Abstract
Acute myeloid leukemia (AML) is a hematological malignancy, which is commonly associated with high incidence and mortality among adult patients. The standard induction regimen for AML has been substantially unchanged over the past 40 years, for which novel nanomedicines have represented a promising strategy in AML therapies. Despite developments of multiple nanoparticles formulated with drugs or genes, less there is not much information available about approaches in AML is available. This review presents an overview of nanomedicines currently being evaluated in AML. First, it briefly summarized conventional chemotherapies in use. Second, nanomedicines presently ongoing in clinical trials or preclinical researches were classified and described, with illustrative examples from recent literatures. Finally, limitations and potential safety issues concerns in clinical translation of AML treatment were discussed as well.
Keywords: nanomedicines, nanoparticles, cancer therapy, acute myeloid leukemia
Introduction
Acute myeloid leukemia (AML) represents as a hematologic malignancy, which is the most common leukemia among adults and more detrimental in older patients, with a higher incidence rate and almost 90% mortality rate for those older than 65 years,1 as well as heterogeneous clinical presentations and subtypes.2,3 It was estimated that there were over 20 000 new cases and 10 670 deaths of patients with AML in the United States in 2018.4
Although progresses have been made in pathobiology and novel therapeutics targets, the standard induction therapies for AML have not been changed substantially for decades.4 Furthermore, with involvement of chemoresistance side effects, such as cardiotoxicity, myelosuppression, and infections, conventional therapeutics always lead to treatment failure or relapse; hence, the overall prognosis remains poor.5,6 Therefore, it is urgent to develop new therapeutic approaches.
Nano-based drug delivery systems have potentials to deliver drugs to specific area more efficiently and reduce side effects.7-9 Therefore, nanomedicines have been highlighted as a new strategy to optimize AML therapies.10-12 Moreover, by conjugated with various ligands, drugs could actively target to AML cell-surface receptors, which may have a tremendous improvement in treatment response or overall survival (OS) rates.13,14
This review attempts to present an overview of nanomedicines for AML currently in experimental researches or clinical trials from published data, with a focus on targeting ligands. This article may not be exhaustive due to the rapid development of new drugs. Rather, we aim to illustrate some potential targeting ligands for nanoparticles (NPs) in AML, which could be utilized in therapeutic nanoplatforms.
Current Treatments in AML
Standard Chemotherapy
The standard induction therapy is the combination of cytarabine and anthracycline (daunorubicin or idarubincin), which has been represented as “7+3 regimen” for more than 40 years.15,16 This regimen is administered as a continuous infusion of cytarabine for 7 days and anthracycline for the following 3 days. Consolidation is then conducted for the next phase after achieving complete remission (CR), which is called postremission therapy. Currently, cladribine combined with “7+3 regimen” is applied as a second standard regimen, which when received leads to an improvement in the CR rate and a 3-year OS.17 Fludarabine and etoposide are also used as an alternative regimen under poor heart functions.16,18 Recently, several new cytotoxic drugs have been utilized in clinic. Azacitidine and decitabine are 2 new DNA methyltransferase inhibitors recommended in the National Comprehensive Cancer Network guidelines.19
Target Therapy
FMS-like tyrosine kinase 3 (FLT3) mutation has been the foremost common change in patients with AML. Midostaurin was the first FLT3 inhibitor proved by the Food and Drug Administration (FDA) and commonly used in FLT3-mutated AML.20 According to an international randomized phase III trial, combined with “7+3 regimen,” midostaurin could significantly improve median OS from 25.6 to 74.7 months in patients with FLT3-mutated AML; however, a large proportion of patients were relapsed within 2 years.21 Quizartinib and cabozantinib were investigated as the second generation FLT3 inhibitors, specifically targeting FLT3 wild-type and FLT3 internal tandem duplications (ITDs) mutation, which achieved higher CR, but still had inevitable drug resistance.22,23 Recently, a novel FLT3 inhibitor gilteritinib has been approved by FDA for relapsed or refractory AML harboring FLT3 mutation, with an achievement of 40% overall response rate and 8% CR.24 Crenolanib is another FLT3 inhibitor enrolled within a phase III trial currently in patients with FLT3-ITD or FLT3-TKD mutated AML (NCT02298166).25
Approximately 20% cases of AML are detected with IDH1 and IDH2 mutations.26 The IDH1 inhibitor enasidenib and IDH2 inhibitor ivosidenib, proved by FDA, were reported achieving an effective overall response for 41.6%27 and 40%,28 respectively. However, acquired clinical resistances were subsequently detected after treatment.
B-cell lymphoma 2 (BCL-2) has been considered as an oncogene and overexpressed in patients with AML.29 A BCL-2 inhibitor venetoclax, which is approved by FDA for chronic lymphocytic leukemia (CLL), has been evaluated in several clinical trials either as a single agent or combined treatment for AML (NCT02203773, NCT02993523, NCT02287233, and NCT03069352).
Immunotherapy
Gemtuzumab ozogamicin, an anti-CD33 monoclonal antibody conjugated with calicheamicin, was first approved by FDA for CD33-positive AML. CD33 is a membrane receptor and potential target, which is highly expressed on leukemic progenitor cells but less on normal hematopoietic stem cells.30 Gemtuzumab ozogamicin was withdrawn in 2010 and ratified again in 2017 with dose and patient population modification. Currently, it is used in combined treatment with induction therapies or as a single agent in relapsed cases.31 However, toxicities in live cases still remain a concern because CD33 was also expressed on hepatocytes.32,33 Other novel anti-CD33 monoclonal antibodies, such as vadastuximab and AMG 330, are under clinical trials at present,34-36 which need further evaluations for dose effect or safety. Moreover, overexpression of CD123 has been observed in patients with resistance or relapsed AML, which is a potential target for the novel monoclonal antibody, SL-401. Currently, several phase I/II studies associated with SL-401 are ongoing.37,38
There are some immune checkpoint inhibitors used to treat AML as well. It has been demonstrated that immune checkpoints, such as cytotoxic T-lymphocyte-associated protein 4 (CTLA4), programmed cell death 1 (PD-1), and programmed cell death-ligand 1 (PD-L1), play unique roles in maintaining malignancy survivals and synergistically inhibiting immune responses against tumors.39 Inhibitors CTLA4 and PD-1/PD-L1 pathways have shown effects on promoting immune-medicated antileukemia responses and increasing survivals in murine AML models.40,41 Clinical trials of the PD-1 inhibitors nivolumab and pembrolizumab, PD-L1 inhibitors durvalumab and atezolizumab, as well as CTLA-4 inhibitor in patients with AML, which have shown well toleration and encouraging response to relapsed AML, are in progress at present.42-45
Chimeric antigen receptor (CAR) T-cell therapy is a novel antitumor immunotherapy that utilizes autologous lymphocytic T cells modified to express CARs, which could target on specific antigen of tumor. Chimeric antigen receptor T-cell therapy has received remarkable outcomes in patients with B-cell malignancies.46 However, the approach has been restricted in AML treatment. Although several target antigens have been studied, there is no ideal molecule and no authority has approved CAR T-cell therapy for AML yet.47,48 In summary, AML is a complex hematological malignancy with high mortality; more studies and clinical trials are required for current chemotreatments to reduce toxicities and improve efficacies.
Approaches of Nanomedicines for AML
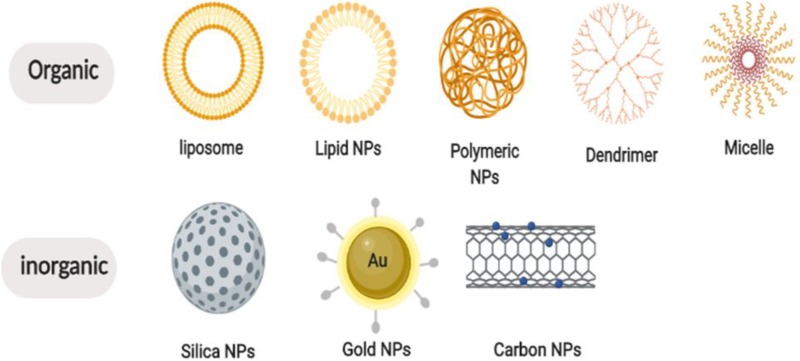
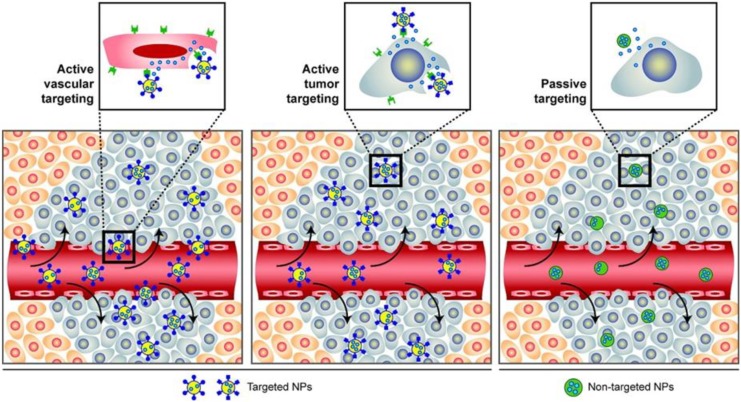
Nanoparticles have been highlighted in cancer therapies, with advantages of enhancing permeability, reducing adverse effects, getting around multidrug resistances (MDRs), improving bioavailability, and prolonging enhanced permeability and retention effects with drug circulations by loading and delivering anticancer agents to tumor site, which have been providing promising approaches to cancer therapies.49,50 Generally, NPs conjugate drugs are already used in AML therapies. Depending on formulation compositions and materials, NPs are mainly constituted with 2 types, organic NPs, such as liposomes, polymeric, micelle, dendrimer, carbon, and so on, and inorganic NPs, such as silica, metal, semiconductor, and so on,51,52 as shown in Figure 1. Generally, there are 2 kinds of classifications for NP-medicated drug deliveries. One is known as passive targeting, which enables different types of NPs passing across capillary endothelium of tumor vessels and accumulating in tumor site. The other is called active targeting, which could recognize cell-surface receptor and target tumor phenotypes directly by utilizing specific ligands (antibodies, proteins, peptides etc.) conjugating with NPs (Figure 2).53 Table 1 presents a summary of clinical trials ongoing in AML, among the 20 clinical trials, of which 18 were identified using liposomal formulations and only 2 were based on polymeric NPs. No other type of NPs is under clinical investigation currently in AML.
Figure 1.
Different classes of nanoparticles.
Figure 2.
A schematic diagram of passive and active targeting. Nanoparticles incorporated with ligands could specifically active target on receptors of blood vessel or cells (left and middle), while the other way is passively targeting and accumulating through enhanced permeability and retention effect (right). Adapted with permission from Farokhzad and Langer.53 Copyright (2009) American Chemical Society.
Table 1.
Ongoing Clinical Trials Involving Nanomedicines in Acute Myeloid Leukemia.
| Class | Compound | Clinical Phase | Trial Name | Status | Identifier |
|---|---|---|---|---|---|
| Liposomes | Annamycin | I/II | Study of Liposomal Annamycin for the Treatment of Subjects With AML | Recruiting | NCT00430443 |
| Doxorubicin | II | Bortezomib and Doxil for the Treatment of Patients With AML | Not yet recruiting | NCT01736943 | |
| Daunorubicin | III | International Randomized Phase III Clinical Trial in Children With AML | Recruiting | NCT02724163 | |
| CPX-351 (daunorubicin-cytarabine) | II | Liposome-encapsulated Daunorubicin-Cytarabine and Venetoclax in Treating Participants With Relapsed, Refractory or Untreated AML | Recruiting | NCT0362917 | |
| I/II | Cytarabine and Daunorubicin in Combination With Ruxolitinib for the Treatment of Secondary AML Transformed From MDS | Recruiting | NCT03878199 | ||
| II | Investigator Initiated Trial of CPX-351 for Untreated AML | Recruiting | NCT03335267 | ||
| I | A Trial to Evaluate the Potential Impact of Renal Impairment on the Pharmacokinetics and Safety of CPX-351 | Recruiting | NCT03555955 | ||
| II | Cytarabine, Idarubicin, Liposome-encapsulated Daunorubicin-Cytarabine or Decitabine in Treating Older Patients With AML | Recruiting | NCT03226418 | ||
| I | CPX-351 and Gemtuzumab Ozogamicin in Treating Patients With Relapsed AML | Not yet recruiting | NCT03904251 | ||
| I | CPX-351+GO in Subjects 55 Years Old, or Older, With AML | Not yet recruiting | NCT03878927 | ||
| IV | The Feasibility of Safely Managing Patients Receiving Induction With Liposomal Daunorubicin and Cytarabine (CPX-351) for AML in an Outpatient Environment | Not yet recruiting | NCT03988205 | ||
| I/II | Phase I/II Trial of CPX-351 + Palbociclib in Patients With AML | Not yet recruiting | NCT03844997 | ||
| II | CPX-351 in Treating Patients With Newly Diagnosed, High-Risk AML | Not recruiting | NCT02286726 | ||
| III | Phase III Study of CPX-351 Versus 7+3 in Patients 60-75 Years Old With Untreated High Risk (Secondary) AML | Not recruiting | NCT01696084 | ||
| BP1001 | II | Clinical Trial of BP1001(Liposomal Grb2 Antisense Oligonucleotide) in Combination With Decitabine in AML/High Risk MDS | Recruiting | NCT02781883 | |
| I/II | Clinical Trial of BP1001 (Liposomal Grb2 Antisense Oligonucleotide) in Combination With Dasatinib in Patients With Ph + CML Who Have Failed TKI, Ph+ AML, Ph+ MDS | Recruiting | NCT02923986 | ||
| I | Recruiting Clinical Trial of BP1001 (L-Grb-2 Antisense Oligonucleotide) in CML, AML, ALL & MDS | Not recruiting | NCT01159028 | ||
| Vincristine | I | EphB4-HSA Fusion Protein and Cytarabine /or Liposomal Vincristine in Patients With Recurrent or Refractory Acute Leukemia | Recruiting | NCT03519984 | |
| Polymeric NPs | AZD2811 | I | A Phase I Study of Safety, Tolerability, and PK of AZD2811 in Patients With Advanced Solid Tumors | NCT02579226 | |
| I/II | Safety, Tolerability, Pharmacokinetics, and Efficacy of AZD2811 Nanoparticles as Monotherapy or in Combination in Acute Myeloid Leukemia Patients | NCT03217838 |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CML, chronic myeloid leukemia; MDS, myelodysplastic syndrome; NPs, nanoparticle; Ph+, Philadelphia chromosome positive; PK, pharmacokinetics.
Passive Targeting Approaches in AML
Liposome-based nanomedicines
Liposomes are spherical vesicles composed of phospholipid bilayers, which could capture either hydrophilic agents by aqueous core or lipophilic agents by lipid bilayer during transportation without structural changes.54,55 For the purpose of increasing solubility, bioactivities, and distributions, liposomes have been considered the most prevalent category. At present, there are 16 liposomal drugs ratified by FDA or/and European Medicines Agency (EMA).56,57
Liposomal nanomedicines approved in AML therapy
CPX-351 (Vyxeos) is a liposomal-encapsulated cytarabine and daunorubicin with a fixed molar ratio (5:1), which was approved by FDA and EMA in 2017 for treating newly diagnosed therapy-related AML and/or AML with myelodysplasia-related changes.58 CPX-351 was initially synthesized and evaluated in in vitro and in vivo studies with leukemia cell lines. The results indicated that the liposomal-encapsulated cytarabine and daunorubicin could display a best synergistic effect and minimum antagonism at the ratio of 5:1, with higher proportions of response rates, more durable remissions, and longer maintenances in bone marrows, compared to a free drug cocktail of cytarabine and daunorubicin with their maximum tolerated doses (MTD).59-61 The therapeutic efficacy and frequency of CPX-351 were then examined using human leukemia xenograft model.61 Moreover, a later study tested in primary blood cells from patients with AML and normal bone marrow donors with CPX-351 and the same ratio of free drugs has shown that normal peripheral blood and bone marrow were more sensitive to free drugs, which illustrated that CPX-351 could preferentially accumulate in AML cells over normal cells.62 Subsequently, phase I study for CPX-351 was conducted in patients with refractory/relapsed AML in 2011, with a dose ranging from 32 U/m2 of the initial response dose to 101 U/m2 of MTD.63 It showed that 9 of 43 patients received CR, accompanied with side effects such as hypertensive crisis, congestive heart failure, and prolonged cytopenias.64 Further, a phase II trial explored efficiency and safety of CPX-351 in 126 older patients with AML and those with secondary AML (sAML) in 2014.65 This trial demonstrated that there was a tendency of higher CR and CR with incomplete blood count recovery for CPX-351 compared to conventional “7+3” therapy (66.7% vs 51.2%, P = .07), especially in sAML subgroup, with a trend toward improving response rate (57.6% vs 31.6%, P = .06), prolonging event-free survival (EFS; hazard ratio [HR], 0.59; P = .08), and OS (HR, 046; P = .01). Although prolonged cytopenias and higher risk of infections were detected as well, there were lower rates in infection-related deaths (3.5% vs 7.3%) and 60-day mortality (4.7% vs 14.6%). With the potential clinical benefit of CPX-351, another phase II study followed up in 2015 comparing CPX-351 to “7+3” induction therapy in 125 patients with first relapsed AML.66 Despite no improvement in 1-year EFS or OS, there was also a higher response rate (39.3% vs 27.6%), lower 60-day mortality rate (16.1% vs 24.1%), improved EFS (HR, 0.63; P = .08), and OS (HR, 0.55; P = .02) for European Prognostic Index–defined, poor-risk patients in CPX-351 group.
Based on these encouraging results, CPX-351 was advanced into phase III clinical studies for further ascertainment. A randomized phase III study comparing first-line CPX-351 (100 U/m2) with “7+3” regimen (daunorubicin, 60 mg/m2; cytarabine, 100 mg/m2) in 309 elderly patients (60-75 years) with high-risk sAML, indicated a significantly improved OS (9.56 months vs 5.95 months), composite response rates (47.7% vs 33.3%), and lower early mortality rates (5.9% and 13.7% vs 10.6% and 21.2%, through 30-day and 60-day, respectively), whereas a comparable frequency and severity of grade 3 to 5 adverse events.67 These encouraging results were presented at 2016 American Society of Clinical Oncology meeting and finally led to FDA approval in 2017. Lancet et al68 further analyzed the data, consistent with these observations; CPX-351 indicated a significant improvement in survival over standard induction chemotherapy for high-risk patients with AML, older patients with sAML, and poor-risk subgroup of patients with AML.
Liposomal nanomedicines under clinical trials in AML therapy
As shown in Table 1, liposomal formulations of vincristine, doxorubicin, annamycin, daunorubicin, and BP1001 are being evaluated in clinical trials at phase I or II stages currently. Liposomal doxorubicin (Doxil) and non-PEGylated liposomal doxorubicin (Myocet) have already been approved for the treatment of AIDS-related Kaposi sarcoma, multiple myeloma (MM), ovarian cancer, and breast cancer.69-71 Melillo et al72 have assessed Myocet combined with fludarabine, cytarabine, and granulocyte colony-stimulating factor (FLAG) in 35 elderly patients with AML, showing a median disease-free survival (DFS) at 12 months, 1-year, and 2-year DFS of 78.9% and 26.7%, and CR and partial remission of 63.8% and 8.5%, respectively, with a 20% resistance and 17% of severe cardiovascular toxicity. Another clinical study utilizing the same regimen was conducted in 18 children with refractory or relapsed AML, which achieved a CR rate of 18% (11/18), OR at 3 years of 38%, EFS at 3 years of 40%, and DFS at 3 years of 58% after hematopoietic stem cell transplantation, with well tolerant and remarkable low toxicity.73 There is one phase II clinical trial ongoing currently (NCT03059615), which is designed to evaluate the safety and efficacy of bortezomib combined with liposomal doxorubicin in patients with relapsed MM, CLL, and non-Hodgkin lymphoma as well as elderly patients with relapsed/refractory AML who are not candidates for standard induction therapy.
Liposomal daunorubicin (DaunoXome) is a non-PEGylated liposomal-encapsulated anthracycline daunorubicin. A phase III study was conducted by the International Berlin-Frankfurt-Münster Study Group in 2013 among pediatric patients with relapsed AML. Patients were randomly assigned to regimens of FLAG and FLAG plus daunorubicin. Although OS and grade 3 to 4 toxicities were similar, FLAG plus daunorubicin regimen showed an improved day 28 BM status (80% vs 70%), higher CR rate (69% vs 59%) compared to FLAG regimen.74 There is an international randomized phase III clinical trial that enrolled liposomal daunorubicin ongoing in children with AML (NCT02724163).
Antisense oligonucleotides, which refer to a class of small interfering RNA (siRNA), microRNA, or short hairpin RNA, have shown a great potential in cancer therapy and been approved for ALL treatment by FDA in 2017.75 However, the clinical application is limited due to instability circulation, inefficiency delivery, and off-target adverse effects. BP1001 is a liposomal formulation of growth factor receptor-bound protein-2 antisense oligodeoxynucleotide (l-Grb-2 antisense oligonucleotide). Previously, a single-center, dose-escalation phase I/Ib clinical trial combined with low-dose cytarabine in patients with refractory or relapsed AML, Philadelphia chromosome–positive (Ph+) chronic myeloid leukemia (CML), Ph+ ALL, or Ph+ myelodysplastic syndrome (MDS) demonstrated a well toleration with an improved therapeutic activity.76 There are 3 clinical trials underway. A phase I trial (NCT01159028) is to evaluate the highest safe dose for patients with AML, Ph+ CML, Ph+ ALL, and Ph+ MDS, in addition to the safety and toxicity in combination with low-dose Ara-C for patients with AML. Another phase I/II trial (NCT02923986) is to determine the efficacy with dose-limiting toxicity and maximal tolerated dose in combination with dasatinib in patients with Ph+ AML, Ph+ CML, or high-risk Ph+ MDS. The third phase II trial (NCT02781883) is to assess the efficacy in combination with decitabine in patients with AML or high-risk MDS.
Liposomal vincristine (Marqibo) was approval by FDA in 2012 for patients with Ph− ALL. There is also a phase I clinical trial ongoing (NCT03519984) that enrolls liposomal vincristine as part of a regimen containing EphB4-HSA fusion protein and cytarabine, for detecting patients with different types of acute leukemia, including AML.
Liposomal nanomedicines investigated in preclinical stage for AML therapy
Other liposomal nanomedicines are mostly being tested in vitro or in vivo. A liposome formulation of safingol was designed and evaluated with antitumor activities in human AML cell lines, patient samples, and murine xenograft models, as well as a longer survival time in U937-inoculated mice.77 Subsequently, a liposomal coencapsulation of safingol/C2-ceramide was developed, which indicated effectivity in vitro and xenograft models, with a dose reduction of 33% compared to liposomal safingol or liposomal C2-ceramide alone.78 Myhren et al79 have reported a PEGylated liposome coencapsulating anthracycline daunorubicin (DNR) and emetine with folate modification, which enhanced loading ability than DNR alone. Leukemia stem cell (LSC) with overexpression of miR-126 has been considered as a potential therapeutic target for AML.80 Dorrance et al81 designed a liposomal formulation containing antagomiR-126 with ligands of transferrin or antibody CD45 on surface. The formulation was tested on murine xenograft models and showed a significant improvement in survival rate with an interference with LSC. GTI-2040 is a 20-mer antisense oligonucleotide complementary to a coding region in the messenger RNA (mRNA) of the R2 small subunit component of human ribonucleotide reductase. Li et al82 designed and evaluated an immunoliposome-encapsulated GTI-2040, grafted with a target ligand of anti-CD33 to AML cells. It substantially downregulated the mRNA and protein expression of R2, reduced 15 times of IC50 than Ara-C, and decreased tumor volumes in Kasumi-1 xenografted model. The delivery efficiency of liposomes can be affected by physicochemical properties, such as particle size, zeta potential, or drug-release kinetics. Liposomes have achieved a series of encouraging results and are in different stages of evaluation. However, it is still unable to predict biological interactions by physicochemical properties accurately, which restrict converting preclinical effects to clinical benefits in AML.83-85
Polymer-based nanomedicines in AML therapy
Polymers containing polymeric particles, micelles, and dendrimers have been mainly investigated in preclinical studies to date. With advantages of biocompatible, tailored release, prolonging circulation, and functionalizing with specific peptide targeting ligands or antibodies, polymers have been the most intensively explored materials in drug delivery systems.86-88
Polymeric NPs could conjugate hydrophobic drugs by encapsulating to solid cores or water-soluble drugs by covalently attaching, which could control drug release, prolong circulation time, or reduce toxicity.89-92 Polylactide (PLA) and poly(lactide-co-glycolide) (PLGA) are commonly used polymers at present. AZD2811 is a polymeric NP loaded with aurora kinase B inhibitor. It has been assessed in AML xenografts model and shown an improved efficacy in inhibiting tumor growth and inducing apoptosis compared to free aurora kinase B inhibitor (AZD1152). Moreover, this formulation also demonstrated a transient cellular reduction in the bone marrow, which may have a potential agent for targeting residual disease.93 There are 2 clinical trials ongoing for evaluating the safety, tolerability, and pharmacokinetics of AZD2811 (NCT02579226, NCT03217838). Poly(lactide-co-glycolide is a FDA-approved polymer widely used as a nanocarrier. Simon et al94 developed a PLGA polymeric NPs-encapsulated all-trans-retinoic acid (ATRA), which prolonged the drug release and induced differentiation as well as inhibited proliferation in AML cells. A PEG-PLGA polymeric micelle loaded with edelfosine and conjugated with transferrin was designed by Sun and Sun.95 This formulation prolonged blood circulation, leading to a continuous drug release and biological activity maintenance, which resulted in a higher cytotoxic effect and apoptosis in K562 cells. Zhu et al96 designed a PLGA and Pluronic85 copolymeric NP encapsulated with doxorubicin and grafted with transferrin, which was further evaluated in AML cell lines and relevant animal models. This formulation led to a reduction of tumor volume in vivo and an enhancement of cytotoxicity in doxorubicin-resistant cells. A polymeric NPs-loaded specific CD-44 siRNA was designed and performed in vitro.97 It was demonstrated to inhibit stem cells–progenitor cells interactions and sensitize chemotherapies by silencing and decreasing CD44 surface levels in AML cell lines, which induced apoptosis and decreased adherence of primary AML cells to bone marrow mesenchymal stem cell. Chandran et al98 developed a PLGA polymer–protein core–shell formulation, loaded with everolimus, sorafenib, and inhibitors of mTOR, MAPK, and STAT5, additionally conjugated with anti-CD33 antibody. The result showed that it could cause synergistic lethality against leukemic cells by simultaneous inhibition, without affecting normal blood cells.98 Recently, another methoxy PEG-PLGA polymeric NP with encapsulated idarubicin was synthesized. The study demonstrated that, compared with free idarubicin, it could decrease cell proliferation and induce apoptosis more remarkable in vivo, and improve the OS more significantly in murine models.99 PCX is a polymeric NP loaded with AMD3100, a FDA-approved CXCR4 inhibitor and carrying siRNA simultaneously. This PCX/siRNA nanomedicine exerted a higher cytotoxic effect on AML cell lines compared to other CXCR4 inhibitors. In addition, it could deliver siRNAs against the transcription factor RUNX1, which was typically required in AML subtypes.100
Micelles are promising copolymers nanomaterial composed of hydrophobic core and hydrophilic corona, which are good candidates for encapsulating hydrophobic anticancer agents and ensuring solubility.101 l-PLA micelle formulation of paclitaxel (Genexol-PM), docetaxel-loaded polymeric micelle (NanoxelM), and paclitaxel micellar (Paclical) have been approved and used in clinic for the treatment of various types of cancers, such as breast cancer, ovarian cancer, and lung cancer, however, not yet in AML.102-104 Dextran is a polymeric micelle loaded with doxorubicin and grafted with folic and retinoic acid. The cytotoxicity of dextran was higher in KG-1 cells than free drug, which reduced approximately half of IC50.105 SP1049C is another micellar formulation of doxorubicin based on pluronic, which has completed phase II trials in patients with advanced esophagus and gastroesophageal junction adenocarcinoma. Alakhova et al106 compared antitumor activities of SP1049C with doxorubicin in P388 murine leukemia ascitic tumor model. The tumor formation frequency and aggression were much more reduced in SP1049C group than that in control group. Further evaluations are needed for clinical application in AML.
Dendrimers are spherical polymers composed of a central core, multibranches, and an outer layer with functional groups, which could conjugate with charged polar molecules through electrostatic interaction by outer layers and encapsulate uncharged molecules by hydrophobic inner.107 These properties of dendrimers enable covalent attach to hydrophobic anticancer agents and increase bioavailability. There is no clinical trial and few in vivo studies for AML associated with dendrimer formulations. The cytotoxicity and apoptosis of a dendrimer NPs encapsulated with cytarabine was assessed in 1301 and HL-60 cell lines previously, which showed an enhancement compared to free drug.108
Metallic nanomedicines in AML therapy
Metal NPs, such as gold or silver, which are inorganic and nontoxic nanomaterials, have been considered to be useful candidates in cancer therapy for attaching and delivering drugs by use of surface plasmon resonances and photophysical properties.109 There is no metallic nanomedicine under clinical trials to date, although quite a few preclinical studies have reported for AML indications. A gold NP loaded with tyrosine kinase inhibitors was designed and showed an increased efficacy compared with free drug.110 Another study newly synthesized a gold NP with adsorbed high-density lipoprotein loaded with BMS309403 (BMS), an AML-promoting factor fatty acid–binding protein 4 inhibitor. The result showed this formulation could induce cell differentiation and reduce progression of AML.111
Lipid-based nanomedicines in AML therapy
Lipid NPs are designed to encapsulate lipophilic drugs.112 Currently, there is no lipid-based nanomedicines approved in clinical trials for AML, as some sporadic experimental reported. It is known that sparingly water solubility has confined clinical applications of etoposide. Khajavinia et al113 synthesized a lipid NP loaded with etoposide and conjugated with transferrin. The result indicated an enhanced cellular uptake and higher cytotoxicity in etoposide lipid–based NPs compared to that in free etoposide. An ATRA-loaded lipid-based NP was obtained and showed a significantly suppression in AML cell lines compared with ATRA in solution.114 Previously, fingolimod, a sphingosine analog that could activate protein phosphatase 2A in leukemia, has been demonstrated to be a potential treatment option for AML.115 A lipid-based NP loaded with fingolimod was therefore designed. Results showed it could induce a higher apoptosis and enhance the oral bioavailability compared with bare fingolimod solution.116
Other types of nanomedicines in AML therapy
Chandran et al117 reported a silico-based nanomedicine loaded with vorinostat, which showed a selective and superior anticancer activity against patient with primary AML cells and AML cell lines. It demonstrated a lower IC50, enhanced histone deacetylase inhibition, apoptosis, and oxidative injury compared to free vorinostat, without toxicity to healthy bone marrow. Carbon-based materials, especially new discovery of grapheme, are another new kind of organic NPs that possess attachment sites on surface of ligands and deliver drugs into cytoplasm of cancer cells by carbon nanotubes. Currently, to our knowledge, there is no carbon base nanomedicine detected in AML.
Active Targeting Approaches in AML
In order to improve delivery efficiency and reduce toxicity to normal cells, there is another type of drug delivery defined as active targeting, with surface of NPs decorated with different ligands.53 Ligands typically include peptides, antibodies, folic acid, retinoic acid, vitamins, and transferrins.118-123 So far, investigations for AML are still in laboratory stage, and summary is presented in Table 2.
Table 2.
Active Targeting Nanoparticles Investigated in AML.
| Nanoparticle | Ligand | Therapeutic Agents | Ref |
|---|---|---|---|
| Liposome | Anti-CD33 mAb | GTI-2040 | 16 |
| Polymer | Anti-CD33 mAb | Ara-C | 17 |
| PLGA polymer | Anti-CD33 mAb | mTOR, MAPK, and STAT5 inhibitor | 18 |
| Liposome | Anti-CD123 mAb | Daunorubicin | 20 |
| Niosome | Anti-CD123 mAb | Daunorubicin | 21 |
| Silica | Anti-B220 mAb | Daunorubicin | 23 |
| Liposome | Anti-CD45 mAb | AntagomiR-126 | 24,25 |
| Polymer | Anti-CD44 mAb | siRNA | 8 |
| Polymer | Transferrin | Doxorubicin | 27 |
| Micelle | Transferrin | Edelfosine | 28 |
Abbreviations: AML, acute myeloid leukemia; PLGA, poly(lactide-co-glycolide); siRNA, small interfering RNA.
Cell-penetrating peptides (CPPs) are composed of 5 to 30 amino acids and able to carry a variety of cargoes, including NPs and antisense oligonucleotides. Cell-penetrating peptides could cross across cell membranes and deliver drugs into cells, with the advantages of highly selective for tumor site and multivalent conjugation to anticancer agents.124 The accurate cellular uptake mechanisms of CPPs remain uncertain, accompanied with 2 major theories. One is energy-independent endocytic process and directly through the lipid bilayer, the other is energy-dependent endocytic process.125 There is no report published on nanomedicines equipped with CPPs in AML to date; however, relevant experimental results may represent potential strategies for further investigations. A specific peptide (CP-EPS8-NLS) derived from the nuclear localization signal of epidermal growth factor receptor pathway substrate no.8 (EPS8) was synthesized and analyzed in AML cells as well as related xenograft models, which showed potential cytotoxicity effects.126 Agarwal et al127 evaluated the specificity and efficacy of a CPP OP449 in antagonizing SET oncoprotein in AML cells and animal models. It has been reported the CPP inhibitor of mucin 1-C-terminal subunit (MUC1-C) oncoprotein could arrest tumor cell growth, induce late apoptosis, and increase the reactive oxygen species, which resulted to induce a terminally differentiated myeloid phenotype in AML cell lines.128 Lastly, a research showed CPP inhibitor GO-203 could depress MUC1-C aberrantly expressed in AML and downregulate the FLT3, which conferred a poor prognosis in AML.129 Li et al130 indicated that a TLR2-binding peptide motif (Pep2) could target and penetrate into AML cells in a dependent manner, thus inducing apoptosis in AML cell lines as well as patient samples and depressing progression in TLR2high AML mice. These results indicated several optional CPPs for further investigations in AML.
Nanomedicines conjugated with antibody can specifically target on cell-surface receptor and delivery drugs into cells.131 GTI-2040 is an antisense oligonucleotide targeting the small subunit R2 of ribonucleotide reductase, which is underevaluation in clinical trials for AML. It has been known that CD33 is a membrane receptor expressed by AML progenitors but absent in normal bone marrow stem cells. GTI-2040-loaded immunoliposomes grafted with an anti-CD33 ligand has been synthesized and observed in AML cells as well as related animal models.82 The result indicated that it significantly downregulated expression of R2 and suppressed cell viabilities in AML cell lines. Moreover, after combining with Ara-C, there was a strengthened inhibition in tumor growth and a prolonged survival time for this immunoliposomal nanomedicine in AML xenograft model. A CD33-targeted pH-sensitive polymeric liposome encapsulated with Ara-C was designed and verified in AML cells, which obviously restrained cell viabilities and successfully internalized into CD33-positive AML cells. On the contrary, limited cellular internalization was found in control liposomes with an isotype antibody.132 As mentioned above, there was also an anti-CD33 incorporated multi-inhibitor-loaded PLGA polymer NPs developed.98 CD123, which is overexpressed on AML cells, has been identified as a potential target for treatment.133 An anti-CD123 PEGylated liposomal encapsulated with daunorubicin (CD123-ILP) was synthesized and assessed in several AML cell lines with different densities of anti-CD123 antibody. CD123-ILP highly slowed down the growth of CD123+ AML cells and showed a stable release in vitro.134 Another anti-CD123 niosome formulation loaded with daunorubicin was formed as well. The result demonstrated improved uptake efficiency than free antibody drug in AML cells with ligand density dependent. Moreover, it resulted in a higher cytotoxicity and prolonged survival time in vitro and in vivo treatment of AML.135 B220, also known as CD45R, is an isoform of CD45. It has been proved that antigen B220 is specifically expressed on LSCs.136 A liposomal formulation containing antagomiR-126 with ligands of transferrin or antibody CD45 has been discussed in previous section.80,81 Recently, an anti-B220-ligand mesoporous silica NP has been designed to deliver daunorubicin. This drug selectively reduced the growth of B220-positive AML LSCs and decreased progression in mice model.137
Transferrin, a single-chain iron-transporting glycoprotein as well as a membrane receptor, is overexpressed on a majority of cancer cells and plays role in mediating intracellular uptake and regulating cancer growth.138 It has reported a copolymeric NP conjugated with transferrin was designed to enhance the antileukemia efficacy of doxorubicin in AML cell lines.96 There was also another polymeric NPs incorporating transferrin and edelfosine, which indicated a higher cytotoxic effect in AML cells.95
Challenges in Clinical Translation of Nanomedicines for AML Treatment
It has been proved that nanomedicines offer abundant benefits, such as improving solubility, biocompatibility, bioavailability, distribution, and stability, as well as reducing toxicity and MDR, which have shown superior efficacy than conventional therapeutics. However, it remains difficult to facilitate the application in clinic. Despite a few nanomedicines approved and used in solid tumors,139-142 CPX-351 is the only liposomal formulation approved by FDA to date. There are some risks and limitations concerning therapeutic effects and safeties due to the intrinsic physicochemical properties of NPs. Sizes are concerned with circulation time and half-life in blood or organs. Previous studies have demonstrated that NPs <4 nm may penetrate vacuoles and interfere cellular processed, such as metabolism, detoxification, transcription, and gene expression, including normal cells.143,144 Particles with sizes ranging from 10 to 250 nm could stay in the liver, spleen, kidneys, or other organs for several months.145,146 Nanoparticles ranging from 15 to 20 nm could penetrate and infiltrate through the blood–brain barrier and blood–retinal barrier, implicating that toxicities should be inevitably taken into a consideration.147 Pharmacokinetics, distributions, and toxicities can also be affected by NPs. Imputable to technology limitations, it is still difficult to evaluated efficacies in loading or release drug exactly. After internalized into cells, NPs often face degradation due to endosomes.148 In addition, there are off-target risks due to some of the NP–antibody ligands that are also target on normal cells.149,150 Additionally, NPs may active immune responses and be cleared by immune cells.151 Therefore, assessing the toxicity and side effects should be taken into account before approval and utilize in clinic. Besides the biological hurdles, there are also technical challenges. It is not an easy approach to fulfill the producing demand in a manufactory rather than in a laboratory, which requires special production equipment and high manufacturing costs. Moreover, each subtype of NPs offers unique features or characteristics, which means general rules could not be laid down and massive efforts have to be made for case-by-case evaluation. In vivo tests are essential for clinical translation. However, the development and progress of tumors in conventional xenograft models are not spontaneous or humanized during nanomedicines evaluation in animal models, which are different from live cases and deviated from realistic situations. Consequently, further evaluations are needed for nanomedicines transferring from bench to bedside in AML.
Conclusions
Acute myeloid leukemia has been considered an intricate hematological malignancy with poor responses and high mortalities. Chemotherapies, targeting therapies, and immunotherapies are the conventional treatment strategies for AML. This review summarized current development and approaches of nanomedicines in AML, including related technologic rationale, efficacies, safety profiles, and limitations, which may provide a novel and highlighted therapeutic option for AML treatment in future. Previous studies have demonstrated a superior for that NPs can be superior in improving bioavailability, reducing adverse effects, and circumventing drug resistances. Liposomal and polymeric NPs have shown especially promising prospects with advantages of strengthened loading ability and drug-release controlling. Chemical formulations and specific ligands such as antibodies or CPPs are also in underdevelopment. On the contrary, due to physical properties, drugs decorated with metal or silica NPs are less biocompatible and biodegradable, which restrict their utilization in AML. However, according to encouraging results in clinical trials, we could inference that NPs may finally become a promising and clinically acceptable option in the treatment of AML.
Footnotes
Author’s Note: Feng Huang is also affiliated with Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing, China.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is supported by grants from Hong Kong Baptist University Special Development Fund (SDF18-0319-P03).
ORCID iD: Xiao Huang  https://orcid.org/0000-0002-6144-2860
https://orcid.org/0000-0002-6144-2860
References
- 1. Howlader N, Noone AM, Krapcho M, et al. (eds). SEER Cancer Statistics Review, 1975–2016. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2014/. Accessed May 15, 2019. [Google Scholar]
- 2. Harris NL, Jaffe ES, Diebold J, et al. The World Health Organization Classification of neoplastic diseases of the haematopoietic and lymphoid tissues: report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1997. Histopathology. 2000;36(1):69–86. [DOI] [PubMed] [Google Scholar]
- 3. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. [DOI] [PubMed] [Google Scholar]
- 4. Vakiti A, Mewawalla P. Cancer, acute myeloid leukemia (AML, erythroid leukemia, myelodysplasia-related leukemia, BCR-ABL chronic leukemia) In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2019. [Google Scholar]
- 5. Swords R, Santini V. In elderly patients with AML, which patients should be considered fit or unfit for standard induction therapy? Hematology Am Soc Hematol Educ Program. 2012;2012:74–75. [DOI] [PubMed] [Google Scholar]
- 6. Yanada M, Naoe T. Acute myeloid leukemia in older adults. Int J Hematol. 2012;96(2):186–193. [DOI] [PubMed] [Google Scholar]
- 7. Jain S, Hirst DG, O’Sullivan JM. Gold nanoparticles as novel agents for cancer therapy. Brit J Radiol. 2012;85(1010):101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou C, Yang Z, Teng L. Nanomedicine based on nucleic acids: pharmacokinetic and pharmacodynamic perspectives. Curr Pharm Biotechnol. 2014;15(9):829–838. [DOI] [PubMed] [Google Scholar]
- 9. Chen Y, Liu X, Yuan H, et al. Therapeutic remodeling of the tumor microenvironment enhances nanoparticle delivery. Adv Sci. 2019;6(5):1802070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Briot T, Roger E, Thepot S, Lagarce F. Advances in treatment formulations for acute myeloid leukemia. Drug Discov Today. 2018;23(12):1936–1949. [DOI] [PubMed] [Google Scholar]
- 11. Guo J, Luan X, Cong Z, et al. The potential for clinical translation of antibody-targeted nanoparticles in the treatment of acute myeloid leukaemia. J Control Release. 2018;286:154–166. [DOI] [PubMed] [Google Scholar]
- 12. Yang Z, Yu B, Zhu J, et al. A microfluidic method to synthesize transferrin-lipid nanoparticles loaded with siRNA LOR-1284 for therapy of acute myeloid leukemia. Nanoscale. 2014;6(16):9742–9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kurrikoff K, Aphkhazava D, Langel U. The future of peptides in cancer treatment. Curr Opin Pharmacol. 2019;47:27–32. [DOI] [PubMed] [Google Scholar]
- 14. Vadevoo SMP, Gurung S, Khan F, et al. Peptide-based targeted therapeutics and apoptosis imaging probes for cancer therapy. Arch Pharm Res. 2019;42(2):150–158. [DOI] [PubMed] [Google Scholar]
- 15. Ferrara F, Vitagliano O. Induction therapy in acute myeloid leukemia: is it time to put aside standard 3 + 7? Hematol Oncol. 2019. doi:10.1002/hon.2615. [DOI] [PubMed] [Google Scholar]
- 16. Lin M, Chen B. Advances in the drug therapies of acute myeloid leukemia (except acute wpromyelocytic leukemia). Drug Des Develop Ther. 2018;12:1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holowiecki J, Grosicki S, Giebel S, et al. Cladribine, but not fludarabine, added to daunorubicin and cytarabine during induction prolongs survival of patients with acute myeloid leukemia: a multicenter, randomized phase III study. J Clin Oncol. 2012;30(20):2441–2448. [DOI] [PubMed] [Google Scholar]
- 18. Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet (Lond Engl). 2018;392(10147):593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28(4):562–569. [DOI] [PubMed] [Google Scholar]
- 20. Kayser S, Levis MJ. Advances in targeted therapy for acute myeloid leukaemia. Br J Haematol. 2018;180(4):484–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stone RM, Larson RA, Dohner H. Midostaurin in FLT3-mutated acute myeloid leukemia. N Engl J Med. 2017;377(19):1903. [DOI] [PubMed] [Google Scholar]
- 22. Ko YC, Hu CY, Liu ZH, et al. Cytarabine-resistant FLT3-ITD leukemia cells are associated with TP53 mutation and multiple pathway alterations-possible therapeutic efficacy of cabozantinib. Int J Mole Sci. 2019;20(5):E1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou F, Ge Z, Chen B. Quizartinib (AC220): a promising option for acute myeloid leukemia. Drug Des Devel Ther. 2019;13:1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee LY, Hernandez D, Rajkhowa T, et al. Preclinical studies of gilteritinib, a next-generation FLT3 inhibitor. Blood. 2017;129(2):257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bohl SR, Bullinger L, Rücker FG. New targeted agents in acute myeloid leukemia: new hope on the rise. Int J Mol Sci. 2019;20(8):E1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DiNardo CD, Ravandi F, Agresta S, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol. 2015;90(8):732–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. DiNardo CD. Ivosidenib in IDH1-mutated acute myeloid leukemia. N Engl J Med. 2018;379(12):1186. [DOI] [PubMed] [Google Scholar]
- 28. Abou Dalle I, DiNardo CD. The role of enasidenib in the treatment of mutant IDH2 acute myeloid leukemia. Ther Adv Hematol. 2018;9(7):163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adams JM, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol. 2007;19(5):488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Norsworthy KJ, Ko CW, Lee JE, et al. FDA approval summary: Mylotarg for treatment of patients with relapsed or refractory CD33-positive acute myeloid leukemia. Oncologist. 2018;23(9):1103–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. FDA approves gemtuzumab ozogamicin for CD33-positive AML. 2017; https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-gemtuzumab-ozogamicin-cd33-positive-aml. Accessed May 20, 2018.
- 32. Gao Z, McAlister VC, Williams GM. Repopulation of liver endothelium by bone-marrow-derived cells. Lancet (London, England). 2001;357(9260):932–933. [DOI] [PubMed] [Google Scholar]
- 33. Thol F, Schlenk RF. Gemtuzumab ozogamicin in acute myeloid leukemia revisited. Expert Opin Biol Ther. 2014;14(8):1185–1195. [DOI] [PubMed] [Google Scholar]
- 34. Fathi AT, Erba HP, Lancet JE, et al. A phase 1 trial of vadastuximab talirine combined with hypomethylating agents in patients with CD33-positive AML. Blood. 2018;132(11):1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walter RB. Investigational CD33-targeted therapeutics for acute myeloid leukemia. Expert Opin Investig Drugs. 2018;27(4):339–348. [DOI] [PubMed] [Google Scholar]
- 36. Aigner M, Feulner J, Schaffer S, et al. T lymphocytes can be effectively recruited for ex vivo and in vivo lysis of AML blasts by a novel CD33/CD3-bispecific BiTE antibody construct. Leukemia. 2013;27(5):1107–1115. [DOI] [PubMed] [Google Scholar]
- 37. Pemmaraju N, Lane AA, Sweet KL, et al. Results from phase 2 trial ongoing expansion stage of SL-401 in patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN). Blood. 2016;128:342–342. [Google Scholar]
- 38. Sweet KL, Pemmaraju N, Lane AA, et al. Lead-in stage results of a pivotal trial of SL-401, an interleukin-3 receptor (IL-3R) targeting biologic, in patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN) or acute myeloid leukemia (AML). Blood. 2015;126(23):3795–3795. [Google Scholar]
- 39. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fevery S, Billiau AD, Sprangers B, et al. CTLA-4 blockade in murine bone marrow chimeras induces a host-derived antileukemic effect without graft-versus-host disease. Leukemia. 2007;21(7):1451–1459. [DOI] [PubMed] [Google Scholar]
- 41. Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood. 2009;114(8):1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zeidner JF, Vincent BG, Ivanova A, et al. Phase II study of high dose cytarabine followed by pembrolizumab in relapsed/refractory acute myeloid leukemia (AML). Blood. 2017;130:1349–1349. [Google Scholar]
- 43. Daver N, Garcia-Manero G, Basu S, et al. Efficacy, safety, and biomarkers of response to azacitidine and nivolumab in relapsed/refractory acute myeloid leukemia: a non-randomized, open-label, phase 2 study. Cancer Discov. 2019;9(3):370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Giannopoulos K. Targeting immune signaling checkpoints in acute myeloid leukemia. J Clin Med. 2019;8(2):E236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. ASH 2018: Azacitidine with nivolumab plus ipilimumab vs azacitidine plus nivolumab in relapsed or refractory AML. 2018; https://www.ascopost.com/News/59523. Accessed May 12, 2019.
- 46. Jetani H, Garcia-Cadenas I, Nerreter T, et al. CAR T-cells targeting FLT3 have potent activity against FLT3(-)ITD(+) AML and act synergistically with the FLT3-inhibitor crenolanib. Leukemia. 2018;32(5):1168–1179. [DOI] [PubMed] [Google Scholar]
- 47. Sauer T, Rooney CM. Current challenges for CAR T-cell therapy of acute myeloid leukemia. Transfusion. 2019;59(4):1171–1173. [DOI] [PubMed] [Google Scholar]
- 48. Cummins KD, Gill S. Will CAR T cell therapy have a role in AML? Promises and pitfalls. Semin Hematol. 2019;56(2):155–163. [DOI] [PubMed] [Google Scholar]
- 49. Sutradhar KB, Amin ML. Nanotechnology in cancer drug delivery and selective targeting. ISRN Nanotechnol. 2014;2014 doi:10.1155/2014/939378. [Google Scholar]
- 50. Dawar S, Singh N, Kanwar RK, et al. Multifunctional and multitargeted nanoparticles for drug delivery to overcome barriers of drug resistance in human cancers. Drug Discov Today. 2013;18(23-24):1292–1300. [DOI] [PubMed] [Google Scholar]
- 51. Fu HB, Yao JN. Size effects on the optical properties of organic nanoparticles. J Am Chem Soc. 2001;123(7):1434–1439. [Google Scholar]
- 52. Scholes GD. Controlling the optical properties of inorganic nanoparticles. Adv Funct Mater. 2008;18(8):1157–1172. [Google Scholar]
- 53. Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3(1):16–20. [DOI] [PubMed] [Google Scholar]
- 54. Yang X, Yang S, Chai H, et al. A novel isoquinoline derivative anticancer agent and its targeted delivery to tumor cells using transferrin-conjugated liposomes. PLoS One. 2015;10(8):e0136649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang X, Huang X, Yang Z, et al. Targeted delivery of tumor suppressor microRNA-1 by transferrin-conjugated lipopolyplex nanoparticles to patient-derived glioblastoma stem cells. Curr Pharm Biotechnol. 2014;15(9):839–846. [DOI] [PubMed] [Google Scholar]
- 56. Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33(10):2373–2387. [DOI] [PubMed] [Google Scholar]
- 57. D’Mello SR, Cruz CN, Chen ML, Kapoor M, Lee SL, Tyner KM. The evolving landscape of drug products containing nanomaterials in the United States. Nat Nanotechnol. 2017;12(6):523–529. [DOI] [PubMed] [Google Scholar]
- 58. Krauss AC, Gao X, Li L, et al. FDA approval summary: (daunorubicin and cytarabine) liposome for injection for the treatment of adults with high-risk acute myeloid leukemia. Clin Cancer Res. 2019;25(9):2685–2690. [DOI] [PubMed] [Google Scholar]
- 59. Tardi P, Johnstone S, Harasym N, et al. In vivo maintenance of synergistic cytarabine: daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res. 2009;33(1):129–139. [DOI] [PubMed] [Google Scholar]
- 60. Lim WS, Tardi PG, Dos Santos N, et al. Leukemia-selective uptake and cytotoxicity of CPX-351, a synergistic fixed-ratio cytarabine: daunorubicin formulation, in bone marrow xenografts. Leuk Res. 2010;34(9):1214–1223. [DOI] [PubMed] [Google Scholar]
- 61. Lim WS, Tardi PG, Xie X, et al. Schedule- and dose-dependency of CPX-351, a synergistic fixed ratio cytarabine: daunorubicin formulation, in consolidation treatment against human leukemia xenografts. Leuk Lymphoma. 2010;51(8):1536–1542. [DOI] [PubMed] [Google Scholar]
- 62. Kim HP, Gerhard B, Harasym TO, Mayer LD, Hogge DE. Liposomal encapsulation of a synergistic molar ratio of cytarabine and daunorubicin enhances selective toxicity for acute myeloid leukemia progenitors as compared to analogous normal hematopoietic cells. Exp Hematol. 2011;39(7):741–750. [DOI] [PubMed] [Google Scholar]
- 63. Feldman EJ, Lancet JE, Kolitz JE, et al. First-in-man study of CPX-351: a liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J Clin Oncol. 2011;29(8):979–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Feldman EJ, Kolitz JE, Trang JM, et al. Pharmacokinetics of CPX-351; a nano-scale liposomal fixed molar ratio formulation of cytarabine:daunorubicin, in patients with advanced leukemia. Leuk Res. 2012;36(10):1283–1289. [DOI] [PubMed] [Google Scholar]
- 65. Lancet JE, Cortes JE, Hogge DE, et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood. 2014;123(21):3239–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cortes JE, Goldberg SL, Feldman EJ, et al. Phase II, multicenter, randomized trial of CPX-351 (cytarabine: daunorubicin) liposome injection versus intensive salvage therapy in adults with first relapse AML. Cancer. 2015;121(2):234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lancet JE, Uy GL, Cortes JE, et al. Final results of a phase III randomized trial of CPX-351 versus 7+3 in older patients with newly diagnosed high risk (secondary) AML. Am Soc Clin Oncol. 2016. doi:10.1200/jco.2016.34.15_suppl.7000. [Google Scholar]
- 68. Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gabizon AA, Patil Y, La-Beck NM. New insights and evolving role of pegylated liposomal doxorubicin in cancer therapy. Drug Resist Update. 2016;29:90–106. [DOI] [PubMed] [Google Scholar]
- 70. Franco YL, Vaidya TR, Ait-Oudhia S. Anticancer and cardio-protective effects of liposomal doxorubicin in the treatment of breast cancer. Breast Cancer (Dove Medical Press). 2018;10:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Huang YF, Kuo MT, Liu YS, Cheng YM, Wu PY, Chou CY. A dose escalation study of trientine plus carboplatin and pegylated liposomal doxorubicin in women with a first relapse of epithelial ovarian, tubal, and peritoneal cancer within 12 months after platinum-based chemotherapy. Front Oncol. 2019;9:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Melillo L, Valente D, D’Arena G, et al. Combination treatment of flag with non-pegylated liposomal doxorubicin (MYOCET(TM)) in elderly patients with acute myeloid leukemia: a single center experience. Int J Immunopathol Pharmacol. 2011;24(3):703–709. [DOI] [PubMed] [Google Scholar]
- 73. Quarello P, Berger M, Rivetti E, et al. FLAG-liposomal doxorubicin (Myocet) regimen for refractory or relapsed acute leukemia pediatric patients. J Pediatr Hematol Oncol. 2012;34(3):208–216. [DOI] [PubMed] [Google Scholar]
- 74. Kaspers GJ, Zimmermann M, Reinhardt D, et al. Improved outcome in pediatric relapsed acute myeloid leukemia: results of a randomized trial on liposomal daunorubicin by the international BFM study group. J Clin Oncol. 2013;31(5):599–607. [DOI] [PubMed] [Google Scholar]
- 75. Chi X, Gatti P, Papoian T. Safety of antisense oligonucleotide and siRNA-based therapeutics. Drug Discov Today. 2017;22(5):823–833. [DOI] [PubMed] [Google Scholar]
- 76. Ohanian M, Tari Ashizawa A, Garcia-Manero G, et al. Liposomal Grb2 antisense oligodeoxynucleotide (BP1001) in patients with refractory or relapsed haematological malignancies: a single-centre, open-label, dose-escalation, phase 1/1b trial. Lancet Haematol. 2018;5(4):e136–e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tan KB, Ling LU, Bunte RM, Chng WJ, Chiu GN. In vivo efficacy of a novel liposomal formulation of safingol in the treatment of acute myeloid leukemia. J Control Release. 2012;160(2):290–298. [DOI] [PubMed] [Google Scholar]
- 78. Tan KB, Ling LU, Bunte RM, Chng WJ, Chiu GN. Liposomal codelivery of a synergistic combination of bioactive lipids in the treatment of acute myeloid leukemia. Nanomedicine (Lond). 2014;9(11):1665–1679. [DOI] [PubMed] [Google Scholar]
- 79. Myhren L, Nilssen IM, Nicolas V, Doskeland SO, Barratt G, Herfindal L. Efficacy of multi-functional liposomes containing daunorubicin and emetine for treatment of acute myeloid leukaemia. Eur J Pharm Biopharm. 2014;88(1):186–193. [DOI] [PubMed] [Google Scholar]
- 80. Pollyea DA, Jordan CT. Therapeutic targeting of acute myeloid leukemia stem cells. Blood. 2017;129(12):1627–1635. [DOI] [PubMed] [Google Scholar]
- 81. Dorrance AM, Neviani P, Ferenchak GJ, et al. Targeting leukemia stem cells in vivo with antagomiR-126 nanoparticles in acute myeloid leukemia. Leukemia. 2015;29(11):2143–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Li H, Xu S, Quan J, et al. CD33-targeted lipid nanoparticles (aCD33LNs) for therapeutic delivery of GTI-2040 to acute myelogenous leukemia. Mol Pharm. 2015;12(6):2010–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Drummond DC, Noble CO, Hayes ME, Park JW, Kirpotin DB. Pharmacokinetics and in vivo drug release rates in liposomal nanocarrier development. J Pharm Sci. 2008;97(11):4696–4740. [DOI] [PubMed] [Google Scholar]
- 84. Sarin H, Kanevsky AS, Wu H, et al. Physiologic upper limit of pore size in the blood–tumor barrier of malignant solid tumors. J Transl Med. 2009;7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zamboni WC, Torchilin V, Patri AK, et al. Best practices in cancer nanotechnology: perspective from NCI nanotechnology alliance. Clin Cancer Res. 2012;18(12):3229–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang Y, Wu H, Wang Z, et al. Optimized synthesis of biodegradable elastomer pegylated poly(glycerol sebacate) and their biomedical application. Polymers. 2019;11(6):E965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hao F, Li Y, Zhu J, et al. Polyethylenimine-based formulations for delivery of oligonucleotides. Curr Med Chem. 2019;26(13):2264–2284. [DOI] [PubMed] [Google Scholar]
- 88. Pan S, Xing H, Fu X, et al. The effect of photothermal therapy on osteosarcoma with polyacrylic acid-coated gold nanorods. Dose-Response. 2018;16(3).doi:10.1177/1559325818789841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sha L, Chen Z, Chen Z, Zhang A, Yang Z. Polylactic acid based nanocomposites: promising safe and biodegradable materials in biomedical field. Int J Polym Sci. 2016;2016:1–11. [Google Scholar]
- 90. Xie J, Teng L, Yang Z, Zhou C, Liu Y, Yung BC, Lee RJ. A polyethylenimine-linoleic acid conjugate for antisense oligonucleotide delivery. Biomed Res Int. 2013;2013 doi:10.1155/2013/710502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sun J, Shen J, Chen S, et al. Nanofiller reinforced biodegradable PLA/PHA composites: current status and future trends. Polymers. 2018;10(5):E505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kang C, Sun Y, Zhu J, et al. Delivery of nanoparticles for treatment of brain tumor. Curr Drug Metab. 2016;17(8):745–754. [DOI] [PubMed] [Google Scholar]
- 93. Floc’h N, Ashton S, Taylor P, et al. Optimizing therapeutic effect of aurora B inhibition in acute myeloid leukemia with AZD2811 nanoparticles. Mol Cancer Ther. 2017;16(6):1031–1040. [DOI] [PubMed] [Google Scholar]
- 94. Simon AM, Jagadeeshan S, Abraham E, et al. Poly (d, l-lactic-co-glycolide) nanoparticles for the improved therapeutic efficacy of all-trans-retinoic acid: a study of acute myeloid leukemia (AML) cell differentiation in vitro. Med Chem. 2012;8(5):805–810. [DOI] [PubMed] [Google Scholar]
- 95. Sun Y, Sun ZL. Transferrin-conjugated polymeric nanomedicine to enhance the anticancer efficacy of edelfosine in acute myeloid leukemia. Biomed Pharmacother. 2016;83:51–57. [DOI] [PubMed] [Google Scholar]
- 96. Zhu B, Zhang H, Yu L. Novel transferrin modified and doxorubicin loaded pluronic 85/lipid-polymeric nanoparticles for the treatment of leukemia: in vitro and in vivo therapeutic effect evaluation. Biomed Pharmacother. 2017;86:547–554. [DOI] [PubMed] [Google Scholar]
- 97. Gul-Uludag H, Valencia-Serna J, Kucharski C, et al. Polymeric nanoparticle-mediated silencing of CD44 receptor in CD34+ acute myeloid leukemia cells. Leuk Res. 2014;38(11):1299–1308. [DOI] [PubMed] [Google Scholar]
- 98. Chandran P, Gupta N, Retnakumari AP, et al. Simultaneous inhibition of aberrant cancer kinome using rationally designed polymer-protein core-shell nanomedicine. Nanomedicine. 2013;9(8):1317–1327. [DOI] [PubMed] [Google Scholar]
- 99. Liang B, Li N, Zhang S, et al. Idarubicin-loaded methoxy poly (ethylene glycol)-b-poly(l-lactide-co-glycolide) nanoparticles for enhancing cellular uptake and promoting antileukemia activity. Int J Nanomed. 2019;14:543–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wang Y, Xie Y, Williams J, et al. Use of polymeric CXCR4 inhibitors as siRNA delivery vehicles for the treatment of acute myeloid leukemia. Cancer Gene Ther. 2019. [DOI] [PubMed] [Google Scholar]
- 101. Gui R, Wan A, Liu X, Jin H. Intracellular fluorescent thermometry and photothermal-triggered drug release developed from gold nanoclusters and doxorubicin dual-loaded liposomes. Chem Commun. 2014;50(13):1546–1548. [DOI] [PubMed] [Google Scholar]
- 102. Ventola CL. Progress in nanomedicine: approved and investigational nanodrugs. P T. 2017;42(12):742–755. [PMC free article] [PubMed] [Google Scholar]
- 103. Do VQ, Park KH, Park JM, Lee MY. Comparative in vitro toxicity study of docetaxel and nanoxel, a docetaxel-loaded micellar formulation using cultured and blood cells. Toxicol Res. 2019;35(2):201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bernabeu E, Cagel M, Lagomarsino E, Moretton M, Chiappetta DA. Paclitaxel: what has been done and the challenges remain ahead. Int J Pharm. 2017;526(1-2):474–495. [DOI] [PubMed] [Google Scholar]
- 105. Varshosaz J, Hassanzadeh F, Sadeghi Aliabadi H, Nayebsadrian M, Banitalebi M, Rostami M. Synthesis and characterization of folate-targeted dextran/retinoic acid micelles for doxorubicin delivery in acute leukemia. Biomed Res Int. 2014;2014:525684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Alakhova DY, Zhao Y, Li S, Kabanov AV. Effect of doxorubicin/pluronic SP1049C on tumorigenicity, aggressiveness, DNA methylation and stem cell markers in murine leukemia. PLoS One. 2013;8(8): e72238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lim J, Simanek EE. Triazine dendrimers as drug delivery systems: from synthesis to therapy. Adv Drug Deliv Rev. 2012;64(9):826–835. [DOI] [PubMed] [Google Scholar]
- 108. Szulc A, Pulaski L, Appelhans D, Voit B, Klajnert-Maculewicz B. Sugar-modified poly(propylene imine) dendrimers as drug delivery agents for cytarabine to overcome drug resistance. Int J Pharm. 2016;513(1-2):572–583. [DOI] [PubMed] [Google Scholar]
- 109. Sharma H, Mishra PK, Talegaonkar S, Vaidya B. Metal nanoparticles: a theranostic nanotool against cancer. Drug Discov Today. 2015;20(9):1143–1151. [DOI] [PubMed] [Google Scholar]
- 110. Petrushev B, Boca S, Simon T, et al. Gold nanoparticles enhance the effect of tyrosine kinase inhibitors in acute myeloid leukemia therapy. Int J Nanomed. 2016;11:641–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Shen N, Yan F, Pang J, et al. HDL-AuNPs-BMS nanoparticle conjugates as molecularly targeted therapy for leukemia. ACS Appl Mater Interfaces. 2018;10(17):14454–14462. [DOI] [PubMed] [Google Scholar]
- 112. Huynh NT, Passirani C, Saulnier P, Benoit JP. Lipid nanocapsules: a new platform for nanomedicine. Int J Pharm. 2009;379(2):201–209. [DOI] [PubMed] [Google Scholar]
- 113. Khajavinia A, Varshosaz J, Dehkordi AJ. Targeting etoposide to acute myelogenous leukaemia cells using nanostructured lipid carriers coated with transferrin. Nanotechnology. 2012;23(40):405101. [DOI] [PubMed] [Google Scholar]
- 114. Silva EL, Lima FA, Carneiro G, et al. Improved in vitro antileukemic activity of all-trans retinoic acid loaded in cholesteryl butyrate solid lipid nanoparticles. J Nanosci Nanotechnol. 2016;16(2):1291–1300. [DOI] [PubMed] [Google Scholar]
- 115. Enjeti AK, D’Crus A, Melville K, Verrills NM, Rowlings P. A systematic evaluation of the safety and toxicity of fingolimod for its potential use in the treatment of acute myeloid leukaemia. Anti-Cancer Drugs. 2016;27(6):560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Estella-Hermoso de Mendoza A, Castello-Cros R, Imbuluzqueta Eet al. Lipid nanosystems enhance the bioavailability and the therapeutic efficacy of FTY720 in acute myeloid leukemia. J Biomed Nanotechnol. 2015;11(4):691–701. [DOI] [PubMed] [Google Scholar]
- 117. Chandran P, Kavalakatt A, Malarvizhi GL, et al. Epigenetics targeted protein-vorinostat nanomedicine inducing apoptosis in heterogeneous population of primary acute myeloid leukemia cells including refractory and relapsed cases. Nanomedicine. 2014;10(4):721–732. [DOI] [PubMed] [Google Scholar]
- 118. Narmani A, Rezvani M, Farhood B, et al. Folic acid functionalized nanoparticles as pharmaceutical carriers in drug delivery systems. Drug Dev Res. 2019;80(4):404–424. [DOI] [PubMed] [Google Scholar]
- 119. Kalmouni M, Al-Hosani S, Magzoub M. Cancer targeting peptides. Cell Mol Life Sci. 2019;76(11):2171–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Carter T, Mulholland P, Chester K. Antibody-targeted nanoparticles for cancer treatment. Immunotherapy. 2016;8(8):941–958. [DOI] [PubMed] [Google Scholar]
- 121. Ngo B, Van Riper JM, Cantley LC, Yun J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat Rev Cancer. 2019;19(5):271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Choudhury H, Pandey M, Chin PX, et al. Transferrin receptors-targeting nanocarriers for efficient targeted delivery and transcytosis of drugs into the brain tumors: a review of recent advancements and emerging trends. Drug Deliv Transl Res. 2018;8(5):1545–1563. [DOI] [PubMed] [Google Scholar]
- 123. Tortorella S, Karagiannis TC. Transferrin receptor-mediated endocytosis: a useful target for cancer therapy. J Membr Biol. 2014;247(4):291–307. [DOI] [PubMed] [Google Scholar]
- 124. Ye J, Liu E, Yu Z, et al. CPP-assisted intracellular drug delivery, what is next? Int J Mol Sci. 2016;17(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Regberg J, Srimanee A, Langel U. Applications of cell-penetrating peptides for tumor targeting and future cancer therapies. Pharmaceuticals (Basel). 2012;5(9):991–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Chen Y, Xie X, Wu A, et al. A synthetic cell-penetrating peptide derived from nuclear localization signal of EPS8 exerts anticancer activity against acute myeloid leukemia. J Exp Clin Cancer Res. 2018;37(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Agarwal A, MacKenzie RJ, Pippa R, et al. Antagonism of SET using OP449 enhances the efficacy of tyrosine kinase inhibitors and overcomes drug resistance in myeloid leukemia. Clin Cancer Res. 2014;20(8):2092–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Yin L, Wu Z, Avigan D, et al. MUC1-C oncoprotein suppresses reactive oxygen species-induced terminal differentiation of acute myelogenous leukemia cells. Blood. 2011;117(18):4863–4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Liu S, Yin L, Stroopinsky D, et al. MUC1-C oncoprotein promotes FLT3 receptor activation in acute myeloid leukemia cells. Blood. 2014;123(5):734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Li K, Lv XX, Hua F, et al. Targeting acute myeloid leukemia with a proapoptotic peptide conjugated to a Toll-like receptor 2-mediated cell-penetrating peptide. Int J Cancer. 2014;134(3):692–702. [DOI] [PubMed] [Google Scholar]
- 131. Hughes B. Antibody–drug conjugates for cancer: poised to deliver? Nat Rev Drug Discov. 2010;9:665. [DOI] [PubMed] [Google Scholar]
- 132. Simard P, Leroux JC. . PH-sensitive immunoliposomes specific to the CD33 cell surface antigen of leukemic cells. Int J Pharm. 2009;381(2):86–96. [DOI] [PubMed] [Google Scholar]
- 133. Al-Hussaini M, Rettig MP, Ritchey JK, et al. Targeting CD123 in acute myeloid leukemia using a T-cell-directed dual-affinity retargeting platform. Blood. 2016;127(1):122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wang Y, Liu F, Wang Q, et al. A novel immunoliposome mediated by CD123 antibody targeting to acute myeloid leukemia cells. Int J Pharm. 2017;529(1-2):531–542. [DOI] [PubMed] [Google Scholar]
- 135. Liu FR, Jin H, Wang Y, et al. Anti-CD123 antibody-modified niosomes for targeted delivery of daunorubicin against acute myeloid leukemia. Drug Deliv. 2017;24(1):882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Deshpande AJ, Buske C. Lymphoid progenitors as candidate cancer stem cells in AML: new perspectives. Cell Cycle (Georgetown, Tex). 2007;6(5):543–545. [DOI] [PubMed] [Google Scholar]
- 137. Mandal T, Beck M, Kirsten N, Linden M, Buske C. Targeting murine leukemic stem cells by antibody functionalized mesoporous silica nanoparticles. Sci Rep. 2018;8(1):989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Daniels TR, Delgado T, Rodriguez JA, Helguera G, Penichet ML. The transferrin receptor part I: biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol. 2006;121(2):144–158. [DOI] [PubMed] [Google Scholar]
- 139. Al-Hatamleh MAI, Ahmad S, Boer JC, et al. A perspective review on the role of nanomedicine in the modulation of TNF-TNFR2 axis in breast cancer immunotherapy. J Oncol. 2019. doi:10.1155/2019/6313242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Gudbergsson JM, Jonsson K, Simonsen JB, Johnsen KB. Systematic review of targeted extracellular vesicles for drug delivery—considerations on methodological and biological heterogeneity. J Control Release. 2019;306:108–120. [DOI] [PubMed] [Google Scholar]
- 141. Liu JF, Jang B, Issadore D, Tsourkas A. Use of magnetic fields and nanoparticles to trigger drug release and improve tumor targeting. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11(6):e1571 doi:10.1002/wnan.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Xiong Y, Wang Y, Tiruthani K. Tumor immune microenvironment and nano-immunotherapeutics in colorectal cancer. Nanomedicine. 2019;21:102034 doi:10.1016/j.nano.2019.102034. [DOI] [PubMed] [Google Scholar]
- 143. Liu Y, Meyer Zaika W, Franzka S, Schmid G, Tsoli M, Kuhn H. Gold-cluster degradation by the transition of B-DNA into A-DNA and the formation of nanowires. Angew Chem Int Ed Engl. 2003;42(25):2853–2857. [DOI] [PubMed] [Google Scholar]
- 144. Semmler Behnke M, Kreyling WG, Lipka J, et al. Biodistribution of 1.4- and 18-nm gold particles in rats. Small. 2008;4(12):2108–2111. [DOI] [PubMed] [Google Scholar]
- 145. De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJ, Geertsma RE. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29(12):1912–1919. [DOI] [PubMed] [Google Scholar]
- 146. Zhang G, Yang Z, Lu W, et al. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials. 2009;30(10):1928–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kim JH, Kim JH, Kim KW, Kim MH, Yu YS. Intravenously administered gold nanoparticles pass through the blood–retinal barrier depending on the particle size, and induce no retinal toxicity. Nanotechnology. 2009;20(50):505101. [DOI] [PubMed] [Google Scholar]
- 148. Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J Control Release. 2010;145(3):182–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016;6(10):1106–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Truong NP, Whittaker MR, Mak CW, Davis TP. The importance of nanoparticle shape in cancer drug delivery. Expert Opin Drug Deliv. 2015;12(1):129–142. [DOI] [PubMed] [Google Scholar]
- 151. Butcher NJ, Mortimer GM, Minchin RF. Drug delivery: unravelling the stealth effect. Nat Nanotechnol. 2016;11(4):310. [DOI] [PubMed] [Google Scholar]