Abstract
While a known link between prenatal cannabis exposure and anencephaly exists, the relationship of prenatal cannabis exposure with neural tube defects (NTDs) generally has not been defined. Published data from Canada Health and Statistics Canada were used to assess this relationship. Both cannabis use and NTDs were shown to follow an east-west and north-south gradient. Last year cannabis consumption was significantly associated (P < .0001; cannabis use–time interaction P < .0001). These results were confirmed when estimates of termination for anomaly were used. Canada Health population data allowed the calculation of an NTD odds ratio) of 1.27 (95% confidence interval = 1.19-1.37; P < 10−11) for high-risk provinces versus the remainder with an attributable fraction in exposed populations of 16.52% (95% confidence interval = 12.22-20.62). Data show a robust positive statistical association between cannabis consumption as both a qualitative and quantitative variable and NTDs on a background of declining NTD incidence. In the context of multiple mechanistic pathways these strong statistical findings implicate causal mechanisms.
Keywords: cannabinioids, anencephalus, spina bifida cystica, cannabis, neural tube defects
Introduction
Neural tube closure defects (NTDs) are usually understood to include lumbosacral myelomeningocele, anencephaly (including exencephaly, cranioraschisis, and acrania), and encephalocele together with a variety of rarer severe defects.1,2 They are responsible for an estimated 300 000 major congenital anomalies worldwide annually, and approximately 88 000 deaths and 8.6 million disability-adjusted life years across the globe.3 They are a major cause of death in small children under 5 years of age.
A number of risk factors have been identified for NTDs including maternal obesity, diabetes, especially poorly controlled insulin-dependent diabetes, vitamin B12 deficiency, some genetic anomalies of the folic acid metabolism pathway, and the use of some drugs.4-6 Gestational use of a variety of drugs has been associated with increased rates of NTDs including anticonvulsants (carbamazepine, phenytoin, valproic acid), antidepressants (sertraline, fluoxetine), mood stabilizers (lithium), and folic acid antagonists (methotrexate, trimethoprim).7,8 Hence, these drugs are relatively contraindicated during parturition and are only used in selected cases where it is felt that the benefits outweigh the known risks.
Dietary supplementation with folic acid has been shown to dramatically reduce the incidence of NTDs by up to 72% in 3 randomized controlled trials from the United States, China, and Europe.9-11 This has rightly been hailed as one of the major neonatal public health triumphs of our time and places NTDs fairly in the group of largely preventable disorders. Unfortunately, their rate continues to be very high in some of the less developed parts of the world, with rates exceeding 50 cases/10 000 live births in parts of India, Africa, the Middle East, and up to 200/10 000 in some of the northern regions of China.3,12,13
The practice of therapeutic early termination of pregnancy for anomaly (ETOPFA) has also been rising from 1980 to the present time in many places including the Canadian province of British Colombia,14 Sweden,15 Europe,16 the United States,17 and Western Australia18 and is thought to be the primary cause of the falling rates of NTDs in various registries.16-19 The issue is very important in relation to NTDs as ETOPFA rates of 80% have been reported for anencephaly, 46% for spina bifida, and 56% for encephalocoele in Canada in recent years.1 Therefore, epidemiological studies that consider data for live births may miss 80% to 90% of the true defect rate in some congenital anomaly registries.18
The incidence of NTDs in Canada as a function of births (= stillbirths plus live births but excluding ETOPFAs) dropped from 1989 to 2007 from a mean of 11.1 to 4.1/10 000 births.20,21
It is said to be difficult to differentiate the relative contribution of dietary folic acid supplementation compared with ETOPFA as an explanation for the falling rates of NTDs in Canada.20
Interestingly, several papers have been issued by the National Birth Defects Prevention Network (NBDPN) of the Centers for Disease Control and Prevention (CDC) Atlanta, Georgia, which have noted that the rate of anencephaly is almost doubled after cannabis exposure.22,23 It is well known that cannabis is the most commonly used illicit drug with 183 million individuals worldwide reporting its use.24-26 Indeed, 161 000 pregnant women in the United States reported cannabis use while pregnant,27-29 24% of pregnant Californian teenagers tested positive for cannabinoids,30 and indeed in 69% of cases cannabis dispensaries in Colorado recommend the use of cannabis to pregnant mothers.31 Cannabis use by females in Canada rose from 7% in 2013 to 10% in 2015.32 Canadian data show that 72% most of the cannabis users who have used cannabis in the last year have used it in the previous 3 months.33 All 4 of the National Cannabis Surveys conducted by Statistics Canada in 2018 show that cannabis is used overwhelmingly and disproportionately by young adults in the reproductive age group.33 Cannabis edibles are used by 41% of females in the preceding 3 months compared with only 26% of males, representing a 1.57-fold higher rate of use.32 Hence, cannabis use during pregnancy is a major public health issue. And as noted above, anencephalus is one of the classical NTDs. These data broaden the context of the cannabis-anencephalus link, which has been reported several times by the CDC from a closely constrained discussion of anencephalus alone and places it in the wider context of NTDs generally since they all share a similar pathophysiology in terms of the zipper-like closure of the neural tube during early fetal development.
This sets up an interesting dichotomy of population health forces in the child-bearing population with folic acid augmentation on the one hand tending to reduce NTDs and drug use on the other tending to increase them.
Canada forms a promising nation in which to study the public health implications of these issues as data pertaining to both NTD incidence and cannabis use has been made available. The Canadian government recently published large reviews of the national experience of NTDs and a number of surveys document drug use by the Canadian populace at the provincial level. Indeed, one survey suggests a 3-fold variation in cannabis use across Canadian provinces from 11% in Quebec to 33% in Nunavut.34 Significant variance in each parameter separately naturally raises the issue of the extent to which the observed variation in cannabis use accounts for the observed variation in NTD incidence. This forms an ideal opportunity to study these modern neonatal epidemiological-ecological trends at the associational level.
This study was performed to examine the relationship between cannabis use and NTD incidence to determine if the causal relationship previously identified by the National Birth Defects Prevention Network (NBDPN) of the CDC in relation to anencephaly22,23 could be identified in the national teratological profile of NTDs in Canada.
Methods
Data Sources
Data on neural tube defect rates excluding terminations for defects by province was taken from a Canadian government publication (Tables B3.2A, B3.2B, B3.2C, pages 106-107, in Public Health Agency of Canada, Health Canada35). Data for NTD rates by province including termination for defects for the same 3 periods was taken from Supplementary Table 2 in De Wals et al.1
Data on cannabis use were taken from 2 sources. The University of Waterloo performed a commissioned survey of Canadian provinces for Canada Health.36 Statistics Canada has also performed surveys of Canadian cannabis use by province and territory.34 The survey for the second quarter of 2018 had complete data for all Canadian provinces and territories.34
Cannabis Use Group Allocation
The 5 highest provinces in the University of Waterloo dataset were assigned to high cannabis states, namely, Newfoundland, Prince Edward Island, Nova Scotia, Manitoba, and Saskatchewan. Statistics Canada indicated that the provinces of Newfoundland, Nova Scotia, and Ontario were in the high-use group together with the territories of Nunavut, Northwest, and Yukon.
ETOPFA Estimation
The existence of a dataset for Canadian NTDs with ETOPFAs included1 and one without20 covering very similar time periods clearly provide an opportunity to derive the applicable ETOPFA rates by period from the difference between these 2 rates. The arithmetical details for these calculations are shown in Supplementary Table 1 (available online). From these data one is able to derive an average ETOPFA rate in each of the 3 periods. Since the data for Prince Edward Island is incomplete in Health Canada,20 this data point was omitted in calculating the average for the 3 periods.
Statistics
Data were processed in “R” v3.5.2 and “R Studio” v1.1.463 from the Central “R” Archive Network. Graphs and maps were drawn in “ggplot”. Data were log transformed to improve compliance with normality assumptions based on the results of the Shapiro-Wilks test. Risk ratios were calculated using the “epiR” package. P < .05 was considered significant.
Ethical Approval
Ethical approval for this study was received from the Human Research Ethics Committee (HREC) of the Southcity Medical Centre and the University of Western Australia. The approval from Southcity Medical Centre was dated May 31, 2018, and the approval from the University of Western Australia was dated April 1, 2019, and numbered RA/4/20/4724.
Results
A total of 3919 cases of NTDs were recorded from 1991 to 2007 among 6 092 250 live births in the Health Canada Reference report.35
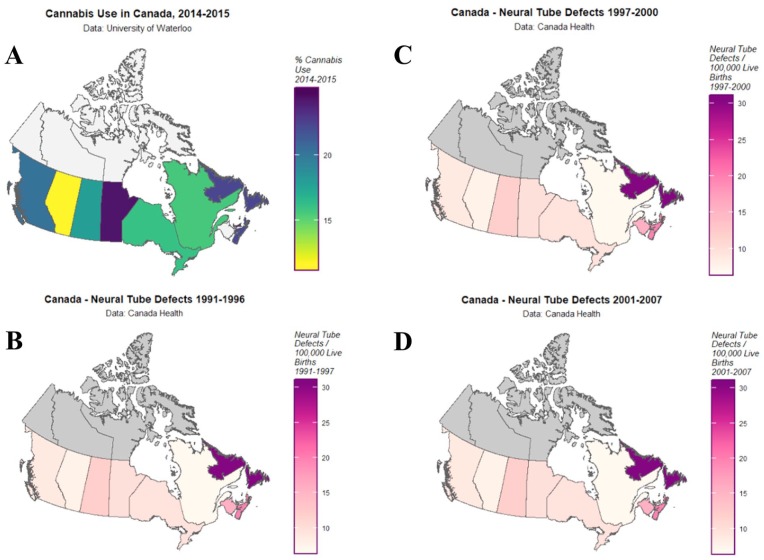
Folic acid augmentation into the grain staples in Canada commenced in 1997 and became mandatory in September 2000. Hence, the NTD incidence data across this period naturally falls into 3 periods: before, during, and after this transitional period. Figure 1 maps the distribution of cannabis use in 2015 and of NTDs in the 3 periods from 1991 to 1996, 1997 to 2000, and 2001 to 2007 across Canada.
Figure 1.
Maps of cannabis and neural tube defect (NTD) distribution. (A) Last year cannabis use rates by province, 2015. (B) NTD rates by province from 1991 to 1996. (C) NTD rates by province from 1997 to 2000. (D) NTD rates by province from 2001 to 2007.
One notes that these datasets relate to differing time periods. While this is an issue, survey data of cannabis use prevalence across Canada is very rare and this University of Waterloo survey is the earliest dataset we were able to identify. It is used here as we feel that due to spatiotemporal autocorrelation whatever cannabis use was at an earlier time period was related in some manner to cannabis use at this earliest documented period.
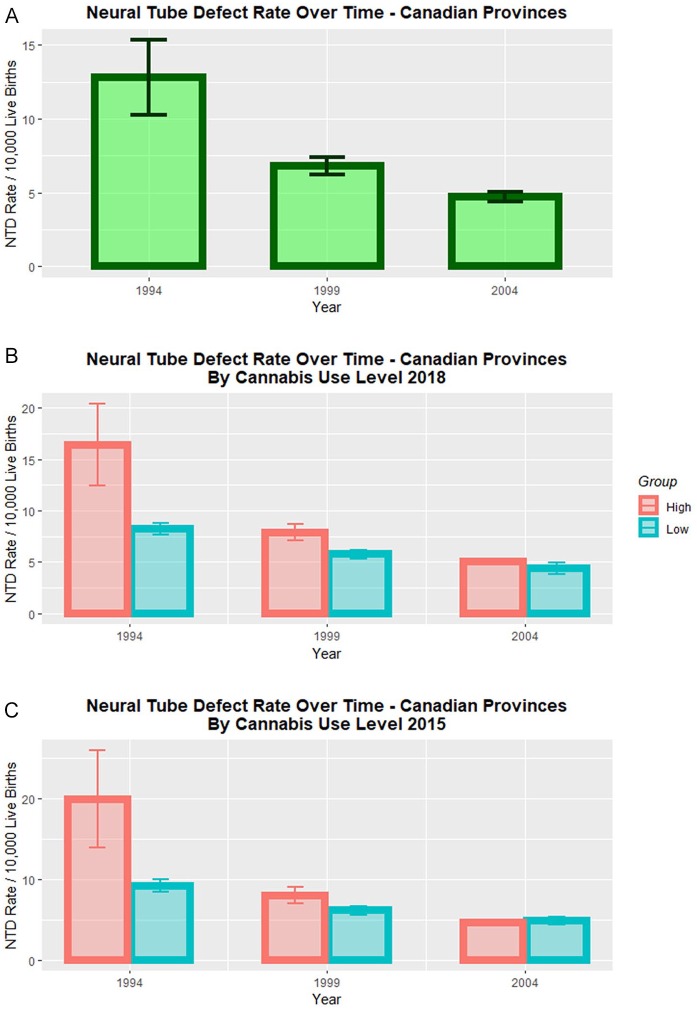
Figure 2A presents a scatterplot of the NTD rate by time. Data have been horizontally “jittered” to prevent overplotting, and data points are positioned about the midpoint of the 3 intervals: 1991 to 1996, 1997 to 2000, and 2001 to 2007. The highest levels of NTD s occurred in Newfoundland and Nova Scotia in the 1991 to 1996 period, with NTD rates of 30.5 and 19.8/10 000, respectively. The obvious downward trend over time is clear.
Figure 2.
Neural tube defect rates over time. (A) Neural tube defect rates over time overall data. (B) Neural tube defect rates over time by high and low cannabis use provinces (2015 data). (C) Neural tube defect rates over time by high and low cannabis use provinces (2018 data).
Figure 2B re-plots these data after dividing the provinces into high and low cannabis use areas ranked from the University of Waterloo survey of 2014-2015.36 A clear separation of the high and low cannabis use provinces is apparent. Figure 2C does the same thing following the assignment distribution of Statistics Canada for 2018.34 One notes in Figure 2B and C that the incidence of NTDs in both high- and low-prevalence provinces is similar in 2004. This appears to be due to a greater reduction in the high cannabis use provinces and territories than in the low prevalence areas; however, in the absence of accurate ETOPFA data one cannot be sure if an increase in ETOPFA practice might also have been implicated. Again Figure 2C shows a clear separation of the 2 regression lines. This is quantified in the Table 1 where cannabis use in 2015 is shown to be significant (P = .0063), and for cannabis use in 2018 both cannabis use itself, and cannabis use in interaction with time are shown to be highly significant (both P < .0001).
Table 1.
Linear Regressions of Live Born Neural Tube Defect (NTD) Rates.
| Input Parameters | Parameter Analyses |
Model Parameters |
||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | t Value | Pr(>|t|) | Adjusted R2 | F | df | P | |
| NTD rate by cannabis use by era | ||||||||
| Era 1 | ||||||||
| Cannabis use (annual) | 0.0509 | 0.0353 | 1.442 | .1925 | 0.1189 | 2.079 | 1.7 | .1925 |
| Era 2 | ||||||||
| Cannabis use (annual) | 0.0507 | 0.0096 | 5.293 | .0018 | 0.7942 | 28.02 | 1.6 | .0018 |
| Era 3 | ||||||||
| Cannabis use (annual) | 0.1365 | 0.0614 | 2.225 | .0678 | 0.3607 | 4.949 | 1.6 | .0678 |
| NTD rate by cannabis use by time and parameter | ||||||||
| Rate ~ year * group (2015) | ||||||||
| Year | −0.0897 | 0.0123 | −7.312 | .0000 | 0.6986 | 32.29 | 2.25 | 1.2E-07 |
| Group | 0.3174 | 0.1064 | 2.984 | .0063 | ||||
| Rate ~ year * cannabis use (annual) | ||||||||
| Year | −0.0826 | 0.0128 | −6.470 | .0000 | 0.6925 | 28.03 | 2.22 | 8.9E-07 |
| Cannabis use (annual) | 0.0447 | 0.0135 | 3.311 | .0032 | ||||
| Rate ~ year * cannabis use (month) | ||||||||
| Year | −0.0836 | 0.0152 | −5.506 | .0000 | 0.6369 | 19.41 | 2.19 | 2.6E-05 |
| Cannabis use (month) | 0.0449 | 0.0172 | 2.611 | .0172 | ||||
| Rate ~ year * age initiation | ||||||||
| Year: age initiation | −0.0060 | 0.0011 | −5.532 | .0000 | 0.5788 | 15.43 | 2.19 | .0001 |
| Age initiation | 11.8219 | 2.1569 | 5.481 | .0000 | ||||
| Rate ~ year * cannabis index | ||||||||
| Year | −0.0850 | 0.0140 | −6.060 | .0000 | 0.6714 | 22.46 | 2.19 | 9.9E-06 |
| Cannabis index | 0.5046 | 0.1998 | 2.526 | .0206 | ||||
| Rate ~ year * group (2018) | ||||||||
| Year | −0.1301 | 0.0213 | −6.108 | .0000 | 0.7122 | 23.27 | 3.24 | 2.8E-07 |
| Group | 110.5561 | 51.4954 | 2.147 | .0421 | ||||
| Year: group | −0.0552 | 0.0258 | −2.141 | .0426 | ||||
| Rate ~ year * cannabis use (3 months, 2018) | ||||||||
| Cannabis use (3 months) | 11.1933 | 1.5533 | 7.206 | .0000 | 0.6753 | 29.08 | 2.25 | 3.0E-07 |
| Year: cannabis use (3 months) | −0.0056 | 0.0008 | −7.179 | .0000 | ||||
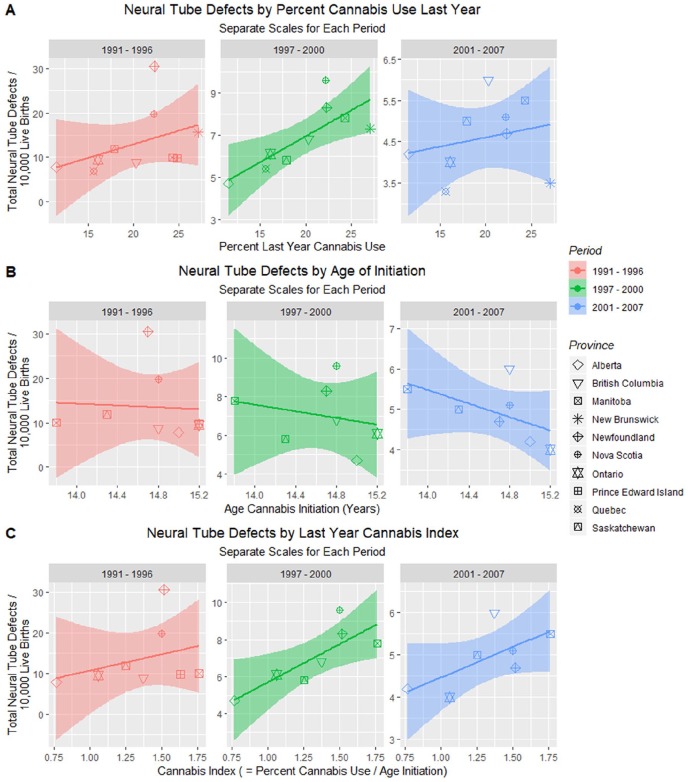
Figure 3 graphs the NTD rates against (1) last year cannabis use; (2) age of initiation of cannabis use; and (3) an index that is derived as the percent of cannabis use divided by the age of initiation for the University of Waterloo data.36 Close examination of panel A shows that the scales in each of the 3 graphs is falling, consistent with the drop in overall levels of NTDs by each period. In the middle panels it appears that rising age of initiation is associated with falling rates of NTDs; however, these changes are not significant. Results for the Cannabis Index parallel those for the percent cannabis use.
Figure 3.
Neural tube defect (NTD) rates by cannabis metrics. (A) NTD rate by last year cannabis use percentage. (B) NTD rates by age of cannabis initiation. (C) NTD rates by cannabis index (= last year cannabis use/age of initiation).
These various effects are quantified in Table 1. One notes that when the whole dataset is considered together there are many highly significant terms involving cannabis use relating to group assignment, last month and last year cannabis use, age of initiation, and the cannabis index all in 2015, and cannabis use in 2018 both as a qualitative variable by way of group assignment and as a quantitative continuous variable.
As noted above, Gilbert et al20 the Canadian provincial NTD data without ETOPFAs and1 lists the same dataset with ETOPFAs included. The difference in these 2 rates therefore logically represents the rate of ETOPFA for NTDs in the various listed Canadian provinces. Data from external sources indicate that the NTD rate has been relatively constant and stable in Canada prior to and after the transitional period of the introduction of folic acid.20,21 Supplementary Table 1 provides the arithmetical details of how these calculations were performed. Informatively one notes that the average ETOPFA rate in the 3 periods rose from 32.56% in the first period, to 35.05% and 43.74% in the second and third periods, respectively. These average rates were then used to estimate an NTD rate inclusive of ETOPFAs for Saskatchewan, Ontario, and New Brunswick, whose data were not provided.1
These estimates of the NTD rates including the ETOPFA rate were then regressed against cannabis use variables with the highly significant results (Table 2).
Table 2.
Linear Regressions of Total NTD Rates Including Estimated ETOPFA Rates.
| Input Parameters | Parameter Analyses |
Model Parameters |
||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SD | t Value | Pr(>|t|) | Adjusted R2 | F | df | P | |
| Rate ~ year * cannabis use, 2015 | ||||||||
| Cannabis use (annual) | 7.2497 | 1.4232 | 5.094 | .0000 | 0.5459 | 16.03 | 2.23 | 4.4E-05 |
| Year: cannabis use (annual) | −0.0036 | 0.0007 | −5.069 | .0000 | ||||
| Rate ~ year * age initiation | ||||||||
| Age of initiation | 10.0201 | 2.3703 | 4.227 | .0004 | 0.4210 | 9.00 | 2.20 | .0016 |
| Year: age of initiation | −0.0050 | 0.0012 | −4.242 | .0004 | ||||
| Rate ~ Year * cannabis index | ||||||||
| Cannabis index | 106.0551 | 22.5859 | 4.696 | .0001 | 0.5427 | 14.05 | 2.20 | .0002 |
| Year: cannabis index | −0.0528 | 0.0113 | −4.672 | .0001 | ||||
| Rate ~ year * cannabis use, 2018 | ||||||||
| Cannabis use (3 months) | 9.4836 | 1.6749 | 5.662 | .0000 | 0.5282 | 16.67 | 2.26 | 2.2E-05 |
| Year: cannabis use (3 months) | −0.0047 | 0.0008 | −5.650 | .0000 | ||||
Abbreviations: NTD, neural tube defect; ETOPFA, early termination of pregnancy for anomaly.
Data presented Public Health Agency of Canada, Health Canada,35 allow one to calculate the birth populations at risk. When populations were divided based on the University of Waterloo dataset it is calculated that 1096 of 1 423 104 births had NTDs in high-risk states and 2823 of 4 667 146 births in low cannabis using states had NTDs, which provides a point estimate for the odds ratio (OR) of 1.274 (95% confidence interval [CI] = 1.19-1.37, P = 9.19 × 10−12). This is equivalent to an attributable risk in the exposed population of 16.52% (95% CI = 12.22% to 20.62%). When provinces were assigned based on the Statistics Canada grouping, very similar results were obtained (OR = 1.25, 95% CI = 1.16-1.35, P = 2.47 × 10−9), attributable risk in exposed population of 16.35% (95% CI = 11.40% to 21.01%).
Discussion
This study shows that, notwithstanding the overall positive response of the maternal population to folic acid supplementation there are statistical associational signals within the Canadian perinatal teratological data on NTDs indicating a positive relationship to cannabis use. Highly statistically significant relationships between NTD incidence both in relation to birth defect rates, and also after correction for estimates of the effect of early abortions for defects were studied, and the relationship remained robust to various categorization protocols, and various metrics of cannabis use.
An OR of 1.27 (95% CI = 1.19-1.37) was calculated for high compared with low cannabis use provinces with an attributable risk in high cannabis use provinces of 16.52% (95% CI = 12.22% to 20.62%).
As such it would appear from this analysis that the different patterns in cannabis consumption across Canada may well explain the east-west gradient of NTDs, which has long been known and has long been of interest to neonatal epidemiologists.
Calculations performed in the present work indicate that the rate of ETOPFA across Canada increased between 1991 and 2007 from a mean of 32.3% to 43.2%. The detailed analysis of NTD rates in Canada of De Wals et al1 included ETOPFA data and showed that a reduction in NTD rate could be demonstrated when ETOPFA data were included. However, Gilbert and colleagues,20 writing several years later, presumably with access to much of the data of the earlier study, felt that they could not say with any certainty how much of the decline in NTD rates were due to rising ETOPFA rates and how much was due to folate supplementation. The present work does not have access to sufficient data to comment on this interesting dilemma.
The greatest drop in Canada NTD rates occurred in high-risk provinces after folate supplementation.1 In Australia this also happened and a dramatic drop in indigenous Australian Aborigines was also documented following folate supplementation.37
Beyond the fascinating pattern of epidemiology, which has been uncovered by the above statistical calculations, one of the most intriguing aspects of this study is the obvious gaps in the data in relation to the Canadian territories. The maps in Figure 1 clearly show that the data tabled provide no data whatsoever for the territories of Nunavut, Yukon, and Northwest and these fascinating circumpolar parts of arctic Canada remain undefined in this regard.
Other reports define high rates of total congenital anomalies, congenital heart disease, Down’s syndrome, orofacial clefts, and gastroschisis in Nunavut, most of which are statistically significantly elevated above the national rates,35 together with high rates of premature birth, infant mortality, and post-neonatal death elevated 3 times and more above the national average.21 One might realistically conjecture, therefore, that when the numbers become available high rates of NTD may also be shown in such remote northern regions. Nunavut area is also described as having very high rates of chronic hunger, homelessness, poverty, violent crime, suicide, substance abuse, and sexually transmissible disease.38 Clearly untangling this complex etiological web of potential causes in the challenging climate of the arctic north among a scattered population is a task that would require considerable resources and dedication.
In considering this bewildering array of complex psychosocial adversity, the present studies would strongly suggest that cannabis should not be forgotten as a potential cause. Indeed it is reported that the use of cannabis by many adult age groups is virtually holoendemic in this region.39 It would seem to us that filling in these datasets would have far-reaching implications potentially well beyond those relating to circumpolar health.
Given robust previous results from leading US schools of public health linking increasingly liberal legislative paradigms with increased cannabis use,40 the present findings must raise concerns that increased cannabis use in Canada related to cannabis legalization in that nation may be shown in time to be associated with unforeseen trends in NTDs among other congenital defects.
While the effect demonstrated in this article is at the level of a statistical association it achieves considerable importance in light of the numerous documented mechanistic pathways linking cannabis with multiple mechanisms of teratogenesis. Space precludes a detailed exploration of this subject in this forum, but the following comments may form a useful point of departure for future workers. It is known that NTDs represent failure of closure of the embryonic neural tube usually at its upper and lower ends. Therefore, pathways that impede cell growth may be implicated. Cannabis has been shown on proteomic screen to interfere with actin and tubulin synthesis.41 Actin is a key molecule for the cytoskeleton involved in cell signaling and cell shape change in cell division and cell migration. Tubulin is the monomer from which microtubules form, which form the rails of cell division. Cannabis has long been known to test positive in the micronuclear assay and this is believed to be on the basis of its interference with microtubular function.42-44 Cannabis also perturbs notch signaling, which is a key morphogen for both embryonic neuraxis and cardiovascular formation.45,46
Closure of the neural tube initiates at the level of the human hindbrain on post-fertilization day 22 and proceeds bidirectionally cephalad and caudal. It also initiates from the rostral neuropore bidirectionally. Closure occurs at the anterior neuropore on day 24 and over the sacrum on day 26.2 Over 200 genes involved in pathways such as cytoskeletal regulation, cell proliferation, transcriptional control, one carbon transfer, epigenetic regulation, and interference with sphingosine phosphate metabolism (by the fungal metabolite fumonisin) have been implicated.2 Molecular signaling pathways including hedgehog, bone morphogenetic proteins, and retinoid signaling have also been implicated.2 Interestingly, cannabinoids interact with and inhibit each of these major pathways with cannabinoid-hedgehog,47 cannabinoid-bone morphogenetic proteins (both directly and by vanilloid mediation),48 and cannabinoid-retinoid interactions49-52 having been previously described.
Cannabis adversely affects robo/slit signaling, which is a key receptor ligand pair that has major dual actions in brain and nerve formation.53 Low levels of robo are key to the hyperproliferation of subventricular zone embryonic neuroblasts and formation of the large human neocortex.54,55 And robo/slit pairs also form key guidance signals for both axon guidance and vascular sprouting and direction.46 Slit2 is also a tumor suppressor.56 Cannabis interferes with neurexin and neuroligin synthesis and signaling this receptor ligand pair forms the basic scaffold of the neuronal synapse and plays a key role in synaptic formation and organization.57,58 Cannabinoids have numerous immune interactions and immune activity is a key sculptor of the embryonic and developing neuraxis.59 Cannabis has widespread epigenetic actions with effects particularly on the brain, immune systems, and sperm.60-64 Interestingly, folic acid also works epigenetically by acting as a methyl donor in N-methylation reactions for DNA and histones via S-adenosyl-methionine.65 Cannabis acts via at least 7 receptors in the body.59 The type 1 cannabinoid receptor (CB1R) is widely distributed in many tissues including brain from very early in fetal life and is thus likely to have downstream consequences.66,67 Cannabinoid signaling via CB1Rs is a key regulator of cerebral microvascular function and is directly responsible for the BOLD signal seen on functional magnetic resonance imaging with neuronal activity.68 The brain’s microvasculature forms a key regulator of brain neurogenic niches.67,69-71 Brain formation and circuit wiring is highly dependent on neuronal activity. In that cannabis is well known to suppress neuronal firing, this implies that it will have major morphological and long-term developmental consequences. And CB1Rs are also found at moderate density in bone.72,73
Cannabinoids are also highly toxic to eggs and spermatids.74-78 Both ova and mature spermatids lack most of the molecular genetic machinery to repair DNA damage. The very real possibility exists therefore that damage to nonrenewable ova may be long term or even permanent.
Interestingly, prenatal cannabis exposure was recently shown to affect the methylation status of 4 genes involved in Wnt signaling, which is a major body morphogen, namely, 3A, 5A, 9A, and 10A.63 Wnt signaling has been shown to control closure of the anterior neuropore,79 a finding which may relate directly to the elevated risk of encephalocoele which has been linked epidemiologically with prenatal cannabis use.80
In summary, while it is often said that cannabis is a “natural product,” the reality is that it has been well detmonstrated in botanical science that cannabinoids form part of a potent plant defense mechanism against both other plants—including other cannabis plants—and animals.81,82 That is to say cannabinoids are in fact a natural plant poison.
The strengths of this study are that it takes national and publicly available data from 2 leading sources, uses 2 metrics of cannabis consumption and 2 categorization protocols to conduct a secondary analysis of national data at the provincial level. The analyses are simple and straightforward and are clearly in close accordance with the demonstrated graphical and map displays. Many of the probability levels reported are at high or very high levels of statistical significance.
Most of the limitations of this study relate to its design as an ecological secondary analysis. Individual case-control data were not available to this study. Territory congenital anomaly and covariate data are not yet publicly available for Canada, and that is likely to be some of the most important data of all. It has not been possible here to take into account any of the covariates such as race, diet, and education that might be studied in a larger investigation as these data were not available for our analysis. Given that much of the north of Canada is very cold and dark most of the year, dietary factors may be very important, as may genetic allele frequencies of native and indigenous people groups. From a methodological perspective, it is important to note that to simply add in covariates such as ethnicity, in the presence of well-documented and adequately substantiated major racial differences in the prevalence of drug use, is to make an opposite error of over-controlling and in fact erroneously regressing out important differences. In this regard, advanced statistical techniques such as generalized 2-stage regression with appropriate instrumental variables may be a more versatile tool. Ideally geospatiotemporal models at high geographic resolution, which take into account all of these various factors using appropriate adjustment and advanced statistical methods, may be most appropriate for future investigations once a sufficient dataset can be assembled. ETOPFA data were not available longitudinally, which would be preferable to conduct a formal study. Notwithstanding this we see our work as important and path finding and showing the way for future more detailed and more complex studies both in Canada and internationally.
Conclusion
The epidemiological relationship that we have demonstrated between cannabis use and NTD incidence within the context of falling overall NTD rates is interesting, provocative, and intriguing. The ecological association is seen in both live born statistics and also in estimates of the complete dataset including ETOPFA data, which are important to complete the holistic picture of the true epidemiological incidence of NTDs; it is seen with 2 metrics of cannabis use and with 2 categorization algorithms for classifying the provinces. Our work is consistent with earlier reports from the CDC in the United States relating to the links between cannabis use and the doubled incidence of anencephaly. In the context of multiple known molecular mechanistic pathways such compelling and robust statistical associations necessarily implicate causality. We would be keen to see this relationship studied in other places along with the all-important applicable ETOPFA and covariate data preferably in case-control designs. The arctic regions of Canada are almost certainly of particular importance to the neonatal epidemiology of NTDs with far-reaching and likely global implications and require further detailed investigation.
Research Data
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
Canada_NTD_Data_-_Export for Cannabis Consumption Patterns Explain the East-West Gradient in Canadian Neural Tube Defect Incidence: An Ecological Study by Albert Stuart Reece and Gary Kenneth Hulse in Global Pediatric Health
Supplemental Material
Supplemental material, Supplementary_Table_1 for Cannabis Consumption Patterns Explain the East-West Gradient in Canadian Neural Tube Defect Incidence: An Ecological Study by Albert Stuart Reece and Gary Kenneth Hulse in Global Pediatric Health
Footnotes
Author Contributions: ASR: Contributed to conception and design; contributed to acquisition, analysis, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
GKH: Contributed to conception; contributed to interpretation; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Albert Stuart Reece  https://orcid.org/0000-0002-3256-720X
https://orcid.org/0000-0002-3256-720X
Supplementary Material: Supplemental material for this article is available online.
References
- 1. De Wals P, Tairou F, Van Allen MI, et al. Reduction in neural-tube defects after folic acid fortification in Canada. N Engl J Med. 2007;357:135-142. [DOI] [PubMed] [Google Scholar]
- 2. Copp AJ, Adzick NS, Chitty LS, Fletcher JM, Holmbeck GN, Shaw GM. Spina bifida. Nat Rev Dis Primers. 2015;1:15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zaganjor I, Sekkarie A, Tsang BL, et al. Describing the prevalence of neural tube defects worldwide: a systematic literature review. PLoS One. 2016;11:e0151586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shaw GM, Quach T, Nelson V, et al. Neural tube defects associated with maternal periconceptional dietary intake of simple sugars and glycemic index. Am J Clin Nutr. 2003;78:972-978. [DOI] [PubMed] [Google Scholar]
- 5. Shaw GM, Todoroff K, Velie EM, Lammer EJ. Maternal illness, including fever and medication use as risk factors for neural tube defects. Teratology. 1998;57:1-7. [DOI] [PubMed] [Google Scholar]
- 6. Yazdy MM, Liu S, Mitchell AA, Werler MM. Maternal dietary glycemic intake and the risk of neural tube defects. Am J Epidemiol. 2010;171:407-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hernandez-Diaz S, Werler MM, Walker AM, Mitchell AA. Neural tube defects in relation to use of folic acid antagonists during pregnancy. Am J Epidemiol. 2001;153:961-968. [DOI] [PubMed] [Google Scholar]
- 8. Lambie DG, Johnson RH. Drugs and folate metabolism. Drugs. 1985;30:145-155. [DOI] [PubMed] [Google Scholar]
- 9. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991;338:131-137. [PubMed] [Google Scholar]
- 10. Berry RJ, Li Z, Erickson JD, et al. Prevention of neural-tube defects with folic acid in China. China-US collaborative project for neural tube defect prevention. N Engl J Med. 1999;341:1485-1490. [DOI] [PubMed] [Google Scholar]
- 11. Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832-1835. [DOI] [PubMed] [Google Scholar]
- 12. Blencowe H, Kancherla V, Moorthie S, Darlison MW, Modell B. Estimates of global and regional prevalence of neural tube defects for 2015: a systematic analysis. Ann N Y Acad Sci. 2018;1414:31-46. [DOI] [PubMed] [Google Scholar]
- 13. Kancherla V, Black RE. Historical perspective on folic acid and challenges in estimating global prevalence of neural tube defects. Ann N Y Acad Sci. 2018;1414:20-30. [DOI] [PubMed] [Google Scholar]
- 14. Van Allen MI, Boyle E, Thiessen P, et al. The impact of prenatal diagnosis on neural tube defect (NTD) pregnancy versus birth incidence in British Columbia. J Appl Genet. 2006;47:151-158. [DOI] [PubMed] [Google Scholar]
- 15. Nikkila A, Rydhstrom H, Kallen B. The incidence of spina bifida in Sweden 1973-2003: the effect of prenatal diagnosis. Eur J Public Health. 2006;16:660-662. [DOI] [PubMed] [Google Scholar]
- 16. Prevalence of neural tube defects in 20 regions of Europe and the impact of prenatal diagnosis, 1980-1986. EUROCAT Working Group. J Epidemiol Community Health. 1991;45:52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cragan JD, Roberts HE, Edmonds LD, et al. Surveillance for anencephaly and spina bifida and the impact of prenatal diagnosis—United States, 1985-1994. MMWR CDC Surveill Summ. 1995;44:1-13. [PubMed] [Google Scholar]
- 18. Bower C, Raymond M, Lumley J, Bury G. Trends in neural tube defects 1980-1989. Med J Aust. 1993;158:152-154. [DOI] [PubMed] [Google Scholar]
- 19. Rosano A, Smithells D, Cacciani L, et al. Time trends in neural tube defects prevalence in relation to preventive strategies: an international study. J Epidemiol Community Health. 1999;53:630-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gilbert NL., De Wals P, Leon JA, Evans JA. Congenital Anomalies in Canada 2013: Chapter 3: Neural Tube Defects. Vol 1 Ottawa, Ontario, Canada: Public Health Agency of Canada; 2013:119. [Google Scholar]
- 21. Health Canada. A Perinatal Health Report, 2002. Vol 1 Ottawa, Ontario, Canada: Health Canada; 2002:1-87. [Google Scholar]
- 22. Van Gelder MMHJ, Donders ART, Devine O, Roeleveld N, Reefhuis J; National Birth Defects Prevention Study. Using Bayesian models to assess the effects of under-reporting of cannabis use on the association with birth defects, national birth defects prevention study, 1997-2005. Paediatr Perinat Epidemiol. 2014;28:424-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Gelder MMHJ, Reefhuis J, Caton AR, Werler MM, Druschel CM, Roeleveld N. Maternal periconceptional illicit drug use and the risk of congenital malformations. Epidemiology. 2009;20:60-66. [DOI] [PubMed] [Google Scholar]
- 24. Degenhardt L, Ferrari AJ, Calabria B, et al. The global epidemiology and contribution of cannabis use and dependence to the global burden of disease: results from the GBD 2010 study. PLoS One. 2013;8:e76635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575-1586. [DOI] [PubMed] [Google Scholar]
- 26. Whiteford HA, Ferrari AJ, Degenhardt L, Feigin V, Vos T. The global burden of mental, neurological and substance use disorders: an analysis from the Global Burden of Disease Study 2010. PLoS One. 2015;10:e0116820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCantz-Katz E. 2017 Annual Report Snippets, NSDUH, SAMHSA, USA DHHS—Selected Streamlined Trends. Vol 1 Rockville, MD: Substance Abuse and Mental Health Services; 2018. [Google Scholar]
- 28. McCantz-Katz E. Urgent and emerging issues in prevention: marijuana, kratom and E-cigarettes. https://www.samhsa.gov/sites/default/files/samhsas_15th_annual_prevention_day_afternoon_plenary_recording.pdf. Accessed November 28, 2019.
- 29. Substance Abuse and Mental Health Services Administration. National Survey on Drug Use and Health (NSDUH). https://www.samhsa.gov/data/data-we-collect/nsduh-national-survey-drug-use-and-health. Accessed November 28, 2019.
- 30. Young-Wolff KC, Tucker L, Alexeeff S, et al. Trends in self-reported and biochemically tested marijuana use among pregnant females in California from 2009-2016. JAMA. 2017;318:2490-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dickson B, Mansfield C, Guiahi M, et al. Recommendations from cannabis dispensaries about first-trimester cannabis use. Obstet Gynecol. 2018;131:1031-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Government of Canada. Canadian tobacco alcohol and drugs (CTADS): 2015 summary. https://www.canada.ca/en/health-canada/services/canadian-tobacco-alcohol-drugs-survey/2015-summary.html. Accessed November 28, 2019.
- 33. Statistics of Canada. Cannabis stats hub. https://www150.statcan.gc.ca/n1/pub/13-610-x/cannabis-eng.htm. Accessed November 28, 2019.
- 34. Statistics Canada. National Cannabis Survey, Second Quarter, 2018. https://www.facebook.com/StatisticsCanada/posts/1636405843137586:0. Accessed November 28, 2019.
- 35. Public Health Agency of Canada, Health Canada. Congenital anomalies in Canada, 2013: a perinatal health surveillance report. https://www.canada.ca/en/public-health/services/health-promotion/what-is-ccasn/congenital-anomalies-canada-2013-a-perinatal-health-surveillance-report.html. Accessed November 28, 2019.
- 36. Leos-Toro C, Reid JL, Madill CL, Rynard VL, Manske SR, Hammond D. Tobacco use in Canada: patterns and trends. Special supplement: cannabis in Canada. https://uwaterloo.ca/tobacco-use-canada/sites/ca.tobacco-use-canada/files/uploads/files/cannabissupplement_2017_final_accessible.pdf. Accessed November 28, 2019.
- 37. Hilder L. Neural tube defects in Australia, 2007-2011: before and after implementation of mandatory folic acid fortification standard. https://npesu.unsw.edu.au/sites/default/files/npesu/surveillances/NTD%20Australia%200711_1.pdf. Accessed November 28, 2019.
- 38. Kilpatrick R. A primer on Nunavut, 5th edition. https://www.nunavutcourts.ca/phocadownload/EN/Primer/PrimerNunavut2015-2016.pdf. Accessed November 28, 2019.
- 39. Nunatsiaq News. Cannabis regulation: Nunavut must take its time. http://old.nunatsiaq.com/stories/article/65674cannabis_regulation_nunavut_must_take_its_time/. Published April 19, 2017. Accessed November 28, 2019.
- 40. Hasin DS, Sarvet al, Cerda M, et al. US adult illicit cannabis use, cannabis use disorder, and medical marijuana laws: 1991-1992 to 2012-2013. JAMA Psychiatry. 2017;74:579-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang J, Yuan W, Li MD. Genes and pathways co-associated with the exposure to multiple drugs of abuse, including alcohol, amphetamine/methamphetamine, cocaine, marijuana, morphine, and/or nicotine: a review of proteomics analyses. Mol Neurobiol. 2011;44:269-286. [DOI] [PubMed] [Google Scholar]
- 42. Piatti E, Rizzi R, Re F, Chiesara E. Genotoxicity of heroin and cannabinoids in humans. Pharmacol Res. 1989;21(suppl 1):59-60. [DOI] [PubMed] [Google Scholar]
- 43. Reece AS, Hulse GK. Chromothripsis and epigenomics complete causality criteria for cannabis- and addiction-connected carcinogenicity, congenital toxicity and heritable genotoxicity. Mutat Res. 2016;789:15-25. [DOI] [PubMed] [Google Scholar]
- 44. Van Went GF. Mutagenicity testing of 3 hallucinogens: LSD, psilocybin and delta 9-THC, using the micronucleus test. Experientia. 1978;34:324-325. [DOI] [PubMed] [Google Scholar]
- 45. Tanveer R, Gowran A, Noonan J, Keating SE, Bowie AG, Campbell VA. The endocannabinoid, anandamide, augments Notch-1 signaling in cultured cortical neurons exposed to amyloid-beta and in the cortex of aged rats. J Biol Chem. 2012;287:34709-34721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carlson BM. Human Embryology and Developmental Biology. Philadelphia, PA: Elsevier; 2014. [Google Scholar]
- 47. Khaliullina H, Bilgin M, Sampaio JL, Shevchenko A, Eaton S. Endocannabinoids are conserved inhibitors of the Hedgehog pathway. Proc Natl Acad Sci U S A. 2015;112:3415-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Birerdinc A, Jarrar M, Stotish T, Randhawa M, Baranova A. Manipulating molecular switches in brown adipocytes and their precursors: a therapeutic potential. Prog Lipid Res. 2013;52:51-61. [DOI] [PubMed] [Google Scholar]
- 49. Jung KM, Astarita G, Thongkham D, Piomelli D. Diacylglycerol lipase-alpha and -beta control neurite outgrowth in neuro-2a cells through distinct molecular mechanisms. Mol Pharmacol. 2011;80:60-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee YS, Jeong WI. Retinoic acids and hepatic stellate cells in liver disease. J Gastroenterol Hepatol. 2012;27(suppl 2):75-79. [DOI] [PubMed] [Google Scholar]
- 51. Mukhopadhyay B, Liu J, Osei-Hyiaman D, et al. Transcriptional regulation of cannabinoid receptor-1 expression in the liver by retinoic acid acting via retinoic acid receptor-gamma. J Biol Chem. 2010;285:19002-19011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fraher D, Ellis MK, Morrison S, et al. Lipid abundance in zebrafish embryos is regulated by complementary actions of the endocannabinoid system and retinoic acid pathway. Endocrinology. 2015;156:3596-3609. [DOI] [PubMed] [Google Scholar]
- 53. Alpar A, Tortoriello G, Calvigioni D, et al. Endocannabinoids modulate cortical development by configuring Slit2/Robo1 signalling. Nat Commun. 2014;5:4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Borrell V, Cardenas A, Ciceri G, et al. Slit/Robo signaling modulates the proliferation of central nervous system progenitors. Neuron. 2012;76:338-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cardenas A, Villalba A, de Juan Romero C, et al. Evolution of cortical neurogenesis in amniotes controlled by robo signaling levels. Cell. 2018;174:590-606.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vaz M, Hwang SY, Kagiampakis I, et al. Chronic cigarette smoke-induced epigenomic changes precede sensitization of bronchial epithelial cells to single-step transformation by KRAS mutations. Cancer Cell. 2017;32:360-376.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Anderson GR, Aoto J, Tabuchi K, et al. beta-neurexins control neural circuits by regulating synaptic endocannabinoid signaling. Cell. 2015;162:593-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang H. Endocannabinoid mediates excitatory synaptic function of β-neurexins. commentary: β-neurexins control neural circuits by regulating synaptic endocannabinoid signaling. Front Neurosci. 2016;10:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cutando L, Maldonado R, Ozaita A. Microglial activation and cannabis exposure. In: Preedy V, ed. Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis and Treatment. New York, NY: Academic Press; 2017:401-412. [Google Scholar]
- 60. Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93-109. [DOI] [PubMed] [Google Scholar]
- 61. Carpentier PA, Palmer TD. Immune influence on adult neural stem cell regulation and function. Neuron. 2009;64:79-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. DiNieri JA, Wang X, Szutorisz H, et al. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol Psychiatry. 2011;70:763-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Murphy SK, Itchon-Ramos N, Visco Z, et al. Cannabinoid exposure and altered DNA methylation in rat and human sperm. Epigenetics. 2018;13:1208-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zumbrun EE, Sido JM, Nagarkatti PS, Nagarkatti M. Epigenetic regulation of immunological alterations following prenatal exposure to marijuana cannabinoids and its long term consequences in offspring. J Neuroimmune Pharmacol. 2015;10:245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chakraborty AA, Laukka T, Myllykoski M, et al. Histone demethylase KDM6A directly senses oxygen to control chromatin and cell fate. Science. 2019;363:1217-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Romero J, Garcia-Palomero E, Berrendero F, et al. Atypical location of cannabinoid receptors in white matter areas during rat brain development. Synapse. 1997;26:317-323. [DOI] [PubMed] [Google Scholar]
- 67. Diaz-Alonso J, Guzman M, Galve-Roperh I. Endocannabinoids via CB(1) receptors act as neurogenic niche cues during cortical development. Philos Trans R Soc Lond B Biol Sci. 2012;367:3229-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Benyo Z, Ruisanchez E, Leszl-Ishiguro M, Sandor P, Pacher P. Endocannabinoids in cerebrovascular regulation. Am J Physiol Heart Circ Physiol. 2016;310:H785-H801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Aguado T, Monory K, Palazuelos J, et al. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 2005;19:1704-1706. [DOI] [PubMed] [Google Scholar]
- 70. Noonan MA, Eisch AJ. Regulation of adult neurogenesis by cannabinoids. Chemistry Today. 2006;24:84-88. [Google Scholar]
- 71. Tan C, Lu NN, Wang CK, et al. Endothelium-derived semaphorin 3G regulates hippocampal synaptic structure and plasticity via neuropilin-2/plexinA4. Neuron. 2019;101:920-937.e13. [DOI] [PubMed] [Google Scholar]
- 72. Bab IA. Regulation of skeletal remodeling by the endocannabinoid system. Ann N Y Acad Sci. 2007;1116:414-422. [DOI] [PubMed] [Google Scholar]
- 73. Tam J, Trembovler V, Di Marzo V, et al. The cannabinoid CB1 receptor regulates bone formation by modulating adrenergic signaling. FASEB J. 2008;22:285-294. [DOI] [PubMed] [Google Scholar]
- 74. Hembree WC, 3rd, Nahas GG, Zeidenberg P, Huang HF. Changes in human spermatozoa associated with high dose marihuana smoking. Adv Biosci. 1978;22-23:429-439. [DOI] [PubMed] [Google Scholar]
- 75. Huang HFS, Nahas GG, Hembree WC. Effects of marijuana inhalation on spermatogenesis of the rat. In: Nahas GG, Sutin KM, Harvey DJ, Agurell S, ed. Marijuana and Medicine. Totowa, NJ: Humana Press; 1999:359-366. [Google Scholar]
- 76. Morishima A. Effects of cannabis and natural cannabinoids on chromosomes and ova. NIDA Res Monogr. 1984;44:25-45. [PubMed] [Google Scholar]
- 77. Zimmerman AM, Zimmerman S, Raj AY. Effects of cannabinoids on spermatogenesis in mice. In: Nahas GG, Sutin KM, Harvey DJ, Agurell S, eds. Marihuana and Medicine. Totowa, NJ: Humana Press; 1999:347-358. [Google Scholar]
- 78. Zimmerman AM, Bruce WR, Zimmerman S. Effects of cannabinoids on sperm morphology. Pharmacology. 1979;18:143-148. [DOI] [PubMed] [Google Scholar]
- 79. Liu W, Komiya Y, Mezzacappa C, Khadka DK, Runnels L, Habas R. MIM regulates vertebrate neural tube closure. Development. 2011;138:2035-2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Forrester MB, Merz RD. Risk of selected birth defects with prenatal illicit drug use, Hawaii, 1986-2002. J Toxicol Environ Health A. 2007;70:7-18. [DOI] [PubMed] [Google Scholar]
- 81. Morimoto S, Tanaka Y, Sasaki K, et al. Identification and characterization of cannabinoids that induce cell death through mitochondrial permeability transition in Cannabis leaf cells. J Biol Chem. 2007;282:20739-20751. [DOI] [PubMed] [Google Scholar]
- 82. Shoyama Y, Sugawa C, Tanaka H, Morimoto S. Cannabinoids act as necrosis-inducing factors in Cannabis sativa. Plant Signal Behav. 2008;3:1111-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
Canada_NTD_Data_-_Export for Cannabis Consumption Patterns Explain the East-West Gradient in Canadian Neural Tube Defect Incidence: An Ecological Study by Albert Stuart Reece and Gary Kenneth Hulse in Global Pediatric Health
Supplemental material, Supplementary_Table_1 for Cannabis Consumption Patterns Explain the East-West Gradient in Canadian Neural Tube Defect Incidence: An Ecological Study by Albert Stuart Reece and Gary Kenneth Hulse in Global Pediatric Health