Short abstract
Background
Processing speed is frequently reduced in patients suffering from multiple sclerosis (MS). Reduced processing speed can also lead to impaired working memory capacity (WMC) in adult MS patients. Less is known about the interplay of cognitive deficits in paediatric MS patients.
Objectives
In the present study, we investigated whether processing speed and WMC are reduced in paediatric MS patients compared with healthy controls and whether reduced processing speed and WMC might explain potential differences in psychometric intelligence between MS patients and healthy controls.
Methods
Twenty-one paediatric MS patients and 21 healthy controls completed a reaction time (RT) task, a working memory task, and Cattell’s Culture Fair Test (CFT20-R).
Results
Patients with MS had slower RT and lower intelligence scores than healthy controls. We could find no significant differences for WMC. An analysis of covariance revealed that group differences in intelligence could be partially explained by processing speed differences.
Conclusion
The results indicate that processing speed is a good marker for MS-related impaired efficiency and increased error-proneness of the central nervous system in higher-order cognition as required by Cattell’s CFT20-R.
Keywords: Cognitive impairments, multiple sclerosis, processing speed, psychometric intelligence
Introduction
Multiple Sclerosis (MS) is a chronic inflammatory disorder of the central nervous system, attacking the myelinated axons and leading to grey matter damage and/or loss of cell connectivity due to white matter lesions and degeneration.1 Typically, MS presents in young adults between 20 and 45 years of age, but occurs in 3–5% of patients before their 16th birthday2 and is then diagnosed as paediatric MS.
Cognitive impairment is commonly observed in many adult3 and paediatric MS patients,2 with processing speed (PS), defined as the time needed to execute a cognitive task, most consistently impaired by MS.4 Results in experimental reaction time (RT) paradigms indicate that MS-related neural noise leads to a general slowing of PS.5 Consequently, when a cognitive task requires a high number of cognitive processes to be executed (and each process is delayed), speed differences between patients with MS and healthy controls are more pronounced than in less complex cognitive tasks. PS deficits have been reported also for paediatric MS patients, but were primarily investigated by means of psychometric neuropsychological tests rather than experimental RT tasks.2,6,7
Another area of cognitive research in MS investigated possible deficits in the capacity of working memory (WMC). Working memory (WM) is conceptualized as a system providing accessibility, maintenance and simultaneous manipulation of mental representations.8 WMC deficits in adult MS patients have been frequently reported3 but might be less pronounced when a given WM task provides sufficient time for a response.9,10 Thus, MS patients’ deficits in PS might underlie WMC deficits, as explicitly stated in DeLuca et al.’s (2004) relative consequence model.11 For paediatric MS patients, Wuerfel et al.7 observed impaired WMC only with more difficult but not with easier WM tasks, while Holland et al.12 did not find WMC deficits in paediatric MS patients.
The present study focuses on psychometric intelligence as a global measure of cognitive functioning13 with well-established relationships to everyday criteria of cognitive functioning such as scholastic achievement.14 Lower psychometric intelligence scores in patients with MS than healthy controls have been reported for adult15,16 as well as for paediatric MS patients.2,7,17–19 Furthermore, both PS20 and WMC21 are well-established cognitive correlates of psychometric intelligence in healthy individuals. But this well-established relationship in healthy individuals does not allow for the conclusion that impaired PS and WMC lead to, or even are functionally related to, lower psychometric intelligence in MS patients, as suggested by Kail’s5 neural noise hypothesis or DeLuca et al.’s11 relative consequence model. In the present study, therefore, we tested empirically whether MS-related deficits in psychometric intelligence can be explained by deficits in PS and/or WMC. We used experimental RT and WM tasks with conditions of different complexity to investigate whether a potential explanation of MS-related deficits in psychometric intelligence by PS and/or WMC might depend on the complexity of the respective experimental task.
Methods
Participants
Twenty-four (20 females) paediatric patients with diagnosed MS according to the McDonald criteria22 were recruited from different hospitals in Germany. Due to technical problems, data from only 21 patients (17 females) could be analysed. Demographic characteristics are given in Table 1.
Table 1.
Number (N), mean (M) and standard deviation (SD) of sample characteristics of 21 children suffering from MS and 21 healthy controls. Also given are t tests (t) with effect size Cohen’s d as well as chi square tests (χ2) with effect size Cramér’s V for the comparison of both groups.
| Paediatric MS | Healthy controls | t/χ² (df) | d/V | |
|---|---|---|---|---|
| Age (in years) | M (SD) = 15.5 (1.8) | M (SD) = 15.8 (1.8) | t(40) = –0.598 | d = −.181 |
| Gender | χ²(1) = 0.000 | V = .000 | ||
| Female | N = 17 | N = 17 | ||
| Male | N = 4 | N = 4 | ||
| School | χ²(2) = 3.135 | V = .273 | ||
| Comprehensive school [Gesamtschule] | N = 1 | N = 1 | ||
| Secondary school [Realschule] | N = 8 | N =3 | ||
| High school [Gymnasium] | N = 12 | N =17 | ||
| IQ | M (SD) = 97.71 (8.24) | M (SD) = 111.57 (13.20) | t(40) = –4.081*** | d = −1.260 |
| Fatigue (MFIS) | M (SD) = 32.52 (17.22) | M (SD) = 23.10 (12.75) | t(40) = 2.017 | d = .820 |
| Depression (DIKJ) | M (SD) = 12.86 (5.74) | M (SD) = 13.67 (8.34) | t(40) = –0.366 | d = −.113 |
| Age at disease onset (in years) | M (SD) = 14.33 (1.79) | |||
| Disease duration (in months) | M (SD) = 18.23 (12.66) | |||
| Number of relapses | M (SD) = 2.58 (1.02) | |||
| Time between previous relapse and assessment (in months) | M (SD) = 4.81 (6.84) | |||
| Neurological disability (EDSS) | M (SD) = 1.55 (1.72) | |||
| Therapy | ||||
| Interferon | N = 18 | |||
| Glatiramer acetate | N = 2 | |||
| No therapy | N = 1 |
***p < 0.001 (two-tailed)
MFIS: Modified Fatigue Impact Scale; DIKJ: Depressionsinventar für Kinder und Jugendliche (engl. Depression Inventory for Children and Adolescents); EDSS: Expanded Disability Status Scale.
In addition, 66 healthy pupils were recruited from different local schools. Due to technical problems, only data from 63 pupils were available. Using the R package MatchIt with the nearest neighbour method,23 21 healthy controls were matched to the 21 MS patients according to gender, age, and type of school (aspired school graduation). The two groups did not differ significantly in age, gender and type of school (see Table 1).
All participants reported normal hearing and normal or corrected-to-normal vision. Prior to testing, all participants and all parents of adolescents younger than 18 years were informed about the study protocol and signed informed consent. The study was approved by the local ethical committee of the Witten/Herdecke University (Nr:173/2016).
Culture Fair Test 20-R (CFT20-R)
The German version of Cattell’s CFT20-R24 was administered as a reliable24 and valid25 measure of psychometric intelligence. This paper and pencil test consisted of two parts, and each part embraced the four subtests Series (continuing a series of elements according to a to-be-identified rule), Classifications (finding a matching figure due to specific features), Matrices (identifying the underlying rule and completing the matrix accordingly), and Topologies (identifying a formation of elements, which is topologically similar to a reference formation). Each of the subtests Series, Classifications and Matrices consisted of 15 items in the first part and of 12 items in the second part. The Subtest Topologies contained 11 items in the first and nine items in the second part.
In the first part, Series and Classifications had a time limit of 5 min, Matrices and Topologies of 4 min. In the second part, the time limit was 3 min for each subtest.
For each participant, the number of correctly answered items was determined and transformed into IQ equivalents according to age-specific norms reported in the manual.
Depression Inventory for Children and Adolescents (DIKJ)
Severity of depressive symptoms was assessed with the German DIKJ.26 The 29 items refer to the most important DSM-IV symptoms of depression. Internal consistency is high ranging between Cronbach’s α = 0.87 and α = 0.92.
Modified Fatigue Impact Scale (MFIS)
With the German version of the MFIS,27 patients with MS and healthy controls self-reported physical, cognitive and psychosocial impairments due to fatigue on a 5-point Likert scale ranging from 0 (not affected by fatigue) to 4 (strongly affected by fatigue). Internal consistency is α = 0.81.27 The dependent variable was the sum score of responses on the 21 items. One MS patient omitted one item and another one two items. The total scores of these two individuals were estimated by computing the mean score of the 19 or 20 answered items and multiplying this mean score by 21.28
Expanded Disability Status Scale (EDSS)
On the EDSS developed by Kurtzke,29 the attending doctors assessed the severity of disability in patients with MS at the time of the survey. EDSS scores could range from 0 to 10.
RT task
Apparatus and stimuli
Stimuli were presented on a Lenovo notebook (L540) with a 15” monitor. Stimulus presentation and response registration were controlled by the experimental software Eprime 2.0. Stimuli were arrows (>and<) and a fixation cross (+) with a height of 1 cm and a width of 1 cm. All stimuli were presented in white font (Courier New, size: 30) on a black background (see Figure 1).
Figure 1.
Examples for the simple RT condition (1), the choice RT condition (2), and the flanker RT condition (3) of the RT task.
RT: Reaction time.
Procedure
The RT task consisted of a simple, a choice, and a flanker RT condition. In the simple and in the choice RT condition, 32 trials were presented, respectively, and 64 trials in the flanker RT condition. Each trial began with a central fixation cross (+) presented for 500 ms followed by a stimulus, which remained on the screen until the participant’s response.
In the simple RT condition, participant pressed a designated key on a Cedrus® response pad (Model RB-40) with the forefinger of their preferred hand as soon as an arrow appeared on the screen – irrespective of the direction of the arrow. In the choice RT condition, the task was to respond to the direction of the arrow by pressing a left or right key.
In the flanker RT condition, adapted from Scheres et al.,30 five arrows were presented on each trial. The flanker arrows were either congruent (e.g. >>>>>) or incongruent (e.g. <<><<) with the middle arrow (see Figure 1). Direction of the middle arrow and congruence/incongruence of the flankers were randomized. The participants’ task was to respond to the direction of the middle arrow.
Mean RT for correctly responded trials was determined for each condition. Only trials with RTs between 100 ms and 2500 ms were used.
WM task
Apparatus and stimuli
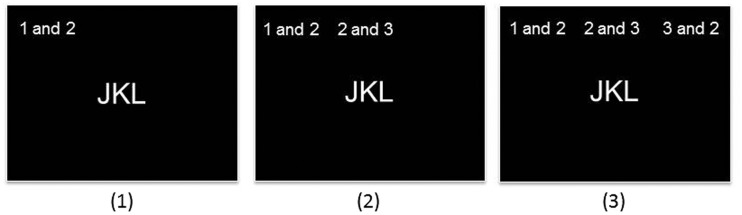
The WM task was adapted from Stankov and Crawford31 to measure WMC with three different levels of task demands. Stimuli were the letters J, K and L, presented centrally in Arial, point size 28, and the ‘swap’ commands at the top of the screen in Arial, point size 20 (see Figure 2). All stimuli were presented in white font on a black background.
Figure 2.
Examples of the 1-swap, 2-swaps, and 3-swaps condition in the WM task.
In the administered version the German ‘und’ was used instead of ‘and’.
WM: working memory.
Procedure
The three task conditions differed in the number of required swaps and, thereby, the number of interim results to be kept in mind. Each condition contained 12 trials. In each trial, the three letters J, K and L were presented in the centre of the monitor screen in different order. At the top of the screen, a command instructed the participant about the required swap(s) (e.g. ‘swap 1 and 2’). Participants had to mentally swap the letters according to the instruction and to type in the final solution via the keypad of the notebook with the forefinger of their preferred hand without time limitation. In the easiest condition only one swap had to be conducted (1-swap condition), but 2 and 3 swaps in the 2-swaps and 3-swaps condition, respectively. An example trial of the 2-swaps condition is given in panel 2 of Figure 2. In this example, the first instruction was to change the first and the second position of the given letters (J-K-L) resulting in an interim result of K-J-L, and to be kept in mind. The second instruction was to change the second and third position of the interim result leading to K-L-J as the final result, which had to be typed in. The trials of the three conditions were randomly interleaved. As dependent variable, hit rate (mean number of correct responses) was computed for each condition.
Procedure of the testing session
Prior to the experimental tasks, participants were informed about the study, signed the informed consent and completed the paper-pencil questionnaires. The experimental testing session started with the RT task (about 10 min) followed by the WM task (about 15 min) and the CFT20-R (about 60 min). All tasks were preceded by written and vocal instructions as well as practice trials. Between each task, participants had breaks of 3–5 min. The session ended with two other experimental tasks irrelevant for the present study and to be reported elsewhere. Each participant was tested individually. The total testing took about 120 min.
Statistical analyses
SPSS (23.0) was used for statistical analyses. To compare patients with MS and healthy controls regarding IQ, age and symptoms of fatigue and depression, t tests (effect size Cohen’s d) were computed. Nominal data (gender and type of school) were compared by means of χ2 tests (effect size Cramr’s V).
The RT and the WM task were analysed separately by two-way analyses of variance (ANOVA) with MS patients and healthy controls being two levels of a between-subjects factor ‘Group’ and the three conditions of the RT or the WM task as three levels of a repeated-measures factor ‘Condition’. RT in the RT task and hit rate in the WM task were the dependent variables, respectively. Effect sizes were computed as ηp2 and Bonferroni-adjusted post-hoc analyses determined the nature of significant effects.
To investigate whether differences in RT explained differences in IQ between patients with MS and healthy controls, a two-step procedure was used. In the first step, the IQ difference between the groups was investigated by a one-way ANOVA with the two groups as two levels of a between-subjects factor and IQ as the dependent variable. In a second step, an analysis of covariance (ANCOVA) was conducted by submitting RT as covariate to the previous ANOVA.
As a post-hoc analysis, we also investigated whether a differential pattern of results could be observed for the number of incorrectly solved items in the CFT20-R (as distinguished from the number of not-reached items). Therefore, the same two-step procedure of ANOVA and ANCOVA as described above for the IQ scores was repeated for the number of incorrectly solved items.
Results
Descriptive statistics of CFT20-R, DIKJ, and MFIS scores are presented in Table 1 separately for MS patients and healthy controls. DIKJ scores did not differ significantly between the two groups, while the tendency of higher MFIS scores in MS patients compared with healthy controls just failed to reach statistical significance, p = 0.050.
Most important for the purpose of the present study, MS patients' CFT20-R scores were significantly lower than those of healthy controls. CFT20-R scores in patients with MS correlated significantly and negatively with the number of relapses, r = –0.45, p = 0.038, and EDSS scores, r = –0.58, p = 0.006 but not with other characteristics of the disease (all p-values >0.153).
Descriptive statistics of RT in the RT task conditions are given in Table 2. For the two-way ANOVA Greenhouse–Geisser correction was used with ε = 0.657 because of violated sphericity. The main effect Condition yielded statistical significance, F(1.311, 52.443) = 129.116, p < 0.001, ηp2 = 0.763. Across both groups, simple RT, 302 ± 47 ms, was significantly shorter than choice RT, 407 ± 69 ms, and choice RT was significantly shorter than RT in the flanker condition, 590 ± 147 ms, all p-values <0.001. The main effect Group was also significant, F(1,40) = 8.854, p = 0.005, ηp2 = 0.181, indicating significantly longer overall mean RT in paediatric MS patients, 462 ± 79 ms, than healthy controls, 402 ± 44 ms. The interaction between Condition and Group was not statistically significant, F(1.311, 52.443) = 1.220, p = 0.290, ηp2 = 0.030.
Table 2.
Mean (M) and standard deviation (SD) of the reaction times (RTs) in the three RT task conditions, hit rates in the three working memory (WM) task conditions for 21 children with MS and 21 healthy controls as well as t tests (t) and Cohen’s d for the comparison of both groups.
|
Paediatric MS |
Healthy controls |
|||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | t(40) | d | |
| RT task condition | ||||||
| Simple Reaction [ms] | 322 | 47 | 282 | 39 | 2.997** | 0.925 |
| Choice Reaction [ms] | 430 | 75 | 384 | 57 | 2.208* | 0.681 |
| Flanker Condition [ms] | 636 | 177 | 544 | 92 | 2.101* | 0.648 |
| WM task condition | ||||||
| 1-Swap [hit rate] | 0.92 | 0.10 | 0.91 | 0.10 | 0.219 | 0.068 |
| 2-Swaps [hit rate] | 0.79 | 0.13 | 0.78 | 0.13 | 0.331 | 0.102 |
| 3-Swaps [hit rate] | 0.72 | 0.21 | 0.73 | 0.24 | –0.117 | –0.034 |
*p < 0.05; **p < 0.01 (two-tailed)
Hit rate: percentage of correctly responded trials; ms: milliseconds.
Table 2 contains descriptive statistics of hit rates in the WM task. The two-way ANOVA revealed a significant main effect Condition, F(2,80) = 20.382, p < 0.001, ηp2 = 0.338. Hit rate decreased significantly from the 1-swap, 0.912 ± 0.097, to the 2-swaps condition, 0.787 ± 0.129, p < 0.001, but only marginally from the 2-swaps to the 3-swaps condition, 0.725 ± 0.221, p = 0.053. Main effect Group, F(1,40) = 0.013, p = 0.911, ηp2 = 0.000, and the interaction effect were not statistically significant, F(2,80) = 0.067, p = 0.935, ηp2 = 0.002. Thus, regardless of task demands, WMC did not differ between patients with MS and healthy controls.
As WMC did not differ between the two groups, only PS was investigated as a possible source underlying the difference in psychometric intelligence between patients with MS and healthy controls. In accordance with the t test, the main effect Group in a one-way ANOVA on CFT20-R scores was statistically significant, F(1,40) = 16.658, p < 0.001, ηp2 =0.294. In a next step, mean RT across all three RT conditions was computed. Since it correlated significantly with normed CFT20-R scores, r = –0.463, p = 0.002, it was submitted to the ANOVA as covariate. The ANCOVA revealed that the difference between MS patients' and healthy controls' normed CFT20-R scores was still significant when controlled for RT, F(1,39) = 9.524, p = 0.004, ηp2 =0.196. The effect of mean RT yielded statistical significance, F(1,39) = 4.624, p = 0.038, ηp2 = 0.106. Effect size ηp2 of the main effect Group decreased from 0.294 in the ANOVA to ηp2 = 0.196 in the ANCOVA, indicating that about 33% of the variance in normed CFT20-R scores between MS patients and healthy controls was explained by RT differences.
A possible explanation of the finding that MS-related deficits in CFT20-R scores could be explained partially by PS is the time limitation of the CFT20-R, which forces speeded test performance and, thus, might disadvantage MS patients. We explored this idea by separately analysing the sum of incorrect items (processed but incorrect response) and of omitted and not-reached items. With mean ( ± SD) values of 5.857 ± 5.659 and 2.714 ± 4.911, the two groups did not differ significantly in the number of not-reached items, t(40) = 1.922, p = 0.062, d = 0.593. MS patients, however, gave significantly more incorrect responses (28.810 ±8.909) than healthy controls (21.810 ± 8.583), t(40) = 2.593, p = 0.013, d = 0.800. Mean RT was significantly positively related to the number of incorrect responses, r = 0.599, p <0.001, but not to the number of not-reached or omitted items, r = –0.017, p = 0.916.
The number of incorrectly solved CFT20-R items also differed significantly between MS patients and healthy controls in a one-way ANOVA, F(1,40) = 6.724, p = 0.013, ηp2 = 0.144. When RT was submitted to this analysis as covariate, the difference between the two groups was no longer significant, F(1,39) = 1.585, p = 0.216, ηp2 = 0.039. The effect of the covariate was significant, F(1,40) = 15.201, p <0.001, ηp2 = 0.280. A comparison of the effect sizes revealed that about 73% of the variance in the incorrect responses between MS patients and healthy controls could be explained by RT differences.
Discussion
In the present study, paediatric MS patients had lower CFT20-R scores and slower RTs but similar WMC as healthy controls. Most importantly, about 33% of the variance in psychometric intelligence between patients with MS and healthy controls was explained by MS patients’ slower PS.
Wuerfel et al.7 proposed that WM deficits in paediatric MS patients might only be observed when the WM task is sufficiently demanding. Here, however, we could not observe WMC differences between paediatric MS patients and healthy controls – irrespective of task demands. These results are consistent with previous studies, which reported no WMC deficits in adult MS patients when the WM task provided enough processing time, as in the present study.9–11 To note, our results indicate that WMC cannot account for the difference in CFT20-R performance between patients with MS and healthy controls. This does not question the important role of WMC for psychometric intelligence.21
Our finding of longer RTs in paediatric MS patients than healthy controls confirms previous reports of impaired PS in MS.2,6,7 Differences in RT ranging from 40 ms to 92 ms between paediatric MS patients and healthy controls do not appear very large. The effect sizes, however, indicate medium to large effects32 when contrasting between-group with intragroup variability (see Table 2) and are similar to those obtained with neuropsychological test batteries.6,7
Kail5,33 reported that PS differences between adult MS patients and healthy controls increased with increasing task demands. In the present study, the increasing task demands led to the expected increase of RT. However, although the difference in RT between MS patients and healthy controls nominally increased from the less to the most demanding task condition, this trend was not statistically significant.
The focus of the present study was on MS-related deficits in psychometric intelligence. Consistent with previous studies,2,7,17–19 paediatric MS patients had lower scores on an established measure of psychometric intelligence than healthy controls in the present study. The main result of this study is that these differences could be partially explained by MS patients’ slower PS.
The difference in intelligence scores was due to a higher number of incorrectly solved CFT20-R items in patients with MS than healthy controls. The number of omitted and unreached items did not differ between the two groups, so that time limitation of the CFT20-R is an unlikely explanation of the IQ differences. Furthermore, RT was associated with the number of incorrectly solved items, but not with the number of not-reached and omitted items. Thus, PS affected MS patients’ response quality rather than their speed of test taking.
Coyle34 proposed that (healthy) developmental changes during childhood in white matter integrity lead to faster and more consistent neural transmissions of information in the central nervous system. These developmental changes might be directly related to faster RT and less error-prone information processing, resulting in better performance on intelligence tests. This assumption is supported by Penke et al.,35 who found white matter integrity to be related to psychometric intelligence and that this relationship was completely mediated by PS.
MS leads to fundamental neural changes over time affecting white matter but also cortical and subcortical structures.5,33 Thus, a tentative explanation of the present findings is that MS-related impairments of white matter integrity result in slower and more error-prone processing of information. The reason for the higher number of mistakes in the CFT20-R in paediatric MS than healthy controls might be the more error-prone processing due to impaired white matter integrity. Impaired white matter integrity also leads to slower PS, and thereby constitutes a relationship between PS (as measured by RT) and processing accuracy (as measures by incorrectly solved items in the CFT20-R).
Limitations and implications
Our results are limited by the small sample size. Furthermore, mean IQ of healthy controls was quite high. The majority of MS patients (and, consequently, of healthy controls) attended schools with graduation providing access to universities and visited primarily by individuals with above average IQ scores.36 It cannot be ruled out, however, that individuals with a higher IQ were more likely to volunteer as healthy control in the present study. Thus, differences in psychometric intelligence between patients with MS and healthy controls have to be interpreted carefully.
The negative correlations between CFT20-R scores and the number of relapses as well as the EDSS scores indicated that MS had adverse effects on psychometric intelligence. Given the close association between psychometric intelligence and scholastic achievement,13 those potential deficits need attention in clinical practice, primarily in patients with continuing disease activity despite disease-modifying therapy.
PS as measured by RT seems to be a sensitive marker of deficits in the development of psychometric intelligence. However, further prospective and longitudinal studies on the association between RT and psychometric intelligence in paediatric MS in conjunction with more differentiated intelligence tests are recommended to confirm the value of RT as a measure of cognitive functioning in clinical settings of paediatric MS.
Contributor Information
Tugba Kapanci, Department of Psychology and Psychotherapy, University of Witten/Herdecke, Witten, Germany.
Kevin Rostásy, Pediatric Neurology, University of Witten/Herdecke, Children’s Hospital Datteln, Datteln, Germany.
Martin Georg Häusler, Division of Neuropediatrics and Social Pediatrics, Department of Pediatrics, University Hospital RWTH Aachen, Aachen, Germany.
Tobias Geis, Department of Pediatric Neurology, Klinik St. Hedwig, University Children’s Hospital Regensburg (KUNO), Regensburg, Germany.
Mareike Schimmel, Pediatric Neurology, University Children’s Hospital Augsburg, Augsburg, Germany.
Christiane Elpers, Children’s Hospital of the University Medical Center, University of Münster, Münster, Germany.
Jonas H. Kreth, Pediatric Neurology, Hospital for Children and Adolescents, gGmbH Klinikum Leverkusen, Leverkusen, Germany
Charlotte Thiels, Department of Pediatrics and Pediatric Neurology, Ruhr University Bochum, Bochum, Germany.
Stefan J Troche, Department of Psychology, University of Bern, Bern, Switzerland.
Conflict of Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
Author names: Tugba Kapanci, Kevin Rostásy, Martin Georg Häusler, Tobias Geis, Mareike Schimmel, Jonas H. Kreth, Charlotte Thiels, Stefan J. Troche
The author whose name is listed immediately below report the following details of affiliation or involvement in an organization or entity with a financial or non-financial interest in the subject matter or materials discussed in this manuscript.
Christiane Elpers: I declare to have financial and non-financial relations to following industrial companies: Bayer Health Care; Novartis Deutschland GmbH; Actelion Pharmaceuticals Ltd., Merck Serono GmbH, Biogen MA Inc.
Funding
The author(s) received no financial support for theresearch, authorship, and/or publication of this article.
ORCID iD
Stefan J Troche https://orcid.org/0000-0002-0961-1081
References
- 1.Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med 2018; 378: 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ekmekci O. Pediatric multiple sclerosis and cognition: A review of clinical, neuropsychologic, and neuroradiologic features. Behav Neurol. Epub ahead of print 25 December 2017. DOI: 10.1155/2017/1463570.: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genova HM, Sumowski JF, Chiaravalloti N, et al. Cognition in multiple sclerosis: A review of neuropsychological and fMRI research. Front Biosci 2009; 14: 1730–1744. [DOI] [PubMed] [Google Scholar]
- 4.Costa SL, Genova HM, DeLuca J, et al. Information processing speed in multiple sclerosis: Past, present, and future. Mult Scler 2017; 23: 772–789. [DOI] [PubMed] [Google Scholar]
- 5.Kail R. Speed of information processing in patients with multiple sclerosis. J Clin Exp Neuropsychol 1998; 20: 1–9. [DOI] [PubMed] [Google Scholar]
- 6.Till C, Ghassemi R, Aubert-Broche B, et al. MRI correlates of cognitive impairment in childhood-onset multiple sclerosis. Neuropsychology 2011; 25: 319–332. [DOI] [PubMed] [Google Scholar]
- 7.Wuerfel E, Weddige A, Hagmayer Y, et al. Cognitive deficits including executive functioning in relation to clinical parameters in paediatric MS patients. PloS One 2018; 13: e0194873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrouillet P, Bernardin S, Camos V. Time constraints and resource sharing in adults' working memory spans. J Exp Psychol Gen 2004; 133: 83–100. [DOI] [PubMed] [Google Scholar]
- 9.Demaree HA, DeLuca J, Gaudino EA, et al. Speed of information processing as a key deficit in multiple sclerosis: Implications for rehabilitation. J Neurol Neurosurg Psychiatry 1999; 67: 661–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lengenfelder J, Bryant D, Diamond BJ, et al. Processing speed interacts with working memory efficiency in multiple sclerosis. Arch Clin Neuropsychol 2006; 21: 229–238. [DOI] [PubMed] [Google Scholar]
- 11.DeLuca J, Chelune GJ, Tulsky DS, et al. Is speed of processing or working memory the primary information processing deficit in multiple sclerosis? J Clin Exp Neuropsychol 2004; 26: 550–562. [DOI] [PubMed] [Google Scholar]
- 12.Holland AA, Graves D, Greenberg BM, et al. Fatigue, emotional functioning, and executive dysfunction in pediatric multiple sclerosis. Child Neuropsychol 2014; 20: 71–85. [DOI] [PubMed] [Google Scholar]
- 13.Suppiej A, Cainelli E. Cognitive dysfunction in pediatric multiple sclerosis. Neuropsychiatr Dis Treat 2014; 10: 1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roth B, Becker N, Romeyke S, et al. Intelligence and school grades: A meta-analysis. Intelligence 2015; 53: 118–137. [Google Scholar]
- 15.Cortese M, Riise T, Bjørnevik K, et al. Preclinical disease activity in multiple sclerosis: A prospective study of cognitive performance prior to first symptom. Ann Neurol 2016; 80: 616–624. [DOI] [PubMed] [Google Scholar]
- 16.Leavitt VM, Lengenfelder J, Moore NB, et al. The relative contributions of processing speed and cognitive load to working memory accuracy in multiple sclerosis. J Clin Exp Neuropsychol 2011; 33: 580–586. [DOI] [PubMed] [Google Scholar]
- 17.Amato MP, Goretti B, Ghezzi A, et al. Cognitive and psychosocial features of childhood and juvenile MS. Neurology 2008; 70: 1891–1897. [DOI] [PubMed] [Google Scholar]
- 18.Banwell BL, Anderson PE. The cognitive burden of multiple sclerosis in children. Neurology 2005; 64: 891–894. [DOI] [PubMed] [Google Scholar]
- 19.MacAllister WS, Belman AL, Milazzo M, et al. Cognitive functioning in children and adolescents with multiple sclerosis. Neurology 2005; 64: 1422–1425. [DOI] [PubMed] [Google Scholar]
- 20.Jensen AR. Human Evolution, Behavior, and Intelligence. The g Factor: The Science of Mental Ability. Westport, CT, US: Praeger Publishers/Greenwood Publishing Group, 1998. [Google Scholar]
- 21.Oberauer K, Schulze R, Wilhelm O, et al. Working memory and intelligence – their correlation and their relation: Comment on Ackerman, Beier, and Boyle. Psychol Bull 2005; 131: 61–65. [DOI] [PubMed] [Google Scholar]
- 22.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 23.Randolph JJ, Falbe K, Manuel AK, et al. A step-by-step guide to propensity score matching in R. Pract Assess Res Eval 2014; 19: 1–6. [Google Scholar]
- 24.Weiß RH. CFT20-R – Grundintelligenztest Skala 2 [The Culture Fair Intelligence Test, Scale 2]. Göttingen, Germany: Hogrefe, 2006. [Google Scholar]
- 25.Johnson W, te Nijenhuis J, Bouchard TJ., Jr. Still just 1 g: Consistent results from five test batteries. Intelligence 2008; 36: 81–95. [Google Scholar]
- 26.Stiensmayer-Pelster J, Schürmann M, Duda K. Depressionsinventar für Kinder und Jugendliche (DIKJ). Handanweisung. Göttingen: Hogrefe, 1989. [Google Scholar]
- 27.Fisk JD, Ritvo PG, Ross L, et al. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis 1994; 18: 79–83. [DOI] [PubMed] [Google Scholar]
- 28.Schlomer GL, Baumann S, Card NA. Best practices for missing data management in counseling psychology. J Counsel Psychol 2010; 57: 1–10. [DOI] [PubMed] [Google Scholar]
- 29.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 30.Scheres A, Oosterlaan J, Swanson J, et al. The effect of methylphenidate on three forms of response inhibition in boys with AD/HD. J Abnorm Child Psychol 2003; 31: 105–120. [DOI] [PubMed] [Google Scholar]
- 31.Stankov L and, Crawford JD. Ingredients of complexity in fluid intelligence. Learn Indiv Diff 1993; 5: 73–111. [Google Scholar]
- 32.Cohen J. Statistical Power Analysis for the Behavioral Sciences (2nd ed). Hillsdale, N.J: L. Erlbaum Associates, 1988. [Google Scholar]
- 33.Kail R. The neural noise hypothesis: Evidence from processing speed in adults with multiple sclerosis. Aging Neuropsychol Cogn 1997; 4: 157–165. [Google Scholar]
- 34.Coyle TR. A differential-developmental model (DDM): Mental speed, attention lapses, and general intelligence (g). J Intell 2017; 5: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penke L, Muños Maniega S, Bastin ME, et al. Brain white matter tract integrity as a neural foundation for general intelligence. Mol Psychiatry 2012; 17: 1026–1030. [DOI] [PubMed] [Google Scholar]
- 36.Schneider W, Niklas F, Schmiedeler S. Intellectual development from early childhood to early adulthood: The impact of early IQ differences on stability and change over time. Learn Indiv Diff 2014; 32: 156–162. [Google Scholar]