Abstract
Objectives:
Pleomorphic adenomas are benign tumors that rarely involve nonsalivary glands. We report an uncommon case of ala nasi pleomorphic adenoma. We discuss the clinical and histopathologic characteristics, and review the literature on nasal pleomorphic adenoma.
Method:
A 20-year-old man presented with a painless slow growing lobulated mass located on the right ala nasi extending into the nasal vestibule.
Results:
Complete surgical excision was performed. Histologic examination found a mixed cellular component: epithelial and myoepithelial cells with chondromyxoid stroma. This was consistent with the diagnosis of a typical pleomorphic adenoma. There was no evidence of recurrence at 18 months after the surgery.
Conclusions:
Pleomorphic adenomas located in the external nose are extremely rare. In such case, pleomorphic adenoma could originate from ectopic minor salivary gland. Complete surgical excision with long-term follow-up is recommended due to the potential risk of recurrence and malignant transformation.
Keywords: Pleomorhpic adenoma, ala nasi, tumor, nose
Introduction
Pleomorphic adenoma (PA) is a benign tumor involving usually the major salivary glands such as the parotid and the submandibular glands. It originates less frequently from the minor salivary glands found in the mucosa of the palate, lip, floor of mouth, tongue, tonsil, pharynx, retromolar area, and nasal cavity.1 Most of the PAs involving the nose arise from the minor salivary glands of the nasal mucosa of the septum or the lateral wall, those that are located in the external nose are extremely rare, and originate from ectopic minor salivary gland of the skin.1 We present an uncommon case of PA originating from the ala nasi. We discuss the clinical and histopathologic features of this tumor, and review the literature on this rare entity.
Case Report
A 20-year-old male patient presented with a painless and gradually increasing mass of the right ala nasi of 6 months evolution. Examination of the nose revealed a firm and lobulated mass located in the lateral part of the right ala nasi, of approximately 2 cm × 1 cm in size, covered by an adherent and thinned skin, extending into the nasal vestibule (Figure 1). No punctum or skin extension was noted. Anterior rhinoscopy found a whitish nonobstructive mass, not bleeding on touch, covered by a normal vestibular skin (Figure 2). There were no other abnormalities in the nasal cavities, and the remainder of the physical examination was normal, in particular, concerning the major salivary glands and the neck.
Figure 1.

Lobulated mass located on the right ala nasi, of approximately 2 cm × 1 cm in size, covered by adherent and thinned skin.
Figure 2.

Endonasal view of a nonobstructive mass covered by a normal vestibular skin.
The tumor was completely resected under general anesthesia. Intranasal vestibular incision was performed parallel to the right alar rim. The tumor was carefully dissected without damaging the capsule, but due to its adherence to the ala nasi skin, it was necessary to remove the tumor along with the covering skin (Figure 3). The subsequent defect was closed up without reconstruction. The postoperative period was uneventful.
Figure 3.

Intraoperative view: complete tumor dissection removing adherent skin (blue arrow) via intranasal vestibular incision.
Pathologic examination of the excised tumor found a pale-white, irregular nodular mass measuring 2 × 1.2 × 1 cm. The cut-section was solid, pale-white, homogeneous, and firm in consistency. On histologic examination, the tumor was composed of a mixture of calcified ductal and tubular formations and spindle myoepithelial cells into chondromyxoid stroma (Figure 4). There were no areas of malignant transformation. On immunohistochemistry, epithelial membrane antigen (EMA), cytokeratin 7 (CK7), and cytokeratin 19 (CK 19) phenotype expression were positive (Figure 5). The histopathologic analysis confirmed the diagnosis of PA with clear surgical margins. There were no signs of local recurrence with 18 months follow-up.
Figure 4.
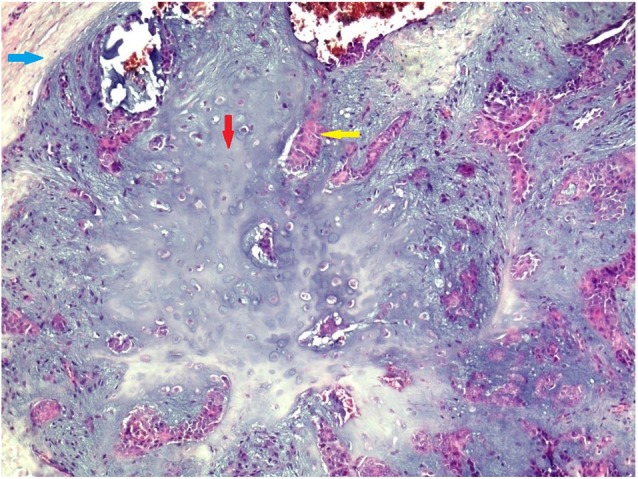
Histopathologic photomicrograph showing epithelial and myoepithlial cells in myxoid stromal component (H&E; x200). Blue arrow indicates fibrous capsule; red arrow, mesenchymatous component; yellow arrow, epithelial and myoepithelial component.
Figure 5.

Immunohistochemistry photomicrograph revealing myoepithelial cells with positive cytokeratin 7 expression (red arrow) (H&E; x400).
Discussion
Pleomorphic adenomas are benign tumors that rarely involve area other than the major salivary glands. In the upper respiratory tract, PAs affect commonly the nasal cavity.2,3 The first reported case of PA of the nasal cavity was by Denker and Kahler (1929). Although, earlier, nasal septum PA were published by Zarniko in 1910, in a monograph on “Diseases of Nose and Nasopharynx.”4 Larger studies of intranasal PAs include 40 cases reported by Compagno and Wong,5 59 cases reported by Batsakis,6 and 41 cases by Suzuki et al.4,7
In our case, the tumor originated from the nasal ala skin which is very uncommon. In the head and neck region, such tumors are described in different locations including the scalp, eyelids, cheek, upper lip, external auditory canal, and the nose.8 Cases of nasal columella and ala nasi PAs were reported, respectively, by Ceylan et al1 and Sung et al.9
It would appear that in such cases, the tumor may originate from sebaceous glands, sweat glands, or ectopic salivary gland.5,8,10,11 In our case, PA seemed to develop from ectopic also named heterotopic salivary gland tissue. Various theories suggested that ectopic salivary tissue is a result of developmental disorders such as differentiation of remnants of primitive embryologic structures and misplaced embryonic epithelial cells derived from ectoderm.12
The clinical features of external nose PAs are nonspecific, usually the tumor is painless, and slow growing over a long period. Due to this lack of specificity, pathologic examination is necessary to confirm the diagnosis of PA and rule out other skin lesions such as dermoids, sebaceous cysts, and neurofibromas.
Pleomorphic adenomas should be differentiated from chondroid syringomas also known as mixed tumors of the skin. These tumors arising from sweat glands are most often benign, with a risk of local recurrences and malignant transformation.13
They have both epithelial and mesenchymal components and share histologic similarities with PAs, which are mixed tumors arising from the salivary glands. Epithelial cells show differentiation toward adnexal structures in chondroid syringomas, this feature is not common in PAs.13
Furthermore, the degree of myoepithelial differentiation is lower in chondroid syringomas, expression of desmin and actin are almost negative which differentiate this tumor from PAs.14
In our case, immunohistochemical examination showed positive CK19 and EMA phenotypes expression, which is characteristic of ductal component. Also, smooth muscle actin and CK7 expressed by myoepithelial cells was positive. As these cells are not usually found in the chondroid syringoma, diagnosis of PA was thus confirmed.
The treatment of choice for nasal PA is complete surgical excision with clear margins.
Recurrences and malignant transformation could occur in 6%.15 The reported recurrence rate is ranging from 2.4% to 10%.7,16 The risk of recurrence is increased by initial incomplete resection which causes seeding of the tumor; it also could be related to some histologic characteristics. Krolls and Boyers17 suggested that myxoid stromas, which might easily spill into the surgical field, are most frequently associated with recurrent PAs. Compagno and Wong5 attributed the low recurrence rate of intranasal PAs to its high cellularity and little myxoid stroma. In our patient, there were no evidence of recurrence after 18 months follow-up. Due to the potential risk of recurrence and malignant transformation of nasal PAs, routine examination and long-term follow-up are recommended.
Conclusions
External nose localization of PA is very rare with only few cases reported in the literature. This tumor does not show specific clinical features and presents usually as a slow growing painless mass. In our case, histopathologic examination confirmed the diagnosis of typical PA with both epithelial and mesenchymal components.
Although these tumors are benign, careful follow-up is recommended due to the potential risk of recurrence and malignant transformation.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: AM: wrote the manuscript, performed the surgical treatment, provided patient’s data (clinical and surgical), reviewed the literature;
SL: performed the histological examination, provided patient’s data (pathological data), reviewed the literature;
AS: contributed to the histological examination, and data analysis;
OZ: contributed to data analysis.
Informed consent: Informed consent was obtained from the patient for publication of this case report and accompanying images.
ORCID iD: Amina Mouzali  https://orcid.org/0000-0002-5386-7834
https://orcid.org/0000-0002-5386-7834
References
- 1. Ceylan A, Celenk F, Poyraz A, Uslu S. Pleomorphic adenoma of the nasal columella. Pathol Res Pract. 2008;204:273-276. [DOI] [PubMed] [Google Scholar]
- 2. Dubey SP, Banerjee S, Ghosh LM, Roy S. Benign pleomorphic adenoma of the larynx: report of a case and review and analysis of 20 additional cases in the literature. Ear Nose Throat J. 1997;76:548-550. [PubMed] [Google Scholar]
- 3. Golz A, Ben-Arie Y, Fradis M. Pleomorphic nasoseptal adenoma. J Otolaryngol. 1997;26:399-401. [PubMed] [Google Scholar]
- 4. Kamal A. Pleomorphic adenoma of the nose: a clinical case. J Laryngol Otol. 1984;98:917-923. [DOI] [PubMed] [Google Scholar]
- 5. Compagno J, Wong RT. Intranasal mixed tumors (pleomorphic adenomas): a clinicopathologic study of 40 cases. Am J Clin Pathol. 1977;68:213-218. [DOI] [PubMed] [Google Scholar]
- 6. Batsakis JW. Tumors of the Head and Neck: Clinical and Pathological Considerations. Baltimore, MD: Lippincott Williams & Wilkins; 1979. [Google Scholar]
- 7. Suzuki K, Moribe K, Baba S. A rare case of pleomorphic adenoma of lateral wall of nasal cavity, with special reference of statistical observation of pleomorphic adenoma of nasal cavity in Japan. Nihon Jibiinkoka Gakkai Kaiho. 1990;93:740-745. [DOI] [PubMed] [Google Scholar]
- 8. Stout AP, Gorman JG. Mixed tumors of the skin of the salivary gland type. Cancer. 1959;12:537-543. [DOI] [PubMed] [Google Scholar]
- 9. Sung KY, Kim YH, Lee SK, Lee SY. An unusual presentation of pleomorphic adenoma: nasal ala. J Craniofac Surg. 2012;23:e641-e642. [DOI] [PubMed] [Google Scholar]
- 10. Nishimura S, Murofushi T, Sugasawa M. Pleomorphic adenoma of the auricle. Eur Arch Otorhinolaryngol. 1999;256:22-24. [DOI] [PubMed] [Google Scholar]
- 11. Pons Vicente O, Almendros Marqués N, Berini Aytés L, Gay Escoda C. Minor salivary gland tumors: a clinicopathological study of 18 cases. Med Oral Patol Oral Cir Bucal. 2008;13:E582-E588. [PubMed] [Google Scholar]
- 12. Willis RA. Some unusual developmental heterotopias. Br Med J. 1968;3:267-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reddy PB, Nandini DB, Sreedevi R, Deepak BS. Benign chondroid syringoma affecting the upper lip: report of a rare case and review of literature. J Oral Maxillofac Pathol. 2018;22:401-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wan H, Xu M, Xia T. Clinical and pathological study on mixed tumors of the skin. Medicine (Baltimore). 2018;97:e12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lo SH, Huang SH, Chang YL. Pleomorphic adenoma of the nasal septum: a case report. Tzu Chi Med J. 2005;17:47-49. [Google Scholar]
- 16. Sciandra D, Dispenza F, Porcasi R, et al. Pleomorphic adenoma of the lateral nasal wall: case report. Acta Otorhinolaryngol Ital. 2008;28:150-153. [PMC free article] [PubMed] [Google Scholar]
- 17. Krolls SO, Boyers RC. Mixed tumors of salivary glands. Long term follow up. Cancer. 1972;30(1):276-81. [DOI] [PubMed] [Google Scholar]


