Abstract
In vivo mesenchymal stem cell (MSC) survival is relevant to therapeutic applications requiring engraftment and potentially to non-engraftment applications as well. MSCs are a mixture of progenitors at different stages of cellular aging, but the contribution of this heterogeneity to the survival of MSC implants is unknown. Here, we employ a biomarker of cellular aging, the decoy TRAIL receptor CD264, to compare the survival kinetics of two cell populations in human bone marrow MSC (hBM-MSC) cultures. Sorted CD264+ hBM-MSCs from two age-matched donors have elevated β-galactosidase activity, decreased differentiation potential and form in vitro colonies inefficiently relative to CD264− hBM-MSCs. Counterintuitive to their aging phenotype, CD264+ hBM-MSCs exhibited comparable survival to matched CD264− hBM-MSCs from the same culture during in vitro colony formation and in vivo when implanted ectopically in immunodeficient NIH III mice. In vitro and in vivo survival of these two cell populations were independent of colony-forming efficiency. These findings have ramifications for the preparation of hBM-MSC therapies given the prevalence of aging CD264+ cells in hBM-MSC cultures and the popularity of colony-forming efficiency as a quality control metric in preclinical and clinical studies with MSCs.
Keywords: Aging, Mesenchymal Stem Cells, Survival, Decoy TRAIL Receptor 2 (CD264)
1. Introduction
Mesenchymal stem cells (MSCs) possess a broad spectrum of regenerative properties (Caplan & Dennis, 2006; Pittenger et al., 1999), which are being deployed in clinical trials to treat numerous disorders (Squillaro, Peluso, & Galderisi, 2016). MSC applications range from repairing articular cartilage defects (Haleem et al., 2010) to improving neurological function after a stroke (Lee et al., 2010). In vivo survival of MSCs is essential for these cells to engraft and replace damaged tissue (Barrilleaux, Phinney, Prockop, & O’Connor, 2006). In addition, MSCs that survive longer after implantation may deposit more trophic factors to promote tissue repair (Caplan, 2007). Accordingly, in vivo MSC survival is relevant to therapeutic applications requiring engraftment and potentially to non-engraftment applications as well.
At present, there is considerable variation in MSC survival following in vivo implantation. To illustrate, the amount of rat BM-MSCs in allografts decreased over 70% after 3 days in vivo according to one study (Zimmermann et al., 2011) but experienced only a 50% loss after 35 days according to another report (Leijs et al., 2017). Previous attempts to improve in vivo human and mouse BM-MSC survival include preconditioning the stem cells prior to implantation with growth factors and hypoxia (Beegle et al., 2015; Lu, Ashraf, & Haider, 2012). Other strategies to retain viable human and rat BM-MSC implants have focused on manipulating the concentration and attachment of bioactive molecules in the stem cell microenvironment (Blocki et al., 2015; Nuschke et al., 2016).
The literature is silent on the contribution of cellular heterogeneity to the survival of MSC implants. Human (h)BM-MSC cultures are a heterogeneous mixture of progenitors with different regenerative potentials (Crigler, Robey, Asawachaicharn, Gaupp, & Phinney, 2006; Russell et al., 2010) at different stages of cellular aging (Madsen et al., 2017; Stenderup, Justesen, Clausen, & Kassem, 2003). Long-term culture of hBM-MSCs revealed continuous and incremental changes to their global gene expression profile towards a senescent phenotype (Wagner et al., 2008). Cellular aging is a result of accumulated DNA damage from replicative stress and can result in a functional change that is detrimental to the regenerative properties of MSCs (Rando, 2006). Although stem cell aging is being studied extensively in vitro, to date, there has been no work to investigate the in vivo survival of aging MSCs of any kind. This is a critical knowledge gap in light of the importance of cell survival to MSC therapies.
Our research group recently identified the decoy TRAIL receptor CD264 as the first known surface marker of cellular aging for hBM-MSCs. CD264 is upregulated concomitantly with p21 at an intermediate stage of cellular aging and remains upregulated through senescence (Madsen et al., 2017). We detected CD264+ cells in all hBM-MSC cultures in our study: CD264+ cell content ranged from 20 – 35% in hBM-MSC cultures from young donors (ages 20 – 40 years old) and 20 – 75% in cultures from older donors (ages 45 – 60 years old) (Madsen et al., 2017). This is consistent with previous reports of CD264 expression for hBM-MSCs (Secchiero et al., 2008; Yulyana et al., 2013). In addition, we showed a strong inverse correlation of CD264+ cell content in hBM-MSC cultures with their in vitro proliferation and differentiation potential (Madsen et al., 2017). The correlation agrees with the link between cellular aging and loss of stem cell fitness observed by others (Bertolo, Baur, Guerrero, Pötzel, & Stoyanov, 2019; Yang, Ogando, Wang See, Chang, & Barabino, 2018). In vivo translational studies of aging CD264+ hBM-MSCs are warranted given their prevalence in hBM-MSC cultures and their diminished in vitro stem cell fitness.
As a first step toward this end, we quantify the in vivo survival kinetics of aging CD264+ hBM-MSCs relative to matched CD264− hBM-MSCs from the same culture. We observed that matched populations of CD264+ and CD264− hBM-MSCs have similar in vivo survival kinetics despite a significant difference in colony-forming efficiency. We provide insight into this counterintuitive finding by investigating in vitro colony formation at the single-cell level. Our results have ramifications for the preparation of hBM-MSC therapies given the prevalence of CD264+ cells in hBM-MSC cultures (Madsen et al., 2017; Secchiero et al., 2008), and the popularity of colony-forming efficiency as a quality control metric in preclinical and clinical studies with MSCs (Chaput et al., 2018; Kuznetsov, Mankani, Bianco, & Robey, 2009).
2. Methods
2.1. hBM-MSC cultures
Primary hBM-MSCs were isolated from iliac crest bone marrow aspirate. The cultures were selected to match the chronological age of the donor to within one year: donor 1 (female, 36 years old) and donor 2 (male, 37 years old). Both donors were healthy adult volunteers who had given their informed consent prior to bone marrow aspiration. The harvesting protocol was approved by the Tulane Institutional Review Board and conformed to the US Federal Policy for the Protection of Human Subjects (Department of Health & Human Services, Washington, DC, USA). Plastic-adherent hBM-MSCs prior to expansion were designated as passage 0 (P0). Donor hBM-MSC cultures employed in this study satisfied the criteria established by the International Society for Cellular Therapy for defining human MSCs based on plastic-adherence, immunophenotype and differentiation (Dominici et al., 2006). Unless otherwise noted, all cell culture supplies were obtained from Thermo Fisher Scientific (Waltham, MA, USA). hBM-MSCs were routinely cultured in T-flasks using complete culture medium with antibiotics (CCMA): α-MEM with 2 mM L-glutamine supplemented with an additional 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 20% fetal bovine serum (FBS) (Sekiya et al., 2002). For cell expansion, hBM-MSC cultures had an initial cell density ≥ 100 cells/cm2 and were maintained at 37°C and 5% CO2 in a humidified incubator. Medium was completely exchanged every 3–4 days. At 50% confluence, cultures were subcultured using 0.25% trypsin/ 1 mM EDTA. hBM-MSCs in this study were amplified to passage 5 (P5), which is within the range of passage numbers for hBM-MSCs in clinical trials (Rizk et al., 2016).
2.2. Other cultures
COLO205 (ATCC CCL-222, Manassas, VA, USA) and G361 (ATCC CRL-1424) human cell lines were used for positive controls for cell surface expression of CD264 and a pericyte marker, neuron-glial antigen 2 (NG2), respectively (Supporting Information Figure S1). These cells were cultured according to supplier’s instructions.
2.3. Lentiviral transduction of hBM-MSCs
hBM-MSCs were transduced using either copGFP Lentiviral Particles (Santa Cruz Biotechnology, Dallas, TX, USA) to express a bright GFP variant (copGFP hBM-MSCs) or with RediFect Red FLuc-GFP Lentiviral Particles (PerkinElmer, Waltham, MA, USA) to express red-shifted Luciola Italica luciferase fused by a T2A self-cleaving linker peptide to enhanced GFP (eGFP-FLuc hBM-MSCs). P2 hBM-MSC cultures were seeded at an initial cell density of 1000 cells/cm2, and CCMA was replaced 24 h later with transduction medium: 100 μg/ml protamine sulfate (Sigma Aldrich, St. Louis, MO, USA) in complete culture media containing no antibiotics (CCM). Medium volume was half of that for routine cultivation to promote transduction. Cultures were infected at a multiplicity of infection (MOI) of 20–25 and gently rocked a few times to evenly distribute viral particles over the cells (Lin et al., 2012). Spent medium was replaced with fresh transduction medium after 24 h, and a second dose of viral particles at the same MOI was added. Medium was replaced the following day with fresh CCM at standard volume. Transduction efficiency was 50–60%. After 3 days, GFP-positive cells were collected by fluorescence-activated cell sorting (FACS) and cultured in CCMA until cryopreserved at passage 3. Our transduced cells retained plastic adherence, an MSC immunophenotype and robust trilineage differentiation (Supporting Information Figures S2 and S3). Following differentiation, transduced hBM-MSCs maintained their bioluminescence and fluorescence (Supporting Information Figure S3) to enable evaluation of their in vivo survival.
2.4. Flow cytometry
hBM-MSC cultures were amplified to P5 prior to flow cytometric analysis and FACS. Antibodies to detect human CD264 (PE-conjugated, FAB633P) and NG2 (APC-conjugated, FAB2585A) were obtained from R&D Systems (Minneapolis, MN, USA). Antibodies to detect standard hBM-MSC markers were acquired as previously described (Madsen et al., 2017). Following trypsinization with 0.25% trypsin/ 1 mM EDTA for 3 minutes and deactivation with CCMA, hBM-MSCs were resuspended in PBS at 0.5 – 1 × 107 cells/ml. Cell suspensions were incubated with antibody at saturating conditions for 30 min in the dark and on ice. Labeled cells were washed with 1x PBS and 1× 4% FBS in PBS, and then resuspended at 2.5 × 106 cells/ml in chilled 4% FBS in PBS for analysis and sorting.
Flow cytometry was performed with a BD FACSAria Fusion flow cytometer equipped with FACSDiva software (version 8.0.1, BD Biosciences, Franklin Lakes, NJ, USA). Transduced hBM-MSCs were analyzed and sorted in tandem with matched isotype and mock-infected controls. Spectral overlap was corrected with multicolor compensation. Samples were gated to eliminate cellular debris and exclude doublets. hBM-MSCs were labeled with Fixable Viability Stain 780 (BD Biosciences) to assess viability, which was ≥ 90%. CD264− and CD264+ populations were sorted in purity mode by capturing hBM-MSCs with the bottom and top 10% of PE fluorescence, respectively. Aliquots of sorted cells were reanalyzed for PE fluorescence to validate sort purity. hBM-MSCs were sorted into chilled CCMA and then allowed to recover in T-flasks containing CCMA for 36 h prior to further experimentation.
Post hoc flow cytometric analysis was done with Kaluza software (version 1.3, Beckman Coulter, Brea, CA, USA). hBM-MSCs with fluorescence greater than the 99th percentile of the fluorescence distribution for the isotype control were designated positive for antigen expression. Mean fluorescence intensity (MFI) ratios were reported as the MFI for the labeled sample relative to that of the isotype control.
2.5. Construct preparation
Each construct was prepared with 40 mg of 15% hydroxyapatite/85% β-tricalcium phosphate (HA/TCP) granules (Mastergraft Mini Granules, Medtronic, Memphis, TN, USA) (Robey, Kuznetsov, Riminucci, & Bianco, 2014) aliquoted into 50 ml vented conical tubes (CELLTREAT, Pepperell, MA, USA). The porous HA/TCP granules are the recommended scaffold material for ectopic hBM-MSC implants (Robey et al., 2014). Granules were washed with 1x PBS and 1x CCMA, and then stored in 10 ml of CCMA overnight. After removing the medium, granules were seeded with 1 × 106 hBM-MSCs in 1 ml of prewarmed CCMA (Becquart et al., 2012). Cells were mixed with the granules at 50–60 rpm for 6 h at 37°C in a CO2 incubator. The hBM-MSC construct was centrifuged at 1000 x g for 8 minutes, and supernatant was removed. Cell attachment was quantified by measuring (1) cells remaining in solution and (2) DNA content on the granules using the PureLink Genomic DNA Mini Kit (Thermo Fisher Scientific). To bind granules together, 15 μl of mouse fibrinogen (3.2 mg/ml in PBS, Oxford Biomedical Research, Rochester Hills, MI) and 15 μl of mouse thrombin (25 U/ml in 2% CaCl2, Oxford Biomedical Research) were added to each construct and allowed to coagulate for 1 min in a CO2 incubator (Mankani, Kuznetsov, Marshall, & Robey, 2008). After 1 ml of fresh CCMA was added to each tube, the construct was implanted.
2.6. In vivo survival assay
This project employed a well-established in vivo survival model that monitors bioluminescence from subcutaneous implants of eGFP-FLuc hBM-MSCs on the dorsum of immunodeficient mice (Figure 1; Manassero et al., 2016; Robey et al., 2014). All protocols and procedures employed in the in vivo survival assay were ethically reviewed and approved by Tulane’s Institutional Animal Care and Use Committee and were in compliance with the Guide for the Care and Use of Laboratory Animals (National Research Council, Washington, DC, USA). Two- to four-month-old male and female NIH-III nude homozygous mice (Charles River Laboratories, Wilmington, MA, USA) were implanted with hBM-MSC constructs. The animals were fed defined Purina LabDiet 5V5R (St. Louis, MO, USA) starting 2 weeks prior to surgery and throughout the assay. Mice were anesthetized using isoflurane (MWI Animal Health, Boise, ID, USA) and administered 5 mg/kg meloxicam (MWI Animal Health) subcutaneously prior to surgery. Each mouse was implanted with 3 hBM-MSC constructs: (1) CD264−, (2) CD264+, and (3) an hBM-MSC-free control. Small incisions (1–2 cm) were made on the dorsal skin surface, and a subcutaneous pocket was created by blunt dissection (Kuznetsov et al., 1997). A single construct was inserted into each pocket. Incisions were closed with simple interrupted sutures and covered with Vetbond Tissue Adhesive (3M, Maplewood, MN, USA). The mice were examined with bioluminescence imaging over 31 days and then humanely sacrificed using CO2 asphyxiation followed by cervical dislocation. Implanted constructs were excised for further analysis.
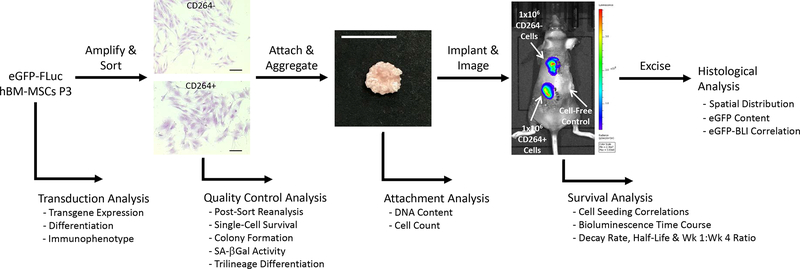
Figure 1.
Overview of project design to quantify the in vivo survival of aging CD264+ hBM-MSCs. Following lentiviral transduction, hBM-MSCs were evaluated for transgene expression and stem cell fitness. eGFP-FLuc hBM-MSCs were amplified and then sorted into CD264+ and control CD264− populations. Quality control assessment of CD264-sorted cells evaluated sort purity, aging phenotype and single-cell survival. Attachment of CD264+ and CD264− eGFP-FLuc hBM-MSCs to HA/TCP porous scaffolds was quantified, and then seeded scaffolds were aggregated with mouse thrombin and fibrinogen. Aggregated hBM-MSC constructs were implanted subcutaneously on the dorsal surface of immunodeficient mice, and bioluminescence imaging was performed every 3–4 days for 31 days. Bioluminescent signal was used to determine the survival kinetics of CD264-sorted hBM-MSCs. On the final day of imaging, implants were excised for histological analysis. Scale bars: 200 μm (cells); 1 cm (construct). Abbreviations: BLI: bioluminescence imaging; eGFP: enhanced green fluorescent protein; FLuc: firefly luciferase; HA/TCP: 15% hydroxyapatite/85% β-tricalcium phosphate; hBM-MSCs: human bone marrow mesenchymal stem cells; P3: passage 3; SA β-Gal: senescence-associated β-galactosidase.
2.7. In vitro survival assays
The assay for single-cell survival was adapted from Russell et al. (2013). Briefly, hBM-MSCs were stained with 10 μM CellTracker Green (Thermo Fisher Scientific) and plated by limited dilution into 96-well plates with 100 μl conditioned media. Wells containing a single cell were detected by fluorescence microscopy. Cells were restained with CellTracker Green after 3 days and with crystal violet (Sigma Aldrich) after 1 week to identify single cells that survived and formed colonies (≥ 10 cells). In vitro survival of hBM-MSC constructs was monitored with bioluminescence imaging. Constructs were cultured in 50 ml vented conical tubes with constant mixing at 50–60 RPM and complete medium exchange every 2–3 days. Every 2 weeks for 2 months, the constructs were transferred to 24-well plates containing CCMA for bioluminescence imaging.
2.8. Bioluminescence imaging (BLI)
Bioluminescence of cell cultures and implants was measured using an IVIS Lumina XRMS In vivo Imaging System (PerkinElmer) with Living Image software (version 4.4, PerkinElmer). For in vivo imaging, mice were sedated with isoflurane and administered 100 μl Xenolight D-luciferin (30 mg/ml, PerkinElmer) subcutaneously adjacent to each implant. For in vitro imaging, hBM-MSC constructs were exposed to 300 μg/ml D-luciferin in CCMA. Bioluminescence was recorded every 5 min after luciferin addition using the automatic exposure settings until the BLI signal decreased. Maximum radiance in the region of interest (ROI) around each construct was measured every 3–4 days for 31 days and background corrected (Bhaumik & Gambhir, 2002). When grouped together, representative bioluminescence images were placed on an identical radiance color scale. An exponential regression was performed on the radiance data. The exponential coefficient corresponds to the rate of radiance decay. BLI signal half-life (t1/2) was calculated from the decay rate (λ) using the following formula: . The week 4: week 1 luminescence signal was calculated from the ratio of the final luminescence (sum of day 28 and day 31) to the initial luminescence (sum of day 0 and day 4).
2.9. Histology
Excised implants and in vitro controls were fixed in 10% neutral-buffered formalin for 7 days, decalcified with Immunocal (StatLab, McKinney, TX, USA) for 2–3 days, and embedded in paraffin prior to sectioning. Five-micron sections from the center and edge of the implants were baked on adhesive microscope slides and deparaffinized. Heat-induced epitope retrieval was performed overnight at 60°C. Samples were washed 3x with PBS-Tween (PBST) and protein blocked using 10% goat serum before overnight incubation at 4°C with the primary antibody (chicken anti-eGFP, 4 μg/ml, NB100–1614, Novus Biologics, Centennial, CO, USA). After washing 3x with PBST, samples were incubated with the fluorophore-conjugated secondary antibody (goat anti-chicken-AF647, 4 μg/ml, 103-605-155, Jackson ImmunoResearch, West Grove, PA, USA) and DAPI (1:2500) at room temperature for 45 min. Slides were washed, dried and treated with ProLong Diamond Antifade Mountant (Thermo Fisher Scientific) before applying coverslips. Standard H&E staining was performed on a serial section of each sample.
Images of immunofluorescence were acquired on a Nikon A1 Plus confocal microscope (NIS Elements software, version 5.02.00, Nikon Instruments, Melville, NY, USA) equipped with 405 nm, 488 nm, and 638 nm excitation lasers to detect DAPI, autofluorescence, and AlexaFluor-647 signals, respectively. The “scan large image” function was used to take images of the entire implant section. ROIs were drawn along the edge of the implant to determine its total area and subsequently drawn around areas containing eGFP fluorescence to calculate the area fraction of eGFP+ cells within the implant.
2.10. Other assays
Colony-forming efficiency was evaluated according to Barrilleaux et al. (2009). hBM-MSCs were plated at a clonogenic level of 100 ± 10 cells in a 10 cm cell culture dish with 15 ml CCMA. Samples were cultured undisturbed for 14 days and then stained with crystal violet to detect cell colonies (≥ 50 cells). Senescence-associated β-galactosidase (SA β-Gal) activity at pH 6.0 was assessed in subconfluent hBM-MSC cultures using Senescence Cells Histochemical Staining Kit (Sigma Aldrich). hBM-MSCs stained at pH 5.0 served as a positive β-Gal control (Chen, Ozanne, & Hales, 2007). Osteo-, adipo-, and chondrogenesis were induced in hBM-MSCs and evaluated after 21 days of differentiation as described in Russell et al. (2011). Alizarin Red S (Sigma Aldrich) detected calcified extracellular matrix in osteogenic samples, AdipoRed (Lonza, Walkersville, MD, USA) stained lipid droplets in adipogenic cells, and Alcian Blue (Sigma Aldrich) identified matrix deposition of sulfated glycosaminoglycans during chondrogenesis. Sulfated glycosaminoglycans deposited by hBM-MSCs were quantified using 1,9-dimethyl-methylene blue (Sigma Aldrich) and compared against a standard curve of known chondroitin sulfate concentrations. Quantitative differentiation values are reported per microgram DNA with the Quant-iT PicoGreen dsDNA assay kit and relative to monolayer cultures cultivated in CCMA for 21 days.
2.11. Statistical analysis
Unless stated otherwise, two-way ANOVA was applied to all data to assess differences between CD264-sorted groups and the hBM-MSC donor. Two-tailed Student’s t-test was used to evaluate differences in NG2 surface expression for donor hBM-MSC cultures. The Pearson’s correlation coefficient (r) was calculated to characterize the relationship between in vivo BLI data and ex vivo histology analysis for all hBM-MSC implants. Statistical analysis was performed using GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Aging phenotype of CD264-sorted populations
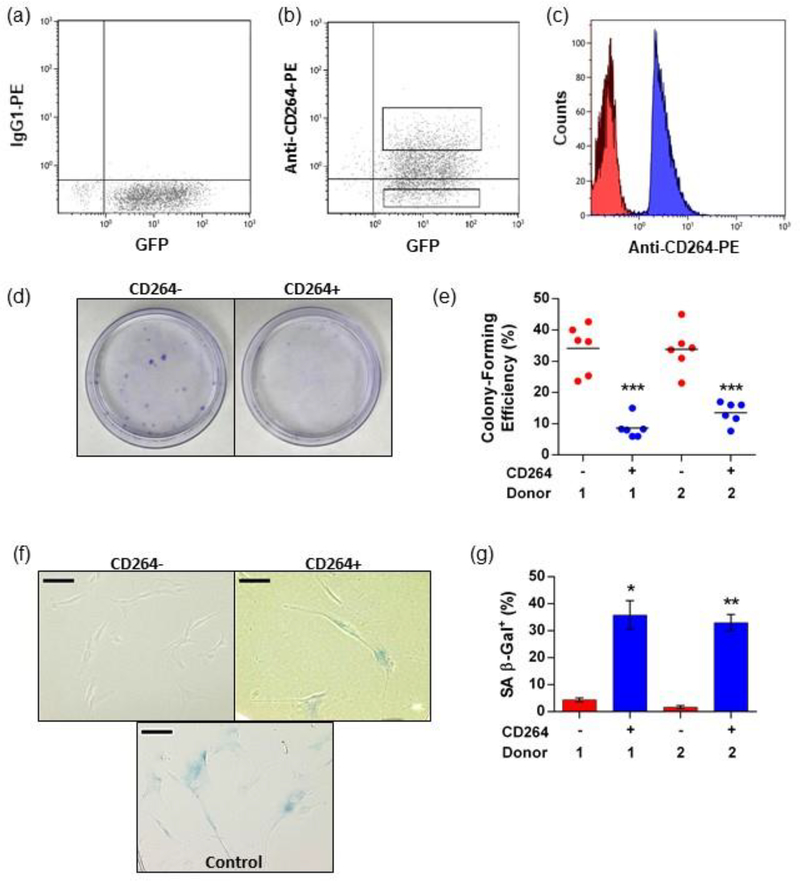
Cellular aging of hBM-MSCs was detected by expression of CD264, as we previously reported (Madsen et al., 2017). Heterogeneous cultures of transduced hBM-MSCs were ~35% positive for CD264 expression based on a 1% isotype cutoff (Figure 2a,b), consistent with the content of CD264+ cells that we have observed for robust hBM-MSC cultures (Madsen et al., 2017). P5 hBM-MSCs were sorted into aging CD264+ and control CD264− populations immediately prior to implantation to avoid artifacts in survival from differences in expansion and sorting conditions. Post-sort reanalysis confirmed distinct fluorescent separation between CD264+ and CD264− hBM-MSCs (Figure 2c). As a quality control assessment, we measured the colony-forming efficiency of each sorted population to be implanted (Figure 2d,e). Control CD264− cells formed colonies with a mean efficiency between 30–40% for both donors (Figure 2e), a value that typifies early-passage hBM-MSC cultures (Sekiya et al., 2002). Colony-forming efficiency of CD264+ hBM-MSCs was 2.5–4.0 times less than their CD264− counterparts (p < 0.0001, n = 6, Figure 2e), indicative of a loss of proliferation potential with cellular aging. Of the two populations, CD264+ hBM-MSCs exhibited decreased differentiation potential (Supporting Information Figure S4), which is a hallmark of aging stem cells (Rando, 2006). Relative to the CD264− control, CD264+ hBM-MSCs had an enlarged size that is emblematic of an aging morphology (Wagner et al., 2008) (Figure 2f). Select batches of sorted cells were assayed for SA β-Gal activity, a biomarker of cellular senescence (Dimri et al., 1995), which was elevated in CD264+ hBM-MSCs and negligible for CD264− hBM-MSCs (p < 0.05 for donor 1 , p < 0.01 for donor 2, n = 3, Figure 2f,g). Increased levels of senescence in the CD264+ hBM-MSCs compared with the CD264− cells is consistent with a phenotype of cellular aging (Wagner et al., 2008). These findings agree with our prior observations that CD264 is first upregulated in hBM-MSCs at an intermediate stage of cellular aging, which is characterized by diminished proliferation potential, and remains upregulated as aging progresses into senescence (Madsen et al., 2017).
Figure 2.
Quality control analysis of sorted hBM-MSCs and aging CD264 phenotype. P5 eGFP-FLuc hBM-MSCs were sorted using FACS into CD264+ and CD264− populations, and analysis was performed in parallel with in vivo assays. (a, b) eGFP-FLuc hBM-MSCs were surface labeled with anti-CD264-PE monoclonal antibodies and sorted into CD264+ and CD264− groups based on the isotype labeling with the gates shown. (c) Reanalysis of CD264-sorted populations was performed immediately after each sort. (d, e) Colony-forming efficiency was evaluated in 10 cm tissue culture plates using crystal violet staining for CD264+ and CD264− hBM-MSCs from each sort (n = 6 biological replicates per group). Mean values are depicted as bars. (f, g) Select CD264 sorts from both hBM-MSC donors were evaluated for SA β-Gal activity at pH 6.0. Data are reported as mean ± SEM (n = 3 biological replicates). Scale bars: 100 μm. *p <0.05, **p < 0.01, and ***p < 0.0001 vs donor-matched CD264− hBM-MSCs. Abbreviations: 1: donor 1; 2: donor 2; eGFP: enhanced green fluorescent protein; FACS: fluorescence-activated cell sorting; FLuc: firefly luciferase; hBM-MSCs: human bone marrow mesenchymal stem cells; P5: passage 5, PE: phycoerythrin; SA β-Gal: senescence-associated β-galactosidase; SEM: standard error of the mean.
3.2. In vitro single-cell survival
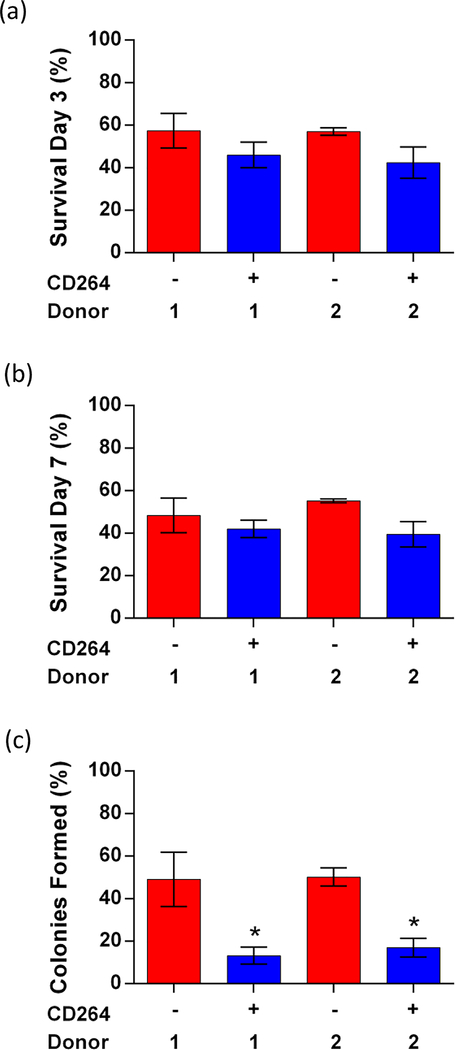
Single-cell survival was comparable between sorted CD264+ and CD264− hBM-MSCs when assessed by limiting dilution into 96-well plates (Figure 3). For each sort group, the inoculum had high viability > 90% and a single-cell plating efficiency > 30% (data not shown), consistent with our previous results as reported in Russell et al. (2013). In total, 90 −110 single-cell wells were analyzed per sort group for each donor. We measured the percentage of single cells that survived on days 3 and 7 (Figure 3a,b), as well as the percentage of single cell-derived colonies containing at least 10 cells by day 7 (Figure 3c). The percentage of single cells that survived on day 3 and day 7 was similar for matched CD264+ and CD264– hBM-MSCs: ~50% on average (Figure 3a,b). hBM-MSCs from donor 1 and donor 2 had similar in vitro survival on days 3 and 7. Nearly half of the single CD264− cells that survived on day 7 formed colonies ≥ 10 cells (Figure 3c). Surviving CD264+ hBM-MSCs formed colonies less efficiently at ~15% (p < 0.05, n = 3 replicates, 30–40 single cells/replicate, Figure 3c). The consistent trend in colony-forming efficiencies shown in Figures 2 and 3 is supportive of the veracity of our single-cell survival data. Figure 3 indicates that aging CD264+ hBM-MSCs have similar in vitro single-cell survival to control CD264− hBM-MSCs, but they form colonies less efficiently due to compromised cell proliferation.
Figure 3.
In vitro single-cell survival of CD264+ and CD264− hBM-MSCs. CD264 sorted cells were plated at an initial density of one cell/well by limiting dilution. Single cells were identified using CellTracker Green fluorescence and confirmed by phase-contrast microscopy. Single-cell wells containing CD264-sorted hBM-MSCs from both donors were assessed for (a) cell survival on day 3, (b) cell survival on day 7, and (c) colonies formed (≥ 10 cells) from surviving cells on day 7. Data are expressed as the mean ± SEM for n = 3 biological replicates (30 – 40 single cells/replicate). *p < 0.05 vs donor-matched CD264− hBM-MSCs. Abbreviations: 1: donor 1; 2: donor 2; hBM-MSCs: human bone marrow mesenchymal stem cells, SEM: standard error of the mean.
3.3. Cell attachment to scaffold
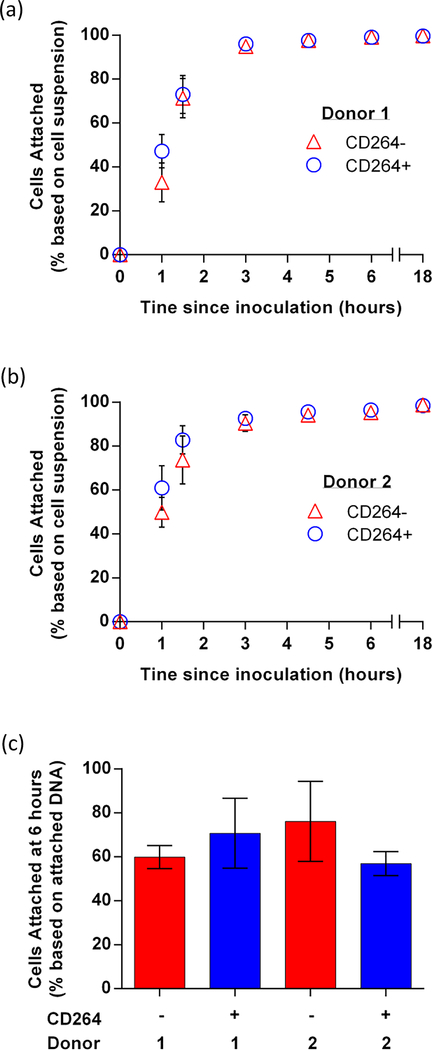
A seeding density of 1×106 hBM-MSCs/40 mg granules was found previously to be optimal for hBM-MSC survival (Becquart et al., 2012) and was selected for our implants. At this seeding density, all hBM-MSC preparations in this study attached efficiently to the scaffold (Figure 4). Cell attachment to the HA/TCP granules was quantified over 18 h of mixing. We estimated that > 95% of hBM-MSCs from both donors attached to the granules after 6 h based on cells remaining in solution (Figure 4a,b). This method can overestimate attachment from cells that have settled on the scaffold but not yet attached. In addition, we estimated cell attachment by measuring DNA content on the scaffold, which can underestimate attachment due to incomplete cell lysing during DNA isolation. According to the second method, 60–75% of the hBM-MSCs had attached by 6 h (Figure 4c). Independent of the method used, CD264+ and CD264− hBM-MSCs had similar attachment efficiencies. Fluorescence microscopy was used to visualize GFP expression and confirm cell attachment to the granules (Supporting Information Figure S5a–c) After 6 h of mixing, the seeded granules were bound together with mouse thrombin and fibrinogen into a larger 3D construct (< 1 cm in diameter) for efficient implantation (Supporting Information Figure S5d,e).
Figure 4.
hBM-MSCs attachment to HA/TCP granules. Attachment of 106 CD264+ and CD264− hBM-MSCs from (a) donor 1 and (b) donor 2 evaluated by cells remaining in suspension (mean ± SEM, n = 3 biological replicates) or (c) DNA content of attached cells after 6 hours (mean ± SEM, n = 3 biological replicates). Abbreviations: 1: donor 1; 2: donor 2; HA/TCP: 15% hydroxyapatite/85% β-tricalcium phosphate; hBM-MSCs: human bone marrow mesenchymal stem cells.
3.4. In vivo survival kinetics
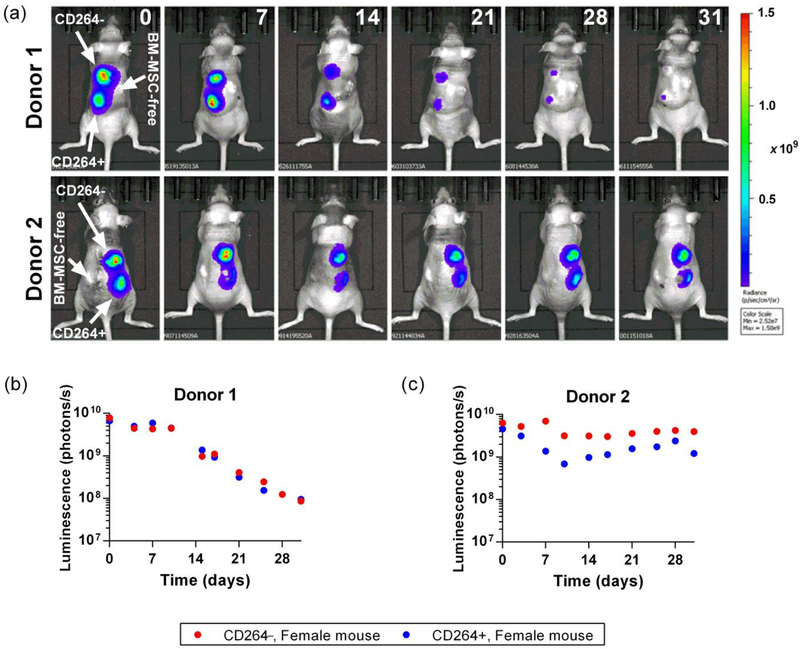
Bioluminescence imaging indicates that ectopic implants of aging CD264+ hBM-MSCs had similar survival kinetics to matched CD264− hBM-MSCs from the same culture (Figures 5 and 6). We estimated hBM-MSC survival based on the linear relationship between the number of implanted eGFP-FLuc hBM-MSCs and bioluminescence from the implant (Supporting Information Figure S6). Figure 5 displays representative image sequences (Figure 5a) and the temporal profiles that correspond to these images (Figure 5b,c) for intra-mouse comparisons of bioluminescence from matched pairs of CD264+ and CD264− implants. These whole-animal images demonstrate the hBM-MSCs remained at the site of implantation.
Figure 5.
In vivo survival of CD264-sorted hBM-MSCs. Mice were implanted with 106 CD264+ and CD264− eGFP-FLuc hBM-MSCs/ 40 mg HA/TCP scaffold and a control scaffold without human cells. Images of the bioluminescent signal radiating from the mice were acquired every 3–4 days for a period of 31 days. (a) Image sequences of representative mice from both hBM-MSCs donors implanted with CD264-sorted hBM-MSCs. The number in the upper right corner of image columns indicates the number of days post-implantation. (b, c) Background-corrected bioluminescent signal from CD264+ and CD264− implants that corresponds to the representative image sequences. Abbreviations: eGFP: enhanced green fluorescent protein; FLuc: firefly luciferase; HA/TCP: 15% hydroxyapatite/85% β-tricalcium phosphate; hBM-MSCs: human bone marrow mesenchymal stem cells.
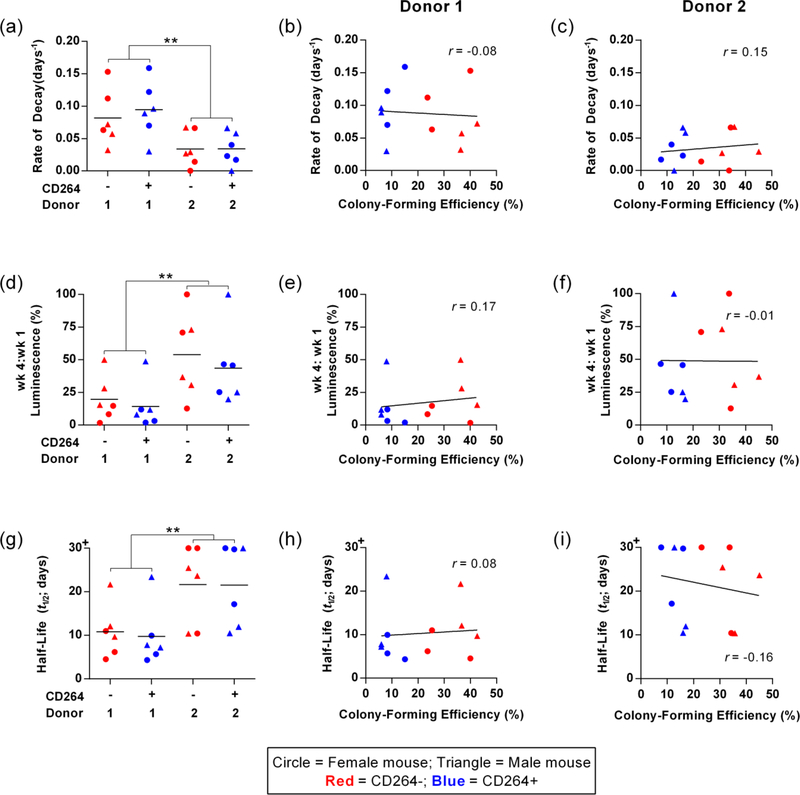
Figure 6.
In vivo survival metrics of CD264-sorted hBM-MSCs and correlations with colony-forming efficiency. (a-c) Rate of signal decay, (d-f) week 4 to week 1 signal ratio, and (g-i) signal half-life of the bioluminescence from CD264+ and CD264− hBM-MSCs for each donor in male and female mice (n = 6 biological replicates per group). Correlations of each survival metric with the colony-forming efficiency of hBM-MSC populations are presented for donor 1 (b, e, h) and donor 2 (c, f, i). Mean values depicted as bars. Linear regression lines and Pearson’s correlation coefficients are presented on each bivariate graph. **p < 0.01 vs donor 1. Abbreviations: 1: donor 1; 2: donor 2; hBM-MSCs: human bone marrow mesenchymal stem cells.
For each implant, we calculated the following survival metrics: rate of BLI signal decay, week 4 to week 1 signal ratio, and signal half-life (Figure 6). Supporting Information Figure S7 provides sample calculations for these metrics. The decay rate characterizes the exponential decrease in luminescence over time (Figure 6a–c). The week 4 to week 1 signal ratio estimates the percent of hBM-MSCs that survived after 1 month (Figure 6d–f). Signal half-life was determined for each sample to allow for intuitive interpretation of survival data and to facilitate meta-analysis across published in vivo MSC survival studies (Figure 6g–i). For each of these parameters, CD264+ and CD264− implants from the same donor had comparable survival kinetics (Figure 6a,d,g). All survival metrics were independent of the colony-forming efficiency of the hBM-MSCs that were implanted (Figure 6b,c,e,f,h,i). The mean half-lifes in our study were among the highest values in the literature, indicating the robust quality of our implant preparations (Table 1).
Table 1.
Half-life of implanted mesenchymal stem cells (MSCs).
| Species | Tissue† | Seeding Density | Scaffold‡ | Attachment Period | Binder/ Gel | Animal | Implant Site§ | Mean Half-Life | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Sheep | BM | 1.0E+06 | HA & HA/Collagen Foam | 1 h | Fibrin | Mouse | SC Ectopic | 2 days | Giannoni et al., 2010 |
| Rat | BM | 5.0E+05 | HA/TCP Granules | 12 days | N.A. | Rat | SC Ectopic | < 3 days | Zimmermann et al., 2011 |
| Human | BM | 1.0E+06 | Coral Block | 4 h | N.A. | Mouse | SC Ectopic & Femoral Defect | Ectopic: 2 days; | Manassero et al., 2016 |
| Orthotopic: 5 days | |||||||||
| Human | AD | 5.0E+05 | None | N.A. | N.A. | Mouse | IM & IV | IM: < 7 days; | Vilalta et al., 2008 |
| IV: < 2 days | |||||||||
| Rat | AD & BM | 5.0E+06 | None | N.A. | N.A. | Rat | Calvarial Defect | AD: 6 days; | Freitas et al., 2017 |
| BM: 8 days | |||||||||
| Human | BM | 1.0E+06 | HA/TCP Granules | 6 h | Fibrin | Mouse | SC Ectopic | Donor 1: 10 days; Donor 2: 22 days | Current Study |
| Rabbit | BM | 1.0E+06 | PLA Disc | 2 h with | N.A. | Rabbit | Osteochondral Defect | < 28 days | Oshima et al., 2009 |
| 1 wk culture | |||||||||
| Rat | BM | 6.0E+05 | N.A. | None | Alginate | Rat | SC Ectopic | ~ 28–35 days | Leijs et al., 2017 |
Tissue of MSC origin. AD: adipose; BM: bone marrow.
Scaffold material. HA: hydroxyapatite; HA/TCP: hydroxyapatite β-tricalcium phosphate; PLA: polylactic acid.
Implant site or administration method. IM: intramuscular; IV: intravenous; SC: subcutaneous.
In contrast to the comparable survival of CD264-sorted BM-MSCs, there was significant differences in the survival kinetics of hBM-MSC implants between donor 1 and donor 2. hBM-MSC implants from donor 2 survived longer with > 2-fold difference in mean values of the kinetics parameters between the two donors (p < 0.01, n = 12 replicates per donor, Figure 6a,d,g). For example, the mean half-life of hBM-MSC implants from donor 2 was > 20 days vs. ~10 days for implants from donor 1 (Figure 6g). Flow cytometric analysis revealed increased NG2 surface expression for high-survival hBM-MSCs from donor 2 relative to donor 1 hBM-MSCs (Supporting Information Figure S8). We previously identified NG2 as a marker of in vitro survival of hBM-MSCs (Russell et al., 2013). We detected no dependence of implant survival on the sex of the mouse (Figure 6a,d,g). There was no significant decrease in BLI signal for control hBM-MSC-scaffold constructs during the first month of in vitro culture (Supplemental Information Figure S9). This implies that the decrease in implant survival reported in Figures 5 and 6 is a consequence of the in vivo microenvironment and not scaffold composition. The similarity in the in vivo survival of CD264+ and CD264− implants in Figures 5 and 6 is supported by analogous findings on the in vitro single-cell survival of CD264-sorted populations in Figure 2. Together, these data indicate matched CD264+ and CD264− BM-MSCs from the same culture have comparable in vitro and in vivo survival, which is independent of colony-forming efficiency.
3.5. hBM-MSC content in excised implants
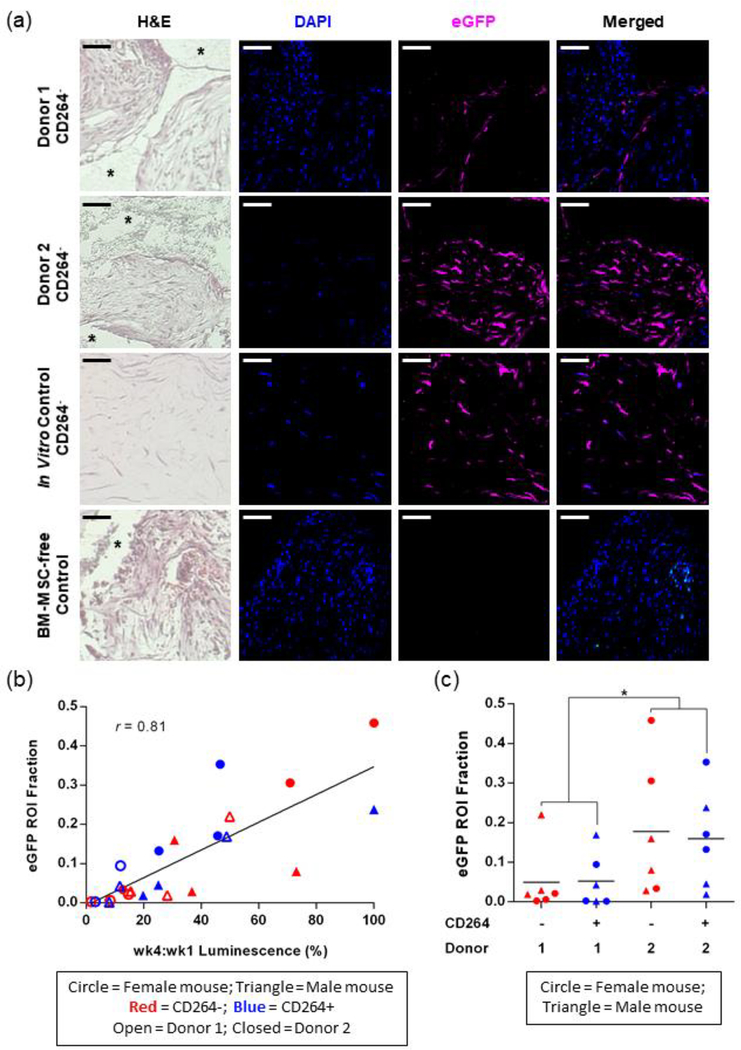
Indirect immunofluorescence of excised implants confirmed the presence of eGFP-FLuc hBM-MSCs (Figure 7). Representative images in Figure 7a were taken from CD264− implants. A 31-day endpoint was selected to excise the implants so that eGFP-positive cells could still be detected in low-survival implants. eGFP-positive hBM-MSCs were abundant and often located within the interstitial space between the solid scaffold granules in high-survival implants from donor 2; whereas, hBM-MSCs were found near the granule surface and the interstitial space showed a greater degree of host cell invasion in low-survival implants from donor 1 (Figure 7a). The presence of eGFP in the in vitro hBM-MSC control and its absence in the hBM-MSC-free implant confirmed the specific labeling of implanted eGFP-positive hBM-MSCs (Figure 7a). The area fraction occupied by eGFP-positive cells in tissue sections (ROI fraction) was strongly correlated with the percent survival of implanted hBM-MSCs for both hBM-MSC donors and CD264-sorted populations (r = 0.81, n = 24, Figure 7b). There was no correlation of eGFP content with CD31, a marker of angiogenesis (data not shown). Consistent with the trends in in vivo BLI data (Figure 6), we found that the mean eGFP ROI fraction was 3x greater for the hBM-MSCs from donor 2 as compared with donor 1 (p < 0.05, n = 12 replicates per donor, Figure 7c). For a given donor, there was no significant difference in eGFP content between (1) aging CD264+ and the control CD264− implants and (2) male and female mice (Figure 7c). The ex vivo immunofluorescence of excised implants validates our in vivo BLI results and provides insight into the spatial distribution of surviving hBM-MSCs.
Figure 7.
Histological analysis of excised hBM-MSC implants. After one month in vivo, mice were sacrificed and the hBM-MSC implants were excised for indirect immunofluorescent detection of eGFP. (a) Representative histology images of CD264− implants from both hBM-MSC donors. Each column corresponds to a stain, specific fluorescence channel, or the merged fluorescence images. Controls: in vitro culture of hBM-MSC construct; hBM-MSC-free implant. Asterisks indicate the area occupied by HA/TCP granules prior to decalcification. Scale bars = 50 μm. (b) Correlation of eGFP ROI area fraction with week 4 to week 1 BLI signal ratio for CD264+ and CD264− hBM-MSC implants from donor 1 and donor 2 in male and female mice. Linear regression line shown for n = 24 implants. (c) eGFP ROI fraction of CD264+ and CD264− implants from each hBM-MSC donor in male and female mice. Mean values depicted as bars. *p < 0.05 vs donor 1. Abbreviations: BLI: bioluminescence imaging; DAPI: nuclear counterstain; 1: donor 1; 2: donor 2; eGFP: enhanced green fluorescent protein; H&E: hematoxylin and eosin; HA/TCP: 15% hydroxyapatite/85% β-tricalcium phosphate; hBM-MSCs: human bone marrow mesenchymal stem cells; ROI: region of interest.
4. Discussion
4.1. Aging CD264+ hBM-MSC survival
The in vitro and in vivo survival of aging CD264+ hBM-MSCs was comparable to matched CD264− hBM-MSCs from the same donor, despite elevated SA β-Gal levels in the CD264+ population. Previous reports describe the isolation and, by inference, in vivo survival of aging hBM-MSCs and other stem cells (Rossi et al., 2005; Wagner et al., 2009). A unique contribution of our work is the longevity of viable CD264+ hBM-MSC implants, particularly from donor 2. This surprising persistence is consistent with the well-documented accumulation of aging cells, including BM-MSCs, during serial passage and in aging organisms (Jeyapalan, Ferreira, Sedivy, & Herbig, 2007; Krishnamurthy et al., 2004; Madsen et al., 2017; Ren et al., 2011). In these cases, the net increase of aging stem cells reflects a decreased rate of depletion relative to their rate of formation. The survival of CD264+ hBM-MSCs that we observed could contribute to this effect.
CD264 may be upregulated in aging hBM-MSCs as a potential stress response to facilitate cell survival. When exposed to adverse conditions, cells undergo a characteristic stress response to cope with the unfavorable environment (Kourtis & Tavernarakis, 2011). For example, the heat shock response pathway becomes activated in cells to combat cytotoxicity of misfolded proteins under heat, oxidative, or proteotoxic stress (Morimoto, 2008). These programmed responses to stress enable cells to survive potentially lethal stimuli. The upregulation of CD264 has been noted in several stress responses including ischemic preconditioning, oxidative stress, and inflammatory signaling (López-Gómez et al., 2016; Panneerselvam et al., 2011; Zhu et al., 2011). Previous work suggests that CD264 expression may have a pro-survival effect on cancer cells by mediating anti-apoptotic signaling (Ehrhardt et al., 2003; Lalaoui et al., 2011). In this context, CD264 may function to counteract the replicative stress of cellular aging in hBM-MSCs by promoting survival, as evidenced by the persistence of these cells following implantation.
4.2. CD264+/− hBM-MSC survival is independent of colony-forming efficiency
This study examined the relationship of the survival of CD264+/− hBM-MSCs and their colony-forming efficiency. An accepted norm is to describe colony formation in cell culture as a measure of in vitro cell survival (Franken, Rodermond, Stap, Haveman, & van Bree, 2006; Ngo, Chan, Ge, Samson, & Engelward, 2019). This popular notion has been extended to the in vivo survival of MSCs. Previous work noted the similarity of the colony-forming efficiency of sheep BM-MSCs to the percentage of the cells that survived after implantation at an arbitrary time (Giannoni et al., 2010). Contrary to this status quo, we observed that the in vitro and in vivo survival of CD264+/− hBM-MSC survival is independent of colony-forming efficiency. Analysis of colony formation at the single-cell level provided insight into these counterintuitive results. The traditional colony-forming assay conflates cell survival and proliferation (DiGirolamo et al., 1999). We employed a single-cell assay to deconstruct colony formation into these two distinct cell processes. By monitoring the fate of single hBM-MSCs, we found no difference in the in vitro survival of aging CD264+ hBM-MSCs and the CD264− control, despite significant differences in their colony-forming efficiency from cell proliferation. Results from our single-cell assay support our findings that CD264+ and CD264− implants from the same donor have comparable in vivo survival that is independent of colony-forming efficiency.
4.3. Applications
The in vivo persistence of aging CD264+ hBM-MSCs reported here has ramifications for the preparation of hBM-MSC therapies. Previous work in our lab has shown that CD264+ cells are present in hBM-MSC cultures from young and older donors to different degrees (Madsen et al., 2017). Our current results indicate that aging CD264+ hBM-MSCs will have comparable survival to healthy CD264− cells. Given the prevalence and persistence of CD264+ cells, it is likely that implanted hBM-MSCs will be a mixture of aging CD264+ and CD264− cells. The presence of CD264+ cells in implants could reduce the therapeutic efficacy of hBM-MSCs. The literature has established that aging and senescent hBM-MSCs, including CD264+ cells, have weakened regenerative potential (Bertolo et al., 2019; Madsen et al., 2017; von Bahr et al., 2012). As a case in point, late-passage hBM-MSCs are less effective in treating patients with graft-versus-host disease than early-passage hBM-MSCs (von Bahr et al., 2012). Due to the in vivo persistence and poor regenerative properties of CD264+ hBM-MSCs, it may be necessary to remove these aging cells from heterogeneous hBM-MSC cultures prior to implantation to improve their therapeutic potential.
Prolonged in vivo survival of aging CD264+ hBM-MSCs can be used to exploit the potential therapeutic effects of the senescence-associated secretory phenotype (SASP). Senescence dramatically changes the bioactive molecules secreted by cells to produce a characteristic SASP: a complex mixture of proinflammatory cytokines, chemokines, growth factors and proteases (McHugh & Gil, 2018). A growing body of data describe potential therapeutic applications of the SASP, such as the treatment of liver fibrosis, wound healing, immune cell recruitment and tissue regeneration (Demaria et al., 2014; Kang et al., 2011; Krizhanovsky et al., 2008; Ritschka et al., 2017). As shown here, CD264+ hBM-MSCs contain senescent cells that can be employed to produce an implant with the desired SASP. Our findings suggest that implanting CD264+ hBM-MSCs is feasible because of their prolonged survival. Creating an implantable construct containing aging CD264+ hBM-MSCs with predictable in vivo survival could be a reliable method to consistently deliver the SASP to a targeted tissue.
Results from this study indicate that hBM-MSC survival is independent of the established colony-forming assay. Colony-forming efficiency is a frequently used in vitro functional assay in preclinical and clinical studies with MSCs as an indicator of proliferation potential and stem cell fitness (Chaput et al., 2018; Daltro et al., 2015; Kuznetsov et al., 2009). In vitro assays that are predictive of in vivo function facilitate the production of effective stem cell therapies. These assays can serve as screening tools to identify highly regenerative MSCs and can monitor MSC properties during manufacturing. Our findings suggest caution should be exercised when interpreting results from a colony-forming assay. We demonstrated that colony-forming efficiency is not a reliable metric of the survival of hBM-MSCs in implants. More work is warranted to develop a functional in vitro assay that is predictive of in vivo survival for MSCs.
Supplementary Material
Figure S1 Positive controls for surface expression of CD264 and NG2. Flow cytometric analysis of CD264 expression on COLO205 human colorectal adenocarcinoma cells and NG2 expression on G361 human melanoma cells. Overlay histograms depict the positive control (dark grey) and the corresponding isotype (light grey). Sample size: n = 10,000 events/group. Abbreviations: APC: allophycocyanin; NG2: neuron-glial antigen 2; PE: phycoerythrin.
Figure S2 Immunophenotype of transduced hBM-MSC cultures. Transduced hBM-MSCs were labeled with mAbs against CD73, CD90, and CD105 (dark grey) or the corresponding isotype (white). Sample size: n = 10,000 events/group. Abbreviations: APC: allophycocyanin; hBM-MSCs: human bone marrow mesenchymal stem cells; PE: phycoerythrin.
Figure S3 Differentiation potential and transgene expression of transduced hBM-MSCs. Mock and transduced hBM-MSCs were cultured for 21 days in either adipo-, osteo-, or chondrogenic medium. Control cultures were maintained in growth medium. AdipoRed detected lipid accumulation during adipogenesis; Alizarin Red S, calcified extracellular matrix during osteogenesis; and Alcian Blue, sulfated glycosaminoglycans during chondrogenesis. Scale bars = 100 μm. Fluorescence microscopy and bioluminescence imaging confirmed GFP and luciferase expression, respectively, in differentiated hBM-MSCs. Scale bars: 100 μm (GFP); 5 mm (luciferase). Abbreviations: GFP: green fluorescent protein; hBM-MSCs: human bone marrow mesenchymal stem cells.
Figure S4 Differentiation potential of CD264-sorted hBM-MSCs. CD264+ and CD264− populations of hBM-MSCs from both donors were cultured for 21 days in either osteo-, adipo-, or chondrogenic medium. Control cultures were maintained in growth medium (a, d, k, n, u, x). To visualize differentiation, Alizarin Red S was used to detect calcified extracellular matrix during osteogenesis (a-f); AdipoRed, lipid accumulation during adipogenesis (k-p); and Alcian Blue, sulfated glycosaminoglycans during chondrogenesis in micromass cultures (u-z). To quantify osteo-, adipo- and chondrogenic differentiation, Alizarin Red S was extracted and absorbance was read at 562 nm (g-j); AdipoRed fluorescence was excited at 485 nm and emission was measured at 572 nm (q-t); and 1,9-dimethyl-methylene blue absorbance of digested pellet cultures was measured at 656 nm after dye decomplexation and compared against a standard curve from known chondroitin sulfate concentrations (aa-ad). Relative differentiation values are reported per microgram DNA and relative to the control cultures. Data are expressed as the mean ± SEM for n = 4 biological replicates. *p < 0.05 and **p < 0.01 vs donor-matched CD264− hBM-MSCs. Scale bars = 200 μm. Abbreviations: GAG: glycosaminoglycans; hBM-MSCs: human bone marrow mesenchymal stem cells; SEM: standard error of the mean.
Figure S5 Visualizing hBM-MSC attachment to HA/TCP granules and scaffold aggregation. (a-c) Fluorescence images of transduced hBM-MSCs that were cultured on 40 mg porous HA/TCP granules for 6 hours at the stated inoculum. Scale bars = 200 μm. Arrows indicate diameter of inner pore (125 μm, a) and outer shell (500 μm, b). (d, e) Scaffold architecture before and after aggregation with mouse fibrinogen and thrombin. Scale bars = 1 cm. Abbreviations: HA/TCP: 15% hydroxyapatite/85% β-tricalcium phosphate; hBM-MSCs: human bone marrow mesenchymal stem cells.
Figure S6 Dependence of implant bioluminescence on hBM-MSC seeding density. Constructs were prepared with 40 mg HA/TCP granules that were seeded with eGFP-FLuc hBM-MSCs. Representative images and background-corrected bioluminescence for the following seeding conditions: (a, b) CD264− hBM-MSCs at three different seeding densities per mouse (n = 3 mice), and (c, d) CD264+ (black) and CD264− (white) hBM-MSCs at the same seeding density per mouse for both 1×105 and 1×106 cells/40 mg granules (n = 3 mice per seeding density). Each mouse is denoted by a different symbol (Δ, □, ○). Abbreviations: eGFP: enhanced green fluorescent protein; FLuc: firefly luciferase; HA/TCP: 15% hydroxyapatite/85% β-tricalcium phosphate; hBM-MSCs: human bone marrow mesenchymal stem cells.
Figure S7 Sample calculation of survival metrics from bioluminescence imaging data. An exponential regression of the bioluminescence signals over 31 days was performed, and the rate of decay was obtained from the exponential coefficient. The implant half-life was calculated from the rate of decay. The week 4: week 1 luminescence signal was calculated from the ratio of the final luminescence (sum of day 28 and day 31) to the initial luminescence (sum of day 0 and day 4). Abbreviations: s: seconds; wk: week.
Figure S8 NG2 surface expression on hBM-MSCs. (a) Representative histogram and (b) mean fluorescence intensity ratio (mean ± SEM, n = 3 biological replicates) from flow cytometric analysis of NG2 surface expression for both donor 1 (blue) and donor 2 (red) relative to isotype (white). The NG2 MFI ratio for donor 2 hBM-MSCs was on average > 1.5 times the value for donor 1 hBM-MSCs. *p < 0.05 vs donor 1. Abbreviations: APC: allophycocyanin; hBM-MSCs: human bone marrow mesenchymal stem cells; MFI: mean fluorescence intensity; NG2: neuron-glial antigen 2; SEM: standard error of the mean.
Figure S9 In vitro survival of CD264+ and CD264− hBM-MSCs. CD264-sorted eGFP-FLuc hBM-MSCs were seeded on 40 mg HA/TCP granules, aggregated with mouse fibrinogen and thrombin, and cultured in vitro for two months. (a) Initial and final bioluminescence images and (b) temporal profile of background-corrected bioluminescence from a representative culture of donor 1 hBM-MSCs. Abbreviations: eGFP: enhanced green fluorescent protein; FLuc: firefly luciferase; HA/TCP: 15% hydroxyapatite/85% β-tricalcium phosphate; hBM-MSCs: human bone marrow mesenchymal stem cells.
Acknowledgements
We are grateful for technical assistance from the Histology Core and Flow Cytometry Core in the Center for Stem Cell Research and Regenerative Medicine at Tulane University, Flow Cytometry Core at the Louisiana Cancer Research Consortium, and Microscopy and Imaging Core at Louisiana State University Health Sciences Center. This research was funded by the National Science Foundation (CBET-1604129, KO), National Institutes of Health (P51OD011104, BB), Tulane University Department of Comparative Medicine, Residency Research Program (KO), and Tulane University Carol Lavin Bernick Faculty Grant Program (KO). Any opinions expressed in this article are those of the authors and do not necessarily reflect the views of the National Science Foundation, National Institutes of Health or Tulane University.
Funding information
National Science Foundation, Grant number: CBET-1604129;
National Institutes of Health, Grant number: P51OD011104
Footnotes
Conflicts of Interest
KO, SM and BB are inventors listed on a provisional patent application entitled “Methods to improve the in vivo survival of mesenchymal stem cells” that has been filed on behalf of Tulane University. All other authors declare that they have no competing interests.
References
- Barrilleaux BL, Phinney DG, Fischer-Valuck BW, Russell KC, Wang G, Prockop DJ, & O’Connor KC (2009). Small-molecule antagonist of macrophage migration inhibitory factor enhances migratory response of mesenchymal stem cells to bronchial epithelial cells. Tissue Engineering. Part A, 15(9), 2335–2346. 10.1089/ten.tea.2008.0434 [DOI] [PubMed] [Google Scholar]
- Barrilleaux BL, Phinney DG, Prockop DJ, & O’Connor KC (2006). Review: ex vivo engineering of living tissues with adult stem cells. Tissue Engineering, 12(11), 3007–3019. 10.1089/ten.2006.12.ft-276 [DOI] [PubMed] [Google Scholar]
- Becquart P, Cambon-Binder A, Monfoulet L-E, Bourguignon M, Vandamme K, Bensidhoum M, … Logeart-Avramoglou D (2012). Ischemia is the prime but not the only cause of human multipotent stromal cell death in tissue-engineered constructs in vivo. Tissue Engineering. Part A, 18(19–20), 2084–2094. 10.1089/ten.TEA.2011.0690 [DOI] [PubMed] [Google Scholar]
- Beegle J, Lakatos K, Kalomoiris S, Stewart H, Isseroff RR, Nolta JA, & Fierro FA (2015). Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells (Dayton, Ohio), 33(6), 1818–1828. 10.1002/stem.1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolo A, Baur M, Guerrero J, Pötzel T, & Stoyanov J (2019). Autofluorescence is a reliable in vitro marker of cellular senescence in human mesenchymal stromal cells. Scientific Reports, 9(1), 2074 10.1038/s41598-019-38546-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaumik S, & Gambhir SS (2002). Optical imaging of Renilla luciferase reporter gene expression in living mice. Proceedings of the National Academy of Sciences of the United States of America, 99(1), 377–382. 10.1073/pnas.012611099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blocki A, Beyer S, Dewavrin J-Y, Goralczyk A, Wang Y, Peh P, … Bhakoo KK (2015). Microcapsules engineered to support mesenchymal stem cell (MSC) survival and proliferation enable long-term retention of MSCs in infarcted myocardium. Biomaterials, 53, 12–24. 10.1016/j.biomaterials.2015.02.075 [DOI] [PubMed] [Google Scholar]
- Caplan AI (2007). Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. Journal of Cellular Physiology, 213(2), 341–347. 10.1002/jcp.21200 [DOI] [PubMed] [Google Scholar]
- Caplan AI, & Dennis JE (2006). Mesenchymal stem cells as trophic mediators. Journal of Cellular Biochemistry, 98(5), 1076–1084. 10.1002/jcb.20886 [DOI] [PubMed] [Google Scholar]
- Chaput CD, Shar A, Jupiter D, Hubert Z, Clough B, Krause U, & Gregory CA (2018). How stem cell composition in bone marrow aspirate relates to clinical outcomes when used for cervical spine fusion. PLoS ONE, 13(9), 1–17. 10.1371/journal.pone.0203714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J-H, Ozanne SE, & Hales CN (2007). Methods of cellular senescence induction using oxidative stress. Methods in Molecular Biology (Clifton, N.J.), 371, 179–189. 10.1007/978-1-59745-361-5_14 [DOI] [PubMed] [Google Scholar]
- Crigler L, Robey RC, Asawachaicharn A, Gaupp D, & Phinney DG (2006). Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Experimental Neurology, 198(1), 54–64. 10.1016/j.expneurol.2005.10.029 [DOI] [PubMed] [Google Scholar]
- Daltro GC, Fortuna V, De Souza ES, Salles MM, Carreira AC, Meyer R, … Borojevic R (2015). Efficacy of autologous stem cell-based therapy for osteonecrosis of the femoral head in sickle cell disease: A five-year follow-up study. Stem Cell Research and Therapy, 6(1), 1–18. 10.1186/s13287-015-0105-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, … Campisi J (2014). An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Developmental Cell, 31(6), 722–733. 10.1016/j.devcel.2014.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digirolamo CM, Stokes D, Colter D, Phinney DG, Class R, & Prockop DJ (1999). Propagation and senescence of human marrow stromal cells in culture: A simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. British Journal of Haematology, 107(2), 275–281. 10.1046/j.1365-2141.1999.01715.x [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, … Pereira-Smith O (1995). A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proceedings of the National Academy of Sciences of the United States of America, 92(20), 9363–9367. 10.1073/pnas.92.20.9363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, … Horwitz EM (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8(4), 315–317. 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]
- Ehrhardt H, Fulda S, Schmid I, Hiscott J, Debatin KM, & Jeremias I (2003). TRAIL induced survival and proliferation in cancer cells resistant towards TRAIL-induced apoptosis mediated by NF-kappaB. Oncogene, 22(25), 3842–3852. 10.1038/sj.onc.1206520 [DOI] [PubMed] [Google Scholar]
- Franken NAP, Rodermond HM, Stap J, Haveman J, & van Bree C (2006). Clonogenic assay of cells in vitro. Nature Protocols, 1(5), 2315–2319. 10.1038/nprot.2006.339 [DOI] [PubMed] [Google Scholar]
- Freitas GP, Lopes HB, Almeida ALG, Abuna RPF, Gimenes R, Souza LEB, … Rosa AL (2017). Potential of osteoblastic cells derived from bone marrow and adipose tissue associated with a polymer/ceramic composite to repair bone tissue. Calcified Tissue International, 101(3), 312–320. 10.1007/s00223-017-0282-3 [DOI] [PubMed] [Google Scholar]
- Giannoni P, Scaglione S, Daga A, Ilengo C, Cilli M, & Quarto R (2010). Short-time survival and engraftment of bone marrow stromal cells in an ectopic model of bone regeneration. Tissue Engineering. Part A, 16(2), 489–499. 10.1089/ten.TEA.2009.0041 [DOI] [PubMed] [Google Scholar]
- Haleem AM, Singergy A. A. El, Sabry D, Atta HM, Rashed LA, Chu CR, … Abdel Aziz MT (2010). The clinical use of human culture-expanded autologous bone marrow mesenchymal stem sells transplanted on platelet-rich fibrin glue in the treatment of articular cartilage defects: a pilot study and preliminary results. Cartilage, 1(4), 253–261. 10.1177/1947603510366027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyapalan JC, Ferreira M, Sedivy JM, & Herbig U (2007). Accumulation of senescent cells in mitotic tissue of aging primates. Mechanisms of Ageing and Development, 128(1), 36–44. 10.1016/j.mad.2006.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang T, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, … Zender L (2011). Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature, 479(7374), 547–551. 10.1038/nature10599 [DOI] [PubMed] [Google Scholar]
- Kourtis N, & Tavernarakis N (2011). Cellular stress response pathways and ageing: intricate molecular relationships. The EMBO Journal, 30(13), 2520–2531. 10.1038/emboj.2011.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, & Sharpless NE (2004). Ink4a/Arf expression is a biomarker of aging. The Journal of Clinical Investigation, 114(9), 1299–1307. 10.1172/JCI22475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, … Lowe SW (2008). Senescence of activated stellate cells limits liver fibrosis. Cell, 134(4), 657–667. 10.1016/j.cell.2008.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov SA, Krebsbach PH, Satomura K, Kerr J, Riminucci M, Benayahu D, & Robey PG (1997). Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. Journal of Bone and Mineral Research, 12(9), 1335–1347. 10.1359/jbmr.1997.12.9.1335 [DOI] [PubMed] [Google Scholar]
- Kuznetsov SA, Mankani MH, Bianco P, & Robey PG (2009). Enumeration of the colony-forming units-fibroblast from mouse and human bone marrow in normal and pathological conditions. Stem Cell Research, 2(1), 83–94. 10.1016/j.scr.2008.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalaoui N, Morlé A, Mérino D, Jacquemin G, Iessi E, Morizot A, … Micheau O (2011). TRAIL-R4 promotes tumor growth and resistance to apoptosis in cervical carcinoma HeLa cells through AKT. PloS One, 6(5), e19679 10.1371/journal.pone.0019679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY, & STARTING collaborators. (2010). A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells (Dayton, Ohio), 28(6), 1099–1106. 10.1002/stem.430 [DOI] [PubMed] [Google Scholar]
- Leijs MJ, Villafuertes E, Haeck JC, Koevoet WJ, Fernandez-Gutierrez B, Hoogduijn MJ, … van Osch GJ (2017). Encapsulation of allogeneic mesenchymal stem cells in alginate extends local presence and therapeutic function. European Cells & Materials, 33(February), 43–58. 10.22203/eCM.v033a04 [DOI] [PubMed] [Google Scholar]
- Lin P, Lin Y, Lennon DP, Correa D, Schluchter M, & Caplan AI (2012). Efficient lentiviral transduction of human mesenchymal stem cells that preserves proliferation and differentiation capabilities. Stem Cells Translational Medicine, 1(12), 886–897. 10.5966/sctm.2012-0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Gómez C, Oliver-Martos B, Pinto-Medel M-J, Suardiaz M, Reyes-Garrido V, Urbaneja P, … Leyva L (2016). TRAIL and TRAIL receptors splice variants during long-term interferon β treatment of patients with multiple sclerosis: evaluation as biomarkers for therapeutic response. Journal of Neurology, Neurosurgery, and Psychiatry, 87(2), 130–137. 10.1136/jnnp-2014-309932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G, Ashraf M, & Haider KH (2012). Insulin-like growth factor-1 preconditioning accentuates intrinsic survival mechanism in stem cells to resist ischemic injury by orchestrating protein kinase cα-erk1/2 activation. Antioxidants & Redox Signaling, 16(3), 217–227. 10.1089/ars.2011.4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen SD, Russell KC, Tucker HA, Glowacki J, Bunnell BA, & O’Connor KC (2017). Decoy TRAIL receptor CD264: A cell surface marker of cellular aging for human bone marrow-derived mesenchymal stem cells. Stem Cell Research & Therapy, 8(1), 201 10.1186/s13287-017-0649-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manassero M, Paquet J, Deschepper M, Viateau V, Retortillo J, Bensidhoum M, … Petite H (2016). Comparison of survival and osteogenic ability of human mesenchymal stem cells in orthotopic and ectopic sites in mice. Tissue Engineering. Part A, 22(5–6), 534–544. 10.1089/ten.TEA.2015.0346 [DOI] [PubMed] [Google Scholar]
- Mankani MH, Kuznetsov SA, Marshall GW, & Robey PG (2008). Creation of new bone by the percutaneous injection of human bone marrow stromal cell and HA/TCP suspensions. Tissue Engineering. Part A, 14(12), 1949–1958. 10.1089/ten.tea.2007.0348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D, & Gil J (2018). Senescence and aging: Causes, consequences, and therapeutic avenues. The Journal of Cell Biology, 217(1), 65–77. 10.1083/jcb.201708092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI (2008). Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes & Development, 22(11), 1427–1438. 10.1101/gad.1657108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo LP, Chan TK, Ge J, Samson LD, & Engelward BP (2019). Microcolony size distribution assay enables high-throughput cell survival quantitation. Cell Reports, 26(6), 1668–1678.e4. 10.1016/j.celrep.2019.01.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuschke A, Rodrigues M, Rivera J, Yates C, Whaley D, Stolz D, … Wells A (2016). Epidermal growth factor tethered to β-tricalcium phosphate bone scaffolds via a high-affinity binding peptide enhances survival of human mesenchymal stem cells/multipotent stromal cells in an immune-competent parafascial implantation assay in mice. Stem Cells Translational Medicine, 5(11), 1580–1586. 10.5966/sctm.2015-0326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima Y, Harwood FL, Coutts RD, Kubo T, & Amiel D (2009). Variation of mesenchymal cells in polylactic acid scaffold in an osteochondral repair model. Tissue Engineering. Part C, Methods, 15(4), 595–604. 10.1089/ten.TEC.2008.0487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneerselvam M, Patel PM, Roth DM, Kidd MW, Chin-Lee B, Head BP, … Davis DP (2011). Role of decoy molecules in neuronal ischemic preconditioning. Life Sciences, 88(15–16), 670–674. 10.1016/j.lfs.2011.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, … Marshak DR (1999). Multilineage potential of adult human mesenchymal stem cells. Science (New York, N.Y.), 284(5411), 143–147. 10.1126/science.284.5411.143 [DOI] [PubMed] [Google Scholar]
- Rando TA (2006). Stem cells, ageing and the quest for immortality. Nature, 441(7097), 1080–1086. 10.1038/nature04958 [DOI] [PubMed] [Google Scholar]
- Ren J, Stroncek D, Jin P, Castiello L, Tran K, Balakumaran A, … Sabatino M (2011). Senescence of cultured bone marrow stromal cells. Biology of Blood and Marrow Transplant, 17(2), S216–S217. 10.1016/j.bbmt.2010.12.194 [DOI] [Google Scholar]
- Ritschka B, Storer M, Mas A, Heinzmann F, Ortells MC, Morton JP, … Keyes WM (2017). The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes & Development, 31(2), 172–183. 10.1101/gad.290635.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk M, Monaghan M, Shorr R, Kekre N, Bredeson CN, & Allan DS (2016). Heterogeneity in studies of mesenchymal stromal cells to treat or prevent graft-versus-host disease: A scoping review of the evidence. Biology of Blood and Marrow Transplantation, 22(8), 1416–1423. 10.1016/j.bbmt.2016.04.010 [DOI] [PubMed] [Google Scholar]
- Robey PG, Kuznetsov SA, Riminucci M, & Bianco P (2014). Bone marrow stromal cell assays: in vitro and in vivo. Methods in Molecular Biology (Clifton, N.J.), 1130(6), 279–293. 10.1007/978-1-62703-989-5_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, & Weissman IL (2005). Cell intrinsic alterations underlie hematopoietic stem cell aging. Proceedings of the National Academy of Sciences of the United States of America, 102(26), 9194–9199. 10.1073/pnas.0503280102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell KC, Phinney DG, Lacey MR, Barrilleaux BL, Meyertholen KE, & O’Connor KC (2010). In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells, 28(4), 788–798. 10.1002/stem.312 [DOI] [PubMed] [Google Scholar]
- Russell, Katie C, Lacey MR, Gilliam JK, Tucker HA, Phinney DG, & O’Connor KC (2011). Clonal analysis of the proliferation potential of human bone marrow mesenchymal stem cells as a function of potency. Biotechnology and Bioengineering, 108(11), 2716–2726. 10.1002/bit.23193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, Katie C, Tucker HA, Bunnell BA, Andreeff M, Schober W, Gaynor AS, … O’Connor KC (2013). Cell-surface expression of neuron-glial antigen 2 (NG2) and melanoma cell adhesion molecule (CD146) in heterogeneous cultures of marrow-derived mesenchymal stem cells. Tissue Engineering. Part A, 19(19–20), 2253–2266. 10.1089/ten.TEA.2012.0649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secchiero P, Melloni E, Corallini F, Beltrami AP, Alviano F, Milani D, … Zauli G (2008). Tumor necrosis factor-related apoptosis-inducing ligand promotes migration of human bone marrow multipotent stromal cells. Stem Cells (Dayton, Ohio), 26(11), 2955–2963. 10.1634/stemcells.2008-0512 [DOI] [PubMed] [Google Scholar]
- Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, & Prockop DJ (2002). Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells (Dayton, Ohio), 20(6), 530–541. 10.1634/stemcells.20-6-530 [DOI] [PubMed] [Google Scholar]
- Squillaro T, Peluso G, & Galderisi U (2016). Clinical trials with mesenchymal stem cells: An update. Cell Transplantation, 25(5), 829–848. 10.3727/096368915X689622 [DOI] [PubMed] [Google Scholar]
- Stenderup K, Justesen J, Clausen C, & Kassem M (2003). Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone, 33(6), 919–926. 10.1016/j.bone.2003.07.005 [DOI] [PubMed] [Google Scholar]
- Vilalta M, Dégano IR, Bagó J, Gould D, Santos M, García-Arranz M, … Blanco J (2008). Biodistribution, long-term survival, and safety of human adipose tissue-derived mesenchymal stem cells transplanted in nude mice by high sensitivity non-invasive bioluminescence imaging. Stem Cells and Development, 17(5), 993–1003. 10.1089/scd.2007.0201 [DOI] [PubMed] [Google Scholar]
- von Bahr L, Sundberg B, Lönnies L, Sander B, Karbach H, Hägglund H, … Ringdén O (2012). Long-term complications, immunologic effects, and role of passage for outcome in mesenchymal stromal cell therapy. Biology of Blood and Marrow Transplantation : Journal of the American Society for Blood and Marrow Transplantation, 18(4), 557–564. 10.1016/j.bbmt.2011.07.023 [DOI] [PubMed] [Google Scholar]
- Wagner W, Bork S, Horn P, Krunic D, Walenda T, Diehlmann A, … Ho AD (2009). Aging and replicative senescence have related effects on human stem and progenitor cells. PloS One, 4(6), e5846 10.1371/journal.pone.0005846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, … Ho AD (2008). Replicative senescence of mesenchymal stem cells: a continuous and organized process. PloS One, 3(5), e2213 10.1371/journal.pone.0002213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YK, Ogando CR, Wang See C, Chang T, & Barabino GA (2018). Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Research & Therapy, 9(1), 131 10.1186/s13287-018-0876-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yulyana Y, Endaya BB, Ng WH, Guo CM, Hui KM, Lam PYP, & Ho I. a W. (2013). Carbenoxolone enhances TRAIL-induced apoptosis through the upregulation of death receptor 5 and inhibition of gap junction intercellular communication in human glioma. Stem Cells and Development, 22(13), 1870–1882. 10.1089/scd.2012.0529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Zhang L, Zheng Y, Xu J, Song J, Rolfe BE, & Campbell JH (2011). Effects of estrogen on stress-induced premature senescence of vascular smooth muscle cells: a novel mechanism for the “time window theory” of menopausal hormone therapy. Atherosclerosis, 215(2), 294–300. 10.1016/j.atherosclerosis.2010.12.025 [DOI] [PubMed] [Google Scholar]
- Zimmermann CE, Gierloff M, Hedderich J, Açil Y, Wiltfang J, & Terheyden H (2011). Survival of transplanted rat bone marrow-derived osteogenic stem cells in vivo. Tissue Engineering. Part A, 17(7–8), 1147–1156. 10.1089/ten.TEA.2009.0577 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Positive controls for surface expression of CD264 and NG2. Flow cytometric analysis of CD264 expression on COLO205 human colorectal adenocarcinoma cells and NG2 expression on G361 human melanoma cells. Overlay histograms depict the positive control (dark grey) and the corresponding isotype (light grey). Sample size: n = 10,000 events/group. Abbreviations: APC: allophycocyanin; NG2: neuron-glial antigen 2; PE: phycoerythrin.
Figure S2 Immunophenotype of transduced hBM-MSC cultures. Transduced hBM-MSCs were labeled with mAbs against CD73, CD90, and CD105 (dark grey) or the corresponding isotype (white). Sample size: n = 10,000 events/group. Abbreviations: APC: allophycocyanin; hBM-MSCs: human bone marrow mesenchymal stem cells; PE: phycoerythrin.
Figure S3 Differentiation potential and transgene expression of transduced hBM-MSCs. Mock and transduced hBM-MSCs were cultured for 21 days in either adipo-, osteo-, or chondrogenic medium. Control cultures were maintained in growth medium. AdipoRed detected lipid accumulation during adipogenesis; Alizarin Red S, calcified extracellular matrix during osteogenesis; and Alcian Blue, sulfated glycosaminoglycans during chondrogenesis. Scale bars = 100 μm. Fluorescence microscopy and bioluminescence imaging confirmed GFP and luciferase expression, respectively, in differentiated hBM-MSCs. Scale bars: 100 μm (GFP); 5 mm (luciferase). Abbreviations: GFP: green fluorescent protein; hBM-MSCs: human bone marrow mesenchymal stem cells.
Figure S4 Differentiation potential of CD264-sorted hBM-MSCs. CD264+ and CD264− populations of hBM-MSCs from both donors were cultured for 21 days in either osteo-, adipo-, or chondrogenic medium. Control cultures were maintained in growth medium (a, d, k, n, u, x). To visualize differentiation, Alizarin Red S was used to detect calcified extracellular matrix during osteogenesis (a-f); AdipoRed, lipid accumulation during adipogenesis (k-p); and Alcian Blue, sulfated glycosaminoglycans during chondrogenesis in micromass cultures (u-z). To quantify osteo-, adipo- and chondrogenic differentiation, Alizarin Red S was extracted and absorbance was read at 562 nm (g-j); AdipoRed fluorescence was excited at 485 nm and emission was measured at 572 nm (q-t); and 1,9-dimethyl-methylene blue absorbance of digested pellet cultures was measured at 656 nm after dye decomplexation and compared against a standard curve from known chondroitin sulfate concentrations (aa-ad). Relative differentiation values are reported per microgram DNA and relative to the control cultures. Data are expressed as the mean ± SEM for n = 4 biological replicates. *p < 0.05 and **p < 0.01 vs donor-matched CD264− hBM-MSCs. Scale bars = 200 μm. Abbreviations: GAG: glycosaminoglycans; hBM-MSCs: human bone marrow mesenchymal stem cells; SEM: standard error of the mean.
Figure S5 Visualizing hBM-MSC attachment to HA/TCP granules and scaffold aggregation. (a-c) Fluorescence images of transduced hBM-MSCs that were cultured on 40 mg porous HA/TCP granules for 6 hours at the stated inoculum. Scale bars = 200 μm. Arrows indicate diameter of inner pore (125 μm, a) and outer shell (500 μm, b). (d, e) Scaffold architecture before and after aggregation with mouse fibrinogen and thrombin. Scale bars = 1 cm. Abbreviations: HA/TCP: 15% hydroxyapatite/85% β-tricalcium phosphate; hBM-MSCs: human bone marrow mesenchymal stem cells.
Figure S6 Dependence of implant bioluminescence on hBM-MSC seeding density. Constructs were prepared with 40 mg HA/TCP granules that were seeded with eGFP-FLuc hBM-MSCs. Representative images and background-corrected bioluminescence for the following seeding conditions: (a, b) CD264− hBM-MSCs at three different seeding densities per mouse (n = 3 mice), and (c, d) CD264+ (black) and CD264− (white) hBM-MSCs at the same seeding density per mouse for both 1×105 and 1×106 cells/40 mg granules (n = 3 mice per seeding density). Each mouse is denoted by a different symbol (Δ, □, ○). Abbreviations: eGFP: enhanced green fluorescent protein; FLuc: firefly luciferase; HA/TCP: 15% hydroxyapatite/85% β-tricalcium phosphate; hBM-MSCs: human bone marrow mesenchymal stem cells.
Figure S7 Sample calculation of survival metrics from bioluminescence imaging data. An exponential regression of the bioluminescence signals over 31 days was performed, and the rate of decay was obtained from the exponential coefficient. The implant half-life was calculated from the rate of decay. The week 4: week 1 luminescence signal was calculated from the ratio of the final luminescence (sum of day 28 and day 31) to the initial luminescence (sum of day 0 and day 4). Abbreviations: s: seconds; wk: week.
Figure S8 NG2 surface expression on hBM-MSCs. (a) Representative histogram and (b) mean fluorescence intensity ratio (mean ± SEM, n = 3 biological replicates) from flow cytometric analysis of NG2 surface expression for both donor 1 (blue) and donor 2 (red) relative to isotype (white). The NG2 MFI ratio for donor 2 hBM-MSCs was on average > 1.5 times the value for donor 1 hBM-MSCs. *p < 0.05 vs donor 1. Abbreviations: APC: allophycocyanin; hBM-MSCs: human bone marrow mesenchymal stem cells; MFI: mean fluorescence intensity; NG2: neuron-glial antigen 2; SEM: standard error of the mean.
Figure S9 In vitro survival of CD264+ and CD264− hBM-MSCs. CD264-sorted eGFP-FLuc hBM-MSCs were seeded on 40 mg HA/TCP granules, aggregated with mouse fibrinogen and thrombin, and cultured in vitro for two months. (a) Initial and final bioluminescence images and (b) temporal profile of background-corrected bioluminescence from a representative culture of donor 1 hBM-MSCs. Abbreviations: eGFP: enhanced green fluorescent protein; FLuc: firefly luciferase; HA/TCP: 15% hydroxyapatite/85% β-tricalcium phosphate; hBM-MSCs: human bone marrow mesenchymal stem cells.