Abstract
Background:
Elevated neutrophil to lymphocyte ratio (NLR) is associated with poor survival in cancer patients, including patients treated with immunotherapies. We sought to investigate NLR as a biomarker of treatment outcomes in melanoma patients treated with PD-1 inhibition.
Methods:
Patients undergoing initial treatment with PD-1 inhibitor monotherapy for stage IV melanoma at a single center from 2012–2015 were included. Clinical characteristics and NLR at baseline and prior to subsequent treatment cycles were collected. Time to treatment failure (TTF) and overall survival (OS) were evaluated using Kaplan-Meier and landmark analyses.
Results:
Among 224 study patients, 63 (28%) had a baseline NLR ≥ 5. Baseline NLR was significantly associated with ECOG performance status and the number of involved metastatic sites. With a median follow-up of 39 months in survivors, baseline NLR ≥ 5 was independently associated with shorter OS (HR 2.0, 95% CI 1.3–2.9) and TTF (HR 1.7, 95% CI 1.2–2.4). NLR increase of ≥ 30% during the first two cycles of treatment was associated with worse OS (median 47 vs. 13.5 months, p < 0.001) and a trend toward shorter TTF (12.8 vs. 5.9 months, p = 0.05). Combining baseline NLR ≥ 5 and NLR increase of ≥ 30% identified a small cohort with markedly shortened OS (median 5.8 months) and TTF (median 1.8 months).
Conclusions:
Elevated baseline NLR and NLR increase early during treatment are prognostic for TTF and OS in melanoma patients treated with PD-1 inhibitor monotherapy. Combined, these biomarkers can widely risk-stratify patients for treatment failure and survival.
Keywords: neutrophil-to-lymphocyte ratio, melanoma, checkpoint inhibitor, PD-1 inhibitor
Precis:
Pre-treatment neutrophil-to-lymphocyte ratio (NLR) ≥ 5 and increase in NLR ≥ 30% are independently prognostic for treatment failure and worse overall survival in patients with melanoma treated with PD-1 inhibitor monotherapy.
Introduction
The neutrophil to lymphocyte ratio (NLR) is biomarker of systemic inflammation that can be readily obtained from a peripheral complete blood count. NLR has consistently been found to be prognostic of survival in patients with cancer, other diseases, and the general population.1–5 In patients without cancer, NLR increases with age; the median value plateaus between 3 and 4 from the 3rd to 6th decades, then continues to rise in the elderly.6 In contrast, patients with metastatic cancer often have substantially higher values. An NLR cutoff of ≥ 5 is most commonly used to define abnormal elevation in the metastatic setting.1,2
Patients with cancer and an NLR above the defined cutoff have consistently been found to have worse outcomes than patients with a lower NLR across cancer types.1 In melanoma, elevated NLR is associated with poorer prognosis in both localized and metastatic disease.7–10 In other cancers, NLR has been hypothesized to be a marker of disease burden, yet NLR appears to be independently prognostic after adjustment for disease stage, sites of metastatic disease, and established tumor markers.11–13 Alternatively, an elevated NLR may reflect an innately dysfunctional host immune response, a byproduct of tumor-related immune suppression, or some combination of the two.
The prognostic significance of this immune-based marker is particularly interesting in light of the clinical success of immunotherapy in melanoma. Recent studies have demonstrated that an elevated baseline NLR is prognostic for worse overall and progression-free survival (OS and PFS) prior to checkpoint inhibitor therapy with CTLA-4 and/or PD-1 blockade.14–20 While the prognostic value of NLR is clear, how this translates into clinical utility remains undefined. Although an elevated NLR is associated with worse outcomes, not all patients treated with immune therapy in the setting of an elevated NLR do poorly, and as such the prognostic value of the pretreatment NLR alone does not appear to be sufficient to influence therapeutic decision-making.
In a cohort of patients treated with ipilimumab, however, our group has previously demonstrated that an elevated NLR is prognostic both at baseline and prior to each cycle of the CTLA-4 blocker ipilimumab, while NLR during treatment was not prognostic in patients treated with BRAF inhibitors.14 Moreover, an NLR increase of 30% or more at any point while on treatment was associated with markedly worse outcomes in patients treated with ipilimumab. The restriction of this prognostic power to ipilimumab-treated patients suggests a potentially predictive role of NLR for response to ipilimumab. We sought to more thoroughly characterize NLR in patients with metastatic melanoma, and further to determine the prognostic value of baseline NLR as well as change in NLR early during treatment with PD-1 blockade.
Methods
Patient selection
After approval from the Memorial Sloan Kettering Cancer Center (MSKCC) institutional review board, the institution’s prospectively maintained melanoma database was queried for patients with stage IV melanoma (i.e., detectable metastases) treated with first course PD-1 inhibitor monotherapy between January 1, 2012 and December 31, 2015. Patients with ocular melanoma and those receiving initial PD-1 inhibition in an adjuvant setting were excluded. The choice of PD-1 inhibitor was at the discretion of the treating oncologist; typically, nivolumab was given every 2 weeks and pembrolizumab every 3 weeks.
Clinicopathologic variables
Clinicopathologic variables collected included age, sex, Eastern Cooperative Oncology Group (ECOG) performance status (0, 1, 2+), site of primary melanoma (cutaneous, mucosal, unknown primary), receipt of prior anti CTLA-4 (ipilimumab), BRAF mutation status, LDH (> 246 defined as elevated), metastatic stage (AJCC 8th edition), and number of metastatic sites (1, 2–3, 4+). Number of metastatic sites was the sum of all anatomic sites affected by disease. Sites captured by the database included adrenal, bone, brain, leptomeninges, liver, lung, lymph nodes, mesentery, other, skin/soft tissue, small bowel, spine, and/or spleen. Given the high degree of missingness for pretreatment LDH, this variable was not included in the survival analyses.
The independent variable of interest was the neutrophil to lymphocyte ratio (NLR), which was calculated by dividing the absolute number of neutrophils by the absolute number of lymphocytes obtained from a peripheral complete blood count (CBC) with differential. Laboratory studies (including a CBC) were obtained from all patients prior to the start of treatment and subsequently on the day of each cycle of treatment prior to administration of therapy. For the purposes of this study, the baseline NLR was calculated prior to the initial treatment with PD-1 blockade, and NLR was collected for as many as 6 subsequent cycles of treatment. An elevated NLR was defined as ≥ 5, consistent with prior studies.1 An increase in NLR of ≥30% during treatment was defined as significant, according to our previous cut-point analysis in patients treated with ipilimumab.14
Statistical analyses
Overall survival (OS) was the primary outcome and was calculated from the date of the first dose of PD-1 inhibitor monotherapy until death or date of last follow-up. Time to treatment failure (TTF) was defined as the interval between the date of the first dose of PD-1 inhibition and the earliest occurrence of either clinical progression, initiation of new therapy (local or systemic), or death.21
Wilcoxon rank sum and Fisher’s exact tests were employed to assess the association of baseline NLR (< 5 vs. ≥ 5) with clinical characteristics. A multivariate logistic regression model was fitted based on these univariate associations. Statistical associations of baseline factors with OS and TTF were assessed using Kaplan-Meier plots, log-rank testing, and Cox proportional hazards modeling. AJCC stage was not included in multivariate analyses of OS and TTF due to its co-linearity with number of metastatic sites. To analyze the effects of early changes in NLR while on treatment, landmark analyses of OS and TTF were performed. OS and TTF were defined beginning from the landmark of 1 month after the start of treatment, as most patients would have received two doses of PD-1 inhibitor by that time point. Patients who died or were lost to follow-up prior to 1 month were not included in the OS analysis. Likewise, patients who experienced a treatment failure or were lost to follow-up prior to 1 month were not included in the TTF analysis. All analyses were performed using SAS 9.4 and R 3.5.1 software. A p-value < 0.05 was considered statistically significant.
Results
Characteristics of study cohort
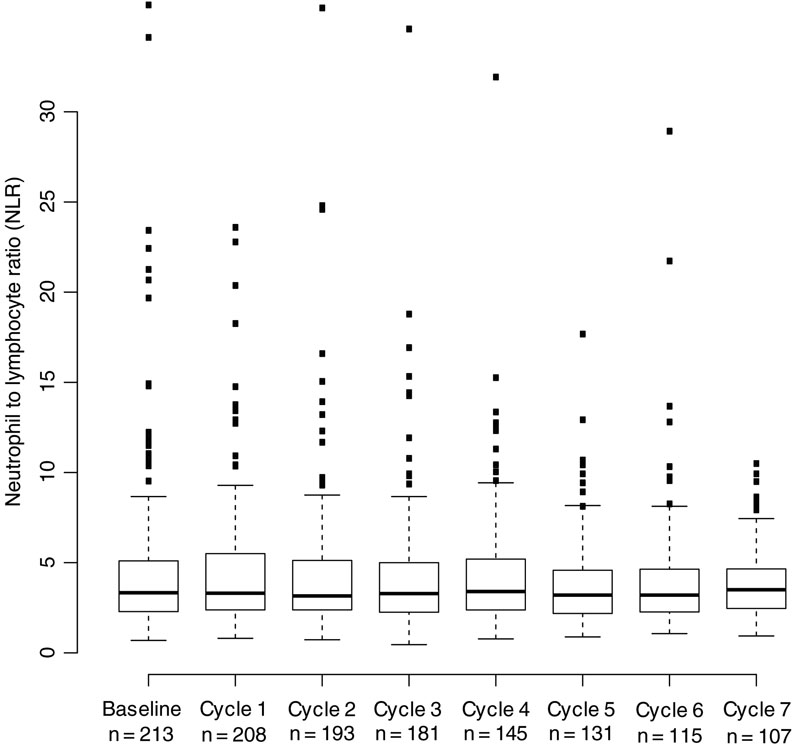
Overall, 224 patients with stage IV metastatic melanoma treated with initial doses of PD-1 inhibition were identified for inclusion in the study. Median patient age was 66 years and the majority were male (66%, Table 1). In most patients, the primary site was cutaneous; 12% of patients had mucosal melanoma, and 15% melanoma of unknown primary. A large majority (87%) of patients had a pre-treatment ECOG performance status of 0 or 1. Pretreatment M1c disease was most common (46%), and only a small minority (10%) of patients presented with M1a disease. More than one anatomic site was affected in 77% of patients. Prior treatment with ipilimumab was common (73%). The PD-1 inhibitor used most frequently was pembrolizumab (79%). The baseline median NLR of the cohort was 3.4, and 28% of patients had an elevated NLR (≥ 5). Patients received a median of 6 cycles of PD-1 inhibition. The median NLR by cycle is shown in Figure 1.
Table 1.
Characteristics of patients with elevated (≥ 5) or non-elevated baseline NLR. Data are n (% of column total for overall or % of row total for NLR categories) unless otherwise indicated.
| Characteristic | Overall (n = 224) | NLR < 5 (n = 161) | NLR ≥ 5 (n = 63) | p value | |
|---|---|---|---|---|---|
| Age | Median (range) | 65.5 (24–94) | 64.7 (24–94) | 67.4 (28–91) | 0.113 |
| Sex | Male | 147 (66) | 103 (70) | 44 (30) | 0.438 |
| Female | 77 (34) | 58 (75) | 19 (25) | -- | |
| Pretreatment ECOG | 0 | 108 (48) | 93 (86) | 15 (14) | <0.001 |
| 1 | 87 (39) | 55 (63) | 32 (37) | -- | |
| 2+ | 19 (9) | 6 (32) | 13 (68) | -- | |
| Unknown | 10 (5) | 7 (70) | 3 (30) | -- | |
| Primary site | Cutaneous | 163 (73) | 123 (75) | 40 (25) | 0.112 |
| Mucosal | 27 (12) | 18 (67) | 9 (33) | -- | |
| Unknown primary | 34 (15) | 20 (59) | 14 (41) | -- | |
| Number of metastatic sites | 1 | 51 (23) | 46 (90) | 5 (10) | 0.001 |
| 2–3 | 116 (52) | 81 (70) | 35 (30) | -- | |
| 4+ | 57 (26) | 34 (60) | 23 (40) | -- | |
| AJCC M-Stage | M1a | 23 (10) | 19 (83) | 4 (17) | 0.012 |
| M1b | 39 (17) | 35 (90) | 4 (10) | -- | |
| M1c | 104 (46) | 69 (66) | 35 (34) | -- | |
| M1d | 58 (26) | 38 (66) | 20 (34) | -- | |
| BRAF Mutation | No | 134 (60) | 96 (72) | 38 (28) | 1.0 |
| Yes | 87 (39) | 63 (72) | 24 (28) | -- | |
| Unknown | 3 (1) | 2 (67) | 1 (33) | -- | |
| LDH | Normal | 119 (53) | 89 (75) | 30 (25) | 0.216 |
| Elevated | 53 (24) | 33 (62) | 20 (38) | -- | |
| Unknown | 52 (23) | 39 (75) | 13 (25) | -- | |
| Prior ipilimumab | No | 63 (27) | 46 (73) | 17 (27) | 0.870 |
| Yes | 161 (73) | 115 (71) | 46 (29) | -- | |
| Treatment | Nivolumab | 47 (21) | 33 (70) | 14 (30) | 0.855 |
| Pembrolizumab | 177 (79) | 128 (72) | 49 (28) | -- |
Figure 1. Distribution of NLR values by cycle of therapy.
N indicates the number of patients with an evaluable NLR after receiving the specified dose of therapy.
Elevated NLR is associated with unknown primary, greater disease burden, and decreased performance status
Several clinicopathologic factors were significantly associated with an elevated baseline NLR (Table 1). Among patients with an ECOG performance status of 0, 14% had an NLR ≥ 5, compared with 37% of patients with an ECOG of 1, and 68% of patients with an ECOG of ≥ 2 (p < 0.001). NLR was rare among patients with a single metastatic site (10%) and increasingly common in patients with more affected sites (30% with 2–3, 40% with ≥ 4, p = 0.001). Similarly, increasing AJCC sub-stage was associated with elevated NLR (p = 0.012). Elevated NLR was more frequent among patients with a non-cutaneous primary; 25% of patients with cutaneous melanoma had an NLR ≥ 5, compared with 33% of patients with mucosal melanomas and 41% of melanoma of unknown primary patients, but this was not statistically significant on univariate analysis (p = 0.112). In a multivariate analysis, lower ECOG performance status (ECOG 1 HR 3.19, 95% CI 1.54–6.61; ECOG 2+ HR 10.62, 95% CI 3.31–34.12), and greater number of metastatic sites (2–3 sites HR 3.20, 95% CI 1.09–9.40; 4+ sites HR 5.43, 95% CI 1.71–17.26) remained independently associated with NLR ≥ 5. Unknown primary site trended toward an association with elevated NLR (HR 2.43, 95% CI 1.00–5.89).
Elevated baseline NLR is associated with shorter OS and TTF
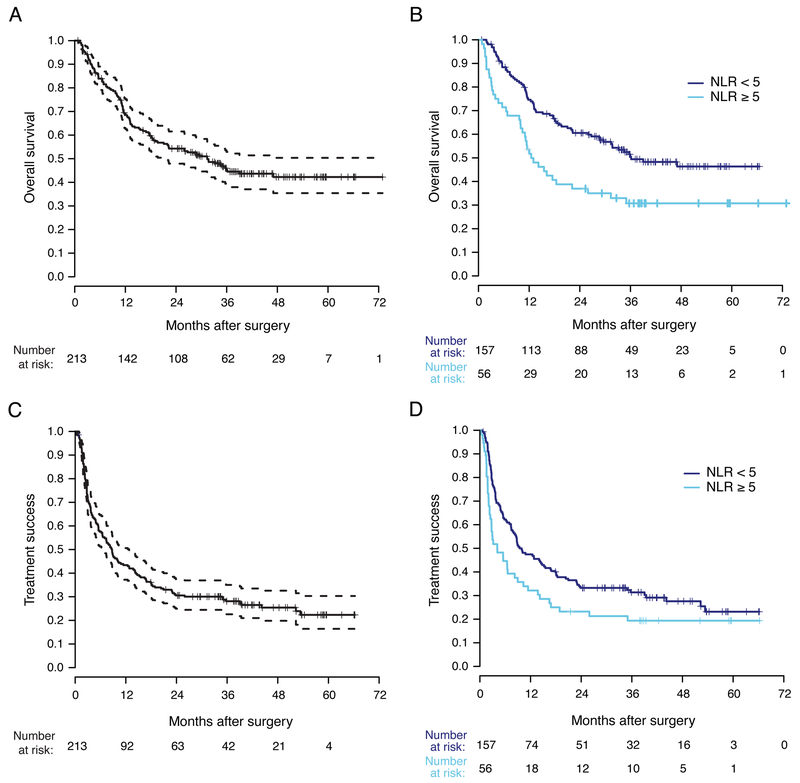
With a median follow-up in survivors of 39 months, the median OS was 28.6 months (95% CI 18.4–36.2, Figure 2A). In a univariate analysis, elevated baseline NLR (Figure 2B), mucosal melanoma, ECOG performance status, higher AJCC sub-stage, and greater number of metastatic sites were significantly associated with OS. In a multivariate model, mucosal melanoma, more metastatic sites, and NLR ≥ 5 remained significantly associated with OS (Table 2).
Figure 2. Outcomes in patients with metastatic melanoma treated with PD-1 monotherapy.
A) Overall survival for the entire cohort (dashed lines indicate 95% CI). B) Overall survival stratified by baseline NLR. C) Time to treatment failure (dashed lines indicate 95% CI). D) Time to treatment failure stratified by baseline NLR.
Table 2.
Univariate and multivariate analyses of factors associated with overall survival and time to treatment failure.
| Characteristic* | 2-year OS | Univariate HR | 95% CI | Univariate p value | Multivariate HR | 95% CI | Multivariate p value | |
| NLR | < 5 | 60% | Ref | <0.001 | Ref | -- | ||
| ≥ 5 | 33% | 2.05 | 1.42–2.97 | 1.95 | 1.33–2.86 | 0.001 | ||
| Primary site | Cutaneous | 52% | Ref | 0.002 | Ref | -- | ||
| Mucosal | 28% | 2.05 | 1.27–3.31 | 2.08 | 1.28–3.37 | 0.003 | ||
| Unknown primary | 71% | 0.68 | 0.40–1.19 | 0.58 | 0.33–1.01 | 0.054 | ||
| Number of metastatic sites | 1 | 71% | Ref | <0.001 | Ref | -- | ||
| 2–3 | 51% | 2.12 | 1.24–3.61 | 1.85 | 1.08–3.17 | 0.026 | ||
| ≥ 4 | 38% | 3.04 | 1.72–5.38 | 2.61 | 1.15–4.67 | 0.001 | ||
| ECOG status | 0 | 62% | Ref | 0.003 | -- | -- | ||
| 1 | 44% | 1.52 | 1.04–2.23 | -- | -- | |||
| 2+ | 32% | 2.55 | 1.41–4.62 | -- | -- | |||
| AJCC M stage | M1a | 64% | Ref | 0.003 | -- | -- | ||
| M1b | 76% | 0.74 | 0.33–1.65 | -- | -- | |||
| M1c | 46% | 1.63 | 0.85–3.09 | -- | -- | |||
| M1d | 43% | 2.09 | 1.07–4.08 | -- | -- | |||
| Characteristic* | 2-year TTF | Univariate HR | 95% CI | Univariate p value | Multivariate HR | 95% CI | Multivariate p value | |
| NLR | < 5 | 32% | Ref | 0.003 | Ref | -- | ||
| ≥ 5 | 21% | 1.65 | 1.19–2.29 | 1.73 | 1.24–2.41 | 0.001 | ||
| Primary site | Cutaneous | 31% | Ref | 0.005 | Ref | -- | ||
| Mucosal | 7% | 1.86 | 1.20–2.87 | 1.80 | 1.16–2.78 | 0.008 | ||
| Unknown primary | 38% | 0.78 | 0.5–1.21 | 0.69 | 0.44–1.08 | 0.109 | ||
| ECOG status | 0 | 30% | Ref | 0.035 | -- | -- | ||
| 1 | 30% | 1.23 | 0.88–1.70 | -- | -- | |||
| 2+ | 21% | 1.99 | 1.16–3.41 | -- | -- |
Variables not listed were not significantly associated with the given outcome in univariate analysis. For both models, ECOG status was not significant after multivariate adjustment and thus was excluded from the final models. AJCC M stage was not included in multivariate analysis, as it was co-linear with number of metastatic sites.
The median TTF was 8.0 months (95% CI 5.5–10.1, Figure 2C). In a univariate analysis, NLR ≥ 5 (Figure 2D), ECOG performance status, and mucosal melanoma were significantly associated with shorter TTF. NLR ≥ 5 and mucosal melanoma remained independently associated with shortened TTF after adjustment in a multivariate model (Table 2).
Increase in NLR during treatment is associated with shorter OS and TTF
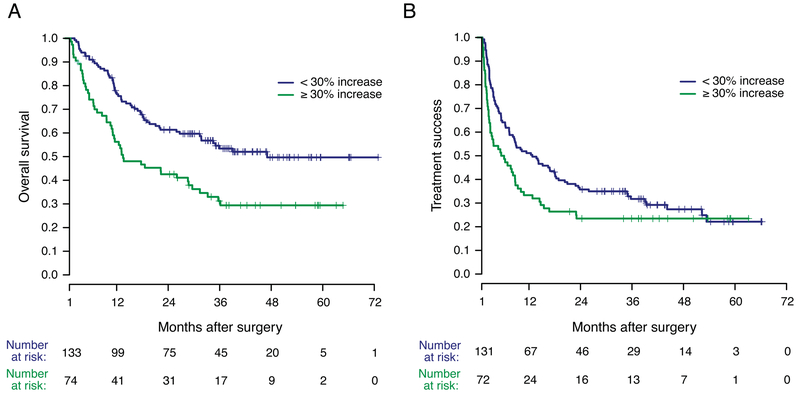
To determine whether change in NLR during treatment might serve as an early biomarker of treatment failure, NLR values were evaluated at baseline and prior to subsequent cycles of PD-1 inhibitor administration in a landmark analysis. An increase of ≥ 30% from the baseline NLR during the first two treatments was significantly associated with shorter OS (median OS: 47 vs. 13.5 months, p < 0.001, Figure 3A). An increase of ≥ 30% in NLR during the first two treatments was associated with a trend toward shorter TTF (median 12.5 months vs. 5.9 months, p = 0.05, Figure 3B).
Figure 3. Outcomes stratified by change in NLR during the first two cycles of PD-1 inhibition.
A) OS stratified by increase in NLR. B) TTF stratified by increase in NLR. Survival analysis was landmarked at 30 days to include only those patients whose change in NLR during the first two cycles of treatment could be assessed.
Baseline NLR and change in NLR during treatment provide additive risk stratification
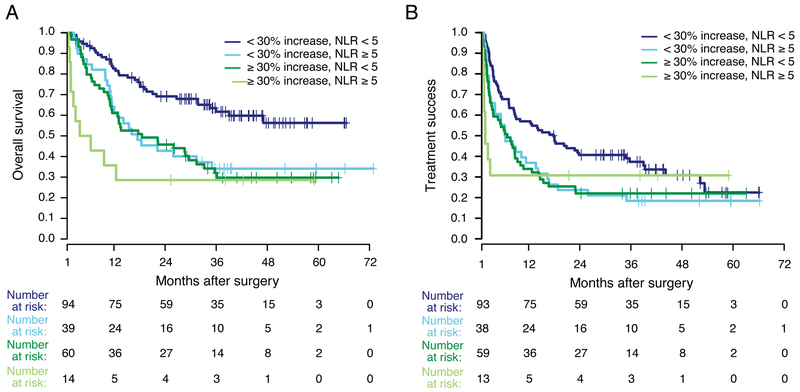
Patients were then stratified by both baseline NLR and change in NLR during the first two cycles of treatment to determine whether the prognostic impact was additive. The combination of both prognostic factors improved risk stratification for OS (p < 0.001, Figure 4A). In a landmark analysis, survival of patients with baseline NLR < 5 and a < 30% increase during the first two cycles (n = 95) was longest (median OS not reached). For patients with one adverse prognostic feature, survival was intermediate (median OS for NLR ≥ 5 and increase < 30%, 16.2 months; for NLR < 5 and increase of ≥ 30%, 18.6 months). Patients with baseline NLR ≥ 5 and an increase of ≥ 30% during early treatment (n=14) faced particularly high risk, with a median OS of 5.3 months and 64% of patients dying within the first year.
Figure 4. Outcomes stratified by baseline NLR (< 5 or ≥ 5) and change in NLR (< 30% or ≥ 30%) during the first two cycles of PD-1 inhibitor treatment.
A) OS stratified by baseline NLR and NLR increase. B) TTF stratified by baseline NLR and NLR increase. Survival analysis was landmarked at 30 days to include only those patients whose change in NLR during the first two cycles of treatment could be assessed.
Similarly, baseline NLR < 5 and a < 30% increase during the first two cycles was associated with prolonged TTF (median TTF = 17.4 months, p < 0.001, Figure 4B). TTF among patients with one adverse prognostic feature was intermediate (median for NLR ≥ 5 and increase < 30%, 6.5 months; for NLR < 5 and increase of ≥ 30%, 7.0 months). For patients with a baseline NLR ≥ 5 and an increase of ≥ 30% during the first two cycles, median TTF was just 1.8 months.
Discussion
We investigated the prognostic significance of NLR as a biomarker in a large cohort of patients with metastatic melanoma undergoing initial systemic treatment with PD-1 inhibitor monotherapy. Baseline NLR was found to be associated with both patient factors (ECOG performance status) as well as tumor factors (number of metastatic sites, unknown primary site [trend toward association]). Despite these associations, baseline elevation in the NLR was independently associated with shorter TTF and shorter OS. Furthermore, an increase in NLR during the first two cycles of treatment appears to serve as a dynamic prognostic biomarker for shorter TTF and OS.
Our findings may help explain the biologic relationship between NLR and prognosis in cancer patients. The prognostic significance of an elevated NLR is observed with remarkable consistency across many cancer types and stages of disease.1,2,7,13,14,22 What remains unclear is whether NLR elevation represents a reaction to immunosuppressive factors from the tumor, or alternatively reflects an intrinsic host variable (i.e. dysfunctional immune response) that leads to a more aggressive clinical course. We find that NLR shows a trend toward association with a primary tumor factor (primary melanoma site), and a significant association with a potential host factor (ECOG performance status), and a factor likely associated with the interaction of the two (number of involved metastatic sites). These associations indirectly support that NLR is reflective of a complex and individualized interaction between both host and tumor factors.
The mechanistic basis for the prognostic importance of NLR remains unclear. The lymphocyte has long been considered the primary effector cell of the antitumor response, and indeed the abundance of tumor-infiltrating lymphocytes appears to be prognostic as well as predictive of response to immune therapies in multiple cancer types.23–25 The role of the neutrophil in cancer is more controversial, with evidence suggesting that neutrophils can either inhibit or promote tumor growth.26,27 A recent study by Patel et al. proposed a potential mechanism to explain these seemingly incongruent findings by identifying distinct subpopulations of neutrophils in mouse models and patients with cancer. While a particularly immunosuppressive neutrophil phenotype is enriched in animal models of metastatic disease, early in the development of cancer, neutrophils appear to have increased migratory activity but lack immunosuppressive function.28 The mechanism driving this phenomenon remains to be fully defined, but this increased migratory activity outside of the bone marrow may explain the neutrophilia seen in many patients with cancer and help establish the immunosuppressive population of neutrophils that ultimately promotes tumor growth and underlies the poor prognosis associated with high NLR.
We show that baseline NLR ≥ 5 is independently prognostic for both TTF and OS in patients with stage IV metastatic melanoma undergoing initial PD-1 inhibition. These findings are consistent with the prognostic significance of NLR, particularly in melanoma.7,8,14,16,17,19 Despite the large clinical magnitude of the difference in survival and its independent significance, an elevated baseline NLR does not preclude long-term survival (30% of patients with an elevated baseline NLR survived >3 years, Figure 2B). These findings are consistent with other studies of NLR in the setting of checkpoint inhibition, which find that elevated baseline NLR alone does not appear to have a sufficient prognostic magnitude to merit withholding PD-1 inhibitor therapy.14,16,17,19,29 Rather, an elevated NLR may identify a patient population of particular interest for clinical trials testing PD-1 inhibition in combination with additional agents.
Our findings directly corroborate those of a recent study by Capone et al. examining the outcomes of 97 patients treated with nivolumab stratified by NLR.29 In this study, which used the same NLR cutoff, a very similar proportion of patients (~25%) demonstrated an elevated baseline NLR, and baseline NLR was highly prognostic for both OS and PFS. We report similar results in a large, independent cohort from an international center. Taken together, these studies provide strong evidence that baseline NLR can serve as a readily available additional prognostic marker in patients with metastatic melanoma undergoing PD-1 inhibition.
We sought to build on the prognostic utility of baseline NLR by additionally evaluating the association with early change in NLR during treatment. Our initial study in patients treated with ipilimumab suggested that an increase of 30% in NLR during treatment was strongly associated with worse OS, but this change was not prognostic in patients treated with BRAF inhibitors.14 In the current study, the difference in OS was striking, with a 47-month median OS in patients whose NLR did not increase ≥ 30% after the first two cycles, compared with 13.5 months in those whose NLR did rise above the cut-point. Other than our previous study in patients treated with ipilimumab, we are not aware of groups who have analyzed change in NLR on treatment in melanoma. A recent study in patients treated with immune checkpoint blockade for renal cell carcinoma, however, demonstrated quite similarly that an increase in NLR of ≥ 25% was strongly associated with worse PFS and OS.30
In the current study, differences in TTF between patient groups stratified by change in NLR were not nearly as pronounced as those seen in OS. Although the difference in median TTF (12.8 vs. 5.9 months) was quite substantial, the landmark analysis demonstrated only a trend toward statistical significance (p=0.05), and the curves appear to converge around the 3-year time point (Figure 3B), such that approximately 25% of patients experienced long-term treatment success regardless of early change in NLR. Thus, while change in NLR does appear likely to be prognostic, it is not universally associated with failure of checkpoint inhibition.
Combining baseline NLR and change in NLR enhances the prognostic impact. Among patients whose NLR did not increase by ≥ 30%, those with low baseline NLR (45% of patients) had especially favorable prognoses. Not only was the median OS of this cohort prolonged (not reached), but the plateau in the OS curve remained nearly 30% above that for other patient groups. Conversely, outcomes among patients with both elevated baseline NLR and an early on-treatment increase of ≥ 30% (7% of patients) were extremely poor (median OS 5.3 months). Although this cohort of patients was small, these findings may support an early transition to alternative therapy among those with both biomarkers. Even in this very high-risk group, however, a subset of patients did survive long-term. To our knowledge, baseline NLR and on-treatment change in NLR have not previously been combined to risk-stratify melanoma patients treated with PD-1 inhibition. Our prior study suggests that change in NLR during treatment may be a biomarker specific to predicting outcomes of checkpoint inhibitor therapy. Future study to elucidate this specificity will further our understanding of the prognostic value of NLR.
The most notable limitations of the study are related to its retrospective nature. For example, patients who progressed rapidly on PD-1 inhibition were transitioned to other treatments, meaning that patients with NLR values available after 6 treatments (n=108 of 224) were those who received clinical benefit from treatment. To control for this, we limited the prognostic analysis of change in NLR to only include the first two cycles of treatment. This choice also meant that we assessed the prognostic value of change in NLR early enough in treatment that it could serve as a clinically useful time point for prediction of response. To further control for the time bias introduced by continued treatment, survival statistics utilized a landmark analysis. Finally, the endpoint was TTF, which is slightly more subjective than the more commonly reported progression-free survival (PFS) or overall response rate (ORR). Indeed, PFS or ORR would be preferred measures of treatment efficacy in the prospective setting, but retrospective assessment of these outcomes is fraught with challenges, especially when RECIST or irRECIST-defined progression are not recorded, as in our institutional database.21 Other studies have found baseline NLR to be associated with PFS and/or ORR in melanoma patients treated with a checkpoint inhibition;14,17,19,29 it remains to be determined if change in NLR during treatment would be similarly associated.
Conclusion
In a cohort of patients with metastatic melanoma treated with initial PD-1 inhibition, both baseline NLR and change in NLR early during treatment are strongly prognostic of survival, and prognostication is improved when both biomarkers are used together. The combination of an elevated baseline NLR and an increase in NLR early during treatment is associated with extremely poor outcomes and may be sufficient reason to change therapy in this small subgroup. Baseline NLR is strongly associated with both tumor and host factors, suggesting that NLR in patients with cancer reflects a complex interaction between the tumor and the host immune response.
Acknowledgments:
The authors would like to thank Jessica Moore for her expert editorial assistance with both the manuscript and figures.
Funding: This study was supported in part by Cancer Center Support Grant NIH/NCI P30 CA008748.
Disclosures: KSP reports stock ownership in Johnson and Johnson, Pfizer, Catalyst Biotech, Viking Therapeutics. MAP reports consulting fees from BMS, Merck, Array BioPharma, Novartis, Incyte, NewLink Genetics, and Aduro; honoraria from BMS and Merck; institutional support from RGenix, Infinity, BMS, Merck, Array BioPharma, Novartis, and AstraZeneca.
Footnotes
The remaining authors have no financial relationships to disclose.
References
- 1.Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. [DOI] [PubMed] [Google Scholar]
- 2.Bowen RC, Little NAB, Harmer JR, et al. Neutrophil-to-lymphocyte ratio as prognostic indicator in gastrointestinal cancers: a systematic review and meta-analysis. Oncotarget. 2017;8(19):32171–32189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Proctor MJ, McMillan DC, Horgan PG, Fletcher CD, Talwar D, Morrison DS. Systemic inflammation predicts all-cause mortality: a glasgow inflammation outcome study. PLoS One. 2015;10(3):e0116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillum RF, Mussolino ME, Madans JH. Counts of neutrophils, lymphocytes, and monocytes, cause-specific mortality and coronary heart disease: the NHANES-I epidemiologic follow-up study. Ann Epidemiol. 2005;15(4):266–271. [DOI] [PubMed] [Google Scholar]
- 5.Leitch EF, Chakrabarti M, Crozier JE, et al. Comparison of the prognostic value of selected markers of the systemic inflammatory response in patients with colorectal cancer. Br J Cancer. 2007;97(9):1266–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis JL, Moutinho V, Jr., Panageas KS, Coit DG. A peripheral blood biomarker estimates probability of survival: the neutrophil-lymphocyte ratio in noncancer patients. Biomark Med. 2016;10(9):953–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis JL, Langan RC, Panageas KS, et al. Elevated Blood Neutrophil-to-Lymphocyte Ratio: A Readily Available Biomarker Associated with Death due to Disease in High Risk Nonmetastatic Melanoma. Ann Surg Oncol. 2017;24(7):1989–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma J, Kuzman J, Ray A, et al. Neutrophil-to-lymphocyte Ratio (NLR) as a predictor for recurrence in patients with stage III melanoma. Sci Rep. 2018;8(1):4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cananzi FC, Dalgleish A, Mudan S. Surgical management of intraabdominal metastases from melanoma: role of the neutrophil to lymphocyte ratio as a potential prognostic factor. World J Surg. 2014;38(6):1542–1550. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt H, Bastholt L, Geertsen P, et al. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. Br J Cancer. 2005;93(3):273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng Z, Wen H, Bi R, et al. Preoperative Neutrophil-to-Lymphocyte Ratio as a Predictive and Prognostic Factor for High-Grade Serous Ovarian Cancer. PLoS One. 2016;11(5):e0156101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nuhn P, Vaghasia AM, Goyal J, et al. Association of pretreatment neutrophil-to-lymphocyte ratio (NLR) and overall survival (OS) in patients with metastatic castration-resistant prostate cancer (mCRPC) treated with first-line docetaxel. BJU Int. 2014;114(6b):E11–E17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chrom P, Stec R, Semeniuk-Wojtas A, Bodnar L, Spencer NJ, Szczylik C. Fuhrman Grade and Neutrophil-To-Lymphocyte Ratio Influence on Survival in Patients With Metastatic Renal Cell Carcinoma Treated With First-Line Tyrosine Kinase Inhibitors. Clin Genitourin Cancer. 2016;14(5):457–464. [DOI] [PubMed] [Google Scholar]
- 14.Cassidy MR, Wolchok RE, Zheng J, et al. Neutrophil to Lymphocyte Ratio is Associated With Outcome During Ipilimumab Treatment. EBioMedicine. 2017;18:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sacdalan DB, Lucero JA, Sacdalan DL. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. Onco Targets Ther. 2018;11:955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaragoza J, Caille A, Beneton N, et al. High neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanoma. Br J Dermatol. 2016;174(1):146–151. [DOI] [PubMed] [Google Scholar]
- 17.Rosner S, Kwong E, Shoushtari AN, et al. Peripheral blood clinical laboratory variables associated with outcomes following combination nivolumab and ipilimumab immunotherapy in melanoma. Cancer Med. 2018;7(3):690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura Y, Kitano S, Takahashi A, et al. Nivolumab for advanced melanoma: pretreatment prognostic factors and early outcome markers during therapy. Oncotarget. 2016;7(47):77404–77415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrucci PF, Ascierto PA, Pigozzo J, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. 2016;27(4):732–738. [DOI] [PubMed] [Google Scholar]
- 20.Bagley SJ, Kothari S, Aggarwal C, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1–7. [DOI] [PubMed] [Google Scholar]
- 21.Shoushtari AN, Friedman CF, Navid-Azarbaijani P, et al. Measuring Toxic Effects and Time to Treatment Failure for Nivolumab Plus Ipilimumab in Melanoma. JAMA Oncol. 2018;4(1):98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rochet NM, Kottschade LA, Grotz TE, Porrata LF, Markovic SN. The prognostic role of the preoperative absolute lymphocyte count and absolute monocyte count in patients with resected advanced melanoma. Am J Clin Oncol. 2015;38(3):252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pages F, Mlecnik B, Marliot F, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391(10135):2128–2139. [DOI] [PubMed] [Google Scholar]
- 25.Trujillo JA, Sweis RF, Bao R, Luke JJ. T Cell-Inflamed versus Non-T Cell-Inflamed Tumors: A Conceptual Framework for Cancer Immunotherapy Drug Development and Combination Therapy Selection. Cancer Immunol Res. 2018;6(9):990–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16(7):431–446. [DOI] [PubMed] [Google Scholar]
- 27.Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest. 2014;124(12):5466–5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel S, Fu S, Mastio J, et al. Unique pattern of neutrophil migration and function during tumor progression. Nat Immunol. 2018;19(11):1236–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Capone M, Giannarelli D, Mallardo D, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. 2018;6(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lalani AA, Xie W, Martini DJ, et al. Change in Neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J Immunother Cancer. 2018;6(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]