Abstract
Reward history is a powerful determinant of what we pay attention to. This influence of reward on attention varies substantially across individuals, being related to a variety of personality variables and clinical conditions. Currently, the ability to measure and quantify attention-to-reward is restricted to the use of psychophysical laboratory tasks, which limits research into the construct in a variety of ways. In the present study, we introduce a questionnaire designed to provide a brief and accessible means of assessing attention-to-reward. Scores on the questionnaire correlate with other measures known to be related to attention-to-reward and predict performance on multiple laboratory tasks measuring the construct. In demonstrating this relationship, we also provide evidence that attention-to-reward as measured in the lab, an automatic and implicit bias in information processing, is related to overt behaviors and motivations in everyday life as assessed via the questionnaire. Variation in scores on the questionnaire is additionally associated with a distinct biomarker in brain connectivity, and the questionnaire exhibits acceptable test-retest reliability. Overall, the value-driven attention questionnaire (VDAQ) provides a useful proxy-measure of attention-to-reward that is much more accessible than typical laboratory assessments.
Keywords: attention, reward learning, personality, questionnaire, functional connectivity, fMRI
1. Introduction
The world is filled with a vast array of perceptual information, far more than we are capable of representing in our brains at any one moment in time (e.g., Desimone & Duncan, 1995; Mack & Rock, 1998; Rensink et al., 1997). In this sense, what we pay attention to determines what we have mental access to when we assess our environment and arrive at behavioral decisions. Attention can be divided into different, dissociable components, including alerting, orienting, and executive attention (Fan et al., 2002, 2005). In the present study, we focus specifically on the orienting of attention, or selective attention, and subsequent references to attention should be interpreted within this context.
What we pay attention to is determined by several different factors, including our current goals (i.e., what we are looking for; Folk et al., 1992; Wolfe et al., 1989), motivations (e.g., Kiss et al., 2009; Jimura et al., 2010; Navalpakkam et al., 2009, 2010; Pessoa & Engelmann, 2010), and the physical salience of different stimuli (e.g., brightness and color contrast; Theeuwes, 1992, 2010; Yantis & Jonides, 1984). More recently, it has been shown that reward history can serve as a powerful determinant of attention (e.g., Anderson et al., 2011a; Bourgeois et al., 2017; Della Libera & Chelazzi, 2009; Hickey et al., 2010a; Raymond & O’Brien, 2009), independently of the other established attention mechanisms (Anderson et al., 2011b). That is, attention is automatically drawn to stimuli previously associated with reward (see Anderson, 2013, 2016a; Bourgeois et al., 2016; Failing & Theeuwes, 2018, for reviews).
It is not the case that reward history affects attention similarly across individuals. Rather, substantial variation in how susceptible an individual is to the influence of reward history on attention has been observed, with some individuals showing a very large effect of reward and others showing no measurable effect at all (e.g., Anderson et al., 2011b, 2013a, 2014b, 2016b; Anderson & Yantis, 2012; Hickey et al., 2010b). Variability in this attention measure has been linked to a variety of personality traits, behaviors, and psychopathologies. These include trait impulsiveness (Anderson et al., 2011b, 2013a, 2016b), behavioral activation system (BAS) traits (Hickey et al., 2010b; Hickey & Peelen, 2015, 2017; Qi et al., 2013), drug addiction (Albertella et al., 2017; Anderson, 2016b; Anderson et al., 2013a, 2016a), impulsive, high-risk behaviors (Anderson et al., 2016a), depression (Anderson et al., 2014b, 2017a), and attention-deficit hyperactivity disorder (ADHD; Sali et al., 2018). Such evidence suggests that attention-to-reward either contributes to these individual characteristics, or itself reflects an individual characteristic that is related to them.
These prior studies suggest that attention-to-reward offers a promising individual differences measure that may be useful in predicting a variety of behavioral, clinical, and life outcomes on a broader scale. Examination of the nature of this attention construct and its predictive power, however, is severely hampered by several interrelated factors. The measurement of attention-to-reward is currently limited to performance in a psychophysical laboratory task (e.g., Anderson et al., 2011b; Anderson & Kim, 2018a, in press; Anderson & Yantis, 2012). Such tasks require specialized equipment and training to administer and analyze the resulting data, making them inaccessible to researchers without the appropriate expertise and equipment. Furthermore, such tasks require dedicated testing space appropriate for psychophysical measurement (e.g., light and sound attenuated, apparatus for stabilizing head position), and typically take an hour or more to administer, both of which substantially limit the sample of participants that can be tested in a reasonable timeframe. These sorts of laboratory tasks are often not well suited for individuals with cognitive or motor deficits, as they assume the ability to respond rapidly and accurately to visual events, and modifications to the task are typical in order to better accommodate patient populations (e.g., Anderson et al., 2013a, 2016b; Sali et al., 2018).
Given these limitations, the ability to obtain a measure of attention-to-reward for an individual that (a) requires minimal training and resources to administer, and (b) can be administered quickly and easily to a large and diverse sample of participants, would be desirable. To this end, in the present study, we introduce a simple questionnaire assessment that provides a useful proxy-measure of attention-to-reward. Experimental measures of attention-to-reward have their own unique advantages, most notably the direct assessment of visual information processing (with visual information processing being central to the construct), and we do not mean to suggest that such measures could be supplanted with a questionnaire approach. Rather, we acknowledge that experimental measures are not always feasible given certain research questions or for laboratories without access to relevant equipment and expertise, and in these cases a proxy-measure would have clear utility.
The questionnaire approach adopted in the present study also provides an opportunity to examine the relationship between attention-to-reward and specific behaviors and motivations in everyday life. Attention-to-reward, as measured in a psychophysical experiment, reflects an automatic/involuntary process (e.g., Anderson et al., 2011b; Anderson, 2016a, 2017a) to which participants have minimal conscious access (e.g., Anderson, 2015a, 2015b; Leganes-Fonteneau, et al. 2018; Seitz et al., 2009). Under certain circumstances, attention-to-reward biases can persist even after the reward associated with a stimulus has been devalued (De Tommaso et al., 2017, but see Pool et al., 2014). In this sense, attention-to-reward cannot be reduced to a consequence of explicit reward-seeking. Theories of incentive salience posit that reward pursuit behaviors, such as drug-seeking, can dissociate from the explicit goals of the individual, such as the goal to maintain abstinence from a drug of abuse, and automatic attentional processes are believed to play a role in facilitating such pursuit (e.g., Berridge, 2012; Berridge & Robinson, 2016).
It remains an open question the extent to which attention-to-reward, as automatic and implicit as it can be, is related to overt reward-seeking behaviors and conscious motivations and experiences (e.g., cue-driven excitement or desire). Evidence suggestive of such a relationship in the literature can be found in the link between attention-to-reward and personality measures probing explicit attitudes and experiences. As is evident from the examples provided below, however, existing questionnaires probe general traits and dispositions rather than specific behaviors, situations, and internal conscious experiences. Demonstrating an association between attention-to-reward as measured in a psychophysical experiment and real-life indicators of reward-seeking and biased information processing would speak to both the ecological validity of the experimental construct of attention-to-reward and the relationship between automatic attentional processes and explicit behaviors.
We begin by providing an overview of the development of the questionnaire we created, which was then validated across six experiments. In Experiment 1, we establish convergent and discriminant validity. In Experiment 2, we show that scores on this questionnaire account for a significant portion of variance in task performance specifically attributable to reward’s influence on attention. In Experiments 3 and 4, we probe the generalizability of the measure and predict how eye movements are affected by reward history in a free-viewing task (Experiment 3) and the influence of rewards on decision-making when rewards and punishments simultaneously compete for attention (Experiment 4). In Experiment 5, we use resting-state functional magnetic resonance imaging (fMRI) to establish a biomarker of attention-to-reward as assessed via the questionnaire, and in Experiment 6 we establish test-retest reliability.
2. Questionnaire Development
Currently, there is no questionnaire assessment dedicated to the construct of attention-to-reward. Given their conceptual overlap, the two questionnaires that have most frequently been related to attention-to-reward are the BIS/BAS scale (Carver & White, 1994) and the Barratt Impulsiveness Scale (BIS-11; Patton et al., 1995). The BIS/BAS scale contains several items assessing both the experience of reward (e.g., “when good things happen to me, it affects me strongly,” “when I get something I want, I feel excited and energized”), and the role of reward as a motivator (e.g., “when I want something, I usually go all-out to get it,” “I go out of my way to get things I want”), which comprise the reward responsiveness and reward drive sub-scales of the measure, respectively. Such constructs should play an important role in attention-to-reward, but have no direct relationship to attention, especially not attentional bias (paying attention to something even though it is not consistent with current goal considerations, such as paying attention to high-calorie food in spite of one’s goal to eat healthy). Attention-to-reward reflects the degree to which reward influences the attention system specifically (rather than behavior more broadly) and especially the degree to which reward considerations can overpower current goal considerations in information processing, neither of which are captured by the BIS/BAS scale. Correspondingly, individual differences in a measure of controlled information processing, working memory capacity—which is unrelated to reward processing per se—have also been shown to be correlated with attention-to-reward (Anderson et al., 2011b, 2013a, 2016b; Anderson & Yantis, 2012), demonstrating that attention-to-reward is not reducible to a nonspecific influence of reward on behavior.
Similarly, the Barratt Impulsiveness Scale (Patton et al., 1995) does not contain questions probing attention-to-reward. Although the questionnaire does contain items relevant to the construct of attention, these questions probe general difficulty remaining focused (e.g., “I don’t ‘pay attention,’” “I concentrate easily,” “I ‘squirm’ at plays or lectures,” “I can only think about one thing at a time”). It is therefore unclear to what degree reward considerations are responsible for the responses that a person gives to such items, or to what degree broader attentional or impulse control issues might be at play.
In the present study, we sought to develop a questionnaire that specifically probes the influence of reward on attention. To this end, we created sixteen questions that were designed to capture specific reward-related looking behaviors (e.g., “when I see an attractive person, I have a hard time taking my eyes off of them”), the specific content of extraneous thoughts/attention (e.g., “when I daydream, it is often about things I want”), the degree to which reward considerations draw attention in an unwanted way akin to distraction (e.g., “if the TV is on in the background, I find it very distracting”), and how potentially reward-related visual and auditory events affect a person (e.g., “when tasty food is placed in the open, I find it very tempting”). The resulting questionnaire (see Appendix A) was intended to reflect this breadth of attention-to-reward in everyday life and assumes a single underlying construct by which reward-related stimuli are found to (1) be attractive and highly noticeable (e.g., Anderson, 2016a; Della Libera & Chelazzi, 2009; Hickey et al., 2010b), (2) be difficult to ignore (e.g., Anderson et al., 2011b), and (3) exert a strong influence on approach behavior that is difficult to suppress (e.g., Anderson, 2017a; Anderson et al., 2016a; Kim & Anderson, 2019). The sixteen items of the questionnaire probe the cue-triggered wanting of reward; these items do not directly probe the perceptual learning aspects of attention-to-reward by which sensory representations are modulated by reward history (see, e.g., Anderson & Kim, 2019; Hickey & Peelen, 2015; Seitz et al., 2009; Serences, 2008; Serences & Saproo, 2010; see Anderson, 2019, for a review), given that participants presumably have minimal conscious access to such learning-dependent changes (e.g., Anderson, 2015a, 2015b; Leganes-Fonteneau, et al. 2018; Seitz et al., 2009).
To examine whether the sixteen items fit with the hypothesized single-construct structure, and to assess the reliability of the measure, we had 363 undergraduate students complete the questionnaire (251 female, ages 18–35). The brief 16-item measure exhibited acceptable reliability, Cronbach’s α = 0.76. None of the items proved detrimental to the reliability of the measure, such that Cronbach’s α was never higher with any single item removed. Exploratory factor analysis revealed that each question loaded positively onto a single factor, which explained 23% of the total variance. No other factor explained more than 8% of the remaining variance, and maximum-likelihood extraction with a single factor indicated that this one factor adequately explained the covariance among items, χ2(104) = 190.3, p < 0.001. Therefore, all sixteen questions were retained for the purposes of assessing the validity of the measure.
3. Experiment 1
Individual variability in attention-to-reward has been related to trait impulsiveness (Anderson et al., 2011b, 2013a, 2016b) using the Barratt Impulsiveness Scale (BIS-11; Patton et al., 1995) and both the reward responsiveness (Hickey & Peelen, 2015) and reward drive (Hickey et al., 2010b; Qi et al., 2013) components of the BIS/BAS scale (Carver & White, 1994). Therefore, a questionnaire that successfully captures variability in attention-to-reward should similarly covary with these personality measures. In Experiment 1, we administered the 16-item questionnaire that we created to provide a proxy measure of reward’s influence on attention, which we refer to as the value-driven attention questionnaire (VDAQ). We also administered the BIS/BAS scale (Carver & White, 1994) and the BIS-11 (Patton et al., 1995). As a measure of discriminant validity, the autism quotient (AQ; Baron-Cohen et al., 2001) scale was also included, as attention-to-reward was not predicted to be related to autistic traits (see Anderson & Kim, 2018b).
3.1. Methods
3.1.1. Participants.
226 participants, ages 18–35 (gender: 54 male, 167 female, 5 not reported) were recruited from the Texas A&M University community and participated in exchange for course credit. The present study was added to other study protocols then active in the lab, unrelated to the present study (and thus not reported here), in order to maximize efficiency in data collection. In each case, the participant completed the present study before completing other experimental tasks. All participants in all experiments provided informed consent, and the procedures were approved by the Texas A&M University Institutional Review Board and conformed to the principles outlined in the Declaration of Helsinki.
3.1.2. Questionnaire Assessments.
Participants completed the VDAQ (see Appendix A), BIS/BAS scale (Carver & White, 1994), BIS-11 (Patton et al., 1995) and AQ scale (Baron-Cohen et al., 2001). Total score on the BIS-11 was computed as the measure of trait impulsiveness, and total BAS score was sub-divided into the drive, responsiveness, and fun-seeking components as advocated by Carver and White (1994) and in prior studies assessing attention-to-reward (Hickey et al., 2010b; Hickey & Peelen, 2015; Qi et al., 2013). A participant’s AQ was computed as the total number of autistic traits endorsed by the individual (Baron-Cohen et al., 2001). A total VDAQ score was calculated by summing the scores across the 16 individual items using a 4-point Likert scale ranging from 1 “The opposite of me” to 4 “Very true of me.” Given six different dependent measures with which the VDAQ was correlated, we applied Bonferroni correction when determining statistical significance (α = 0.008).
3.1.3. Procedure.
All questionnaires were completed privately in a small testing room. Participants completed paper-and-pencil implementations of the questionnaires. Each paper-and-pencil questionnaire was scored by two individuals, and any disagreement in score was reconciled by a third individual.
3.2. Results
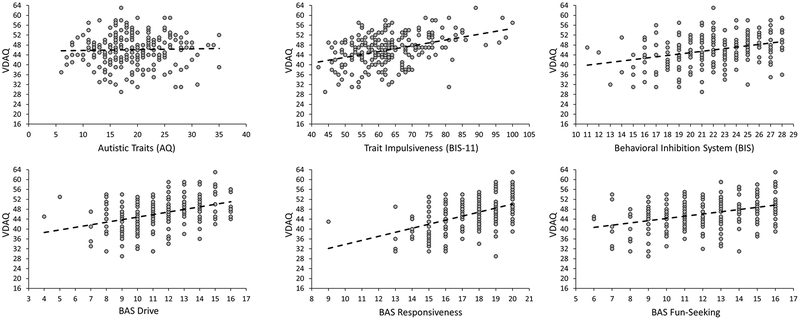
Scores on the VDAQ were significantly correlated with trait impulsiveness, r = 0.401, p < 0.001, and all three BAS traits, BAS drive: r = 0.381, p < 0.001, BAS responsiveness: r = 0.501, p < 0.001, BAS fun-seeking: r = 0.356, p < 0.001. Unexpectedly, VDAQ scores also correlated with behavioral inhibition system (BIS) traits, r = 0.330, p < 0.001; however, total BAS score was a significantly better predictor of VDAQ score than BIS score, z = 2.66, p = 0.008. As expected, VDAQ score was unrelated to autistic traits, r = 0.030, p = 0.654. Scatterplots for these correlations are depicted in Figure 1.
Figure 1.
Scatterplots showing the relationship between VDAQ score and the other questionnaire assessments obtained.
A multiple regression model with all three BAS component scores, BIS score, and trait impulsiveness as predictors revealed that BAS drive, BAS responsiveness, BIS traits, and trait impulsiveness all predicted unique variance in VDAQ score, βs > 0.209, ps < 0.001, with a combined R2 = 0.472. BAS fun-seeking did not contribute unique variance, β = 0.042, p = 0.498, which is consistent with prior studies that have only implicated the other two BAS traits in attention-to-reward (Hickey et al., 2010b, Hickey & Peelen, 2015; Qi et al., 2013); further analysis revealed that trait impulsiveness was significantly correlated with fun-seeking, r = 0.458, p < 0.001, more so than with any other predictor, zs > 3.95, ps < 0.001. BIS traits were also significantly correlated with BAS drive, r = 0.259, p < 0.001, which may partly explain the relationship between BIS traits and VDAQ score in our sample.
Females scored marginally higher than males on the VDAQ (46.5 vs 44.4), t(219) = 2.09, p = 0.038, BAS responsiveness (17.7 vs 17.1), t(219) = 1.90, p = 0.059, and BAS fun-seeking (12.2 vs 11.5), t(219) = 1.98, p = 0.049, although none of these passed corrections for multiple comparisons. Only the BIS showed a significant difference (22.7 vs 20.8), t(219) = 3.34, p = 0.001 (other measures: t < 1).
3.3. Discussion
Scores on the VDAQ significantly correlated with three personality traits previously demonstrated to predict the magnitude of attentional capture by reward cues: trait impulsiveness (Anderson et al., 2011b, 2013a, 2016b) and the reward drive (Hickey et al., 2010b; Qi et al., 2013) and reward responsiveness (Hickey & Peelen, 2015) components of the BAS. The sizes of these correlations were generally in the moderate range, ranging from 0.33 to 0.5. As a measure of discriminant validity, we observed that VDAQ scores were unrelated to autistic traits (see Anderson & Kim, 2018b). No single measure accounted for more than 26% of the variance in VDAQ score, and when taken together these measures only accounted for 47.2% of the variance, suggesting that VDAQ scores were not redundant with any of these predictors. The results indicate that VDAQ scores are related to the traits that would be expected if this questionnaire assessment were to provide a proxy measure of attention-to-reward as assessed from performance in a psychophysical experiment.
4. Experiment 2
In Experiment 2, we assess the ability of the VDAQ to predict distraction by reward cues in a well-validated implementation of the value-driven attentional capture paradigm (Anderson et al., 2011b; Anderson & Kim, 2019, in press; Anderson & Yantis, 2012). Participants first learned to associate red and green colored circles with reward, receiving a small amount of money each time they fixated the color-defined target. In a subsequent test phase, participants oriented to a uniquely-shaped target in a task in which the color of shapes was explicitly task-irrelevant and rewards were no longer available. Value-driven attentional capture, our measure of attention-to-reward, was defined as the probability of fixating the high-value distractor relative to a value-neutral non-target. This measure of attention-to-reward shows high test-retest reliability (Anderson & Kim, in press), making it well suited to examine individual differences. To focus our analysis on the influence of reward in biasing attention, rather than distractibility more generally, we also recorded a measure of attentional capture by physically salient stimuli not previously associated with reward and included the corresponding measure of distraction as a covariate (see Anderson & Kim, 2019).
4.1. Methods
4.1.1. Participants.
Fifty participants (18–35 years of age, M = 21.2y (SD = 2.8y), 32 female, 46 right-handed) were recruited from the Texas A&M University community. The sample size was determined a priori to allow significant correlations as small as r = 0.28, and smaller correlations would fall below our goals for the predictive power of the VDAQ. Participants were compensated with money earned in the experimental task. All reported normal or corrected-to-normal visual acuity and normal color vision. Data from nine participants were replaced due an inability to reliably track eye position (resulting in a failure to register a target fixation on >25% of trials). All procedures were approved by the Texas A&M University Institutional Review Board and conformed with the principles outlined in the Declaration of Helsinki.
4.1.2. Apparatus.
A Dell OptiPlex equipped with MATLAB software and Psychophysics Toolbox extensions (Brainard, 1997) was used to present the stimuli on a Dell P2717H monitor. The participants viewed the monitor from a distance of approximately 70 cm in a dimly lit room. Eye position was monitored using an EyeLink 1000 Plus desktop mount eye tracker (SR Research). Head position was maintained using an adjustable chin rest (SR Research).
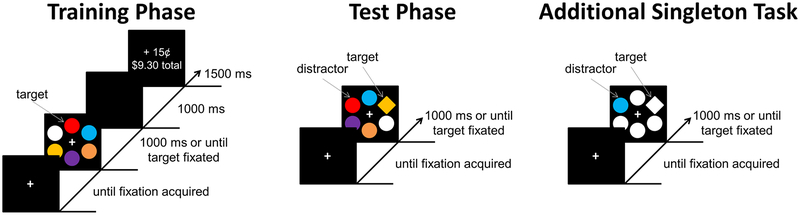
4.1.3. Training Phase.
Each trial consisted of a fixation display, a search array, and a reward feedback display (see Figure 2). The fixation display remained on screen until eye position was registered within 1.1° of the center of the fixation cross for a continuous period of 500 ms. The search array was then presented for 1000 ms or until a fixation on the target was registered. The search array consisted of six colored circles, one of which was red (CIE: u’ = 0.485, v’ = 0.523, cd/m2 = 40.63) or green (CIE: u’ = 0.135, v’ = 0.566, cd/m2 = 146.58) on each trial. The color of the other five circles was drawn randomly from the set {blue (CIE: u’ = 0.152, v’ = 0.302, cd/m2 = 47.81), cyan (CIE: u’ = 0.142, v’ = 0.451, cd/m2 = 164.02), purple (CIE: u’ = 0.299, v’ = 0.317, cd/m2 = 57.79), orange (CIE: u’ = 0.341, v’ = 0.541, cd/m2 = 71.61), yellow (CIE: u’ = 0.216, v’ = 0.557, cd/m2 = 187.53), white (CIE: u’ = 0.202, v’ = 0.463, cd/m2 = 205.12)} on each trial without replacement. Each circle was approximately 3.6° visual angle in diameter, placed at equal intervals along an imaginary circle with a radius of 10.2°. The reward feedback display was presented for 1500 ms and consisted of the money earned on the current trial along with the updated total earnings. If the participant failed to fixate the target before the timeout limit, the word “Miss” was presented in place of the money earned. A 1000 ms blank screen was inserted between the search and feedback displays, and each trial concluded with a 500 ms blank interval.
Figure 2.
Example trial for each phase of Experiment 2. Participants were rewarded for fixating color-defined targets in the Training Phase. These reward-associated colors then served as task-irrelevant distractors during the Test Phase, in which participants searched for the uniquely-shaped target. Finally, susceptibility to distraction by non-reward-related stimuli was measured using the Additional Singleton Task, which was similar to the test phase except that the critical distractor was a uniquely-colored item not previously associated with reward.
Participants were instructed to fixate (“look directly at”) the red or green circle on each trial and were informed that they would earn a small amount of money for every trial on which they fixated the target before the time-out limit. Red and green target circles appeared equally-often across trials within a block, with either color appearing equally-often in each of the six stimulus positions. Correctly fixating one color target (red or green, counterbalanced across participants) was associated with an 80% probability of a high reward of 15¢ and a 20% probability of a low reward of 3¢ (high-value color), while for the other target color these percentages were reversed (low-value color). Each block consisted of 60 trials, the order of which was randomized, and participants completed four blocks for a total of 240 trials.
4.1.4. Test Phase.
Each trial consisted of a fixation display (until fixation was acquired for a continuous period of 500 ms), a search array (1000 ms or until a fixation on the target was registered), a 1000 ms blank interval, and, in the event of an incorrect response, a feedback display (1000 ms). For correct responses, the feedback display was omitted. Each trial concluded with a 500 ms blank interval (Figure 2). The target was now defined as the unique shape, either a diamond among circles or a circle among diamonds (equally-often), on which participants were instructed to fixate. The colors of the shapes were irrelevant to the task, and participants were instructed to ignore color. The feedback display consisted of the word “Miss” presented at the center of the screen. To maximize sensitivity to attentional capture by the distractors, participants were not required to fixate the target first to avoid receiving “Miss” feedback (i.e., they only needed to fixate the target within the 1000 ms limit).
One of the non-target shapes was rendered in the color of the formerly high-value target (high-value distractor) on one-third of the trials, and likewise in the color of the formerly low-value target (low-value distractor) on another third of the trials. On the remaining one-third of trials, none of the shapes were rendered in the color of a formerly reward-predictive target (distractor-absent trials). Stimuli other than the critical distractor were drawn from the same color set used for non-targets in the test phase, and the same stimulus positions were used. Targets and distractors appeared equally-often in each of the six possible stimulus positions across trials within a block. Each block consisted of 90 trials, the order of which was randomized, and participants completed three blocks for a total of 270 trials.
4.1.5. Additional Singleton Task.
The additional singleton task (see Theeuwes, 1992) was similar to the test phase, except that on distractor-absent trials, the all of the shapes were either blue or white (Figure 2). On distractor-present trials, one of the non-targets was rendered in the color not used for the other shapes on that trial (e.g., blue circle among four white circles and a white diamond). The distractor was present on half of all trials within a block, and was blue and white equally-often. The target was blue and white equally-often. Targets and distractors appeared equally-often in each of the six possible stimulus positions across trials within a block. Each block consisted of 60 trials, the order of which was randomized, and participants completed three blocks for a total of 180 trials.
4.1.6. Procedure.
Participants completed the VDAQ first, prior to completing the experimental tasks. As participants are generally unaware of attentional capture and indeed value-driven attentional capture can even occur in the absence of awareness of the reward contingencies (see, e.g., Anderson, 2015a, 2015b; Leganes-Fonteneau, et al. 2018; Seitz et al., 2009), it is unlikely that participants were intentionally more or less distractible in an effort to behave in a self-consistent manner. Participants completed the training phase, test phase, and additional singleton task in that order.
For eye tracking, the position of the right eye was monitored at 1000 Hz. Eye position was calibrated prior to each block of trials using 9-point calibration, and was manually drift corrected by the experimenter as necessary (the next trial could not begin until 500 ms of continuous fixation was registered at the center of the screen). Each of the three experimental tasks was preceded by interactive instructions that included practice trials with and without the timeout limit. Participants were paid the amount of money earned in the training phase at the completion of the experiment.
4.1.7. Data Analysis.
We measured which of the six shape stimuli was initially fixated on each trial, as well as response time (RT, time to fixate the target). Fixation of a stimulus was registered if eye position remained within a region extending 0.7° around the stimulus for a continuous period of at least 50 ms (100 ms on the target to trigger the termination of the stimulus array). On distractor-absent trials, in order to quantify the probability of initially fixating a distractor for the sake of comparison, one of the non-targets was dummy-coded as the critical distractor on each trial using the same parameters that were used to define the position of the critical distractors on distractor-present trials (i.e., same counterbalance of position relative to the target position; note that averaging across all non-target fixations produces the same pattern of results). Value-driven attentional capture was quantified as the probability of fixating a high-value distractor minus the probability of fixating a non-target, as in prior studies examining individual differences in the construct (e.g., Anderson & Yantis, 2012; see Anderson et al., 2011b, 2013a, 2014b, 2016b, 2016c, 2017b; Anderson & Kim, in press, for an analogous measure using RT as the dependent variable). RT was measured from stimulus onset until gaze first entered the fixation window surrounding the target. Salience-driven attentional capture was quantified as the probability of fixating the color singleton distractor (as in Anderson & Kim, 2019); to verify that the salient distractor indeed captured attention, we also compared this probability to the probability of fixating a non-salient non-target on distractor-absent trials. For the purposes of individual differences analyses, fixation measures were used over RT measures given their superior test-retest reliability (Anderson & Kim, in press).
4.2. Results and Discussion
4.2.1. Training Phase.
A fixation on the target within the timeout limit was registered on 95.6% of all trials. RT to fixate the target did not differ between high-value (M = 367 ms, SD = 69 ms) and low-value targets (M = 378 ms, SD = 61 ms), t(49) = 1.17, p = 0.249.
4.2.2. Test Phase.
A fixation on the target within the timeout limit was registered on 93% of all trials. The frequency of errant distractor fixations differed across the three distractor conditions, F(2,98) = 15.80, p < 0.001, η2p = 0.244. Robust value-driven attentional capture was evident across participants when comparing high-value distractor (M = 8.4%, SD = 6.5%) to distractor-absent trials (M = 3.7%, SD = 2.2%), t(49) = 5.10, p < 0.001, d = 0.72. Errant fixations on low-value distractor trials (M = 7.2%, SD = 5.3%) also differed significantly from errant fixations on distractor-absent trials, t(49) = 5.01, p < 0.001, d = 0.71, but not high-value distractor trials, t(49) = 1.29, p = 0.203.
Similar results were obtained using RT. RT differed significantly by distractor condition, F(2,98) = 4.43, p = 0.014, η2p = 0.083. RT on high-value distractor trials (M = 412 ms, SD = 59 ms) differed significantly from RT on distractor-absent trials (M = 403 ms, SD = 62 ms), t(49) = 2.88, p = 0.006, d = 0.41. RT on low-value distractor trials (M = 407 ms, SD = 58 ms) did not significantly differ from RT on either high-value, t(49) = 1.76, p = 0.085, or distractor-absent trials, t(49) = 1.27, p = 0.211.
4.2.3. Additional Singleton Task.
A fixation on the target within the timeout limit was registered on 90.3% of all trials. The physically salient distractor was initially fixated on 45% of trials (SD = 16.1%); this differed substantially from the probability of fixating a non-salient non-target on distractor-absent trials (M = 1.9%, SD = 1.3%), t(49) = 19.61, p < 0.001, d = 2.77, indicating robust salience-driven attentional capture. RT also differed significantly between distractor-present and distractor-absent trials (Mdiff = 144 ms, SDdiff = 41 ms), t(49) = 24.59, p < 0.001, d = 3.48.
4.2.4. Analysis of Individual Differences.
Value-driven attentional capture was significantly correlated with salience-driven attentional capture, r = 0.404, p = 0.004 (see Section 4.1.7. for how each measure was quantified), suggesting a common component to susceptibility to distraction in general. The magnitude of salience-driven attentional capture served as a measure of general attention abilities (i.e., the ability to ignore any potentially distracting stimulus, regardless of its relationship with reward) and was used as a covariate in order to better isolate the specific influence of reward learning on attention. VDAQ scores were significantly predictive of attentional capture by high-value distractors, β = 0.272, p = 0.048.
The results of Experiment 2 demonstrate that VDAQ scores are related to a well-validated experimental measure of attention-to-reward. Although the relationship was modest in magnitude, the VDAQ offers a proxy measure that has some explanatory power.
5. Experiment 3
In Experiment 3, we assess the generalizability of the VDAQ in predicting attention-to-reward as measured from behavior. In this experiment, participants were initially presented with pictures of real-world scenes and were provided with feedback each time they moved the mouse cursor and clicked somewhere within the scene. Within each scene, one quadrant of that scene yielded positive feedback when clicked on, while the others did not. Participants then completed a second task in which they freely viewed the same scenes while eye position was measured using an eye tracker. Total time fixating the previously high-value quadrant, relative to the other three quadrants, was measured across scenes and related to VDAQ score.
5.1. Methods
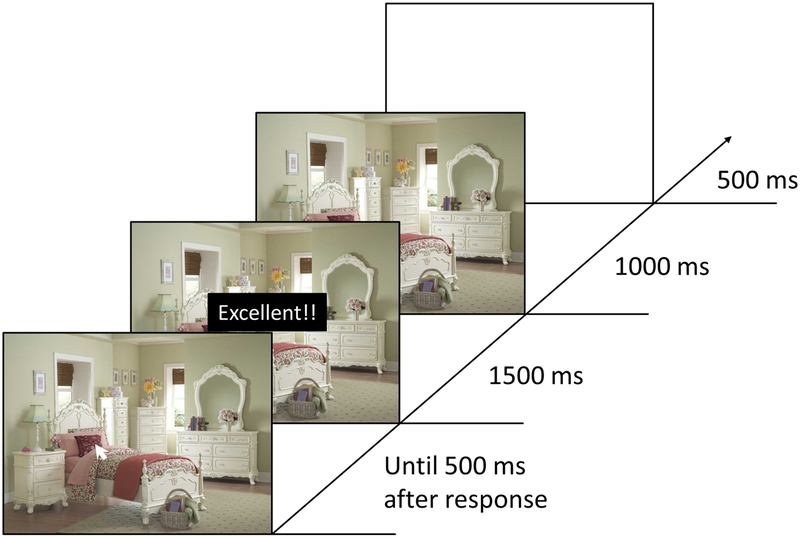
The experimental methods were exactly identical to those reported in Anderson and Kim (2018a). In addition to the original sample of 36 participants, 14 more participants (ages 18–21, M = 18.6y (SD = 1.1y), 12 female, 13 right-handed) were recruited to produce a total sample size of 50 participants, matching the sample size of Experiment 2 given the same power considerations. A detailed description of the methods is provided in Anderson & Kim (2018a). Briefly, during the training phase, a scene image was presented on each trial, and participants clicked on a location within the scene using the mouse cursor (see Figure 3). One quadrant of the scene yielded positive feedback when clicked on (‘Good!’ towards the edges of the quadrant and ‘Excellent!!’ in the center of the quadrant), while the other quadrants did not (‘Not good’). Eight different scenes were used, and each quadrant served as the high-value quadrant equally-often across scenes (with the scene-to-quadrant mapping counterbalanced across participants). During a subsequent test phase, participants freely viewed the scenes while their eye position was recorded using an eye tracker. As in Experiment 2, the position of the right eye was monitored at 1000 Hz and eye position was calibrated prior to each block of trials using 9-point calibration. We measured the total time fixating the high-value quadrant compared to the average of the other three quadrants as a measure of attention-to-reward. As in Experiment 2, the VDAQ was completed prior to the attention task.
Figure 3.
Example trial for the Training Phase of Experiment 3. Participants clicked on different areas of scenes using the mouse cursor and received positive or negative feedback depending on where they clicked. In the test phase, participants freely viewed these same scenes while eye position was measured using an eye tracker.
5.2. Results and Discussion
Overall, participants exhibited a robust gaze preference for the high-value quadrant (M = 1391 ms, SD = 589 ms) relative to a low-value quadrant (M = 379 ms, SD = 170 ms), t(49) = 9.49, p < 0.001, d = 1.34. Participants shifted their gaze between two quadrants an average of 3.9 times per trial. The magnitude of the observed gaze preference was significantly correlated with VDAQ score, r = 0.298, p = 0.035. That is, higher VDAQ scores were associated with a stronger gaze preference for the regions of a scene previously associated with high reward during free viewing (i.e., longer total fixation time). Experiment 3 provides converging evidence for the ability of the VDAQ to predict attention-to-reward as measured in a psychophysical experiment, this time using a more naturalistic task in which participants free-viewed scenes.
6. Experiment 4
In Experiment 4, we further assess the generalizability of the VDAQ in predicting attention-to-reward as measured from behavior. In this experiment, participants completed a single task in which they selected one of four different options represented by four differently colored boxes. Each color was associated with a different probability of a monetary reward and an aversive electric shock when selected. Reward and shock could occur simultaneously within a single trial. Thus, reward and punishment information compete for attention during the learning process (Desimone & Duncan, 1995), and attention-to-reward has been shown to bias actions directed towards high-value stimuli (see Anderson, 2017a, for a review). We quantified the degree to which decisions were influenced by rewards and punishments and asked whether the preference for reward would be captured by VDAQ score.
6.1. Methods
6.1.1. Participants.
Fifty participants (19 females) between the ages of 18 and 35 were recruited from the Texas A&M University community, matching the sample size of Experiments 2 and 3. All participants reported normal or corrected-to-normal visual acuity and normal color vision. Written informed consent was obtained for each participant. All procedures were approved by the Texas A&M Institutional Review Board.
6.1.2. Apparatus.
A Dell OptiPlex 7040 (Dell, Round Rock, TX, USA) equipped with MATLAB software (Mathworks, Natick, MA, USA) and Psychophysics Toolbox extensions (Brainard, 1997) was used to present the stimuli on a Dell P2717H monitor. The participants viewed the monitor from a distance of approximately 70 cm in a dimly lit room. Manual responses were entered using a standard computer mouse. Paired electrodes (EL500, BioPac Systems, Inc., Goleta, CA, USA) were attached to the left forearm of each participant. Electric shocks were delivered through an isolated linear stimulator under the constant current setting (STMISOLA, BioPac Systems), which was controlled by custom MATLAB scripts.
6.1.3. Stimuli.
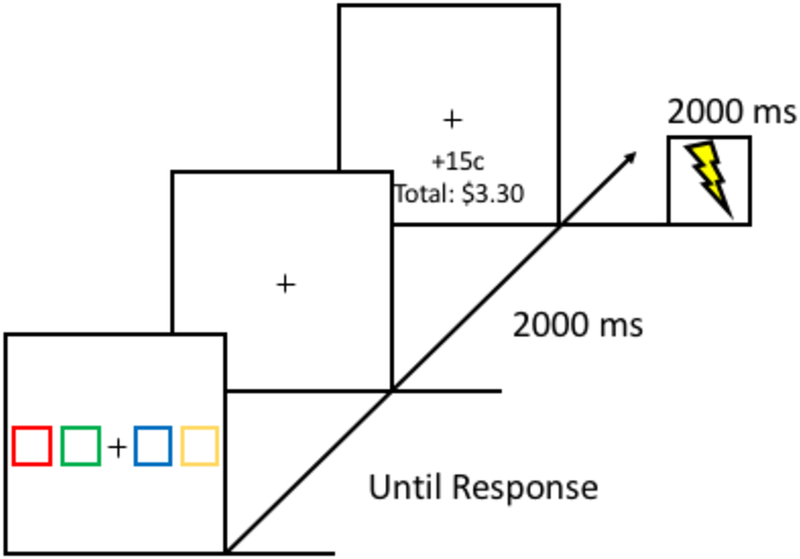
Each trial consisted of a choice array, a fixation display, and a feedback display (see Figure 4). The choice array consisted of a white fixation cross (0.8° × 0.8° visual angle) flanked by two boxes to the left and right (each box 3.7° × 4.8°). The two inner boxes were 4.9° center-to-center from the fixation cross and the two outer boxes were 7.4° center-to-center from the neighboring box. The color of each box was drawn from the following set without replacement: {red (CIE: u’ = 0.485, v’ = 0.523, cd/m2 = 40.63), green (CIE: u’ = 0.135, v’ = 0.566, cd/m2 = 146.58), blue (CIE: u’ = 0.160, v’ = 0.165, cd/m2 = 17.26), yellow (CIE: u’ = 0.216, v’ = 0.557, cd/m2 = 187.53)}. The mouse cursor was always visible. After a color box was chosen, all four boxes disappeared, and only the fixation cross remained visible. The feedback display, which consisted of the fixation cross, earnings from the current trial, and the current bank total, was then presented. Electric shock, if administered on that trial, was delivered simultaneously with the onset of the feedback display.
Figure 4.
Example trials for the decision-making task used for Experiment 4. Participants clicked on one of four colored boxes and received a monetary reward, an electric shock, both, or neither for their choice. Different colors were associated with different probabilities of reward and shock.
6.1.4. Design.
Each color was associated with a specific probability of receiving money and a specific probability of receiving an electric shock when chosen. The possible monetary reward and shock punishment combinations were 25% money/0% shock, 25% money/30% shock, 50% money/30% shock, and 75% money/75% shock (high-valence option). The probabilities chosen were designed to create variability in performance depending on whether a participant’s choices are influenced more by reward or punishment. Our considerations were (a) have a high-valence option for which both shock and reward are equally-likely (the 75/75 option), thereby forcing a preference for seeking reward vs avoiding shock, (b) offer an above-average-value option for which shock was much less likely (the 50/30 option), to serve as the main alternative to the high-valence option for people tending towards shock avoidance, and (c) offer two explicitly less-desirable options that differed with respect to shock probability (the other two options). The location of each color was determined randomly on each trial, and which color was associated with which pair of reward and shock probabilities was randomly determined for each participant.
6.1.5. Procedure.
First, an electronic implementation of the VDAQ was administered. Then a shock calibration procedure was conducted for each participant to achieve a level that was “unpleasant, but not painful” (Anderson & Britton, in press; Murty et al., 2012; Schmidt et al., 2015, 2017). Participants were initially administered a very weak shock of 2 ms in duration, and the shock intensity was gradually increased by 1 ma and the shock re-administered until the participant first reported pain, after which the intensity was reduced by one increment (1 ma) and used for the experiment.
The decision-making task consisted of 200 trials. Each trial began with the presentation of four color boxes, which remained onscreen until a box was selected using the mouse. After a decision was made, a fixation display would appear for two seconds. Then the feedback display, which indicated a monetary reward gained (+15¢) or not (+0¢) and the participant’s total earnings, was presented for 2 seconds. If a shock was administered based on the probability assigned to the selected stimulus, the shock would be delivered concurrent with the onset of the feedback display. Whether a monetary gain was indicated in the feedback display and whether a shock was administered during the feedback display were independently determined on each trial based on the probabilities assigned to the selected stimulus. Participants were not explicitly informed of the underlying probabilities, only that decisions would sometimes result in reward, sometimes in shock, or sometimes both. At the end of the experiment, participants were paid the total monetary reward obtained during the decision-making task.
6.1.6. Data Analysis.
Data from two participants were excluded from analyses because they chose randomly, showing no sensitivity to either reward or punishment in the task (each color selected ≤ ±2% from chance, or 25%). Exclusion of these two participants did not change any of the conclusions. We devised an empirical model that would quantify the overall impact of reward on choice. Beginning at 50% (equally likely to be rewarded or not), the value of each color in terms of its associated reward was updated based on the proportion of trials on which it was rewarded using a sliding window encompassing the last 5 trials on which the color was chosen (similar results were obtained using a sliding window of 10 and 20 trials). On each trial, the participant’s choice was coded in terms of the proportion of associated reward across all four colors reflected in that choice; we then averaged the resulting value of choices over trials to arrive at a relative measure of the amount of available reward pursued by the participant.
6.2. Results and Discussion
The probability of selecting a color stimulus differed across the four value/shock conditions, F(3,144) = 35.27, p < 0.001, η2p = 0.429. Across participants, the high-valence color was chosen significantly more often than any of the other three options, ts > 4.14, ps < 0.001, ds > 0.59. The 50% reward/30% shock color was chosen significantly more frequently than the two lowest-value colors, ts > 3.65, ps < 0.001, ds > 0.52, which themselves did not significantly differ, t(47) = 0.49, p = 0.626 (see Table 1). The fact that participants robustly preferred the 50% reward/30% shock color over these other two lower-value colors provides clear evidence of reward learning in the task.
Table 1.
Mean percentage each color box was selected across participants as a function of the reward and shock probabilities associated with that box (standard deviations are in parentheses).
| 25% reward/0% shock | 25% reward/30% shock | 50% reward/30% shock | 75% reward/75% shock |
|---|---|---|---|
| 15% (8.2%) | 15.7% (9.1%) | 25.2% (14.6%) | 44.2% (19.9%) |
The overall influence of reward on decision-making was robustly correlated with VDAQ score, r = 0.402, p = 0.005. Thus, VDAQ score predicts the influence of reward feedback on decision-making when reward and punishment feedback compete for attention during the learning process.
7. Combined Analysis
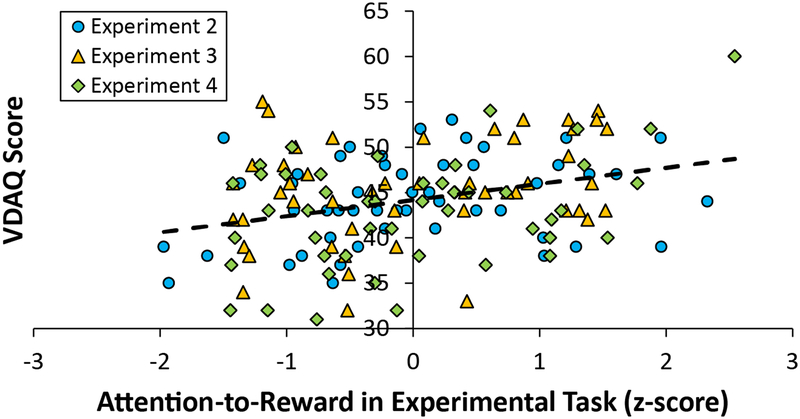
To obtain an overall picture of the ability of the VDAQ to predict attention-to-reward as measured in a laboratory task, we combined the data from Experiments 2–4 and computed the correlation between these two measures. In each experiment, attention-to-reward (the attention measure correlated with VDAQ scores in each experiment) was z-scored to the mean of that experiment to place the measures on a common scale, with attention-to-reward scores in Experiment 2 again adjusted for general distractibility as in the main analysis. This combined analysis revealed a robust relationship between VDAQ score and experimental measures of attention-to-reward, r = 0.321, p < 0.001 (Figure 5), supporting the validity of the questionnaire.
Figure 5.
Scatterplot showing the relationship between attention-to-reward across experiments (z-scored separately for each experiment) and VDAQ score.
8. Experiment 5
In Experiment 5, we relate VDAQ score to variation in brain connectivity as measured in the resting state using functional magnetic resonance imaging (fMRI). Participants completed an electronic implementation of the VDAQ and subsequently completed an MRI study that included a resting state scan in which participants passively viewed a fixation display. We chose as seed regions the ventral tegmental area (VTA) and the nucleus accumbens (NAcc, which receives input from the VTA), which code dopaminergic reward-prediction errors (e.g., O’Doherty, 2004; Schultz et al., 1997; Waelti et al., 2001). Such reward-prediction errors are thought to shape responses in visual areas, resulting in elevated responses evoked by learned predictors of reward post-conditioning (e.g., Anderson, 2016a, 2017b; Anderson et al., 2013b, 2017b; Hickey et al., 2010a; Sali et al., 2014; van Koningsbruggen et al., 2016). Given the well-validated role of the visual cortex and caudate tail (e.g., Anderson, 2017b; Anderson et al., 2014a, 2016c; Hickey & Peelen, 2015, 2017; Kim & Anderson, in press; Yamamoto et al., 2013) in value-driven attentional orienting, and theories concerning the neural mechanisms of value-driven attention that hypothesize teaching signals from the VTA/NAcc to these regions (Anderson, 2017b, 2019) we hypothesized that higher VDAQ scores would be associated with stronger connectivity between these areas and the VTA/NAcc. Although resting-state functional connectivity has generally not been investigated in the context of behavioral measures of value-driven attentional capture, there is one study linking connectivity between the ventral striatum and regions involved in the signaling of value-based attentional priority to a behavioral measure of attention-to-reward (Wang et al., 2015).
8.1. Methods
8.1.1. Participants.
Eighty-two participants (38 females) between the ages of 18 and 35 (M = 22.5y, SD = 4.3y) were recruited from the Texas A&M University community. All participants reported normal or corrected-to-normal visual acuity and normal color vision. Written informed consent was obtained for each participant. All procedures were approved by the Texas A&M Institutional Review Board. Participants were recruited to participate in one of multiple currently active study protocols, to which the VDAQ and a resting state scan were added for the present purposes.
8.1.2. Apparatus, Stimuli, and Task Instruction.
Participants viewed a white fixation cross presented in the center of the screen against a black background. Stimulus presentation was controlled by an Invivo SensaVue display system. The resting state scan lasted 6 min, during which participants were instructed to lie still and keep their eyes open and fixated at the center of the screen. Resting state scans were completed towards the middle of a larger study protocol lasting 1.25–1.75 hrs, during which brain scans addressing other research questions were conducted.
8.1.3. MRI Data Acquisition.
MRI data were acquired with a Siemens 3-Tesla MAGNETOM Verio scanner and a 32-channel head coil at the Texas A&M Institute for Preclinical Studies (TIPS). An anatomical scan was acquired using a T1-weighted magnetization prepared rapid gradient echo (MPRAGE) sequence (150 coronal slices, TR = 7.9 ms, TE = 3.65 ms, flip angle = 8°, voxel size = 1 mm isotropic with no gap). Whole-brain functional images were acquired using a T2*-weighted multiband echo planar imaging (EPI) sequence (56 axial slices, TR = 600ms, TE = 29ms, flip angle = 52°, image matrix = 96×96, voxel size = 2.5mm isotropic with no gap). The functional scan began with 14 dummy pulses that were not recorded in order to allow magnetization to reach steady-state, after which 600 volumes were acquired over a single 6 min scan.
8.1.4. Preprocessing of MRI data.
Preprocessing and analysis were conducted using the AFNI software package (Cox, 1996). All functional images were motion corrected using the first image (which immediately followed the anatomical scan in acquisition) as a reference. Functional images were then co-registered to the anatomical image of each participant and warped to the Talairach brain (Talairach & Tournoux, 1998) using 3dQwarp. The images were converted into percent signal change normalized to the mean of each run and spatially smoothed to a resulting 5 mm full-width half-maximum using 3dBlurToFWHM. Finally, a band-pass filter (0.01 – 0.10 Hz) was applied to the functional data, and the anatomical image was segmented into white matter (WM), grey matter, and cerebrospinal fluid (CSF).
8.1.5. Data Analysis.
Time series data were extracted from masks of the segmented WM and CSF, as well as the signal averaged over the whole brain, and used as continuous regressors of non-interest in a general linear model (GLM). Additional nuisance regressors included six degrees of head motion and drift in the scanner signal. Data from four participants were eliminated from further analysis due to maximal single-frame motion displacement that exceeded 2.5 SD of the group mean (Van Dijk et al., 2012). The regressor of interest was the time series data extracted from the VTA/NAcc, which was defined using three spheres following the parameters established by Zhang et al. (2015). Frames on which head motion exceeded 1 mm in any direction, in addition to the frame preceding and following each such instance of head motion, were censored from analysis.
Clusters of voxels where the connectivity with the VTA/NAcc (beta value from the VTA/NAcc regressor) was predicted by VDAQ score (used as a subject-level covariate) were assessed for statistical significance using 3dClustSim. For the cluster simulation, the smoothness of the data was estimated using the ACF method on the residuals. Small volume correction was applied to the resulting statistical parametric map, with one region of interest (ROI) representing the visual cortex and another representing the caudate tail. Each ROI was defined using the anatomical labels in the Talairach Daemon provided in AFNI (the visual cortex was conservatively defined as regions of the occipital lobe, 5492 voxels, while the caudate tail was defined as the correspondingly labeled region in the Talairach Daemon, in both cases bilaterally), with the caudate tail mask dilated by 5 mm to account for its very small size (with the final mask of the caudate tail comprising 1769 voxels). The clusterwise p threshold was set to 0.025 to accommodate two independent tests (voxelwise p < 0.01).
8.2. Results and Discussion
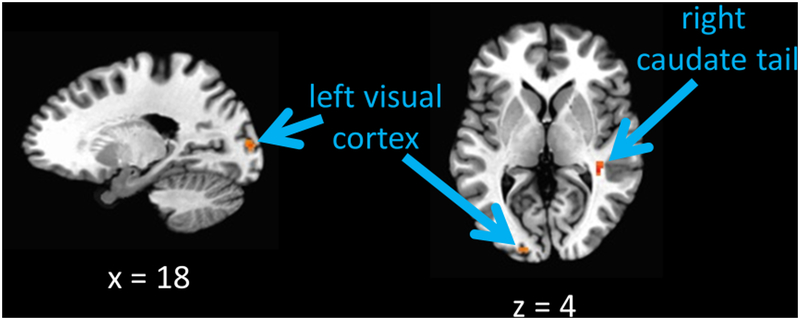
VDAQ score predicted functional connectivity between the VTA/NAcc and a region of the left visual cortex and right caudate tail (Figure 6), with higher VDAQ scores associated with stronger functional connectivity. These relationships are consistent with theories concerning the neural mechanisms underlying attention-to-reward, which posit that dopaminergic reward prediction-error signals modulate stimulus representations in the visual cortex and caudate tail, biasing competition in the visual system and oculomotor selection, respectively (Anderson, 2016a, 2017b, 2019; Anderson et al., 2014a, 2016c, 2017b; Hickey et al., 2010a; Hickey & Peelen, 2015, 2017; Kim & Anderson, in press; Sali et al., 2014; van Koningsbruggen et al., 2016; Yamamoto et al., 2012, 2013). A follow-up whole-brain analysis did not reveal any additional clusters.
Figure 6.
Regions of the visual cortex and caudate tail where functional connectivity with the VTA/NAcc seed was predicted by VDAQ score, with higher VDAQ scores indicating greater functional connectivity.
9. Experiment 6
Attention-to-reward, as measured in a psychophysical task, shows marked persistence long after stimulus-reward associations have been learned (Anderson & Yantis, 2013; Della Libera & Chelazzi, 2009), and psychophysical measurements of attention-to-reward have strong test-retest reliability when assessed using eye tracking methods (Anderson & Kim, in press). In Experiment 6, we examined the test-retest reliability of the VDAQ. A subset of participants who had previously completed Experiment 2 of the present study were invited to complete the VDAQ a second time, four weeks after initial participation. Data were obtained for 28 of these individuals, and scores across the two assessments were correlated. Initial scores were robustly correlated with scores obtained four weeks later, r = 0.690, p < 0.001, indicating acceptable test-retest reliability. This test-retest reliability was a bit lower than that for the eye-tracking measure of value-driven attentional capture (r = 0.798) as estimated in prior research (Anderson & Kim, in press).
10. General Discussion
Attention-to-reward, as assessed in psychophysical laboratory tasks, provides a rich individual differences measure that is related to a variety of personality characteristics and psychopathologies (e.g., Albertella et al., 2017; Anderson, 2016b; Anderson et al., 2011b, 2013a, 2016b, 2014b, 2017a; Hickey et al., 2010b; Hickey & Peelen, 2015, 2017; Qi et al., 2013; Sali et al., 2018). Exploration of the predictive power of this source of individual variability, however, is severely hampered by the burden of its measurement, which currently requires the use of psychophysical techniques, specialized equipment, dedicated testing space, and considerable time to administer (approximately one hour per measurement). The present study assessed the ability to capture individual differences in attention-to-reward using a 16-item questionnaire-based assessment.
The questionnaire that we developed, the VDAQ, reliably predicted performance on three different tasks measuring the impact of reward on selective attention. Although its predictive power was rather modest in each case, the variety of tasks attests to the breadth of this questionnaire in capturing a core element of the construct. This was reflected in a robust relationship between VDAQ scores and across-experiment measures of attention-to-reward. Scores on the VDAQ further correlate with personality characteristics previously linked to attention-to-reward, demonstrating both convergent and discriminant validity. Compellingly, scores on the VDAQ covary with brain connectivity, in a manner predicted by prior findings concerning the neural mechanisms underlying value-based attentional orienting (Anderson, 2016a, 2017b, 2019; Anderson et al., 2014a, 2016c, 2017b; Hickey et al., 2010a; Hickey & Peelen, 2015, 2017; Sali et al., 2014; van Koningsbruggen et al., 2016; Yamamoto et al., 2013). This converging evidence all points to the validity of the VDAQ as an accessible proxy-measure of attention-to-reward.
It is important to note that the questionnaire we developed probes the putative influence of attention-to-reward in everyday life, which is much broader than the highly controlled context used for psychophysical assessment. Although psychophysical assessments of attention-to-reward are predictive of real-world outcomes, including psychopathology (Albertella et al., 2017; Anderson, 2016b; Anderson et al., 2013a, 2014b, 2016b, 2017a; Sali et al., 2018) and the thoughts and behaviors indicative of impulsiveness (Anderson et al., 2011b, 2013a, 2016b) and behavioral activation system traits (Hickey et al., 2010b; Hickey & Peelen, 2015, 2017; Qi et al., 2013), the degree to which such laboratory assessments generalize to attention in the real world is unclear. Our questionnaire offers a potentially more direct assessment of the influence of attention in more naturalistic settings, with greater face validity. This may, in part, explain why the observed relationships between VDAQ scores and psychophysical assessments of attention-to-reward were not more robust. The breadth with which were able to predict different laboratory measures of attention-to-reward is consistent with the idea that our questionnaire captures some degree of the diversity with which reward learning can influence information processing across a variety of situations.
10.1. Limitations
Several limitations and weaknesses of the VDAQ are important to acknowledge. Sixteen items were developed on the basis of their apparent face validity and were shown to exhibit acceptable reliability and the hypothesized single factor structure. A stronger approach would be to develop a much larger number of candidate items, whittle that item pool down empirically to provide the strongest independent predictor of a behavioral measure of attention-to-reward (above-and-beyond other available measures such as the BIS/BAS scale and BIS-11), and then validate the retained items using independent data.
The VDAQ probes aspects of attention-to-reward to which participants have conscious access, with particular emphasis on how presumed attention-to-reward influences overt behavior or explicit motivations. Although this approach has the strength of linking attention-to-reward with behavior in everyday life, it ignores perceptual aspects of attention-to-reward evident in psychophysical assessments but minimally accessible to consciousness, which reflects a limitation of any questionnaire approach. In this sense, the VDAQ is likely only measuring one of at least two dimensions of attention-to-reward, and VDAQ scores should be interpreted accordingly.
Although the VDAQ correlated with the other measures that it was expected to, demonstrating convergent validity, it also unexpectedly correlated with BIS scores, which reflects undesired variance. Furthermore, as the ability of the VDAQ to predict behavioral measures of attention-to-reward was not directly compared to other available measures such as the BIS/BAS scale (Carver & White, 1994) and BIS-11 (Patton et al., 1995), it is unclear just how much of an improvement the VDAQ might be in terms of predictive power. At present, the primary value of the VDAQ in relation to these other measures lies in its face validity and the breadth with which it has been shown to predict different measures of attention-to-reward in the present study. In this sense, the present study offers a proof-of-concept with a variety of data to support the practical utility of the questionnaire, and future research should revise and strengthen the assessment tool along these lines.
10.2. Conclusions
The VDAQ offers a widely accessible means by which researchers can assess attention-to-reward as an individual trait, without the demands and limitations that are typical of psychophysical laboratory techniques. Personality researchers can use this measure to explore a variety of questions that might otherwise be inaccessible. Even for research questions that could theoretically be addressed using psychophysical measures of attention-to-reward, the VDAQ offers the potential to measure the construct on a scale that would otherwise be prohibitive. Very large samples can be obtained in an effort to predict a variety of clinical and life outcomes, as well as data from individuals whose condition or situation is difficult to accommodate using typical psychophysical tasks and laboratory assessments. In this way, the VDAQ opens up new vistas in research into individual differences, offering a bridge to insights that have been developed through the psychophysics literature.
Funding.
This study was supported by grants from the Brain & Behavior Research Foundation [NARSAD 26008] and NIH [R01-DA046410] to BAA.
Appendix A. The Value-Driven Attention Questionnaire (VDAQ)
| The opposite of me | Does not describe me well | Somewhat true of me | Very true of me | |
|---|---|---|---|---|
| 1. When I see an attractive person, I have a hard time taking my eyes off of them. | O | O | O | O |
| 2. If the TV is on in the background, I find it very distracting. | O | O | O | O |
| 3. I like to go window shopping (or browse items for sale on the internet) and look at items even if I know I cannot afford them. | O | O | O | O |
| 4. When I daydream, it is often about things I want. | O | O | O | O |
| 5. When tasty food is placed in the open, I find it very tempting. | O | O | O | O |
| 6. I have an eye for beauty. | O | O | O | O |
| 7. I tend to focus on the things I want more than the things I need or know are good for me. | O | O | O | O |
| 8. Billboards really draw my attention. | O | O | O | O |
| 9. The sights and sounds of a place like Las Vegas excite me. | O | O | O | O |
| 10. When I see other people doing an activity I like, it makes me want to drop everything and join in. | O | O | O | O |
| 11. I am much more likely to pursue something when the opportunity is right in front of me. | O | O | O | O |
| 12. Seeing something makes me want it more. | O | O | O | O |
| 13. I find it hard to focus on work when something fun or interesting is going on around me. | O | O | O | O |
| 14. I never miss the text message sound on my phone or the email chime on my computer, and I read it right away even if it takes me away from what I am doing. | O | O | O | O |
| 15. I am likely to buy one of the items featured on display at a store, even though I was not planning to when I entered the store. | O | O | O | O |
| 16. I find unrewarding tasks to be boring and not worth paying much attention to when I do them. | O | O | O | O |
Footnotes
Conflict of Interest. The authors declare no conflict of interest.
Ethical Approval. All procedures were conducted in accordance with the ethical standards of the Texas A&M University Institutional Review Board and with the 1964 Helsinki declaration and its later amendments.
Informed consent. Informed consent was obtained from all individual participants included in the study.
12. References
- Albertella L, Copeland D, Pearson D, Watson P, Wiers RW, & Le Pelley ME (2017). Selective attention moderates the relationship between attentional capture by signals of nondrug reward and illicit drug use. Drug and Alcohol Dependence, 175, 99–105. [DOI] [PubMed] [Google Scholar]
- Anderson BA (2013). A value-driven mechanism of attentional selection. Journal of Vision, 13(3):7, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA (2015a). Value-driven attentional capture is modulated by spatial context. Visual Cognition, 23, 67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA (2015b). Value-driven attentional priority is context specific. Psychonomic Bulletin and Review, 22, 750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA (2016a). The attention habit: How reward learning shapes attentional selection. Annals of the New York Academy of Sciences, 1369, 24–39. [DOI] [PubMed] [Google Scholar]
- Anderson BA (2016b). What is abnormal about addiction-related attentional biases? Drug and Alcohol Dependence, 167, 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA (2017a). Going for it: The economics of automaticity in perception and action. Current Directions in Psychological Science, 26, 140–145. [Google Scholar]
- Anderson BA (2017b). Reward processing in the value-driven attention network: Reward signals tracking cue identity and location. Social, Cognitive, and Affective Neuroscience, 12, 461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA (2019). Neurobiology of value-driven attention. Current Opinion in Psychology, 29, 27–33. [DOI] [PubMed] [Google Scholar]
- Anderson BA, & Britton MK (in press). On the automaticity of attentional orienting to threatening stimuli. Emotion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Chiu M, DiBartolo MM, & Leal SL (2017a). On the distinction between value-driven attention and selection history: Evidence from individuals with depressive symptoms. Psychonomic Bulletin and Review, 24, 1636–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Faulkner ML, Rilee JJ, Yantis S, & Marvel CL (2013a). Attentional bias for non-drug reward is magnified in addiction. Experimental and Clinical Psychopharmacology, 21, 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Folk CL, Garrison R, Rogers L (2016a). Mechanisms of habitual approach: Failure to suppress irrelevant responses evoked by previously reward-associated stimuli. Journal of Experimental Psychology: General, 145, 796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, & Kim H (2018a). Mechanisms of value-learning in the guidance of spatial attention. Cognition, 178, 26–36. [DOI] [PubMed] [Google Scholar]
- Anderson BA, & Kim H (2018b). Relating attentional biases for stimuli associated with social reward and punishment to autistic traits. Collabra: Psychology, 4(1), article 10. [Google Scholar]
- Anderson BA, & Kim H (2019). On the relationship between value-driven and stimulus-driven attentional capture. Attention, Perception, and Psychophysics, 81, 607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, & Kim H (in press). Test-retest reliability of value-driven attentional capture. Behavior Research Methods. [DOI] [PubMed] [Google Scholar]
- Anderson BA, Kronemer SI, Rilee JJ, Sacktor N, & Marvel CL (2016b). Reward, attention, and HIV-related risk in HIV+ individuals. Neurobiology of Disease, 92, 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Kuwabara H, Wong DF, Gean EG, Rahmim A, Brasic JR, George N, Frolov B, Courtney SM, & Yantis S (2016c). The role of dopamine in value-based attentional orienting. Current Biology, 26, 550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Kuwabara H, Wong DF, Roberts J, Rahmim A, Brasic JR, & Courtney SM (2017b). Linking dopaminergic reward signals to the development of attentional bias: A positron emission tomographic study. NeuroImage, 157, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, & Yantis S (2011a). Learned value magnifies salience-based attentional capture. PLoS ONE, 6, e27926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, & Yantis S (2011b). Value-driven attentional capture. Proceedings of the National Academy of Sciences USA, 108, 10367–10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, & Yantis S (2013b). Reward predictions bias attentional selection. Frontiers in Human Neuroscience, 7:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, & Yantis S (2014a). Value-driven attentional priority signals in human basal ganglia and visual cortex. Brain Research, 1587, 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Leal SL, Hall MG, Yassa MA, & Yantis S (2014b). The attribution of value-based attentional priority in individuals with depressive symptoms. Cognitive, Affective, and Behavioral Neuroscience, 14, 1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, & Yantis S (2012). Value-driven attentional and oculomotor capture during goal-directed, unconstrained viewing. Attention, Perception, and Psychophysics, 74, 1644–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, & Yantis S (2013). Persistence of value-driven attentional capture. Journal of Experimental Psychology: Human Perception and Performance, 39, 6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, & Clubly E (2001). The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders, 31, 5–17. [DOI] [PubMed] [Google Scholar]
- Berridge KC (2012). From prediction error to incentive salience: Mesolimbic computation of reward motivation. European Journal of Neuroscience, 35, 1124–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, & Robinson TE (2016). Liking, wanting, and the incentive-sensitization theory of addiction. American Psychologist, 71, 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois A, Chelazzi L, & Vuilleumier P (2016). How motivation and reward learning modulate selective attention. Progress in Brain Research, 229, 325–342. [DOI] [PubMed] [Google Scholar]
- Bourgeois A, Neveu R, Bayle DJ, & Vuilleumier P (2017). How does reward compete with goal-directed and stimulus-driven shifts of attention? Cognition and Emotion, 31, 109–118. [DOI] [PubMed] [Google Scholar]
- Brainard DH (1997). The psychophysics toolbox. Spatial Vision, 10, 433–436. [PubMed] [Google Scholar]
- Carver CS, & White TL (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. Journal of Personality and Social Psychology, 67, 319–333. [Google Scholar]
- De Tommaso M, Mastropasqua T, & Turatto M (2017). The salience of a reward cue can outlast reward devaluation. Behavioral Neuroscience, 131, 226–234. [DOI] [PubMed] [Google Scholar]
- Della Libera C, & Chelazzi L (2009). Learning to attend and to ignore is a matter of gains and losses. Psychological Science, 20, 778–784. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J (1995). Neural mechanisms of selective visual attention. Annual Review of Neuroscience, 18, 193–222. [DOI] [PubMed] [Google Scholar]
- Failing M, & Theeuwes J (2018). Selection history: How reward modulates selectivity of visual attention. Psychonomic Bulletin and Review, 25, 514–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, & Posner MI (2005). The activation of attention networks. NeuroImage, 26, 471–479. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, & Posner MI (2002). Testing the efficacy and independence of attentional networks. Journal of Cognitive Neuroscience, 14, 340–347. [DOI] [PubMed] [Google Scholar]
- Folk CL, Remington RW, & Johnston JC (1992). Involuntary covert orienting is contingent on attentional control settings. Journal of Experimental Psychology: Human Perception and Performance, 18, 1030–1044. [PubMed] [Google Scholar]
- Hickey C, Chelazzi L, & Theeuwes J (2010a). Reward changes salience in human vision via the anterior cingulate. Journal of Neuroscience, 30, 11096–11103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C, Chelazzi L, & Theeuwes J (2010b). Reward guides vision when it’s your thing: Trait reward-seeking in reward-mediated visual priming. PLOS ONE, 5, e14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C, & Peelen MV (2015). Neural mechanisms of incentive salience in naturalistic human vision. Neuron, 85, 512–518. [DOI] [PubMed] [Google Scholar]
- Hickey C, & Peelen MV (2017). Reward selectively modulates the lingering neural representation of recently attended objects in natural scenes. Journal of Neuroscience, 37, 7297–7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K, Locke HS, & Braver TS (2010). Prefrontal cortex mediation of cognitive enhancement in rewarding motivational contexts. Proceedings of the National Academy of Sciences USA, 107, 8871–8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AJ, & Anderson BA (in press). Neural correlates of attentional capture by stimuli previously associated with social reward. Cognitive Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, & Anderson BA (2019). Neural evidence for automatic value-modulated approach behavior. NeuroImage, 189, 150–158. [DOI] [PubMed] [Google Scholar]
- Kiss M, Driver J, & Eimer M (2009). Reward priority of visual target singletons modulates event-related potential signatures of attentional selection. Psychological Science, 20, 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leganes-Fonteneau M, Scott R, & Duka T (2018). Attentional responses to stimuli associated with a reward can occur in the absence of knowledge of their predictive values. Behavioural Brain Research, 341, 26–36. [DOI] [PubMed] [Google Scholar]
- Mack A, & Rock I (1998). Inattentional Blindness. Cambridge, MA: MIT Press. [Google Scholar]
- Murty VP, Labar KS, & Adcock RA (2012). Threat of punishment motivates memory encoding via amygdala, not midbrain, interactions with the medial temporal lobe. Journal of Neuroscience, 32, 8969–8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navalpakkam V, Koch C, & Perona P (2009). Homo economicus in visual search. Journal of Vision, 9(1):31, 1–16. [DOI] [PubMed] [Google Scholar]
- Navalpakkam V, Koch C, Rangel A, & Perona P (2010). Optimal reward harvesting in complex perceptual environments. Proceedings of the National Academy of Sciences, USA, 107, 5232–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty JP (2004). Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology, 14, 769–776. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, & Barratt ES (1995). Factor structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology, 51, 768–774. [DOI] [PubMed] [Google Scholar]
- Pessoa L, & Engelmann JB (2010). Embedding reward signals into perception and cognition. Frontiers Neuroscience, 4(17), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool E, Brosch T, Delplanque S, & Sander D (2014). Where is the chocolate? Rapid spatial orienting toward stimuli associated with primary rewards. Cognition, 130, 348–359. [DOI] [PubMed] [Google Scholar]
- Qi S, Zeng Q, Ding C, & Li H (2013). Neural correlates of reward-driven attentional capture in visual search. Brain Research, 1532, 32–43. [DOI] [PubMed] [Google Scholar]
- Raymond JE, & O’Brien JL (2009). Selective visual attention and motivation: The consequences of value learning in an attentional blink task. Psychological Science, 20, 981–988. [DOI] [PubMed] [Google Scholar]
- Sali AW, Anderson BA, Yantis S (2014). The role of reward prediction in the control of attention. Journal of Experimental Psychology: Human Perception and Performance, 40, 1654–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sali AW, Anderson BA, Yantis S, Mostofsky SH, & Rosch KS (2018). Reduced value-driven attentional capture among children with ADHD compared to typically developing controls. Journal of Abnormal Child Psychology, 46, 1187–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt LJ, Belopolsky AV, & Theeuwes J (2015). Attentional capture by signals of threat. Cognition and Emotion, 29, 687–694. [DOI] [PubMed] [Google Scholar]
- Schmidt LJ, Belopolsky AV, & Theeuwes J (2017). The time course of attentional bias to cues of threat and safety. Cognition and Emotion, 31, 845–857. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, & Montague PR (1997). A neural substrate of prediction and reward. Science, 275, 1593–1599. [DOI] [PubMed] [Google Scholar]
- Seitz AR, Kim D, & Watanabe T (2009). Rewards evoke learning of unconsciously processed visual stimuli in adult humans. Neuron, 61, 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT (2008). Value-based modulations in human visual cortex. Neuron, 60, 1169–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, & Saproo S (2010). Population response profiles in early visual cortex are biased in favor of more valuable stimuli. Journal of Neurophysiology, 104, 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeuwes J (1992). Perceptual selectivity for color and form. Perception and Psychophysics, 51, 599–606. [DOI] [PubMed] [Google Scholar]
- Theeuwes J (2010). Top-down and bottom-up control of visual selection. Acta Psychologica, 135, 77–99. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, & Buckner RL (2012). The influence of head motion on intrinsic functional connectivity MRI. NeuroImage, 59, 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Koningsbruggen MG, Ficarella SC, Battelli L, & Hickey C (2016). Transcranial random noise stimulation of visual cortex potentiates value-driven attentional capture. Social, Cognitive, and Affective Neuroscience, 11, 1481–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelti P, Dickinson A, & Schultz W (2001). Dopamine responses comply with basic assumptions of formal learning theory. Nature, 412, 43–48. [DOI] [PubMed] [Google Scholar]
- Wang L, Yu H, Hu J, Theeuwes J, Gong X, Xiang Y, Jiang C, & Zhou X (2015). Reward breaks through center-surround inhibition via anterior insula. Human Brain Mapping, 36, 5233–5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe JM, Cave KR, Franzel SL (1989). Guided Search: An alternative to the feature integration model for visual search. Journal of Experimental Psychology: Human Perception and Performance, 15, 419–433. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Kim HF, & Hikosaka O (2013). Reward value-contingent changes of visual responses in the primate caudate tail associated with a visuomotor skill. The Journal of Neuroscience, 33, 11227–11238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Monosov IE, Yasuda M, & Hikosaka O (2012). What and where information in the caudate tail guides saccades to visual objects. The Journal of Neuroscience, 32, 11005–11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S, & Jonides J (1984). Abrupt visual onsets and selective attention: Evidence from visual search. Journal of Experimental Psychology: Human Perception and Performance, 10, 350–374. [DOI] [PubMed] [Google Scholar]
- Zhang J-T, Ma S-S, Yip SW, Wang L-J, Chen C, Yan C-G, Liu L, Liu B, Deng L-Y, Liu Q-X, & Fang X-Y (2015). Decreased functional connectivity between ventral tegmental area and nucleus accumbens in internet gaming disorder: evidence from resting state functional magnetic resonance imaging. Behavioral and Brain Functions, 11:37, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]