Abstract
During viral infections, significant numbers of T cells are activated in a T cell receptor-independent and cytokine-dependent manner, a phenomenon referred to as “bystander activation.” Cytokines, including type I interferons, interleukin-18, and interleukin-15, are the most important factors that induce bystander activation of T cells, each of which plays a somewhat different role. Bystander T cells lack specificity for the pathogen, but can nevertheless impact the course of the immune response to the infection. For example, bystander-activated CD8+ T cells can participate in protective immunity by secreting cytokines, such as interferon-γ. They also mediate host injury by exerting cytotoxicity that is facilitated by natural killer cell-activating receptors, such as NKG2D, and cytolytic molecules, such as granzyme B. Interestingly, it has been recently reported that there is a strong association between the cytolytic function of bystander-activated CD8+ T cells and host tissue injury in patients with acute hepatitis A virus infection. The current review addresses the induction of bystander CD8+ T cells, their effector functions, and their potential roles in immunity to infection, immunopathology, and autoimmunity.
Subject terms: Viral infection, Viral hepatitis
Viral infection: Inciting an immunological mob
Immune cells that are non-specifically activated during infection can offer protection, but may also inflict collateral damage on infected patients. T cells normally mount an antigen-specific immune response, but certain T cells can become stimulated during viral infection without selective activation by a particular antigen. Tae-Shin Kim and Eui-Cheol Shin at KAIST in Daejon, South Korea, have reviewed current insights into this ‘bystander activation’ phenomenon. They explore how the immune response to viruses such as influenza and hepatitis A produces molecular signals that induce bystander activation of ‘killer’ T cells. In some scenarios, this leads to stronger immune protection, but these cells can also damage host tissues, or contribute to disease progression. Modulating this nonspecific response could prove valuable in managing the severity of viral disease.
Introduction
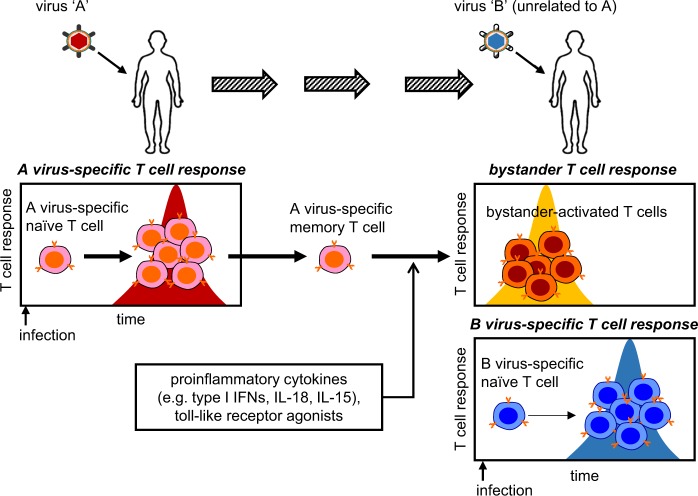
During the course of a viral infection, various immune cells are sequentially activated to eliminate the invading virus. While these immune responses are generally beneficial, they can also cause collateral damage to the host, referred to as “immunopathology”1. The nature of the immunopathological response can be significantly impacted by the remnants of the immune response to previous unrelated infections, that is, heterologous immunity2. Heterologous immune responses can include both antigen-dependent T cell activation by cross-reactive memory T cells and antigen-independent activation by cytokines (i.e., bystander activation)3,4. While intensive studies have revealed the nature and pathophysiological significance of T cell cross-reactivity2, relatively little is known about the induction and function of bystander T cells. In this review, we will discuss various aspects of the bystander CD8+ T cell response (Fig. 1), including the underlying mechanisms of T cell activation, the pathophysiological impact of activated bystander T cells during infection, and the longer-term clinical implications.
Fig. 1. Bystander activation of memory T cells during viral infections.
Upon infection with a virus “A,” naive T cells specific for the virus undergo a T cell receptor (TCR)-dependent clonal expansion and then a contraction, leaving memory T cells. Next, infection with an unrelated virus “B” induces TCR-dependent responses as well as TCR-independent “bystander activation” of virus A-specific memory T cells. This bystander response is rapidly induced by cytokines or Toll-like receptor agonists. Type I IFNs, type I interferons; IL-18, interleukin-18; IL-15, interleukin-15
Bystander activation of CD8+ T cells during viral infections
Acute hepatitis A virus infection
Infection of adults with hepatitis A virus (HAV) can result in acute hepatitis A (AHA) and severe liver injury. It was previously hypothesized that liver injury resulted from an excessive virus-specific T cell response during AHA5,6. Consistent with this hypothesis, HAV-specific CD8+ T cells were detected by both HLA-A2 tetramer binding and intracellular cytokine staining in acutely infected patients7. However, a study using chimpanzees challenged with HAV showed that functional HAV-specific CD8+ T cells increased only after viremia and liver injury began to decline6.
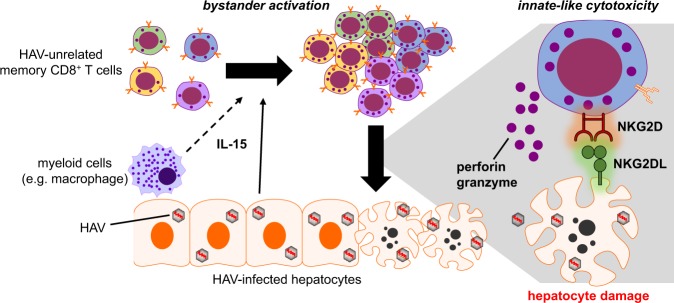
Recently, our group demonstrated that CD8+ T cells specific for pathogens other than HAV are activated by a T cell receptor (TCR)-independent but interleukin-15 (IL-15)-dependent mechanism during acute HAV infection8 (Fig. 2). These bystander-activated CD8+ T cells expressed high levels of cytotoxic molecules (perforin and granzyme B) and natural killer (NK) cell-activating receptors (NKG2D and NKp30) and exhibited innate-like cytotoxicity against hepatocytes. Furthermore, the number of bystander CD38+HLA-DR+ (activated) CD8+ T cells but not that of HAV-specific CD8+ T cells was strongly correlated with the level of liver injury during AHA8. These activated, HAV-unrelated CD8+ T cells were specific for a number of unrelated viruses, including human cytomegalovirus (HCMV), Epstein–Barr virus (EBV), influenza A virus (IAV), respiratory syncytial virus, and vaccinia virus8. It is unlikely that these cells were activated by TCR-dependent cross-reactivity given the very small HAV RNA genome (7.5 kb)5 and the limited amino acid sequence homology between HAV proteins and epitope peptides used in the tetramer detection of HAV-unrelated viruses8. In addition, the significant increase in NKG2D expression on HAV-unrelated memory CD8+ T cells compared to that on HAV-specific CD8+ T cells in AHA patients further supports bystander activation rather than TCR-dependent activation. Treatment of peripheral blood mononuclear cells from healthy donors with IL-15 increases the level of NKG2D expression in memory CD8+ T cells, whereas TCR stimulation with an anti-CD3 antibody or cognate peptide does not. Interestingly, the expression of NKG2D is not significantly increased when the cells are stimulated with both IL-15 and anti-CD3 antibodies8, suggesting that NKG2D upregulation on memory CD8+ T cells reflects activation by IL-15 in the absence of TCR stimulation8. Taken together, these findings provide considerable evidence that the activation of HAV-unrelated CD8+ T cells is mediated by an antigen-independent bystander mechanism.
Fig. 2. Pathological role of bystander-activated CD8+ T cells in acute hepatitis A virus (HAV) infection.
During acute hepatitis A, memory CD8+ T cells specific for HAV-unrelated viruses undergo IL-15-dependent bystander activation. IL-15 is produced by hepatocytes and possibly myeloid cells in the infected liver. These activated, HAV-unrelated CD8+ T cells exhibit “innate-like cytotoxicity” to hepatocytes, which is triggered by the ligation of natural killer cell-activating receptors (e.g., NKG2D) with their ligands
Hepatitis B virus and hepatitis C virus infections
Nonspecific T cell responses have not been extensively analyzed during hepatitis B virus (HBV) and hepatitis C virus (HCV) infections. Unlike HAV, both HBV and HCV can establish chronic persistent infection in the host9. Because virus-specific CD8+ T cells become functionally exhausted during the chronic stages of viral infection, it has been postulated that bystander CD8+ T cells might contribute to immunopathologic liver injury during chronic HBV or HCV infections9.
Maini et al.10 examined the frequency and function of HBV-specific CD8+ T cells in patients with chronic HBV infection. They unexpectedly found that liver injury was not associated with an increase in the frequency of HBV-specific CD8+ T cells. Instead, their data suggested that HBV-unrelated CD8+ T cells infiltrating the liver contribute to pathological injury10. In support of this idea, Sandalova et al.11 have reported that CD8+ T cells specific for HBV-unrelated pathogens (HCMV and EBV) were activated in the absence of evidence for HCMV or EBV reactivation in 20 patients with acute HBV infection.
In the case of HCV infection, there are no published reports of bystander activation. However, it has been shown that naive CD8+ T cells from patients with chronic hepatitis C exhibited hyperactivation (accompanied by a decreased expression of CD5) and differentiated into memory-phenotype cells12. The relationship between this phenomenon and bystander activation remains unknown.
Human immunodeficiency virus infection
Systemic immune activation, specifically for T and B cells, is one of the hallmarks of untreated chronic human immunodeficiency virus (HIV) infection13,14. Although it has been hypothesized that both antigen-dependent and antigen-independent mechanisms mediate HIV-associated immune activation, the exact mechanism remains unclear13.
Recent studies have provided evidence for bystander activation during HIV infection. The Oxenius group reported that HIV-1 rebound due to the interruption of antiretroviral therapy (ART) led to the activation and expansion of CD8+ T cells irrespective of their antigen specificities15. Their data further suggested that myeloid dendritic cell (DC) activation and the resulting IL-15 production drive bystander activation of CD8+ T cells during HIV-1 infection15. Younes et al.16 examined the T cell repertoire of untreated HIV-infected patients and found that the TCR diversity of cycling effector/memory CD8+ T cells reflected that of the entire effector/memory CD8+ population. The authors concluded that the activation and expansion of the CD8+ T cells was driven by nonspecific, bystander activation16. In fact, bystander activation of CD8+ T cells appears to occur early in HIV infection. During primary HIV infection, activation markers (e.g., CD38 and HLA-DR) are upregulated in the total CD8+ T cell population and, more importantly, in the CD8+ T cells specific for HIV-unrelated viruses, such as EBV, HCMV, and IAV17,18.
Immune activation is an important factor contributing to disease progression during HIV infection. Importantly, bystander activation of CD8+ T cells may be one of the drivers of the disease, as a strong correlation between the activation of CD8+ T cells and the rate of CD4+ T cell loss has been reported in untreated patients19. Even in patients undergoing ART, the persistent activation of CD8+ T cells was associated with decreased recovery of CD4+ T cells20 and an increased risk of non-acquired immunodeficiency syndrome (AIDS)-related clinical events21. Furthermore, a study conducted in sooty mangabey monkeys offered interesting insight into the role of bystander immune activation in HIV pathogenesis. Sooty mangabeys are natural hosts for simian immunodeficiency virus (SIV) infection, but do not develop AIDS despite a high level of viral replication22. This nonpathogenic infection in sooty mangabeys is accompanied by low levels of immune activation compared to that in HIV-infected humans22.
Influenza A virus infection
It is well established that antigen-specific T cells play a central role in controlling IAV infection23–27. However, much less is known about the impact of activated bystander memory CD8+ T cells and their possible contribution to pathogenesis28–30.
Early studies showed that IAV infections draw bystander memory CD8+ T cells into the lung airways from the circulation28,31. Despite not being specific for IAV, these memory CD8+ T cells express strong cytolytic capacity29,30. However, it remains unclear whether these recruited bystander cells have an essential role during a primary infection, although they may accelerate the induction of inflammation during a recall response29,30. In humans, Sandalova et al.11 have shown that memory CD8+ T cells specific for HCMV or EBV exhibited an activated phenotype (CD38+HLA-DR+) during acute IAV infection, although the number of patients analyzed was small. Recent studies have shown that mucosal-associated invariant T (MAIT) cells can also be activated in a bystander manner during influenza infection and participate in protective immune response both in humans and mice32,33.
Overall, a significant degree of bystander activation of memory T cells occurs during influenza infection, although its impact on the course of the infection, if any, is not clear. It is also unclear whether bystander-activated CD8+ T cells contribute to immunopathology in influenza.
Factors inducing bystander activation
The cytokines that induce bystander activation generally overlap with those that regulate the activation of antigen-specific CD8+ T cells. Specifically, innate inflammatory cytokines seem to be crucial for inducing bystander activation during infection. Pathogen-associated molecular pattern (PAMP) signaling through Toll-like receptors (TLRs) also supplies important signals for bystander activation.
Type I interferons
Sprent’s group was the first to report the importance of type I interferons (IFNs) in nonspecific T cell proliferation upon viral infection or lipopolysaccharide injection4,34. Indeed, type I IFNs may act directly on CD8+ T cells and drive the expansion of T cells during infection with lymphocytic choriomeningitis virus35. Moreover, an intriguing study on the pathogenesis of HIV infection suggested that type I IFNs are the key drivers of T cell activation and disease progression in patients with persistent HIV infection36. Blocking-type I IFN signaling in humanized mouse models of chronic HIV infection results in a reduction in hyperactivation of T cells and their functional recovery37,38.
The mechanism of immune bystander activation of memory CD8+ T cells by type I IFNs is not known. When antigen-specific memory CD8+ T cells are treated in vitro with type I IFNs, they do not exhibit significant functional activation unless also treated with other cytokines, such as IL-1839. This result suggests that type I IFNs require additional secondary signals or accessory cells to fully activate bystander T cells. Accordingly, type I IFN signaling may induce IL-15 production by accessory cells40,41 and increase T cell responsiveness to IL-1842. Both IL-15 and IL-18 are notable mediators of bystander activation.
It is also possible that type I IFNs may have negative effects on the bystander activation of memory T cells during viral infection. A series of experiments performed by Welsh and co-workers43,44 revealed that virus-induced type I IFNs mediate rapid attrition of bystander CD8+ T cells, especially those with a memory phenotype. This finding suggests that weak rather than strong-type I IFN responses may be optimal for inducing bystander CD8+ T cells. In this regard, it is interesting to note that acute HAV infection elicits a relatively weak type I IFN-stimulated gene (ISG) response45 and is accompanied by vigorous immunopathology mediated by bystander-activated CD8+ T cells8.
Interleukin-18
IL-18, a member of the IL-1 family of cytokines, is one of the most well-characterized cytokines that induce antigen-independent IFN-γ production by effector and memory CD8+ T cells during microbial infections46. Effector and memory CD8+ T cells that are treated with cytokine combinations, including both type I IFNs and IL-18, exhibit an activated phenotype (i.e., CD69+) and high levels of IFN-γ production39. In addition, studies in vitro and in murine infection models have demonstrated a dramatic synergism between IL-18 and other proinflammatory cytokines (e.g., IL-12, IL-2, IL-15, and IL-21) for inducing antigen-nonspecific IFN-γ production39,47–49. Thus, it appears that IL-18 cooperates with a wide range of cytokines in the inflammatory milieu to induce bystander activation of T cells.
IL-18 responsiveness by effector and memory CD8+ T cells but not naive CD8+ T cells results from selective expression of the IL-18 receptor48,49. Recently, Martin et al.50 showed that memory CD8+ T cells exhibited a gradual reduction in the expression of IL-12 and IL-18 receptors following initial antigen stimulation. Consistent with this finding is a decrease in the ability of the cells to be activated by bystander signals. The opposite is true in the situation when cells are repeatedly stimulated with the antigen.
Interleukin-15
IL-15, a member of the common γ-chain family of cytokines, is another key factor involved in mediating bystander activation of CD8+ T cells in both mice and humans8,15,16,51,52. IL-15 has been shown to function in various aspects of lymphoid biology, including the development of NK and invariant NK T (iNKT) cells, the activation of NK cells, and the homeostatic maintenance of memory CD8+ T cells (reviewed elsewhere53). Importantly, IL-15 can potently induce the activation of murine effector and memory CD8+ T cells when synergizing with IL-12, IL-18, or type I IFNs39. We and others have shown that memory CD8+ T cells from healthy human individuals strongly respond to IL-15 by expressing markers of activation (CD38 and HLA-DR), proliferation (Ki-67), and cytotoxic activity (such as granzyme B)8,11,16. In the pathologic conditions such as AHA and untreated HIV-1 infection, the level of IL-15 is elevated in the serum and lymph nodes, respectively. This finding suggests that IL-15 drives bystander activation of CD8+ T cells in pathologic situations8,16.
The remarkable feature of IL-15 compared to IL-18 is that it confers cytolytic ability on memory CD8+ T cells, which can be triggered by NK-activating receptors, such as NKG2D. This phenomenon is referred to as “innate-like (or NK-like) cytotoxicity.” In addition, IL-15 promotes the expression of cytolytic molecules (e.g., granzyme B and perforin)8,52,54. A dual control mechanism for the two major effector functions of bystander-activated CD8+ T cells, cytokine secretion and cytolytic function, has been suggested by Soudja et al.52 whereby IL-18 induces IFN-γ secretion and IL-15 induces the expression of cytolytic molecules (Fig. 3).
Fig. 3. Effector functions of bystander-activated CD8+ T cells.
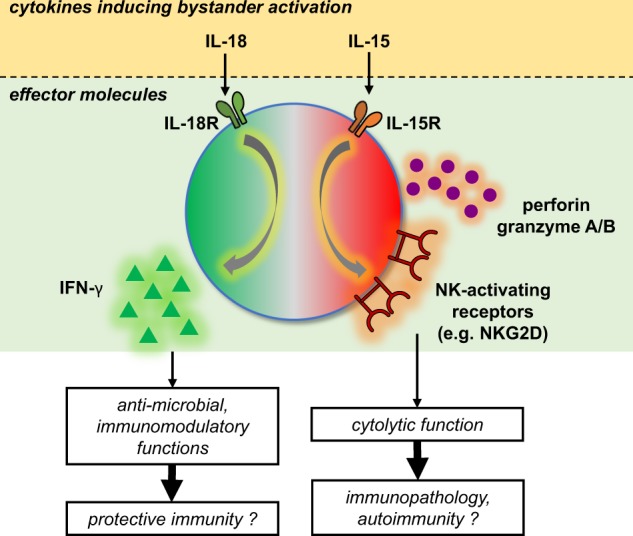
Innate inflammatory cytokines, such as IL-18 and IL-15, are crucial for inducing bystander activation during infection. While signaling through IL-18R may contribute to protective immunity via IFN-γ secretion, signaling through IL-15R may lead to immunopathology via NKG2D-dependent cytotoxicity. IL-18R, interleukin-18 receptor; IL-15R, interleukin-15 receptor
Sentinel myeloid cells such as DCs and inflammatory monocytes have been considered the source of IL-15 and IL-18 during viral, bacterial, and fungal infections52,55. However, many other cell types, including both hematopoietic and nonhematopoietic cells, can also express IL-1556. Indeed, epithelial cells such as enterocytes and hepatocytes have been suggested as the main source of IL-15 in the case of celiac disease and AHA, respectively8,57. Further research is needed to determine the relative contributions and functional differences between myeloid and epithelial cells in the production of bystander activation-inducing cytokines during viral infections.
Toll-like receptors
TLRs are the key receptors in innate immune cells that detect PAMPs and initiate the innate immune response. However, TLRs also function in cells of the adaptive immune system, including T cells58. Earlier studies revealed that murine and human effector/memory CD8+ T cells express TLR1/2/6 and TLR3, respectively, which function as costimulatory receptors, lowering the threshold for TCR activation59,60. More recently, Salerno et al.61 discovered that murine memory CD8+ T cells can be directly stimulated with the ligands for TLR2 and TLR7 to produce IFN-γ. However, TLR2 or TLR7 stimuli in combination with TCR triggering drive cells to produce IFN-γ, tumor necrosis factor-α (TNF-α), and IL-262.
Given that TLR2, TLR3, and TLR7 react with various types of ligands derived from a wide range of pathogens63, these data imply that TLR-mediated bystander activation can occur during various kinds of infections64. Indeed, TLR2-mediated bystander activation of T cells has been demonstrated to contribute to the development of arthritis in mice infected with Borrelia burgdorferi, a causative bacterial pathogen for Lyme arthritis65. However, evidence of TLR-dependent bystander activation is still lacking for viral infections. TLR3 and TLR7/8 recognize double- and single-stranded RNAs, respectively, which are the PAMPs usually associated with viruses63. Thus, future studies need to focus on the role of virus-related TLRs in the bystander activation of T cells.
Effector functions of bystander-activated CD8+ T cells
Bystander-activated CD8+ T cells share many effector functions with antigen-specific CD8+ T cells, such as cytotoxicity and cytokine secretion.
Cytotoxicity and NKG2D
NKG2D encoded by KLRK1 was first identified in human NK cells as one of the NK cell-activating receptors and was subsequently shown to be expressed by many other lymphoid cells, such as iNKT cells, γδ T cells, and CD8+ αβ T cells66. In CD8+ T cells, it has been suggested that NKG2D mediates a costimulatory function in the presence of TCR engagement67–70. Engagement of the TCR and NKG2D results in enhanced cytokine production and proliferation in CD8+ T cells with effector67 and memory phenotypes68,69. The expression of NKG2D ligands is upregulated in multiple tissues during stress conditions, such as viral infection and cellular transformation71, and it has been hypothesized that signaling downstream of NKG2D–NKG2DL interactions regulates the activation of antigen-stimulated effector/memory T cells in the local tissue environment67.
NKG2D signaling can also elicit cytolytic function in the absence of TCR engagement72. For example, freshly isolated CD8+ TCRαβ+ intraepithelial lymphocytes (IELs) from patients with active celiac disease or IELs prestimulated with IL-15 exhibit NKG2D-mediated cytotoxicity without TCR engagement67,73. Moreover, bystander-activated CD8+ T cells in patients with AHA exert innate-like cytotoxicity against hepatocytes via a TCR-independent, NKG2D-dependent manner8. This effect is reminiscent of what occurs in patients with celiac disease73. It is noteworthy that excessive killing of target cells by bystander-activated CD8+ T cells may initiate and propagate the cycle of inflammation and immunopathology74. The role of NKG2D in mediating the effector function of bystander-activated CD8+ T cells has also been demonstrated in mouse models of bacterial and parasitic infections75,76.
It should be noted that signaling through the IL-15 receptor induces the upregulation of NKG2D expression8,54,67,73. Intriguingly, concurrent TCR activation abrogates IL-15-induced upregulation of NKG2D on the surface of memory CD8+ T cells8. This result supports the idea that NKG2D preferentially acts in the absence of TCR stimulation. Another important signal that downregulates NKG2D expression during viral infection is type I IFN77. Thus, NKG2D expression in bystander-activated CD8+ T cells is probably modulated through the balanced action of various proinflammatory cytokines (e.g., IL-15 and type I IFNs) and TCR signaling.
Interferon-γ
Cytotoxic activity and cytokine production are the major effector mechanisms mediated by CD8+ T cells during viral infection78–80. The best-known examples of CD8+ T cell-derived cytokines are IFN-γ and TNF-α61,81. While the expression of both IFN-γ and TNF-α is induced in CD8+ T cells stimulated with peptide antigen, only IFN-γ is induced upon treatment with cytokines such as IL-12 and IL-1881. Similarly, antigen-experienced murine CD8+ T cells stimulated with TLR ligands produce IFN-γ but not TNF-α61.
In line with these data, several studies using various infection models (e.g., bacteria, viruses, and parasites) showed that memory CD8+ cells underwent bystander activation with rapid upregulation of IFN-γ47,48,52. As expected, the induction of IFN-γ in bystander-activated CD8+ T cells conferred enhanced control over the challenging bacterial pathogen48,52. Although yet to be confirmed, bystander-derived IFN-γ may also have a protective effect against viral pathogens82. The protective action of IFN-γ produced by bystander-activated T cells clearly contrasts with the pathological consequences of NKG2D-mediated cytotoxicity observed during AHA in humans or during Leishmania infection in a mouse model8,76.
A recent paper using high-throughput single-cell analysis of CD8+ T cells offered insight into how the same CD8+ T cells can exhibit different functional consequences according to the context. When antigen-specific CD8+ T cells were stimulated with cognate antigens, they exhibited either cytokine secretion or cytolytic activity (but rarely both), indicating that these two functions are independently regulated83. This functional differentiation may also be true of bystander-activated T cells. Indeed, while both IFN-γ secretion and NKG2D-mediated cytolysis are observed in bystander-activated CD8+ T cells during Listeria infection48,52, only NKG2D-mediated cytolysis and consequent immunopathology are noticeable during Leishmania infection76,84. The factors contributing to this functional difference are currently unclear, but may include the pathogen load, chronicity of inflammation76, location of CD8+ T cells84, and surrounding cytokine milieu52.
Clinical implications of bystander activation
Tough et al.4 who first identified bystander activation during viral infection, predicted that the physiological role of bystander activation is to maintain memory CD8+ T cells in vivo in the absence of further cognate antigenic stimulation. The hypothesis seemed plausible; however, it has not been demonstrated experimentally. Although bystander-activated CD8+ T cells express functional effectors, the precise role in host immunity at the time of infection or thereafter has not been clearly defined.
Protective vs. pathological role
Bystander activation of T cells during the early stages of infections may contribute to an overall protective immune response. Compared to the antigen-specific T cell response, which takes several days to develop, bystander activation of memory T cells can occur rapidly in response to innate cytokines (e.g., type I IFNs, IL-18, and IL-15), establishing a primary line of defense4,47–49,52. Despite lacking specificity for the invading pathogen, these cells may engage an inflammatory process that accelerates immune recruitment to the site and helps to control pathogen loads through the rapid production of IFN-γ, which has direct antimicrobial and immunomodulatory functions82,85 (Fig. 3). Indeed, the protective function of adoptively transferred bystander memory T cells was especially evident in IFN-γ-deficient recipient mice86.
Perhaps, a more clinically important question is the role of bystander activation in contributing to immunopathology. As described above, bystander-activated T cell-mediated immunopathology is observed in mainly local tissues (e.g., hepatocytes in AHA and skin lesions in Leishmania infection) and after sustained inflammation8,76 (Fig. 2). These results suggest that bystander-activated CD8+ T cells have different phenotypic and functional characteristics depending on their location and duration of exposure to inflammation. More studies are needed to clarify the conditions that induce bystander-activated CD8+ T cells involved in immunopathology.
Implications for autoimmunity and antitumor immunity
What would happen if CD8+ T cells specific for self-antigens were activated via a bystander manner during infections? In fact, both microbial infections and bystander T cell activation have long been suggested as contributing factors for autoimmune diseases14,87,88. In this regard, a scenario in which bystander activation of T cells triggered by viral infections accelerates the onset of type 1 diabetes has been supported in animal models, although clinical data are lacking89. Interestingly, autoreactive T cells are dependent on IL-15 for their maintenance and antigen-independent activation90. Furthermore, autoreactive CD8+ T cells primed with IL-15 and IL-21 are able to induce disease in a murine model of autoimmune diabetes91. Recently, memory CD4+ T cells have been shown to undergo bystander activation92 and increase the susceptibility of mice to experimental autoimmune encephalomyelitis, a model for multiple sclerosis93. In the future, it will be interesting to investigate the relationship between viral infections with strong bystander activation, such as AHA, and the development of subsequent autoimmune complications.
Bystander activation of CD8+ T cells may play a role in antitumor immune responses. In mice treated with highly active immunotherapeutic agents, such as a CD40 agonist and IL-2, memory CD8+ T cells underwent bystander activation with upregulation of NKG2D and granzyme B94. In addition, recent elegant studies have revealed an abundance of intratumoral bystander CD8+ T cells without tumor antigen specificity in various types of human cancer, although their roles are not yet clear95–97. These findings suggest that bystander CD8+ T cells may participate in antitumor immune responses.
Conclusion and future perspectives
Bystander activation of memory CD8+ T cells by cytokine stimulation is an important aspect of immune responses to pathogens. Antigen-independent activation of these T cells may either contribute to protection or initiate aberrant immune responses, such as immunopathology or autoimmunity. Despite ample evidence demonstrating the occurrence of bystander activation during viral infections, its pathophysiological role remains poorly understood.
Many aspects of bystander activation regarding its induction and function have been revealed using animal models. Recently, reported “dirty” mouse models—laboratory mice that have been co-housed with pet store mice or that have undergone sequential infection—are an example of how a host’s infection history can change the immune response in subsequent events and provide a realistic model to examine bystander activation98,99.
In the future, we need to consider bystander-activated T cells as a therapeutic target to alleviate severe immunopathology during viral diseases and prevent autoimmunity following viral infections.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2018R1A6A3A01010977) and by research grants through the NRF funded by the Ministry of Science and ICT (NRF-2017R1A2A1A17069782 and NRF-2018M3A9D3079498).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Graham AL, Allen JE, Read AF. Evolutionary causes and consequences of immunopathology. Annu. Rev. Ecol. Evol. Sci. 2005;36:373–397. doi: 10.1146/annurev.ecolsys.36.102003.152622. [DOI] [Google Scholar]
- 2.Welsh RM, Che JW, Brehm MA, Selin LK. Heterologous immunity between viruses. Immunol. Rev. 2010;235:244–266. doi: 10.1111/j.0105-2896.2010.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welsh RM, Selin LK. No one is naive: the significance of heterologous T-cell immunity. Nat. Rev. Immunol. 2002;2:417–426. doi: 10.1038/nri820. [DOI] [PubMed] [Google Scholar]
- 4.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 5.Lemon SM, Ott JJ, Van Damme P, Shouval D. Type A viral hepatitis: a summary and update on the molecular virology, epidemiology, pathogenesis and prevention. J. Hepatol. 2018;68:167–184. doi: 10.1016/j.jhep.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y, et al. Dominance of the CD4(+) T helper cell response during acute resolving hepatitis A virus infection. J. Exp. Med. 2012;209:1481–1492. doi: 10.1084/jem.20111906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulte I, et al. Characterization of CD8+ T-cell response in acute and resolved hepatitis A virus infection. J. Hepatol. 2011;54:201–208. doi: 10.1016/j.jhep.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Kim J, et al. Innate-like cytotoxic function of bystander-activated CD8(+) T cells is associated with liver injury in acute hepatitis A. Immunity. 2018;48:161–173. doi: 10.1016/j.immuni.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 9.Shin EC, Sung PS, Park SH. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat. Rev. Immunol. 2016;16:509–523. doi: 10.1038/nri.2016.69. [DOI] [PubMed] [Google Scholar]
- 10.Maini MK, et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J. Exp. Med. 2000;191:1269–1280. doi: 10.1084/jem.191.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandalova E, et al. Contribution of herpesvirus specific CD8 T cells to anti-viral T cell response in humans. PLoS Pathog. 2010;6:e1001051. doi: 10.1371/journal.ppat.1001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alanio C, et al. Bystander hyperactivation of preimmune CD8+ T cells in chronic HCV patients. Elife. 2015;4:e07916. doi: 10.7554/eLife.07916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haas A, Zimmermann K, Oxenius A. Antigen-dependent and -independent mechanisms of T and B cell hyperactivation during chronic HIV-1 infection. J. Virol. 2011;85:12102–12113. doi: 10.1128/JVI.05607-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bangs SC, McMichael AJ, Xu XN. Bystander T cell activation—implications for HIV infection and other diseases. Trends Immunol. 2006;27:518–524. doi: 10.1016/j.it.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Bastidas S, et al. CD8+ T cells are activated in an antigen-independent manner in HIV-infected individuals. J. Immunol. 2014;192:1732–1744. doi: 10.4049/jimmunol.1302027. [DOI] [PubMed] [Google Scholar]
- 16.Younes SA, et al. IL-15 promotes activation and expansion of CD8+ T cells in HIV-1 infection. J. Clin. Invest. 2016;126:2745–2756. doi: 10.1172/JCI85996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalod M, et al. Weak anti-HIV CD8(+) T-cell effector activity in HIV primary infection. J. Clin. Invest. 1999;104:1431–1439. doi: 10.1172/JCI7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doisne JM, et al. CD8+ T cells specific for EBV, cytomegalovirus, and influenza virus are activated during primary HIV infection. J. Immunol. 2004;173:2410–2418. doi: 10.4049/jimmunol.173.4.2410. [DOI] [PubMed] [Google Scholar]
- 19.Deeks SG, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 20.Hunt PW, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J. Infect. Dis. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 21.Cao W, Mehraj V, Kaufmann DE, Li T, Routy JP. Elevation and persistence of CD8 T-cells in HIV infection: the Achilles heel in the ART era. J. Int. AIDS Soc. 2016;19:20697. doi: 10.7448/IAS.19.1.20697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silvestri G, et al. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18:441–452. doi: 10.1016/S1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 23.McMaster SR, Wilson JJ, Wang H, Kohlmeier JE. Airway-resident memory CD8 T cells provide antigen-specific protection against respiratory virus challenge through rapid IFN-gamma production. J. Immunol. 2015;195:203–209. doi: 10.4049/jimmunol.1402975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flynn KJ, et al. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/S1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 25.Piet B, et al. CD8(+) T cells with an intraepithelial phenotype upregulate cytotoxic function upon influenza infection in human lung. J. Clin. Invest. 2011;121:2254–2263. doi: 10.1172/JCI44675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamada H, et al. Multiple redundant effector mechanisms of CD8+ T cells protect against influenza infection. J. Immunol. 2013;190:296–306. doi: 10.4049/jimmunol.1200571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun J, Braciale TJ. Role of T cell immunity in recovery from influenza virus infection. Curr. Opin. Virol. 2013;3:425–429. doi: 10.1016/j.coviro.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ely KH, et al. Nonspecific recruitment of memory CD8+ T cells to the lung airways during respiratory virus infections. J. Immunol. 2003;170:1423–1429. doi: 10.4049/jimmunol.170.3.1423. [DOI] [PubMed] [Google Scholar]
- 29.Kohlmeier JE, Cookenham T, Roberts AD, Miller SC, Woodland DL. Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity. 2010;33:96–105. doi: 10.1016/j.immuni.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sckisel GD, et al. Influenza infection results in local expansion of memory CD8(+) T cells with antigen non-specific phenotype and function. Clin. Exp. Immunol. 2014;175:79–91. doi: 10.1111/cei.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Topham DJ, Castrucci MR, Wingo FS, Belz GT, Doherty PC. The role of antigen in the localization of naive, acutely activated, and memory CD8(+) T cells to the lung during influenza pneumonia. J. Immunol. 2001;167:6983–6990. doi: 10.4049/jimmunol.167.12.6983. [DOI] [PubMed] [Google Scholar]
- 32.Loh L, et al. Human mucosal-associated invariant T cells contribute to antiviral influenza immunity via IL-18-dependent activation. Proc. Natl Acad. Sci. USA. 2016;113:10133–10138. doi: 10.1073/pnas.1610750113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilgenburg BV, et al. MAIT cells contribute to protection against lethal influenza infection in vivo. Nat. Commun. 2018;9:4706. doi: 10.1038/s41467-018-07207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tough DF, Sun S, Sprent J. T cell stimulation in vivo by lipopolysaccharide (LPS) J. Exp. Med. 1997;185:2089–2094. doi: 10.1084/jem.185.12.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardy GA, et al. Interferon-alpha is the primary plasma type-I IFN in HIV-1 infection and correlates with immune activation and disease markers. PLoS ONE. 2013;8:e56527. doi: 10.1371/journal.pone.0056527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng L, et al. Blocking type I interferon signaling enhances T cell recovery and reduces HIV-1 reservoirs. J. Clin. Invest. 2017;127:269–279. doi: 10.1172/JCI90745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhen A, et al. Targeting type I interferon-mediated activation restores immune function in chronic HIV infection. J. Clin. Invest. 2017;127:260–268. doi: 10.1172/JCI89488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freeman BE, Hammarlund E, Raue HP, Slifka MK. Regulation of innate CD8+ T-cell activation mediated by cytokines. Proc. Natl Acad. Sci. USA. 2012;109:9971–9976. doi: 10.1073/pnas.1203543109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mattei F, Schiavoni G, Belardelli F, Tough DF. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J. Immunol. 2001;167:1179–1187. doi: 10.4049/jimmunol.167.3.1179. [DOI] [PubMed] [Google Scholar]
- 41.Colpitts SL, et al. Cutting edge: the role of IFN-alpha receptor and MyD88 signaling in induction of IL-15 expression in vivo. J. Immunol. 2012;188:2483–2487. doi: 10.4049/jimmunol.1103609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sareneva T, Julkunen I, Matikainen S. IFN-alpha and IL-12 induce IL-18 receptor gene expression in human NK and T cells. J. Immunol. 2000;165:1933–1938. doi: 10.4049/jimmunol.165.4.1933. [DOI] [PubMed] [Google Scholar]
- 43.McNally JM, et al. Attrition of bystander CD8 T cells during virus-induced T-cell and interferon responses. J. Virol. 2001;75:5965–5976. doi: 10.1128/JVI.75.13.5965-5976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bahl K, et al. IFN-induced attrition of CD8 T cells in the presence or absence of cognate antigen during the early stages of viral infections. J. Immunol. 2006;176:4284–4295. doi: 10.4049/jimmunol.176.7.4284. [DOI] [PubMed] [Google Scholar]
- 45.Lanford RE, et al. Acute hepatitis A virus infection is associated with a limited type I interferon response and persistence of intrahepatic viral RNA. Proc. Natl. Acad. Sci. USA. 2011;108:11223–11228. doi: 10.1073/pnas.1101939108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cox MA, Kahan SM, Zajac AJ. Anti-viral CD8 T cells and the cytokines that they love. Virology. 2013;435:157–169. doi: 10.1016/j.virol.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lertmemongkolchai G, Cai G, Hunter CA, Bancroft GJ. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J. Immunol. 2001;166:1097–1105. doi: 10.4049/jimmunol.166.2.1097. [DOI] [PubMed] [Google Scholar]
- 48.Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J. Exp. Med. 2003;198:1583–1593. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raue HP, Brien JD, Hammarlund E, Slifka MK. Activation of virus-specific CD8+ T cells by lipopolysaccharide-induced IL-12 and IL-18. J. Immunol. 2004;173:6873–6881. doi: 10.4049/jimmunol.173.11.6873. [DOI] [PubMed] [Google Scholar]
- 50.Martin MD, Shan Q, Xue HH, Badovinac VP. Time and antigen-stimulation history influence memory CD8 T cell bystander responses. Front. Immunol. 2017;8:634. doi: 10.3389/fimmu.2017.00634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/S1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 52.Soudja SM, Ruiz AL, Marie JC, Lauvau G. Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity. 2012;37:549–562. doi: 10.1016/j.immuni.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castillo EF, Schluns KS. Regulating the immune system via IL-15 transpresentation. Cytokine. 2012;59:479–490. doi: 10.1016/j.cyto.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Correia MP, Costa AV, Uhrberg M, Cardoso EM, Arosa FA. IL-15 induces CD8+ T cells to acquire functional NK receptors capable of modulating cytotoxicity and cytokine secretion. Immunobiology. 2011;216:604–612. doi: 10.1016/j.imbio.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 55.Dominguez-Andres J, et al. Inflammatory Ly6C(high) monocytes protect against Candidiasis through IL-15-driven NK cell/neutrophil activation. Immunity. 2017;46:1059–1072. doi: 10.1016/j.immuni.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 56.Jabri B, Abadie V. IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction. Nat. Rev. Immunol. 2015;15:771–783. doi: 10.1038/nri3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Di Sabatino A, et al. Epithelium derived interleukin 15 regulates intraepithelial lymphocyte Th1 cytokine production, cytotoxicity, and survival in coeliac disease. Gut. 2006;55:469–477. doi: 10.1136/gut.2005.068684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reynolds JM, Dong C. Toll-like receptor regulation of effector T lymphocyte function. Trends Immunol. 2013;34:511–519. doi: 10.1016/j.it.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 59.Tabiasco J, et al. Human effector CD8+ T lymphocytes express TLR3 as a functional coreceptor. J. Immunol. 2006;177:8708–8713. doi: 10.4049/jimmunol.177.12.8708. [DOI] [PubMed] [Google Scholar]
- 60.Cottalorda A, et al. TLR2 engagement on CD8 T cells lowers the threshold for optimal antigen-induced T cell activation. Eur. J. Immunol. 2006;36:1684–1693. doi: 10.1002/eji.200636181. [DOI] [PubMed] [Google Scholar]
- 61.Salerno F, Guislain A, Cansever D, Wolkers MC. TLR-mediated innate production of IFN-gamma by CD8+ T cells is independent of glycolysis. J. Immunol. 2016;196:3695–3705. doi: 10.4049/jimmunol.1501997. [DOI] [PubMed] [Google Scholar]
- 62.Salerno F, Freen-van Heeren JJ, Guislain A, Nicolet BP, Wolkers MC. Costimulation through TLR2 drives polyfunctional CD8(+) T cell responses. J. Immunol. 2019;202:714–723. doi: 10.4049/jimmunol.1801026. [DOI] [PubMed] [Google Scholar]
- 63.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 64.Whiteside SK, Snook JP, Williams MA, Weis JJ. Bystander T cells: a balancing act of friends and foes. Trends Immunol. 2018;39:1021–1035. doi: 10.1016/j.it.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Whiteside SK, et al. IL-10 deficiency reveals a role for TLR2-dependent bystander activation of T cells in Lyme arthritis. J. Immunol. 2018;200:1457–1470. doi: 10.4049/jimmunol.1701248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wensveen FM, Jelencic V, Polic B. NKG2D: a master regulator of immune cell responsiveness. Front. Immunol. 2018;9:441. doi: 10.3389/fimmu.2018.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roberts AI, et al. NKG2D receptors induced by IL-15 costimulate CD28-negative effector CTL in the tissue microenvironment. J. Immunol. 2001;167:5527–5530. doi: 10.4049/jimmunol.167.10.5527. [DOI] [PubMed] [Google Scholar]
- 68.Groh V, et al. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat. Immunol. 2001;2:255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 69.Jamieson AM, et al. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. doi: 10.1016/S1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 70.Maasho K, Opoku-Anane J, Marusina AI, Coligan JE, Borrego F. NKG2D is a costimulatory receptor for human naive CD8+ T cells. J. Immunol. 2005;174:4480–4484. doi: 10.4049/jimmunol.174.8.4480. [DOI] [PubMed] [Google Scholar]
- 71.Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol. Rev. 2010;235:267–285. doi: 10.1111/j.0105-2896.2010.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lanier LL. NKG2D receptor and its ligands in host defense. Cancer Immunol. Res. 2015;3:575–582. doi: 10.1158/2326-6066.CIR-15-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meresse B, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357–366. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 74.Crosby EJ, Clark M, Novais FO, Wherry EJ, Scott P. Lymphocytic choriomeningitis virus expands a population of NKG2D+CD8+ T cells that exacerbates disease in mice coinfected with Leishmania major. J. Immunol. 2015;195:3301–3310. doi: 10.4049/jimmunol.1500855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chu T, et al. Bystander-activated memory CD8 T cells control early pathogen load in an innate-like, NKG2D-dependent manner. Cell Rep. 2013;3:701–708. doi: 10.1016/j.celrep.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Crosby EJ, Goldschmidt MH, Wherry EJ, Scott P. Engagement of NKG2D on bystander memory CD8 T cells promotes increased immunopathology following Leishmania major infection. PLoS Pathog. 2014;10:e1003970. doi: 10.1371/journal.ppat.1003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muntasell A, Magri G, Pende D, Angulo A, Lopez-Botet M. Inhibition of NKG2D expression in NK cells by cytokines secreted in response to human cytomegalovirus infection. Blood. 2010;115:5170–5179. doi: 10.1182/blood-2009-11-256479. [DOI] [PubMed] [Google Scholar]
- 78.Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 79.Phillips S, et al. CD8(+) T cell control of hepatitis B virus replication: direct comparison between cytolytic and noncytolytic functions. J. Immunol. 2010;184:287–295. doi: 10.4049/jimmunol.0902761. [DOI] [PubMed] [Google Scholar]
- 80.Jo J, et al. Analysis of CD8+ T-cell-mediated inhibition of hepatitis C virus replication using a novel immunological model. Gastroenterology. 2009;136:1391–1401. doi: 10.1053/j.gastro.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 81.Beadling C, Slifka MK. Differential regulation of virus-specific T-cell effector functions following activation by peptide or innate cytokines. Blood. 2005;105:1179–1186. doi: 10.1182/blood-2004-07-2833. [DOI] [PubMed] [Google Scholar]
- 82.Kang S, Brown HM, Hwang S. Direct antiviral mechanisms of interferon-gamma. Immune Netw. 2018;18:e33. doi: 10.4110/in.2018.18.e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Varadarajan N, et al. A high-throughput single-cell analysis of human CD8(+) T cell functions reveals discordance for cytokine secretion and cytolysis. J. Clin. Invest. 2011;121:4322–4331. doi: 10.1172/JCI58653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Novais FO, Wong AC, Villareal DO, Beiting DP, Scott P. CD8(+) T cells lack local signals to produce IFN-gamma in the skin during Leishmania infection. J. Immunol. 2018;200:1737–1745. doi: 10.4049/jimmunol.1701597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Soudja SM, et al. Memory-T-cell-derived interferon-gamma instructs potent innate cell activation for protective immunity. Immunity. 2014;40:974–988. doi: 10.1016/j.immuni.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martin MD, Badovinac VP. Antigen-dependent and -independent contributions to primary memory CD8 T cell activation and protection following infection. Sci. Rep. 2015;5:18022. doi: 10.1038/srep18022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gravano DM, Hoyer KK. Promotion and prevention of autoimmune disease by CD8+ T cells. J. Autoimmun. 2013;45:68–79. doi: 10.1016/j.jaut.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 88.Munz C, Lunemann JD, Getts MT, Miller SD. Antiviral immune responses: triggers of or triggered by autoimmunity? Nat. Rev. Immunol. 2009;9:246–258. doi: 10.1038/nri2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pane JA, Coulson BS. Lessons from the mouse: potential contribution of bystander lymphocyte activation by viruses to human type 1 diabetes. Diabetologia. 2015;58:1149–1159. doi: 10.1007/s00125-015-3562-3. [DOI] [PubMed] [Google Scholar]
- 90.Itsumi M, Yoshikai Y, Yamada H. IL-15 is critical for the maintenance and innate functions of self-specific CD8(+) T cells. Eur. J. Immunol. 2009;39:1784–1793. doi: 10.1002/eji.200839106. [DOI] [PubMed] [Google Scholar]
- 91.Ramanathan S, et al. Exposure to IL-15 and IL-21 enables autoreactive CD8 T cells to respond to weak antigens and cause disease in a mouse model of autoimmune diabetes. J. Immunol. 2011;186:5131–5141. doi: 10.4049/jimmunol.1001221. [DOI] [PubMed] [Google Scholar]
- 92.van Aalst S, Ludwig IS, van der Zee R, van Eden W, Broere F. Bystander activation of irrelevant CD4+ T cells following antigen-specific vaccination occurs in the presence and absence of adjuvant. PLoS ONE. 2017;12:e0177365. doi: 10.1371/journal.pone.0177365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee HG, et al. Pathogenic function of bystander-activated memory-like CD4(+) T cells in autoimmune encephalomyelitis. Nat. Commun. 2019;10:709. doi: 10.1038/s41467-019-08482-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tietze JK, et al. Delineation of antigen-specific and antigen-nonspecific CD8(+) memory T-cell responses after cytokine-based cancer immunotherapy. Blood. 2012;119:3073–3083. doi: 10.1182/blood-2011-07-369736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Simoni Y, et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557:575–579. doi: 10.1038/s41586-018-0130-2. [DOI] [PubMed] [Google Scholar]
- 96.Scheper W, et al. Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat. Med. 2019;25:89–94. doi: 10.1038/s41591-018-0266-5. [DOI] [PubMed] [Google Scholar]
- 97.Duhen T, et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun. 2018;9:2724. doi: 10.1038/s41467-018-05072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beura LK, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532:512–516. doi: 10.1038/nature17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reese TA, et al. Sequential infection with common pathogens promotes human-like immune gene expression and altered vaccine response. Cell Host Microbe. 2016;19:713–719. doi: 10.1016/j.chom.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]