Abstract
We previously found that artificial glycosphingolipids (artGSLs) containing very-long-chain fatty acids behave as strong immunogens in mice and promote the production of antibodies recognizing the oligosaccharide portion of artGSLs as the epitope. Here, we report that the oligosaccharide structure of artGSLs influences these immunogenic properties. We evaluated the antibody-inducing activity of artGSLs with different oligosaccharide structures in mice and found strong IgG-inducing activity only with an artGSL containing a core-fucosylated tetraoligosaccharide (Manβ1,4GlcNAcβ1,4[Fucα1,6]GlcNAc). To characterize the immunogenic properties of this artGSL, we analyzed various derivatives and found that the non-reducing terminal mannose structure was critical for the antibody-inducing activity. These artGSLs also exhibited IgG-inducing activity dependent on co-administration of lipid A adjuvant, but no cytokine-inducing activity similar to α-galactosylceramide was detected. Furthermore, repetitive immunization with the artGSL promoted the production of antibodies against a core-fucosylated α-fetoprotein isoform (AFP-L3) known as a hepatocellular carcinoma–specific antigen. These results indicate that the newly designed artGSLs specifically induce adaptive immune responses and promote antibody production by B cells, which can be utilized to develop anti-glycoconjugate antibodies and cancer vaccines targeting tumor-associated carbohydrate antigens.
Subject terms: Antibody generation, Conjugate vaccines
Introduction
Of the glycoconjugates synthesized in mammalian cells, glycosphingolipids (GSLs) are known to exhibit immunogenicity in immunized animals1. This activity was identified by analyzing anti-glycoconjugate monoclonal antibodies isolated from animals immunized with cancer cells1–3, which revealed that intracellular GSLs function as efficient immunogens for inducing antibody production. Recent studies also revealed that α-linked monosaccharyl ceramides such as α-galactosylceramide (αGalCer) isolated from marine sponges and bacteria activate mammalian natural killer T (NKT) cells and promote cytokine production4–7. In addition, CD1d expressed by antigen-presenting cells (APCs) was identified as the receptor for these NKT cell ligand GSLs5,7. Rodent GSLs with an α-galactosyl structure that exhibit this activity7–9 are considered natural ligands necessary for the development of NKT cells. Although the role of GSLs in innate immunity has been extensively investigated, the antibody-inducing activity of mammalian GSLs has received relatively little research attention.
We previously demonstrated that stimulation of human vascular endothelial cells with tumor necrosis factor-α promotes GSL synthesis and that the synthesized GSLs, particularly globotetraosylceramide (globoside/Gb4Cer), predominantly contain C24 very-long-chain fatty acids in the ceramide portion10,11. Surprisingly, we found that C24-Gb4Cer exhibits potent antibody-inducing activity in mice, including efficient induction of anti-Gb4Cer IgG synthesis12,13. Using immunized mice, we obtained monoclonal IgM and IgG3 antibodies that specifically recognize Gb4Cer with high affinity12–14. Further modeling experiments using newly designed artificial glycosphingolipids (artGSLs) composed of simple ceramide mimetics and 6′-sialyl LacNAc revealed that the C24 fatty acid structure is associated with the high antibody-inducing activity of the artGSLs1,12. Mice immunized with the artGSLs efficiently produced antibodies recognizing glycoproteins containing 6′-sialyl LacNAc12.
Of the ceramide mimetics we generated, C12L exhibited the highest immunogenicity (Fig. 1). In this study, we synthesized several artGSLs composed of C12L and various oligosaccharides and analyzed the effect of the oligosaccharide portion on antibody-inducing activity. The effect of altering the sphingosine portion was also analyzed. We found that artGSLs containing a core-fucosylated tetrasaccharide (CF4: Manβ1,4GlcNAcβ1,4[Fucα1,6]GlcNAc) exhibited potent IgG-inducing activity that was dependent on the non-reducing terminal mannose structure and co-administration of lipid A adjuvant. Unlike αGalCer, these artGSLs did not induce cytokine production in mice. Furthermore, repetitive immunization with CF4-C12L induced the production of antibodies against a core-fucosylated α-fetoprotein isoform (AFP-L3) known to be a hepatocellular carcinoma–specific antigen15. Collectively, these results indicate that the newly designed artGSLs specifically induce adaptive immune responses and promote antibody production by B cells, which can be exploited to develop anti-glycoconjugate antibodies and cancer vaccines targeting tumor-associated carbohydrate antigens.
Figure 1.
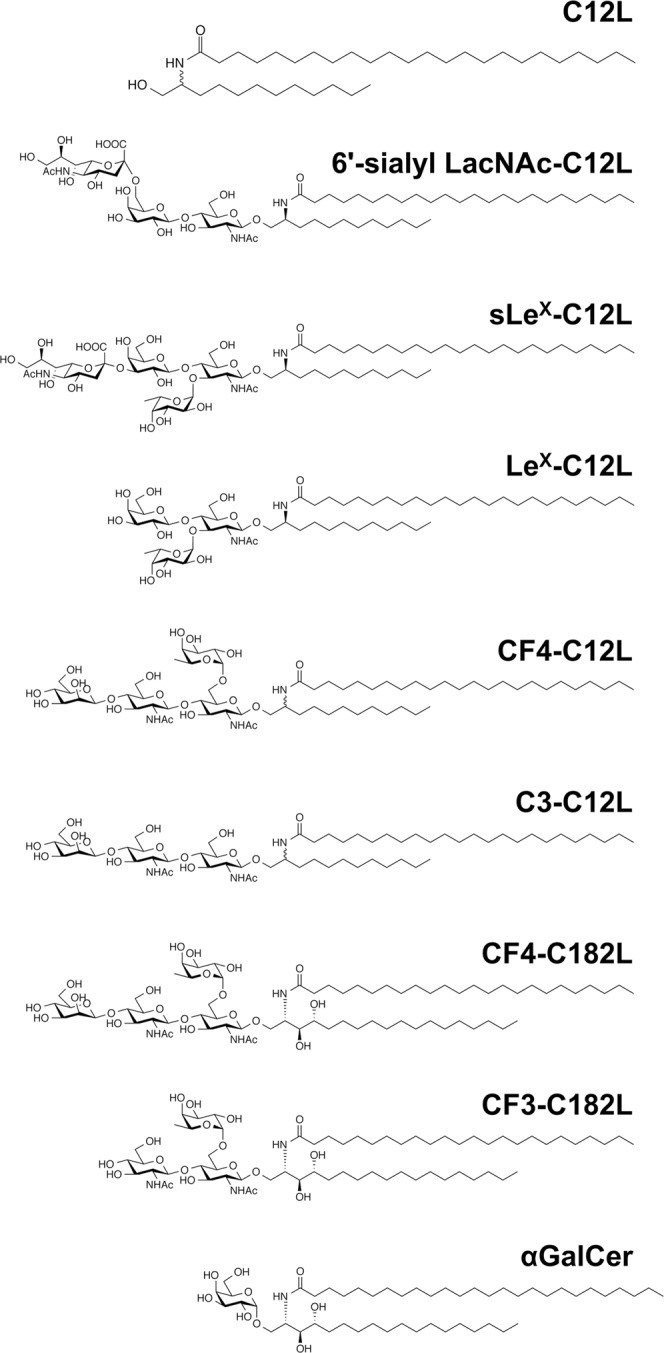
Chemical structures of artGSLs. The structures of C12L, artGSLs, and αGalCer used in this study are shown. The ceramide mimetic C12L is composed of a saturated C12-sphingosine mimetic and lignoceric acid (C24:0). The ceramide mimetic C182L is composed of C18-phytosphingosine and lignoceric acid. These ceramide mimetics are bound to the oligosaccharide via a β-linkage. The ceramide in αGalCer is composed of C18-phytosphingosine and hexacosanoic acid (C26:0) and bound to galactose via an α-linkage. Abbreviations: 6′-sialyl LacNAc, Neu5Acα2,6Galβ1,4GlcNAc; sLeX, sialyl LewisX/Neu5Acα2,3Galβ1,4(Fucα1,3)GlcNAc; LeX, LewisX/Galβ1,4(Fucα1,3)GlcNAc; CF4, core-fucosylated tetrasaccharide/Manβ1,4GlcNAcβ1,4(Fucα1,6)GlcNAc.
Results
Design of new artGSLs containing very-long-chain fatty acids
We previously analyzed the antibody-inducing activity artGSLs composed of 6′-sialyl LacNAc (Neu5Acα2,6Galβ1,4GlcNAc) and C12L (Fig. 1) or C12L analogues and found that the very-long-chain fatty acid structure of C12L is essential for the induction of potent antibody responses by artGSLs1,12. In this study, we analyzed the effect of the oligosaccharide structure of various C12L conjugates on antibody-inducing activity in comparison with 6′-sialyl LacNAc-C12L and several oligosaccharide conjugates of C12L. From a practical perspective, oligosaccharides containing a sialyl LewisX (sLeX), LewisX (LeX), or core fucose epitope known to be carcinoembryonic antigens were selected (Fig. 1). sLeX is expressed on lymphocytes and in gastrointestinal tumors16–18 and exhibits adhesive properties that play a role in the metastasis of these cancers. LeX, also known as SSEA-1, is a precursor of sLeX and a well-characterized cell surface marker of mouse embryo and stem cells19. Core fucose forms the core structure of N-linked oligosaccharides in mammalian glycoproteins such as α-fetoprotein isoform AFP-L3, a hepatocellular carcinoma–specific antigen15. The core fucose structure of immunoglobulins has also been associated with anti-cancer activity20,21. To further analyze core fucose, we designed and prepared a C12L conjugate of core-fucosylated tetrasaccharide (CF4-C12L) and a non-fucosylated analogue (C3-C12L) as a negative control. For further analysis of CF4-C12L, we also prepared a phytosphingosine analogue (CF4-C182L) and trisaccharide analogue (CF3-C182L). As tri or tetrasaccharide length target oligosaccharide was enough for induction of antibodies that recognize the target oligosaccharide with high specificity12–14, we designed artGSLs containing tri or tetrasaccharides in this study.
Antibody-inducing activity of artGSLs containing various oligosaccharides
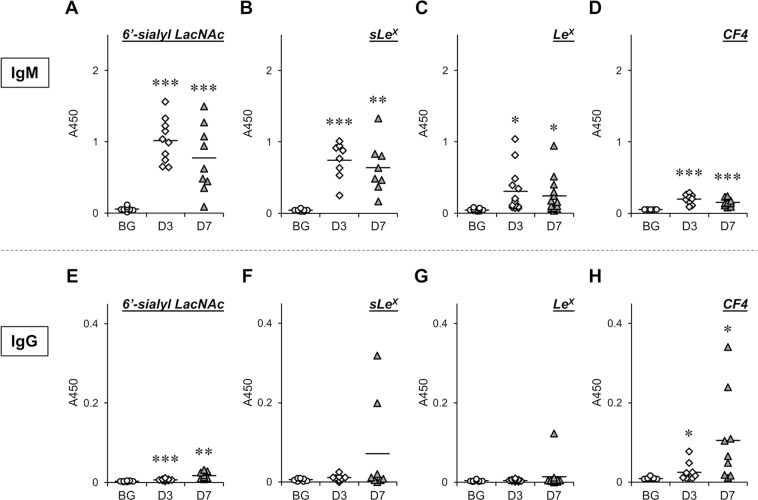
Mice were immunized with C12L conjugates containing 6′-sialyl LacNAc, sLeX, LeX, and CF4 using a liposome immunization method established by Brodin et al.22. The second booster immunization was performed intraperitoneally 2 weeks after the first subcutaneous immunization, and the antibody titer in the serum was evaluated by enzyme-linked immunosorbent assay (ELISA) on days 3 and 7 after the second immunization (Figs. 2, 3). Although our previous study using 6′-sialyl LacNAc-C12L showed that immunization of mice with this artGSL immediately induced the production of serum IgM and IgG against 6′-sialyl LacNAc as the epitope, the serum IgG titer was markedly lower than that of IgM12. In the present study, we found that the ratio of IgG to IgM depended on the oligosaccharide structure of the artGSLs. Figure 2A–D shows that 6′-sialyl LacNAc-C12L, sLeX-C12L, and LeX-C12L strongly induced the generation of IgM against these respective immunogens. While serum IgG titers appeared rapidly after the second immunization (Fig. 2E–H), these titers were relatively low (6′-sialyl LacNA-C12L) or exhibited large individual differences between mice (sLeX-C12L and LeX-C12L). However, CF4-C12L strongly induced the generation of serum IgG against the immunogen in almost all mice (Fig. 2H). The anti-immunogen IgG titers of serum from CF4-C12L–immunized mice significantly increased from 3 days after booster immunization, and the average value on the 7 days after was 6 times higher than that of 6′-sialyl LacNA-C12L. The IgG titer of 6 of 9 mice showed over 5 times higher than that of average level of backgrounds, such immunogenicity was not observed in other artGSLs. In contrast, IgM titers were relatively lower compared with the other artGSL-immunized mice (Fig. 2D).
Figure 2.
Titers of immunogen-specific serum IgM and IgG in artGSL-immunized mice. Mice were immunized with 6′-sialyl LacNAc-C12L (A,E), sLeX-C12L (B,F), LeX-C12L (C,G), or CF4-C12L (D,H), and serum was prepared 3 days (D3) or 7 days (D7) after the final immunization. BG, serum from non-treated mice. Titers of serum IgM (A–D) and IgG (E–H) against artGSLs used for immunization were determined by ELISA. Average absorbance values are indicated as horizontal bars. *P < 0.05, **P < 0.01, **P < 0.001 BG vs. D3 or D7 (n = 8–13).
Figure 3.
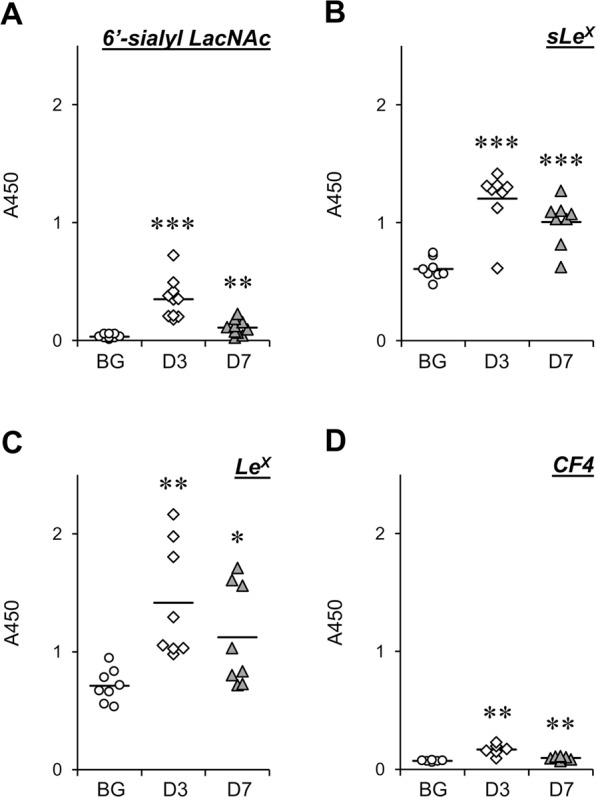
Titers of serum glycoprotein-specific IgM in artGSL-immunized mice. Mice were immunized with 6′-sialyl LacNAc-C12L (A), sLeX-C12L (B), LeX-C12L (C), or CF4-C12L (D), and serum was prepared 3 days (D3) or 7 days (D7) after the final immunization. BG, serum from non-treated mice. Titers of serum IgM against glycoproteins containing oligosaccharides of identical structure as artGSLs used for immunization were determined by ELISA. Glycoproteins containing 6′-sialyl LacNAc (fetuin [A]), sLeX and LeX (glycoproteins extracted from HL-60 cells [B,C]), and CF4 (thyroglobulin [D]) were used as immobilized antigens for ELISA, respectively. Average absorbance values are indicated as horizontal bars. *P < 0.05, **P < 0.01, **P < 0.001 BG vs. D3 or D7 (n = 8–10).
Our previous study also showed that artGSL-induced antibodies react with glycoproteins with oligosaccharide structures identical to those of the artGSLs used for immunization. Thus, we also analyzed the titers of serum antibodies against glycoproteins containing 6′-sialyl LacNAc (fetuin), sLeX and LeX (glycoproteins extracted from HL-60 cells), and CF4 (thyroglobulin)16,23,24 in artGSL-immunized mice using ELISAs (Fig. 3). Although no induction of IgG was detected under the experimental conditions used, significant induction of IgM against glycoproteins containing oligosaccharides identical to those of the immunogens was observed in all artGSL-immunized mice. Although significant induction of IgM against thyroglobulin was detected in mice immunized with CF4-C12L, the titers were lower than those of other artGSL-immunized mice (Fig. 3D). As only core fucose forms the root structure of N-linked oligosaccharides, while the other oligosaccharides are terminal structures, it was considered that the low IgM titers were related to structural issues. Thus, to confirm that the antibodies generated in CF4-C12L–immunized mice recognized core fucose as the epitope, we analyzed the reactivity of the antibodies to the precursor, C3-C12L, which does not contain core fucose (Fig. 4). The reactivity of IgM generated in response to CF4-C12L was significantly higher than that of the IgM generated in response to C3-C12L; a similar tendency was observed with IgG.
Figure 4.
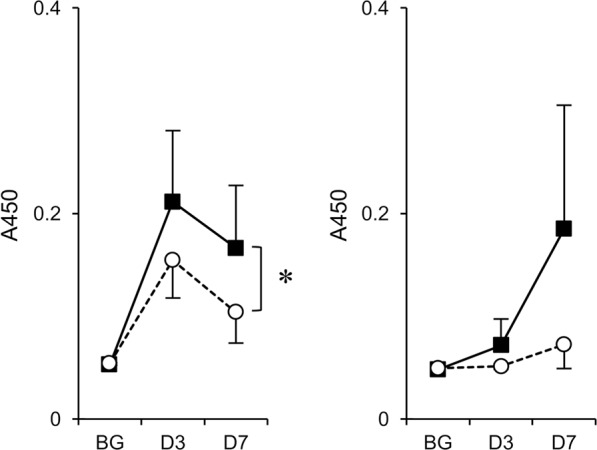
Comparison of serum titers of antibodies against CF4-C12L and C3-C12L in mice immunized with CF4-C12L. Mice were immunized with CF4-C12L, and serum was prepared 3 days (D3) or 7 days (D7) after the final immunization. BG, serum from non-treated mice. Titers of serum IgM (left panel) and IgG (right panel) against CF4-C12L (closed squares with solid line) and C3-C12L (open circles with dotted line) were determined by ELISA. Error bars: mean ± S.D. (n = 6). *P < 0.05 anti-CF4-C12L vs. anti-CF3-C12L.
These results demonstrated that conjugation of C12L efficiently enhances the immunogenicity of various oligosaccharides in mice and that conjugates/artGSLs are efficient immunogens for inducing the generation of antibodies against glycoproteins containing target oligosaccharides. Furthermore, we found that the oligosaccharide structure influences the ratio of IgG to IgM induction, and more specifically, class switching.
Characterization of the immunogenic properties of CF4-C12L and its derivatives
To determine the key structure mediating the high IgG-inducing activity of CF4-C12L, we prepared various CF4-C12L derivatives (Fig. 1, CF4-C182L and CF3-C182L) and analyzed their immunogenic properties. As our previous analysis using 6′-sialyl LacNAc-C12L revealed that the C24:0 lignoceric acid of C12L was essential for IgG-inducing activity12, in the present study, we examined the effect of altering the sphingoid base or oligosaccharide structure of CF4-C12L. First, we examined the antibody-inducing activity of CF4-C182L, a phytosphingosine analogue of CF4-C12L, in comparison to CF4-C12L (Fig. 5). We previously examined how the length of the alkyl chain of the sphingosine mimetic portion of C12L affects the immunogenicity of 6′-sialyl LacNAc-C12L and found that the C12L conjugate exhibited higher immunogenicity than the short (8 carbon length) and long (16 carbon length) sphingosine analogues12. As the hydroxyl group of the phytosphingosine portion is known to play an important role in determining the immunogenicity of αGalCer25–27, in the present study, we examined the immunogenicity of the phytosphingosine analogue of C12L. In Fig. 5B, we observed a significant enhancement of serum IgG titers against immunogen in mice immunized with CF4-C182L compared to that in CF4-C12L–immunized mice (D3, P < 0.05; D7, P < 0.01; anti-CF4-C12L vs. anti-CF4-C182L). Further analysis of the endpoint titers in the serum from three mice with high IgG titer against CF4-conjugates (Fig. S1) also showed that the endopoint titer of CF4-C182L tended to be lower than that of CF4-C12L (CF4-C12L, from 1000-fold to 2000-fold; CF4-C182L, 2000-fold).
Figure 5.
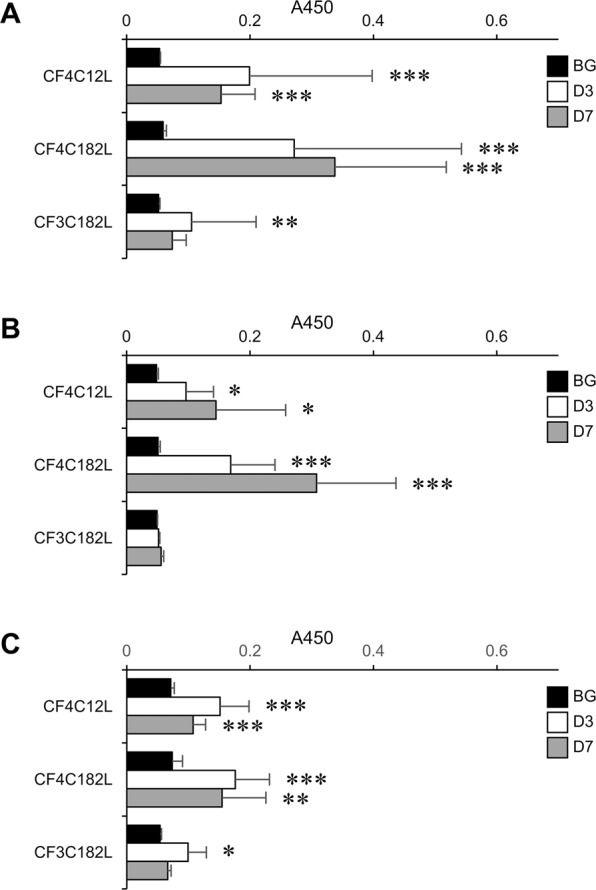
Serum antibody titers in CF4-C182L– and CF3-C182L–immunized mice. Mice were immunized with CF4-C182L or CF3-C182L, and serum was prepared 3 (open bars) or 7 (gray bars) days after the final immunization. Closed bars, serum from non-treated mice. Titers of serum IgM (A) and IgG (B) against each immunogen were determined by ELISA. (C) Titers of serum IgM against thyroglobulin. Titers of serum antibodies in CF4-C12L–immunized mice are shown for reference. Error bars: mean ± S.D. (n = 6–7). *P < 0.05, **P < 0.01, **P < 0.001 BG vs. D3 or D7.
Next, we prepared an oligosaccharide analogue of CF4-C182L by removing the non-reducing terminal mannose (CF3-C182L) and analyzed its antibody-inducing activity in mice. Surprisingly, we found that removal of the terminal mannose resulted in a marked reduction in the antibody-inducing activity of CF3-C182L; in particular, the IgG titer was markedly reduced by removal of the terminal mannose (Fig. 5B). This result indicates that the terminal mannose plays a critical role in antibody induction, particularly the IgG-inducing activity of the CF4 conjugates.
As conjugates of CF4 were found to induce IgG with high efficiency, we hypothesized that the conjugates induce the production of cytokines related to class switching, similar to αGalCer4,5,7. Thus, we examined serum levels of IL-4 and IFN-γ, representative cytokines induced by αGalCer, in mice administered CF4 conjugates. In the C3H/HeN mice used in the immunization experiment, αGalCer administration transiently increased the IL-4 level at 3 hours and gradually increased the IFN-γ level between 9 and 24 hours post-administration (Fig. 6A). We therefore examined serum levels of IL-4 and IFN-γ at 3 and 24 hours after administration of the CF4 conjugates (Fig. 6B,C). In contrast to αGalCer, no production of these cytokines was observed in mice administered the CF4 conjugates. In addition, we also did not detect the production of antibodies against αGalCer in αGalCer-immunized mice (Fig. S2). These results indicate that the CF4 conjugates do not stimulate innate immunity, but they do induce an adaptive immune response.
Figure 6.
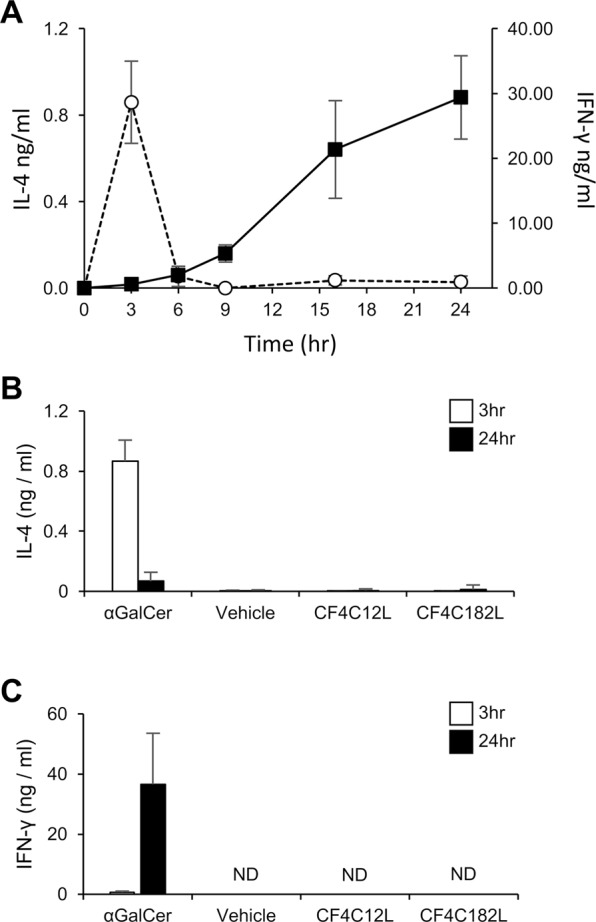
Comparison of cytokine-inducing activity of αGalCer and artGSLs. Time course of change in serum IL-4 (open circles with dotted line) and IFN-γ (closed squares with solid line) levels in mice administered αGalCer (A). Serum levels of IL-4 (B) and IFN-γ (C) at 3 (open bars) or 24 (closed bars) hours after administration of CF4-C12L and CF4-C182L. Vehicle indicates serum cytokine levels in mice not administered glycosphingolipid-containing liposomes. Error bars: mean ± S.D. (n = 5). ND, not detected.
To examine antibody induction, we simultaneously administered immunogens with lipid A as an adjuvant. In order to analyze the antibody-inducing activity of CF4 conjugates alone, mice were immunized with liposomes containing CF4 conjugates but without lipid A, and the serum antibodies titers were then determined. Although IgM-inducing activity remained in the absence of lipid A, IgG-inducing activity was almost abolished (Fig. 7), indicating that the IgG-inducing activity of the CF4 conjugates is dependent on co-immunization with lipid A.
Figure 7.
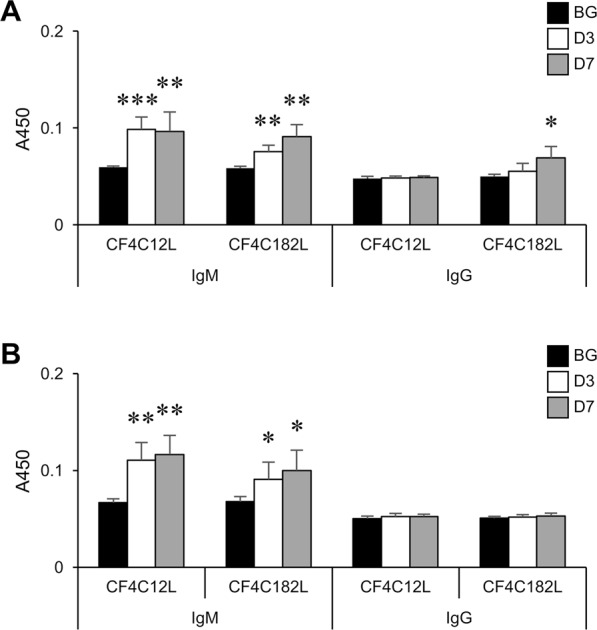
Effect of lipid A on antibody-inducing activity of CF4 conjugates. Mice were immunized with CF4-C12L or CF4-C182L without lipid A, and serum was prepared 3 (open bars) or 7 (gray bars) days after the final immunization. Closed bars, serum from non-treated mice. Titers of serum IgM and IgG against each immunogen (upper panel) or thyroglobulin (lower panel) were determined by ELISA. Error bars: mean ± S.D. (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 BG vs. D3 or D7.
Induction of antibodies to cancer-specific glycoproteins by immunization with CF4-C12L
AFP-L3 is a core-fucosylated α-fetoprotein isoform and hepatocellular carcinoma–specific marker15. If immunization with CF4 conjugates could induce the generation of antibodies that specifically react with AFP-L3, it would be possible to produce monoclonal antibodies against AFP-L3 for diagnostic use, and this could also facilitate the development of vaccines to prevent this type of cancer.
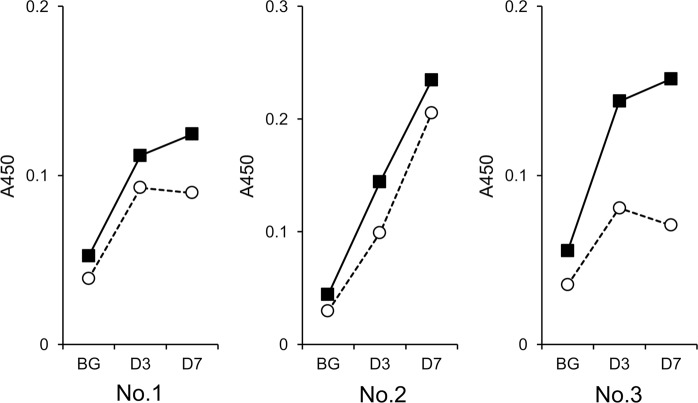
To examine the generation of antibodies against AFP-L3, mice were repeatedly immunized with CF4-C12L, and titers of serum antibodies against AFP-L3 were analyzed by ELISA. As preliminary experiments showed that the antibody-inducing activity of CF4-C182L was not enhanced by repetitive immunization (Fig. S3), we used CF4-C12L for this experiment. We started this experiment using 6 mice, and finally analyzed 3 mice that showed strong antibody titers against immunogen. After repetitive immunization with CF4-C12L, antibodies against AFP-L3 were clearly detected in three mice, as measured by an increase in total immunoglobulin (Fig. 8). Particularly high reactivity to AFP-L3 relative to AFP-L1, the non-fucosylated α-fetoprotein isoform, was observed in one of the three mice (Fig. 8, No. 3). This result suggests that CF4-C12L could be an effective immunogen for practical use in the diagnosis and treatment of hepatocellular carcinoma. In further preliminary experiments using serum from mouse No. 3, IgM and IgG3 in which the κ light chain was detected were detected as the major serum immunoglobulins; other IgG subclasses were detected as minor components (Fig. S4). As the subclass of monoclonal antibodies obtained previously using a GSL with similar structure of CF4-conjugates was also IgG313, these results suggest that the immune responses induced by the CF4-conjugates may be a T-independent response.
Figure 8.
Titers of serum antibodies against AFP-L3 in CF4-C12L–immunized mice. Serum was prepared from mice immunized more than 4 times with CF4-C12L at 3 days (D3) or 7 days (D7) after the final immunization. BG, serum from non-treated mice. Total titer of serum immunoglobulin against AFP-L3 (closed squares with solid line) or AFP-L1 (open circles with dotted line) was determined by ELISA. Three mice (Nos. 1, 2, and 3) were analyzed.
Discussion
The results of this study demonstrate that altering the oligosaccharide structure of mammalian GSL analogues enhances their IgG-inducing activity. We previously found that both mammalian and artificial GSLs containing very-long-chain fatty acids induce the generation of IgG that reacts with these oligosaccharides as the epitope, but the IgG-inducing activity was relatively lower than the IgM-inducing activity12,13. In contrast, newly designed artGSL derivatives containing CF4 exhibited strong IgG-inducing activity, and the finding that certain structural features of the oligosaccharide portion of GSLs are associated with their IgG-inducing activity is novel. In previous studies of innate immunity using αGalCer analogues, elongation of the monosaccharides to oligosaccharides was thought to weaken the immunogenicity of the analogues28. However, our study demonstrates that certain oligosaccharides can induce a specific adaptive immune response, which overturns a conventional concept of mammalian immunology. This finding not only contributes to progress in immunology research but might also facilitate advances in drug development, such as the design of novel glycoconjugate immunogens and vaccines.
Of the artGSL oligosaccharide structures examined in this study, CF4 exhibited the highest IgG-inducing activity (Fig. 2). This activity was abolished by removal of the non-reducing terminal mannose (Fig. 5B), indicating that this structure is necessary for the IgG-inducing activity of artGSLs. IgG production by B cells involves class switching via APC-mediated interaction between B and T cells. As the mannose receptor, a C-type lectin present on the surface of APCs, recognizes the mannose structure of glycoconjugates29, APCs could recognize artGSLs containing mannose more efficiently. Furthermore, the very-long-chain fatty acid structure of αGalCer is known to play an important role in recognition involving CD1d, an antigen-presenting molecule expressed by APCs7,30–32. A previous model study also reported that very-long-chain alkyl structures of synthetic lipids are required for transport to lysosomes, where GSLs dock with CD1d33. These observations indicate that CF4-C12L and CF4-C182L possess several structural advantages that facilitate more-efficient recognition by APCs, resulting in strong induction of IgG responses.
Our results also indicate that GSLs containing very-long-chain fatty acids exhibit strong antibody-inducible activity in mice, where they can become self-antigens. Further elucidation of the physiologic functions of anti-GSL antibodies may resolve this issue. We previously reported that GSLs containing very-long-chain fatty acids derived from human vascular endothelial cells also exhibit potent antibody-inducing activity12,13, and the results of the present study demonstrate that the potent antibody-inducing activity of artGSLs containing very-long-chain fatty acid depends on the co-administration of lipid A, a component of lipopolysaccharide (LPS) (Fig. 7). In mice administered LPS, levels of GSLs containing very-long-chain fatty acids increase in vascular endothelial cells10,11,34,35, which is thought to result in the suppression of inflammation35. These results suggest the possibility that levels of GSLs containing very-long-chain fatty acids increase following infection with LPS-producing gram-negative bacteria, which in turn induces the production of IgM and IgG that exert anti-inflammatory effects in coordination with lipid A derived from LPS.
Tumor necrosis factor-α promotes the synthesis of GSLs containing very-long-chain fatty acids in human vascular endothelial cells through the transcriptional up-regulation of GSL synthesis–related genes10,11, including the ceramide synthase 2 gene. As mice deficient in this gene develop hepatocellular carcinoma36, ceramide synthase 2 is considered a tumor suppressor gene. Although the function of this gene in tumor suppression remains unclear, we speculate that in mammals, oncogenesis-associated up-regulation of ceramide synthase 2 promotes the synthesis of GSLs containing very-long-chain fatty acids and the production of antibodies that react with them, resulting in the suppression of oncogenesis. This hypothesis is also supported by the results of the present study demonstrating that the antibodies induced by immunization with CF4-C12L react with a glycoprotein specifically produced in hepatocellular carcinoma (Fig. 8). These results also indicate that CF4-C12L could serve as a seed compound in the development of vaccines against liver cancer.
In conclusion, the results of the present study revealed that artGSLs containing C12L and C182L can induce a specific adaptive immune response and the production of antibodies against the oligosaccharide as the epitope, and certain oligosaccharide structures, such as a terminal mannose, can enhance the IgG-inducing activity. As artGSLs incorporating these structural features efficiently induce the production of antibodies reactive with various oligosaccharides, they could be used to develop anti-glycoconjugate antibodies and vaccines for cancers associated with aberrant glycosylation.
Methods
Materials
Synthesis of artGSLs via organic chemistry was carried out by Wako Pure Chemical Industries, Ltd. (Osaka, Japan) and Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Details of the artGSL synthesis procedure are found in Japan patent 6143240 and United States patent US10307471B2. Lipid A from Salmonella enterica, fetuin from fetal calf serum, and thyroglobulin from porcine thyroid gland were obtained from Sigma-Aldrich (St. Louis, MO, USA). The presence of 6′-sialyl LacNAc in fetuin was confirmed as reported previously12, and the core fucose epitope of thyroglobulin was confirmed using horseradish peroxidase (HRP)-linked Lens culinaris agglutinin (LCA, J-OIL MILLS, Tokyo, Japan). AFP-L3 and AFP-L1 were obtained from Wako Pure Chemical Industries. Synthetic αGalCer was purchased from Tokyo Chemical Industry. Glycoprotein extraction was conducted according to a previously reported method37–39 with slight modifications. Glycoproteins containing sLeX and LeX epitopes were obtained by extraction from 1 × 107 HL-60 cells (provided by the RIKEN Cell Bank, Tsukuba, Japan) in 1 ml of 20 mM HEPES/0.25 M sucrose buffer (pH 7.5) supplemented with 0.1 mM phenylmethylsulfonyl fluoride and a proteinase inhibitor cocktail (Complete mini EDTA-free; Roche Diagnostics Gmbh, Mannheim, Germany) using a tight-fitting Dounce homogenizer (total of 25 strokes). After centrifugation of the homogenates at 800 g for 15 min at 4 °C, the supernatants were filtered using an Ultrafree-MC (0.45 μm) centrifugal device (Millipore, Billerica, MA, USA) at 13,000 g for 3 min at 4 °C. The filtrates were used as glycoprotein samples in subsequent experiments. The concentration of protein in each sample was determined using a Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA, USA). sLeX and LeX epitopes in this sample were confirmed by ELISA using anti-sLeX (KM93, Millipore) and anti-LeX antibodies (MC-480, R&D Systems, Minneapolis, MN, USA), respectively.
Immunization and preparation of serum
C3H/HeN mice (CLEA Japan, Tokyo) were used in this study. Mice were immunized with artGSLs according to a liposome immunization method12,22. In brief, 100 μg of artGSL was mixed with 10 μg of lipid A, 0.5 μmol of cholesterol, and 0.5 μmol of dipalmitoylphosphatidylcholine. The mixture was then dissolved in PBS and used as an immunogen. Mice were initially immunized subcutaneously and then intraperitoneally 2 weeks after the first immunization. Serum was prepared from blood collected from the tail vein 3 or 7 days after the second immunization. The Committee for Experiments Involving Animals of the National Institute of Advanced Industrial Science and Technology (AIST) approved all animal experiments, and all experiments were performed in accordance with relevant guidelines and regulations.
ELISAs
ELISAs were performed as described previously12. In brief, 500 ng of artGSL, 500 ng of AFP-L1 and -L3, and 1 μg of other glycoproteins was applied to the wells of a 96-well microtiter plate and were incubated overnight. After washing twice with PBS, blocking buffer (1% bovine serum albumin in PBS) was added to each well and incubated for 15 min at room temperature, followed by the addition of diluted serum (1:50 for IgG, 1:100 for IgM and total immunoglobulin). After incubation for 3 h at room temperature, the wells were washed with 0.1% Tween 20 in PBS, and then an HRP-linked secondary antibody (anti-IgM or anti-IgG or anti-Ig) was added. Antibody binding was detected using an HRP substrate (1-Step Ultra TMB-ELISA Substrate; Thermo Fisher Scientific, Waltham, MA, USA) and measurement of absorbance at 450 nm. The immobilization efficiency of 6′-sialyl LacNAc-C12L derivatives on the microtiter plate were shown in Fig. S5. The result indicates that C24 fatty acid structure is important for the efficiency but the sphingosine structures are hardly influenced to the efficiency.
Serum cytokine analysis
Liposomes composed of 0.5 μmol of cholesterol, 0.5 μmol of dipalmitoylphosphatidylcholine, and 10 μg of αGalCer or 100 μg of artGSL were dissolved in PBS and injected intraperitoneally into C3H/HeN mice. Blood was collected from the tail vein of mice at specific time points (see Fig. 6), and then serum samples were prepared. Levels of IL-4 and INF-γ in the serum were determined using mouse IL-4 and INF-γ ELISA kits (Thermo Fisher Scientific).
Characterization of serum immunoglobulin isotypes
Serum was diluted 500-fold with 1% bovine serum albumin in PBS, and the isotype of antibodies present in the serum was determined using a mouse monoclonal antibody isotyping kit (Roche Diagnostics GmbH) according to the manufacturer’s instructions.
Statistical analysis
After determination of variance using the F-test, the statistical significance of differences in data was evaluated using the two-tailed Student’s t-test, with statistical significance defined as follows: *P < 0.05, **P < 0.01, and ***P < 0.001. Samples with similar average values between groups were excluded from this test.
Supplementary information
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (JSPS KAKENHI Grant number 15H02907, 15K15201, and 19K11810) and THE JAPAN SCIENCE SOCIETY (Sasakawa Grants for Science Fellows Grant Number F19-234). We thank A. Fukui and I. Nagashima for technical assistance.
Author contributions
T.O. conceived, designed, performed, analyzed the experiments and wrote the paper. K.S. performed the ELISA experiment for AFP-L3. S.H. and M.D. prepared materials (CF4-C12L and C3-C12L).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
National Institute of Advanced Industrial Science and Technology (AIST) has a patent JP6143240 issued, a patent JP6172687 issued, a patent US9796785 issued, a patent US10307471 issued, a patent EP2993184 issued, and a patent EP14791926.0 pending, which relates to the materials reported in this article. Authors declare no other competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-55088-9.
References
- 1.Okuda T. Design of Carrier Molecules Suitable for Inducing Immunity to Oligosaccharide Antigens: Application to Anti-Glycoprotein Monoclonal Antibodies. Trends. Glycosci. Glycotechnol. 2018;30:E113–E116. doi: 10.4052/tigg.1762.1E. [DOI] [Google Scholar]
- 2.Koprowski H, Herlyn M, Steplewski Z, Sears HF. Specific antigen in serum of patients with colon carcinoma. Science. 1981;212:53–55. doi: 10.1126/science.6163212. [DOI] [PubMed] [Google Scholar]
- 3.Magnani JL, et al. A monosialoganglioside is a monoclonal antibody-defined antigen of colon carcinoma. Science. 1981;212:55–56. doi: 10.1126/science.7209516. [DOI] [PubMed] [Google Scholar]
- 4.Morita M, et al. Structure-activity relationship of a-galactosylceramides against B16-bearing mice. J. Med. Chem. 1995;38:2176–2187. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- 5.Kawano T, et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 6.Kinjo Y, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 7.Rossjohn J, Pellicci DG, Patel O, Gapin L, Godfrey D. Recognition of CD1d-restricted antigens by natural killer T cells. Nat. Rev. Immunol. 2012;12:845–857. doi: 10.1038/nri3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou D, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 9.Mattner J, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 10.Okuda T, Nakakita S, Nakayama K. Structural characterization and dynamics of globotetraosylceramide in vascular endothelial cells under TNF-α stimulation. Glycoconj. J. 2010;27:287–296. doi: 10.1007/s10719-009-9277-2. [DOI] [PubMed] [Google Scholar]
- 11.Okuda T. Data set for characterization of TNF-α-inducible glycosphingolipids in vascular endothelial cells. Data Brief. 2018;21:29–35. doi: 10.1016/j.dib.2018.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okuda T, Fukui A. Generation of anti-oligosaccharide antibodies that recognize mammalian glycoproteins by immunization with a novel artificial glycosphingolipid. Biochem. Biophys. Res. Commun. 2018;497:983–989. doi: 10.1016/j.bbrc.2018.02.113. [DOI] [PubMed] [Google Scholar]
- 13.Okuda T. Data on immunoglobulin G antibodies induced by immunization of mice with globoside carrying very long-chain fatty acids. Data Brief. 2018;19:256–260. doi: 10.1016/j.dib.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okuda T. PUGNAc treatment provokes globotetraosylceramide accumulation in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 2017;487:76–82. doi: 10.1016/j.bbrc.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Aoyagi Y, et al. Fucosylation of serum α-fetoprotein in patients with primary hepatocellular carcinoma. Biochim. Biophys. Acta. 1985;830:217–223. doi: 10.1016/0167-4838(85)90277-8. [DOI] [PubMed] [Google Scholar]
- 16.Walz G, Aruffo A, Kolanus W, Bevilacqua M, Seed B. Recognition by ELAM-1 of the sialyl-LeX determinant on myeloid and tumor cells. Science. 1990;250:1132–1135. doi: 10.1126/science.1701275. [DOI] [PubMed] [Google Scholar]
- 17.Maly P, et al. The α(1,3) fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/S0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- 18.Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc. Natl. Acad. Sci. USA. 2002;99:10231–10233. doi: 10.1073/pnas.172380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muramatsu T. Embryoglycan: a highly branched poly-N-acetyllactosamine in pluripotent stem cells and early embryonic cells. Glycoconj. J. 2017;34:701–712. doi: 10.1007/s10719-016-9673-3. [DOI] [PubMed] [Google Scholar]
- 20.Shields RL, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 21.Shinkawa T, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 2003;278:3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 22.Brodin T, Thurin J, Strömberg N, Karlsson KA, Sjögren HO. Production of oligosaccharide-binding monoclonal antibodies of diverse specificities by immunization with purified tumor-associated glycolipids inserted into liposomes with lipid A. Eur. J. Immunol. 1986;16:951–956. doi: 10.1002/eji.1830160813. [DOI] [PubMed] [Google Scholar]
- 23.Green ED, Adelt G, Baenziger JU, Wilson S, Van Halbeek H. The asparagine-linked oligosaccharides on bovine fetuin. Structural analysis of N-glycanase-released oligosaccharides by 500-megahertz 1H NMR spectroscopy. J. Biol. Chem. 1988;263:18253–18268. [PubMed] [Google Scholar]
- 24.Yamamoto K, Tsuji T, Irimura T, Osawa T. The structure of carbohydrate unit B of porcine thyroglobulin. Biochem. J. 1981;195:701–713. doi: 10.1042/bj1950701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 26.Borg NA, et al. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 27.Pellicci DG, et al. Differential recognition of CD1d-α-galactosyl ceramide by the Vβ8.2 and Vβ7 semi-invariant NKT T cell receptors. Immunity. 2009;31:47–59. doi: 10.1016/j.immuni.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinjo Y, et al. Natural Sphingomonas glycolipids vary greatly in their ability to activate natural killer T cells. Chem. Biol. 2008;15:654–664. doi: 10.1016/j.chembiol.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKenzie EJ, Su YP, Luisa MP. The mannose receptor, a bi-functional lectin with roles in homeostasis and immunity. Trends. Glycosci. Glycotechnol. 2002;14:273–283. doi: 10.4052/tigg.14.273. [DOI] [Google Scholar]
- 30.Goff RD, et al. Effects of lipid chain lengths in α-galactosylceramides on cytokine release by natural killer T cells. J. Am. Chem. Soc. 2004;126:13602–13603. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 31.Zajonc DM, et al. Structure and function of a potent agonist for the semi-invariant natural killer T cell receptor. Nat. Immunol. 2005;6:810–818. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koch M, et al. The crystal structure of human CD1d with and without α-galactosylceramide. Nat. Immunol. 2005;6:819–826. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 33.Mukherjee S, Soe TT, Maxfield FR. Endocytic sorting of lipid analogues differing solely in the chemistry of their hydrophobic tails. J. Cell. Biol. 1999;144:1271–1284. doi: 10.1083/jcb.144.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okuda T, et al. Targeted disruption of Gb3/CD77 synthase gene resulted in the complete deletion of globo-series glycosphingolipids and loss of sensitivity to verotoxins. J. Biol. Chem. 2006;281:10230–10235. doi: 10.1074/jbc.M600057200. [DOI] [PubMed] [Google Scholar]
- 35.Kondo Y, et al. TLR4-MD-2 complex is negatively regulated by an endogenous ligand, globotetraosylceramide. Proc. Natl. Acad. Sci. USA. 2013;110:4714–4719. doi: 10.1073/pnas.1218508110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imgrund S, et al. Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas. J. Biol. Chem. 2009;284:33549–33560. doi: 10.1074/jbc.M109.031971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okuda T, Morita N. A very low carbohydrate ketogenic diet prevents the progression of hepatic steatosis caused by hyperglycemia in a juvenile obese mouse model. Nutr. Diabetes. 2012;2:e50. doi: 10.1038/nutd.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okuda T, Fukui A, Morita N. Altered expression of O-GlcNAc-modified proteins in a mouse model whose glycemic status is controlled by a low carbohydrate ketogenic diet. Glycoconj. J. 2013;30:781–789. doi: 10.1007/s10719-013-9482-x. [DOI] [PubMed] [Google Scholar]
- 39.Okuda T. Western blot data using two distinct anti-O-GlcNAc monoclonal antibodies showing unique glycosylation status on cellular proteins under 2-deoxy-D-glucose treatment. Data Brief. 2017;10:449–453. doi: 10.1016/j.dib.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.