13.65 | 59.24 ± 14.76 | 53.91 ± 13.24 | 54.18 ± 16.30 | 49.34 ± 16.28 | 49.15 ± 16.45 | 54.69 ± 16.22 | 54.31 ± 18.02 |
| Race | ||||||||||
| White | 2175 (48.91) | 893 (48.59) | 1247 (63.01) | 568 (67.94) | 268 (37.75) | 68 (26.36) | 361 (36.24) | 143 (34.29) | 299 (39.24) | 114 (34.86) |
| Black | 426 (9.58) | 163 (8.87) | 209 (10.56) | 69 (8.25) | 60 (8.45) | 28 (10.85) | 85 (8.53) | 41 (9.83) | 72 (9.45) | 25 (7.65) |
| Asian/Pacific Islander | 1813 (40.77) | 761 (41.40) | 510 (25.77) | 194 (23.21) | 377 (53.10) | 156 (60.47) | 543 (54.52) | 227 (54.44) | 383 (50.26) | 184 (56.27) |
| American Indian/Alaska Native | 33 (0.74) | 21 (1.14) | 13 (0.66) | 5 (0.60) | 5 (0.70) | 6 (2.33) | 7 (0.70) | 6 (1.44) | 8 (1.05) | 4 (1.22) |
| Stage | ||||||||||
| Localized | 388 (8.72) | 175 (9.52) | 202 (10.21) | 103 (12.32) | 47 (6.62) | 12 (4.65) | 91 (9.14) | 43 (10.31) | 48 (6.30) | 17 (5.20) |
| Regional | 1786 (40.16) | 759 (41.29) | 848 (42.85) | 366 (43.78) | 235 (33.10) | 90 (34.88) | 441 (44.28) | 180 (43.17) | 262 (34.38) | 123 (37.61) |
| Distant | 493 (11.09) | 190 (10.34) | 281 (14.20) | 102 (12.20) | 54 (7.61) | 10 (3.88) | 100 (10.04) | 52 (12.47) | 58 (7.61) | 26 (7.95) |
| Unstaged | 247 (5.55) | 132 (7.18) | 122 (6.16) | 75 (8.97) | 11 (1.55) | 4 (1.55) | 47 (4.72) | 19 (4.56) | 67 (8.79) | 34 (10.40) |
| Cases diagnosed ≥2004 | 1533 (34.47) | 582 (31.66) | 526 (26.58) | 190 (22.73) | 363 (51.13) | 142 (55.04) | 317 (31.83) | 123 (29.50) | 327 (42.91) | 127 (38.84) |
Abbreviations: NOS, not otherwise specified; NPC, nasopharyngeal carcinoma.
Although incidence rates for the overall population appeared to have remained relatively stagnant among males (APC, −0.2%; 95% CI, −0.4%, 0.1%) and females (APC, −0.4%; 95% CI, −0.8%, 0.0%), these trends varied greatly across both race and histology. At the beginning of the study period, the expected age-standardized rates (EASRs) for white and black males were 0.67 and 0.66 per 100,000, respectively. White and black females both had notably lower rates, with EASRs of 0.34 and 0.16 per 100,000, respectively. The other race category, largely comprised of Asians/Pacific Islanders, exhibited the highest NPC rates, with EASRs of 4.79 and 1.76 per 100,000 among males and females, respectively. Temporal assessment of NPC incidence revealed significant decreases among white males (APC, −1.2%; 95% CI, −1.5%, −0.8%) and females (APC, −1.6%; 95% CI, −2.1%, −1.0%), black females (APC, −1.4%; 95% CI, −2.5%, −0.4%), as well as males (APC, −1.0%; 95% CI, −1.4%, −0.6%) and females (APC, −1.3%; 95% CI, −1.9%, −0.7%) in the other race category (Table 2).
TABLE 2.
Joinpoint Analyses of Nasopharyngeal Carcinoma Trends by Sex and Morphology
| APC (95% CI), % | ||||||||
|---|---|---|---|---|---|---|---|---|
| All | White | Black | Other | |||||
| Male | Female | Male | Female | Male | Female | Male | Female | |
| Overall NPC | −0.2 (−0.4, 0.1) | −0.4 (−0.8, 0.0) | −1.2a (−1.5, −0.8) | −1.6a (−2.1, −1.0) | −0.1 (−1.0, 0.7) | −1.4a (−2.5, −0.4) | −1.0a (−1.4, −0.6) | −1.3a (−1.9, −0.7) |
| Keratinizing | 1.1 (−1.2, 3.5)b; −2.6a (−3.2, −2.1)c | −2.4a (−3.0, −1.7) | −2.1a (−2.6, −1.6) | −2.4a (−3.2, −1.6) | −1.2 (−2.4, 0.0) | −2.3a (−4.1, −0.4) | −3.4a (−4.1, −2.7) | −4.2a (−5.1, −3.2) |
| Nonkeratinizing | 1,0a (0.5–1.5) | 1.2a (0.5–1.8) | 0.5 (−0.2, 1.1) | −0.0 (−1.1, 1.1) | 0.6 (−0.8, 2.0) | −0.6 (−2.1,0.9) | −0.8a (−1.5, −0.2) | −0.6 (−1.5, 0.2) |
| Differentiated | 3.0a (2.1–3.9) | 4.4a (3.0–5.9) | 3.8a (2.5–5.2) | 4.0a (1.9–6.2) | 3.9a (2.0–5.7) | 2.7a (0.8–4.6) | −0.1 (−1.3, 1.0) | 1,8a (0.4–3.3) |
| Undifferentiated | −0.5 (−1.1, 0.1) | −0.8a (−1.5, −0.0) | −2.1a (−2.8, −1.3) | −1.8a (−3.0, −0.6) | −1.6 (−3.3, 0.2) | −2.7a (−4.2, −1.1) | −1.5a (−2.2, −0.6) | −2.4a (−3.5, −1.4) |
Abbreviations: APC, annual percent change; NPC, nasopharyngeal carcinoma.
This value indicates statistical significance at α = .05.
These values are for the period from 1973 through 1985.
These values are for the period from 1985 through 2015.
These observed decreases in overall NPC were largely attributable to declines in the predominant keratinizing squamous cell carcinoma subtype. Joinpoint regression results for the overall population exhibited nonsignificant increases in keratinizing carcinoma among men from 1973 through 1985 (APC, 1.1%; 95% CI, −1.2%, 3.5%), followed by a significant decrease from 1985 to 2015 (APC, −2.6%, 95% CI, −3.2%, −2.1%), and similar declines were observed among women (APC, −2.4%; 95% CI, −3.0%, −1.7%). Although this pattern of reduced keratinizing NPC was seen across all races, it was not found to be significant among black males and demonstrated the greatest impact among males (APC, −3.4%; 95% CI, −4.1%, −2.7%) and females (APC, −4.2%; 95% CI, −5.1%, −3.2%) in the other race category (Table 2).
Nonkeratinizing NPC revealed 2 very different patterns in incidence trends, depending on histological subtype. Undifferentiated carcinomas significantly decreased, with an APC of approximately −2% among white males (95% CI, −2.8%, −1.3%) and females (95% CI, −3.0%, −0.6%), −2.7% among black females (95% CI, −4.2%, −1.1%), −1.5% among males in the other race category (95% CI, −2.2%, −0.6%), and −2.4% among women in the other race category (95% CI, −3.5%, −1.4%). The differentiated subtype, conversely, increased starkly in incidence, with an APC of approximately 4% among white males (95% CI, 2.5%–5.2%), white females (95% CI, 1.9% differentiated and undifferentiated led to an attenuation in the overall trends of nonkeratinizing NPC (Table 2).6.2%), and black males (95% CI, 2.0% differentiated and undifferentiated led to an attenuation in the overall trends of nonkeratinizing NPC (Table 2).5.7%); 2.7% among black females (95% CI, 0.8%–4.6%); and 1.8% among women in the other race category (95% CI, 0.4%–3.3%). This dichotomy in incidence between differentiated and undifferentiated led to an attenuation in the overall trends of nonkeratinizing NPC (Table 2).
Exploratory analyses of incidence trends in extranodal NK/T-cell lymphoma, nasal type, revealed trends very similar to those apparent in differentiated nonkeratinizing NPC. Between 2001 and 2015, incidence increased for among both sexes, with an APC of 3.2% (95% CI, 0.7%–5.8%) among males and 3.9% (95% CI, 0.2%–7.8%) among females. Race-stratified analysis demonstrated even steeper increases among white males (APC, 3.8%; 95% CI, 0.6%–7.1%) and females (APC, 4.1%; 95% CI, 0.7%–7.6%). No significant increases were noted for the other race groups, likely because of small sample sizes within these groups.
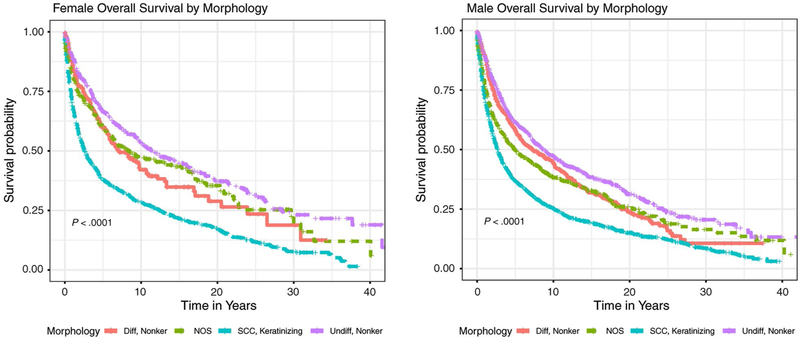
Five-year relative survival estimated by race, sex, and histological subtype can be found in Table 3. For all NPC types, 5-year relative survival was 53.0% among males and 55.8% among females. Figure 1 displays sex-stratified Kaplan-Meier survival plots by histological subgrouping. Both male and female patients who had keratinizing squamous cell carcinoma had the worst survival compared with those who had the other histological subtypes (log-rank P < .001). The 5-year relative survival rates for keratinizing squamous cell carcinoma were 41.9% and 41.5% for males and females of all races, respectively. Whereas black males had consistently poorer survival across all histological subtypes, individuals in the other race category, particularly females, had the highest 5-year relative survival estimates (Table 3).
TABLE 3.
Five-Year Relative Survival by Sex and Race
| Cell Type | 5-Year Relative Survival (95% CI), % | |||||||
|---|---|---|---|---|---|---|---|---|
| All | White | Black | Other | |||||
| Male | Female | Male | Female | Male | Female | Male | Female | |
| Overall NPC | 53.0 (51.3–54.7) | 55.8 (53.2–58.4) | 47.7 (45.2–50.2) | 45.7 (41.8–49.6) | 44.4 (38.7–49.9) | 56.5 (47.1–64.8) | 60.8 (58.2–63.2) | 66.1 (62.2–69.7) |
| Keratinizing | 41.9 (39.4–44.4) | 41.5 (37.6–45.4) | 37.7 (34.6–40.9) | 37.1 (32.3–41.8) | 35.5 (27.7–43.4) | 35.9 (22.9–49.0) | 53.2 (48.3–57.7) | 54.3 (46.3–61.6) |
| Nonkeratinizing | 64.7 (62.0–67.2) | 67.7 (63.5–71.6) | 64.4 (59.8–68.6) | 61.0 (52.7–68.3) | 54.4 (44.4–63.3) | 70.6 (55.8–81.2) | 66.4 (62.8–69.7) | 70.5 (65.1–75.2) |
| Differentiated | 63.1 (58.7–67.1) | 63.4 (56.0–69.9) | 62.3 (55.0–68.7) | 51.6 (36.2–65.0) | 49.5 (32.8–64.2) | 66.5 (41.2–82.9) | 65.3 (59.4–70.5) | 67.3 (58.1–74.9) |
| Undifferentiated | 65.8 (62.4–69.0) | 70.1 (64.8–74.8) | 65.8 (59.7–71.1) | 65.0 (55.1–73.3) | 57.1 (44.5–67.9) | 72.6 (53.2–85.0) | 67.1 (62.5–71.3) | 72.5 (65.6–78.3) |
| NOS | 54.6 (50.4–58.6) | 66.2 (59.7–71.9) | 51.9 (45.0–58.4) | 58.6 (46.5–68.9) | 47.7 (34.3–59.9) | 69.1 (42.9–85.1) | 57.5 (51.8–62.9) | 69.9 (61.6–76.7) |
Abbreviations: NOS, not otherwise specified; NPC, nasopharyngeal carcinoma.
Figure 1.
Kaplan-Meier survival curves illustrate overall survival by sex and morphology. NOS indicates not otherwise specified; SCC, squamous cell carcinoma.
DISCUSSION
This is the first study, to our knowledge, to assess temporal changes in NPC incidence in the United States. Although overall NPC incidence appears to be decreasing in the United States, the EBV-related differentiated nonkeratinizing NPC subtype is increasing at a concerning rate among all race groups and sexes in the United States. NPC has long been considered a relatively rare disease in the United States, with a reported incidence rate <1 per 100,000.2,33,34 Nevertheless, in endemic regions, NPC remains a major public health problem, with annual incidence rates exceeding 20 per 100,000 individuals.33 Several studies have previously reported decreased incidence of NPC in successive generations of Chinese migrants to the United States35; however, the EASR among those belonging to the other race category, of which 98% identify as Asians/Pacific Islanders, remain >5 times higher in females and 7 times higher among males compared with their white counterparts. In addition, the rate of change in overall NPC incidence, represented as the APC, between the white and other race individuals remained comparable throughout the study period, leading to continued disparity among this race group across time.
EBV, a prominent etiological factor in NPC, is a ubiquitous virus that infects and persists latently in >90% of the global population.36 Although primary infection is often subclinical, EBV replication can occur in oropharyngeal epithelial cells37 as well as B lymphocytes in normal and malignant nasopharyngeal tissue,38 EBV-encoded RNA has repeatedly been detected in both premalignant and malignant tissues of primarily nonkeratinizing NPC39–41; nevertheless, few studies, particularly in nonendemic countries, have characterized the potential differences in etiology between differentiated and undifferentiated NPC. It has also been demonstrated that antibody titers, particularly for IgA, not only precede cancer development42 but are associated with prognosis and recurrence.43 A considerable amount of research has been conducted to determine whether particular strains of EBV may explain, at least in part, the international patterns of NPC.44–47 Several different sequence variations have been detected in the oncogenic viral latent membrane protein 1 (LMP1) of EBV in NPC tumors44,48–50; however, there remains no strong evidence to suggest an increased risk associated with different EBV variants.33,49–52 In the current study, we found that differentiated nonkeratinizing NPC incidence was increasing among all sex and race groups, leading to speculation of an increased role of EBV in NPC in the United States. Exploratory analyses into SEER incidence trends of extranodal NK/T-cell lymphoma, a form of non-Hodgkin’s lymphoma consistently associated with EBV,53–67 demonstrated significant increases in this cancer type among both sexes over this time period, further supporting the possibility of an increasing role in EBV etiology in US malignancies (see Supporting Fig. 1).
In certain endemic populations, such as the Cantonese, early age incidence peaks in NPC are generally associated with childhood consumption of, or even traditional weaning by,68 salted fish and other preserved foods high in volatile N-nitrosamines.1 Early life infection by EBV,69,70 as well as genetic predisposition, have both been proposed as possible risk factors for early onset NPC, but little consensus has been reached regarding the etiology of such cases in the United States. Although the average age at diagnosis in our study was 55 years for both sexes, we noted a bimodal distribution in cases among blacks, with 25% of patients being diagnosed before age 40 years compared with 12% of their white counterparts. Whereas this distribution has been observed previously,35 we noted that this disparity was particularly apparent in the nonkeratinizing subtype, in which 32% of cases among blacks occurred before age 40 years (see Supporting Fig. 2). Although we are unable to definitively conclude that the increase in these young-onset cases is caused by EBV alone, EBV seroprevalence has been reported to be significantly higher among African American youth compared with their white peers.71,72 Further studies are needed to properly assess the role childhood infection by EBV as well as possible gene-environment interactions in the US population.
The majority of case-control studies report a 2-fold to 4-fold increased risk of NPC among smokers.15,16,18–22 Vaughan et al estimated that two-thirds of keratinizing NPCs in the United States are attributable to smoking, whereas both subtypes of nonkeratinizing carcinoma failed to demonstrate significant associations.15 Decreases in smoking prevalence in the United States73 may explain the decreases in keratinizing squamous cell carcinoma seen in our analyses. Diet, which has been the primary nonviral exposure associated with NPC in endemic regions, has been postulated to affect NPC primarily through the consumption of preformed nitrosamines or nitrosamine precursors.74 Although cultural differences in dietary patterns preclude early and repeated exposure to salted fish,75 harissa, quaddid, and touklia76 from US dietary etiology, preserved meats, which contain high levels of added nitrites, have been studied and have not been significantly associated with NPC carcinogenesis.74 Although that particular study did not assess genetic variation, it has been shown that individuals possessing the c2/c2 metabolic genotype for CYP2E1, a catalytic enzyme for the metabolic activation of low-molecular-weight nitrosamines, experience a 2.6-fold increased risk of NPC compared with those possessing 1 or 2 copies of the wild-type allele.77 Moreover, alcohol, in addition to its innate role as a carcinogen,15,18 has been shown in animal models to increase the carcinogenicity of ingested nitrosamines,78,79 further stressing the importance of dietary interaction studies in the understanding of nasopharyngeal carcinogenesis.
Our study confirmed previous reports of a survival advantage of the undifferentiated nonkeratinizing morphology over the differentiated nonkeratinizing and keratinizing squamous cell NPCs.5,35 In addition, we found that the other racial grouping had consistently improved survival compared with white and black patients, particularly among those presenting with keratinizing squamous cell carcinoma; these findings are consistent with other reports of a survival advantage among Chinese/Asian patients.5,35,80 One proposed rationale for this observation may be the high proportion of polymorphisms in the epidermal growth factor receptor (EGFR) found in the Chinese/Asian population, leading to lower EGFR expression and, consequently, improved prognosis.5,81–84
Several limitations are present in the current study. Although the use of SEER-9 data allows for temporal analysis of cancer trends since 1973, it represents only one-half of the participating registries to date. In addition, population estimates used for trends analyses were only available for the white, black and other race categories until 1990, resulting in our inability to assess age-adjusted NPC incidence trends in specific other races (American Indian/Alaska Native and Asian/Pacific Islander) or by Hispanic ethnicity for the entire study period. Treatment variables, particularly those pertinent to NPC, such as chemotherapy and radiation, are not suggested for use by SEER because of known data gaps for these variables. The absence of data regarding comorbidities, smoking, drinking behavior, and EBV prevalence limits our ability to make definitive statements regarding the underlying etiology influencing the observed trends. Although more precise treatment and comorbidity variables are available by linking the SEER database to the Medicare claims database, the majority of patients with NPC in this study were too young to qualify for Medicare (aged <65 years). Trends in adenocarcinoma of the nasopharynx, largely associated with occupational exposures like wood dust,85–87 were not able to be assessed because of small sample sizes. Finally, the WHO classification system for NPC had changed several times throughout the study period, consequently leading to concerns regarding the interpretability of subtype-specific results. That said, the changes within the WHO guidelines were made in the grouping of the subtypes, not the pathology; this study uses morphology codes to define NPC subtypes; therefore, the changes in WHO classifications have no effect on the study or the conclusions.
Although NPC remains relatively rare in the overall US population, the EBV-related differentiated subtype is increasing across all sexes and races. Many studies assessing nonkeratinizing NPC, particularly in the United States, aggregate differentiated and undifferentiated carcinoma because of the lack of a sufficient sample size; the results of the current study highlight the need for additional research to elucidate the potential etiological differences between these histological subgroups, consequently addressing the rationale for the disparity in the observed incidence trends. Because prospective cohort studies with comprehensive exposure assessment would require decades to recruit a sufficient sample size to assess gene-environment interactions, multicenter studies or case-control studies in high-incidence regions may prove to be a more feasible method to improve understanding of NPC etiology. In addition, studies evaluating the biological mechanism by which EBV is involved in NPC are needed to move EBV preventative and targeted therapeutic methods forward, such as immunotherapy or even an EBV vaccine. Recent interest in the possible role of HPV in NPC, likely representing subepithelial extension from the oropharynx because of the lack of anatomical constraints in Waldeyer ring,1 also may aid in understanding incidence trends and NPC pathogenesis. Finally, a comprehensive characterization of risk factors among young adults is needed to reveal the cause for the adolescent incidence peak observed in African American populations in this study.
Supplementary Material
FUNDING SUPPORT
This work was supported by grant T32AG027708 from the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Katie R. Zarins reports grants from Brooklyn ImmunoTherapeutics to the University of Michigan outside the submitted work. The remaining authors made no disclosures.
REFERENCES
- 1.Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2014;88:580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong TS, Kwong DL, Sham J, Wei WI, Kwong YL, Yuen AP. Elevation of plasma osteopontin level in patients with undifferentiated nasopharyngeal carcinoma. Eur J Surg Oncol. 2005;31:555–558. [DOI] [PubMed] [Google Scholar]
- 3.Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Pathology and Genetics of Head and Neck Tumours. World Health Organization Classification of Tumours. 3rd ed Vol. 9 International Agency of Research on Cancer; 2005. [Google Scholar]
- 4.Punwaney R, Brandwein MS, Zhang DY, et al. Human papillomavirus may be common within nasopharyngeal carcinoma of Caucasian Americans: investigation of Epstein-Barr virus and human papilloma-virus in eastern and western nasopharyngeal carcinoma using ligation-dependent polymerase chain reaction. Head Neck. 1999;21:21–29. [DOI] [PubMed] [Google Scholar]
- 5.Ou SH, Zell JA, Ziogas A, Anton-Culver H. Epidemiology of nasopharyngeal carcinoma in the United States: improved survival of Chinese patients within the keratinizing squamous cell carcinoma histology. Ann Oncol. 2007;18:29–35. [DOI] [PubMed] [Google Scholar]
- 6.Thompson LD. Update on nasopharyngeal carcinoma. Head Neck Pathol. 2007;1:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Zhang Y, Ma S. Racial differences in nasopharyngeal carcinoma in the United States. Cancer Epidemiol. 2013;37:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parkin DM, Whelan SL, Ferlay J, Raymond L, Young J, eds. Cancer Incidence in Five Continents Vol. VII IARC Scientific Publications No. 143. International Agency for Research on Cancer; 1997. [Google Scholar]
- 9.Pathmanathan R, Prasad U, Sadler R, Flynn K, Raab-Traub N. Clonal proliferations of cells infected with Epstein-Barr virus in pre-invasive lesions related to nasopharyngeal carcinoma. N Engl J Med. 1995;333:693–698. [DOI] [PubMed] [Google Scholar]
- 10.Chan AS, To KF, Lo KW, et al. High frequency of chromosome 3p deletion in histologically normal nasopharyngeal epithelia from southern Chinese. Cancer Res. 2000;60:5365–5370. [PubMed] [Google Scholar]
- 11.Chan AS, To KF, Lo KW, et al. Frequent chromosome 9p losses in histologically normal nasopharyngeal epithelia from southern Chinese. Int J Cancer. 2002;102:300–303. [DOI] [PubMed] [Google Scholar]
- 12.Fan SQ, Ma J, Zhou J, et al. Differential expression of Epstein-Barr virus-encoded RNA and several tumor-related genes in various types of nasopharyngeal epithelial lesions and nasopharyngeal carcinoma using tissue microarray analysis. Hum Pathol. 2006;37:593–605. [DOI] [PubMed] [Google Scholar]
- 13.Mimi CY, Yuan JM. Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12:421–429. [DOI] [PubMed] [Google Scholar]
- 14.Tricker AR, Preussmann R. Carcinogenic N-nitrosamines in the diet: occurrence, formation, mechanisms and carcinogenic potential. Mutat Res. 1991;259:277–289. [DOI] [PubMed] [Google Scholar]
- 15.Vaughan TL, Shapiro JA, Burt RD, et al. Nasopharyngeal cancer in a low-risk population: defining risk factors by histological type. Cancer Epidemiol Biomarkers Prev. 1996;5:587–593. [PubMed] [Google Scholar]
- 16.Yuan JM, Wang XL, Xiang YB, Gao YT, Ross RK, Yu MC. Non-dietary risk factors for nasopharyngeal carcinoma in Shanghai, China. Int J Cancer. 2000;85:364–369. [PubMed] [Google Scholar]
- 17.Yu MC, Garabrant DH, Huang TB, Henderson BE. Occupational and other non-dietary risk factors for nasopharyngeal carcinoma in Guangzhou, China. Int J Cancer. 1990;45:1033–1039. [DOI] [PubMed] [Google Scholar]
- 18.Nam J, McLaughlin JK, Blot WJ. Cigarette smoking, alcohol, and nasopharyngeal carcinoma: a case-control study among US whites. J Natl Cancer Inst. 1992;84:619–622. [DOI] [PubMed] [Google Scholar]
- 19.West S, Hildesheim A, Dosemeci M. Non-viral risk factors for nasopharyngeal carcinoma in the Philippines: results from a case-control study. Int J Cancer. 1993;55:722–727. [DOI] [PubMed] [Google Scholar]
- 20.Chow WH, McLaughlin JK, Hrubec Z, Nam JM, Blot WJ. Tobacco use and nasopharyngeal carcinoma in a cohort of US veterans. Int J Cancer. 1993;55:538–540. [DOI] [PubMed] [Google Scholar]
- 21.Zhu K, Levine RS, Brann EA, Gnepp DR, Baum MK. A population-based case-control study of the relationship between cigarette smoking and nasopharyngeal cancer (United States). Cancer Causes Control. 1995;6:507–512. [DOI] [PubMed] [Google Scholar]
- 22.Cheng YJ, Hildesheim A, Hsu M-M, et al. Cigarette smoking, alcohol consumption and risk of nasopharyngeal carcinoma in Taiwan. Cancer Causes Control. 1999;10:201–207. [DOI] [PubMed] [Google Scholar]
- 23.Giannoudis A, Ergazaki M, Segas J, et al. Detection of Epstein-Barr virus and human papillomavirus in nasopharyngeal carcinoma by the polymerase chain reaction technique. Cancer Lett. 1995;89: 177–181. [DOI] [PubMed] [Google Scholar]
- 24.Hording U, Nielsen HW, Daugaard S, Albeck H. Human papilloma-virus types 11 and 16 detected in nasopharyngeal carcinomas by the polymerase chain reaction. Laryngoscope. 1994;104:99–102. [DOI] [PubMed] [Google Scholar]
- 25.Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32:562–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rassekh CH, Rady PL, Arany I, et al. Combined Epstein-Barr virus and human papillomavirus infection in nasopharyngeal carcinoma. Laryngoscope. 1998;108:362–367. [DOI] [PubMed] [Google Scholar]
- 27.Heck JE, Berthiller J, Vaccarella S, et al. Sexual behaviours and the risk of head and neck cancers: a pooled analysis in the International Head and Neck Cancer Epidemiology (INHANCE) Consortium. Int J Epidemiol. 2009;39:166–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Populations-Total U.S. (1969–2016) <Katrina/Rita Adjustment>-Linked To County Attributes-Total U.S., 1969–2016 Counties. National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program; 2018. [Google Scholar]
- 29.Chan J, Bray F, McCarron P, et al. Nasopharyngeal carcinoma In: Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Pathology and Genetics of Head and Neck Tumours. 3rd ed World Health Organization Classification of Tumours. IARC Press; 2005:85–97. [Google Scholar]
- 30.Segi M, Kyokai NT, Igakubu TD, eds. Cancer Mortality for Selected Sites in 24 Countries. Japan Cancer Society; 1967. Accessed March 9, 2018 https://www.ncbi.nlm.nih.gov/nlmcatalog/7900272 [Google Scholar]
- 31.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. [DOI] [PubMed] [Google Scholar]
- 32.Demanelis K, Sriplung H, Meza R, et al. Differences in childhood leukemia incidence and survival between Southern Thailand and the United States: a population-based analysis. Pediatr Blood Cancer. 2015;62:1790–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765–1777. [DOI] [PubMed] [Google Scholar]
- 34.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. [DOI] [PubMed] [Google Scholar]
- 35.Burt RD, Vaughan TL, McKnight B. Descriptive epidemiology and survival analysis of nasopharyngeal carcinoma in the United States. Int J Cancer. 1992;52:549–556. [DOI] [PubMed] [Google Scholar]
- 36.Fields BN, Knipe DM, Howley PM, Griffin DE. Fields Virology. 4th ed Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 37.Miller G, Niederman JC, Andrews LL. Prolonged oropharyngeal excretion of Epstein-Barr virus after infectious mononucleosis. N Engl J Med. 1973;288:229–232. [DOI] [PubMed] [Google Scholar]
- 38.Chen CL, Hsu MM, Hsu HC. Differential expression of EBER1 in nontumor nasopharyngeal biopsies and nontumor component of nasopharyngeal carcinoma. Intervirology. 1996;39:230–235. [DOI] [PubMed] [Google Scholar]
- 39.Aguilar Madrid G, Beaudry M, Bell W, et al. Statement in response to asbestos industry efforts to prevent a ban on asbestos in Pakistan: chrysotile asbestos use is not safe and must be banned. Arch Environ Occup Health. 2013;68:243–249. [DOI] [PubMed] [Google Scholar]
- 40.Tsao SW, Tsang CM, Lo KW. Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos Trans R Soc Lond B Biol Sci. 2017;372:20160270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshizaki T, Ito M, Murono S, Wakisaka N, Kondo S, Endo K. Current understanding and management of nasopharyngeal carcinoma. Auris Nasus Larynx. 2012;39:137–144. [DOI] [PubMed] [Google Scholar]
- 42.Chien YC, Chen JY, Liu MY, et al. Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med. 2001;345:1877–1882. [DOI] [PubMed] [Google Scholar]
- 43.de Vathaire F, Sancho-Garner H, de The H, et al. Prognostic value of EBV markers in the clinical management of nasopharyngeal carcinoma (NPC): a multicenter follow-up study. Int J Cancer. 1988;42: 176–181. [DOI] [PubMed] [Google Scholar]
- 44.Hu LF, Zabarovsky ER, Chen F, et al. Isolation and sequencing of the Epstein-Barr virus BNLF-1 gene (LMP1) from a Chinese nasopharyngeal carcinoma. J Gen Virol. 1991;72:2399–2409. [DOI] [PubMed] [Google Scholar]
- 45.Abdel-Hamid M, Chen JJ, Constantine N, Massoud M, Raab-Traub N. EBV strain variation: geographical distribution and relation to disease state. Virology. 1992;190:168–175. [DOI] [PubMed] [Google Scholar]
- 46.Bouzid M, Djennaoui D, Dubreuil J, et al. Epstein-Barr virus genotypes in NPC biopsies from North Africa. Int J Cancer. 1994;56:468–473. [DOI] [PubMed] [Google Scholar]
- 47.Chen ML, Tsai C, Liang CL, et al. Cloning and characterization of the latent membrane protein (LMP) of a specific Epstein-Barr virus variant derived from the nasopharyngeal carcinoma in the Taiwanese population. Oncogene. 1992;7:2131–2140. [PubMed] [Google Scholar]
- 48.Miller WE, Edwards RH, Walling DM, Raab-Traub N. Sequence variation in the Epstein-Barr virus latent membrane protein 1. J Gen Virol. 1994;75:2729–2740. [DOI] [PubMed] [Google Scholar]
- 49.Edwards RH, Seillier-Moiseiwitsch F, Raab-Traub N. Signature amino acid changes in latent membrane protein 1 distinguish Epstein-Barr virus strains. Virology. 1999;261:79–95. [DOI] [PubMed] [Google Scholar]
- 50.Cheung ST, Lo KW, Leung S, et al. Prevalence of LMP1 deletion variant of Epstein-Barr virus in nasopharyngeal carcinoma and gastric tumors in Hong Kong. Int J Cancer. 1996;66:711–712. [DOI] [PubMed] [Google Scholar]
- 51.Zhang XS, Song KH, Mai HQ, et al. The 30-bp deletion variant: a polymorphism of latent membrane protein 1 prevalent in endemic and non-endemic areas of nasopharyngeal carcinomas in China. Cancer Lett. 2002;176:65–73. [DOI] [PubMed] [Google Scholar]
- 52.Sandvej K, Gratama JW, Munch M, et al. Sequence analysis of the Epstein-Barr virus (EBV) latent membrane protein-1 gene and promoter region: identification of four variants among wild-type EBV isolates. Blood. 1997;90:323–330. [PubMed] [Google Scholar]
- 53.Proceedings of the IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Epstein-Barr Virus and Kaposi’s Sarcoma Herpesvirus/Human Herpesvirus 8. Lyon, France, 17–24 June 1997. IARC Monogr Eval Carcinog Risks Hum. 1997;70:1–492.9705682 [Google Scholar]
- 54.Thompson MP, Kurzrock R. Epstein-Barr virus and cancer. Clin Cancer Res. 2004;10:803–821. [DOI] [PubMed] [Google Scholar]
- 55.Arber DA, Weiss LM, Albujar PF, Chen YY, Jaffe ES. Nasal lymphomas in Peru. High incidence of T-cell immunophenotype and Epstein-Barr virus infection. Am J Surg Pathol. 1993;17:392–399. [PubMed] [Google Scholar]
- 56.Chan JK, Yip TT, Tsang WY, et al. Detection of Epstein-Barr viral RNA in malignant lymphomas of the upper aerodigestive tract. Am J Surg Pathol. 1994;18:938–946. [DOI] [PubMed] [Google Scholar]
- 57.Ott G, Kalla J, Ott MM, Muller-Hermelink HK. The Epstein-Barr virus in malignant non-Hodgkin’s lymphoma of the upper aerodigestive tract. Diagn Mol Pathol. 1997;6:134–139. [DOI] [PubMed] [Google Scholar]
- 58.Hamilton-Dutoit SJ, Pallesen G. A survey of Epstein-Barr virus gene expression in sporadic non-Hodgkin’s lymphomas. Detection of Epstein-Barr virus in a subset of peripheral T-cell lymphomas. Am J Pathol. 1992;140:1315–1325. [PMC free article] [PubMed] [Google Scholar]
- 59.van Gorp J, Brink A, Oudejans JJ, et al. Expression of Epstein-Barr virus encoded latent genes in nasal T cell lymphomas. J Clin Pathol. 1996;49:72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohshima K, Suzumiya J, Tasiro K, et al. Epstein-Barr virus infection and associated products (LMP, EBNA2, vIL-10) in nodal non-Hodgkin’s lymphoma of human immunodeficiency virus-negative Japanese. Am J Hematol. 1996;52:21–28. [DOI] [PubMed] [Google Scholar]
- 61.Tsuchiyama J, Yoshino T, Mori M, et al. Characterization of a novel human natural killer-cell line (NK-YS) established from natural killer cell lymphoma/leukemia associated with Epstein-Barr virus infection. Blood. 1998;92:1374–1383. [PubMed] [Google Scholar]
- 62.Kanegane H, Yachie A, Miyawaki T, Tosato G. EBV-NK cells interactions and lymphoproliferative disorders. Leuk Lymphoma. 1998;29:491–498. [DOI] [PubMed] [Google Scholar]
- 63.Borisch B, Hennig I, Laeng RH, Waelti ER, Kraft R, Laissue J. Association of the subtype 2 of the Epstein-Barr virus with T-cell non-Hodgkin’s lymphoma of the midline granuloma type. Blood. 1993;82:858–864. [PubMed] [Google Scholar]
- 64.Kwong YL, Chan AC, Liang RH. Natural killer cell lymphoma/leukemia: pathology and treatment. Hematol Oncol. 1997;15:71–79. [DOI] [PubMed] [Google Scholar]
- 65.d’Amore F, Johansen P, Houmand A, Weisenburger DD, Mortensen LS. Epstein-Barr virus genome in non-Hodgkin’s lymphomas occurring in immunocompetent patients: highest prevalence in nonlymphoblastic T-cell lymphoma and correlation with a poor prognosis. Danish Lymphoma Study Group, LYFO. Blood. 1996;87:1045–1055. [PubMed] [Google Scholar]
- 66.Hsu JL, Glaser SL. Epstein-Barr virus-associated malignancies: epidemiologic patterns and etiologic implications. Crit Rev Oncol Hematol. 2000;34:27–53. [DOI] [PubMed] [Google Scholar]
- 67.Castillo JJ, Beltran BE, Miranda RN, Paydas S, Winer ES, Butera JN. Epstein-Barr virus–positive diffuse large B-cell lymphoma of the elderly: what we know so far. Oncologist. 2011;16:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu MC, Huang TB, Henderson BE. Diet and nasopharyngeal carcinoma: a case-control study in Guangzhou, China. Int J Cancer. 1989;43:1077–1082. [DOI] [PubMed] [Google Scholar]
- 69.Cannon T, Zanation AM, Lai V, Weissler MC. Nasopharyngeal carcinoma in young patients: a systematic review of racial demographics. Laryngoscope. 2006;116:1021–1026. [DOI] [PubMed] [Google Scholar]
- 70.Ayan I, Kaytan E, Ayan N. Childhood nasopharyngeal carcinoma: from biology to treatment. Lancet Oncol. 2003;4:13–21. [DOI] [PubMed] [Google Scholar]
- 71.Ford JL, Stowe RP. Racial-ethnic differences in Epstein-Barr virus antibody titers among US children and adolescents. Ann Epidemiol. 2013;23:275–280. [DOI] [PubMed] [Google Scholar]
- 72.Dowd JB, Palermo T, Brite J, McDade TW, Aiello A. Seroprevalence of Epstein-Barr virus infection in US children ages 6–19, 2003–2010. PLoS One. 2013;8:e64921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alamar B, Glantz SA. Effect of increased social unacceptability of cigarette smoking on reduction in cigarette consumption. Am J Public Health. 2006;96:1359–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Farrow DC, Vaughan TL, Berwick M, Lynch CF, Swanson GM, Lyon JL. Diet and nasopharyngeal cancer in a low-risk population. Int J Cancer. 1998;78:675–679. [DOI] [PubMed] [Google Scholar]
- 75.Zou XN, Lu SH, Liu B. Volatile N-nitrosamines and their precursors in Chinese salted fish—a possible etological factor for NPC in China. Int J Cancer. 1994;59:155–158. [DOI] [PubMed] [Google Scholar]
- 76.Jeannel D, Hubert A, De Vathaire F, et al. Diet, living conditions and nasopharyngeal carcinoma in Tunisia—a case-control study. Int J Cancer. 1990;46:421–425. [DOI] [PubMed] [Google Scholar]
- 77.Hildesheim A, Anderson LM, Chen CJ, et al. CYP2E1 genetic polymorphisms and risk of nasopharyngeal carcinoma in Taiwan. J Natl Cancer Inst. 1997;89:1207–1212. [DOI] [PubMed] [Google Scholar]
- 78.Swann PF. Effect of ethanol on nitrosamine metabolism and distribution. Implications for the role of nitrosamines in human cancer and for the influence of alcohol consumption on cancer incidence. IARC Sci Publ. 1984;57:501–512. [PubMed] [Google Scholar]
- 79.International Agency for Research on Cancer (IARC). Alcohol Drinking. IARC Monographs on the Evaluation of Carcinogenic Risk to Humans. Volume 44 IARC; 1988. [Google Scholar]
- 80.Bhattacharyya N The impact of race on survival in nasopharyngeal carcinoma: a matched analysis. Am J Otolaryngol. 2004;25: 94–97. [DOI] [PubMed] [Google Scholar]
- 81.Ma BB, Poon TC, To KF, et al. Prognostic significance of tumor angiogenesis, Ki 67, p53 oncoprotein, epidermal growth factor receptor and HER2 receptor protein expression in undifferentiated nasopharyngeal carcinoma—a prospective study. Head Neck. 2003;25:864–872. [DOI] [PubMed] [Google Scholar]
- 82.Chua DT, Nicholls JM, Sham JS, Au GK. Prognostic value of epidermal growth factor receptor expression in patients with advanced stage nasopharyngeal carcinoma treated with induction chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:11–20. [DOI] [PubMed] [Google Scholar]
- 83.Liu W, Innocenti F, Chen P, Das S, Cook EH, Ratain MJ. Interethnic difference in the allelic distribution of human epidermal growth factor receptor intron 1 polymorphism. Clin Cancer Res. 2003;9:1009–1012. [PubMed] [Google Scholar]
- 84.Liu W, Innocenti F, Wu MH, et al. A functional common polymorphism in a Sp1 recognition site of the epidermal growth factor receptor gene promoter. Cancer Res. 2005;65:46–53. [PubMed] [Google Scholar]
- 85.Leclerc A, Cortes MM, Gerin M, Luce D, Brugere J. Sinonasal cancer and wood dust exposure: results from a case-control study. Am J Epidemiol. 1994;140:340–349. [DOI] [PubMed] [Google Scholar]
- 86.Delzell E Wood dust and formaldehyde. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 62. Cancer Causes Control. 1995;6:574–575. [Google Scholar]
- 87.Demers PA, Kogevinas M, Boffetta P, et al. Wood dust and sino-nasal cancer: pooled reanalysis of twelve case-control studies. Am J Ind Med. 1995;28:151–166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.