Abstract
Objective. To review the published literature on women who were intoxicated at delivery and outcomes for their infants. Methods. A systematic literature review was utilized to identify articles meeting our inclusion criteria. After screening using titles and abstracts, we identified 34 articles requiring full-text review. Each of these were reviewed by at least 2 of the authors. We identified 12 articles that met our inclusion criteria. Results. We identified case reports of 16 mothers who delivered with a blood alcohol concentration (BAC) ranging from 42.1 to 473 mg/dL. Three of the pregnancies (18.8%) ended with a stillbirth, 5 (31.3%) were infant deaths, 6 (37.5%) lived, and 2 (12.5%) had no fetal or infant outcome reported. The BAC for the stillborn infants ranged from 120 to 460 mg/dL. The BAC among the infant deaths ranged from 96 to 715 mg/dL. Among surviving infants, the BAC ranged from 38.4 to 246.5 mg/dL. Conclusion. We identified no deaths with a BAC <96 mg/dL. However, it is not clear if this represents the lower level of BAC where mortality risk increases. In this article, we present 9 suggestions to improve detection and management of these mothers and their infants.
Keywords: pregnancy, intoxication, stillbirth, live birth, infant death, alcohol concentrations, delivery
Introduction
In the United States, alcohol use in women of childbearing age is common with more than half of nonpregnant women self-reporting alcohol use, and 13% of these women self-reporting binge drinking.1 Up to 50% of pregnancies within the United States are unplanned, increasing the risk that the developing fetus could be exposed to alcohol in the early phases of gestation prior to pregnancy recognition.1 First trimester exposure rates are 56% for all women and 78.9% for women with recent alcohol dependence.2 The majority of pregnant women consuming alcohol did so during the first month (22.5%), likely prior to pregnancy recognition, but 2.7% of women report drinking throughout their pregnancy.3 About 80 000 women in the United States consume alcohol during all 3 trimesters of pregnancy each year.1,4
Prenatal alcohol exposure increases risk for adverse outcomes including fetal alcohol spectrum disorders (FASDs).4 Two studies have reviewed mortality rates for mothers with high levels of alcohol use during pregnancy and women who had a child diagnosed with FASD.5,6 Both of these meta-analyses found large increases in mortality risk for these mothers. In the most recent review, the mean of the weighted mortality proportion was 11.25%.6 The mortality risk for a mother of a child diagnosed with FASD was increased (odds ratio = 38.9; 95% confidence interval = 23.41-64.57).6 Worldwide, this suggests that as many as 37 800 mothers of children diagnosed with FASD die each year. This is 103 every day or about 4 per hour.
Interestingly, the data examining the number of women drinking during labor or with a positive blood alcohol concentration (BAC) at delivery are very limited and are usually confined to individual case reports or case series studies.4,7,8 This is surprising since a previous publication has suggested that mortality among infants delivered with a high BAC may exceed 50%.9
In this article, we present a comprehensive literature review of women presenting at labor and delivery with a positive BAC. We will present available data from this case series including the BAC of the mother, stillborn infants, live births, infant deaths, and infants who were exhibiting alcohol withdrawal.
Methods
A comprehensive literature search was performed to identify reports of women who gave birth with a positive BAC. The search strategies were designed and implemented by a medical librarian, and the searches were performed between October 5, 2017, and November 13, 2017. We searched all languages and all years.
Searches were conducted in multiple bibliographic databases and search engines, including (in alphabetical order): CINAHL (Cumulative Index of Nursing and Allied Health Literature), Google Scholar, MEDLINE (via the EBSCOhost and PubMed interfaces), ProQuest Newsstand, and Scopus. Searches were conducted using combinations of the following appropriate keywords and subject headings: (1) MH “Labor, Obstetric+,” “Labor, Obstetric”[MeSh], MH “Delivery, Obstetric+,” “Delivery, Obstetric”[MeSh], MH “Obstetric Labor Complications,” “Obstetric Labor Complications”[MeSh], MH “Labor Complications+,” MH “Labor+,” pregna*, labor, deliver*, obstetric*, women, maternal, neonatal, OR childbirth; AND (2) MH “Alcoholic Intoxication,” MH “Ethanol+,” “Ethanol”[nm], “Alcohol Drinking/adverse effects”[MAJR], “Alcoholic Intoxication/complications”[MAJR], “alcohol poisoning,” alcohol, ethanol, drunk, OR intoxicat*; AND (3) MH “Maternal Mortality,” MH “Maternal Death,” MH “Stillbirth,” “Stillbirth”[MeSh], “Fetal Death”[MeSh], MH “Infant Death,” “Infant Death”[MeSh], MH “Infant Mortality,” MH “Perinatal Death,” “Perinatal Death”[MeSh], death, stillborn, stillbirth, OR morbidity.
The study inclusion criteria required that the article must include BAC values for the mother and/or the fetus. Articles that did not meet this criterion were excluded from this review. For example, Kim and Hodgkinson’s article from 1976 was excluded from our review because no BAC values were presented, even though fetal outcome was discussed.10 We then screened the articles using the titles and abstracts to eliminate those articles that would not meet our inclusion criteria. The remaining 34 individual articles were forwarded to the authors for screening and selection. Each of these articles were then reviewed by at least 2 authors.
Statistical Analysis
We utilized summary statistics for this review including medians and interquartile ranges (IQRs) for maternal age, gestational age, fetal and newborn birthweights, head circumference, and BACs.
Since this is a review of published literature, our university institutional review board does not require approval of this study.
Results
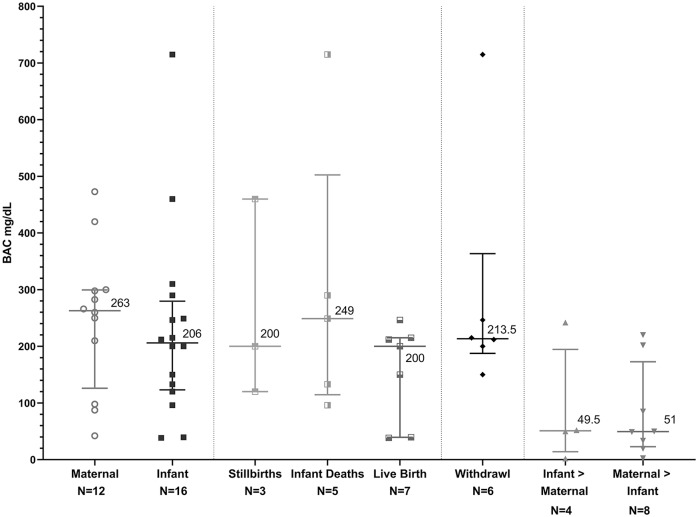
We identified 12 articles meeting our study criteria that included reports of 16 mothers with a reported BAC during labor or delivery (see Table 1). The age of the mothers ranged from 20 to 38 years with a median of 31 years and an IQR of 25.75 to 32 years. Gestational age at time of delivery ranged from 31 to 41 weeks with a median of 35.5 weeks. Infant birth weight ranged from 620 to 3630 g with a mean of 2440 g. Seven cases reported data on head circumference ranging from 29.5 to 34.5 cm (median = 33.5; Table 1). Maternal BAC levels ranged from 42.1 mg/dL to 473 mg/dL, with a median BAC of 126 mg/dL (IQR = 126-299.5 mg/dL; Figure 1).
Table 1.
Summary Data From the 12 Studies Included in This Review.
| Reference | Pregnancy | Maternal Age (Years) | Gestational Age (Weeks) | Actual Fetal/Infant Weight (g) | Expected Infant Weight for Gestational Age (g)a | Actual − Expected Weight (g) | Fetal Head Circumference (cm) | Maternal BAC (mg/dL) | Fetal/Infant BAC (mg/dL) | Maternal − Fetal BAC (mg/dL) | Outcome | Withdrawal |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kuznetsov, 198112 | 34 | 36 | 2150 | 2800 | −650 | 29.5 | 460 | Stillbirth | ||||
| Barinov et al,8 1997 | G4P2A2 | 33 | 40 | 290 | Newborn death | |||||||
| Khodasevich and Rubtsov,9 1989 | G14P2A11 | 35 | 38 | 2900 | 3200 | −300 | 420 | 200 | 220 | Stillbirth | ||
| Khodasevich and Rubtsov,9 1989 | G11 | 35 | 31 | 1470 | 1650 | −180 | 120 | Stillbirth | ||||
| Uzel et al,7 1990 | 35 | 33 | 3630 | 2050 | 1580 | 261 to 304 (average = 282.5) | 238 to 260 (average = 249) | 33.5 | Newborn death | |||
| Uzel et al,7 1990 | 25 | 33 | 2170 | 2050 | 120 | 295 to 301 (average = 298) | 96 | 202 | Newborn death | |||
| Fitzsimons, 198113 | 28 | 40 | 2440 | 3600 | −1160 | 50th percentile (estimated 34.7) | 250 (estimated) | 200 at 8 hours | 50 | Live birth | + | |
| Jung et al, 198014 | G1P0 | 20 | 32 | 1420 | 1850 | −430 | 473 at 6 hours | 715 at 5 hours | −242 | Neonatal death at 56 hours | + | |
| Cook, 197515 | G8P9 | 30 | 41 | 2920 | 3750 | −830 | 34.5 | 98 at 1 hour | 150 at 1 hour | −52 | Live birth | + |
| Kloppel and Weiler, 198916 | 133 | Neonatal death | ||||||||||
| Beattie,11 1986 | G2P1 | 29 | 37+ | 2440 | 3000 | −560 | 32 | 210 | 212 | −2 | Live birth | + |
| Fischer et al, 200317 | G4P1 | 35 | 2840 | 2500 | 340 | 260 | 310 | −50 | Cardiac arrest, resuscitated | |||
| Kvigne et al, 201218 | G6 | 26 | 40 | 3470 | 3600 | −130 | 34.5 | 87.4 at −66 minutes | 38.4 at 129 minutes | 49 | Live birth | |
| Kvigne et al, 201218 | G7 | 27 | 34 | 2240 | 2300 | −60 | 32 | 42.1 at 1 hour | 39.5 at 1 hour | 2.6 | Live birth | |
| Kvigne et al, 201218 | G8 | 32 | 40 | 2450 | 3600 | −1150 | 33.5 | 265.9 at −27 minutes | 246.5 at 67 minutes | 19.4 | Live birth | + |
| Silva et al, 198719 | G9P5A3 | 38 | 32 | 620 | 1850 | −1230 | 300 at −2 hours | 215 (from cord blood) | 85 | Live birth | + | |
| Median | 31 | 35.5 | 2440 | 2650 | −365 | 33.5 | 263 | 206 | 26.45 | |||
| IQR | 26.75 to 35 | 32.75 to 40 | 1980 to 2905 | 2000 to 3600 | −910 to −15 | 32 to 34.5 | 126 to 299.5 | 123.3 to 279.8 | −38 to 76.25 |
Abbreviations: G, gravida; P, para; A, abortion; IQR, interquartile range.
Weights based on values from Fenton.20
Figure 1.
Graphic summary of blood alcohol concentrations (BACs) for mothers, stillbirths, infant deaths, infants who lived, cases where infant BAC exceeded maternal levels, and cases where maternal levels exceeded infants BACs. BAC presented in milligrams per deciliter (mg/dL) as medians and interquartile ranges.
We then grouped the births into 4 unique outcome groups. The 16 pregnancies resulted in 3 (19%) late-term stillbirths (Table 1). Among the stillborn infants, the BAC ranged from 120 to 460 mg/dL (median BAC = 200 mg/dL; Figure 1). The gestational age of the stillborn infants ranged from 31 to 38 weeks (median = 36 weeks). The birthweights ranged from 1470 to 2900 g (median = 2150 g).
Five cases (31%) resulted in infant deaths (Table 1). The BAC levels ranged from 96 to 715 mg/dL (median = 249 mg/dL; Figure 1). The gestational age of these infants ranged from 32 weeks to 40 weeks (median = 33 weeks; Table 1). One infant died at 56 hours of age. The age at time of death for the other 4 infants was not specified in the case reports.
We found 6 (38%) infants who lived (Table 1). The BAC for the living infants ranged from 38.4 to 246.5 mg/dL (median of 200 mg/dL; Figure1). The gestational age ranged from 32 weeks to 40 weeks with a median of 40 weeks. The birthweights of the living infants ranged from 620 g to 3470 g (mean = 2440 g). Outcomes were not reported for 2 infants (13%; Table 1).
Six of the neonates (38%) exhibited symptoms of acute alcohol withdrawal (Table 1 and Figure 1). The BAC for these infants ranged from 150 to 715 mg/dL (median = 213.5 mg/dL). Among the infants with alcohol withdrawal, 4 infants had known outcomes including one (25%) death. The characteristics of alcohol withdrawal from this review are presented in Table 2. In this small sample, a BAC >150 mg/dL was associated with withdrawal (Figure 1). The maternal BAC levels ranged from 98 to 473 mg/dL (median = 263 mg/dL; Figure 1). Three of the mothers had BACs exceeding the infants’ BAC and 3 of the infants had BACs exceeding the mothers’ levels.
Table 2.
Symptoms of Neonatal Alcohol Withdrawal.
| Article | Reported Symptoms |
|---|---|
| Beattie,11 1986 | Tremors, irritability, tachypnea, hypertonia, excessive crying, exaggerated mouthing behavior, opisthotonos, and seizures |
| Cook, 197515 | Shallow respirations, lethargy, hypotonia, abnormal reflexes, irritability, jitteriness, apnea, temperature instability, hypoglycemia, and vomiting |
| Fitzsimons, 198113 | Hypotonia and depressed reflexes |
| Jung, 198014 | Bradycardia, flaccidity, and cyanosis |
| Kvigne, 201218 | Hypotonia and jitteriness |
| Silva, 198719 | Irritability, tremors, and abdominal distention |
| Pierog et al,21 1977 | Tremors, seizures, irritability, abdominal distention, and opisthotonos |
| Most prevalent signs | Tremors/jitteriness, hypotonia, and irritability |
In 8 cases, infant BACs exceeded maternal levels ranging from 2.6 to 220 mg/dL. This suggests that as maternal BACs decrease, the changes in the infants’ BAC may have a lag time. In 4 cases, maternal BAC exceeded infant BAC by 2 to 242 mg/dL. As a result, it may be important to obtain a BAC for the infant independent of the maternal level. Since it may not be clear if the maternal BAC is stable, decreasing, or increasing, obtaining a second BAC for the mothers a couple of hours later would be important, especially if the mother is still in labor.
No identifiable management protocols were located in our literature search. In the absence of published research, every occurrence of alcohol exposure during labor and at delivery is a unique patient management decision made by a clinician.
Discussion
From the 16 pregnancies with a reported BAC during labor or at birth, we were unable to establish a BAC predicting either mortality or survival. However, Khodasevich and Rubstov previously reported that a BAC between 200 and 300 mg/dL is lethal for 50% of newborns.9 In this review, we identified 5 deaths with BACs >200 mg/dL, ranging from 200 to 715 mg/dL (mean = 380 mg/dL). We found 3 infants who died with a BAC <200 mg/dL (96, 120, and 133 mg/dL, with a group mean of 116 mg/dL). It seems likely based on these limited data that a BAC >96 mg/dL should be considered to represent increased mortality risk.
We identified 4 infants with a BAC >200 mg/dL who were reported to live (one required resuscitation). The BACs ranged from 212 to 310 mg/dL (mean = 245 mg/dL). Among infants who lived, we found BACs as low as 38.4 mg/dL and as high as 246.5 mg/dL. Conversely, a newborn and a neonatal death was reported with a BAC as low as 96 mg/dL and as high as 715 mg/dL.
Table 1 shows the difference in fetal versus maternal BAC levels. Infant alcohol dehydrogenase enzyme activity is diminished compared with maternal alcohol dehydrogenase.1,4 Beattie reported that an infant’s alcohol clearance rate is approximately half of its mother’s rate.11 We have reported that the infant’s alcohol elimination rate increases to about 85% of the mother’s within a few hours after birth.1,4 There was modest overall correlation between maternal and fetal BAC in this study, and maternal BAC was not necessarily predictive of fetal BAC. This may depend on the timing of the BAC collection along the exposure curve. It is also possible that this may reflect individual differences in alcohol dispersion into the fetal compartment and differences in alcohol elimination rates from the fetal compartment.1,4,22
It is widely known that alcohol exposure during pregnancy increases the likelihood of preterm birth23,24 (Table 1). This review supports this concept, with an average gestational age of 36 weeks at time of delivery. Low birthweight was a common occurrence in the reviewed cases, with the infants weighing an average of 331 g less than expected for gestational age.20
Of the infants with known fetal outcomes, 4 were reported to have symptoms of alcohol withdrawal. However, the phenotype of withdrawal for the fetus or newborn is largely unknown. Pierog et al and Robe et al summarized differences between the neonatal alcohol withdrawal phenotype from neonatal opioid withdrawal.21,25 Three symptoms were reported to suggest alcohol not opioid withdrawal (seizures, abdominal dilation, and opisthotonos).20,21 One infant (25%) of those with withdrawal symptoms died at 56 hours of age from alcohol poisoning. Alcohol withdrawal is associated with increased mortality risk in adults.26 The mortality proportion in this study may approximate rates for adults undergoing alcohol withdrawal.26 Since the onset and recognition of withdrawal can present hours to 1 or 2 days after delivery, clinicians may want to consider management of infants with a positive BAC in a neonatal intensive care unit for 72 hours.
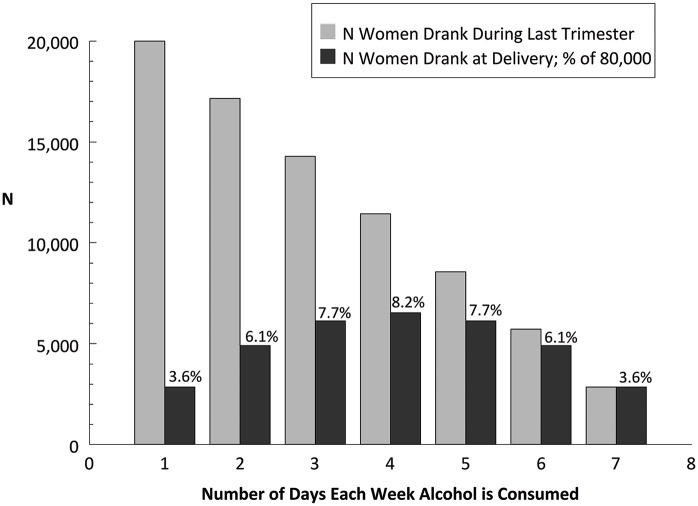
It is difficult to explain the absence of literature or evidence-based guidance for patient management in what appears to be a fairly common event. In Figure 2, we present a hypothetical model of women drinking at the end of pregnancy and the proportion who will be drinking on their delivery day. The United States has 3.9 million births annually,27 and 2% of pregnant women drink throughout pregnancy. These 80 000 women were divided into 7 groups, and we assumed that fewer women will drink every day than women who drink 1, 2, or 3 days per week. The bars in Figure 2 show the hypothetical distribution of women drinking on the day of delivery. For example, 14 286 women were expected to drink 3 days a week, and 6122 or 7.7% were expected to be drinking on the day of delivery. The model predicts that 42.9% of women drinking at the end of pregnancy will be drinking on their delivery day. As a result, they would be expected to have a detectable BAC during labor and delivery. We are unaware of any published reports of prevalence studies of maternal alcohol use or intoxication on presentation for labor or at delivery.
Figure 2.
A model of women drinking near delivery. In this hypothetical model, we assume that the number of women drinking only 1 day a week is more than the number of women drinking 7 days a week (it decreases linearly). The solid bars decrease from left to right (20 000 to 2857) as the number of drinking days per week increases.
Limitations
This study is limited by the data available for analysis. All articles included represent case reports with no population-based studies. Recent data suggest that women’s self-report of substance use is typically underreporting. In one study, for every woman reporting alcohol use during pregnancy, 5 were found to have a positive biomarker for alcohol exposure during pregnancy.28 Recognition of alcohol use by labor and delivery personnel and neonatal intensive care unit staff may also be unreliable. Another alternative could be that alcohol intoxication is often recognized but rarely reported. In this study, we did not include data on smoking, which may have important effects on outcome since combined exposure to both smoking and alcohol use during pregnancy has a multiplicative effect on risk for some adverse outcomes.29
Conclusions
Every year in the United States alone, we estimate that nearly 35 000 women deliver who have been drinking that day. It is likely that several thousand women each year present to labor and delivery while intoxicated in the United States alone. As such, a higher index of suspicion and development of a protocol for management of these women and their infants would likely have an important impact on improving maternal, fetal, and infant outcomes.
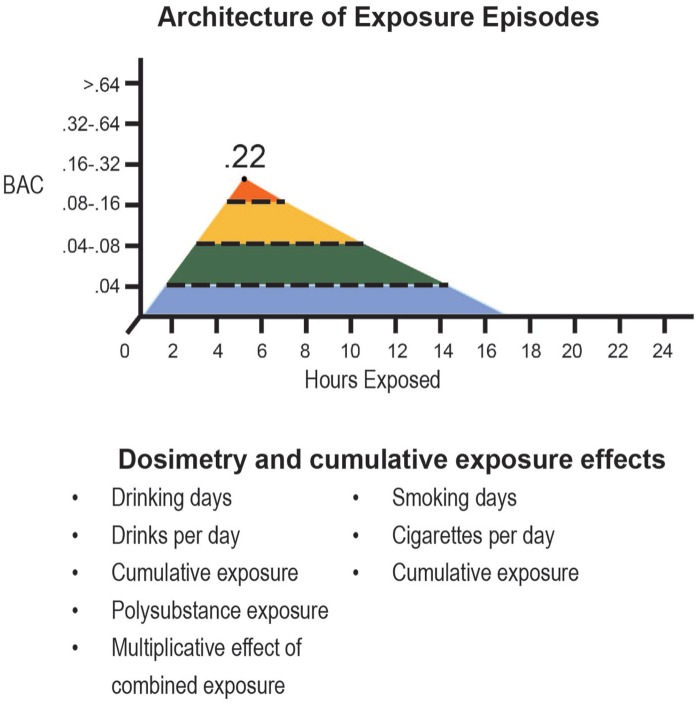
In several previous articles, we have described the essentials of exposure assessment in pregnancy. This included detection risk stratification (drinking after pregnancy confirmation), dosimetry (drinking days during pregnancy), drinks per drinking day, binge days (4 or more), and estimates of cumulative exposure during pregnancy (Figure 3).
Figure 3.
Graphic depiction of exposure assessment. This graphic depicts the key components used to estimate alcohol exposure during pregnancy. These data can be collected during or after pregnancy.
The absence of evidence-based management protocols may have important consequences for infants born to mothers who are drinking at the end of pregnancy. Several important outcomes should be considered:
Every year in the United States alone, 34 285 women deliver who have been drinking on that day (93 per day).
The infant’s alcohol elimination rate rises to about 85% of the mother within a few hours after birth.1,4
The mortality proportion in this study for infants with alcohol withdrawal (25%) study approximates rates for adults undergoing alcohol with-drawal.26
Three symptoms were thought to suggest alcohol not opioid withdrawal (seizures, abdominal dilation, and opisthomas).
Since the onset and recognition of withdrawal can present hours to 1 or 2 days after delivery, clinicians may want to consider management of infants with a positive BAC in a neonatal intensive care unit for 72 hours.
When the fetal or infant BAC was >200 mg/dL, the mortality rate was 50% (including stillbirths and newborn deaths).
Identification of women drinking at the end of pregnancy and referral for treatment could reduce the risk of exposure for subsequent pregnancies.
The lack of data for such a common event suggests the need for an ambitious research agenda to more carefully examine this complex public health problem. This should include improved exposure detection using biomarker panels and enhancing exposure assessments by studying the use of breathalyzers during pregnancy and during labor and delivery. Greatly increased rates of screening in neonatal intensive care nurseries is urgently required.
Footnotes
Author Contributions: Elizabeth Schaff assisted with review of literature, manuscript composition and revision, and statistical analysis. Marcos Moreno assisted with review of literature and manuscript revision. Katrina Foster assisted with conception of the study, review of literature, and manuscript preparation. Marilyn Klug assisted with statistical analysis and generation of figures. Larry Burd assisted with review of literature, manuscript revision, and statistical analysis.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Larry Burd  https://orcid.org/0000-0001-7199-2711
https://orcid.org/0000-0001-7199-2711
References
- 1. Paintner A, Williams AD, Burd L. Fetal alcohol spectrum disorders—implications for child neurology, part 1: prenatal exposure and dosimetry. J Child Neurol. 2012;27:258-263. [DOI] [PubMed] [Google Scholar]
- 2. Substance Abuse and Mental Health Data Archive. National Survey on Drug Use and Health: 10-year substate R-DAS (NSDUH-2002-2011-DS0001). https://www.datafiles.samhsa.gov/study-dataset/national-survey-drug-use-and-health-10-year-substate-r-das-nsduh-2002-2011-ds0001. Accessed November 28, 2019.
- 3. Ethen MK, Ramadhani TA, Scheuerle AE, et al. ; National Birth Defects Prevention Study. Alcohol consumption by women before and during pregnancy. Matern Child Health J. 2009;13:274-285. doi: 10.1007/s10995-008-0328-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burd L, Blair J, Dropps K. Prenatal alcohol exposure, blood alcohol concentrations and alcohol elimination rates for the mother, fetus and newborn. J Perinatol. 2012;32:652-659. [DOI] [PubMed] [Google Scholar]
- 5. Li Q, Fisher WW, Peng CZ, Williams AD, Burd L. Fetal alcohol spectrum disorders: a population based study of premature mortality rates in the mothers. Matern Child Health J. 2012;16:1332-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schwartz M, Hart B, Weyrauch D, Benson P, Klug MG, Burd L. The hidden face of fetal alcohol spectrum disorder. Curr Womens Health Rev. 2017;13:96-102. doi: 10.2174/1573404813666170418114243 [DOI] [Google Scholar]
- 7. Uzel R, Grumlik R, Uhlir F. Labor in a drunk mother—fatal risk for the neonate [in Czech]. Cesk Gynekol. 1990;55:132-134. [PubMed] [Google Scholar]
- 8. Barinov EH, Burago JI, Bulanakova AB. The death of newborn infant from ethanol poisoning [in Russian]. Sud Med Ekspert. 1997;40:45. [PubMed] [Google Scholar]
- 9. Khodasevich LS, Rubtsov VK. Morphologic diagnosis of alcoholic embryofetopathy [in Russian]. Sud Med Ekspert. 1989;32:51-52. [PubMed] [Google Scholar]
- 10. Kim SS, Hodgkinson R. Acute ethanol intoxication and its prolonged effect on a full-term neonate. Anesth Analg. 1976;55:602-603. [DOI] [PubMed] [Google Scholar]
- 11. Beattie JO. Transplacental alcohol intoxication. Alcohol Alcohol. 1986;21:163-166. [PubMed] [Google Scholar]
- 12. Kuznetsov LE. Fetal death from acute ethanol poisoning. Forensic Medical Expertise [Sudebno-Meditsinskaia Ekspertiza]. 1981;24(3):56-57. [PubMed] [Google Scholar]
- 13. Fitzsimons RB, Mahony MJ, Cussen GH. Ethanol intoxication of the newborn: A case report and review of the literature. Ir Med J. 1981;74(8):230-231. [PubMed] [Google Scholar]
- 14. Jung AL, Roan Y, Temple AR. Neonatal death associated with acute transplacental ethanol intoxication. Am J Dis Child. 1980;134(4):419-420. [DOI] [PubMed] [Google Scholar]
- 15. Cook LN, Shott RJ, Andrews BF. Acute transplacental ethanol intoxication. Am J Dis Child. 1975;129(9):1075-1076. doi: 10.1001/archpedi.1975.02120460057014 [DOI] [PubMed] [Google Scholar]
- 16. Kloppel A, Weiler G. Diaplacental poisoning with narcotics and alcohol in newborn infants. Beitr Gerichtl Med. 1989;47:77-79. [PubMed] [Google Scholar]
- 17. Fischer D, Solbach C, Kitz R, Ahr A, Veldman A. Acute ethanol intoxication during pregnancy and consecutive fetal cardiac arrest: A case report. J Perinat Med. 2003;31(4):343-344. doi: 10.1515/JPM.2003.050 [doi]. [DOI] [PubMed] [Google Scholar]
- 18. Kvigne VL, Randall B, Simanton EG, Brenneman G, Welty TK. Blood alcohol levels for american indian mothers and newborns. Pediatrics. 2012;130(4):e1015-e1018. DOI:10.1542/peds.2011-1400 [DOI] [PubMed] [Google Scholar]
- 19. Silva PD, Miller KD, Madden J, Keegan KA., Jr Abnormal fetal heart rate pattern associated with severe intrapartum maternal ethanol intoxication. A case report. J Reprod Med. 1987;32(2):144-146. [PubMed] [Google Scholar]
- 20. Fenton TR. A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format. BMC Pediatr. 2003;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pierog S, Chandavasu O, Wexler I. Withdrawal symptoms in infants with the fetal alcohol syndrome. J Pediatr. 1977;90:630-633. [DOI] [PubMed] [Google Scholar]
- 22. Longhurst W, Ernst J, Burd L. Fetal alcohol exposure and development of the integument. Res Rep Neonatol. 2016;6:25-32. [Google Scholar]
- 23. Bailey BA, Sokol RJ. Prenatal alcohol exposure and miscarriage, stillbirth, preterm delivery, and sudden infant death syndrome. Alcohol Res Health. 2011;34:86-91. [PMC free article] [PubMed] [Google Scholar]
- 24. Sokol RJ, Janisse JJ, Louis JM, et al. Extreme prematurity: an alcohol-related birth effect. Alcohol Clin Exp Res. 2007;31:1031-1037. [DOI] [PubMed] [Google Scholar]
- 25. Robe LB, Gromisch DS, Iosub S. Symptoms of neonatal ethanol withdrawal. Curr Alcohol. 1981;8:485-493. [PubMed] [Google Scholar]
- 26. Trevisan LA, Boutros N, Petrakis IL, Krystal JH. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22:61-66. [PMC free article] [PubMed] [Google Scholar]
- 27. Centers for Disease Control and Prevention. National Center for Health Statistics: births and natality. https://www.cdc.gov/nchs/fastats/births.htm. Accessed November 28, 2019.
- 28. Lange S, Shield K, Koren G, Rehm J, Popova S. A comparison of the prevalence of prenatal alcohol exposure obtained via maternal self-reports versus meconium testing: a systematic literature review and meta-analysis. BMC Pregnancy Childbirth. 2014;14:12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Odendaal HJ, Steyn DW, Elliott A, Burd L. Combined effects of cigarette smoking and alcohol consumption on perinatal outcome. Gynecol Obstet Investig. 2009;67:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burd L. Fetal alcohol spectrum disorder: complexity from comorbidity. Lancet. 2016;387:926-927. [DOI] [PubMed] [Google Scholar]
- 31. Williams AD, Nkombo Y, Nkodia G, Leonardson G, Burd L. Prenatal alcohol exposure in the Republic of the Congo: prevalence and screening strategies. Birth Defects Res A Clin Mol Teratol. 2013;97:489-496. [DOI] [PubMed] [Google Scholar]