Abstract
Tissue homeostasis depends on a balance of synthesis and degradation of constituent proteins, with use of a given protein potentially regulating its turnover. Extracellular matrix (ECM) is predominantly composed of fibrillar collagens that exhibit tension-sensitive degradation, which we review here at different levels of hierarchy. Past experiments and recent proteomics measurements together suggest that mechanical strain stabilizes collagen against enzymatic degradation at the scale of tissues and fibrils whereas isolated collagen molecules exhibit a biphasic behavior that depends on load magnitude. Within a Michaelis-Menten framework, collagenases at constant concentration effectively exhibit a low activity on substrate fibrils when the fibrils are strained by tension. Mechanisms of such mechanosensitive regulation are surveyed together with relevant interactions of collagen fibrils with cells.
Keywords: Tissue, Extracellular Matrix, Collagen, Collagenase, Matrix metalloproteinases (MMPs), Degradation, Strain
1. Introduction
Extracellular matrix (ECM) proteins are the most abundant proteins in animals and form a structural and functional framework for tissues and of course the cells within tissues. ECM proteins undergo varying extents of enzymatic degradation and synthesis under homeostatic conditions, but ECM also establishes the stiffness of solid tissues. This latter fact is easily demonstrated by the fluidization of tissue that occurs rapidly upon addition of a collagenase enzyme. Physical properties of polymer systems such as elasticity and viscosity generally scale with polymer concentration [1], which is true for collagen-I (e.g. [2]), and polymer interactions, assembly, and crosslinking generally modulate such physical properties [3]. Tissue stiffness also scales with the levels of collagen type-I and several other fibrillar collagens as:
Soft tissues such as brain and bone marrow (E ~ 0.1 – 1 kPa) indeed possess much less collagen than stiffer tissues such as muscle (E ~ 10 kPa) or bone [4]. The multi-tissue proteomic approach that revealed this scaling relationship has also been applied to developing heart in chick embryos, which exhibits similar scaling as collagen accumulates and stiffens the heart during its first days of beating [5]. Mechanical stresses sustained and/or generated by stiffer tissues tend to be higher than in softer tissues, consistent with an inter-relationship between: (i) tissue stiffness, (ii) the typical stress in the tissue (i.e. tissue stress ~ E), and (iii) the homeostatic balance of collagen synthesis and degradation. Mathematical modeling of this inter-relationship is rooted in the concept of ‘use it or lose it’ [4] [5] which implies that amino acids not needed for the structure and integrity of a tissue (i.e. ECM) can be recycled after proteolysis for use in other proteins or pathways of greater importance.
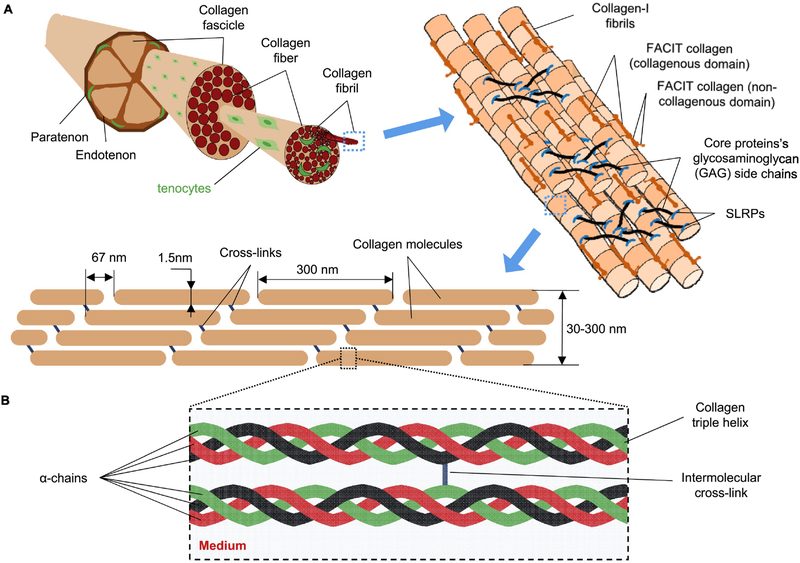
The ECM of load-bearing connective tissues such as tendon exhibit a structural hierarchy (Fig. 1) that is built up primarily from multiple highly-conserved, triple-helical collagens (types I, II, III, V and XI) in the form of fibrils [6] [7] [8] [9] [10] plus other proteins that include small leucine rich proteoglycans (SLRPs) such as decorin, biglycan, fibromodulin, and their associated glycosaminoglycan (GAG) chains. Procollagens are secreted by cells, and end-terminal cleavage (via peptidase) yields collagen molecules that readily self-assemble into the higher-order collagen fibrils, with assembly aided by lysyl oxidase LOX enzymes. Collagen fibrils are generally seen to be oriented in the direction of applied mechanical stress, even during growth. Collagen fibril assemblies present in tissues thus seem adapting to stresses and/or strains in physiological and pathological conditions [11], whether skeletal muscle [12] [13] [14] [15], bone and cartilage [16], or lung [17].
Fig. 1:
Tendon tissue at different length scales, with various factors affecting its enzymatic degradation. (A) Collagen-I fibrils establish the architecture of the tissue. (B) Enzymatic degradation of collagen fibrils depends on conformation of collagen molecules (regulated by intermolecular cross-links between collagen molecules) as well as solution physicochemical factors including temperature, pH, and concentrations of any type of collagenase and collagenase inhibitors present in the tissue.
Mechanical stimulation influences the regulation of various ECM factors in addition to the fibrillar collagens [18] [19], including proteoglycans [20], aggrecans [21], laminins and type IV collagens in basement membranes [22]. Mechanical forces on fibroblasts [23] among other cell types activate expression of collagens, fibronectin and proteoglycans, ultimately resulting in ECM that carries collagen fibrils aligned in the direction of load [24] [25] [26] [27]. The actomyosin cytoskeleton within embryonic tendon cells facilitates deposition and alignment of collagen fibrils in the ECM [28], but roles for cell-generated forces remain unclear. Mechanical loads also affect expression of matrix metalloproteinases (MMPs) and tissue inhibitors of matrix metalloproteinases (TIMPs) in mesenchymal stem cells (MSCs) [29], fibroblasts [30] [25] [31], osteoblasts [32] [33] and chondrocytes [34] [35]. For 3D collagen gels subjected to mechanical loading that is changed in its direction, embedded fibroblasts up-regulate expression of MMPs (MMP-1, 2, 3), which preferentially degrade unloaded collagen fibrils [25]. Such regulation seems to be one aspect of mechanically induced differentiation in vitro [36] [37] and in vivo in tendon [38] [39] [40] [41] [42] [43] and cartilage [44] [45]. These MMPs act as primary mediators of tissue remodeling via collagen degradation; for example, tendons that are injured by load are remodeled by MMPs [46] [47] [48].
Direct mechano-sensitivity of collagen fibrils independent of cells was first reported decades ago [49], with subsequent studies of enzymatic cleavage under mechanical strain largely done with bacterial collagenase (BC) (i.e. Clostridium histoliticum collagenase, available as Col-G and Col-H forms [50] [51]) rather than MMPs that are difficult to purify, activate, and keep stable [52] [53]. Here, it is important to keep in mind that despite ~100 fold higher enzyme-substrate affinity of MMP-1 towards fibrillar than monomeric collagen (indicated by Michaelis constant) [52], MMP-1 catalyzes degradation of purified collagen type-I (from guinea pig) in monomer [54] as well as fibrillar [55] forms at similar rates (~25 collagen molecules per molecule of MMP-1 per hour). Interestingly, the packing of monomers into fibrils exposes only ~10% of total available collagen molecules (i.e. surface molecules depending upon fibril diameter) to MMP-1 and reduces “effective” degradation rate. Thus looking at architecture of ECM, mechanical load on it could in principle control collagen degradation (Fig. 2) by either: (i) changing collagen’s molecular conformation, (ii) changing collagenase mobility within ECM, and/or (iii) changing collagenase conformation.
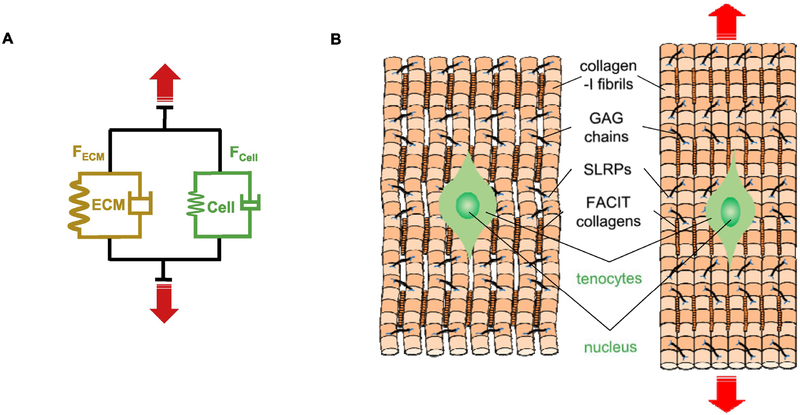
Fig. 2:
Mechanical response of ECM and cells of tendon-type tissues. (A) Spring-dashpot representation of forces shared by ECM (FECM) and Cells (FCell) which depend on the type of tissue (tendon, muscle, heart, etc.). ECM components include structural proteins such as collagen (various types) and also adhesion proteins (such as Fibronectin, Vitronectin, Osteonectin) that couple cells to ECM through adhesion receptors. (B) Mechanical strain on tendon tissue is primarily sustained by collagen fibrils present in collagen fibers which results in straightening of their crimps, but strain is also transferred to tenocytes via adhesion receptors.
Structural changes in collagen molecules or fibrils under load are certainly likely given the spectrum of collagen flexibility as revealed across different experimental approaches [56] [57] [58] [59] [60] [61] [62]. However, molecular scale simulations provide perhaps the clearest notions for how single collagen molecules respond, and conformational changes under forces [63] [64] [65] exhibit a dramatic transition at a tensile force of ~10 pN [66] [67]. A similar level of force unfolds and exposes reactive residues (cysteines) within the helix-rich cytoskeletal protein spectrin in sheared cells [68], and so the collagen molecule simulations potentially provide realistic structural rearrangements at the molecular level. While intermolecular collagen crosslinks oppose enzymatic degradation of unloaded collagen, molecular scale simulations show forces still disrupt native conformation which might increase the susceptibility to degradation [69].
Collagen molecules are ~1.5 nm in diameter but further assembly into fibrils increases the diameter from 10’s to 100’s of nanometers, whereas collagenases have Stoke’s radii of a few nanometers [70]. The larger effective pore sizes of fibrillar collagen gels (few tens of nm’s) as compared to non-fibrillar collagen (4–6 nm) perhaps rules out mechanisms of hindered diffusion or collagenase deformation in mechanically strained ECM. Additionally, flexibility of collagen fibrils is determined by bendability of constituting collagen molecules [63], and therefore homotrimers and heterotrimer collagen molecules carrying distinct flexibilities result into two different type of fibrils carrying entirely different molecular packing densities as well as flexibilities [71] but ultimately result in ECMs carrying similar effective pore-sizes. Although under mechanical load, these ECMs may not facilitate significantly different diffusion from each other but at least underscore the importance of amino-acid sequence on overall physicochemical behavior of collagen fibrils.
To keep our brief review of the main topic systematic while also keeping in mind the limitations of past methods, we first discuss mechanical strain-dependent degradation of fibrillar collagen at various levels of structural hierarchy. We then describe molecular mechanisms of cleavage by different collagenases and briefly discuss the likely influence of cells on collagen molecule organization. Unfortunately, past studies on “enzyme mechano-kinetics” of tissues have used conventional stress (or load)–relaxation tests [49] [72] that fit a three-parameter relaxation function [73], which is generally difficult to interpret directly in terms of tissue strain and degradation rate. These measurements are further complicated by dependencies on sample size, protein distribution, and estimations of strain [72], which might include “end effects” of local tissue drying and stress concentrations. We do not review such studies in quantitative detail, but try to point out a need for further innovative experiments.
2. Mechanical force suppresses enzymatic degradation of collagenous ECM of tissues
The rate of in vitro enzymatic degradation of mechanically stretched collagen fibers extracted from bovine achilles tendon correlates with the extent of in vivo collagen degradation upon subcutaneous implantation in guinea pigs [74]. Yannas and co-workers provided the earliest, surprising evidence that mechanical load suppresses the turnover and degradation of ECM [49]. In these studies, tendon collagen was reconstituted as a tape (~3.2 μm thick, ~130 μm wide), stretched in a solution of BC, and the rate of force relaxation was used to approximate the rate of degradation. The degradation rate appeared to (i) decrease up to a fiber strain of ~4% and (ii) depend linearly on BC concentration, which is consistent with standard Michaelis-Menten enzyme kinetics. The degradation rate of the tendon collagen tape also was found: (iii) higher upon collagen denaturation which indicates the importance of molecular conformation, (iv) inhibited by collagen intermolecular cross-linking, (v) to follow an Arrhenius relationship for temperatures of 10–56°C, (vi) inhibited by EDTA, O-phenanthroline, 2,3-dimercaptopropanol, and D,L,-cysteine, and (vii) to be maximal at neutral pH 7–8.
Michaelis-Menten kinetics is the standard model of enzyme kinetics, and it is useful to sketch out how it might apply in the present context. If a Protease (P) has a dissociation constant K (or Michaelis constant) that increases with strain (ε) applied [75] [76] to the collagen fibril substrate (S), then the initial enzyme-mediated degradation rate (V) of the substrate decreases according to:
where V(ε) and K(ε) are functions of strain. If strains are small such as in the first experiments on strain-modulated degradation rate (ε << 1), then it is reasonable to make a Taylor series expansion around ε = 0 (such as for an exponential function, Ko exp(A ε)), which gives K(ε) = Ko + A ε (Fig. 3A–i) where Ko reflects the activity at zero strain and A > 0 is a constant that reflects reduced activity with strain. Before much degradation of S occurs, the rate at zero strain is Vo ≈ c P S / (Ko+S). On the other hand, for sufficiently high strains (A ε >> Ko+S), V(ε) ≈ c P S / (A ε + ε2 term) asymptotically approaches zero (Fig. 3A–ii). For pure collagen fibrillar gels treated with MMP-8 and locally strained (or not) by micro-needles [77], the initial degradation rate (when S ~ constant) is strain-suppressed locally from Vo to V’ by a factor of ~2–3-fold (Fig. 3A-iii). Subsequent studies with micro-needles by the same group produced more measurements and estimated a ‘force per monomer’ of ~pN that gave a similar hyperbolic decay in ‘cleavage rate’ [78]. The Michaelis-Menten model thus seems appropriate.
Fig. 3:
Molecular kinetics and mechanism for strain-suppressed enzymatic degradation of collagen–I molecule within collagen fibrils. (A-i) Michaelis-Menten model states collagenase-collagen dissociation constant (K) increases with tensile strain on collagen fibrils and, (A-ii) collagen fibril degradation rate is suppressed with increasing tensile strain which is evident from (A-iii) respective degradation rates of collagen fibrils (mechanically strained or unstrained) when exposed to MMP-8, obtained using slopes of initial region of collagen conc. vs time curve (adopted from Flynn et al 2010 [77]) (B) Differences between MMP cleavage and BC-driven proteolysis. MMPs have smaller catalytic domains than BC and cut each α-chain of mechanically unloaded collagen molecule sequentially. The only vulnerable cleavage site of a partially unfolded α2-chain (for MMP-1, −8 & −13) is sequestered by mechanical strain. (C) BC cuts all α-chains of unstrained collagen molecule in parallel but the many cleavage sites are sequestered by mechanical strain.
Within a Michaelis-Menten equilibrium approximation, K(ε) is a ratio of enzyme off-rate and on-rate in binding to substrate collagen, so that strain could increase the off-rate, or decrease the on-rate, or both. At lowest order as above, koff(ε) = koff-0 + a ε, and kon(ε) = kon-0 – b ε for which a, b ≥ 0. Given the linearity of K(ε) = koff(ε) / kon(ε), the simplest model is a > 0 and b = 0, which stipulates that only the off-rate is strain sensitive. Such issues have yet to be fully resolved it seems, but the seminal studies to date ([78], for example) motivate many more studies.
Subsequent to the very first studies that showed strain inhibits collagen fiber proteolysis [74], the collagen degradation rates in strained tissues were measured by additional methods. One optics-based method for measuring strain [72] complemented conventional displacement measurements, and the degradation rate of collagen fibers present in rat tail tendon fascicles based on conventional stress-time response was observed to decrease linearly with strain; the results were interpreted as further indicating that strain-induced conformational changes in collagen molecules inhibit its enzymatic cleavage. Osmotic effects on the measured equilibrium stress in the conventional load-relaxation test with exposure of the fascicle to BC (or dextran) were also evident only for strains < 4% but subsided quickly as compared to duration of experiments. Additionally, chemically cross-linked tendon fascicle samples carrying glycation cross-links (i.e. nonenzymatic cross-linking) were > 5 times more resistant to degradation by BC than non-cross-linked ones in unloaded configuration but became highly susceptible to degradation under tensile deformation [79]. For various tissues, mechanical strains of various types (e.g. uniaxial tension, biaxial tension, etc.) often are reported to suppress degradation rate, with a few exceptions (Table 1). It seems conceivable that high local strains in complex tissues cause physical rupture and relaxation of some fibrils that are then degraded.
Table 1:
Evidence of mechanical load dependent enzymatic degradation of various tissues
| # | Tissue & Collagen type | Loading | Enzyme | Degr. rate |
Ref. |
|---|---|---|---|---|---|
| 1 | Cornea & I | Uniaxial Tension | BC | ↓ | [152] [153] [154] |
| 2 | Anulus fibrosus & I and II | Compression | MMP-1 | ↓ | [155] |
| 3 | Lungs & I and III | Uniaxial Tension | BC | ↓ & ↑ | [156] |
| 4 | Pericardium & I and III | Uni- & Biaxial Tension |
BC | ↓ | [157]; |
| 5 | Carotid arteries & I and III | Uniaxial Tension | BC** | ↓ & ↑ | [158] |
| 6 | Pericardium & I and III | Uniaxial Tension | BC | ↓ | [159] |
BC (Bacterial Collagenase or CHC or Clostridium histolicitum);
Crude and purified CHC (targets collagen only)
3. Mechanical strain suppresses collagen degradation at the fibril level
The micro-needle studies cited above focused on gels formed by reconstituted bovine collagen-I and revealed locally slowed fibril degradation by both BC [80] and MMP-8 [77]. The average force acting per collagen molecule in the fibril was estimated from the micro-needle forces as well as the diameter and elastic modulus of collagen fibrils [78]. As mentioned, the results seem consistent with Michaelis-Menten kinetics.
Proteolysis by MMP-1 is also suggested to be affected by mechanical loading of collagen fibrils obtained from rat tail tendon [81]. Because MMP cleavage sites of collagen molecules in fibrils are normally inaccessible due to molecular packing [82] [83], fluorescent MMPs (MMP-1 and MMP-9) bind at positions with a highly regular spatial periodicity of ~1 μm [84]. This periodicity and fluctuations of the binding regions of fluorescently labelled MMP-1 on collagen fibrils has indicated that in unloaded collagen fibrils (Fig. 3B), the “buckled” conformation exposes the otherwise protected [83] but thermally labile MMP-1 cleavage site of collagen molecules [85] [86] [87] [88], creating an entry point for initiation of proteolysis [84]. Binding to the buckled site often occurs without subsequent cleavage but is an obligatory and rate-limiting step in the proteolysis of fibrillar collagen [84]. Application of tensile load on fibril removes the buckled site in a switch-like manner and thereby inhibits degradation. The results seem consistent with the aforementioned Michaelis-Menten equilibrium approximation: K(ε) = koff(ε) / kon, in which strain increases only the off-rate.
4. Mechanical strain-dependent degradation of individual collagen molecules
Whether strain-dependent effects on fibril degradation also occur with isolated collagen molecules has remained ambiguous, even though fibrillar collagens fray to produce partially exposed collagen molecules during breakdown [89, 90]. The uncertainty has implications for the many non-fibrillar collagens in diverse tissues, including collagen-IV trimers in basement membrane ECM [91]. This is relevant to various MMPs on plasma membranes, which co-localize with integrins that bind ECM [92]; such co-localization is required for degradation of both fibrillar collagen [93] and localized gelatin, i.e. partially denatured collagen [94]. Given that single integrin receptors can transmit >20 pN of force on the ECM [95], a combination of cell-generated traction forces and MMP activity might thus contribute to the degradation of isolated or exposed collagen molecules and possibly other ECM molecules.
The susceptibility of individual homotrimeric collagen type-I (α1(I)3) molecule to enzymatic cleavage is different from that of its heterotrimeric (α1(I)2 α2) form, even upon application of tensile force. Collagen type-I molecules exist as heterotrimers in normal tissues, while the atypical homotrimeric form (α1(I)3) that is very resistant to MMP-1 cleavage is present in embryonic and cancer tissues [96]. Homotrimeric type-I (α1(I)3) molecules are also more resistant to cleavage by both MMP-1 and trypsin than other homotrimeric molecules including collagen type-II (α1(II)3) and collagen type-III (α1(III)3); this resistance is thought to be a function of the extent of hydrogen bonding in these homotrimeric forms [97]. Although type-I homotrimers are more flexible than type-I heterotrimers and thus form kinks [71], the cleavage region on the unloaded heterotrimer is thought to be locally unfolded in its equilibrium state, making it more susceptible to MMP-1 breakdown [96]. In particular, the locally unfolded region is found on the α2 chain near an MMP-1 cleavage site [98] as a result of disrupted hydrogen bonding in the native configuration [97]. This makes the α2 chain of heterotrimer more prone to MMP-1 cleavage than α1 chains [99], even in homotrimeric form (α2(I)3) [97]. Upon application of force, the naturally unfolded region of α2(I) chain in the heterotrimer refolds into a triple helical structure, potentially protecting the molecule against MMP-1 degradation. In contrast, a type-I collagen homotrimer retains a triple helical structure under no load which makes it more resistant to enzyme cleavage but applied force unfolds molecule and makes it prone to degradation [100]. These findings are consistent with the results of Dunn and coworkers [101] [102] as well as those of Nagase and coworkers [97] that have shown unloaded type-I heterotrimers are more susceptible to MMP cleavage than type-I homotrimers, and application of force increases the cleavage rate of the type-I heterotrimers by ~8 fold compared to ~100-fold for type-I homotrimers. Various studies have further focused on the tensile load-dependent enzymatic degradation for different trimeric forms of collagen and enzymes as well as tensile load magnitudes (Table 2), but more studies than not show that molecular cleavage of collagen is increased by force and strain unlike the results for collagen fibrils.
Table 2:
Experimental results for enzymatic degradation of collagen trimers under tensile load
| # | Collagen trimer | Enzyme | Force (pN) |
Degr. rate |
Ref. |
|---|---|---|---|---|---|
| 1 | Recombinant human collagen type I | BC | 3–10 | ↓ (10X) | [103] |
| 2 | Recombinant, post-translationally modified human collagen type I | BC | ~16 | - | [102] |
| 3 | Single collagen trimer peptide α1 (GPQGIAGQRGVVGL) | MMP-1 | ~10 | ↑ (100X) |
[101] |
| 4 | Recombinant, post-translationally modified human collagen I | MMP-1 | ~16 | ↑ (8X) | [102] |
| 5 | Recombinant human type III collagen | Trypsin | ~ 9 | ↑ (10–20X) | [108]. |
Given the tight coupling observed between tensile forces and degradation rate of single collagen molecules, precise measurements of local molecular strain and conformational state(s) would ideally help identify the structural changes occurring in regions carrying specific cleavage sites, perhaps ultimately enabling predictions of collagen turnover rate in various regions of load bearing tissues. To date, however, all of the reported studies are limited to descriptions of force and collagenase activity. Forces that are consistently in the range of ~1 to ~10 pN do provide a reasonable reference for single collagen molecule studies and are reportedly based on a transition from entropic to energetic elasticity [66] [67] [63] [65]. A bi-phasic response to strain appears plausible: tensile loads of ~3 to ~10 pN could suppress degradation of type-I collagen molecules by BC [103] through local conformational changes, whereas greater magnitudes of ~16 pN could in contrast enhance degradation rate [102] by stretching its peptide backbone and unwinding the triple helix [100].
Technical limitations are of some concern in considering single molecule level studies of different collagen trimer forms and strain-dependent enzymatic degradation. Model peptide sequences lack some properties of full-length proteins [67] [101] [97], and even in atomistic simulations, choice of force field affects triple-helical stability [67] [104] [63] [105], so that many factors such as sequence could influence conformation-dependent processes. Collagenases used to investigate force-induced deformations [101] [102] [103] begin to cleave once added [97] [106] [107], which makes it difficult to set a reference degradation rate for absolute measurements whereas enzymes such as trypsin [108] likely target only denatured collagen. Although trypsin does not belong to the metalloproteinase family, one of its 81 possible cleavage sites [109] located in the “loose” region of the triple helix is shared by the unique recognition sequence of MMPs [88]. The force-enhancement of collagen molecule proteolysis by trypsin, as with MMP-1, may thus be a result of both enzymes probing the same region of the collagen triple helix.
5. Cleavage mechanisms of BC versus MMPs
MMPs are far less efficient in degrading collagen fibrils than the highly studied BC [52]. In general, MMPs (MMP-1, MMP-8, and MMP-13) cleave collagen molecules at a single site (Fig. 3B) approximately ¾ of the length from its N-terminus [110], although binding may also occur at other sites [111]. More specifically, both MMP-1 and MMP-8 have been shown to cleave native type-1 collagen α1 chains between Gly775 and lle776 and α2 chain between Gly775 and Leu776 [88]. BC on the other hand cleaves collagen molecules at multiple sites (Fig. 3C) but does show preferences for certain sites [112] [113] [114], for example Pro–X–Gly–Pro, where X is often a neutral amino acid targeted by BC [115]. Both BC and MMPs cleave all three chains within a collagen molecule, albeit through distinct predicted mechanisms. MMP-1 cannot dock all three chains simultaneously and instead unwinds the helical chains one by one near the target site prior to cleavage [102] [101] [106] [116] [117] [118]. Unlike MMPs, the catalytic domain of BC is large enough to surround, unwind, and cleave the three chains of a single collagen molecule at once [102] [107]. Brandstetter and colleagues [107] used structural and functional data to provide an integrated mechanistic model of collagen recognition and unraveling of collagen micro-fibrils into triple helices, as well as a model of triple helix cleavage by BC driven by opening and closing of its cleavage domain. More recently, AFM-based studies of BC movement along fibrils [119] revealed that intermolecular interactions between collagen molecules within a fibril prevent BC from engaging. BC molecules exhibit catalytic, zinc-dependent, and polarized movement along the fibril axis toward the collagen N-terminus, suggesting that their movement and function are tightly coupled. As BC degrades collagen, collagen molecules are occasionally rearranged and incorporated into neighboring fibrils. In contrast, measurements of MMPs (MMP-1, MMP-2, MT1-MMP) moving on fibrillar collagen through fluorescence correlation spectroscopy [120] [121] [122] and, more recently, direct single-molecule tracking of MMP-1 and MMP-9 [84], revealed that MMPs undergo one-dimensional diffusion on collagen fibril, frequently interrupted by transient binding. Degradation by MMP-1 initiates from a small subset of these binding events, with the initial cleavage event followed in rapid succession by ~15 or more cleavage reactions resulting in processive bursts of MMP-1 motion along the collagen fibril [84] and rearrangement of collagen molecules surrounding cleavage site [83]. Cleavage followed by fibril relaxation and further cleavage (cleave-relax-cleave) is a burning bridge type mechanism [123] that would be limited by fibril length and some type of tethered diffusion (perhaps by collagen) to the next binding site before dissociation. Collectively, these findings suggest the collagenase-driven cleavage mechanisms of collagen molecules within fibrils are primarily dictated by collagen intermolecular interactions, which can be directly regulated by mechanical strain.
6. Collagen’s response to cellular forces
The idea expressed at the outset of this review that tissue stiffness scales with tissue stress and with collagen levels might apply locally to forces at cell membrane adhesions. Some MMPs are certainly membrane bound, and cells can exert ~pN level tensions on ECM [95], which is relevant to affect degradation. Collagen molecules present in ECM have indeed been reported to directly interact with cells through various integrin receptors (i.e. α- and β-integrin isoforms e.g. α1β1, α2β1) [124] [125] [126] [127], discoidin domain receptors (DDR1, DDR2) [128] [129], plasma membrane glycoproteins (GPIIIb, GPIV, GPVI/p62) [130] [131], immunoglobulin-like receptors (e.g. LAIR-1) [132] [133], Annexin-A5 (also known as anchorin II) [134] [135], glycosaminoglycan chains present in transmembrane proteins such as CD44 (and in proteoglycans) [136] [137] [138] [139], and also indirectly via fibronectin involving α5β1 integrins [140]. Some of these receptors might mediate strong application of force to collagen fibrils, but more such measurements are needed, especially in relation to integrity of local fibrils.
In general, collagen receptor(s) bind to the collagen triple helix at particular site(s) carrying either a highly specific motif (present in a particular collagen type) or a common sequence (in various collagen types), although some receptors also show affinity towards cryptic binding sites and/or non-collagenous domains present in collagen molecules [141]. Cells exert contractile forces on collagen molecules [23] [24] [142] [143] [144] via focal-adhesions containing clustered integrins. Moreover, force magnitudes somehow orchestrate focal-adhesion size in a reversible manner, and this occurs on a time scale of seconds to minutes (not hours) while maintaining overall constant traction force per unit area of focal adhesion [145]. This force-dependent focal adhesion size dynamics is notably similar to force-dependent assembly and disassembly of fibronectin molecules into fibrils [146] [147] which are well known to mediate cell-collagen interactions using α5β1 integrins [6] [140]. Although the role of various collagen-receptors in dictating collagen molecular packing and organization is not clear but certainly relevant to the ‘force per fibril’, fibronectin [148] [149] and particular integrin subtypes (e.g. α5β1, α2β1) [150] [151] are implicated in collagen fibrillogenesis [6].
7. Concluding Prospectus
As homeostasis and adaptation of tissues is partially governed by the mechanosensitive nature of ECM with collagen protein as its primary component, it is of fundamental importance to understand collagen’s force-dependent degradation by enzymes. In addition to large tensile stress bearing tissue such as tendon, other tissues also show tension-dependent enzymatic degradation – although precise measurements have been limited by complexities associated with load-relaxation tests and unknown strain relationships among various levels of the structural hierarchy. Tissue level studies show tension-inhibited degradation by BC, but because of the differences of their catalytic domain sizes and target sites on collagen molecules as well as physiological importance, the use of MMPs in tissue level studies would likely be more interesting. Similar to tissues, individual collagen fibrils show strain-inhibited degradation against both BC and some MMPs – but at the molecular level, collagen seems to show biphasic behavior with smaller loads conferring resistance against cleavage and larger loads conversely making them more susceptible to degradation. Thus, improved and standardized characterization of how mechanical force, at various magnitudes, regulates collagen ECM remodeling at each structural hierarchy level appears necessary. Furthermore, mechanical strain dependent exposure of collagen cryptic sites as well as sequestration of recognition sites for collagen receptors could dramatically change cell-ECM interactions and downstream pathways including cell fate and function. Understanding the very local processes of cell adhesion and collagen stability could indeed shed light on mechanisms of mechanosensing matrix elasticity and phenotype. Molecular and systems-level mechanistic insight gained from such studies will likely further benefit the treatment of a broad range of ECM-associated pathological conditions, which range from cardiovascular injury/disease to numerous solid cancers.
Highlights.
Enzymatic degradation of tissues is dependent on mechanical load
Tensile strain inhibits enzymatic degradation of ECM’s collagen at different structural hierarchy levels of most tissues
At molecular level, collagen degradation rate show mechanical load magnitude dependent biphasic behavior
Tensile load suppresses enzymatic degradation of collagen by MMPs and BC
Acknowledgements:
The authors in this study were supported by the National Institutes of Health National Cancer Institute under Physical Sciences Oncology Center Award U54 CA193417, Human Frontiers Sciences Program grant RGP0024, National Heart, Lung, and Blood Institute award R01 HL124106, SERB Indo-US Postdoctoral Fellowship 2016–157, the US–Israel Binational Science Foundation, National Science Foundation Materials Science and Engineering Center grant to the University of Pennsylvania and also grant agreement CMMI 15–48571. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other granting agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest, financial or otherwise, are declared by the author(s).
References:
- 1.Gennes P.G.d., Scaling concepts in polymer physics. 1979, Ithaca, N.Y.: Cornell University Press; 324 p. [Google Scholar]
- 2.Yang YL, Leone LM, and Kaufman LJ, Elastic moduli of collagen gels can be predicted from two-dimensional confocal microscopy. Biophys J, 2009. 97(7): p. 2051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doi M and Edwards SF, The theory of polymer dynamics. Vol. 73 1988: oxford university press. [Google Scholar]
- 4.Swift J, et al. , Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science, 2013. 341(6149): p. 1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho S, et al. , Mechanosensing by the lamina protects against nuclear rupture, DNA damage, and cell cycle arrest. bioRxiv, 2019: p. 583179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadler KE, Hill A, and Canty-Laird EG, Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol, 2008. 20(5): p. 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gelse K, Poschl E, and Aigner T, Collagens--structure, function, and biosynthesis. Adv Drug Deliv Rev, 2003. 55(12): p. 1531–46. [DOI] [PubMed] [Google Scholar]
- 8.Lodish HF, Molecular cell biology. 5th ed 2003, New York: W.H. Freeman and Company; xxxiii, 973, 79 p. [Google Scholar]
- 9.Holmes DF, et al. , Collagen Fibril Assembly and Function. Curr Top Dev Biol, 2018. 130: p. 107–142. [DOI] [PubMed] [Google Scholar]
- 10.Kadler KE, Fell Muir Lecture: Collagen fibril formation in vitro and in vivo. Int J Exp Pathol, 2017. 98(1): p. 4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laurent GJ, Dynamic state of collagen: pathways of collagen degradation in vivo and their possible role in regulation of collagen mass. Am J Physiol, 1987. 252(1 Pt 1): p. C1–9. [DOI] [PubMed] [Google Scholar]
- 12.Doris MS, Chapter 5 - the Role of Tension in Muscle Growth. 1972: p. 77 – 100. [Google Scholar]
- 13.Goldspink DF, The influence of passive stretch on the growth and protein turnover of the denervated extensor digitorum longus muscle. Biochem J, 1978. 174(2): p. 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg AL, Protein synthesis during work-induced growth of skeletal muscle. J Cell Biol, 1968. 36(3): p. 653–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldspink DF, The influence of immobilization and stretch on protein turnover of rat skeletal muscle. J Physiol, 1977. 264(1): p. 267–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Binderman I, Somjen D, and Shimshoni Z, Growth Induction of bone and cartilage cells by physical forces, in Tissue nutrition and viability. 1986, Springer; p. 121–133. [Google Scholar]
- 17.Rannels DE, Role of physical forces in compensatory growth of the lung. Am J Physiol, 1989. 257(4 Pt 1): p. L179–89. [DOI] [PubMed] [Google Scholar]
- 18.Bishop JE and Lindahl G, Regulation of cardiovascular collagen synthesis by mechanical load. Cardiovasc Res, 1999. 42(1): p. 27–44. [DOI] [PubMed] [Google Scholar]
- 19.Parsons M, et al. , Mechanical load enhances procollagen processing in dermal fibroblasts by regulating levels of procollagen C-proteinase. Exp Cell Res, 1999. 252(2): p. 319–31. [DOI] [PubMed] [Google Scholar]
- 20.Robbins JR, Evanko SP, and Vogel KG, Mechanical loading and TGF-beta regulate proteoglycan synthesis in tendon. Arch Biochem Biophys, 1997. 342(2): p. 203–11. [DOI] [PubMed] [Google Scholar]
- 21.Ragan PM, et al. , Down-regulation of chondrocyte aggrecan and type-II collagen gene expression correlates with increases in static compression magnitude and duration. J Orthop Res, 1999. 17(6): p. 836–42. [DOI] [PubMed] [Google Scholar]
- 22.Streuli CH and Bissell MJ, Expression of extracellular matrix components is regulated by substratum. J Cell Biol, 1990. 110(4): p. 1405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiquet M, et al. , How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biol, 2003. 22(1): p. 73–80. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen TD, et al. , Effects of cell seeding and cyclic stretch on the fiber remodeling in an extracellular matrix-derived bioscaffold. Tissue Eng Part A, 2009. 15(4): p. 957–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mudera VC, et al. , Molecular responses of human dermal fibroblasts to dual cues: contact guidance and mechanical load. Cell Motil Cytoskeleton, 2000. 45(1): p. 1–9. [DOI] [PubMed] [Google Scholar]
- 26.Ejim OS, Blunn GW, and Brown RA, Production of artificial-orientated mats and strands from plasma fibronectin: a morphological study. Biomaterials, 1993. 14(10): p. 743–8. [DOI] [PubMed] [Google Scholar]
- 27.Guido S and Tranquillo RT, A methodology for the systematic and quantitative study of cell contact guidance in oriented collagen gels. Correlation of fibroblast orientation and gel birefringence. J Cell Sci, 1993. 105 ( Pt 2): p. 317–31. [DOI] [PubMed] [Google Scholar]
- 28.Canty EG, et al. , Actin filaments are required for fibripositor-mediated collagen fibril alignment in tendon. J Biol Chem, 2006. 281(50): p. 38592–8. [DOI] [PubMed] [Google Scholar]
- 29.Chen YJ, et al. , Effects of cyclic mechanical stretching on the mRNA expression of tendon/ligament-related and osteoblast-specific genes in human mesenchymal stem cells. Connect Tissue Res, 2008. 49(1): p. 7–14. [DOI] [PubMed] [Google Scholar]
- 30.Prajapati RT, et al. , Mechanical loading regulates protease production by fibroblasts in three-dimensional collagen substrates. Wound Repair Regen, 2000. 8(3): p. 226–37. [DOI] [PubMed] [Google Scholar]
- 31.Yang G, Im HJ, and Wang JH, Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene, 2005. 363: p. 166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karasawa Y, et al. , Tension Force Downregulates Matrix Metalloproteinase Expression and Upregulates the Expression of Their Inhibitors through MAPK Signaling Pathways in MC3T3-E1 cells. Int J Med Sci, 2015. 12(11): p. 905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, et al. , Upregulation of MMP-13 and TIMP-1 expression in response to mechanical strain in MC3T3-E1 osteoblastic cells. BMC Res Notes, 2010. 3: p. 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicodemus GD and Bryant SJ, Mechanical loading regimes affect the anabolic and catabolic activities by chondrocytes encapsulated in PEG hydrogels. Osteoarthritis Cartilage, 2010. 18(1): p. 126–37. [DOI] [PubMed] [Google Scholar]
- 35.Wong M, Siegrist M, and Goodwin K, Cyclic tensile strain and cyclic hydrostatic pressure differentially regulate expression of hypertrophic markers in primary chondrocytes. Bone, 2003. 33(4): p. 685–93. [DOI] [PubMed] [Google Scholar]
- 36.Engler AJ, et al. , Matrix elasticity directs stem cell lineage specification. Cell, 2006. 126(4): p. 677–89. [DOI] [PubMed] [Google Scholar]
- 37.Altman GH, et al. , Cell differentiation by mechanical stress. FASEB J, 2002. 16(2): p. 270–2. [DOI] [PubMed] [Google Scholar]
- 38.Koskinen SO, et al. , Physical exercise can influence local levels of matrix metalloproteinases and their inhibitors in tendon-related connective tissue. J Appl Physiol (1985), 2004. 96(3): p. 861–4. [DOI] [PubMed] [Google Scholar]
- 39.Arnoczky SP, et al. , Matrix metalloproteinase inhibitors prevent a decrease in the mechanical properties of stress-deprived tendons: an in vitro experimental study. Am J Sports Med, 2007. 35(5): p. 763–9. [DOI] [PubMed] [Google Scholar]
- 40.Thornton GM, et al. , Changes in mechanical loading lead to tendonspecific alterations in MMP and TIMP expression: influence of stress deprivation and intermittent cyclic hydrostatic compression on rat supraspinatus and Achilles tendons. Br J Sports Med, 2010. 44(10): p. 698–703. [DOI] [PubMed] [Google Scholar]
- 41.Fu SC, et al. , Increased expression of matrix metalloproteinase 1 (MMP1) in 11 patients with patellar tendinosis. Acta Orthop Scand, 2002. 73(6): p. 658–62. [DOI] [PubMed] [Google Scholar]
- 42.Sun HB, et al. , Coordinate regulation of IL-1beta and MMP-13 in rat tendons following subrupture fatigue damage. Clin Orthop Relat Res, 2008. 466(7): p. 1555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnoczky SP, Lavagnino M, and Egerbacher M, The mechanobiological aetiopathogenesis of tendinopathy: is it the over-stimulation or the under-stimulation of tendon cells? Int J Exp Pathol, 2007. 88(4): p. 217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blain EJ, et al. , Up-regulation of matrix metalloproteinase expression and activation following cyclical compressive loading of articular cartilage in vitro. Arch Biochem Biophys, 2001. 396(1): p. 49–55. [DOI] [PubMed] [Google Scholar]
- 45.Sun HB, Mechanical loading, cartilage degradation, and arthritis. Ann N Y Acad Sci, 2010. 1211: p. 37–50. [DOI] [PubMed] [Google Scholar]
- 46.Cousineau-Pelletier P and Langelier E, Relative contributions of mechanical degradation, enzymatic degradation, and repair of the extracellular matrix on the response of tendons when subjected to under- and over- mechanical stimulations in vitro. J Orthop Res, 2010. 28(2): p. 204–10. [DOI] [PubMed] [Google Scholar]
- 47.Walden G, et al. , A Clinical, Biological, and Biomaterials Perspective into Tendon Injuries and Regeneration. Tissue Eng Part B Rev, 2017. 23(1): p. 44–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snedeker JG and Foolen J, Tendon injury and repair - A perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomater, 2017. 63: p. 18–36. [DOI] [PubMed] [Google Scholar]
- 49.Huang C and Yannas IV, Mechanochemical studies of enzymatic degradation of insoluble collagen fibers. J Biomed Mater Res, 1977. 11(1): p. 137–54. [DOI] [PubMed] [Google Scholar]
- 50.Matsushita O, et al. , Gene duplication and multiplicity of collagenases in Clostridium histolyticum. J Bacteriol, 1999. 181(3): p. 923–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshihara K, et al. , Cloning and nucleotide sequence analysis of the colH gene from Clostridium histolyticum encoding a collagenase and a gelatinase. J Bacteriol, 1994. 176(21): p. 6489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Welgus HG, Jeffrey JJ, and Eisen AZ, Human skin fibroblast collagenase. Assessment of activation energy and deuterium isotope effect with collagenous substrates. J Biol Chem, 1981. 256(18): p. 9516–21. [PubMed] [Google Scholar]
- 53.Duarte AS, Correia A, and Esteves AC, Bacterial collagenases - A review. Crit Rev Microbiol, 2016. 42(1): p. 106–26. [DOI] [PubMed] [Google Scholar]
- 54.Welgus HG, Jeffrey JJ, and Eisen AZ, The collagen substrate specificity of human skin fibroblast collagenase. J Biol Chem, 1981. 256(18): p. 9511–5. [PubMed] [Google Scholar]
- 55.Welgus HG, et al. , Characteristics of the action of human skin fibroblast collagenase on fibrillar collagen. J Biol Chem, 1980. 255(14): p. 6806–13. [PubMed] [Google Scholar]
- 56.Utiyama H, et al. , Flexibility of tropocollagen from sedimentation and viscosity. Biopolymers, 1973. 12(1): p. 53–64. [DOI] [PubMed] [Google Scholar]
- 57.Nestler FH, et al. , Flexibility of collagen determined from dilute solution viscoelastic measurements. Biopolymers, 1983. 22(7): p. 1747–58. [DOI] [PubMed] [Google Scholar]
- 58.Saito T, et al. , Semiflexibility of collagens in solution. Biopolymers, 1982. 21(4): p. 715–28. [DOI] [PubMed] [Google Scholar]
- 59.Hofmann H, et al. , Localization of flexible sites in thread-like molecules from electron micrographs. Comparison of interstitial, basement membrane and intima collagens. J Mol Biol, 1984. 172(3): p. 325–43. [DOI] [PubMed] [Google Scholar]
- 60.Sun YL, Luo ZP, and An KN, Stretching short biopolymers using optical tweezers. Biochem Biophys Res Commun, 2001. 286(4): p. 826–30. [DOI] [PubMed] [Google Scholar]
- 61.Sun YL, et al. , Direct quantification of the flexibility of type I collagen monomer. Biochem Biophys Res Commun, 2002. 295(2): p. 382–6. [DOI] [PubMed] [Google Scholar]
- 62.Sun YL, et al. , Stretching type II collagen with optical tweezers. J Biomech, 2004. 37(11): p. 1665–9. [DOI] [PubMed] [Google Scholar]
- 63.Buehler MJ and Wong SY, Entropic elasticity controls nanomechanics of single tropocollagen molecules. Biophys J, 2007. 93(1): p. 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Egorov SA, et al. , A new insight into the isotropic-nematic phase transition in lyotropic solutions of semiflexible polymers: density-functional theory tested by molecular dynamics. Soft Matter, 2016. 12(22): p. 4944–4959. [DOI] [PubMed] [Google Scholar]
- 65.Saini K and Kumar N, Mechanical response of collagen molecule under hydrostatic compression. Mater Sci Eng C Mater Biol Appl, 2015. 49: p. 720–726. [DOI] [PubMed] [Google Scholar]
- 66.Wieczorek A, et al. , Development and characterization of a eukaryotic expression system for human type II procollagen. BMC Biotechnol, 2015. 15: p. 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang SW and Buehler MJ, Molecular biomechanics of collagen molecules. Materials Today, 2014. 17(2): p. 70–76. [Google Scholar]
- 68.Johnson CP, et al. , Forced unfolding of proteins within cells. Science, 2007. 317(5838): p. 663–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bourne JW and Torzilli PA, Molecular simulations predict novel collagen conformations during cross-link loading. Matrix Biol, 2011. 30(5–6): p. 356–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tyn MT and Gusek TW, Prediction of diffusion coefficients of proteins. Biotechnol Bioeng, 1990. 35(4): p. 327–38. [DOI] [PubMed] [Google Scholar]
- 71.Chang SW, Shefelbine SJ, and Buehler MJ, Structural and mechanical differences between collagen homo- and heterotrimers: relevance for the molecular origin of brittle bone disease. Biophys J, 2012. 102(3): p. 640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wyatt KE, Bourne JW, and Torzilli PA, Deformation-dependent enzyme mechanokinetic cleavage of type I collagen. J Biomech Eng, 2009. 131(5): p. 051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Findley WN, Lai JS, and Onaran K, Creep and relaxation of nonlinear viscoelastic materials : with an introduction to linear viscoelasticity Dover books on engineering. 1989, New York: Dover; xii, 371 p. [Google Scholar]
- 74.Yannas IV, et al. , Correlation of in vivo collagen degradation rate with in vitro measurements. J Biomed Mater Res, 1975. 9(6): p. 623–8. [DOI] [PubMed] [Google Scholar]
- 75.Dingal PC and Discher DE, Systems mechanobiology: tension-inhibited protein turnover is sufficient to physically control gene circuits. Biophys J, 2014. 107(11): p. 2734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buxboim A, et al. , Matrix elasticity regulates lamin-A,C phosphorylation and turnover with feedback to actomyosin. Curr Biol, 2014. 24(16): p. 1909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Flynn BP, et al. , Mechanical strain stabilizes reconstituted collagen fibrils against enzymatic degradation by mammalian collagenase matrix metalloproteinase 8 (MMP-8). PLoS One, 2010. 5(8): p. e12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Flynn BP, Tilburey GE, and Ruberti JW, Highly sensitive single-fibril erosion assay demonstrates mechanochemical switch in native collagen fibrils. Biomech Model Mechanobiol, 2013. 12(2): p. 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bourne JW, Lippell JM, and Torzilli PA, Glycation cross-linking induced mechanical-enzymatic cleavage of microscale tendon fibers. Matrix Biol, 2014. 34: p. 179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bhole AP, et al. , Mechanical strain enhances survivability of collagen micronetworks in the presence of collagenase: implications for load-bearing matrix growth and stability. Philos Trans A Math Phys Eng Sci, 2009. 367(1902): p. 3339–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dittmore A, et al. , Internal strain drives spontaneous periodic buckling in collagen and regulates remodeling. Proc Natl Acad Sci U S A, 2016. 113(30): p. 8436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Orgel JP, et al. , Microfibrillar structure of type I collagen in situ. Proc Natl Acad Sci U S A, 2006. 103(24): p. 9001–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perumal S, Antipova O, and Orgel JP, Collagen fibril architecture, domain organization, and triple-helical conformation govern its proteolysis. Proc Natl Acad Sci U S A, 2008. 105(8): p. 2824–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sarkar SK, et al. , Single-molecule tracking of collagenase on native type I collagen fibrils reveals degradation mechanism. Curr Biol, 2012. 22(12): p. 1047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lu KG and Stultz CM, Insight into the degradation of type-I collagen fibrils by MMP-8. J Mol Biol, 2013. 425(10): p. 1815–25. [DOI] [PubMed] [Google Scholar]
- 86.Leikina E, et al. , Type I collagen is thermally unstable at body temperature. Proc Natl Acad Sci U S A, 2002. 99(3): p. 1314–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stultz CM and Edelman ER, A structural model that explains the effects of hyperglycemia on collagenolysis. Biophys J, 2003. 85(4): p. 2198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fields GB, A model for interstitial collagen catabolism by mammalian collagenases. J Theor Biol, 1991. 153(4): p. 585–602. [DOI] [PubMed] [Google Scholar]
- 89.Asuwa N, Collagen degradation in the rabbit skin during short-term tissue culture. Virchows Arch B Cell Pathol Incl Mol Pathol, 1988. 55(6): p. 345–54. [DOI] [PubMed] [Google Scholar]
- 90.Woolley DE, et al. , Purification of rheumatoid synovial collagenase and its action on soluble and insoluble collagen. Eur J Biochem, 1975. 54(2): p. 611–22. [DOI] [PubMed] [Google Scholar]
- 91.Paulsson M, Basement membrane proteins: structure, assembly, and cellular interactions. Crit Rev Biochem Mol Biol, 1992. 27(1–2): p. 93–127. [DOI] [PubMed] [Google Scholar]
- 92.Gonzalo P, et al. , MT1-MMP and integrins: Hand-to-hand in cell communication. Biofactors, 2010. 36(4): p. 248–54. [DOI] [PubMed] [Google Scholar]
- 93.Wolf K, et al. , Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol, 2007. 9(8): p. 893–904. [DOI] [PubMed] [Google Scholar]
- 94.Wang Y and McNiven MA, Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex. J Cell Biol, 2012. 196(3): p. 375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li F, et al. , Force measurements of the alpha5beta1 integrin-fibronectin interaction. Biophys J, 2003. 84(2 Pt 1): p. 1252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Han S, et al. , Molecular mechanism of type I collagen homotrimer resistance to mammalian collagenases. J Biol Chem, 2010. 285(29): p. 22276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mekkat A, et al. , Effects of flexibility of the alpha2 chain of type I collagen on collagenase cleavage. J Struct Biol, 2018. 203(3): p. 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nerenberg PS and Stultz CM, Differential unfolding of alpha1 and alpha2 chains in type I collagen and collagenolysis. J Mol Biol, 2008. 382(1): p. 246–56. [DOI] [PubMed] [Google Scholar]
- 99.Wu H, et al. , Generation of collagenase-resistant collagen by site-directed mutagenesis of murine pro alpha 1(I) collagen gene. Proc Natl Acad Sci U S A, 1990. 87(15): p. 5888–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chang SW, et al. , Molecular mechanism of force induced stabilization of collagen against enzymatic breakdown. Biomaterials, 2012. 33(15): p. 3852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Adhikari AS, Chai J, and Dunn AR, Mechanical load induces a 100-fold increase in the rate of collagen proteolysis by MMP-1. J Am Chem Soc, 2011. 133(6): p. 1686–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Adhikari AS, Glassey E, and Dunn AR, Conformational dynamics accompanying the proteolytic degradation of trimeric collagen I by collagenases. J Am Chem Soc, 2012. 134(32): p. 13259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Camp RJ, et al. , Molecular mechanochemistry: low force switch slows enzymatic cleavage of human type I collagen monomer. J Am Chem Soc, 2011. 133(11): p. 4073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zitnay JL, et al. , Molecular level detection and localization of mechanical damage in collagen enabled by collagen hybridizing peptides. Nat Commun, 2017. 8: p. 14913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Varma S, et al. , Effect of intrinsic and extrinsic factors on the simulated D-band length of type I collagen. Proteins, 2015. 83(10): p. 1800–12. [DOI] [PubMed] [Google Scholar]
- 106.Chung L, et al. , Collagenase unwinds triple-helical collagen prior to peptide bond hydrolysis. EMBO J, 2004. 23(15): p. 3020–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eckhard U, et al. , Structure of collagenase G reveals a chew-and-digest mechanism of bacterial collagenolysis. Nat Struct Mol Biol, 2011. 18(10): p. 1109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kirkness MWH and Forde NR, Single-Molecule Assay for Proteolytic Susceptibility: Force-Induced Collagen Destabilization. Biophys J, 2018. 114(3): p. 570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miller EJ, et al. , Specific cleavage of the native type III collagen molecule with trypsin. Similarity of the cleavage products to collagenase-produced fragments and primary structure at the cleavage site. Arch Biochem Biophys, 1976. 173(2): p. 631–7. [DOI] [PubMed] [Google Scholar]
- 110.Fields GB, Interstitial collagen catabolism. J Biol Chem, 2013. 288(13): p. 8785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sun HB, et al. , Atomic force microscopy-based detection of binding and cleavage site of matrix metalloproteinase on individual type II collagen helices. Anal Biochem, 2000. 283(2): p. 153–8. [DOI] [PubMed] [Google Scholar]
- 112.French MF, Mookhtiar KA, and Van Wart HE, Limited proteolysis of type I collagen at hyperreactive sites by class I and II Clostridium histolyticum collagenases: complementary digestion patterns. Biochemistry, 1987. 26(3): p. 681–7. [DOI] [PubMed] [Google Scholar]
- 113.French MF, Bhown A, and Van Wart HE, Identification of Clostridium histolyticum collagenase hyperreactive sites in type I, II, and III collagens: lack of correlation with local triple helical stability. J Protein Chem, 1992. 11(1): p. 83–97. [DOI] [PubMed] [Google Scholar]
- 114.Toyoshima T, et al. , Collagen-binding domain of a Clostridium histolyticum collagenase exhibits a broad substrate spectrum both in vitro and in vivo. Connect Tissue Res, 2001. 42(4): p. 281–90. [DOI] [PubMed] [Google Scholar]
- 115.Haralson MA and Hassell JR, Extracellular matrix : a practical approach The Practical approach series. 1995, Oxford ; New York: IRL Press; xxvii, 404 p. [Google Scholar]
- 116.Bertini I, et al. , Structural basis for matrix metalloproteinase 1-catalyzed collagenolysis. J Am Chem Soc, 2012. 134(4): p. 2100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jakowski J, et al. , Theoretical assessment of the nuclear quantum effects on polymer crystallinity via perturbation theory and dynamics. International Journal of Quantum Chemistry, 2018. 118(20). [Google Scholar]
- 118.Desai PR, Sinha S, and Das S, Compression of polymer brushes in the weak interpenetration regime: scaling theory and molecular dynamics simulations. Soft Matter, 2017. 13(22): p. 4159–4166. [DOI] [PubMed] [Google Scholar]
- 119.Watanabe-Nakayama T, et al. , High-speed atomic force microscopy reveals strongly polarized movement of clostridial collagenase along collagen fibrils. Sci Rep, 2016. 6: p. 28975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Collier IE, et al. , Substrate recognition by gelatinase A: the C-terminal domain facilitates surface diffusion. Biophys J, 2001. 81(4): p. 2370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Collier IE, et al. , Diffusion of MMPs on the surface of collagen fibrils: the mobile cell surface-collagen substratum interface. PLoS One, 2011. 6(9): p. e24029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Saffarian S, et al. , Interstitial collagenase is a Brownian ratchet driven by proteolysis of collagen. Science, 2004. 306(5693): p. 108–11. [DOI] [PubMed] [Google Scholar]
- 123.Saffarian S, et al. , Powering a burnt bridges Brownian ratchet: a model for an extracellular motor driven by proteolysis of collagen. Phys Rev E Stat Nonlin Soft Matter Phys, 2006. 73(4 Pt 1): p. 041909. [DOI] [PubMed] [Google Scholar]
- 124.Huhtala M, et al. , Integrin evolution: insights from ascidian and teleost fish genomes. Matrix Biol, 2005. 24(2): p. 83–95. [DOI] [PubMed] [Google Scholar]
- 125.Hynes RO, Integrins: bidirectional, allosteric signaling machines. Cell, 2002. 110(6): p. 673–87. [DOI] [PubMed] [Google Scholar]
- 126.Takada Y, Ye X, and Simon S, The integrins. Genome Biol, 2007. 8(5): p. 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Choi CK, et al. , Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol, 2008. 10(9): p. 1039–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vogel W, et al. , The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell, 1997. 1(1): p. 13–23. [DOI] [PubMed] [Google Scholar]
- 129.Shrivastava A, et al. , An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell, 1997. 1(1): p. 25–34. [DOI] [PubMed] [Google Scholar]
- 130.Moroi M and Jung SM, Platelet glycoprotein VI: its structure and function. Thromb Res, 2004. 114(4): p. 221–33. [DOI] [PubMed] [Google Scholar]
- 131.Tandon NN, Kralisz U, and Jamieson GA, Identification of glycoprotein IV (CD36) as a primary receptor for platelet-collagen adhesion. J Biol Chem, 1989. 264(13): p. 7576–83. [PubMed] [Google Scholar]
- 132.Lebbink RJ, et al. , Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J Exp Med, 2006. 203(6): p. 1419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Curino AC, et al. , Intracellular collagen degradation mediated by uPARAP/Endo180 is a major pathway of extracellular matrix turnover during malignancy. J Cell Biol, 2005. 169(6): p. 977–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mollenhauer J and von der Mark K, Isolation and characterization of a collagen-binding glycoprotein from chondrocyte membranes. EMBO J, 1983. 2(1): p. 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kirsch T, et al. , The roles of annexins and types II and X collagen in matrix vesicle-mediated mineralization of growth plate cartilage. J Biol Chem, 2000. 275(45): p. 35577–83. [DOI] [PubMed] [Google Scholar]
- 136.Ponta H, Sherman L, and Herrlich PA, CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol, 2003. 4(1): p. 33–45. [DOI] [PubMed] [Google Scholar]
- 137.Jalkanen S and Jalkanen M, Lymphocyte CD44 binds the COOH-terminal heparin-binding domain of fibronectin. J Cell Biol, 1992. 116(3): p. 817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Elenius K, et al. , Binding of human syndecan to extracellular matrix proteins. J Biol Chem, 1990. 265(29): p. 17837–43. [PubMed] [Google Scholar]
- 139.Liu W, et al. , Heparan sulfate proteoglycans as adhesive and anti-invasive molecules. Syndecans and glypican have distinct functions. J Biol Chem, 1998. 273(35): p. 22825–32. [DOI] [PubMed] [Google Scholar]
- 140.Dalton SL, Marcantonio EE, and Assoian RK, Cell attachment controls fibronectin and alpha 5 beta 1 integrin levels in fibroblasts. Implications for anchorage-dependent and -independent growth. J Biol Chem, 1992. 267(12): p. 8186–91. [PubMed] [Google Scholar]
- 141.Heino J, The collagen family members as cell adhesion proteins. Bioessays, 2007. 29(10): p. 1001–10. [DOI] [PubMed] [Google Scholar]
- 142.Riveline D, et al. , Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol, 2001. 153(6): p. 1175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zaidel-Bar R, et al. , Hierarchical assembly of cell-matrix adhesion complexes. Biochem Soc Trans, 2004. 32(Pt3): p. 416–20. [DOI] [PubMed] [Google Scholar]
- 144.Geiger B and Yamada KM, Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol, 2011. 3(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Balaban NQ, et al. , Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol, 2001. 3(5): p. 466–72. [DOI] [PubMed] [Google Scholar]
- 146.Zhong C, et al. , Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol, 1998. 141(2): p. 539–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gao M, et al. , Structure and functional significance of mechanically unfolded fibronectin type III1 intermediates. Proc Natl Acad Sci U S A, 2003. 100(25): p. 14784–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McDonald JA, Kelley DG, and Broekelmann TJ, Role of fibronectin in collagen deposition: Fab’ to the gelatin-binding domain of fibronectin inhibits both fibronectin and collagen organization in fibroblast extracellular matrix. J Cell Biol, 1982. 92(2): p. 485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dzamba BJ, et al. , Fibronectin binding site in type I collagen regulates fibronectin fibril formation. J Cell Biol, 1993. 121(5): p. 1165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li S, et al. , Vascular smooth muscle cells orchestrate the assembly of type I collagen via alpha2beta1 integrin, RhoA, and fibronectin polymerization. Am J Pathol, 2003. 163(3): p. 1045–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jokinen J, et al. , Integrin-mediated cell adhesion to type I collagen fibrils. J Biol Chem, 2004. 279(30): p. 31956–63. [DOI] [PubMed] [Google Scholar]
- 152.Robitaille MC, et al. , Small-angle light scattering to detect strain-directed collagen degradation in native tissue. Interface Focus, 2011. 1(5): p. 767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zareian R, et al. , Probing collagen/enzyme mechanochemistry in native tissue with dynamic, enzyme-induced creep. Langmuir, 2010. 26(12): p. 9917–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ruberti JW and Hallab NJ, Strain-controlled enzymatic cleavage of collagen in loaded matrix. Biochem Biophys Res Commun, 2005. 336(2): p. 483–9. [DOI] [PubMed] [Google Scholar]
- 155.Lotz JC, et al. , Anulus fibrosus tension inhibits degenerative structural changes in lamellar collagen. Eur Spine J, 2008. 17(9): p. 1149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yi E, et al. , Mechanical Forces Accelerate Collagen Digestion by Bacterial Collagenase in Lung Tissue Strips. Front Physiol, 2016. 7: p. 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ghazanfari S, et al. , Modulation of collagen fiber orientation by strain-controlled enzymatic degradation. Acta Biomater, 2016. 35: p. 118–26. [DOI] [PubMed] [Google Scholar]
- 158.Gaul RT, et al. , Strain mediated enzymatic degradation of arterial tissue: Insights into the role of the non-collagenous tissue matrix and collagen crimp. Acta Biomater, 2018. 77: p. 301–310. [DOI] [PubMed] [Google Scholar]
- 159.Ellsmere JC, Khanna RA, and Lee JM, Mechanical loading of bovine pericardium accelerates enzymatic degradation. Biomaterials, 1999. 20(12): p. 1143–50. [DOI] [PubMed] [Google Scholar]